Abstract
The American Diabetes Association and the European Association for the Study of Diabetes convened a panel to update the previous consensus statements on the management of hyperglycemia in type 2 diabetes in adults, published since 2006 and last updated in 2019. The target audience is the full spectrum of the professional health care team providing diabetes care in the U.S. and Europe. A systematic examination of publications since 2018 informed new recommendations. These include additional focus on social determinants of health, the health care system, and physical activity behaviors, including sleep. There is a greater emphasis on weight management as part of the holistic approach to diabetes management. The results of cardiovascular and kidney outcomes trials involving sodium–glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists, including assessment of subgroups, inform broader recommendations for cardiorenal protection in people with diabetes at high risk of cardiorenal disease. After a summary listing of consensus recommendations, practical tips for implementation are provided.
Graphical Abstract
Introduction
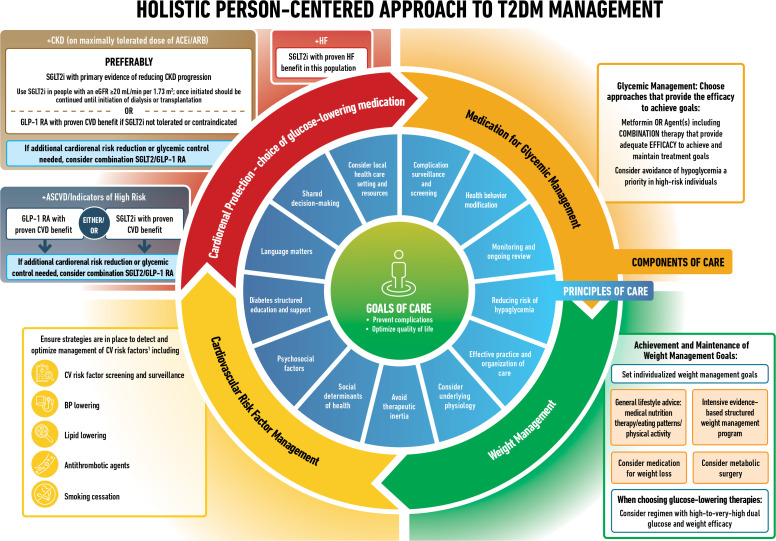
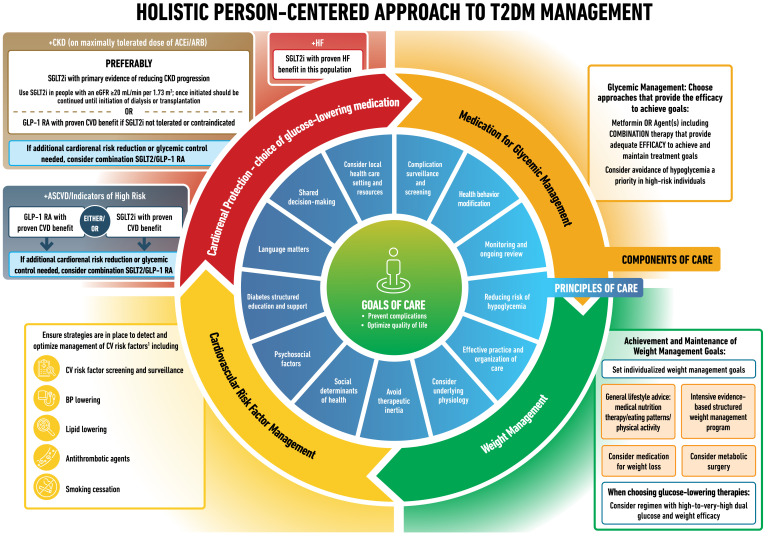
Type 2 diabetes is a chronic complex disease, and management requires multifactorial behavioral and pharmacological treatments to prevent or delay complications and maintain quality of life (Fig. 1). This includes management of blood glucose levels, weight, cardiovascular risk factors, comorbidities, and complications. This necessitates that care be delivered in an organized and structured way, such as described in the chronic care model, and includes a person-centered approach to enhance engagement in self-care activities (1). Careful consideration of social determinants of health and the preferences of people living with diabetes must inform individualization of treatment goals and strategies (2).
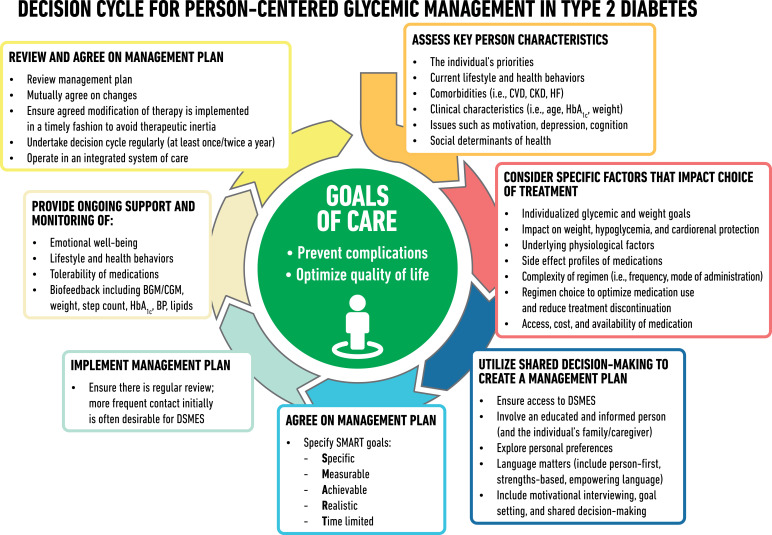
Figure 1.
Decision cycle for person-centered glycemic management in type 2 diabetes. Adapted from Davies et al. (5) with permission. BGM, blood glucose monitoring; BP, blood pressure; CGM, continuous glucose monitoring; CKD, chronic kidney disease; CVD, atherosclerotic cardiovascular disease; DSMES, diabetes self-management education and support; HF, heart failure.
This consensus report addresses the approaches to management of blood glucose levels in nonpregnant adults with type 2 diabetes. The principles and approach for achieving this are summarized in Fig. 1. These recommendations are not generally applicable to individuals with diabetes due to other causes, for example, monogenic diabetes, secondary diabetes, and type 1 diabetes, or to children.
Data Sources, Searches, and Study Selection
The writing group members were appointed by the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD). The group largely worked virtually, with regular teleconferences from September 2021, a 3-day workshop in January 2022, and a face-to-face 2-day meeting in April 2022. The writing group accepted the 2012 (3), 2015 (4), 2018 (5), and 2019 (6) editions of this consensus report as a starting point. To identify newer evidence, a search was conducted on PubMed for randomized control trials (RCTs), systematic reviews, and meta-analyses published in English between 28 January 2018 and 13 June 2022; eligible publications examined the effectiveness or safety of pharmacological or nonpharmacological interventions in adults with type 2 diabetes. Reference lists in eligible reports were scanned to identify additional relevant articles. Details of the keywords and the search strategy are available at https://data.mendeley.com/datasets/h5rcnxpk8w/2. Papers were grouped according to subject, and the authors reviewed this new evidence. Up-to-date meta-analyses evaluating the effects of therapeutic interventions across clinically important subgroup populations were assessed in terms of their credibility using relevant guidance (7,8). Evidence appraisal was informed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines on the formulation of clinical practice recommendations (9,10). The draft consensus recommendations were evaluated by invited reviewers and presented for public comment. Suggestions were incorporated as deemed appropriate by the authors (see Acknowledgments). Nevertheless, although evidence based with stakeholder input, the recommendations presented herein reflect the values and preferences of the consensus group.
The Rationale, Importance, and Context of Glucose-Lowering Treatment
Fundamental aspects of diabetes care include promoting healthy behaviors through medical nutrition therapy (MNT), physical activity, and psychological support, as well as weight management and tobacco/substance abuse counseling as needed. This is often delivered in the context of diabetes self-management education and support (DSMES). The expanding number of glucose-lowering interventions—from behavioral interventions to pharmacological interventions, devices, and surgery—and growing information about their benefits and risks provide more options for people with diabetes and providers but complicate decision-making. The demonstrated benefits for high-risk individuals with atherosclerotic cardiovascular disease (CVD), heart failure (HF), or chronic kidney disease (CKD) afforded by the glucagon-like peptide 1 receptor agonists (GLP-1 RA) and sodium–glucose cotransporter 2 inhibitors (SGLT2i) provide important progress in treatment aimed at reducing the progression and burden of diabetes and its complications. These benefits are largely independent of their glucose-lowering effects. These treatments were initially introduced as glucose-lowering agents but are now also prescribed for organ protection. In this consensus report, we summarize a large body of recent evidence for practitioners in the U.S. and Europe with the aim of simplifying clinical decision-making and focusing our efforts on providing holistic person-centered care.
Attaining recommended glycemic targets yields substantial and enduring reductions in the onset and progression of microvascular complications (11,12), and early intervention is essential (13). The greatest absolute risk reduction comes from improving very elevated glycemic levels, and a more modest reduction results from near normalization of plasma glucose levels (2,14). The impact of glucose control on macrovascular complications is less certain but is supported by multiple meta-analyses and epidemiological studies. Because the benefits of intensive glucose control emerge slowly while the harms can be immediate, people with longer life expectancy have more to gain from early intensive glycemic management. A reasonable HbA1c target for most nonpregnant adults with sufficient life expectancy to see microvascular benefits (generally ∼10 years) is around 53 mmol/mol (7%) or less (2). Aiming for a lower HbA1c level than this may have value if it can be achieved safely without significant hypoglycemia or other adverse treatment effects. A lower target may be reasonable, particularly when using pharmacological agents that are not associated with hypoglycemic risk. Higher targets can be appropriate in cases of limited life expectancy, advanced complications, or poor tolerability or if other factors such as frailty are present. Thus, glycemic treatment targets should be tailored based on an individual’s preferences and characteristics, including younger age (i.e., age <40 years), risk of complications, frailty and comorbid conditions (2,15–17), and the impact of these features on the risk of adverse effects of therapy (e.g., hypoglycemia and weight gain).
Principles of Care
Language Matters
Communication between people living with type 2 diabetes and health care team members is at the core of integrated care, and clinicians must recognize how language matters. Language in diabetes care should be neutral, free of stigma, and based on facts; be strength-based (focus on what is working), respectful, and inclusive; encourage collaboration; and be person-centered (18). People living with diabetes should not be referred to as “diabetics” or described as “noncompliant” or blamed for their health condition.
Diabetes Self-Management Education and Support
DSMES is a key intervention, as important to the treatment plan as the selection of pharmacotherapy (19–21). DSMES is central to establishing and implementing the principles of care (Fig. 1). DSMES programs usually involve face-to-face contact in group or individual sessions with trained educators, and key components of DSMES are shown in Supplementary Table 1 (19–24). Given the ever-changing nature of type 2 diabetes, DSMES should be offered on an ongoing basis. Critical junctures when DSMES should be provided include at diagnosis, annually, when complications arise, and during transitions in life and care (Supplementary Table 1) (22).
High-quality evidence has consistently shown that DSMES significantly improves knowledge, glycemic levels, and clinical and psychological outcomes, reduces hospital admissions and all-cause mortality, and is cost-effective (22,25–30). DSMES is delivered through structured educational programs provided by trained diabetes care and education specialists (termed DCES in the U.S.; hereafter referred to as diabetes educators) that focus particularly on the following: lifestyle behaviors (healthy eating, physical activity, and weight management), medication-taking behavior, self-monitoring when needed, self-efficacy, coping, and problem solving.
Importantly, DSMES is tailored to the individual’s context, which includes their beliefs and preferences. DSMES can be provided using multiple approaches and in a variety of settings (20,31), and it is important for the care team to know how to access local DSMES resources. DSMES supports the psychosocial care of people with diabetes but is not a replacement for referral for mental health services when they are warranted, for example, when diabetes distress remains after DSMES. Psychiatric disorders, including disordered eating behaviors, are common, often unrecognized, and contribute to poor outcomes in diabetes (32).
The best outcomes from DSMES are achieved through programs with a theory-based and structured curriculum and with contact time of over 10 h (26). While online programs may reinforce learning, a comprehensive approach to education using multiple methods may be more effective (26). Emerging evidence demonstrates the benefits of telehealth or web-based DSMES programs (33), and these were used with success during the coronavirus disease 2019 (COVID-19) pandemic (34–36). Technologies such as mobile apps, simulation tools, digital coaching, and digital self-management interventions can be used to deliver DSMES and extend its reach to a broader segment of the population with diabetes and provide comparable or even better outcomes (37). Greater HbA1c reductions are demonstrated with increased engagement of people with diabetes (35,38). However, data from trials of digital strategies to support behavior change are still preliminary in nature and quite heterogeneous (22,37).
Individualized and Personalized Approach
Type 2 diabetes is a very heterogeneous disease with variable age at onset, related degree of obesity, insulin resistance, and tendency to develop complications (39,40). Providing person-centered care that addresses multimorbidity and is respectful of and responsive to individual preferences and barriers, including the differential costs of therapies, is essential for effective diabetes management (41). Shared decision-making, facilitated by decision aids that show the absolute benefit and risk of alternative treatment options, is a useful strategy to determine the best treatment course for an individual (42–45). With compelling indications for therapies such as SGLT2i and GLP-1 RA for high-risk individuals with CVD, HF, or CKD, shared decision-making is essential to contextualize the evidence on benefits, safety, and risks. Providers should evaluate the impact of any suggested intervention in the context of cognitive impairment, limited literacy, distinct cultural beliefs, and individual fears or health concerns. The health care system is an important factor in the implementation, evaluation, and development of the personalized approach. Furthermore, social determinants of health—often out of direct control of the individual and potentially representing lifelong risk—contribute to medical and psychosocial outcomes and must be addressed to improve health outcomes. Five social determinants of health areas have been identified: socioeconomic status (education, income, and occupation), living and working conditions, multisector domains (e.g., housing, education, and criminal justice system), sociocultural context (e.g., shared cultural values, practices, and experiences), and sociopolitical context (e.g., societal and political norms that are root-cause ideologies and policies underlying health disparities) (46). More granularity on social determinants of health as they pertain to diabetes is provided in a recent ADA review (47), with a particular focus on the issues faced in the African American population provided in a subsequent report (48). Environmental, social, behavioral, and emotional factors, known as psychosocial factors, also influence living with diabetes and achieving satisfactory medical outcomes and psychological well-being. Thus, these multifaceted domains (heterogeneity across individual characteristics, social determinants of health, and psychosocial factors) challenge individuals with diabetes, their families, and their providers when attempting to integrate diabetes care into daily life (49).
Current principles of, and approaches to, person-centered care in diabetes (Fig. 1) include assessing key characteristics and preferences to determine individualized treatment goals and strategies. Such characteristics include comorbidities, clinical characteristics, and compelling indications for GLP-1 RA or SGLT2i for organ protection (6).
Weight Reduction as a Targeted Intervention
Weight reduction has mostly been seen as a strategy to improve HbA1c and reduce the risk for weight-related complications. However, it was recently suggested that weight loss of 5–15% should be a primary target of management for many people living with type 2 diabetes (50). A higher magnitude of weight loss confers better outcomes. Weight loss of 5–10% confers metabolic improvement; weight loss of 10–15% or more can have a disease-modifying effect and lead to remission of diabetes (50), defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report (51). Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life (50).
Glucose Management: Monitoring
Glycemic management is primarily assessed with the HbA1c test, which was the measure used in trials demonstrating the benefits of glucose lowering (2,52). As with any laboratory test, HbA1c measurement has limitations (2,52). There may be discrepancies between HbA1c results and an individual’s true mean blood glucose levels, particularly in certain racial and ethnic groups and in conditions that alter erythrocyte turnover, such as anemia, end-stage kidney disease (especially with erythropoietin therapy), and pregnancy, or if an HbA1c assay insensitive to hemoglobin variants is used in someone with a hemoglobinopathy. Discrepancies between measured HbA1c levels and measured or reported glucose levels should prompt consideration that one of these may not be reliable (52,53).
Regular blood glucose monitoring (BGM) may help with self-management and medication adjustment, particularly in individuals taking insulin. BGM plans should be individualized. People with type 2 diabetes and the health care team should use the monitoring data in an effective and timely manner. In people with type 2 diabetes not using insulin, routine glucose monitoring is of limited additional clinical benefit while adding burden and cost (54,55). However, for some individuals, glucose monitoring can provide insight into the impact of lifestyle and medication management on blood glucose and symptoms, particularly when combined with education and support (53). Technologies such as intermittently scanned or real-time continuous glucose monitoring (CGM) provide more information and may be useful for people with type 2 diabetes, particularly in those treated with insulin (53,56).
When using CGM, standardized, single-page glucose reports, such as the ambulatory glucose profile, can be uploaded from CGM devices. They should be considered standard metrics for all CGM devices and provide visual cues for management opportunities. Time in range is defined as the percentage of time that CGM readings are in the range 3.9–10.0 mmol/L (70–180 mg/dL). Time in range is associated with the risk of microvascular complications and can be used for assessment of glycemic management (57). Additionally, time above and below range are useful variables for the evaluation of treatment regimens. Particular attention to minimizing the time below range in those with hypoglycemia unawareness may convey benefit. If using the ambulatory glucose profile to assess glycemic management, a goal parallel to an HbA1c level of <53 mmol/mol (<7%) for many is time in range of >70%, with additional recommendations to aim for time below range of <4% and time at <3.0 mmol/L (<54 mg/dL) of <1% (2).
Treatment Behaviors, Persistence, and Adherence
Suboptimal medication-taking behavior and low rates of continued medication use, or what is termed “persistence to therapy plans,” affects almost half of people with type 2 diabetes, leading to suboptimal glycemic and CVD risk factor control as well as increased risks of diabetes complications, mortality, and hospital admissions and increased health care costs (58–62). Although this consensus report focuses on medication-taking behavior, the principles are pertinent to all aspects of diabetes care. Multiple factors contribute to inconsistent medication use and treatment discontinuation among people with diabetes, including perceived lack of medication efficacy, fear of hypoglycemia, lack of access to medication, and adverse effects of medication (63). Focusing on facilitators of adherence, such as social/family/provider support, motivation, education, and access to medications/foods, can provide benefits (64). Observed rates of medication adherence and persistence vary across medication classes and between agents; careful consideration of these differences may help improve outcomes (61). Ultimately, individual preferences are major factors driving the choice of medications. Even when clinical characteristics suggest the use of a particular medication based on the available evidence from clinical trials, preferences regarding route of administration, injection devices, side effects, or cost may prevent use by some individuals (65).
Therapeutic Inertia
Therapeutic (or clinical) inertia describes a lack of treatment intensification when targets or goals are not met. It also includes failure to de-intensify management when people are overtreated. The causes of therapeutic inertia are multifactorial, occurring at the levels of the practitioner, person with diabetes, and/or health care system (66). Interventions targeting therapeutic inertia have facilitated improvements in glycemic management and timely insulin intensification (67,68). For example, the involvement of multidisciplinary teams that include nonphysician providers with authorization to prescribe (e.g., pharmacists, specialist nurses, and advanced practice providers) may reduce therapeutic inertia (69,70).
Therapeutic Options: Lifestyle and Healthy Behavior, Weight Management, and Pharmacotherapy for the Treatment of Type 2 Diabetes
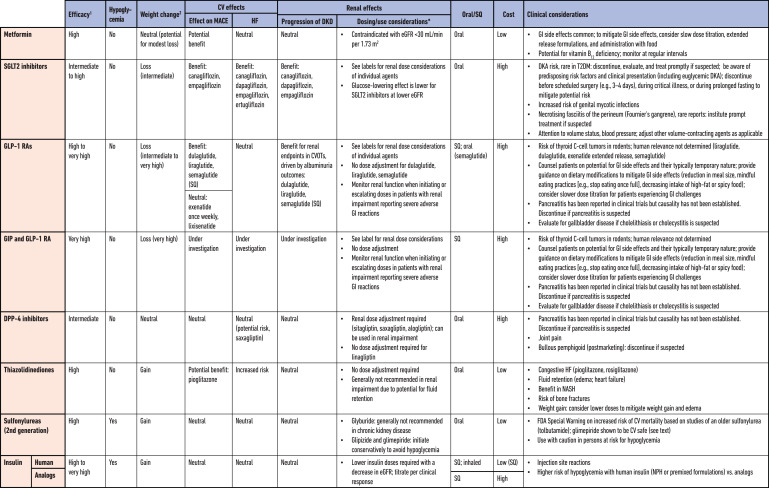
This section summarizes the lifestyle and behavioral therapy, weight management interventions, and pharmacotherapy that support glycemic management in people with type 2 diabetes. Specific pharmacological treatment options are summarized in Table 1. Additional details are available in the previous ADA/EASD consensus report and update (5,6) and the ADA’s 2022 Standards of Medical Care in Diabetes (71).
Table 1.
Medications for lowering glucose, summary of characteristics
CV, cardiovascular; CVOT, cardiovascular outcomes trial; DKA, diabetic ketoacidosis; DKD, diabetic kidney disease; DPP-4, dipeptidyl peptidase 4; eGFR, estimated glomerular filtration rate; GI, gastrointestinal; GIP, gastric inhibitory polypeptide; GLP-1 RA, glucagon-like peptide 1 receptor agonist; HF, heart failure; NASH, nonalcoholic steatohepatitis; MACE, major adverse cardiovascular events; SGLT2, sodium-glucose cotransporter 2; SQ, subcutaneous; T2DM, type 2 diabetes mellitus.
Nutrition Therapy
Nutrition therapy is integral to diabetes management, with goals of promoting and supporting healthy eating patterns, addressing individual nutrition needs, maintaining the pleasure of eating, and providing the person with diabetes with the tools for developing healthy eating (22). MNT provided by a registered dietitian/registered dietitian nutritionist complements DSMES, can significantly reduce HbA1c, and can help prevent, delay, and treat comorbidities related to diabetes (19). Two core dimensions of MNT that can improve glycemic management include dietary quality and energy restriction.
Dietary Quality and Eating Patterns
There is no single ratio of carbohydrate, proteins, and fat intake that is optimal for every person with type 2 diabetes. Instead, individually selected eating patterns that emphasize foods with demonstrated health benefits, minimize foods shown to be harmful, and accommodate individual preferences with the goal of identifying healthy dietary habits that are feasible and sustainable are recommended. A net energy deficit that can be maintained is important for weight loss (5,6,22,72–74).
A network analysis comparing trials of nine dietary approaches of >12 weeks’ duration demonstrated reductions in HbA1c from –9 to –5.1 mmol/mol (–0.82% to –0.47%) with all approaches compared with a control diet. Greater glycemic benefits were seen with the Mediterranean diet and low-carbohydrate diet (75). The greater glycemic benefits of low-carbohydrate diets (<26% of energy) at 3 and 6 months are not evident with longer follow-up (72). In a systematic review of trials of >6 months’ duration, compared with a low-fat diet, the Mediterranean diet demonstrated greater reductions in body weight and HbA1c levels, delayed the requirement for diabetes medication, and provided benefits for cardiovascular health (76,77). Similar benefits have been ascribed to vegan and vegetarian diets (78).
There has been increased interest in time-restricted eating and intermittent fasting to improve metabolic variables, although with mixed, and modest, results. In a meta-analysis there were no differences in the effect of intermittent fasting and continuous energy restriction on HbA1c, with intermittent fasting having a modest effect on weight (–1.70 kg) (79). In a 12-month RCT in adults with type 2 diabetes comparing intermittent energy restriction (2,092–2,510 kJ [500–600 kcal] diet for 2 nonconsecutive days/week followed by the usual diet for 5 days/week) with continuous energy restriction (5,021–6,276 kJ [1,200–1,500 kcal] diet for 7 days/week), glycemic improvements were comparable between the two groups. At 24 months’ follow-up, HbA1c increased in both groups to above baseline (80), while weight loss (–3.9 kg) was maintained in both groups (81). Fasting may increase the rates of hypoglycemia in those treated with insulin and sulfonylureas, highlighting the need for individualized education and proactive medication management during significant dietary changes (82).
