Abstract
Objective
To summarize knowledge and identify gaps in evidence regarding treatment of right ventricular dysfunction (RVD) in acute respiratory distress syndrome (ARDS).
Data sources
We conducted a comprehensive search of MEDLINE, Embase, CINAHL, Web of Science, and the Cochrane Central Register of Controlled Trials.
Study selection
Studies were included if they reported effects of treatments on right ventricular function, whether or not the intent was to modify right ventricular function.
Data extraction
Data extraction was performed independently and in duplicate by two authors. Data items included the study design, patient population, type of intervention, comparison group, and RV-specific outcomes.
Data synthesis
Of 1,430 studies screened, 51 studies reporting on 1,526 patients were included. By frequency, the included studies examined the following interventions: ventilator settings (29.4%), inhaled medications (33.3%), extracorporeal life support (13.7%), intravenous or oral medications (13.7%), and prone positioning (9.8%). The majority of the studies were non-randomized experimental studies (53%), with the next most common being case reports (16%). Only 5.9% of studies were RCTs. In total, 27% of studies were conducted with the goal of modifying RV function.
Conclusions
Given the prevalence of RVD in ARDS and its association with mortality, the dearth of research on this topic is concerning. This review highlights the need for prospective trials aimed at treating RV dysfunction in ARDS.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-023-04395-9.
Keywords: ARDS, Right ventricular dysfunction, Scoping review, Treatment
Introduction
Acute respiratory distress syndrome (ARDS) occurs in approximately 10% of patients admitted to intensive care units (ICUs), carrying a mortality rate of nearly 35% [1]. Determining an accurate incidence and clarifying the epidemiology of right ventricular dysfunction (RVD) incurred during ARDS has been historically complicated by the lack of a universal definition of RVD. RVD occurs in anywhere from 20 to 50% of ARDS cases [2] and is associated with a nearly 50% increase in mortality [3, 4].
The pathophysiology of RVD in ARDS is multifactorial and incompletely understood. ARDS-mediated interstitial pulmonary edema, hypoxemic and hypercapnic pulmonary vasoconstriction, thromboembolism [5], and maladaptive vascular remodeling contribute to increased pulmonary vascular tone and right ventricular (RV) afterload [6, 7]. The alveolar inflation pressures associated with positive pressure ventilation and the use of positive end expiratory pressure (PEEP) to recruit the ARDS lung may also contribute to this increase in RV afterload [8]. The cumulative effect of both disease-specific complications and consequences of therapeutic interventions may ultimately lead to RV dilation and dysfunction [9]. There are no consensus guidelines on the screening, diagnosis, or management of RVD in ARDS. Several management strategies have been suggested under the moniker of “RV protective ventilation.” These include, but are not limited to, the following: prone position ventilation, adjustment of ventilator settings to limit inflation pressures and reduce hypoxemia and hypercapnia, inhaled pulmonary vasodilators, and extracorporeal life support (ECLS).
While previous reviews have described the management of pulmonary hypertension and RVD in adult critical care [2, 10, 11], there are no systematic assessments of the quantity and quality of evidence of interventions directed to manage RVD in ARDS. Given the high prevalence and impact of this condition, we aimed to assess the existing treatment strategies in this population. We hypothesized that the literature consists of a limited number of heterogeneous studies and thus performed a scoping review to describe the existing literature.
Methods
Protocol and registration
The study protocol and search strategy are registered on Open Science Framework (OSF) at the following link (https://osf.io/9kuph). Ethics approval was not necessary for this work. Reporting of this scoping review follows the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines, and the checklist is included in Additional file 1. A scoping review strategy was chosen based on a priori suspicion that there would be few randomized controlled trials or large observational studies to synthesize.
Information sources, search strategy, and eligibility criteria
MEDLINE, Embase, CINAHL, Web of Science, and the Cochrane Central Register of Controlled Trials databases were queried on July 21, 2022. Specific search terms can be found in Additional file 2. In brief, articles describing both ARDS and any mention of right ventricular function or dysfunction were included in the search. Studies in the English language with patients 18 years and older were included from any year. Animal studies were excluded.
Selection of sources of evidence
All study types were eligible for inclusion except for review articles. Conference abstracts were excluded due to high risk of bias. Studies were included if they reported changes in RV function (by any metric) before and after an intervention, whether it was intended to directly influence RV function or not.
Data extraction process and data items
Covidence software (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia) was used for abstract and full-text screening. Abstract screening was completed by two authors (SG and GA) independently, in duplicate, and conflicts were resolved by a third author (MTS). Full-text screening was completed by MTS. Data extraction was performed independently and in duplicate by authors SG and MTS. Data items included the study design, patient population, type of intervention, comparison group (when available), outcome, and any additional information felt useful to describe the literature.
Synthesis of results
Results were summarized by intervention type, including the following categories:
Changes to ventilator settings, such as PEEP, tidal volume (Vt), respiratory rate, or ventilator mode
Prone position ventilation (PP)
Inhaled pulmonary vasodilators
Intravenous or oral medications
ECLS.
Outcome summarization could not be reported uniformly due to heterogeneous use of technologies and parameters to assess RV function. Therefore, we reported the outcomes provided by the individual studies, which typically included transthoracic or transesophageal echocardiographic measurements of RV size and function and/or pulmonary artery catheterization (PAC) determinations. Given the heterogeneity of the literature, no formal synthesis or analyses were performed.
Results
Selection of sources of evidence
A total of 1430 studies were assessed for abstract review after removing duplicates. After exclusion of 1317 due to irrelevance, 113 full-text studies remained. In total, 62 studies were further excluded after a full-text review (Additional file 2: Fig. S1). We report the data on 1526 patients from the remaining 51 studies.