Nonsurgical Energy Restriction for Weight Loss
An overall healthy eating plan that results in an energy deficit, in conjunction with medications and/or metabolic surgery as individually appropriate, should be considered to support glycemic and weight management goals in adults with type 2 diabetes (5,22). Structured nutrition and lifestyle programs may be considered for glycemic benefit and can be adapted for specific cultural indications (83–87).
The Diabetes Remission Clinical Trial (DiRECT) demonstrated greater remission of diabetes with a weight management program than with usual best practice care in adults with type 2 diabetes within 6 years of diagnosis. The structured, primary care-led intensive weight management program involved total diet replacement (3,452–3,569 kJ/day [825–853 kcal/day] for 3–5 months) followed by stepped food reintroduction and structured support for long-term weight loss maintenance. In the whole study population, remission directly varied with degree of weight loss (88). At the 2-year follow-up, sustained remission correlated with extent of sustained weight loss. In the whole study population, of those maintaining at least 10 kg weight loss, 64% achieved diabetes remission. However, only 24% of the participants in the intervention group maintained at least 10 kg weight loss, highlighting both the potential and the challenges of long-term durability of weight loss (89).
The Look AHEAD: Action for Health in Diabetes (Look AHEAD) trial on the longer-term effects of an intensive lifestyle intervention in adults who were overweight/obese with type 2 diabetes showed improvements in diabetes control and complications, depression, physical function, health-related quality of life, sleep apnea, incontinence, brain structure, and health care use and costs, with positive impacts on composite indices of multimorbidity, geriatric syndromes, and disability-free life-years. This should be balanced against potential negative effects on body composition, bone density, and frailty fractures (90,91). Although there was no difference in the primary cardiovascular outcome or mortality rate between the intervention and the control groups, post hoc exploratory analyses suggested potential benefits in certain groups (e.g., in those who achieved at least 10% weight loss in the first year of the study). Progressive metabolic benefits were seen with greater degrees of weight loss from >5% to ≥15%, with an overall suggestion that ≥10% weight loss may be required to see benefits for CVD events and mortality rate and other complications, such as nonalcoholic steatohepatitis (50,90,92–95).
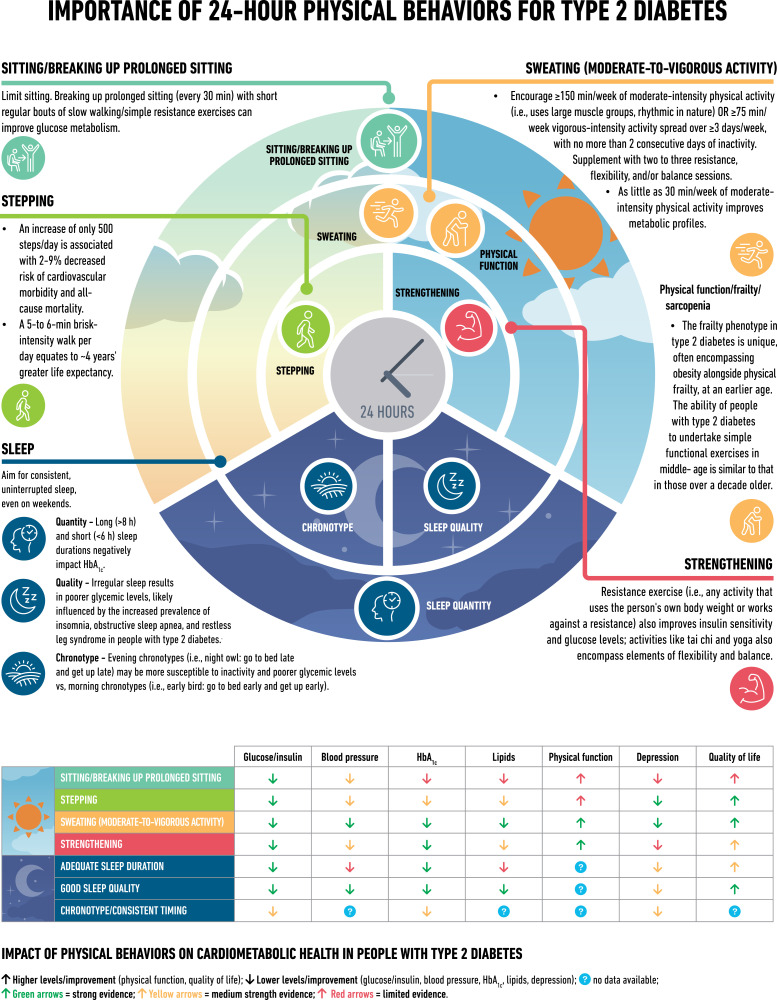
Physical Activity Behaviors, Including Sleep
Physical activity behaviors significantly impact cardiometabolic health in type 2 diabetes (Fig. 2) (96–117). Regular aerobic exercise (i.e., involving large muscle groups and rhythmic in nature) improves glycemic management in adults with type 2 diabetes, resulting in less daily time in hyperglycemia and reductions of ∼7 mmol/mol (∼0.6%) in HbA1c (118), and induces clinically significant benefits in cardiorespiratory fitness (101,110,119). These glycemic effects can be maximized by undertaking activity during the postprandial period and engaging in activities for ≥45 min (101,120). Resistance exercise (i.e., using your own body weight or working against a resistance) also improves blood glucose levels, flexibility, and balance (101,110). This is important given the increased risk of impaired physical function at an earlier age in type 2 diabetes (112).
Figure 2.
Importance of 24-h physical behaviors for type 2 diabetes.
A wide range of physical activities, including leisure time activities, can significantly reduce HbA1c levels (5,22,121,122). Even small, regular changes can make a difference to long-term health, with an increase of only 500 steps/day associated with 2–9% decreased risk of cardiovascular morbidity and all-cause mortality rates (105–107). Beneficial effects are evident across the continuum of human movement, from breaking prolonged sitting with light activity (103) to high-intensity interval training (123).
Sleep
Healthy sleep is considered a key lifestyle component in the management of type 2 diabetes (124), with clinical practice guidelines promoting the importance of sleep hygiene (113). Sleep disorders are common in type 2 diabetes and cause disturbances in the quantity, quality, and timing of sleep and are associated with an increased risk of obesity and impairments in daytime functioning and glucose metabolism (114,115). Additionally, obstructive sleep apnea affects over half of people with type 2 diabetes, and its severity is associated with blood glucose levels (115,116).
The quantity of sleep is known to be associated (in a U-shaped manner) with health outcomes (e.g., obesity and HbA1c), with both long (>8 h) and short (<6 h) sleep durations having negative impacts (97). By extending the sleep duration of short sleepers, it is possible to improve insulin sensitivity and reduce energy intake (117,125). However, “catch-up” weekend sleep alone is not enough to reverse the impact of insufficient sleep (126).
Weight Management Beyond Lifestyle Interventions
Medications for Weight Loss in Type 2 Diabetes
Weight loss medications are effective adjuncts to lifestyle interventions and healthy behaviors for management of weight and have also been found to improve glucose control in people with diabetes (127).
Newer therapies have demonstrated very high efficacy for weight management in people with type 2 diabetes. In the Semaglutide Treatment Effect in People with Obesity 2 (STEP 2) trial, subcutaneous semaglutide 2.4 mg once a week as an adjunct to a lifestyle intervention performed better than either semaglutide 1.0 mg or placebo, with weight loss of 9.6% (6.2% more than with placebo and 2.7% more than with semaglutide 1.0 mg). More than two-thirds of participants in the semaglutide 2.4-mg arm achieved an HbA1c level of ≤48 mmol/mol (≤6.5%) (128). However, the weight loss was less pronounced than the 14.9% weight loss (vs. 2.4% with placebo) seen in the STEP 1 trial in adults with overweight or obesity without diabetes (129). Tirzepatide, a novel glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 RA, at weekly doses of 5 mg, 10 mg, and 15 mg, reduced body weight by 15%, 19.5%, and 20.9%, respectively, compared with 3.1% with placebo at 72 weeks in people with obesity but without diabetes; however, tirzepatide has not yet been approved for weight management by regulatory authorities (130). Studies in adults with overweight or obesity suggest that withdrawing treatment with semaglutide leads to increases in body weight (131), highlighting the chronic nature of, and need for, obesity/weight management.
Metabolic Surgery
Metabolic surgery should be considered as a treatment option in adults with type 2 diabetes who are appropriate surgical candidates (127,132). Metabolic surgery also appears to be effective for diabetes remission in people with type 2 diabetes and a BMI ≥25 kg/m2, although efficacy for both weight loss and diabetes remission appears to vary by surgical type (133–135). One mixed-effects meta-analysis model has estimated a 43% diabetes remission rate (95% CI 34%, 53%) following metabolic surgery in people with type 2 diabetes and a BMI <30 kg/m2 (136), significantly higher than that achieved with traditional medical management (137). However, there is a strong association between duration of diabetes and the likelihood of postoperative diabetes remission. People with more recently diagnosed diabetes are more likely to experience remission after metabolic surgery, and the likelihood of remission decreases significantly with duration of diabetes longer than about 5–8 years (138). Even in people with diabetes who do not achieve postoperative diabetes remission, or relapse after initial remission, metabolic surgery is associated with better metabolic control than medical management (137,139). In the Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently (STAMPEDE) trial, metabolic surgery was also associated with improvements in patient-reported outcomes related to physical health; however, measures of social and psychological quality of life did not improve (140). It is important to note that many of these estimates of benefit included data from nonrandomized studies and compared outcomes with medical treatments for obesity that were less effective than those available today.
Medications For Lowering Glucose
Cardiorenal-Protective Glucose-Lowering Medications
SGLT2i.
The SGLT2i are oral medications that reduce plasma glucose by enhancing urinary excretion of glucose. They have intermediate-to-high glycemic efficacy, with lower glycemic efficacy at lower estimated glomerular filtration rate (eGFR). However, their scope of use has significantly expanded based on cardiovascular and renal outcome studies (5,141). Cardiorenal outcome trials have demonstrated their efficacy in reducing the risk of composite major adverse cardiovascular events (MACE), cardiovascular death, myocardial infarction, hospitalization for heart failure (HHF), and all-cause mortality and improving renal outcomes in individuals with type 2 diabetes with an established/high risk of CVD. This is discussed in the section Personalized Approach to Treatment Based on Individual Characteristics and Comorbidities: Recommended Process for Glucose-Lowering Medication Selection. Evidence supporting their use is summarized in Table 1 (141,142).
Recent data have increased confidence in the safety of the SGLT2i drug class (141,142). Their use is associated with increased risk for mycotic genital infections, which are reported to be typically mild and treatable. While SGLT2i use can increase the risk of diabetic ketoacidosis (DKA), the incidence is low, with a modest incremental absolute risk (142). The SGLT2i cardiovascular outcome trials (CVOTs) have reported DKA rates of 0.1–0.6% compared with rates of <0.1–0.3% with placebo (143–147), with very low rates in the HF (148–151) and CKD (152,153) outcome studies. Risk can be mitigated with education and guidance, including education on signs and symptoms of DKA that should prompt medical attention, and temporary discontinuation of the medication in clinical situations that predispose to ketoacidosis (e.g., during prolonged fasting and acute illness and perioperatively, i.e., 3 days prior to surgery) (154–158). The Dapagliflozin in Respiratory Failure in Patients With COVID-19 (DARE-19) RCT demonstrated a low risk of DKA (0.3% vs. 0% in dapagliflozin-treated vs. placebo-treated participants) with structured monitoring of acid–base balance and kidney function during inpatient use in adults admitted with COVID-19 and at least one cardiometabolic risk factor without evidence of critical illness (159).
While early studies brought attention to several safety areas of interest (acute kidney injury, dehydration, orthostatic hypotension, amputation, and fractures) (5,6), longer-term studies that have prospectively assessed and monitored these events (160,161) have not seen a significant imbalance in risks. Analyses of SGLT2i outcome trial data also suggest that people with type 2 diabetes and peripheral arterial disease derive greater absolute outcome benefits from SGLT2i therapy than those without peripheral arterial disease, without an increase in risk of major adverse limb events (162). In post hoc analyses, SGLT2i use has been associated with reduced incidence of serious and nonserious kidney-related adverse events in people with type 2 diabetes and CKD and greater full recovery from acute kidney injury (163).
GLP-1 RA.
GLP-1 RA augment glucose-dependent insulin secretion and glucagon suppression, decelerate gastric emptying, curb postmeal glycemic increments, and reduce appetite, energy intake, and body weight (5,6,164). Beyond improving HbA1c in adults with type 2 diabetes, specific GLP-1 RA have also been approved for reducing risk of MACE in adults with type 2 diabetes with established CVD (dulaglutide, liraglutide, and subcutaneous semaglutide) or multiple cardiovascular risk factors (dulaglutide) (Table 1) and for chronic weight management (subcutaneous liraglutide titrated to 3.0 mg once daily; subcutaneous semaglutide titrated to 2.4 mg once weekly). This is discussed in the sections Medications for Weight Loss in Type 2 Diabetes and Personalized Approach to Treatment Based on Individual Characteristics and Comorbidities: Recommended Process for Glucose-Lowering Medication Selection. GLP-1 RA are primarily available as injectable therapies (subcutaneous administration), with one oral GLP-1 RA now available (oral semaglutide) (165).
The recent higher-dose GLP-1 RA studies have indicated incremental benefits for glucose and weight at higher doses of GLP-1 RA, with greater proportions of people achieving glycemic targets and the ability of stepwise dose escalation to improve gastrointestinal tolerability. The Assessment of Weekly Administration of LY2189265 (dulaglutide) in Diabetes 11 (AWARD-11) trial evaluated higher doses of dulaglutide (3.0 mg and 4.5 mg weekly) compared with 1.5 mg weekly, demonstrating superior HbA1c reductions (−19.4 vs. −16.8 mmol/mol [–1.77 vs. −1.54%], estimated treatment difference [ETD] –2.6 mmol/mol [−0.24%]) and weight loss (–4.6 vs. –3.0 kg, ETD –1.6 kg) with dulaglutide 4.5 mg compared with 1.5 mg at 36 weeks in people with type 2 diabetes inadequately controlled with metformin (166). Likewise, the SUSTAIN FORTE trial studied higher doses of once-weekly subcutaneous semaglutide (2.0 mg) compared with the previously approved 1.0 mg dose, reporting a mean change in HbA1c from baseline to week 40 of −23 vs. −21 mmol/mol (–2.1 vs. −1.9%, ETD –2 mmol/mol [−0.18%]) and weight change of –6.4 kg with semaglutide 2.0 mg and –5.6 kg with semaglutide 1.0 mg (ETD –0.77 kg [95% CI –1.55, 0.01]) (167).
The most common side effects of GLP-1 RA are gastrointestinal in nature (nausea, vomiting, and diarrhea) and tend to occur during initiation and dose escalation and diminish over time. Gradual up-titration is recommended to mitigate gastrointestinal effects (164,168,169). Education should be provided when initiating GLP-1 RA therapy. GLP-1 RA promote a sense of satiety, facilitating reduction in food intake. It is important to help people distinguish between nausea, a negative sensation, and satiety, a positive sensation that supports weight loss. Mindful eating should be encouraged: eating slowly, stopping eating when full and not eating when not hungry. Smaller meals or snacks, decreasing intake of high-fat and spicy foods, moderating alcohol intake, and increasing water intake are also recommended. Slower or flexible dose escalations can be considered in the setting of gastrointestinal intolerance (168,169).
Data from CVOTs on other safety areas of interest (pancreatitis, pancreatic cancer, and medullary thyroid cancer) indicate that there is no increase in these risks with GLP-1 RA. GLP-1 RA are contraindicated in people at risk for the rare medullary thyroid cancer (164), that is, those with a history or family history of medullary thyroid cancer or multiple endocrine neoplasia type 2, due to thyroid C-cell tumors seen in rodents treated with GLP-1 RA in preclinical studies. Increased retinopathy complications seen in the SUSTAIN 6 CVOT appear attributable to the magnitude and rapidity of HbA1c reductions in individuals with pre-existing diabetic retinopathy and high glycemic levels, as has been seen in previous studies with insulin (170,171). GLP-1 RA are also associated with higher risks of gallbladder and biliary diseases (172).
Other Glucose-Lowering Medications
Metformin.
Because of its high efficacy in lowering HbA1c, minimal hypoglycemia risk when used as monotherapy, weight neutrality with the potential for modest weight loss, good safety profile, and low cost, metformin has traditionally been recommended as first-line glucose-lowering therapy for the management of type 2 diabetes. However, there is ongoing acceptance that other approaches may be appropriate. Notably, the benefits of GLP-1 RA and SGLT2i for cardiovascular and renal outcomes have been found to be independent of metformin use, and thus these agents should be considered in people with established or high risk of CVD, HF, or CKD, independent of metformin use (173–175). Early combination therapy based on the perceived need for additional glycemic efficacy or cardiorenal protection can be considered at treatment initiation to extend the time to treatment failure (176). Metformin should not be used in people with an eGFR <30 ml/min per 1.73 m2, and dose reduction should be considered when the eGFR is <45 ml/min per 1.73 m2 (177). Metformin use may result in lower serum vitamin B12 concentrations and worsening of symptoms of neuropathy; therefore, periodic monitoring and supplementation are generally recommended if levels are deficient, particularly in those with anemia or neuropathy (178,179).
Dipeptidyl Peptidase 4 Inhibitors.
Dipeptidyl peptidase 4 inhibitors (DPP-4i) are oral medications that inhibit the enzymatic inactivation of endogenous incretin hormones, resulting in glucose-dependent insulin release and a decrease in glucagon secretion. They have a more modest glucose-lowering efficacy and a neutral effect on weight and are well tolerated with minimal risk of hypoglycemia. CVOTs have demonstrated the cardiovascular safety without cardiovascular risk reduction of four DPP-4i (saxagliptin, alogliptin, sitagliptin, and linagliptin) (141). Reductions in risk of albuminuria progression were noted with linagliptin in the Cardiovascular and Renal Microvascular Outcome Study With Linagliptin (CARMELINA) trial (180). While generally well tolerated, an increased risk of HHF was found with saxagliptin, which is reflected in its label, and there have been rare reports of arthralgia and hypersensitivity reactions with the DPP-4i class (16).
The high tolerability and modest efficacy of DPP-4i may mean that they are suitable for specific populations and considerations. For example, in a 6-month open-label RCT comparing a DPP-4i (linagliptin) with basal insulin (glargine) in long-term care and skilled nursing facilities, mean daily blood glucose was similar, with fewer hypoglycemic events with linagliptin compared with insulin (181). Treatment of inpatient hyperglycemia with basal insulin plus DPP-4i has been demonstrated to be effective and safe in older adults with type 2 diabetes, with similar mean daily blood glucose but lower glycemic variability and fewer hypoglycemic episodes compared with the basal–bolus insulin regimen (182).
Glucose-Dependent Insulinotropic Polypeptide and GLP-1 RA.
In May 2022, the U.S. Food and Drug Administration (FDA) approved tirzepatide, a GIP and GLP-1 RA, for once-weekly subcutaneous administration to improve glucose control in adults with type 2 diabetes as an addition to healthy eating and exercise. In the Phase III clinical trial program, tirzepatide demonstrated superior glycemic efficacy to placebo (183,184), subcutaneous semaglutide 1.0 mg weekly (185), insulin degludec (186), and insulin glargine (187). For HbA1c, placebo-adjusted reductions of 21 mmol/mol (1.91%), 21 mmol/mol (1.93%), and 23 mmol/mol (2.11%) were demonstrated with tirzepatide 5, 10, and 15 mg weekly, respectively, and mean weight reductions of 7–9.5 kg were seen (183). Additional metabolic benefits included improvements in liver fat content and reduced visceral and subcutaneous abdominal adipose tissue volume (188). Based on meta-analysis findings, tirzepatide was superior to its comparators, including other long-acting GLP-1 RA, in reducing glucose and body weight, but was associated with increased odds for gastrointestinal adverse events, in particular nausea (189). Similar warnings and precautions are included in the prescribing information for tirzepatide as for agents in the GLP-1 RA class. Additionally, current short-term data from RCTs suggest that tirzepatide does not increase the risk of MACE versus comparators; however, robust data on its long-term cardiovascular profile will be available after completion of the SURPASS-CVOT trial (190). Tirzepatide has received a positive opinion in the European Union (EU).
Sulfonylureas.
As per the previous consensus report and update, sulfonylureas are assessed as having high glucose-lowering efficacy, but with a lack of durable effect, and the advantages of being inexpensive and accessible (5,6). However, due to their glucose-independent stimulation of insulin secretion, they are associated with an increased risk for hypoglycemia. Sulfonylureas are also associated with weight gain, which is relatively modest in large cohort studies (191). Use of sulfonylureas or insulin for early intensive blood glucose control in the UK Prospective Diabetes Study (UKPDS) significantly decreased the risk of microvascular complications, underscoring the importance of early and continued glycemic management (192). Adverse cardiovascular outcomes with sulfonylureas in some observational studies have raised concerns, although findings from systematic reviews have found no increase in all-cause mortality rates compared with other active treatments (191). The incidence of cardiovascular events was comparable in those treated with a sulfonylurea or pioglitazone in the Thiazolidinediones or Sulfonylureas and Cardiovascular Accidents Intervention Trial (TOSCA.IT) (193), and no difference in the incidence of MACE was found in people at high cardiovascular risk treated with glimepiride or linagliptin (194), a medication whose cardiovascular safety was demonstrated in a population at high cardiovascular and renal risk (195).
Thiazolidinediones.
Thiazolidinediones (TZDs) are oral medications that increase insulin sensitivity and are of high glucose-lowering efficacy (5,6). TZDs have a high durability of glycemic response, most likely through a potent effect on preserving β-cell function (196). In the Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROactive) in adults with type 2 diabetes and macrovascular disease, a reduction in secondary cardiovascular end points was seen, although significance was not achieved for the primary outcome (197). In the Insulin Resistance Intervention After Stroke (IRIS) study in adults without diabetes but with insulin resistance (HOMA of insulin resistance >3.0) and recent history of stroke or transient ischemic attack, there was a lower risk of stroke or myocardial infarction with pioglitazone versus placebo (198,199). Beneficial effects on nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) have been seen with pioglitazone (200,201). However, these benefits must be balanced against possible side effects of fluid retention and congestive HF (196,197,202), weight gain (196–198,202,203), and bone fracture (204,205). Side effects can be mitigated by using lower doses and combining TZD therapy with other medications (SGLT2i and GLP-1 RA) that promote weight loss and sodium excretion (199,206).
Insulin.