Characteristics of sources of evidence
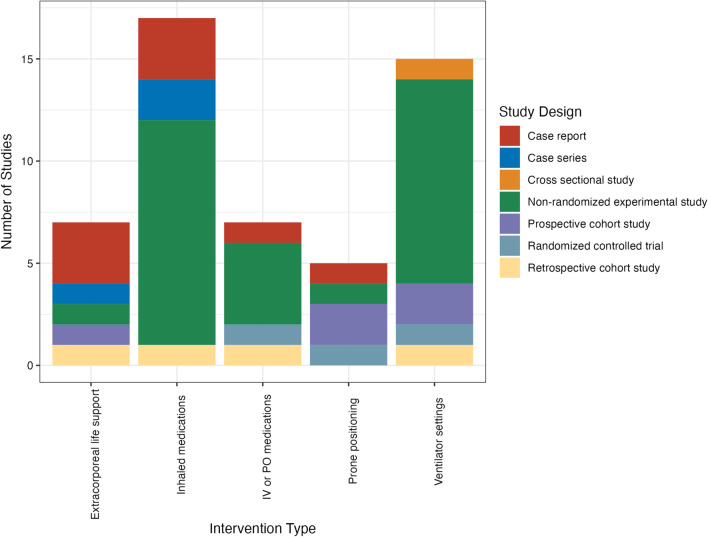
We identified 27 (53%) non-randomized experimental studies, 8 (16%) case reports, 5 (9.8%) prospective cohort studies, 4 (7.8%) retrospective cohort study, 3 (5.9%) case series, 3 (5.9%) randomized controlled trials (RCT), and 1 (2%) cross-sectional study. Fifteen studies (29.4%) evaluated ventilator settings, 17 (33.3%) studied inhaled medications, 7 (13.7%) each tested ECLS and IV/PO medications, and 5 (9.8%) tested prone position ventilation.
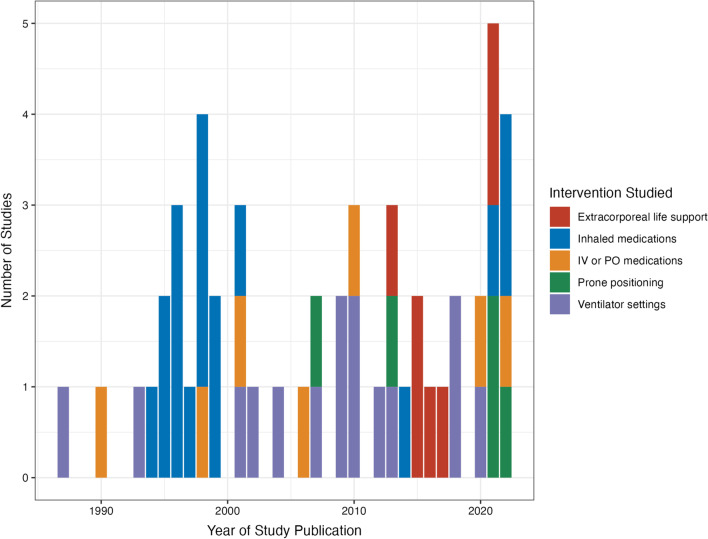
The median number of patients enrolled in all studies was 13 (interquartile range [IQR] 8–20); by intervention: ECLS (n = 6, IQR 1–16), inhaled medications (n = 12, IQR 8–16), IV/PO medications (10, IQR 8–26), PP (18, IQR 9–42), and ventilator settings (n = 16, IQR 12–20) (Fig. 1 and Additional file 2: Table S1). Only 14 studies (27%) were conducted with the goal of treating RVD; 50% of these assessed the effect of inhaled medications, with 29% and 21% assessing IV/PO medications or ECLS, respectively. No studies were conducted with the goal of preventing right ventricular dysfunction. Interventions studied by year are detailed in Fig. 2. Study design by year is detailed in Additional file 2: Fig. S2. A total of six studies (11.8%) reported on patients with COVID-19.
Fig. 1.
Interventions studied, by study design. IV = intravenous, PO = by mouth
Fig. 2.
Interventions studied by year. IV = intravenous, PO = by mouth
RV function was assessed by various means, the most common of which was by PAC measurements (n = 31, 60.7%), followed by transthoracic echocardiography (TTE) (n = 16, 31.4%) and transesophageal echocardiography (TEE) (n = 11, 21.6%) (Additional file 2: Table S2). Other means of RV function assessment included cardiac magnetic resonance imaging, central venous catheter pressure measurements, invasive pressure–volume loop measurements, and transpulmonary thermodilution systems.
Ventilator settings
Out of the 15 studies testing ventilator settings, 9 tested the effect of PEEP on RV function. Full details of the studies are listed in Additional file 2: Table S3. Four studies showed increasing PEEP led to RVD by observing unfavorable responses such as decreased stroke volume (SV), increased right ventricular end diastolic volume (RVEDV), decreased tricuspid annular plane systolic excursion (TAPSE), and decreased cardiac index (CI) [12–15]. One study showed no statistically significant impact of PEEP on CI [16]. Another compared PEEP set to best respiratory system compliance to PEEP set using the lower inflection point of P–V curve, demonstrating better RV function with best compliance PEEP compared to PEEP set using the lower inflection point [17]. One study examined recruitment maneuvers, including a sustained insufflation to 45 cmH2O for 40 s, and showed decreased CI and increased mean pulmonary artery pressure (mPAP) during the maneuver [18]. Two other studies evaluating the impact of recruitment maneuvers in this patient population found that although the RV Tei index, cardiac output (CO), and TAPSE were lower at time of the maneuver, they returned to baseline with decremental PEEP titration to best respiratory compliance [19, 20].
Three studies evaluated high-frequency oscillatory ventilation (HFOV), showing a reduction in CI, increased right ventricular end diastolic area (RVEDA): left ventricular end diastolic area (LVEDA), increased mPAP, and pulmonary vascular resistance (PVR) index during HFOV compared to standard ventilator settings [21–23]. Another investigation compared inverse ratio, pressure control ventilation to volume control ventilation, showing a significant increase in CI in inverse ratio ventilation compared to volume control with no significant change in mPAP [24].
Another study evaluated the impact of various plateau pressures on the incidence of acute cor pulmonale (ACP). It is found that the incidence of ACP as well as mortality was highest in patients with plateau pressures > 35 cmH2O. It is also noted that in patients with plateau pressures ranging from 18 to 26 cmH2O, the presence of ACP did not confer increased mortality compared to those without ACP [25]. One study attempted to measure the impact of respiratory rate on RVD, showing a higher respiratory rate resulted in a lower CI and pulmonary artery (PA) velocity time integral (VTI) [26]. This was due in part to higher alveolar dead space, as well as increase in intrinsic PEEP.
Prone positioning
Out of the 5 studies testing prone positioning, 3 showed improvement in RV function by a reduction in RVEDA:LVEDA ratio and increased CI (Additional file 2: Table S4) [27–29]. In contrast, a case report showed a decrease in SV and RV EF, and increased mPAP as measured with invasive pressure–volume loops [30]. Another study demonstrated no significant impact of prone positioning on RV function as measured by TTE [31].
Inhaled pulmonary vasodilators
Of the 17 studies evaluating inhaled pulmonary vasodilators, 15 tested inhaled nitric oxide (iNO), 3 tested nebulized prostacyclin analogues, and 2 tested nebulized alprostadil (Additional file 2: Table S5). A wide range of iNO doses were used, ranging from 5 to 40 parts per million (ppm). Eleven of the studies with iNO showed a reduction in mPAP and/or PVR/PVRi [32–42], 3 showed improvement in RV EF [33, 35, 38], and 4 showed improvement in CO [37, 41–43]. In contrast, 6 studies failed to find a statistically significant impact of iNO on CO [32–34, 39, 40, 44]. Two investigations reported reduced mortality in patients who responded to iNO (40% vs 67% in one study and 50% vs 65% in the other) [35, 41], and another noted return to baseline hemodynamic measurements postdiscontinuation of iNO [37].
Three studies evaluating nebulized prostacyclin demonstrated a reduction in mPAP. Two of those also showed a reduction in PVR. There was no change in CO/CI in any of the studies [36, 44, 45].
Two studies investigating nebulized alprostadil showed no significant change in CI as measured by PAC, although one showed a decreased in right ventricular end systolic and end diastolic volume index [38, 46].
Intravenous and oral medications
Two studies evaluating enteral sildenafil found a decreased PVR with an increase in CO [47, 48]. Two studies tested IV almitrine; one demonstrated a decrease in mPAP and PVR, an increase in CI, and an improved RV global longitudinal strain at a dose of 4 μg/kg/min [43]. The other investigation found that almitrine increased mPAP, PVRi, and reduced RV ejection fraction (EF) without a significant change in CI at a dose of 16 μg/kg/min [49]. An investigation of IV epoprostenol showed a reduction in mPAP and PVRi, and an increase in RV EF (in patients with abnormal baseline values) and CI [50]. Another study explored the use of IV norepinephrine with iNO compared to iNO alone and found that there was a greater decrease in mPAP, PVR index and right ventricle stroke work (RVSWI) in patients receiving norepinephrine with iNO compared to iNO alone [51]. A single RCT in 35 patients demonstrated that levosimendan decreased mPAP, PVR index, and increased CI (from 3.8 L/min/m2 to 4.2) as well as RVEF [52]. See Additional file 2: Table S6.
Extracorporeal therapies
Three out of the seven studies exploring the impact of ECLS evaluated veno-venous extracorporeal membranous oxygenation (VV-ECMO). These works have heterogeneous findings, with one showing decreased central venous pressures (CVP) and mPAP without a significant change in CO [53], while another case report showed decreased interventricular septal flattening immediately after VV ECMO cannulation, which normalized at time of ICU discharge [54]. Another investigation demonstrated that VV-ECMO combined with ultra-protective tidal volumes (driving pressures of 10 cm H2O) reduced PA systolic pressures; however, it did not result in significant differences in RV size and systolic dysfunction as measured by TTE [55]. In a case series, the addition of an intra-aortic balloon pump (IABP) to VV-ECMO resulted in increased PA pressures and reduced CVP [56]. A case report displayed a reversal of septal flattening and improved RV systolic function on conversion to VAV-ECMO from VV-ECMO [57].
Two studies examined the impact of extracorporeal CO2 removal (ECCO2R) on RV function. One found that ECCO2R decreased RVEDA, improved RVEDA:LVEDA ratio, and increased CO [58]. The second study investigated the impact of ECCO2R with ultra-protective TV (4 ml/kg of ideal body weight) and found no detectable change in CVP, right ventricular end diastolic diameter to left ventricular end diastolic diameter ratio, or PA systolic pressures; however, it did show improvement in TAPSE, see Additional file 2: Table S7 [59].
Discussion
To our knowledge, this is the first scoping review of the literature on the effect of clinical interventions on RV function in ARDS. Despite over 50 years of ARDS research, we found only 51 citations meeting inclusion criteria, only 27% of which intended to directly influence RV function. The strength of evidence overall is low, given the low number of enrolled patients and a paucity of randomized controlled trials—only three in this study. The majority of more recent studies were case reports, case series, or otherwise observational in nature. The sole pharmacologic RCT on RVD in ARDS occurred in 2006 [52]. Overall, the literature on RVD in ARDS suffers from a lack of standardization in definitions, treatment targets, and patient-centered outcomes.