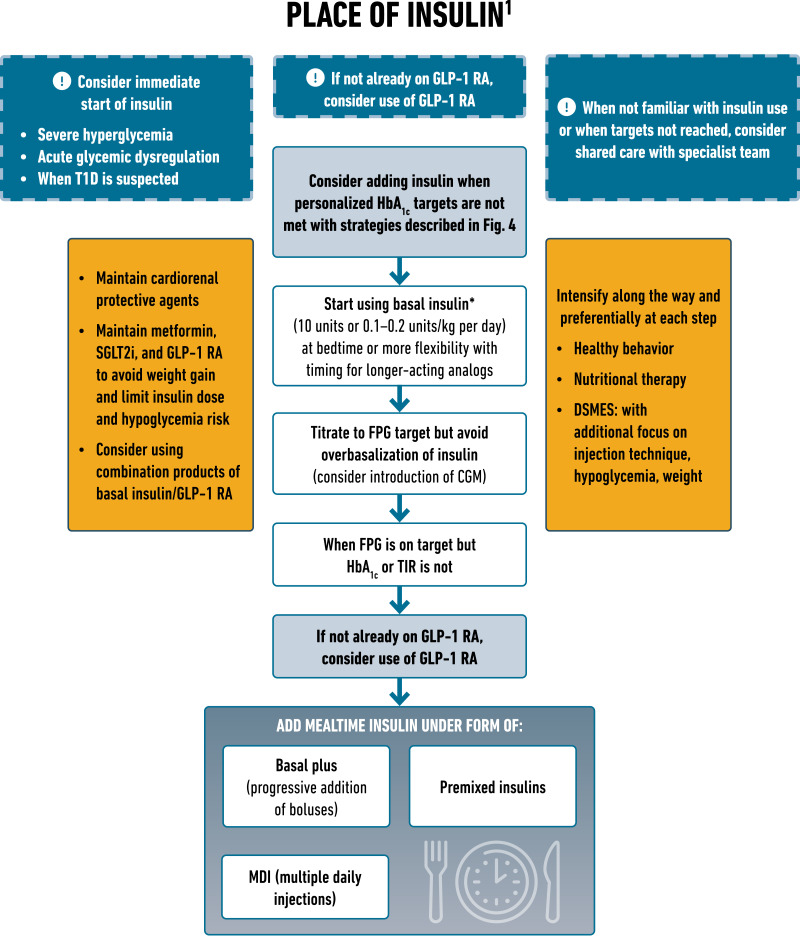
The previous consensus report and update provide detailed descriptions of the different insulins (5,6). The primary advantage of insulin therapy is that it lowers glucose in a dose-dependent manner and thus can address almost any level of blood glucose. However, its efficacy and safety are largely dependent on the education and support provided to facilitate self-management (5,6). Careful consideration should be given to the pharmacokinetic and pharmacodynamic profiles of the available insulins as well as the matching of the dose and timing to an individual’s physiological requirements. Numerous formulations of insulin are available, with advances in therapy geared toward better mimicking physiological insulin release patterns. Challenges of insulin therapy include weight gain, the need for education and titration for optimal efficacy, risk of hypoglycemia, the need for regular glucose monitoring, and cost. The approval of biosimilar insulins may improve accessibility at lower treatment costs. Both insulin glargine U100 and insulin degludec have demonstrated cardiovascular safety in dedicated CVOTs (207,208). Comprehensive education on self-monitoring of blood glucose, diet, injection technique, self-titration of insulin, and prevention and adequate treatment of hypoglycemia are of utmost importance when initiating and intensifying insulin therapy (5,6). Novel formulations and devices, including prefilled syringes, auto-injectors, and intranasal insufflators, are now available to administer glucagon in the setting of severe hypoglycemia and should be considered for those at risk (209).
Starting doses of basal insulin (NPH or analog) are estimated based on body weight (0.1–0.2 units/kg per day) and the degree of hyperglycemia, with individualized titration as needed. A modest but significant reduction in HbA1c and the risk of total and nocturnal hypoglycemia has been observed for basal insulin analogs versus NPH insulin (210). Longer-acting basal insulin analogs have a lower risk of hypoglycemia than earlier generations of basal insulin, although they may cost more. Concentrated insulins allow injection of a reduced volume (5). Cost and access are important considerations and can contribute to treatment discontinuation. Short- and rapid-acting insulin can be added to basal insulin to intensify therapy to address prandial blood glucose levels. Premixed insulins combine basal insulin with mealtime insulin (short- or rapid-acting) in the same vial or pen, retaining the pharmacokinetic properties of the individual components. Premixed insulin may offer convenience for some but reduces treatment flexibility. Rapid-acting insulin analogs are also formulated as premixes, combining mixtures of the insulin with protamine suspension and the rapid-acting insulin. Analog-based mixtures may be timed in closer proximity to meals. Education on the impact of dietary nutrients on glucose levels to reduce the risk of hypoglycemia while using mixed insulin is important. Insulins with different routes of administration (inhaled, bolus-only insulin delivery patch pump) are also available (211–213).
Combination GLP-1–Insulin Therapy.
Two fixed-ratio combinations of GLP-1 RA with basal insulin analogs are available: insulin degludec plus liraglutide (IDegLira) and insulin glargine plus lixisenatide (iGlarLixi). The combination of basal insulin with GLP-1 RA results in greater glycemic lowering efficacy than the monocomponents, with less weight gain and lower rates of hypoglycemia than with intensified insulin regimens, and better gastrointestinal tolerability than with GLP-1 RA alone (214,215). In studies of people with type 2 diabetes inadequately controlled on basal insulin or GLP-1 RA, switching to a fixed-ratio combination of basal insulin and GLP-1 RA demonstrated significant improvements in blood glucose levels and achievement of glycemic goals with fewer hypoglycemic events than with basal insulin alone (216–220).
Less Commonly Used Glucose-Lowering Medications.
α-Glucosidase inhibitors improve glycemic control by reducing postprandial glycemic excursions and glycemic variability and may provide specific benefits in cultures and settings with high carbohydrate consumption or reactive hypoglycemia (221,222). Other glucose-lowering medications (i.e., meglitinides, colesevelam, quick-release bromocriptine, and pramlintide) are not commonly used in the U.S., and most are not licensed in Europe. There was no new evidence that impacts clinical practice.
Comparative Efficacy of Glucose-Lowering Agents
In a network meta-analysis of 453 trials assessing glucose-lowering medications from nine drug classes, the greatest reductions in HbA1c were seen with insulin regimens and GLP-1 RA (223). A network meta-analysis comparing the effects of glucose-lowering therapy on body weight and blood pressure indicates that the greatest efficacy for reducing body weight is seen with subcutaneous semaglutide followed by the other GLP-1 RA and SGLT2i, and the greatest reduction in blood pressure is seen with the SGLT2i and GLP-1 RA classes (224). As discussed above, the novel GIP and GLP-1 RA tirzepatide was associated with greater glycemic and weight loss efficacy than semaglutide 1 mg weekly (185).
Combination Therapy
The underlying pathophysiology of type 2 diabetes is complex, with multiple contributing abnormalities resulting in a naturally progressive disease and increasing HbA1c over time in many. While traditional recommendations have focused on the stepwise addition of therapy, allowing for clear delineation of positive and negative effects of new drugs, there are data to suggest benefits of combination approaches in diabetes care. Combination therapy has several potential advantages, including 1) increased durability of the glycemic effect (225–227), addressing therapeutic inertia, 2) simultaneous targeting of the multiple pathophysiological processes characterized by type 2 diabetes, and 3) impacts on medication burden, medication-taking behavior, and treatment persistence, and 4) complementary clinical benefits (e.g., on glycemic control, weight and cardiovascular risk profiles) (215,228–244).
The Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE) was a multicenter open-label RCT designed to test four different diabetes medication classes in people with type 2 diabetes and compare their ability to achieve and maintain HbA1c levels <53 mmol/mol (<7%). Eligible participants had their metformin therapy optimized and were randomly assigned to receive a sulfonylurea (glimepiride), a DPP-4i (sitagliptin), a GLP-1 RA (liraglutide), or basal insulin (insulin glargine), with the primary outcome being the time to metabolic failure, defined as the time to an initial HbA1c level ≥53 mmol/mol (≥7%) if it was confirmed at the next visit to remain above that threshold. Starting with a mean baseline HbA1c level of 58 mmol/mol (7.5%) before the addition of one of the four medications, over 5 years of follow-up, 71% of the cohort reached the primary metabolic outcome. Insulin glargine and liraglutide were significantly, albeit modestly, more effective at achieving and maintaining HbA1c targets. Liraglutide exhibited a lower risk than the pooled effect of the other three medications on a composite cardiovascular outcome comprising MACE, revascularization, or HF or unstable angina requiring hospitalization (245,246).
Personalized Approach to Treatment Based on Individual Characteristics and Comorbidities: Recommended Process for Glucose-Lowering Medication Selection
People With Cardiorenal Comorbidities
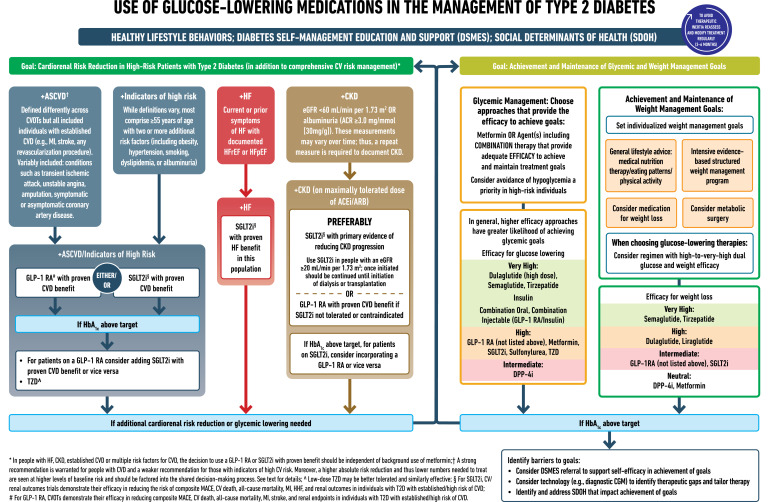
The 2018 ADA/EASD consensus report and 2019 update focused on the consideration of clinically important factors when choosing glucose-lowering therapy. In people with established CVD or with a high risk for CVD, GLP-1 RA were prioritized over SGLT2i. Given their favorable drug class effect in reducing HHF and progression of CKD, SGLT2i were prioritized in people with HF, particularly those with a reduced ejection fraction, or CKD. Since 2019, additional cardiovascular, kidney, and HF outcome trials have been completed, particularly with SGLT2i. In addition, updated meta-analyses have been published that compare subgroup populations based on clinically relevant characteristics, such as presence of CVD, use of background therapy with metformin, stage of CKD, history of HF, and age. Collectively, this new evidence was systematically retrieved and appraised to be incorporated into these clinical practice recommendations (Fig. 3).
Figure 3.
Use of glucose-lowering medications in the management of type 2 diabetes. ACEi, angiotensin-converting enzyme inhibitor; ACR, albumin/creatinine ratio; ARB, angiotensin receptor blocker; ASCVD, atherosclerotic cardiovascular disease; CGM, continuous glucose monitoring; CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; CVOT, cardiovascular outcomes trial; DPP-4i, dipeptidyl peptidase 4 inhibitor; eGFR, estimated glomerular filtration rate; GLP-1 RA, glucagon-like peptide 1 receptor agonist; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HHF, hospitalization for heart failure; MACE, major adverse cardiovascular events; MI, myocardial infarction; SDOH, social determinants of health; SGLT2i, sodium-glucose cotransporter 2 inhibitor; T2D, type 2 diabetes; TZD, thiazolidinedione.
New Evidence From Cardiorenal Outcomes Studies Since the Last Consensus Report
In the Evaluation of Ertugliflozin Efficacy and Safety CVOT (VERTIS CV), which recruited exclusively people with established CVD and type 2 diabetes, ertugliflozin was similar to placebo with respect to the primary MACE outcome and all key secondary outcomes (including a composite kidney outcome) except for HHF (146). The Canagliflozin and Renal End points in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) study included adults with type 2 diabetes with an eGFR from 30 to <90 ml/min per 1.73 m2 and albuminuria (30–500 mg/mmol [300–5,000 mg/g] creatinine) (152). In CREDENCE, canagliflozin treatment significantly reduced the risk of a composite primary outcome of progression to renal replacement therapy, eGFR of <15 ml/min per 1.73 m2, a doubling of serum creatinine level, or death from cardiovascular or kidney causes. The Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial recruited participants with and without type 2 diabetes with an eGFR of 25–75 ml/min per 1.73 m2 and a urinary albumin/creatinine ratio (UACR) of 20–500 mg/mmol [200–5,000 mg/g] (153). Results of the trial demonstrated a clear benefit of dapagliflozin on a composite kidney outcome, on individual kidney-specific outcomes, and on cardiovascular death or HHF, both in the overall population and in the subgroup of people with diabetes (68% of participants). In CREDENCE, the SGTL2i was continued until initiation of dialysis or transplantation.
The Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk (SCORED) trial assessed sotagliflozin (a dual SGLT1i/SGLT2i, currently not approved for type 2 diabetes in the U.S. or the EU) in people with type 2 diabetes who had CKD and additional cardiovascular risk factors (147). Sotagliflozin reduced the composite end point of cardiovascular mortality, HHF, or urgent visits for HF compared with placebo but had no effect on the composite kidney end point.
SGLT2i have been recently assessed in people with HF in dedicated HF outcome trials. In the Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction (EMPEROR-Reduced), empagliflozin reduced the primary composite end point of cardiovascular mortality or HHF in people with HF and a reduced ejection fraction, irrespective of the presence of type 2 diabetes (50% of participants) (149). Notably, this beneficial effect of empagliflozin regardless of diabetes status was consistently evident in those with a preserved ejection fraction (>40%), as demonstrated in the Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction (EMPEROR-Preserved) (151). Additionally, the Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF) trial showed that, in people with type 2 diabetes and worsening HF, sotagliflozin reduced the total number of cardiovascular deaths or hospitalizations or urgent visits for HF compared with placebo regardless of ejection fraction (150). All these data corroborate the salutary drug class effects of SGLT2i on HF-related outcomes in the setting of HF, irrespective of ejection fraction or diabetes status.
Finally, among GLP-1 RA, the Effect of Efpeglenatide on Cardiovascular Outcomes (AMPLITUDE-O) trial demonstrated a beneficial effect of weekly efpeglenatide on MACE and on a composite kidney outcome (decrease in kidney function or severe albuminuria) (247). Of note, an exploratory analysis suggested a possible dose–response effect of efpeglenatide on MACE. In a CVOT of an osmotic minipump delivering exenatide subcutaneously (ITCA 650) over 3–6 months, ITCA 650 had a neutral effect on MACE compared with placebo over 16 months (248). Both trials recruited individuals with type 2 diabetes with an established, or high, risk for CVD. Neither efpeglenatide nor ITCA 650 has received marketing authorization by the FDA or European Medicines Agency. As mentioned previously, the cardiovascular effects of tirzepatide are being assessed in the ongoing SURPASS-CVOT trial, with dulaglutide as an active comparator.
Evidence is emerging regarding the potential benefits of combined treatment with both an SGLT2i and a GLP-1 RA on outcomes. A post hoc analysis of data from the Exenatide Study of Cardiovascular Event Lowering (EXSCEL) has suggested that the combination of exenatide once weekly (EQW) plus open-label SGLT2i reduces all-cause mortality rates and attenuates the decline in eGFR compared with treatment with EQW alone (244). Importantly, a prespecified exploratory analysis of the AMPLITUDE-O trial found comparable benefits of GLP-1 RA treatment in participants who were receiving an SGLT2i as background therapy (15% of the total trial population) and those who were not (241).
Results From Evidence Syntheses
Recent cardiovascular, kidney, and HF outcome trials have been incorporated in updated meta-analyses assessing SGLT2i or GLP-1 RA, both in the overall trial populations and in clinically relevant subgroups. Pairwise meta-analyses of SGLT2i CVOTs verified that SGLT2i reduced MACE, HHF, and a composite kidney outcome in the overall population versus placebo (142,249). Regarding GLP-1 RA, a meta-analysis of relevant CVOTs demonstrated the favorable effect of GLP-1 RA versus placebo on MACE and its individual components, including stroke, HHF, and a composite kidney outcome including severe albuminuria (250,251). It should be noted, however, that the overall effect estimate for HHF seems to have been driven by CVOTs of albiglutide and efpeglenatide, which are not available for clinical use. Similarly, the overall effect estimate for the composite kidney outcome was most likely driven by the effect of GLP-1 RA on severe albuminuria only and not on hard kidney end points. Of note, the beneficial kidney effects of canagliflozin, dapagliflozin, and empagliflozin were also evident for hard kidney outcomes, including chronic dialysis and kidney transplantation (252). When individual components of MACE were analyzed separately, GLP-1 RA reduced all three outcomes, with a more pronounced effect on stroke followed by cardiovascular death and myocardial infarction (253,254). Conversely, SGLT2i, albeit reducing cardiovascular death, had a neutral effect on stroke (142,255).
The applicability of data to support selection of subgroups has been questioned because of a lack of RCTs focusing on specific populations, such as those using versus those not using metformin. This has been examined in subgroup analyses of recent meta-analyses (6). It should be noted that findings of subgroup analyses should not be regarded as conclusive, their credibility should always be formally assessed, and ideally they should be complemented by findings from relevant RCTs (7,8). Recently published subgroup analyses have explored the role of background use of metformin as a potential effect modifier of cardiovascular benefit. For SGLT2i, no differences were observed in MACE, cardiovascular death or HHF, major kidney outcomes, and mortality rates in those using versus those not using metformin (174). Further, for GLP-1 RA, no differences were shown in MACE and mortality outcomes (256–258) in metformin users compared with nonusers. The similarity of the direction and magnitude of the effect estimates between individual trials, the number of trials that contributed data, mostly to within-trial comparisons, and the statistical analyses implemented support the credibility of the conclusions favoring use of SGLT2i or GLP-1 RA in individuals with compelling indications independent of the use of metformin.
Similarly, other subgroup analyses have explored the role of baseline cardiovascular risk as a potential effect modifier regarding the effect of treatment on MACE, HHF, or kidney outcomes. Consistency of findings from between-trial and within-trial comparisons, formal statistical testing verifying the absence of a subgroup effect, and the similarity of baseline cardiovascular risk across different cardiovascular risk categories between individual CVOTs despite the use of seemingly different enrolment criteria suggest the benefits of the use of SGLT2i or GLP-1 RA in people with type 2 diabetes and established CVD and in those at high cardiovascular and/or kidney risk (142,253). Of note, the level of certainty in this recommendation is higher for the former subgroup, because some CVOTs recruited exclusively people with established CVD, while fewer events were recorded for participants with cardiovascular risk factors only in CVOTs that recruited both subgroup populations. In addition, the definition used for risk factors was not identical among CVOTs. However, in general it comprised age ≥55 years plus two or more additional risk factors (including obesity, hypertension, smoking, dyslipidemia, or albuminuria). Furthermore, in terms of absolute effects, the cardiovascular benefits of GLP-1 RA and SGLT2i were less pronounced in people with three or more cardiovascular risk factors than in those with established CVD. This was shown in a network meta-analysis that estimated the absolute effects of treatment with GLP-1 RA or SGLT2i on cardiovascular and kidney outcomes for different categories of baseline cardiovascular risk by combining relative effect estimates with baseline risk estimates (259).
Subgroup meta-analyses based on participants’ kidney function indicated that the salutary effects of SGLT2i on MACE, cardiovascular death or HHF, and a composite kidney outcome (substantial loss of kidney function, end-stage kidney disease, or death due to kidney disease) do not significantly differ among subgroups based on eGFR (142,252). Moreover, the overall effect on MACE and the kidney outcome seemed to be consistent across the three subgroups (normal urine albumin excretion rate [UACR <3.0 mg/mmol (<30 mg/g)], moderate albuminuria [UACR 3.0–30 mg/mmol (30–300 mg/g)], and severe albuminuria [UACR ≥30 mg/mmol (≥300 mg/g)]) (252). In addition, no modification of the effect estimates for MACE, cardiovascular death or HHF, and the composite kidney outcome was observed for SGLT2i in subgroup meta-analyses based on history of HF (142). Regarding GLP-1 RA, a subgroup meta-analysis found that their effect on MACE did not significantly differ between people with an eGFR <60 ml/min per 1.73 m2 and those with an eGFR ≥60 ml/min per 1.73 m2 (253). Moreover, the effect on MACE did not appear to differ between people with lower and higher HbA1c at baseline, both for SGLT2i and for GLP-1 RA (142,253). Nevertheless, the conclusions of all subgroup analyses should be regarded with increased caution because of the small number of trials contributing data to within-trial comparisons, heterogeneity between individual trials, or lack of formal statistical testing.
Comparative Effectiveness Data
While CVOTs and pairwise meta-analyses allow inferences about the overall efficacy and safety of novel glucose-lowering therapies, none of them directly compared SGLT2i with GLP-1 RA. However, the comparative effectiveness of the two drug classes has been assessed in three recent network meta-analyses, which found that, in people with type 2 diabetes, SGLT2i were superior to GLP-1 RA in reducing HHF and a composite kidney outcome, while GLP-1 RA seemed more efficacious in reducing the risk of stroke (223,259,260). No important differences between the two drug classes were evident in terms of mortality rates and other cardiovascular outcomes. These conclusions are further supported by observational data from a large population-based cohort study in the U.S., which showed that SGLT2i reduced HHF compared with GLP-1 RA in people both with CVD (hazard ratio [HR] 0.71; 95% CI 0.64, 0.79) and without CVD (HR 0.69; 95% CI 0.56, 0.81). Differences between the two drug classes with regard to mortality rates and other cardiovascular outcomes were not clinically important (261).
In terms of differences among individual SGLT2i and GLP-1 RA, choice should be based on country-specific label indications and data on efficacy, safety, and outcome benefits considering within-class heterogeneity. No CVOT is available that focuses on people with type 2 diabetes who are at low cardiovascular risk. Some inferences about the effect of glucose-lowering medications as primary cardiovascular prevention in populations with low cardiovascular risk can be made from network meta-analyses, suggesting that no agent or drug class has a notable beneficial effect on cardiovascular events in low-risk individuals with diabetes (223,259).
Additional Clinical Considerations
Age: Older People With Diabetes
Type 2 diabetes represents a model of accelerated biological ageing. As such, type 2 diabetes is associated with declines in physical capacity, underpinned by dysfunction within skeletal muscle. The ability of people with type 2 diabetes to undertake simple functional exercises in middle age has been shown to be like those at least a decade older within the general population. Importantly, this places people living with type 2 diabetes at a high risk of impaired physical function and frailty, which in turn reduces quality of life and increases health care use. As such, frailty is increasingly recognized as a major complication of type 2 diabetes and an important target for treatment (112,262).
Informed decisions regarding treatment of older (>65 years) adults with diabetes are limited by the underrepresentation of such participants in clinical trials. When older individuals have been studied, analyses from trials such as Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) suggested that more frail individuals have worse outcomes and benefit less from intensive control of blood glucose levels and blood pressure (263). However, our confidence in selecting medications to improve outcomes has improved, in part because of regulatory requirements to include older people in trials to determine the efficacy and safety of new drugs for diabetes (264,265). For example, a recent meta-analysis of 11 large outcome trials found that, in those aged 65 years or older, the cardiovascular and/or kidney outcome benefits of GLP-1 RA or SGLT2i therapy were consistent with the effects seen in the overall trial population (266). Therefore, recommendations for the selection of medications to improve cardiovascular and kidney outcomes do not differ for older people. Older age should not be an obstacle to treatment of individuals with established or high risk for CVD. However, medication choices for people who are frail or who have multiple comorbidities may require modification for safety and tolerability. People with diabetes should also understand and be able to appropriately modify use of their prescribed medications during times of illness. Frailty is associated with poorer prognosis, and some attenuation of benefit from intensive glucose-lowering and blood pressure-lowering treatments has been demonstrated in frail individuals (263). Consideration of deprescribing medication to avoid unnecessary medication or medication associated with harm, such as hypoglycemia and hypotension, is important in such populations.
Age: Younger People With Diabetes
Rates of impaired glucose tolerance and/or impaired fasting glucose and type 2 diabetes have increased significantly in the adolescent and young adult population, in concert with increases in obesity (267). It is estimated that one in five adolescents and one in four young adults now have impaired glucose tolerance and/or impaired fasting glucose in the U.S., which in turn increases the risks of progression to type 2 diabetes, CKD, and cardiovascular complications (267). Minority populations are particularly affected, with half or more of newly diagnosed cases of type 2 diabetes in childhood and adolescence occurring in Hispanic, non-Hispanic Black, Asian/Pacific Islander, and American Indian populations (268). Affected young people have a more rapid deterioration in blood glucose levels, an attenuated response to diabetes medication, and more rapid development of diabetes complications (269–273). Early disease onset, higher levels of hyperglycemia, and the multiple cardiometabolic risk factors found in adolescents and young adults with impaired glucose tolerance and/or impaired fasting glucose and diabetes all contribute to an increase in risk of adverse outcomes (267). Most children and adolescents who develop type 2 diabetes will have microvascular complications by young adulthood (274); in addition, a recently identified 25% increase in the risks of hyperglycemic crises, acute myocardial infarction, stroke, and lower extremity amputation over a 5-year period was most notable in people with diabetes aged 18–44 years (275). Younger people with type 2 diabetes should be considered at very high risk for complications and treated correspondingly. Early use of combination therapy may be considered, as the Vildagliptin Efficacy in Combination with Metformin for Early Treatment of Type 2 Diabetes (VERIFY) trial findings suggest that this approach provides superior and more durable effects on blood glucose levels than metformin monotherapy in people with both early-onset (age <40 years) and later-onset diabetes (276). Most of the evidence for health behavior interventions, glucose-lowering approaches, and the effectiveness of medications to improve cardiovascular and kidney outcomes in younger people with diabetes is poorly understood because of the very limited enrollment of this group in completed trials (15). Beyond the scope of this statement, there are data emerging on the use of GLP-1 RA and SGLT2i in children that suggest glycemic benefit; however, the durability of this effect and any impact on cardiorenal outcomes in children and young adults remain unknown.