Significant heterogeneity in the included literature precludes meaningful pooling and systematic synthesis of these results. Many of the interventions tested were applied at different doses and durations with differing inclusion and exclusion criteria. A significant proportion of studies tested inhaled pulmonary vasodilators, which have been repeatedly shown to improve oxygenation, with uncertain effect on pulmonary artery pressures, and no clear mortality benefit [60, 61]. Over the past decade, extracorporeal life support modalities have been tested with increasing frequency, but there is still considerable heterogeneity in the configurations used as well as the quality of evidence.
Furthermore, methods of RV assessment and the definition of RVD differed across studies. The most common assessment was via pulmonary artery catheterization, which largely reflects the era in which most of these studies were completed. Currently, pulmonary artery catheters are used infrequently in patients with ARDS in many centers around the world. While transthoracic echocardiography has become increasingly available across intensive care units, the challenges of acquiring adequate images for RV assessment in patients with ARDS may limit its utility. Furthermore, approximately 30% of included studies were completed before the lung protective ventilation era, where higher tidal volumes (and thereby distending pressures) were used. This may have predisposed to higher rates of RVD [4], perhaps in ways which could modify responsiveness to any of the tested therapies compared to a contemporary cohort.
Priorities
In order to enroll patients in high-quality prospective studies, consensus definitions of RVD and specifically, RVD in ARDS, are needed [62]. These metrics ideally should account for both functional and structural changes in the right ventricle, incorporating features such as systolic and diastolic function, size, morphology, as well as measures of right ventricular pulmonary artery coupling [63]. Reproducible definitions of RVD may be aided by improved cardiovascular phenotyping in ARDS [64, 65]. While the incidence of RVD in ARDS is estimated to be 20–50% [2], both the etiology and the natural history of RVD in ARDS are incompletely understood and could have time-varying characteristics.
Given the mortality risk associated with RVD in ARDS, this review highlights several priority areas for scientific inquiry. There is a clear need for randomized controlled trials of all the major categories of treatments detailed in this review. A “RV-protective ventilation” strategy has been described [7] and would be a low-cost intervention bundle with potentially high benefit. Patients at increased risk for RVD in ARDS (by established acute cor pulmonale risk scores [4]) could be selectively enrolled in trials, even as a preventative strategy. While patients with moderate to severe ARDS seem to benefit from a higher PEEP strategy [66, 67], optimal PEEP strategy may differ in patients at increased risk for RVD. While pulmonary vasodilator medications have not shown benefit in unselected ARDS patients, it may be worthwhile to conduct RCTs in patients with established RVD as a predictive enrichment strategy.
Although previous investigations examining the use of extracorporeal support in ARDS have yielded tepid results [68, 69], RVD is not typically an explicit therapeutic target for ECLS. However, RVD is associated with increased mortality in this group [70]. In addition to the included studies on ECLS, a retrospective analysis of 15 patients with COVID-19 ARDS demonstrated improvement in RV echo parameters after VV-ECMO cannulation (published after our systematic search). In patients who are already receiving ECLS, there are still opportunities for prospective physiologic studies to mitigate RVD, using adjunctive management such as pharmacologic therapies as well as different mechanical ventilation strategies. One recently published retrospective analysis demonstrated an improvement in RVD and hemodynamic parameters when PEEP was optimized to a slightly positive transpulmonary pressure using esophageal manometry [71]. Finally, there may be differences in right ventricular outcomes based on cannulation strategy [72, 73], which should be further investigated in prospective studies. Rescue strategies (including changes to cannulation strategy, among other interventions) for persistent RVD despite ECLS [74] deserve further study but may be challenging to perform systematically across a small number of heterogeneous patients and centers.
Secondly, there are several areas of ARDS management which were unexplored in this review of the literature. For instance, the specific role of tidal volume was not studied. This may be a priority area since it has been demonstrated that higher tidal volumes increase RV afterload even outside of ARDS [75]. The effect of tidal volume on mortality in ARDS may depend on respiratory system elastance [76], and it is unknown if any of this effect is due to impact on RV function. While some alternative modes of ventilation were studied, including inverse-ratio pressure control, no studies on airway pressure release ventilation were found. Finally, the effect of commonly used vasoactive agents which may have varying effects on pulmonary vascular resistance (such as vasopressin and phenylephrine) as well as inotropic agents (such as dobutamine and milrinone) was not specifically assessed.
Limitations
This review has limitations. We systematically and exhaustively searched multiple databases, but relevant articles may have been missed if the broad search criteria did not capture them. Extraction of data from individual studies proved challenging given heterogeneous reporting, which limited our own reporting to a descriptive format.
Conclusions
Given the prevalence of RV dysfunction in ARDS and its association with mortality, the dearth of high-quality research in this area is concerning. The existing literature is characterized by small sample sizes, inconsistent application of treatments across studies, and variable reporting of results. Prospective trials aimed at treating or preventing RV dysfunction should be a research priority for the ARDS scientific community.
Supplementary Information
Additional file 1. PRISMA-ScR Checklist.
Additional file 2. Search Strategy, Supplemental Tables and Figures.
Abbreviations
- RVD
Right ventricular dysfunction
- ARDS
Acute respiratory distress syndrome
- RV
Right ventricle
- PEEP
Positive end expiratory pressure
- ECLS
Extracorporeal life support
- PP
Prone position ventilation
- SV
Stroke volume
- RVEDV
Right ventricular end diastolic volume
- TAPSE
Tricuspid annular plane systolic excursion
- CI
Cardiac index
- mPAP
Mean pulmonary artery pressure
- CO
Cardiac output
- HFOV
High-frequency oscillatory ventilation
- RVEDA
Right ventricular end diastolic area
- LVEDA
Left ventricular end diastolic area
- ACP
Acute cor pulmonale
- PA
Pulmonary artery
- VTI
Velocity time integral
- iNO
Inhaled nitric oxide
- PVR
Pulmonary vascular resistance
- PAC
Pulmonary artery catheter
- VV-ECMO
Veno-venous extracorporeal membranous oxygenation
- ICU
Intensive care unit
- IABP
Intraaortic balloon pump
- CVP
Central venous pressure
- ECCO2R
Extracorporeal CO2 removal
- TV
Tidal volume
- RCT
Randomized controlled trial
- TTE
Transthoracic echocardiography
- TEE
Transesophageal echocardiography
- EF
Ejection fraction
- RVSWI
Right ventricular stroke work index
Author contributions
SG performed screening of abstracts, data extraction, drafting and critical revision of manuscript. GA performed abstract screening and critical revision of the manuscript. MS and MTS devised search strategy. MH, AT, and AD performed critical revisions of the manuscript. MTS conceived the original idea, performed full-text screening, data extraction, and drafting and critical revision of the manuscript. All authors read and approved the final manuscript.