Race and Ethnicity
Although specific populations are disproportionately affected by diabetes, they are consistently underrepresented in outcomes and other trials. A meta-analysis of six large cardiovascular and kidney outcome trials found that non-White participants had higher rates of cardiovascular and other comorbidities than the White cohort but comprised only about 21% of the overall enrolled trial populations. Importantly, both non-White and White subgroups had significant reductions in the risk of cardiovascular death or HHF with SGLT2i therapy compared with placebo (odds ratio 0.66 and 0.82, respectively) (277). The increased burden of complications in underrepresented populations with diabetes should be factored into personalized treatment plans, and beneficial medications should be used irrespective of race or ethnicity. Ongoing and future trials should recruit to be representative of the overall population of people with diabetes so that the effects of interventions in understudied subgroups may be better ascertained (278,279).
Sex Differences
In women with reproductive potential, the use of highly effective contraception should be ensured, such as long-acting reversible contraception (intrauterine device or progesterone implant), prior to prescribing medications that may adversely affect a fetus. Diabetes significantly increases the risk of cardiovascular complications in both sexes, and CVD causes most hospitalizations and deaths in women and men with diabetes (280,281). In the general population, women are at lower risk for cardiovascular events than men of the same age; however, this vascular protection or advantage is reduced in women who develop type 2 diabetes (282,283). In fact, the increase in relative risk of CVD due to type 2 diabetes is greater in women than in men (284–286). Despite this, women have been underrepresented in recent CVOTs in diabetes, comprising between 28.5 and 35.8% of participants (287). This analysis also described differing patterns of cardiovascular complications in women compared with men and poorer management of cardiovascular risk factors in women (287). Within-trial analyses and meta-analyses suggest that there are likely no between-sex differences in outcomes achieved with SGLT2i and GLP-1 RA therapy (288,289). Continued efforts should be made to enroll women in outcomes trials and to identify and address modifiable cardiovascular risk factors in women with diabetes.
Obesity and Weight-Related Comorbidities, Particularly Nafld and Nash
The care of people with diabetes who have weight-related comorbidities such as NAFLD, HF with preserved ejection fraction, or obstructive sleep apnea should include strategies intended to result in weight loss. People with type 2 diabetes frequently have NAFLD and are at increased risk for progression to more severe stages of liver disease, including NASH, hepatic fibrosis, and cirrhosis (290). The management of type 2 diabetes in people with NASH should include lifestyle modification with a goal of weight loss, including strong consideration of medical and/or surgical approaches to weight loss in those at higher risk of hepatic fibrosis (291). Pioglitazone therapy, GLP-1 RA therapy, and metabolic surgery have all been shown to reduce NASH activity; pioglitazone therapy and metabolic surgery may also improve hepatic fibrosis (188,292–298).
Although not licensed for this purpose, it has therefore been suggested that people with type 2 diabetes at intermediate to high risk of fibrosis should be considered for treatment with pioglitazone and/or a GLP-1 RA with evidence of benefit (291,299). Although SGLT2i therapy has also been shown to reduce elevated levels of liver enzymes and hepatic fat content in people with NAFLD, at this time there is less evidence to support use of this class of drug as treatment for NASH (300–302). NAFLD, and in particular NASH, is also associated with an increased risk of cardiovascular complications; therefore, people with NAFLD should have their cardiovascular risk factors assessed and managed to minimize this risk (303).
SGLT2i have been shown to reduce incident obstructive sleep apnea in two SGLT2i CVOTs based on adverse event reporting (304,305). However, it is not clear that the data collected on incident obstructive sleep apnea in these trials were complete or that the benefit is mediated through changes in weight.
Consensus Recommendations
All people with type 2 diabetes should be offered access to ongoing DSMES programs.
Providers and health care systems should prioritize the delivery of person-centered care.
Optimizing medication adherence should be specifically considered when selecting glucose-lowering medications.
MNT focused on identifying healthy dietary habits that are feasible and sustainable is recommended in support of reaching metabolic and weight goals.
Physical activity improves glycemic control and should be an essential component of type 2 diabetes management.
Adults with type 2 diabetes should engage in physical activity regularly (>150 min/week of moderate- to vigorous-intensity aerobic activity) and be encouraged to reduce sedentary time and break up sitting time with frequent activity breaks.
Aerobic activity should be supplemented with two to three resistance, flexibility, and/or balance training sessions/week. Balance training sessions are particularly encouraged for older individuals or those with limited mobility/poor physical function.
Metabolic surgery should be considered as a treatment option in adults with type 2 diabetes who are appropriate surgical candidates with a BMI ≥40.0 kg/m2 (BMI ≥37.5 kg/m2 in people of Asian ancestry) or a BMI of 35.0–39.9 kg/m2 (32.5–37.4 kg/m2 in people of Asian ancestry) who do not achieve durable weight loss and improvement in comorbidities (including hyperglycemia) with nonsurgical methods.
In people with established CVD, a GLP-1 RA with proven benefit should be used to reduce MACE, or an SGLT2i with proven benefit should be used to reduce MACE and HF and improve kidney outcomes.
In people with CKD and an eGFR ≥20 ml/min per 1.73 m2 and a UACR >3.0 mg/mmol (>30 mg/g), an SGLT2i with proven benefit should be initiated to reduce MACE and HF and improve kidney outcomes. Indications and eGFR thresholds may vary by region. If such treatment is not tolerated or is contraindicated, a GLP-1 RA with proven cardiovascular outcome benefit could be considered to reduce MACE and should be continued until kidney replacement therapy is indicated.
In people with HF, SGLT2i should be used because they improve HF and kidney outcomes.
In individuals without established CVD but with multiple cardiovascular risk factors (such as age ≥55 years, obesity, hypertension, smoking, dyslipidemia, or albuminuria), a GLP-1 RA with proven benefit could be used to reduce MACE, or an SGLT2i with proven benefit could be used to reduce MACE and HF and improve kidney outcomes.
In people with HF, CKD, established CVD, or multiple risk factors for CVD, the decision to use a GLP-1 RA or SGLT2i with proven benefit should be independent of background use of metformin.
SGLT2i and GLP-1 RA reduce MACE, which is likely to be independent of baseline HbA1c. In people with HF, CKD, established CVD, or multiple risk factors for CVD, the decision to use a GLP-1 RA or an SGLT2i with proven benefit should be independent of baseline HbA1c.
In general, selection of medications to improve cardiovascular and kidney outcomes should not differ for older people.
In younger people with diabetes (<40 years), consider early combination therapy.
In women with reproductive potential, counseling regarding contraception and taking care to avoid exposure to medications that may adversely affect a fetus are important.
Putting It All Together: Strategies for Implementation
Importance of Integrated Care
The overall goal of the management of type 2 diabetes is to maintain quality of life and avoid complications. The management approach must be holistic and multifactorial and account for the lifelong nature of type 2 diabetes (Figs. 1, 3, and 4). The person living with type 2 diabetes should be at the center of care. The structure and organization of the health care team will vary across systems but generally involves multiple disciplines, including the primary care provider, diabetologist, diabetes care and education specialist, registered dietitian/nutritionist, pharmacists, nurses, and other specialists as needed (e.g., dentist, eye care professional, podiatrist, mental health provider, cardiologist, nephrologist, neurologist, hepatologist, sleep medicine specialist, and pain management specialist) (306). Technology is now an important tool to enhance communication, support, and monitoring. Communication between people living with type 2 diabetes and health care team members is at the core of integrated care, and clinicians must recognize the importance of language in this communication.
Figure 4.
Holistic person-centered approach to T2DM management. 1“Cardiovascular Disease and Risk Management” in Standards of Medical Care in Diabetes—2022 (141). ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers; ASCVD, atherosclerotic cardiovascular disease; BP, blood pressure; CKD, chronic kidney disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; GLP-1 RA, glucagon-like peptide 1 receptor agonist; HF, heart failure; SGLT2i, sodium-glucose cotransporter 2 inhibitor; T2D, type 2 diabetes.
Practical Tips for Clinicians
Acknowledge the lifelong and evolving nature of type 2 diabetes.
Identify and coordinate with the team.
Know your local resources.
Language matters in diabetes care.
See Supplementary Fig. 1.
Individualization of Care
The integrated care of type 2 diabetes must consider the person with diabetes as an individual (Figs. 1, 3, and 4) with respect to specific preferences and values, social determinants of health, barriers to care, comorbid conditions, degree of hyperglycemia, risks of complications, and susceptibility to medication side effects. Attention should be given to how the balance of risks and benefits of each intervention is communicated to each person living with diabetes. Risk estimator tools, especially for CVD risk, may also be helpful, but when using these tools one must be aware that they work best when they are derived from and/or are validated in a population similar to the population in which they are applied (307). These risk estimator tools are often developed in populations that exclude younger and older people and underrepresent women and various minority populations. Finally, shared decision-making is essential to incorporating an individual’s preferences and values when formulating a management plan.
Social determinants of health must be assessed and addressed (47) to achieve health equity in diabetes. Health systems must ensure equity in the delivery of all diabetes care, including access to the more expensive, organ-protecting pharmacotherapies (SGLT2i and GLP-1 RAs) and technologies (e.g., CGM).
Many people living with type 2 diabetes have multiple comorbidities, some related to diabetes, such as obesity, hypertension, dyslipidemia, cardiorenal disease, NASH/NAFLD, and mental health problems. Other important conditions whose relationship to diabetes is not as well established, such as chronic obstructive pulmonary disease and cancer, are prevalent. Attention to these comorbidities should be paid throughout the life span of the person living with diabetes, as such comorbidities may impact the tailoring and implementation of the holistic plan for diabetes management, including choice of glucose-lowering medication.
Importantly, diabetes is associated with cognitive decrements, which can substantially impact management (308,309). Further, long-term hyperglycemia is associated with worsening cognitive decline. Screening for cognitive impairment should be performed when risk factors are identified, such as frequent hypoglycemia, difficulty with diabetes self-management, or unexplained falls. People with cognitive impairment should be referred for additional support. Other conditions, such as serious mental illness and substance use disorders, must also be identified and managed appropriately in the holistic approach to diabetes. Mental illness, including depression, is associated with an increased risk of diabetes and with poorer prognosis but may also complicate diabetes management and be a barrier to self-management.
Practical Tips for Clinicians
Consider each person living with diabetes an individual with specific context, risks, and preferences.
Health care systems should monitor and address inequity in the delivery of evidence-based interventions for type 2 diabetes.
Assess and address social determinants of health for each individual living with diabetes, particularly in those not achieving goals.
Incorporate comorbidities when developing and implementing the management plan.
See Supplementary Fig. 1.
Diabetes Self-Management Education and Support
DSMES is critical to integrated, holistic, person-centered care in type 2 diabetes (19–21,23) and is as important to the management plan as the selection of medication. DSMES should be offered on an ongoing basis, should be provided by trained diabetes care and education specialists, and can be delivered using multiple approaches and in a variety of settings (Supplementary Table 1) (20,31). The care team must be aware of the available local DSMES resources and how to access them. Importantly, DSMES is complementary to but does not replace MNT (see below) (310) or referral for mental health services when they are warranted (49).
Practical Tips for Clinicians
Embrace DSMES as being as important as other aspects of diabetes care, such as pharmacotherapy.
Identify and know how to access your local DSMES resources.
Impress on the person and the health care team the importance of DSMES in the ongoing holistic approach to the management of type 2 diabetes.
Initiate or refer for DSMES at diagnosis, annually, with changes in social or health status, and with transitions of care or life situation.
See Supplementary Fig. 1.
Facilitating Healthy Behaviors and Weight Management
Promotion of healthy behaviors is central to the holistic management of type 2 diabetes and should be addressed at the time of diagnosis and throughout the course of diabetes. Healthy behaviors include healthy nutrition, regular physical activity, adequate sleep, and smoking cessation. Health behaviors should always be assessed and addressed when glycemic targets are not met and when new pharmacotherapy or interventions (e.g., metabolic surgery) are initiated.
All individuals with type 2 diabetes should be offered MNT to develop a personal food plan in the context of diabetes. The need for additional dietary advice should be reevaluated over time (310). There is no single dietary pattern recommended for all individuals with type 2 diabetes; many dietary patterns can be effective for achieving treatment goals, and a structured food plan should be based on an individual person’s preferences and context.
Explicit physical activity and minimization of sedentary time should be the focus of the physical activity regimen for people living with type 2 diabetes (Fig. 2). Individual preferences and circumstances should inform the specific activity regimen. A reasonable target for physical activity is at least 150 min/week. In addition to these activity minutes, breaking up sedentary time with activity breaks (e.g., 5-min activity break every hour) can be beneficial (101). A gradual increase in overall volume and intensity of activity does not require medical clearance (101). Additional clinical assessment may be warranted in those with moderate-to-severe diabetic retinopathy, diabetic kidney disease, peripheral neuropathy, and unstable HF and for those prescribed insulin or with a history of hypoglycemia (101). Individual preferences, motivations, and circumstances should inform choice.
Weight management should be a central focus for individuals with type 2 diabetes with overweight or obesity, with individualized weight loss goals. For most people, a target of at least 5% weight loss is reasonable and can be expected to have clinical benefits. Substantial (>10%) weight loss and weight loss early in the course of type 2 diabetes increase the chance of remission of disease (50). The use of glucose-lowering agents that provide significant weight loss, particularly GLP-1 RA with high weight loss efficacy, should be considered, as they can often provide 10–15% weight loss or more. Metabolic surgery, which is most effective when performed early during diabetes, can be considered in those without a sufficient response to nonsurgical weight loss interventions based on the specific context and preferences and should be accompanied by health behavior interventions. The benefits of metabolic surgery need to be balanced against its potential adverse effects, which vary by procedure and include surgical complications, late metabolic or nutritional complications, and impact on psychological health (5,6,127). People being considered for metabolic surgery should be evaluated for comorbid psychological conditions and social and situational circumstances that may interfere with surgery outcomes. People who undergo metabolic surgery should receive long-term medical and behavioral support. Metabolic surgery should be performed in high-volume centers with experienced multidisciplinary teams (127).
SMART (specific, measurable, attainable, relevant, time-based) goals are more effective for achieving behavior change than nonspecific recommendations (311). An “all or none” approach related to behavioral goals should be avoided, as any improvement in healthy behaviors can have a positive impact in diabetes (93,312). Self-monitoring of achievements (e.g., physical activity monitoring and weight measurement) is crucial to the achievement of health behavior goals (Fig. 1). Behavioral health specialists or psychologists with specific training in behavior change interventions can be of particular value as members of the team to help the person with type 2 diabetes achieve goals.
Practical Tips for Clinicians
Specific health behavior and weight management goals should be agreed on between the person with type 2 diabetes and the care team; shared decision-making is an important component of this discussion.
Emphasize self-monitoring behaviors and review data collected (e.g., glucose monitoring, weight, tracking physical activity) in clinical visits to convey their importance in achieving the desired health behavior goals.
People taking insulin or a sulfonylurea should be educated about the risk, symptoms, and treatment of hypoglycemia when undertaking physical activity or adopting a specific nutritional plan; prescribe glucagon in people at risk for severe hypoglycemia.
DSMES and MNT can help the person living with diabetes to identify and address barriers to implementing healthier behaviors.
See Supplementary Fig. 2.
Choice of Glucose-Lowering Medication
The choice of glucose-lowering agents should be directed by the individual profile of the person with type 2 diabetes, in particular the presence of comorbidities, risk of side effects, preferences, and context (Figs. 3 and 4). Pharmacological treatment of hyperglycemia must be integrated in DSMES and accompanied by a focus on healthy behaviors from diagnosis onwards. This should be integrated as part of a holistic, multifactorial approach to type 2 diabetes that includes weight, blood pressure, and lipid management (Fig. 4).
Whereas the pursuit of glycemic control and the pursuit of organ-specific (e.g., heart and kidney) protection are complementary and not mutually exclusive, clinicians should not confuse the discussion of choice of agents for their glucose-lowering effect with the discussion of choice of specific agents for their direct organ-protecting effect. Some agents, in particular SGLT2i, have been shown to protect organs (heart, kidney) partly independently of their glucose-lowering effect, as this organ protection also occurs in those not affected by type 2 diabetes.
Based on these principles, regardless of HbA1c level or the presence of other glucose-lowering agents, all individuals with diabetes and established or subclinical CVD should be prescribed an agent with proven cardiovascular benefit from the GLP-1 RA class or SGLT2i class (5,6). The evidence for cardiovascular benefits of GLP-1 RA and SGLT2i in those with only risk factors for CVD, based on MACE (myocardial infarction, stroke, or cardiovascular death), is less robust, as fewer people with lower event rates are included in studies (313–315). Furthermore, it is important to recognize that the predicted absolute benefit of an intervention is dependent on the absolute risk, and thus those with prior CVD events are more likely to experience a benefit over intermediate time frames than those with cardiovascular risk factors only. Through shared decision-making, considering an individual’s lifelong CVD risk, introduction of a GLP-1 RA or SGLT2i with proven cardiovascular benefit into the regimen for a person with CVD risk factors can be considered in the context of increased treatment burden and potential side effects with lower absolute risk reduction.
All individuals with diabetes and CKD (eGFR <60 ml/min per 1.73 m2 or UACR >3.0 mg/mmol [>30 mg/g]) should receive an agent with proven kidney benefit from the SGLT2i class (or GLP-1 RA class if SGLT2i are contraindicated or not preferred or their use is not permitted under license). Likewise, those with HF (HF with reduced ejection fraction or HF with preserved ejection fraction) should receive an agent from the SGLT2i class with proven benefit for HF. In both instances, the goal of organ protection with SGLT2i or GLP-1 RA should be independent of background glucose-lowering therapies, current HbA1c level, or target HbA1c level (Figs. 3 and 4).
While there is compelling evidence to support a place for SGLT2i and the GLP-1 RA class in the treatment of many people with type 2 diabetes based on their direct organ-protecting effects, it is acknowledged that to date these agents are expensive. In the setting of resource constraints, prioritization of the highest risk groups for access to these agents may be needed, with consideration of absolute risk reduction in addition to relative risk reductions.
Evidence on specific agents and their effects on other comorbidities, such as NAFLD, is emerging. For those with NAFLD/NASH at high risk of fibrosis, pioglitazone could be considered. There is emerging evidence for benefits of metabolic surgery and three classes of glucose-lowering therapy (GLP-1 RA, SGLT2i, and GIP and GLP-1 RA) (188,292–298,316).
Overall, for treatment of hyperglycemia, metformin remains the agent of choice in most people with diabetes, based on its glucose-lowering efficacy, minimal risk of hypoglycemia, lack of weight increase, and affordability. Often, monotherapy with metformin will not suffice to maintain glucose levels at target. As proposed in the previous consensus report and update (5,6), other classes of agents are useful in combination with metformin or when metformin is contraindicated or not tolerated. Selection of other glucose-lowering agents will be determined by the balance between the glucose-lowering efficacy and the side effect profile of the individual agents (see Table 1).
Special attention needs to be given to populations in which hypoglycemia is most dangerous, for example, people with frailty, in whom agents without risk of hypoglycemia need to be prioritized. If sulfonylureas or insulin are used, consideration of less stringent targets in such settings is prudent and deprescribing if asymptomatic or severe hypoglycemia ensues.
Finally, it needs to be stated that the evidence on organ-protecting or glucose-lowering effects of specific pharmacotherapies in specific subpopulations (e.g., younger and older people, women, and various racial/ethnic groups) continues to be limited. This lack of evidence is, however, not a reason to withhold these medications in these subpopulations, given their proven benefits in large general populations.
Practical Tips for Clinicians
Providers should continually update their knowledge on the efficacy and side effects of diabetes pharmacotherapy (see Table 1).
Identify relevant comorbidities (e.g., obesity, CVD, HF, CKD, NAFLD).
Assess the profile of the person with diabetes (e.g., younger age, frailty, limited life expectancy, cognitive impairment, social determinants of health).
Consider risk factors for medication adverse events (e.g., hypoglycemia, volume depletion, genital infections, history of pancreatitis).
Prioritize the use of organ-protective medications (GLP-1 RA, SGLT2i, TZD) in those with cardiorenal disease or NASH or at high risk.
See Supplementary Fig. 2.
Proactive Care: Avoiding Inertia
Reassessment of individual glycemic targets and their achievement at regular intervals is key (Figs. 1, 3, and 4). When targets are not met, in addition to addressing health behaviors and referral to DSMES, the intensification of glucose-lowering medication by combining agents with complementary mechanisms of action should be pursued. Traditionally, a stepwise approach was advocated, in which a new agent is added to the existing regimen, but evidence is growing to support a more proactive approach in many by combining glucose-lowering agents from initial diagnosis (6).
Early use of combinations of agents allows tighter glucose control than monotherapy with the individual agents, and thus combinations of agents are indicated in those who have HbA1c levels >16.3 mmol/mol (>1.5%) above their target at diagnosis (e.g., ≥70 mmol/mol [8.5%] in most) (6). In particular, among young adults with type 2 diabetes, immediate and sustained glycemic management should be pursued, aiming for HbA1c <53 mmol/mol (7%) (or even lower). This presents the best opportunity to avoid complications of diabetes across the life span. Moreover, the pathophysiology of micro- and macrovascular damage shares more commonality than usually thought, suggesting that the prevention of microvascular disease may, in the long term, contribute to a reduction in macrovascular complications as well (317).
The knowledge base guiding clinicians beyond dual therapy in type 2 diabetes is still limited. In general, intensification of treatment beyond two medications follows the same general principles as the addition of a second medication, with the assumption that the effectiveness of third and fourth medications will be generally less than when they are used alone. Whereas solid evidence exists for combining SGLT2i and GLP-1 RA for weight and glucose lowering, emerging data suggest promise for combined effects on cardiorenal outcomes (228).
As more medications are added, there is an increased treatment burden and risk of adverse effects. It is important to consider medication interactions and whether regimen complexity may become an obstacle to adherence. Fixed-dose combination preparations can improve medication-taking behaviors. Finally, with each additional medication comes increased costs, which can affect medication-taking behavior and medication effectiveness (318–326).
Response to all therapies should be reviewed at regular intervals, including the impact on efficacy (HbA1c, weight), safety, and organ protection. While most people with diabetes require intensification of glucose-lowering medications, some require medication reduction or discontinuation, particularly if the therapy is ineffective or associated with side effects such as hypoglycemia or when glycemic goals have changed because of a change in clinical circumstances (e.g., development of comorbidities or even healthy ageing). Medication should be stopped, or the dose reduced, if there are minimal benefits or if harm outweighs any benefit. Ceasing or reducing the dose of medications that have an increased risk of hypoglycemia is suggested when any new glucose-lowering treatment (behavioral or medication) is started and the individual’s glycemic levels are close to target (66). HbA1c levels below 48 mmol/mol (6.5%) or substantially below the individualized glycemic target as well as any increased risk of hypoglycemia should prompt stopping or reducing the dose of medications associated with an increased risk of hypoglycemia.
Practical Tips for Clinicians
Consider initial combination therapy with glucose-lowering agents, especially in those with high HbA1c at diagnosis (i.e., >70 mmol/mol [>8.5%]), in younger people with type 2 diabetes (regardless of HbA1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.
Avoid therapeutic inertia and reevaluate health behaviors, individuals’ medication-taking behaviors, and side effects of agents at every clinic visit.
When additional glycemic control is needed, incorporate, rather than substitute, glucose-lowering therapies with complementary mechanisms of action.
Consider fixed-dose combinations to reduce prescription burden.
Consider deintensification of therapy, e.g., in frail older adults and in the setting of hypoglycemia-causing medications, in those with glycemic metrics substantially better than target.
See Supplementary Fig. 2.
Place of Insulin in Type 2 Diabetes
Insulin is a useful and effective glucose-lowering agent (Fig. 5). When glycemic measurements do not reach targets, and insulin is the best choice for the individual, its introduction should not be delayed. When clinicians are not familiar with insulin use, referral to specialist care is indicated. However, with the growing evidence supporting use of particular agents in people with type 2 diabetes with specific profiles (comorbidities, overweight/obesity) and with the availability of multiple glucose-lowering agents with good efficacy and acceptable side effect profiles, the initiation of insulin can be postponed in many to later stages of the disease. GLP-1 RA should be considered in all when no contraindications are present before initiation of insulin therapy, as they allow lower glycemic targets to be reached with a lower injection burden and lower risk of hypoglycemia and weight gain than with insulin alone.
Figure 5.
Place of insulin. *NPH insulin or preferably analog to reduce nocturnal hypoglycemia risk. 1More details can be found in Davies et al. (12) and “Pharmacologic Approaches to Glycemic Treatment” in Standards of Medical Care in Diabetes—2022 (16). CGM, continuous glucose monitoring; DSMES, diabetes self-management education and support; FPG, fasting plasma glucose; GLP-1 RA, glucagon-like peptide 1 receptor agonist; SGLT2i, sodium-glucose cotransporter 2 inhibitor; T1D, type 1 diabetes; TIR, time in range.
The preferred way of initiating insulin in people with type 2 diabetes is to add basal insulin to the existing pharmacological therapy in conjunction with revisiting health behaviors and rereferral to DSMES. However, agents that cause hypoglycemia in themselves, such as sulfonylureas, should be discontinued once insulin is started. Technologies allowing continuous monitoring of glucose levels without finger sticking have clear advantages in those on insulin. Other support tools and systems, such as apps guiding insulin dose adaptation or phone-based guidance, can also be helpful.
In specific circumstances, insulin may be the preferred agent for glucose lowering, specifically in the setting of severe hyperglycemia (HbA1c >86 mmol/mol [>10%]), particularly when associated with weight loss or ketonuria/ketosis and with acute glycemic dysregulation (e.g., during hospitalization, surgery, or acute illness), in underweight people, or when the diagnosis of type 1 diabetes is suspected.
If affordable, basal insulin analog formulations are preferred to NPH insulin because of their reduced risk of hypoglycemia, particularly nocturnal hypoglycemia, when titrated to the same fasting glucose target (327). Basal insulins are typically administered before bedtime, but, with newer analogs, more flexibility in the timing of insulin injection is possible (i.e., any time of the day).
In some, as the disease progresses, despite titration of the basal insulin to correct fasting hyperglycemia (typically more than 0.5 U/kg), mealtime insulin may have to be added to meet glycemic targets, particularly postprandial glucose (328). Mealtime insulin may be required to enhance postprandial blood glucose levels and achieve HbA1c targets. Therapeutic inertia in intensification of insulin therapy should be avoided, and, when clinicians are not familiar with multiple daily injection therapy, referral to specialist care and/or DSMES is warranted. A straightforward way to introduce mealtime insulin is to start with a short- or rapid-acting insulin injection before the meal associated with the largest glucose excursion. Adding mealtime rapid-acting insulin requires increased DSMES and self-monitoring of glucose levels and adds complexity and cost to the therapy. In contrast to basal insulin analogs, the evidence supporting the choice of mealtime rapid-acting insulin analogs is less clear (329). Another simpler and still popular way of combining mealtime and basal insulin components is using premixed insulins. Insulin analog-based combinations have the advantage of resulting in fewer hypoglycemic events and weight gain than are typically observed with human premixed insulin (330).
Finally, it needs to be reemphasized that, in all insulin-treated people with type 2 diabetes, agents associated with cardiorenal protection or weight reduction should be kept in the treatment regimen whenever possible (331). The combination of a basal insulin analog and GLP-1 RA in one injection may be a simple way to reduce the burden and complexity of treatment (332).
Practical Tips for Clinicians
The use of a GLP-1 RA should be considered prior to initiation of insulin.
When initiating insulin, start with a basal insulin and intensify the dose in a timely fashion, titrating to achieve an individualized fasting glycemic target set for every person.
When insulin is initiated, continue organ-protective glucose-lowering medications and metformin.
Refer for DSMES when initiating insulin or advancing to basal–bolus therapy.
See Supplementary Fig. 3.
Place of Technology
The use of technology in the therapy of people with type 2 diabetes is increasing through a broad range of approaches, for example, telehealth, remote monitoring systems, CGM, and behavioral aids to support physical activity, meal planning and monitoring, medication-taking behavior, mindfulness, and stress management. Evidence on the impact of these systems is variable and highly dependent on the embedding of the technology in a more comprehensive approach. Evidence for a beneficial impact of telehealth on achieving treatment goals in those living with type 2 diabetes is growing (333,334). During the COVID-19 pandemic, telehealth has proven to be an efficient way of overseeing the treatment of people with type 2 diabetes. In particular, interventions using apps as tools to support DSMES have been shown to have an impact on outcomes (34).
For those needing insulin as part of their treatment, smart insulin pens and insulin pumps (continuous subcutaneous insulin infusion [CSII]) are available. Specific evidence on the benefit of smart pens in people with type 2 diabetes is still scarce. CSII use is associated with small improvements in HbA1c and fewer hypoglycemic events, suggesting that CSII can be considered in people living with type 2 diabetes treated with multiple daily insulin injections and able to manage the device (71). Again, for optimal effect, this technology should be embedded in an integrated approach to type 2 diabetes therapy, specifically to avoid weight gain (335).
In individuals with type 2 diabetes treated with insulin, CGM, both intermittently scanned CGM and real-time CGM, has gained traction, with evidence that CGM results in better overall glucose control as defined by HbA1c and time in range (3.9–10.0 mmol/L [70–180 mg/dL]), fewer hyperglycemic and hypoglycemic episodes, and improvements in diabetes distress (336,337).
As with other wearables, for example, those collecting steps walked or monitoring dietary intake, medication dose administered, or sleep quality, use of CGM has also been proposed as a motivational tool for those with type 2 diabetes not on insulin therapy, but the evidence on this is modest (337).
Finally, to date, no convincing evidence is available on the use of hybrid closed-loop systems specifically in people with type 2 diabetes.
Practical Tips for Clinicians
Technology can be useful in people with type 2 diabetes but needs to be part of a holistic plan of care and supported by DSMES.
Consider CGM in people with type 2 diabetes on insulin.
Adapt the clinic/system to optimize effective use of technology among people with type 2 diabetes, particularly to support behavior change through self-monitoring.
See Supplementary Fig. 3.
Working Within the System to Deliver Improved Care
We are fortunate to have evidence on numerous effective interventions in type 2 diabetes, but translating this evidence into practice cannot rest only with front-line clinicians during individual clinic visits. The systems of care that support front-line clinicians have a significant role in improving diabetes clinical management, outcomes, and experience for people living with diabetes. Front-line clinicians must inform and drive the design of care, but the systems of care should be held accountable for implementation. Supplementary Table 2, informed by the Effective Practice and Organization of Care (EPOC) taxonomy (338), outlines key domains and questions that must be answered to achieve the goal of better care and outcomes for people living with type 2 diabetes. All levels of the care delivery system have a role and responsibility in improving diabetes management. Clinic leaders have a responsibility to improve workflows to make it easy to provide evidence-based care and provide data to inform quality improvement efforts. Continuing education is necessary to ensure evolving evidence reaches people living with type 2 diabetes. Policy makers have a responsibility to ensure that evidence-based interventions are available and affordable to all. Interventions to improve diabetes must also include the health system (including the microsystems within a system) and governmental agencies. Policy makers, together with all stakeholders, should reflect on care delivery. How, where, and by whom is care delivered? Who coordinates care and the management of care processes? Practices and systems must establish enhanced communication technology to improve engagement. Governance arrangements must be implemented specifically around accountability for health professionals, with a focus on training and evaluation of quality of practice. Finally, reflection is needed around implementation strategies at the level of the system, facility, and individual health care workers. These principles are aligned with recommendations outlined in the recent Lancet Commission on diabetes (339).
Practical Tips for Clinicians
Identify and incorporate continuing education activities on the management of type 2 diabetes for all members of the health care team.
Team-based care is required for integrated care of diabetes; this includes coordination between multiple disciplines (diabetes care and education specialist, dietitians, psychologists, etc.) and often other medical specialties (primary care, endocrinology, ophthalmology, nephrology, etc.).
Management of type 2 diabetes requires continuous quality improvement interventions tailored to the local setting.
See Supplementary Fig. 3.
Key Knowledge Gaps and a Call to Action
In this 100th year since the discovery and partial purification of insulin, we should remember the remarkable speed at which this first glucose-lowering medication was developed and distributed as life-saving therapy for people with diabetes. Through our experience in the last few years with the COVID-19 pandemic, we have demonstrated how quickly many governments, industry, health care systems, and academic institutions can respond to global health care crises. Within a year of identification of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, preventive and therapeutic products were not only developed and tested but also administered on a massive scale. The annual global mortality rate directly attributable to diabetes is approximately 1.5 million people, with 540 million people affected (340,341). Although not as spectacular as the impact of COVID-19 on the health of society, diabetes is sure and steady in its burden, increasing in prevalence and with an increase in mortality and morbidity over time.
Two centuries of investigation into the pathophysiology of diabetes have led to the extraordinary advances in treatment of the last two decades. As reviewed in this consensus report, encouraging healthy behaviors, DSMES, medications, devices, technologies, and organization of care all represent effective tools for the management of diabetes to reduce its morbidity and mortality. However, despite the generous approach of Banting and Best in licensing the patent for insulin for one Canadian dollar, it is not yet readily available to all people with diabetes (342,343). Recent events have focused attention on the contribution of social determinants of health and a lack of equity in the delivery of care to disparate and unfavorable outcomes. Today, the major opportunities to improve diabetes outcomes in the near term come from more effective implementation of best evidence through organization of care at all levels (national to individual practices) and from addressing social determinants of health. Every reader of this consensus report has a role to play in better implementation with a focus on equity. For providers, that could involve a focus on shared decision-making to improve adherence to behavioral and medication interventions as well as organizing practice to minimize therapeutic inertia and enhance engagement and support for all people with diabetes. For policy makers, health care systems, payors, and companies with marketed products or services, ensuring equitable access to minimize health disparities should be a priority.
Broad support for basic science is necessary to bring about the next generation of interventions. Implementation science is an essential area for future work, particularly in the context of “learning health care systems,” in which internal data are systematically integrated with published evidence to drive quality improvement (344–346). Precision medicine initiatives, whether omics-based or focused on social determinants of health, aim to optimally target interventions based on the wide heterogeneity of the population affected by diabetes. Precision medicine has tremendous but largely unrealized promise. When these efforts are driven by real-world data, causal inference study design and analysis create greater confidence in the implementation and evaluation of insights. Studies should be conducted to support the better understanding of precision medicine approaches to the full spectrum of diabetes interventions, from medications to behavioral treatments and diabetes support.
Several key areas where further research could better inform future consensus reports were of particular interest to the writing group. For each area, one could add the need for more precision medicine insights and a better understanding of the full spectrum of investigations that are supporting efforts to advance the field from basic to implementation science. With upwards of 10% of the population affected by diabetes and the enormous attendant costs, a focus on individualizing care to make sure that the right person is getting the right therapy at the right time while working to overcome barriers dependent on social determinants of health is essential. Regulatory reform, more efficient study conduct and analysis, coordinated global efforts in defining outcomes and data collection instruments, data sharing, exploration of new forms of health care delivery (e.g., telehealth), and increased efforts to reach underserved populations, as were made to address COVID-19, would accelerate progress in defining and implementing optimal approaches for diabetes care.
Study conduct. Across the spectrum from highly controlled trials to observational studies, paying greater attention to subgroups, in particular vulnerable populations, is essential. Dedicated studies in young adults with type 2 diabetes, or including much larger numbers of younger adults in broader studies, are essential to better understand how to mitigate their high risk of early disability. As more younger adults are being treated with therapies that have been inadequately studied in pregnancy, it is essential to describe the reproductive safety of recommended approaches. Similarly, there have been inadequate studies of frail older people and those aged >75 years with regard to understanding both appropriate targets and interventions to minimize harms and maximize quality of life. Sex balance is another dimension where our present studies fail to be representative. Better recruitment, retention, and analysis to ensure safety and effectiveness in populations historically underrepresented in studies and generally suffering from health inequities is a minimal first step to enhance health justice by sex, race/ethnicity, nationality, etc.
Weight management. With the emergence of more effective behavioral and medical therapies and novel surgical approaches for the treatment of people who are overweight with diabetes, more direct comparisons are required to better target interventions based on impact and cost-effectiveness.
Targets. Studies designed to explicitly examine glucose-centric versus weight-centric approaches to diabetes management are needed. The impact of prioritizing early aggressive therapy to induce remission is unclear.
Cardiorenal protection. Data are required to better inform when to select a GLP-1 RA and/or an SGLT2i in the setting of CVD but without HF or CKD and to fully validate the recommendation for combination therapy in those at high risk who do not meet glycemic targets. As discussed, there is considerable uncertainty about the absolute benefits of GLP-1 RA and SGLT2i for CVD outcomes in those with risk factors only. As a result, there is variability in the recommendations on how to define high-risk people with diabetes, to whom these disease-modifying agents should be prescribed to have the greatest benefit/impact. As all people with diabetes are at high risk of CVD, HF, and CKD over time, real-world evidence and cost-effectiveness studies of GLP-1 RA and SGLT2i in broad populations would help to better target interventions to have the greatest impact on outcomes.
Glucose monitoring. Further studies to understand the role and optimal implementation of CGM and/or episodic CGM in type 2 diabetes are needed.
Comorbidities. There are numerous studies underway to understand the role of interventions in the setting of NAFLD and cognitive impairment. NAFLD is highly prevalent, and thus understanding the impact of interventions on person-centered outcomes and costs is essential. Cognitive impairment is a major burden to people with diabetes, their families, and society; better understanding of the pathophysiology and the impact of interventions is a challenging but high-reward area for investigation. There are virtually no data to inform best practice in the care of people with diabetes and advanced CKD, particularly in dialysis-dependent kidney disease. Additional studies, particularly of GLP-1 RA, GIP and GLP-1 RA, and SGLT2i, will hopefully provide new avenues to reduce mortality in this population, in which there are enormous health disparities.
Screening and prevention. Screening for diabetes and its complications and comorbidities remains inadequate. Early intervention to prevent progression is also generally suboptimal. National health care systems should comprehensively assess the implementation of recommendations and create incentives for effective programs. To optimally target resources, additional studies may be required on natural history and subpopulations, as much of the rationale for screening is based on studies conducted decades ago.
Technology. Remote care, wearables, apps, and decision support aids have exploded in availability, and a clear rationale exists as to why they may be of benefit. However, their optimal application is poorly understood.
Sleep and chronotype. Poor sleep is common and clearly associated with poor outcomes. Further studies are needed to understand behavioral sleep therapy and its benefits more fully as well as the benefits of medication and device aids. As chronotype is potentially modifiable, future research should focus on social and lifestyle factors to optimize interventional responses.
Until science and medicine bring us further insights, we recommend empathic, person-centered decision-making and support informed by an understanding of local resources and individual social determinants of health. Combined with consistent efforts to improve health behaviors (nutrition, activity, sleep, and self-monitoring) and to provide DSMES, these form the foundation of diabetes management. In this context, acceptance of, adherence to, and persistence with medical and behavioral interventions to support cardiorenal health, cardiovascular risk reduction, and attainment of glycemic and weight goals will prevent complications and optimize quality of life. We must establish and refine quality improvement efforts in diabetes care at the local level to equitably implement evidence-based interventions for the benefit of all people with type 2 diabetes.
Article Information
Acknowledgments. F. Zaccardi performed the literature searches and M. Bonar, C. Franklin, and S. Jamal assisted with the conception and execution of figures and tables; T. Yates and J. Henson supported the production and content of Fig. 2 (all from Leicester Diabetes Centre, University of Leicester and the University Hospitals of Leicester NHS Trust). T. Karagiannis (Clinical Research and Evidence-Based Medicine Unit, Aristotle University of Thessaloniki, Thessaloniki, Greece) assisted in the credibility assessment and interpretation of meta-analyses evaluating the effects of glucose-lowering medications across subgroup populations and contributed in applying GRADE guidance in the formulation of respective practice recommendations. D. Bradley (Ohio State University College of Medicine, Columbus, OH), P. Home (Newcastle University, Newcastle, U.K.), M.S. Kirkman (University of North Carolina, Chapel Hill, NC), S. Dinneen (Galway University Hospitals, Galway, Ireland), H.W. Rodbard (Adventis HealthCare Shady Grove Medical Center, Rockville, MD), G. Sesti (Sapienza University of Rome, Rome, Italy), P. Newland-Jones (University of Southampton, Southampton, U.K.), E. Montanya (University of Barcelona, Barcelona, Spain), and M. Nauck (Medical Department I, St. Josef-Hospital [Ruhr-University Bochum], Bochum, Germany) all served as invited reviewers. We acknowledge the support of N.A. El Sayed, R.R. Bannuru, M. Saraco, and M.I. Hill (all ADA, Arlington, VA), P. Niemann and N. Buckley-Mühge (both EASD, Dusseldorf, Germany), the Committee for Clinical Affairs of the EASD, and the Professional Practice Committee of the ADA.
Data Availability. Details of the search strategy and list of identified articles can be found at https://data.mendeley.com/datasets/h5rcnxpk8w/2.
Funding. This activity was funded by the American Diabetes Association and the European Association for the Study of Diabetes.
Duality of Interest. M.J.D. has acted as a consultant, advisory board member, and speaker for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Sanofi, an advisory board member and speaker for AstraZeneca, an advisory board member for Janssen, Lexicon, Pfizer, and ShouTi Pharma, and as a speaker for Napp Pharmaceuticals, Novartis, and Takeda Pharmaceuticals International. Her institution has received grants from Novo Nordisk, Sanofi, Eli Lilly, Boehringer Ingelheim, AstraZeneca, and Janssen. V.R.A. has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi. V.R.A.’s spouse is an employee of Janssen and a former employee of Merck. V.R.A.’s employer institution has received research funding for her role as investigator on clinical trials from Applied Therapeutics, Medpace, Eli Lilly, Fractyl, Premier, Novo Nordisk, and Sanofi. B.S.C. is a nominating work group member of the American Academy of Physician Assistants. R.A.G. is an advisor to Vida and Lark. J.G. is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. She conducts research at her institution for Boehringer Ingelheim/Lilly, Merck, and Roche. N.M.M. is under a license agreement between Johns Hopkins HealthCare Solutions and Johns Hopkins University. She and the university are entitled to royalty distributions related to an online diabetes prevention program. S.E.R. participated in at least one advisory board for Bayer, Traverse, and AstraZeneca. Her employer receives industry research support from Bayer and Astra Zeneca. S.D.P. is a member of the advisory board for Abbott, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Hengrui Pharmaceuticals, Menarini International, Novo Nordisk, Sanofi, and Vertex. He is a participant in a speaker’s bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novo Nordisk, Sanofi, and Takeda. His employer receives research funding from AstraZeneca and Boheringer Ingelheim. C.M. serves or has served on the advisory panel for Novo Nordisk, Sanofi, MSD, Eli Lilly, Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Medtronic, ActoBio Therapeutics, Pfizer, Insulet, and Zealand Pharma. Financial compensation for these activities has been received by KU Leuven. KU Leuven has received research support for C.M. from Medtronic, Novo Nordisk, Sanofi, and ActoBio Therapeutics. C.M. serves or has served on the speaker’s bureau for Novo Nordisk, Sanofi, Eli Lilly, Boehringer Ingelheim, AstraZeneca, and Novartis. Financial compensation for these activities has been received by KU Leuven. G.M. is a consultant to Novo Nordisk, Fractyl, Recor, Keyron, and Metadeq and is on the scientific board of Fractyl. P.R.’s institution has received industry research funding from AstraZeneca and Novo Nordisk. Her institution has also received consultancy fees from AstraZeneca, Bayer, Boehringer Ingelheim, Novo Nordisk, Gilead, and MSD and lecture fees from Sanofi, Astellas, Novo Nordisk, Bayer, and AstraZeneca. T.T. is on the advisory board for Boehringer Ingelheim, AstraZeneca, Sanofi, Novo Nordisk, and Eli Lilly and in the speaker’s bureau for Boehringer Ingelheim, AstraZeneca, Sanofi, Novo Nordisk, Eli Lilly, MSD, Servier, and Merck. A.T. has served on the advisory board for Novo Nordisk and Boehringer Ingelheim, and his university has received research funding. His university also receives funding for educational and research support from Eli Lilly. J.B.B. is a paid consultant to Anji Pharmaceuticals, Boehringer Ingelheim, Eli Lilly, Fortress Biotech, Janssen, Mellitus Health, Moderna, Pendulum Therapeutics, Praetego, ReachMD, Stability Health, and Zealand Pharma. He is a member of the advisory board for Boehringer Ingelheim, Eli Lilly, Mellitus Health, Moderna, Novo Nordisk, Pendulum Therapeutics, Praetego, Stability Health, vTv Therapeutics, and Zealand Pharma. His employer receives research funding from Dexcom, Eli Lilly, NovaTarg, Novo Nordisk, Sanofi, Tolerion, and vTv Therapeutics. He is an investor in Mellitus Health, Pendulum Therapeutics, and PhaseBio.
Author Contributions. All authors were responsible for drafting the article and revising it critically for important intellectual content. All authors approved the version to be published.
Footnotes
This article is being simultaneously published in Diabetologia (https://doi.org/10.1007/s00125-022-05787-2) and Diabetes Care (https://doi.org/10.2337/dci22-0034) by the European Association for the Study of Diabetes and American Diabetes Association.
A consensus report of a particular topic contains a comprehensive examination and is authored by an expert panel and represents the panel’s collective analysis, evaluation and opinion. M.J.D. and J.B.B. were co-chairs for the Consensus Report Writing Group. V.R.A., B.S.C., R.A.G., J.G., N.M.M., and S.E.R. were the writing group members for ADA. S.D.P., C.M., G.M., P.R., T.T., and A.T. were the writing group members for EASD. The article was reviewed for EASD by its Committee on Clinical Affairs and approved by its Executive Board. The article was reviewed for ADA by its Professional Practice Committee.
This article contains supplementary material online at https://doi.org/10.2337/figshare.20800537.