Funding
No funding was received for this work.
Availability of data and materials
All data related to this work are included in the main text or the supplementary materials. For pre-registered search strategy, please see https://osf.io/9kuph.
Declarations
Ethical approval and consent to participate
This work is exempt from IRB review as it is a synthesis of published literature.
Competing interests
The authors have no financial or non-financial competing interests to report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 2.Zochios V, Parhar K, Tunnicliffe W, Roscoe A, Gao F. The right ventricle in ARDS. Chest. 2017;152(1):181–193. doi: 10.1016/j.chest.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Sato R, Dugar S, Cheungpasitporn W, Schleicher M, Collier P, Vallabhajosyula S, et al. The impact of right ventricular injury on the mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care. 2021;25(1):172. doi: 10.1186/s13054-021-03591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mekontso Dessap A, Boissier F, Charron C, Bégot E, Repessé X, Legras A, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med. 2016;42(5):862–870. doi: 10.1007/s00134-015-4141-2. [DOI] [PubMed] [Google Scholar]
- 5.Tomashefski JF, Davies P, Boggis C, Greene R, Zapol WM, Reid LM. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol. 1983;112(1):112–126. [PMC free article] [PubMed] [Google Scholar]
- 6.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol. 2017;69(2):236–243. doi: 10.1016/j.jacc.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 7.Paternot A, Repessé X, Vieillard-Baron A. Rationale and description of right ventricle-protective ventilation in ARDS. Respir Care. 2016;61(10):1391–1396. doi: 10.4187/respcare.04943. [DOI] [PubMed] [Google Scholar]
- 8.Mahmood SS, Pinsky MR. Heart–lung interactions during mechanical ventilation: the basics. Ann Transl Med. 2018;6(18):349. doi: 10.21037/atm.2018.04.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zapol WM, Snider MT. Pulmonary hypertension in severe acute respiratory failure. N Engl J Med. 1977;296(9):476–480. doi: 10.1056/NEJM197703032960903. [DOI] [PubMed] [Google Scholar]
- 10.Price LC, Wort SJ, Finney SJ, Marino PS, Brett SJ. Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care. 2010;14(5):R169. doi: 10.1186/cc9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boucly A, Savale L, Vuillard C, Turpin M, Jaïs X, Montani D, et al. Management of right ventricular failure in pulmonary vascular diseases. Rev Mal Respir. 2020;37(2):171–179. doi: 10.1016/j.rmr.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Martin C, Saux P, Albanese J, Bonneru JJ, Gouin F. Right ventricular function during positive end-expiratory pressure. Thermodilution evaluation and clinical application. Chest. 1987;92(6):999–1004. doi: 10.1378/chest.92.6.999. [DOI] [PubMed] [Google Scholar]
- 13.Mekontso Dessap A, Charron C, Devaquet J, Aboab J, Jardin F, Brochard L, et al. Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med. 2009;35(11):1850–1858. doi: 10.1007/s00134-009-1569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fougères E, Teboul JL, Richard C, Osman D, Chemla D, Monnet X. Hemodynamic impact of a positive end-expiratory pressure setting in acute respiratory distress syndrome: importance of the volume status. Crit Care Med. 2010;38(3):802–807. doi: 10.1097/CCM.0b013e3181c587fd. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Zhang H, Zhang D. Effect of positive end-expiratory pressure on right heart function in mechanically ventilated patients: an ultrasonography based study. Kuwait Med J. 2020;52(2):198–203. [Google Scholar]
- 16.Fares WH, Carson SS, NIH NHLBI ARDS Network The relationship between positive end-expiratory pressure and cardiac index in patients with acute respiratory distress syndrome. J Crit Care. 2013;28(6):992–997. doi: 10.1016/j.jcrc.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitt JM, Vieillard-Baron A, Augarde R, Prin S, Page B, Jardin F. Positive end-expiratory pressure titration in acute respiratory distress syndrome patients: impact on right ventricular outflow impedance evaluated by pulmonary artery Doppler flow velocity measurements. Crit Care Med. 2001;29(6):1154–1158. doi: 10.1097/00003246-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Iannuzzi M, De Sio A, De Robertis E, Piazza O, Servillo G, Tufano R. Different patterns of lung recruitment maneuvers in primary acute respiratory distress syndrome: effects on oxygenation and central hemodynamics. Minerva Anestesiol. 2010;76(9):692–698. [PubMed] [Google Scholar]
- 19.Mercado P, Maizel J, Kontar L, Nalos M, Huang S, Orde S, et al. Moderate and severe acute respiratory distress syndrome: hemodynamic and cardiac effects of an open lung strategy with recruitment maneuver analyzed using echocardiography. Crit Care Med. 2018;46(10):1608–1616. doi: 10.1097/CCM.0000000000003287. [DOI] [PubMed] [Google Scholar]
- 20.Gernoth C, Wagner G, Pelosi P, Luecke T. Respiratory and haemodynamic changes during decremental open lung positive end-expiratory pressure titration in patients with acute respiratory distress syndrome. Crit Care. 2009;13(2):R59. doi: 10.1186/cc7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David M, von Bardeleben RS, Weiler N, Markstaller K, Scholz A, Karmrodt J, et al. Cardiac function and haemodynamics during transition to high-frequency oscillatory ventilation. Eur J Anaesthesiol. 2004;21(12):944–952. doi: 10.1097/00003643-200412000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Guervilly C, Forel JM, Hraiech S, Demory D, Allardet-Servent J, Adda M, et al. Right ventricular function during high-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care Med. 2012;40(5):1539–1545. doi: 10.1097/CCM.0b013e3182451b4a. [DOI] [PubMed] [Google Scholar]
- 23.Mentzelopoulos SD, Anninos H, Malachias S, Zakynthinos SG. ‘Low-’ versus ‘high’-frequency oscillation and right ventricular function in ARDS. A randomized crossover study. J Intensive Care. 2018;6:58. doi: 10.1186/s40560-018-0327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poelaert JI, Visser CA, Everaert JA, Koolen JJ, Colardyn FA. Acute hemodynamic changes of pressure-controlled inverse ratio ventilation in the adult respiratory distress syndrome. A transesophageal echocardiographic and Doppler study. Chest. 1993;104(1):214–219. doi: 10.1378/chest.104.1.214. [DOI] [PubMed] [Google Scholar]
- 25.Jardin F, Vieillard-Baron A. Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive Care Med. 2007;33(3):444–447. doi: 10.1007/s00134-007-0552-z. [DOI] [PubMed] [Google Scholar]
- 26.Vieillard-Baron A, Prin S, Augarde R, Desfonds P, Page B, Beauchet A, et al. Increasing respiratory rate to improve CO2 clearance during mechanical ventilation is not a panacea in acute respiratory failure. Crit Care Med. 2002;30(7):1407–1412. doi: 10.1097/00003246-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Vieillard-Baron A, Charron C, Caille V, Belliard G, Page B, Jardin F. Prone positioning unloads the right ventricle in severe ARDS. Chest. 2007;132(5):1440–1446. doi: 10.1378/chest.07-1013. [DOI] [PubMed] [Google Scholar]
- 28.Jozwiak M, Teboul JL, Anguel N, Persichini R, Silva S, Chemla D, et al. Beneficial hemodynamic effects of prone positioning in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2013;188(12):1428–1433. doi: 10.1164/rccm.201303-0593OC. [DOI] [PubMed] [Google Scholar]
- 29.Lu H, Zhang P, Liu X, Jin L, Zhu H. Effect of prone position ventilation on right heart function in patients with acute respiratory distress syndrome. Clin Respir J. 2021;15(11):1229–1238. doi: 10.1111/crj.13431. [DOI] [PubMed] [Google Scholar]
- 30.Kremer NC, Richter MJ, Rako ZA, Tello K. Acute impact of prone positioning on the right ventricle in COVID-19-associated acute respiratory distress syndrome. Circ Heart Fail. 2021;14(8):e008810. doi: 10.1161/CIRCHEARTFAILURE.121.008810. [DOI] [PubMed] [Google Scholar]
- 31.Temperikidis P, Koroneos A, Xourgia E, Kotanidou A, Siempos II. Abnormal right ventricular free wall strain prior to prone ventilation may be associated with worse outcome of patients with COVID-19-associated acute respiratory distress syndrome. Crit Care Explor. 2022;4(1):e0620. doi: 10.1097/CCE.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bigatello LM, Hurford WE, Kacmarek RM, Roberts JD, Zapol WM. Prolonged inhalation of low concentrations of nitric oxide in patients with severe adult respiratory distress syndrome. Effects on pulmonary hemodynamics and oxygenation. Anesthesiology. 1994;80(4):761–770. doi: 10.1097/00000542-199404000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Rossaint R, Slama K, Steudel W, Gerlach H, Pappert D, Veit S, et al. Effects of inhaled nitric oxide on right ventricular function in severe acute respiratory distress syndrome. Intensive Care Med. 1995;21(3):197–203. doi: 10.1007/BF01701472. [DOI] [PubMed] [Google Scholar]
- 34.Fierobe L, Brunet F, Dhainaut JF, Monchi M, Belghith M, Mira JP, et al. Effect of inhaled nitric oxide on right ventricular function in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151(5):1414–1419. doi: 10.1164/ajrccm.151.5.7735594. [DOI] [PubMed] [Google Scholar]
- 35.Krafft P, Fridrich P, Fitzgerald RD, Koc D, Steltzer H. Effectiveness of nitric oxide inhalation in septic ARDS. Chest. 1996;109(2):486–493. doi: 10.1378/chest.109.2.486. [DOI] [PubMed] [Google Scholar]
- 36.Zwissler B, Kemming G, Habler O, Kleen M, Merkel M, Haller M, et al. Inhaled prostacyclin (PGI2) versus inhaled nitric oxide in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1671–1677. doi: 10.1164/ajrccm.154.6.8970353. [DOI] [PubMed] [Google Scholar]
- 37.Benzing A, Mols G, Beyer U, Geiger K. Large increase in cardiac output in a patient with ARDS and acute right heart failure during inhalation of nitric oxide. Acta Anaesthesiol Scand. 1997;41(5):643–646. doi: 10.1111/j.1399-6576.1997.tb04758.x. [DOI] [PubMed] [Google Scholar]
- 38.Putensen C, Hörmann C, Kleinsasser A, Putensen-Himmer G. Cardiopulmonary effects of aerosolized prostaglandin E1 and nitric oxide inhalation in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1743–1747. doi: 10.1164/ajrccm.157.6.9609017. [DOI] [PubMed] [Google Scholar]
- 39.Gallart L, Lu Q, Puybasset L, Umamaheswara Rao GS, Coriat P, Rouby JJ. Intravenous almitrine combined with inhaled nitric oxide for acute respiratory distress syndrome. The NO Almitrine Study Group. Am J Respir Crit Care Med. 1998;158(6):1770–1777. doi: 10.1164/ajrccm.158.6.9804066. [DOI] [PubMed] [Google Scholar]