References
- 1. Rodriguez-Gutierrez R, Gionfriddo MR, Ospina NS, et al. Shared decision making in endocrinology: present and future directions. Lancet Diabetes Endocrinol 2016;4:706–716 [DOI] [PubMed] [Google Scholar]
- 2. Draznin B, Aroda VR, Bakris G, et al.; American Diabetes Association Professional Practice Committee . 6. Glycemic targets: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S83–S96 [DOI] [PubMed] [Google Scholar]
- 3. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centred approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–1596 [DOI] [PubMed] [Google Scholar]
- 4. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015;58:429–442 [DOI] [PubMed] [Google Scholar]
- 5. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018;61:2461–2498 [DOI] [PubMed] [Google Scholar]
- 6. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2020;63:221–228 [DOI] [PubMed] [Google Scholar]
- 7. Schandelmaier S, Briel M, Varadhan R, et al. Development of the instrument to assess the credibility of effect modification analyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ 2020;192:E901–E906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun X, Ioannidis JPA, Agoritsas T, Alba AC, Guyatt G. How to use a subgroup analysis: users’ guide to the medical literature. JAMA 2014;311:405–411 [DOI] [PubMed] [Google Scholar]
- 9. Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 2013;66:719–725 [DOI] [PubMed] [Google Scholar]
- 10. Santesso N, Glenton C, Dahm P, et al.; GRADE Working Group . GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol 2020;119:126–135 [DOI] [PubMed] [Google Scholar]
- 11. Sun S, Hisland L, Grenet Get al. Reappraisal of the efficacy of intensive glycaemic control on microvascular complications in patients with type 2 diabetes: a meta-analysis of randomised control-trials. Therapie 2021;77:413–423 [DOI] [PubMed] [Google Scholar]
- 12. Agrawal L, Azad N, Bahn GD, et al.; VADT Study Group . Long-term follow-up of intensive glycaemic control on renal outcomes in the Veterans Affairs Diabetes Trial (VADT). Diabetologia 2018;61:295–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lind M, Imberg H, Coleman RL, Nerman O, Holman RR. Historical HbA1c values may explain the type 2 diabetes legacy effect: UKPDS 88. Diabetes Care 2021;44:2231–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Riddle MC, Gerstein HC, Holman RR, et al. A1C targets should be personalized to maximize benefits while limiting risks. Diabetes Care 2018;41:1121–1124 [DOI] [PubMed] [Google Scholar]
- 15. Sargeant JA, Brady EM, Zaccardi F, et al. Adults with early-onset type 2 diabetes (aged 18-39 years) are severely underrepresented in diabetes clinical research trials. Diabetologia 2020;63:1516–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Diabetes Association Professional Practice Committee; Draznin B, Aroda VR, Bakris G, et al. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes–2022. Diabetes Care 2022;45(Suppl. 1):S125–S143 [DOI] [PubMed] [Google Scholar]
- 17. Crabtree T, Ogendo JJ, Vinogradova Y, Gordon J, Idris I. Intensive glycemic control and macrovascular, microvascular, hypoglycemia complications and mortality in older (age ≥60years) or frail adults with type 2 diabetes: a systematic review and meta-analysis from randomized controlled trial and observation studies. Expert Rev Endocrinol Metab 2022;17:255–267 [DOI] [PubMed] [Google Scholar]
- 18. Dickinson JK, Guzman SJ, Maryniuk MD, et al. The use of language in diabetes care and education. Diabetes Care 2017;40:1790–1799 [DOI] [PubMed] [Google Scholar]
- 19. Powers MA, Bardsley JK, Cypress M, et al. Diabetes self-management education and support in adults with type 2 diabetes: a consensus report of the American Diabetes Association, the Association of Diabetes Care & Education Specialists, the Academy of Nutrition and Dietetics, the American Academy of Family Physicians, the American Academy of PAs, the American Association of Nurse Practitioners, and the American Pharmacists Association. Diabetes Care 2020;43:1636–1649 [DOI] [PubMed] [Google Scholar]
- 20. Davis J, Fischl AH, Beck J, et al. 2022 National standards for diabetes self-management education and support. Diabetes Care 2022;45:484–494 [DOI] [PubMed] [Google Scholar]
- 21. National Institute for Health and Care Excellence . Type 2 diabetes in adults: management. Recommendations. NICE guideline [NG28]. London, U.K., National Institute for Health and Care Excellence, 2022. Accessed 4 June 2022. Available from www.nice.org.uk/guidance/ng28/chapter/Recommendations#patient-education
- 22. Draznin B, Aroda VR, Bakris G, et al.; American Diabetes Association Professional Practice Committee . 5. Facilitating behavior change and well-being to improve health outcomes: Standards of Medical Care in Diabetes–2022. Diabetes Care 2022;45(Suppl. 1):S60–S82 [DOI] [PubMed] [Google Scholar]
- 23. Department of Health and Diabetes UK . Structured patient education in diabetes: report from the Patient Education Working Group. London, U.K., Department of Health and Diabetes UK, 2005. Accessed 5 August 2022. Available from www.diabetes.org.uk/resources-s3/2017-11/structuredpatiented.pdf
- 24. National Institute for Health and Clinical Excellence . Diabetes in adults. Quality statements 2 and 3. Quality standard [QS6]. London, U.K., National Institute for Health and Clinical Excellence, 2016. Accessed 18 August 2022. Available from www.nice.org.uk/guidance/qs6
- 25. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns 2016;99:926–943 [DOI] [PubMed] [Google Scholar]
- 26. Pillay J, Armstrong MJ, Butalia S, et al. Behavioral programs for type 2 diabetes mellitus: a systematic review and network meta-analysis. Ann Intern Med 2015;163:848–860 [DOI] [PubMed] [Google Scholar]
- 27. Zhao FF, Suhonen R, Koskinen S, Leino-Kilpi H. Theory-based self-management educational interventions on patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. J Adv Nurs 2017;73:812–833 [DOI] [PubMed] [Google Scholar]
- 28. Odgers-Jewell K, Ball LE, Kelly JT, Isenring EA, Reidlinger DP, Thomas R. Effectiveness of group-based self-management education for individuals with type 2 diabetes: a systematic review with meta-analyses and meta-regression. Diabet Med 2017;34:1027–1039 [DOI] [PubMed] [Google Scholar]
- 29. He X, Li J, Wang B, et al. Diabetes self-management education reduces risk of all-cause mortality in type 2 diabetes patients: a systematic review and meta-analysis. Endocrine 2017;55:712–731 [DOI] [PubMed] [Google Scholar]
- 30. Chatterjee S, Davies MJ, Heller S, Speight J, Snoek FJ, Khunti K. Diabetes structured self-management education programmes: a narrative review and current innovations. Lancet Diabetes Endocrinol 2018;6:130–142 [DOI] [PubMed] [Google Scholar]
- 31. Captieux M, Pearce G, Parke HL, et al. Supported self-management for people with type 2 diabetes: a meta-review of quantitative systematic reviews. BMJ Open 2018;8:e024262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lindekilde N, Scheuer SH, Rutters F, et al. Prevalence of type 2 diabetes in psychiatric disorders: an umbrella review with meta-analysis of 245 observational studies from 32 systematic reviews. Diabetologia 2022;65:440–456 [DOI] [PubMed] [Google Scholar]
- 33. Dening J, Islam SMS, George E, Maddison R. Web-based interventions for dietary behavior in adults with type 2 diabetes: systematic review of randomized controlled trials. J Med Internet Res 2020;22:e16437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nkhoma DE, Soko CJ, Banda KJ, Greenfield D, Li Y-CJ, Iqbal U. Impact of DSMES app interventions on medication adherence in type 2 diabetes mellitus: systematic review and meta-analysis. BMJ Health Care Inform 2021;28:e100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Omar MA, Hasan S, Palaian S, Mahameed S. The impact of a self-management educational program coordinated through WhatsApp on diabetes control. Pharm Pract (Granada) 2020;18:1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quinn LM, Davies MJ, Northern A, et al. Use of MyDesmond digital education programme to support self-management in people with type 2 diabetes during the COVID-19 pandemic. Diabet Med 2021;38:e14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gershkowitz BD, Hillert CJ, Crotty BH. Digital coaching strategies to facilitate behavioral change in type 2 diabetes: a systematic review. J Clin Endocrinol Metab 2021;106:e1513–e1520 [DOI] [PubMed] [Google Scholar]
- 38. Lee MK, Lee DY, Ahn HY, Park CY. A novel user utility score for diabetes management using tailored mobile coaching: secondary analysis of a randomized controlled trial. JMIR Mhealth Uhealth 2021;9:e17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018;6:361–369 [DOI] [PubMed] [Google Scholar]
- 40. Pigeyre M, Hess S, Gomez MF, et al. Validation of the classification for type 2 diabetes into five subgroups: a report from the ORIGIN trial. Diabetologia 2022;65:206–215 [DOI] [PubMed] [Google Scholar]
- 41. American Diabetes Association Professional Practice Committee . 1. Improving care and promoting health in populations: Standards of Medical Care in Diabetes–2022. Diabetes Care 2022;45(Suppl. 1):S8–S16 [DOI] [PubMed] [Google Scholar]
- 42. Kunneman M, Montori VM, Castaneda-Guarderas A, Hess EP. What is shared decision making? (And what it is not). Acad Emerg Med 2016;23:1320–1324 [DOI] [PubMed] [Google Scholar]
- 43. Breslin M, Mullan RJ, Montori VM. The design of a decision aid about diabetes medications for use during the consultation with patients with type 2 diabetes. Patient Educ Couns 2008;73:465–472 [DOI] [PubMed] [Google Scholar]
- 44. Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med 2009;169:1560–1568 [DOI] [PubMed] [Google Scholar]
- 45. Stacey D, Légaré F, Lewis Ket al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2017;4:CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Haire-Joshu D, Hill-Briggs F. The next generation of diabetes translation: a path to health equity. Annu Rev Public Health 2019;40:391–410 [DOI] [PubMed] [Google Scholar]
- 47. Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care 2020;44:258–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hill-Briggs F, Ephraim PL, Vrany EA, et al. Social determinants of health, race, and diabetes population health improvement: Black/African Americans as a population exemplar. Curr Diab Rep 2022;22:117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Young-Hyman D, de Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016;39:2126–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lingvay I, Sumithran P, Cohen RV, le Roux CW. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet 2022;399:394–405 [DOI] [PubMed] [Google Scholar]
- 51. Riddle MC, Cefalu WT, Evans PH, et al. Consensus report: definition and interpretation of remission in type 2 diabetes. Diabetes Care 2021;44:2438–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. American Diabetes Association Professional Practice Committee . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S17–S38 [DOI] [PubMed] [Google Scholar]
- 53. Draznin B, Aroda VR, Bakris G, et al.; American Diabetes Association Professional Practice Committee . 7. Diabetes technology: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S97–S112 [DOI] [PubMed] [Google Scholar]
- 54. Mannucci E, Antenore A, Giorgino F, Scavini M. Effects of structured versus unstructured self-monitoring of blood glucose on glucose control in patients with non-insulin-treated type 2 diabetes: a meta-analysis of randomized controlled trials. J Diabetes Sci Technol 2018;12:183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Young LA, Buse JB, Weaver MA, et al.; Monitor Trial Group . Glucose self-monitoring in non-insulin-treated patients with type 2 diabetes in primary care settings: a randomized trial. JAMA Intern Med 2017;177:920–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. National Institute for Health and Care Excellence . Type 2 diabetes in adults: management. Overview. NICE guideline [NG28]. London, U.K., National Institute for Health and Clinical Excellence. Accessed 28 July 2022. Available from www.nice.org.uk/guidance/ng28
- 57. Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 2018;41:2370–2376 [DOI] [PubMed] [Google Scholar]
- 58. Egede LE, Gebregziabher M, Echols C, Lynch CP. Longitudinal effects of medication nonadherence on glycemic control. Ann Pharmacother 2014;48:562–570 [DOI] [PubMed] [Google Scholar]
- 59. Huber CA, Reich O. Medication adherence in patients with diabetes mellitus: does physician drug dispensing enhance quality of care? Evidence from a large health claims database in Switzerland. Patient Prefer Adherence 2016;10:1803–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Iglay K, Cartier SE, Rosen VM, et al. Meta-analysis of studies examining medication adherence, persistence, and discontinuation of oral antihyperglycemic agents in type 2 diabetes. Curr Med Res Opin 2015;31:1283–1296 [DOI] [PubMed] [Google Scholar]
- 61. McGovern A, Tippu Z, Hinton W, Munro N, Whyte M, de Lusignan S. Comparison of medication adherence and persistence in type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes Metab 2018;20:1040–1043 [DOI] [PubMed] [Google Scholar]
- 62. Khunti K, Seidu S, Kunutsor S, Davies M. Association between adherence to pharmacotherapy and outcomes in type 2 diabetes: a meta-analysis. Diabetes Care 2017;40:1588–1596 [DOI] [PubMed] [Google Scholar]
- 63. Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence 2016;10:1299–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Konstantinou P, Kassianos AP, Georgiou G, et al. Barriers, facilitators, and interventions for medication adherence across chronic conditions with the highest non-adherence rates: a scoping review with recommendations for intervention development. Transl Behav Med 2020;10:1390–1398 [DOI] [PubMed] [Google Scholar]
- 65. Lasalvia P, Barahona-Correa JE, Romero-Alvernia DM, et al. Pen devices for insulin self-administration compared with needle and vial: systematic review of the literature and meta-analysis. J Diabetes Sci Technol 2016;10:959–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Khunti K, Davies MJ. Clinical inertia—time to reappraise the terminology? Prim Care Diabetes 2017;11:105–106 [DOI] [PubMed] [Google Scholar]
- 67. Furler J, O’Neal D, Speight J, et al. Supporting insulin initiation in type 2 diabetes in primary care: results of the Stepping Up pragmatic cluster randomised controlled clinical trial. BMJ 2017;356:j783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Manski-Nankervis JA, Furler J, O’Neal D, Ginnivan L, Thuraisingam S, Blackberry I. Overcoming clinical inertia in insulin initiation in primary care for patients with type 2 diabetes: 24-month follow-up of the Stepping Up cluster randomised controlled trial. Prim Care Diabetes 2017;11:474–481 [DOI] [PubMed] [Google Scholar]
- 69. Tabesh M, Magliano DJ, Koye DN, Shaw JE. The effect of nurse prescribers on glycaemic control in type 2 diabetes: a systematic review and meta-analysis. Int J Nurs Stud 2018;78:37–43 [DOI] [PubMed] [Google Scholar]
- 70. Murphy ME, Byrne M, Galvin R, Boland F, Fahey T, Smith SM. Improving risk factor management for patients with poorly controlled type 2 diabetes: a systematic review of healthcare interventions in primary care and community settings. BMJ Open 2017;7:e015135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. American Diabetes Association . Introduction: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S1–S2 [DOI] [PubMed] [Google Scholar]
- 72. Sainsbury E, Kizirian NV, Partridge SR, Gill T, Colagiuri S, Gibson AA. Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract 2018;139:239–252 [DOI] [PubMed] [Google Scholar]
- 73. Snorgaard O, Poulsen GM, Andersen HK, Astrup A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res Care 2017;5:e000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. van Zuuren EJ, Fedorowicz Z, Kuijpers T, Pijl H. Effects of low-carbohydrate- compared with low-fat-diet interventions on metabolic control in people with type 2 diabetes: a systematic review including GRADE assessments. Am J Clin Nutr 2018;108:300–331 [DOI] [PubMed] [Google Scholar]
- 75. Schwingshackl L, Chaimani A, Hoffmann G, Schwedhelm C, Boeing H. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur J Epidemiol 2018;33:157–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Martínez-González MA, Gea A, Ruiz-Canela M. The Mediterranean diet and cardiovascular health. Circ Res 2019;124:779–798 [DOI] [PubMed] [Google Scholar]
- 77. Estruch R, Ros E, Salas-Salvadó J, et al.; PREDIMED Study Investigators . Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 2018;378:e34. [DOI] [PubMed] [Google Scholar]
- 78. Papamichou D, Panagiotakos DB, Itsiopoulos C. Dietary patterns and management of type 2 diabetes: a systematic review of randomised clinical trials. Nutr Metab Cardiovasc Dis 2019;29:531–543 [DOI] [PubMed] [Google Scholar]
- 79. Wang X, Li Q, Liu Y, Jiang H, Chen W. Intermittent fasting versus continuous energy-restricted diet for patients with type 2 diabetes mellitus and metabolic syndrome for glycemic control: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 2021;179:109003. [DOI] [PubMed] [Google Scholar]
- 80. Carter S, Clifton PM, Keogh JB. Effect of intermittent compared with continuous energy restricted diet on glycemic control in patients with type 2 diabetes: a randomized noninferiority trial. JAMA Netw Open 2018;1:e180756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Carter S, Clifton PM, Keogh JB. The effect of intermittent compared with continuous energy restriction on glycaemic control in patients with type 2 diabetes: 24-month follow-up of a randomised noninferiority trial. Diabetes Res Clin Pract 2019;151:11–19 [DOI] [PubMed] [Google Scholar]
- 82. Corley BT, Carroll RW, Hall RM, Weatherall M, Parry-Strong A, Krebs JD. Intermittent fasting in type 2 diabetes mellitus and the risk of hypoglycaemia: a randomized controlled trial. Diabet Med 2018;35:588–594 [DOI] [PubMed] [Google Scholar]
- 83. O’Neil PM, Miller-Kovach K, Tuerk PW, et al. Randomized controlled trial of a nationally available weight control program tailored for adults with type 2 diabetes. Obesity (Silver Spring) 2016;24:2269–2277 [DOI] [PubMed] [Google Scholar]
- 84. Mottalib A, Salsberg V, Mohd-Yusof BN, et al. Effects of nutrition therapy on HbA1c and cardiovascular disease risk factors in overweight and obese patients with type 2 diabetes. Nutr J 2018;17:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chee WSS, Gilcharan Singh HK, Hamdy O, et al. Structured lifestyle intervention based on a trans-cultural diabetes-specific nutrition algorithm (tDNA) in individuals with type 2 diabetes: a randomized controlled trial. BMJ Open Diabetes Res Care 2017;5:e000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mohd Yusof BN, Hasbullah FY, Mohd Shahar AS, et al. Changes in dietary intake improve glycemic control following a structured nutrition therapy during Ramadan in individuals with type 2 diabetes. Clin Nutr ESPEN 2021;46:314–324 [DOI] [PubMed] [Google Scholar]
- 87. Mohd Yusof BN, Wan Zukiman WZHH, Abu Zaid Z, et al. Comparison of structured nutrition therapy for Ramadan with standard care in type 2 diabetes patients. Nutrients 2020;12:E813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 2018;391:541–551 [DOI] [PubMed] [Google Scholar]
- 89. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol 2019;7:344–355 [DOI] [PubMed] [Google Scholar]
- 90. Look AHEAD Research Group . Does lifestyle intervention improve health of adults with overweight/obesity and type 2 diabetes? Findings from the Look AHEAD randomized trial. Obesity (Silver Spring) 2021;29:1246–1258 [DOI] [PubMed] [Google Scholar]
- 91. Houston DK, Neiberg RH, Miller ME, et al. Physical function following a long-term lifestyle intervention among middle aged and older adults with type 2 diabetes: the Look AHEAD study. J Gerontol A Biol Sci Med Sci 2018;73:1552–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Garvey WT. Long-term health benefits of intensive lifestyle intervention in the Look AHEAD study. Obesity (Silver Spring) 2021;29:1242–1243 [DOI] [PubMed] [Google Scholar]
- 93. Wing RR, Bolin P, Brancati FL, et al.; Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gregg EW, Jakicic JM, Blackburn G, et al.; Look AHEAD Research Group . Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2016;4:913–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wing RR, Bray GA, Cassidy-Begay M, et al.; Look AHEAD Research Group . Effects of intensive lifestyle intervention on all-cause mortality in older adults with type 2 diabetes and overweight/obesity: results from the Look AHEAD study. Diabetes Care 2022;45:dc211805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. American Diabetes Association . 4. Comprehensive medical evaluation and assessment of comorbidities: Standards of Medical Care in Diabetes–2021. Diabetes Care 2021;44(Suppl. 1):S40–S52 [DOI] [PubMed] [Google Scholar]
- 97. Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev 2017;31:91–101 [DOI] [PubMed] [Google Scholar]
- 98. Schipper SBJ, Van Veen MM, Elders PJM, et al. Sleep disorders in people with type 2 diabetes and associated health outcomes: a review of the literature. Diabetologia 2021;64:2367–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Navarro DJ, Alpert PT, Cross C. The impact of shift work on diabetes self-management activities. J Dr Nurs Pract 2019;12:66–72 [DOI] [PubMed] [Google Scholar]
- 100. Henson J, Rowlands AV, Baldry E, et al.; CODEC Investigators . Physical behaviors and chronotype in people with type 2 diabetes. BMJ Open Diabetes Res Care 2020;8:e001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kanaley JA, Colberg SR, Corcoran MH, et al. Exercise/physical activity in individuals with type 2 diabetes: a consensus statement from the American College of Sports Medicine. Med Sci Sports Exerc 2022;54:353–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020;54:1451–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Homer AR, Taylor FC, Dempsey PC, et al. Frequency of interruptions to sitting time: benefits for postprandial metabolism in type 2 diabetes. Diabetes Care 2021;44:1254–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dempsey PC, Larsen RN, Sethi P, et al. Benefits for type 2 diabetes of interrupting prolonged sitting with brief bouts of light walking or simple resistance activities. Diabetes Care 2016;39:964–972 [DOI] [PubMed] [Google Scholar]
- 105. Rowlands A, Davies M, Dempsey P, Edwardson C, Razieh C, Yates T. Wrist-worn accelerometers: recommending ∼1.0 mg as the minimum clinically important difference (MCID) in daily average acceleration for inactive adults. Br J Sports Med 2021;55:814–815 [DOI] [PubMed] [Google Scholar]
- 106. Yates T, Haffner SM, Schulte PJ, et al. Association between change in daily ambulatory activity and cardiovascular events in people with impaired glucose tolerance (NAVIGATOR trial): a cohort analysis. Lancet 2014;383:1059–1066 [DOI] [PubMed] [Google Scholar]
- 107. Saint-Maurice PF, Troiano RP, Bassett DR Jr, et al. Association of daily step count and step intensity with mortality among US adults. JAMA 2020;323:1151–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Jayedi A, Emadi A, Shab-Bidar S. Dose-dependent effect of supervised aerobic exercise on HbA1c in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Sports Med 2022;52:1919–1938 [DOI] [PubMed] [Google Scholar]
- 109. Chudasama YV, Khunti KK, Zaccardi F, et al. Physical activity, multimorbidity, and life expectancy: a UK Biobank longitudinal study. BMC Med 2019;17:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pan B, Ge L, Xun YQ, et al. Exercise training modalities in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Int J Behav Nutr Phys Act 2018;15:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mickute M, Henson J, Rowlands AV, et al. Device-measured physical activity and its association with physical function in adults with type 2 diabetes mellitus. Diabet Med 2021;38:e14393. [DOI] [PubMed] [Google Scholar]
- 112. Ahmad E, Sargeant JA, Yates T, Webb DR, Davies MJ. Type 2 diabetes and impaired physical function: a growing problem. Diabetology 2022;3:30–45 [Google Scholar]
- 113. Smyth A, Jenkins M, Dunham M, Kutzer Y, Taheri S, Whitehead L. Systematic review of clinical practice guidelines to identify recommendations for sleep in type 2 diabetes mellitus management. Diabetes Res Clin Pract 2020;170:108532. [DOI] [PubMed] [Google Scholar]
- 114. Zuraikat FM, Makarem N, Redline S, Aggarwal B, Jelic S, St-Onge MP. Sleep regularity and cardiometabolic heath: is variability in sleep patterns a risk factor for excess adiposity and glycemic dysregulation? Curr Diab Rep 2020;20:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. International Diabetes Federation . (2017) The IDF Consensus Statement on sleep apnoea and type 2 diabetes. Brussels, Belgium, International Diabetes Federation, 2017. Accessed 5 June 2022. Available from www.idf.org/our-activities/advocacy-awareness/resources-and-tools/62-idf-consensus-statement-on-sleep-apnoea-and-type-2-diabetes.html
- 116. Fallahi A, Jamil DI, Karimi EB, Baghi V, Gheshlagh RG. Prevalence of obstructive sleep apnea in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Syndr 2019;13:2463–2468 [DOI] [PubMed] [Google Scholar]