- 40.Iotti GA, Olivei MC, Palo A, Galbusera C, Veronesi R, Braschi A. Acute effects of inhaled nitric oxide in adult respiratory distress syndrome. Eur Respir J. 1998;12(5):1164–1171. doi: 10.1183/09031936.98.12051164. [DOI] [PubMed] [Google Scholar]
- 41.Bhorade S, Christenson J, O’connor M, Lavoie A, Pohlman A, Hall JB. Response to inhaled nitric oxide in patients with acute right heart syndrome. Am J Respir Crit Care Med. 1999;159(2):571–579. doi: 10.1164/ajrccm.159.2.9804127. [DOI] [PubMed] [Google Scholar]
- 42.Kuhlen R, Walbert E, Fränkel P, Thaden S, Behrendt W, Rossaint R. Combination of inhaled nitric oxide and intravenous prostacyclin for successful treatment of severe pulmonary hypertension in a patient with acute respiratory distress syndrome. Intensive Care Med. 1999;25(7):752–754. doi: 10.1007/s001340050941. [DOI] [PubMed] [Google Scholar]
- 43.Huette P, Beyls C, Guilbart M, Haye G, Najid FZ, Mestan B, et al. Acute cor pulmonale in COVID-19-related ARDS: improvement with almitrine infusion. JACC Case Rep. 2020;2(9):1311–1314. doi: 10.1016/j.jaccas.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walmrath D, Schneider T, Schermuly R, Olschewski H, Grimminger F, Seeger W. Direct comparison of inhaled nitric oxide and aerosolized prostacyclin in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;153(3):991–996. doi: 10.1164/ajrccm.153.3.8630585. [DOI] [PubMed] [Google Scholar]
- 45.Domenighetti G, Stricker H, Waldispuehl B. Nebulized prostacyclin (PGI2) in acute respiratory distress syndrome: impact of primary (pulmonary injury) and secondary (extrapulmonary injury) disease on gas exchange response. Crit Care Med. 2001;29(1):57–62. doi: 10.1097/00003246-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 46.Seo H, Lopez CN, Succar L, Donahue KR. Evaluation of inhaled alprostadil in hospitalized adult patients. Ann Pharmacother. 2022;56(6):671–678. doi: 10.1177/10600280211042675. [DOI] [PubMed] [Google Scholar]
- 47.Cornet AD, Hofstra JJ, Swart EL, Girbes ARJ, Juffermans NP. Sildenafil attenuates pulmonary arterial pressure but does not improve oxygenation during ARDS. Intensive Care Med. 2010;36(5):758–764. doi: 10.1007/s00134-010-1754-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McFadyen C, Garfield B, Mancio J, Ridge CA, Semple T, Keeling A, et al. Use of sildenafil in patients with severe COVID-19 pneumonitis. Br J Anaesth. 2022;129(1):e18–21. doi: 10.1016/j.bja.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michard F, Wolff MA, Herman B, Wysocki M. Right ventricular response to high-dose almitrine infusion in patients with severe hypoxemia related to acute respiratory distress syndrome. Crit Care Med. 2001;29(1):32–36. doi: 10.1097/00003246-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Radermacher P, Santak B, Wüst HJ, Tarnow J, Falke KJ. Prostacyclin and right ventricular function in patients with pulmonary hypertension associated with ARDS. Intensive Care Med. 1990;16(4):227–232. doi: 10.1007/BF01705156. [DOI] [PubMed] [Google Scholar]
- 51.Papazian L, Bregeon F, Gaillat F, Kaphan E, Thirion X, Saux P, et al. Does norepinephrine modify the effects of inhaled nitric oxide in septic patients with acute respiratory distress syndrome? Anesthesiology. 1998;89(5):1089–1098. doi: 10.1097/00000542-199811000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Morelli A, Teboul JL, Maggiore SM, Vieillard-Baron A, Rocco M, Conti G, et al. Effects of levosimendan on right ventricular afterload in patients with acute respiratory distress syndrome: a pilot study. Crit Care Med. 2006;34(9):2287–2293. doi: 10.1097/01.CCM.0000230244.17174.4F. [DOI] [PubMed] [Google Scholar]
- 53.Reis Miranda D, van Thiel R, Brodie D, Bakker J. Right ventricular unloading after initiation of venovenous extracorporeal membrane oxygenation. Am J Respir Crit Care Med. 2015;191(3):346–348. doi: 10.1164/rccm.201408-1404LE. [DOI] [PubMed] [Google Scholar]
- 54.Mongodi S, Orlando A, Tavazzi G, Pozzi M, Maggio G, Braschi A, et al. Veno-venous extracorporeal membrane oxygenation for acute respiratory distress syndrome in a patient with acute right heart failure. J Cardiothorac Vasc Anesth. 2017;31(4):1374–1377. doi: 10.1053/j.jvca.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 55.Pettenuzzo T, Pichette M, Douflé G, Fan E. Effect of ultraprotective mechanical ventilation on right ventricular function during extracorporeal membrane oxygenation in adults with acute respiratory distress syndrome. J Cardiothorac Vasc Anesth. 2021;35(6):1906–1908. doi: 10.1053/j.jvca.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pappalardo F, Pieri M, De Bonis M, Maj G, Calabrò MG, Ajello S, et al. Cardiac support with IABP during venovenous ECMO for ARDS. Intensive Care Med. 2013;39(6):1152–1153. doi: 10.1007/s00134-013-2886-z. [DOI] [PubMed] [Google Scholar]
- 57.Lee SH, Jung JS, Chung JH, Lee KH, Kim HJ, Son HS, et al. Right heart failure during veno-venous extracorporeal membrane oxygenation for H1N1 induced acute respiratory distress syndrome: case report and literature review. Korean J Thorac Cardiovasc Surg. 2015;48(4):289–293. doi: 10.5090/kjtcs.2015.48.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cherpanath TGV, Landburg PP, Lagrand WK, Schultz MJ, Juffermans NP. Effect of extracorporeal CO2 removal on right ventricular and hemodynamic parameters in a patient with acute respiratory distress syndrome. Perfusion. 2016;31(6):525–529. doi: 10.1177/0267659115621783. [DOI] [PubMed] [Google Scholar]
- 59.Goursaud S, Valette X, Dupeyrat J, Daubin C, du Cheyron D. Ultraprotective ventilation allowed by extracorporeal CO2 removal improves the right ventricular function in acute respiratory distress syndrome patients: a quasi-experimental pilot study. Ann Intensive Care. 2021;11(1):3. doi: 10.1186/s13613-020-00784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fuller BM, Mohr NM, Skrupky L, Fowler S, Kollef MH, Carpenter CR. The use of inhaled prostaglandins in patients with ARDS: a systematic review and meta-analysis. Chest. 2015;147(6):1510–1522. doi: 10.1378/chest.14-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferguson ND. Inhaled nitric oxide for acute respiratory distress syndrome. BMJ Br Med J. 2007;334(7597):757–758. doi: 10.1136/bmj.39168.568692.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dugar S, Sato R, Zochios V, Duggal A, Vallabhajosyula S. Defining right ventricular dysfunction in acute respiratory distress syndrome. J Cardiothorac Vasc Anesth. 2022;36(2):632–634. doi: 10.1053/j.jvca.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 63.Lahm T, Douglas IS, Archer SL, Bogaard HJ, Chesler NC, Haddad F, et al. Assessment of right ventricular function in the research setting: knowledge gaps and pathways forward. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med. 2018;198(4):e15–43. doi: 10.1164/rccm.201806-1160ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chotalia M, Ali M, Alderman JE, Bansal S, Patel JM, Bangash MN, et al. Cardiovascular subphenotypes in acute respiratory distress syndrome. Critical Care Medicine. 9900; https://journals.lww.com/ccmjournal/Fulltext/9900/Cardiovascular_Subphenotypes_in_Acute_Respiratory.84.aspx. [DOI] [PubMed]
- 65.Zochios V, Yusuff H, Schmidt M, on behalf of Protecting the Right Ventricle Network (PRORVnet) Acute right ventricular injury phenotyping in ARDS. Intensive Care Med. 2023;49(1):99–102. doi: 10.1007/s00134-022-06904-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303(9):865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 67.Dianti J, Tisminetzky M, Ferreyro BL, Englesakis M, Del Sorbo L, Sud S, et al. Association of positive end-expiratory pressure and lung recruitment selection strategies with mortality in acute respiratory distress syndrome: a systematic review and network meta-analysis. Am J Respir Crit Care Med. 2022;205(11):1300–1310. doi: 10.1164/rccm.202108-1972OC. [DOI] [PubMed] [Google Scholar]
- 68.Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 69.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 70.Chad T, Yusuff H, Zochios V, Pettenuzzo T, Fan E, Schmidt M, et al. Right ventricular injury increases mortality in patients with acute respiratory distress syndrome on veno-venous extracorporeal membrane oxygenation: a systematic review and meta-analysis. ASAIO J. 2023;69(1):e14–22. doi: 10.1097/MAT.0000000000001854. [DOI] [PubMed] [Google Scholar]
- 71.Estoos EM, Jocham KP, Zhang C, Benson LM, Milas A, Zakhary B. Optimal positive end-expiratory pressure reduces right ventricular dysfunction in COVID-19 patients on venovenous extracorporeal membrane oxygenation: A retrospective single-center study. J Crit Care. 2023;8(75):154274. doi: 10.1016/j.jcrc.2023.154274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saeed O, Stein LH, Cavarocchi N, Tatooles AJ, Mustafa A, Jorde UP, et al. Outcomes by cannulation methods for venovenous extracorporeal membrane oxygenation during COVID-19: a multicenter retrospective study. Artif Organs. 2022;46(8):1659–1668. doi: 10.1111/aor.14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith NJ, Park S, Zundel MT, Dong H, Szabo A, Cain MT, et al. Extracorporeal membrane oxygenation for COVID-19: an evolving experience through multiple waves. Artif Organs. 2022;46(11):2257–2265. doi: 10.1111/aor.14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ivins-O’Keefe KM, Cahill MS, Mielke AR, Sobieszczyk MJ, Sams VG, Mason PE, et al. Percutaneous pulmonary artery cannulation to treat acute secondary right heart failure while on veno-venous extracorporeal membrane oxygenation. ASAIO J. 2022;68(12):1483–1489. doi: 10.1097/MAT.0000000000001692. [DOI] [PubMed] [Google Scholar]
- 75.Slobod D, Assanangkornchai N, Alhazza M, Mettasittigorn P, Magder S. Right ventricular loading by lung inflation during controlled mechanical ventilation. Am J Respir Crit Care Med. 2022;205(11):1311–1319. doi: 10.1164/rccm.202111-2483OC. [DOI] [PubMed] [Google Scholar]
- 76.Goligher EC, Costa ELV, Yarnell CJ, Brochard LJ, Stewart TE, Tomlinson G, et al. Effect of lowering Vt on mortality in acute respiratory distress syndrome varies with respiratory system elastance. Am J Respir Crit Care Med. 2021;203(11):1378–1385. doi: 10.1164/rccm.202009-3536OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. PRISMA-ScR Checklist.
Additional file 2. Search Strategy, Supplemental Tables and Figures.
Data Availability Statement
All data related to this work are included in the main text or the supplementary materials. For pre-registered search strategy, please see https://osf.io/9kuph.