- 117. Sondrup N, Termannsen AD, Eriksen JN, et al. Effects of sleep manipulation on markers of insulin sensitivity: a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev 2022;62:101594. [DOI] [PubMed] [Google Scholar]
- 118. Delevatti RS, Bracht CG, Lisboa SDC, et al. The role of aerobic training variables progression on glycemic control of patients with type 2 diabetes: a systematic review with meta-analysis. Sports Med Open 2019;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Najafipour F, Mobasseri M, Yavari A, et al. Effect of regular exercise training on changes in HbA1c, BMI and VO2max among patients with type 2 diabetes mellitus: an 8-year trial. BMJ Open Diabetes Res Care 2017;5:e000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Borror A, Zieff G, Battaglini C, Stoner L. The effects of postprandial exercise on glucose control in individuals with type 2 diabetes: a systematic review. Sports Med 2018;48:1479–1491 [DOI] [PubMed] [Google Scholar]
- 121. Guo S, Xu Y, Qin J, et al. Effect of tai chi on glycaemic control, lipid metabolism and body composition in adults with type 2 diabetes: a meta-analysis and systematic review. J Rehabil Med 2021;53:jrm00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gupta U, Gupta Y, Jose D, et al. Effectiveness of yoga-based exercise program compared to usual care, in improving HbA1c in individuals with type 2 diabetes: a randomized control trial. Int J Yoga 2020;13:233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. de Mello MB, Righi NC, Schuch FB, Signori LU, da Silva AMV. Effect of high-intensity interval training protocols on VO2max and HbA1c level in people with type 2 diabetes: a systematic review and meta-analysis. Ann Phys Rehabil Med 2022;65:101586. [DOI] [PubMed] [Google Scholar]
- 124. Draznin B, Aroda VR, Bakris G, et al.; American Diabetes Association Professional Practice Committee . 4. Comprehensive medical evaluation and assessment of comorbidities: Standards of Medical Care in Diabetes–2022. Diabetes Care 2022;45(Suppl. 1):S46–S59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tasali E, Wroblewski K, Kahn E, Kilkus J, Schoeller DA. Effect of sleep extension on objectively assessed energy intake among adults with overweight in real-life settings: a randomized clinical trial. JAMA Intern Med 2022;182:365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Depner CM, Melanson EL, Eckel RH, et al. Ad libitum weekend recovery sleep fails to prevent metabolic dysregulation during a repeating pattern of insufficient sleep and weekend recovery sleep. Curr Biol 2019;29:957–967.e4 [DOI] [PubMed] [Google Scholar]
- 127. Draznin B, Aroda VR, Bakris G, et al.; American Diabetes Association Professional Practice Committee . 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: Standards of Medical Care in Diabetes–2022. Diabetes Care 2022;45(Suppl. 1):S113–S124 [DOI] [PubMed] [Google Scholar]
- 128. Davies M, Færch L, Jeppesen OK, et al.; STEP 2 Study Group . Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021;397:971–984 [DOI] [PubMed] [Google Scholar]
- 129. Wilding JPH, Batterham RL, Calanna S, et al.; STEP 1 Study Group . Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med 2021;384:989–1002 [DOI] [PubMed] [Google Scholar]
- 130. Jastreboff AM, Aronne LJ, Ahmad NN, et al.; SURMOUNT-1 Investigators . Tirzepatide once weekly for the treatment of obesity. N Engl J Med 2022;387:205–216 [DOI] [PubMed] [Google Scholar]
- 131. Rubino D, Abrahamsson N, Davies M, et al.; STEP 4 Investigators . Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA 2021;325:1414–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Rubino F, Nathan DM, Eckel RH, et al.; Delegates of the 2nd Diabetes Surgery Summit . Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by International Diabetes Organizations. Diabetes Care 2016;39:861–877 [DOI] [PubMed] [Google Scholar]
- 133. Carmona MN, Santos-Sousa H, Lindeza L, et al.; CRI-O group . Comparative effectiveness of bariatric surgeries in patients with type 2 diabetes mellitus and BMI ≥ 25 kg/m2: a systematic review and network meta-analysis. Obes Surg 2021;31:5312–5321 [DOI] [PubMed] [Google Scholar]
- 134. Currie AC, Askari A, Fangueiro A, Mahawar K. Network meta-analysis of metabolic surgery procedures for the treatment of obesity and diabetes. Obes Surg 2021;31:4528–4541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Cresci B, Cosentino C, Monami M, Mannucci E. Metabolic surgery for the treatment of type 2 diabetes: a network meta-analysis of randomized controlled trials. Diabetes Obes Metab 2020;22:1378–1387 [DOI] [PubMed] [Google Scholar]
- 136. Rubio-Almanza M, Hervás-Marín D, Cámara-Gómez R, Caudet-Esteban J, Merino-Torres JF. Does metabolic surgery lead to diabetes remission in patients with BMI < 30 kg/m2?: A meta-analysis. Obes Surg 2019;29:1105–1116 [DOI] [PubMed] [Google Scholar]
- 137. Khorgami Z, Shoar S, Saber AA, Howard CA, Danaei G, Sclabas GM. Outcomes of bariatric surgery versus medical management for type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Obes Surg 2019;29:964–974 [DOI] [PubMed] [Google Scholar]
- 138. Fultang J, Chinaka U, Rankin J, Bakhshi A, Ali A. Preoperative bariatric surgery predictors of type 2 diabetes remission. J Obes Metab Syndr 2021;30:104–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Mingrone G, Panunzi S, De Gaetano A, et al. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2021;397:293–304 [DOI] [PubMed] [Google Scholar]
- 140. Aminian A, Kashyap SR, Wolski KE, et al. Patient-reported outcomes after metabolic surgery versus medical therapy for diabetes: insights from the STAMPEDE randomized trial. Ann Surg 2021;274:524–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. American Diabetes Association Professional Practice Committee . 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes–2022. Diabetes Care 2022;45(Suppl. 1):S144–S174 [DOI] [PubMed] [Google Scholar]
- 142. McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 2021;6:148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 144. Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 145. Wiviott SD, Raz I, Bonaca MPet al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 146. Cannon CP, Pratley R, Dagogo-Jack S, et al.; VERTIS CV Investigators . Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020;383:1425–1435 [DOI] [PubMed] [Google Scholar]
- 147. Bhatt DL, Szarek M, Pitt B, et al.; SCORED Investigators . Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med 2021;384:129–139 [DOI] [PubMed] [Google Scholar]
- 148. McMurray JJV, Solomon SD, Inzucchi SE, et al.; DAPA-HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008 [DOI] [PubMed] [Google Scholar]
- 149. Packer M, Anker SD, Butler J, et al.; EMPEROR-Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424 [DOI] [PubMed] [Google Scholar]
- 150. Bhatt DL, Szarek M, Steg PG, et al.; SOLOIST-WHF Trial Investigators . Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117–128 [DOI] [PubMed] [Google Scholar]
- 151. Anker SD, Butler J, Filippatos G, et al.; EMPEROR-Preserved Trial Investigators . Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461 [DOI] [PubMed] [Google Scholar]
- 152. Perkovic V, Jardine MJ, Neal B, et al.; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 153. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al.; DAPA-CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446 [DOI] [PubMed] [Google Scholar]
- 154. Boehringer Ingelheim . Prescribing information for JARDIANCE. Ingelheim am Rhein, Germany, 2022. Accessed 20 June 2022. Available from https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Jardiance/jardiance.pdf
- 155. Janssen . Prescribing information for INVOKANA. Beerse, Belgium, Janssen, 2020. Accessed 20 June 2022. Available from www.janssenlabels.com/package-insert/product-monograph/prescribing-information/INVOKANA-pi.pdf
- 156. AstraZeneca . Prescribing information for FARXIGA. Wilmington, DE, 2021. Accessed 20 June 2022. Available from https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/0be9cb1b-3b33-41c7-bfc2-04c9f718e442/0be9cb1b-3b33-41c7-bfc2-04c9f718e442_viewable_rendition__v.pdf
- 157. Merck . Prescribing information for STEGLATRO. Readington, NJ, Merck, 2017. Accessed 20 June 2022. Available from www.accessdata.fda.gov/drugsatfda_docs/label/2017/209803s000lbl.pdf
- 158. Mistry S, Eschler DC. Euglycemic diabetic ketoacidosis caused by SGLT2 inhibitors and a ketogenic diet: a case series and review of literature. AACE Clin Case Rep 2020;7:17–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kosiborod MN, Esterline R, Furtado RHM, et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 2021;9:586–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Qian BB, Chen Q, Li L, Yan CF. Association between combined treatment with SGLT2 inhibitors and metformin for type 2 diabetes mellitus on fracture risk: a meta-analysis of randomized controlled trials. Osteoporos Int 2020;31:2313–2320 [DOI] [PubMed] [Google Scholar]
- 161. Dorsey-Treviño EG, González-González JG, Alvarez-Villalobos N, et al. Sodium-glucose cotransporter 2 (SGLT-2) inhibitors and microvascular outcomes in patients with type 2 diabetes: systematic review and meta-analysis. J Endocrinol Invest 2020;43:289–304 [DOI] [PubMed] [Google Scholar]
- 162. Barraclough JY, Yu J, Figtree GA, et al. Cardiovascular and renal outcomes with canagliflozin in patients with peripheral arterial disease: data from the CANVAS Program and CREDENCE trial. Diabetes Obes Metab 2022;24:1072–1083 [DOI] [PubMed] [Google Scholar]
- 163. Heerspink HJL, Oshima M, Zhang H, et al. Canagliflozin and kidney-related adverse events in type 2 diabetes and CKD: findings from the randomized CREDENCE trial. Am J Kidney Dis 2022;79:244–256.e1 [DOI] [PubMed] [Google Scholar]
- 164. Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes–state-of-the-art. Mol Metab 2021;46:101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Novo Nordisk . Prescribing information for RYBELSUS. Plainsboro, NJ, Novo Nordisk, 2019. Accessed 20 June 2022. Available from www.accessdata.fda.gov/drugsatfda_docs/label/2019/213051s000lbl.pdf
- 166. Frias JP, Bonora E, Nevarez Ruiz L, et al. Efficacy and safety of dulaglutide 3.0 mg and 4.5 mg versus dulaglutide 1.5 mg in metformin-treated patients with type 2 diabetes in a randomized controlled trial (AWARD-11). Diabetes Care 2021;44:765–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Frías JP, Auerbach P, Bajaj HS, et al. Efficacy and safety of once-weekly semaglutide 2·0 mg versus 1·0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial. Lancet Diabetes Endocrinol 2021;9:563–574 [DOI] [PubMed] [Google Scholar]
- 168. Wharton S, Davies M, Dicker D, et al. Managing the gastrointestinal side effects of GLP-1 receptor agonists in obesity: recommendations for clinical practice. Postgrad Med 2022;134:14–19 [DOI] [PubMed] [Google Scholar]
- 169. Peng H, Want LL, Aroda VR. Safety and tolerability of glucagon-like peptide-1 receptor agonists utilizing data from the exenatide clinical trial development program. Curr Diab Rep 2016;16:44. [DOI] [PubMed] [Google Scholar]
- 170. Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab 2018;20:889–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Bethel MA, Diaz R, Castellana N, Bhattacharya I, Gerstein HC, Lakshmanan MC. HbA1c change and diabetic retinopathy during GLP-1 receptor agonist cardiovascular outcome trials: a meta-analysis and meta-regression. Diabetes Care 2021;44:290–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. He L, Wang J, Ping F, et al. Association of glucagon-like peptide-1 receptor agonist use with risk of gallbladder and biliary diseases: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med 2022;182:513–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Crowley MJ, McGuire DK, Alexopoulos AS, et al. Effects of liraglutide on cardiovascular outcomes in type 2 diabetes patients with and without baseline metformin use: post hoc analyses of the LEADER trial. Diabetes Care 2020;43:e108–e110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Neuen BL, Arnott C, Perkovic V, et al. Sodium-glucose co-transporter-2 inhibitors with and without metformin: a meta-analysis of cardiovascular, kidney and mortality outcomes. Diabetes Obes Metab 2021;23:382–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Masson W, Lavalle-Cobo A, Lobo M, Masson G, Molinero G. Novel antidiabetic drugs and risk of cardiovascular events in patients without baseline metformin use: a meta-analysis. Eur J Prev Cardiol 2021;28:69–75 [DOI] [PubMed] [Google Scholar]
- 176. Matthews D, Del Prato S, Mohan V, et al. Insights from VERIFY: early combination therapy provides better glycaemic durability than a stepwise approach in newly diagnosed type 2 diabetes. Diabetes Ther 2020;11:2465–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Lalau JD, Kajbaf F, Bennis Y, Hurtel-Lemaire AS, Belpaire F, De Broe ME. Metformin treatment in patients with type 2 diabetes and chronic kidney disease stages 3A, 3B, or 4. Diabetes Care 2018;41:547–553 [DOI] [PubMed] [Google Scholar]
- 178. Out M, Kooy A, Lehert P, Schalkwijk CA, Stehouwer CDA. Long-term treatment with metformin in type 2 diabetes and methylmalonic acid: post hoc analysis of a randomized controlled 4.3year trial. J Diabetes Complications 2018;32:171–178 [DOI] [PubMed] [Google Scholar]
- 179. Aroda VR, Edelstein SL, Goldberg RB, et al.; Diabetes Prevention Program Research Group . Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab 2016;101:1754–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Perkovic V, Toto R, Cooper ME, et al.; CARMELINA investigators . Effects of linagliptin on cardiovascular and kidney outcomes in people with normal and reduced kidney function: secondary analysis of the CARMELINA randomized trial. Diabetes Care 2020;43:1803–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Umpierrez GE, Cardona S, Chachkhiani D, et al. A randomized controlled study comparing a DPP4 inhibitor (linagliptin) and basal insulin (glargine) in patients with type 2 diabetes in long-term care and skilled nursing facilities: linagliptin-LTC trial. J Am Med Dir Assoc 2018;19:399–404.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Batule S, Ramos A, Pérez-Montes de Oca A, et al. Comparison of glycemic variability and hypoglycemic events in hospitalized older adults treated with basal insulin plus vildagliptin and basal-bolus insulin regimen: a prospective randomized study. J Clin Med 2022;11:2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet 2021;398:143–155 [DOI] [PubMed] [Google Scholar]
- 184. Dahl D, Onishi Y, Norwood P, et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA 2022;327:534–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Frías JP, Davies MJ, Rosenstock J, et al.; SURPASS-2 Investigators . Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 2021;385:503–515 [DOI] [PubMed] [Google Scholar]
- 186. Ludvik B, Giorgino F, Jódar E, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet 2021;398:583–598 [DOI] [PubMed] [Google Scholar]
- 187. Del Prato S, Kahn SE, Pavo I, et al.; SURPASS-4 Investigators . Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 2021;398:1811–1824 [DOI] [PubMed] [Google Scholar]
- 188. Gastaldelli A, Cusi K, Fernández Landó L, Bray R, Brouwers B, Rodríguez Á. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol 2022;10:393–406 [DOI] [PubMed] [Google Scholar]
- 189. Karagiannis T, Avgerinos I, Liakos A, et al. Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: a systematic review and meta-analysis. Diabetologia 2022;65:1251–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Sattar N, McGuire DK, Pavo I, et al. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med 2022;28:591–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Khunti K, Chatterjee S, Gerstein HC, Zoungas S, Davies MJ. Do sulphonylureas still have a place in clinical practice? Lancet Diabetes Endocrinol 2018;6:821–832 [DOI] [PubMed] [Google Scholar]
- 192. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 193. Vaccaro O, Masulli M, Nicolucci A, et al.; Thiazolidinediones Or Sulfonylureas Cardiovascular Accidents Intervention Trial (TOSCA.IT) study group; Italian Diabetes Society . Effects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): a randomised, multicentre trial. Lancet Diabetes Endocrinol 2017;5:887–897 [DOI] [PubMed] [Google Scholar]
- 194. Rosenstock J, Kahn SE, Johansen OE, et al.; CAROLINA Investigators . Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA 2019;322:1155–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Rosenstock J, Perkovic V, Johansen OE, et al.; CARMELINA Investigators . Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA 2019;321:69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Kahn SE, Haffner SM, Heise MA, et al.; ADOPT Study Group . Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 197. Dormandy JA, Charbonnel B, Eckland DJA, et al.; PROactive Investigators . Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279–1289 [DOI] [PubMed] [Google Scholar]
- 198. Kernan WN, Viscoli CM, Furie KL, et al.; IRIS Trial Investigators . Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016;374:1321–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Spence JD, Viscoli CM, Inzucchi SE, et al.; IRIS Investigators . Pioglitazone therapy in patients with stroke and prediabetes: a post hoc analysis of the IRIS randomized clinical trial. JAMA Neurol 2019;76:526–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med 2016;165:305–315 [DOI] [PubMed] [Google Scholar]
- 201. Della Pepa G, Russo M, Vitale M, et al. Pioglitazone even at low dosage improves NAFLD in type 2 diabetes: clinical and pathophysiological insights from a subgroup of the TOSCA.IT randomised trial. Diabetes Res Clin Pract 2021;178:108984. [DOI] [PubMed] [Google Scholar]
- 202. Home PD, Pocock SJ, Beck-Nielsen H, et al.; RECORD Study Team . Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 2009;373:2125–2135 [DOI] [PubMed] [Google Scholar]
- 203. Hanefeld M, Brunetti P, Schernthaner GH, Matthews DR; QUARTET Study Group . One-year glycemic control with a sulfonylurea plus pioglitazone versus a sulfonylurea plus metformin in patients with type 2 diabetes. Diabetes Care 2004;27:141–147 [DOI] [PubMed] [Google Scholar]
- 204. Viscoli CM, Inzucchi SE, Young LH, et al.; IRIS Trial Investigators . Pioglitazone and risk for bone fracture: safety data from a randomized clinical trial. J Clin Endocrinol Metab 2017;102:914–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Kahn SE, Zinman B, Lachin JM, et al.; Diabetes Outcome Progression Trial (ADOPT) Study Group . Rosiglitazone-associated fractures in type 2 diabetes: an analysis from A Diabetes Outcome Progression Trial (ADOPT). Diabetes Care 2008;31:845–851 [DOI] [PubMed] [Google Scholar]
- 206. DeFronzo RA, Inzucchi S, Abdul-Ghani M, Nissen SE. Pioglitazone: the forgotten, cost-effective cardioprotective drug for type 2 diabetes. Diab Vasc Dis Res 2019;16:133–143 [DOI] [PubMed] [Google Scholar]
- 207. Marso SP, McGuire DK, Zinman B, et al.; DEVOTE Study Group . Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med 2017;377:723–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Gerstein HC, Bosch J, Dagenais GR, et al.; ORIGIN Trial Investigators . Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–328 [DOI] [PubMed] [Google Scholar]
- 209. Lowe RN, Williams B, Claus LW. Diabetes: how to manage patients experiencing hypoglycaemia. Drugs Context 2022;11:2021-9-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Mannucci E, Caiulo C, Naletto L, Madama G, Monami M. Efficacy and safety of different basal and prandial insulin analogues for the treatment of type 2 diabetes: a network meta-analysis of randomized controlled trials. Endocrine 2021;74:508–517 [DOI] [PubMed] [Google Scholar]
- 211. Chan J, Cheng-Lai A. Inhaled insulin: a clinical and historical review. Cardiol Rev 2017;25:140–146 [DOI] [PubMed] [Google Scholar]
- 212. Bergenstal RM, Peyrot M, Dreon DM, et al.; Calibra Study Group . Implementation of basal-bolus therapy in type 2 diabetes: a randomized controlled trial comparing bolus insulin delivery using an insulin patch with an insulin pen. Diabetes Technol Ther 2019;21:273–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Heinemann L, Parkin CG. Rethinking the viability and utility of inhaled insulin in clinical practice. J Diabetes Res 2018;2018:4568903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Aroda VR, Arulandu JR, Cannon AJ. Insulin/glucagon-like peptide-1 receptor agonist combination therapy for the treatment of type 2 diabetes: are two agents better than one? Clin Diabetes 2018;36:138–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Maiorino MI, Chiodini P, Bellastella G, et al. Free and fixed-ratio combinations of basal insulin and GLP-1 receptor agonists versus basal insulin intensification in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Obes Metab 2018;20:2309–2313 [DOI] [PubMed] [Google Scholar]
- 216. Blonde L, Rosenstock J, Del Prato S, et al. Switching to iGlarLixi versus continuing daily or weekly GLP-1 RA in type 2 diabetes inadequately controlled by GLP-1 RA and oral antihyperglycemic therapy: the LixiLan-G randomized clinical trial. Diabetes Care 2019;42:2108–2116 [DOI] [PubMed] [Google Scholar]
- 217. Linjawi S, Bode BW, Chaykin LB, et al. The efficacy of IDegLira (insulin degludec/liraglutide combination) in adults with type 2 diabetes inadequately controlled with a GLP-1 receptor agonist and oral therapy: DUAL III randomized clinical trial. Diabetes Ther 2017;8:101–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Aroda VR, Rosenstock J, Wysham C, et al.; LixiLan-L Trial Investigators . Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care 2016;39:1972–1980 [DOI] [PubMed] [Google Scholar]
- 219. Lingvay I, Pérez Manghi F, García-Hernández P, et al.; DUAL V Investigators . Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial. JAMA 2016;315:898–907 [DOI] [PubMed] [Google Scholar]
- 220. Buse JB, Vilsbøll T, Thurman J, et al.; NN9068-3912 (DUAL-II) Trial Investigators . Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care 2014;37:2926–2933 [DOI] [PubMed] [Google Scholar]
- 221. Kasthuri S, Poongothai S, Anjana RM, et al. Comparison of glycemic excursion using flash continuous glucose monitoring in patients with type 2 diabetes mellitus before and after treatment with voglibose. Diabetes Technol Ther 2021;23:213–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Dalsgaard NB, Gasbjerg LS, Hansen LS, et al. The role of GLP-1 in the postprandial effects of acarbose in type 2 diabetes. Eur J Endocrinol 2021;184:383–394 [DOI] [PubMed] [Google Scholar]
- 223. Tsapas A, Avgerinos I, Karagiannis T, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med 2020;173:278–286 [DOI] [PubMed] [Google Scholar]
- 224. Tsapas A, Karagiannis T, Kakotrichi P, et al. Comparative efficacy of glucose-lowering medications on body weight and blood pressure in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Obes Metab 2021;23:2116–2124 [DOI] [PubMed] [Google Scholar]
- 225. Abdul-Ghani M, Puckett C, Adams J, et al. Durability of triple combination therapy versus stepwise addition therapy in patients with new-onset T2DM: 3-year follow-up of EDICT. Diabetes Care 2021;44:433–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Matthews DR, Paldánius PM, Proot P, Chiang Y, Stumvoll M; VERIFY study group . Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, double-blind trial. Lancet 2019;394:1519–1529 [DOI] [PubMed] [Google Scholar]
- 227. Aroda VR, González-Galvez G, Grøn R, et al. Durability of insulin degludec plus liraglutide versus insulin glargine U100 as initial injectable therapy in type 2 diabetes (DUAL VIII): a multicentre, open-label, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol 2019;7:596–605 [DOI] [PubMed] [Google Scholar]
- 228. Mantsiou C, Karagiannis T, Kakotrichi P, et al. Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors as combination therapy for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 2020;22:1857–1868 [DOI] [PubMed] [Google Scholar]
- 229. Li C, Luo J, Jiang M, Wang K. The efficacy and safety of the combination therapy with GLP-1 receptor agonists and SGLT-2 inhibitors in type 2 diabetes mellitus: a systematic review and meta-analysis. Front Pharmacol 2022;13:838277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Guo M, Gu J, Teng F, et al. The efficacy and safety of combinations of SGLT2 inhibitors and GLP-1 receptor agonists in the treatment of type 2 diabetes or obese adults: a systematic review and meta-analysis. Endocrine 2020;67:294–304 [DOI] [PubMed] [Google Scholar]
- 231. Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet 2014;384:2228–2234 [DOI] [PubMed] [Google Scholar]
- 232. Li D, Shi W, Wang T, Tang H. SGLT2 inhibitor plus DPP-4 inhibitor as combination therapy for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 2018;20:1972–1976 [DOI] [PubMed] [Google Scholar]
- 233. Chenchula S, Varthya SB, Padmavathi R. Rationality, efficacy, tolerability of empagliflozin plus linagliptin combination for the management of type 2 diabetes mellitus: a systematic review of randomized controlled trials and observational studies. Curr Diabetes Rev 2022;18:e100921196392. [DOI] [PubMed] [Google Scholar]
- 234. Katsiki N, Ofori-Asenso R, Ferrannini E, Mazidi M. Fixed-dose combination of empagliflozin and linagliptin for the treatment of patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab 2020;22:1001–1005 [DOI] [PubMed] [Google Scholar]
- 235. Min SH, Yoon JH, Moon SJ, Hahn S, Cho YM. Combination of sodium-glucose cotransporter 2 inhibitor and dipeptidyl peptidase-4 inhibitor in type 2 diabetes: a systematic review with meta-analysis. Sci Rep 2018;8:4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Milder TY, Stocker SL, Abdel Shaheed C, et al. Combination therapy with an SGLT2 inhibitor as initial treatment for type 2 diabetes: a systematic review and meta-analysis. J Clin Med 2019;8:E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Castellana M, Cignarelli A, Brescia F, Laviola L, Giorgino F. GLP-1 receptor agonist added to insulin versus basal-plus or basal-bolus insulin therapy in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev 2019;35:e3082. [DOI] [PubMed] [Google Scholar]
- 238. Min SH, Yoon JH, Hahn S, Cho YM. Efficacy and safety of combination therapy with an α-glucosidase inhibitor and a dipeptidyl peptidase-4 inhibitor in patients with type 2 diabetes mellitus: a systematic review with meta-analysis. J Diabetes Investig 2018;9:893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Cai X, Gao X, Yang W, Han X, Ji L. Efficacy and safety of initial combination therapy in treatment-naïve type 2 diabetes patients: a systematic review and meta-analysis. Diabetes Ther 2018;9:1995–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Dave CV, Kim SC, Goldfine AB, Glynn RJ, Tong A, Patorno E. Risk of cardiovascular outcomes in patients with type 2 diabetes after addition of SGLT2 inhibitors versus sulfonylureas to baseline GLP-1RA Therapy. Circulation 2021;143:770–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Lam CSP, Ramasundarahettige C, Branch KRH, et al. Efpeglenatide and clinical outcomes with and without concomitant sodium-glucose cotransporter-2 inhibition use in type 2 diabetes: exploratory analysis of the AMPLITUDE-O trial. Circulation 2022;145:565–574 [DOI] [PubMed] [Google Scholar]
- 242. DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab 2017;19:1353–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Wright AK, Carr MJ, Kontopantelis E, et al. Primary prevention of cardiovascular and heart failure events with SGLT2 inhibitors, GLP-1 receptor agonists, and their combination in type 2 diabetes. Diabetes Care 2022;45:909–918 [DOI] [PubMed] [Google Scholar]
- 244. Clegg LE, Penland RC, Bachina S, et al. Effects of exenatide and open-label SGLT2 inhibitor treatment, given in parallel or sequentially, on mortality and cardiovascular and renal outcomes in type 2 diabetes: insights from the EXSCEL trial. Cardiovasc Diabetol 2019;18:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Nathan DM. Glycemic outcomes in the glycemia reduction approaches in type 2 diabetes: a comparative effectiveness (GRADE) study. N Engl J Med. In press. [Google Scholar]
- 246. Nathan DM. Glycemia reduction approaches in type 2 diabetes: a comparative effectiveness (GRADE) study microvascular and cardiovascular outcomes. N Engl J Med. In press. [Google Scholar]
- 247. Gerstein HC, Sattar N, Rosenstock J, et al.; AMPLITUDE-O Trial Investigators . Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med 2021;385:896–907 [DOI] [PubMed] [Google Scholar]
- 248. Ruff CT, Baron M, Im K, O’Donoghue ML, Fiedorek FT, Sabatine MS. Subcutaneous infusion of exenatide and cardiovascular outcomes in type 2 diabetes: a non-inferiority randomized controlled trial. Nat Med 2022;28:89–95 [DOI] [PubMed] [Google Scholar]
- 249. Giugliano D, Longo M, Caruso P, Maiorino MI, Bellastella G, Esposito K. Sodium-glucose co-transporter-2 inhibitors for the prevention of cardiorenal outcomes in type 2 diabetes: an updated meta-analysis. Diabetes Obes Metab 2021;23:1672–1676 [DOI] [PubMed] [Google Scholar]
- 250. Lee MMY, Kristensen SL, Gerstein HC, McMurray JJV, Sattar N. Cardiovascular and mortality outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a meta-analysis with the FREEDOM cardiovascular outcomes trial. Diabetes Metab Syndr 2022;16:102382. [DOI] [PubMed] [Google Scholar]
- 251. Shaman AM, Bain SC, Bakris GL, et al. Effect of the glucagon-like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: pooled analysis of SUSTAIN 6 and LEADER. Circulation 2022;145:575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019;7:845–854 [DOI] [PubMed] [Google Scholar]
- 253. Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 2021;9:653–662 [DOI] [PubMed] [Google Scholar]
- 254. Tsai WH, Chuang SM, Liu SC, et al. Effects of SGLT2 inhibitors on stroke and its subtypes in patients with type 2 diabetes: a systematic review and meta-analysis. Sci Rep 2021;11:15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Strain WD, Frenkel O, James MAet al. Effects of semaglutide on stroke subtypes in type 2 diabetes: post hoc analysis of the randomized SUSTAIN 6 and PIONEER 6. Stroke 2022;53:2749–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Tsapas A, Karagiannis T, Avgerinos I, Liakos A, Bekiari E. GLP-1 receptor agonists for cardiovascular outcomes with and without metformin. A systematic review and meta-analysis of cardiovascular outcomes trials. Diabetes Res Clin Pract 2021;177:108921. [DOI] [PubMed] [Google Scholar]
- 257. Lavalle-Cobo A, Masson W, Lobo M, Masson G, Molinero G. Glucagon-like peptide-1 receptor agonists and cardioprotective benefit in patients with type 2 diabetes without baseline metformin: a systematic review and update meta-analysis. High Blood Press Cardiovasc Prev 2021;28:605–612 [DOI] [PubMed] [Google Scholar]
- 258. Husain M, Consoli A, De Remigis A, Pettersson Meyer AS, Rasmussen S, Bain S. Semaglutide reduces cardiovascular events regardless of metformin use: a post hoc subgroup analysis of SUSTAIN 6 and PIONEER 6. Cardiovasc Diabetol 2022;21:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ 2021;372:m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Lin DSH, Lee JK, Hung CS, Chen WJ. The efficacy and safety of novel classes of glucose-lowering drugs for cardiovascular outcomes: a network meta-analysis of randomised clinical trials. Diabetologia 2021;64:2676–2686 [DOI] [PubMed] [Google Scholar]
- 261. Patorno E, Htoo PT, Glynn RJ, et al. Sodium-glucose cotransporter-2 inhibitors versus glucagon-like peptide-1 receptor agonists and the risk for cardiovascular outcomes in routine care patients with diabetes across categories of cardiovascular disease. Ann Intern Med 2021;174:1528–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Bahour N, Cortez B, Pan H, Shah H, Doria A, Aguayo-Mazzucato C. Diabetes mellitus correlates with increased biological age as indicated by clinical biomarkers. Geroscience 2022;44:415–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Nguyen TN, Harris K, Woodward M, et al. The impact of frailty on the effectiveness and safety of intensive glucose control and blood pressure-lowering therapy for people with type 2 diabetes: results from the ADVANCE trial. Diabetes Care 2021;44:1622–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. European Medicines Agency . Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. Amsterdam, Netherlands, European Medicines Agency, 2012. Accessed 18 August 2022. Available from www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf
- 265. Food and Drug Administration . Draft guidance for industry on diabetes mellitus: developing drugs and therapeutic biologics for treatment and prevention–guidance document. Silver Spring, MD, Food and Drug Administration, 2008. Accessed 22 June 2022. Available from www.regulations.gov/document/FDA-2008-D-0118-0003
- 266. Karagiannis T, Tsapas A, Athanasiadou E, et al. GLP-1 receptor agonists and SGLT2 inhibitors for older people with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract 2021;174:108737. [DOI] [PubMed] [Google Scholar]
- 267. Andes LJ, Cheng YJ, Rolka DB, Gregg EW, Imperatore G. Prevalence of prediabetes among adolescents and young adults in the United States, 2005-2016. JAMA Pediatr 2020;174:e194498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Dabelea D, Bell RA, D’Agostino RB Jr, et al.; Writing Group for the SEARCH for Diabetes in Youth Study Group . Incidence of diabetes in youth in the United States. JAMA 2007;297:2716–2724 [DOI] [PubMed] [Google Scholar]
- 269. RISE Consortium . Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 2018;41:1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. RISE Consortium . Lack of durable improvements in β-cell function following withdrawal of pharmacological interventions in adults with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 2019;42:1742–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Hannon TS, Arslanian SA. The changing face of diabetes in youth: lessons learned from studies of type 2 diabetes. Ann N Y Acad Sci 2015;1353:113–137 [DOI] [PubMed] [Google Scholar]
- 272. TODAY Study Group . Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care 2013;36:1749–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Jalaludin MY, Deeb A, Zeitler P, et al. Efficacy and safety of the addition of sitagliptin to treatment of youth with type 2 diabetes and inadequate glycemic control on metformin without or with insulin. Pediatr Diabetes 2022;23:183–193 [DOI] [PubMed] [Google Scholar]
- 274. Bjornstad P, Drews KL, Caprio S, et al.; TODAY Study Group . Long-term complications in youth-onset type 2 diabetes. N Engl J Med 2021;385:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Gregg EW, Hora I, Benoit SR. Resurgence in diabetes-related complications. JAMA 2019;321:1867–1868 [DOI] [PubMed] [Google Scholar]
- 276. Chan JCN, Paldánius PM, Mathieu C, Stumvoll M, Matthews DR, Del Prato S. Early combination therapy delayed treatment escalation in newly diagnosed young-onset type 2 diabetes: a subanalysis of the VERIFY study. Diabetes Obes Metab 2021;23:245–251 [DOI] [PubMed] [Google Scholar]
- 277. Bhattarai M, Salih M, Regmi M, et al. Association of sodium-glucose cotransporter 2 inhibitors with cardiovascular outcomes in patients with type 2 diabetes and other risk factors for cardiovascular disease: a meta-analysis. JAMA Netw Open 2022;5:e2142078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Mishriky BM, Powell JR, Wittwer JA, et al. Do GLP-1RAs and SGLT-2is reduce cardiovascular events in black patients with type 2 diabetes? A systematic review and meta-analysis. Diabetes Obes Metab 2019;21:2274–2283 [DOI] [PubMed] [Google Scholar]
- 279. Food and Drug Administration . Diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials; draft guidance for industry; availability. Silver Spring, MD, Food and Drug Administration, 2022. Accessed 22 June 2022. Available from www.fda.gov/regulatory-nformation/isearch-fda-guidance-documents/diversity-plans-improve-enrollment-participants-underrepresented-racial-and-ethnic-populations
- 280. Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 2001;44(Suppl. 2):S14–S21 [DOI] [PubMed] [Google Scholar]
- 281. Regensteiner JG, Golden S, Huebschmann AG, et al.; American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health, Council on Epidemiology and Prevention, Council on Functional Genomics and Translational Biology, and Council on Hypertension . Sex differences in the cardiovascular consequences of diabetes mellitus: a scientific statement from the American Heart Association. Circulation 2015;132:2424–2447 [DOI] [PubMed] [Google Scholar]
- 282. Kannel WB, Wilson PW. Risk factors that attenuate the female coronary disease advantage. Arch Intern Med 1995;155:57–61 [PubMed] [Google Scholar]
- 283. Hu G, Jousilahti P, Qiao Q, Katoh S, Tuomilehto J. Sex differences in cardiovascular and total mortality among diabetic and non-diabetic individuals with or without history of myocardial infarction. Diabetologia 2005;48:856–861 [DOI] [PubMed] [Google Scholar]
- 284. Peters SAE, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 2014;383:1973–1980 [DOI] [PubMed] [Google Scholar]
- 285. Peters SAE, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 2014;57:1542–1551 [DOI] [PubMed] [Google Scholar]
- 286. Peters SAE, Woodward M. Sex differences in the burden and complications of diabetes. Curr Diab Rep 2018;18:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Clemens KK, Woodward M, Neal B, Zinman B. Sex disparities in cardiovascular outcome trials of populations with diabetes: a systematic review and meta-analysis. Diabetes Care 2020;43:1157–1163 [DOI] [PubMed] [Google Scholar]
- 288. Singh AK, Singh R. Gender difference in cardiovascular outcomes with SGLT-2 inhibitors and GLP-1 receptor agonist in type 2 diabetes: a systematic review and meta-analysis of cardio-vascular outcome trials. Diabetes Metab Syndr 2020;14:181–187 [DOI] [PubMed] [Google Scholar]
- 289. Rådholm K, Zhou Z, Clemens K, Neal B, Woodward M. Effects of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes in women versus men. Diabetes Obes Metab 2020;22:263–266 [DOI] [PubMed] [Google Scholar]
- 290. Lazarus JV, Mark HE, Anstee QM, et al.; NAFLD Consensus Consortium . Advancing the global public health agenda for NAFLD: a consensus statement. Nat Rev Gastroenterol Hepatol 2022;19:60–78 [DOI] [PubMed] [Google Scholar]
- 291. Kanwal F, Shubrook JH, Adams LA, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology 2021;161:1657–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Newsome PN, Buchholtz K, Cusi K, et al.; NN9931-4296 Investigators . A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med 2021;384:1113–1124 [DOI] [PubMed] [Google Scholar]
- 293. Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Intern Med 2017;177:633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Panunzi S, Maltese S, Verrastro O, et al. Pioglitazone and bariatric surgery are the most effective treatments for non-alcoholic steatohepatitis: a hierarchical network meta-analysis. Diabetes Obes Metab 2021;23:980–990 [DOI] [PubMed] [Google Scholar]
- 295. Mantovani A, Byrne CD, Scorletti E, Mantzoros CS, Targher G. Efficacy and safety of anti-hyperglycaemic drugs in patients with non-alcoholic fatty liver disease with or without diabetes: An updated systematic review of randomized controlled trials. Diabetes Metab 2020;46:427–441 [DOI] [PubMed] [Google Scholar]
- 296. Lassailly G, Caiazzo R, Ntandja-Wandji LC, et al. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology 2020;159:1290–1301.e5 [DOI] [PubMed] [Google Scholar]
- 297. Russo MF, Lembo E, Mari A, et al. Insulin resistance is central to long-term reversal of histologic nonalcoholic steatohepatitis after metabolic surgery. J Clin Endocrinol Metab 2021;106:750–761 [DOI] [PubMed] [Google Scholar]
- 298. Hartman ML, Sanyal AJ, Loomba R, et al. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care 2020;43:1352–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Ghosal S, Datta D, Sinha B. A meta-analysis of the effects of glucagon-like-peptide 1 receptor agonist (GLP1-RA) in nonalcoholic fatty liver disease (NAFLD) with type 2 diabetes (T2D). Sci Rep 2021;11:22063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Coelho FDS, Borges-Canha M, von Hafe M, et al. Effects of sodium-glucose co-transporter 2 inhibitors on liver parameters and steatosis: a meta-analysis of randomized clinical trials. Diabetes Metab Res Rev 2021;37:e3413. [DOI] [PubMed] [Google Scholar]
- 301. Dwinata M, Putera DD, Hasan I, Raharjo M. SGLT2 inhibitors for improving hepatic fibrosis and steatosis in non-alcoholic fatty liver disease complicated with type 2 diabetes mellitus: a systematic review. Clin Exp Hepatol 2020;6:339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Shao SC, Kuo LT, Chien RN, Hung MJ, Lai ECC. SGLT2 inhibitors in patients with type 2 diabetes with non-alcoholic fatty liver diseases: an umbrella review of systematic reviews. BMJ Open Diabetes Res Care 2020;8:e001956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care 2017;40:419–430 [DOI] [PubMed] [Google Scholar]
- 304. Wojeck BS, Inzucchi SE, Neeland IJ, et al. Ertugliflozin and incident obstructive sleep apnea: an analysis from the VERTIS CV trial. Sleep Breath 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Neeland IJ, Eliasson B, Kasai T, et al.; EMPA-REG OUTCOME Investigators . The impact of empagliflozin on obstructive sleep apnea and cardiovascular and renal outcomes: an exploratory analysis of the EMPA-REG OUTCOME trial. Diabetes Care 2020;43:3007–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Levengood TW, Peng Y, Xiong KZ, et al.; Community Preventive Services Task Force . Team-based care to improve diabetes management: a community guide meta-analysis. Am J Prev Med 2019;57:e17–e26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Chamnan P, Simmons RK, Sharp SJ, Griffin SJ, Wareham NJ. Cardiovascular risk assessment scores for people with diabetes: a systematic review. Diabetologia 2009;52:2001–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Srikanth V, Sinclair AJ, Hill-Briggs F, Moran C, Biessels GJ. Type 2 diabetes and cognitive dysfunction–towards effective management of both comorbidities. Lancet Diabetes Endocrinol 2020;8:535–545 [DOI] [PubMed] [Google Scholar]
- 309. Palta P, Schneider ALC, Biessels GJ, Touradji P, Hill-Briggs F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: a meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J Int Neuropsychol Soc 2014;20:278–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 2019;42:731–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Centers for Disease Control and Prevention . Writing SMART objectives. Atlanta, GA, Centers for Disease Control and Prevention, 2019. Accessed 22 June 2022. Available from www.cdc.gov/dhdsp/evaluation_resources/guides/writing-smart-objectives.htm
- 312. Pi-Sunyer X. The Look AHEAD trial: a review and discussion of its outcomes. Curr Nutr Rep 2014;3:387–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Cahn A, Raz I, Leiter LA, et al. Cardiovascular, renal, and metabolic outcomes of dapagliflozin versus placebo in a primary cardiovascular prevention cohort: analyses from DECLARE-TIMI 58. Diabetes Care 2021;44:1159–1167 [DOI] [PubMed] [Google Scholar]
- 314. Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Gerstein HC, Colhoun HM, Dagenais GR, et al.; REWIND Investigators . Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394:121–130 [DOI] [PubMed] [Google Scholar]
- 316. Kahl S, Ofstad AP, Zinman B, et al. Effects of empagliflozin on markers of liver steatosis and fibrosis and their relationship to cardiorenal outcomes. Diabetes Obes Metab 2022;24:1061–1071 [DOI] [PubMed] [Google Scholar]
- 317. Brownrigg JRW, Hughes CO, Burleigh D, et al. Microvascular disease and risk of cardiovascular events among individuals with type 2 diabetes: a population-level cohort study. Lancet Diabetes Endocrinol 2016;4:588–597 [DOI] [PubMed] [Google Scholar]
- 318. Mearns ES, Saulsberry WJ, White CM, et al. Efficacy and safety of antihyperglycaemic drug regimens added to metformin and sulphonylurea therapy in type 2 diabetes: a network meta-analysis. Diabet Med 2015;32:1530–1540 [DOI] [PubMed] [Google Scholar]
- 319. Zaccardi F, Dhalwani NN, Dales J, et al. Comparison of glucose-lowering agents after dual therapy failure in type 2 diabetes: a systematic review and network meta-analysis of randomized controlled trials. Diabetes Obes Metab 2018;20:985–997 [DOI] [PubMed] [Google Scholar]
- 320. Downes MJ, Bettington EK, Gunton JE, Turkstra E. Triple therapy in type 2 diabetes; a systematic review and network meta-analysis. PeerJ 2015;3:e1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Lee CMY, Woodward M, Colagiuri S. Triple therapy combinations for the treatment of type 2 diabetes–a network meta-analysis. Diabetes Res Clin Pract 2016;116:149–158 [DOI] [PubMed] [Google Scholar]
- 322. Lukashevich V, Del Prato S, Araga M, Kothny W. Efficacy and safety of vildagliptin in patients with type 2 diabetes mellitus inadequately controlled with dual combination of metformin and sulphonylurea. Diabetes Obes Metab 2014;16:403–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Hong AR, Lee J, Ku EJ, et al. Comparison of vildagliptin as an add-on therapy and sulfonylurea dose-increasing therapy in patients with inadequately controlled type 2 diabetes using metformin and sulfonylurea (VISUAL study): a randomized trial. Diabetes Res Clin Pract 2015;109:141–148 [DOI] [PubMed] [Google Scholar]
- 324. Moses RG, Kalra S, Brook D, et al. A randomized controlled trial of the efficacy and safety of saxagliptin as add-on therapy in patients with type 2 diabetes and inadequate glycaemic control on metformin plus a sulphonylurea. Diabetes Obes Metab 2014;16:443–450 [DOI] [PubMed] [Google Scholar]
- 325. Moses RG, Round E, Shentu Y, et al. A randomized clinical trial evaluating the safety and efficacy of sitagliptin added to the combination of sulfonylurea and metformin in patients with type 2 diabetes mellitus and inadequate glycemic control. J Diabetes 2016;8:701–711 [DOI] [PubMed] [Google Scholar]
- 326. American Diabetes Association . Overcoming therapeutic inertia. Alexandria, VA, American Diabetes Association. Accessed 3 September 2019. Available from https://professional.diabetes.org/meeting/other/overcoming-therapeutic-inertia
- 327. Madenidou AV, Paschos P, Karagiannis T, et al. Comparative benefits and harms of basal insulin analogues for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med 2018;169:165–174 [DOI] [PubMed] [Google Scholar]
- 328. Monnier, L, Colette, C. Addition of rapid-acting insulin to basal insulin therapy in type 2 diabetes: indications and modalities. Diabetes Metab 2006;32:7–13 [DOI] [PubMed] [Google Scholar]
- 329. Nicolucci A, Ceriello A, Di Bartolo P, Corcos A, Orsini Federici M. Rapid-acting insulin analogues versus regular human insulin: a meta-analysis of effects on glycemic control in patients with diabetes. Diabetes Ther 2020;11:573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Christiansen JS, Niskanen L, Rasmussen S, Johansen T, Fulcher G. Lower rates of hypoglycemia during maintenance treatment with insulin degludec/insulin aspart versus biphasic insulin aspart 30: a combined analysis of two phase 3a studies in type 2 diabetes. J Diabetes 2016;8:720–728 [DOI] [PubMed] [Google Scholar]
- 331. Gabler M, Picker N, Geier S, et al. Real-world clinical outcomes and costs in type 2 diabetes mellitus patients after initiation of insulin therapy: a German claims data analysis. Diabetes Res Clin Pract 2021;174:108734. [DOI] [PubMed] [Google Scholar]
- 332. McCarty D, Olenik A, McCarty BP. Efficacy and safety of basal insulin/GLP-1 receptor agonist used in combination for type 2 diabetes management. J Pharm Pract 2019;32:671–678 [DOI] [PubMed] [Google Scholar]
- 333. Hangaard S, Laursen SH, Andersen JD, et al. The effectiveness of telemedicine solutions for the management of type 2 diabetes: a systematic review, meta-analysis, and meta-regression. J Diabetes Sci Technol. 26 December 2021 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Eberle C, Stichling S. Effect of telemetric interventions on glycated hemoglobin A1c and management of type 2 diabetes mellitus: systematic meta-review. J Med Internet Res 2021;23:e23252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Dicembrini I, Mannucci E, Monami M, Pala L. Impact of technology on glycaemic control in type 2 diabetes: A meta-analysis of randomized trials on continuous glucose monitoring and continuous subcutaneous insulin infusion. Diabetes Obes Metab 2019;21:2619–2625 [DOI] [PubMed] [Google Scholar]
- 336. Evans M, Welsh Z, Ells S, Seibold A. The impact of flash glucose monitoring on glycaemic control as measured by HbA1c: a meta-analysis of clinical trials and real-world observational studies. Diabetes Ther 2020;11:83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Martens T, Beck RW, Bailey R, et al.; MOBILE Study Group . Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA 2021;325:2262–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Effective Practice and Organisation of Care (EPOC) . (2015) EPOC taxonomy. London, U.K., 2015. Accessed 29 July 2022. Available from https://epoc.Cochrane.org/epoc-taxonomy
- 339. Chan JCN, Lim LL, Wareham NJ, et al. The Lancet Commission on diabetes: using data to transform diabetes care and patient lives. Lancet 2021;396:2019–2082 [DOI] [PubMed] [Google Scholar]
- 340. World Health Organization . Diabetes. Geneva, Switzerland, World Health Organization, 2021. Accessed 1 August 2022. Available from www.who.int/news-room/fact-sheets/detail/diabetes
- 341. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 2022;183:109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Falcetta P, Aragona M, Bertolotto A, et al. Insulin discovery: a pivotal point in medical history. Metabolism 2022;127:154941. [DOI] [PubMed] [Google Scholar]
- 343. Beran D, Lazo-Porras M, Mba CM, Mbanya JC. A global perspective on the issue of access to insulin. Diabetologia 2021;64:954–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Horwitz LI, Kuznetsova M, Jones SA. Creating a learning health system through rapid-cycle, randomized testing. N Engl J Med 2019;381:1175–1179 [DOI] [PubMed] [Google Scholar]
- 345. McGinnis JM, Fineberg HV, Dzau VJ. Advancing the learning health system. N Engl J Med 2021;385:1–5 [DOI] [PubMed] [Google Scholar]
- 346. Sheikh A, Anderson M, Albala S, et al. Health information technology and digital innovation for national learning health and care systems. Lancet Digit Health 2021;3:e383–e396 [DOI] [PubMed] [Google Scholar]