Abstract
Autoimmune glial fibrillar acidic protein (GFAP) astrocytopathy is a rare autoimmune neuroinflammatory disorder that affects the central nervous system. We present a case of GFAP astrocytopathy in a middle-aged male who presented with constitutional symptoms, encephalopathy and lower extremity weakness and numbness. Initially MRI of the spine was normal, but he subsequently developed longitudinally extensive myelitis and meningoencephalitis. Workup for infectious aetiologies was negative and the patient’s clinical course worsened despite broad antimicrobial coverage. Ultimately, he was found to have anti-GFAP antibodies in his cerebral spinal fluid consistent with GFAP astrocytopathy. He was treated with steroids and plasmapheresis with clinical and radiographic improvement. This case demonstrates the temporal evolution of myelitis on MRI in a case of steroid-refractory GFAP astrocytopathy.
Keywords: Spinal cord, Neuroimaging, Infection (neurology), Neurology, Radiology
Background
Autoimmune glial fibrillar acidic protein (GFAP) astrocytopathy is a rare autoimmune neuroinflammatory disorder that affects the central nervous system. Clinical presentation varies, but onset is typically subacute with prodromal influenza-like symptoms.1–3 Patients go on to develop meningoencephalitis characterised by encephalopathy, headache and neck stiffness.1 2 Some patients will develop myelitis, optic disc oedema, tremor, ataxia, psychiatric symptoms and/or autonomic dysfunction.1 2 Characteristic imaging findings include linear radial perivascular enhancement surrounding the lateral ventricles on MRI brain and longitudinally extensive T2 hyperintensities on MRI spine.2 Patients have circulating antibodies against the intermediate filament protein found in astrocytes, GFAP. The antibodies are highly specific to this condition but are unlikely to be pathological themselves since the GFAP protein is intracellular.4 Animal studies suggest that GFAP protein-specific cytotoxic T cells drive the pathogenesis through their interaction with GFAP-expressing astrocytes.4 The exact pathogenesis remains unknown, but some cases are associated with malignancy, with as many as 34% of patients being diagnosed with malignancy within 2 years.2 Other cases are thought to be parainfectious because of the frequent prodromal influenza-like symptoms.1–3 The causal infectious agent is rarely found, however, there are reports implicating HIV, herpes simplex virus (HSV) and varicella zoster virus.1–3 Coexisting autoimmune conditions have also been described with reports of several coexisting autoantibodies: N-methyl-D-aspartic acid, aquaporin 4 (AQP4), glutamic acid decarboxylase 65 and thyroid peroxidase.1 2 Most patients respond to glucocorticoids, the first-line treatment. However, steroids are not effective in all cases and some patients require second-line treatments such as plasmapheresis. Relapse occurs in 20%–50% of cases.5 6 We present a case that illustrates the temporal evolution of myelitis in steroid-refractory GFAP astrocytopathy.
Case presentation
A man in his 50s with a history of paroxysmal atrial fibrillation presented with 1 week of fever, nausea, vomiting and generalised weakness. Two days prior to presentation, he developed confusion, back pain, urinary hesitancy, and lower extremity numbness and weakness. He denied rash, arthralgias, cough, shortness of breath, diarrhoea, saddle anaesthesia or history of back problems. He had recent travel outside the country and worked outdoors in dusty conditions, but denied personal history of mycobacterium tuberculosis (MTB) infection, sick contacts or MTB exposure.
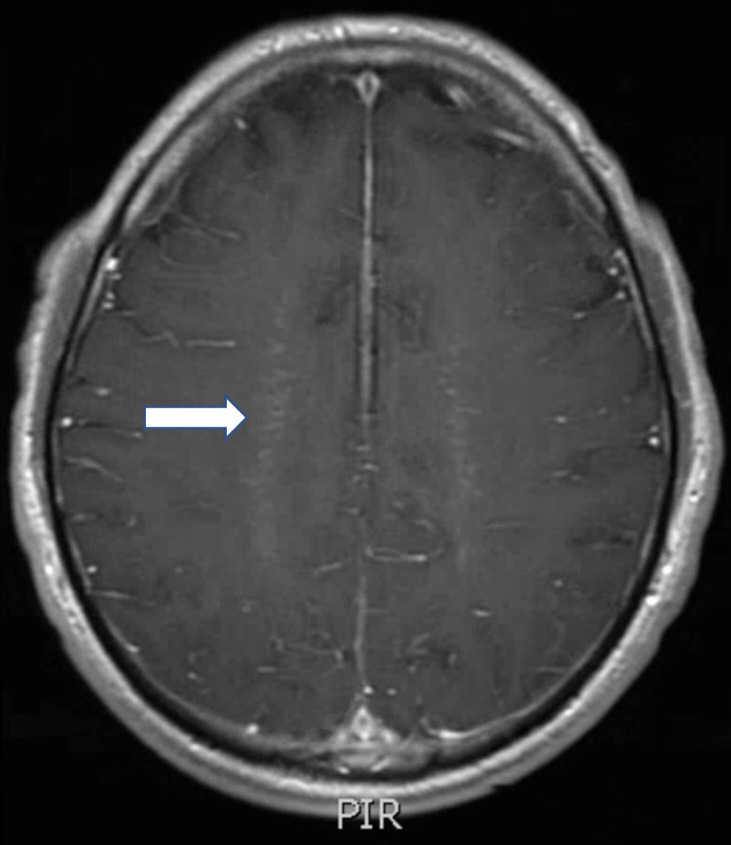
At presentation, the patient was febrile to 38.9°C, disoriented to situation and had impaired concentration. He had 4+/5 strength in the hip and knee flexors, ankle clonus and upgoing toes bilaterally. Upper extremity strength, cranial nerves and coordination were intact. MRI of the thoracic and lumbar spine showed normal signal throughout the spinal cord parenchyma and was read as normal (figure 1). On retrospective review, subtle contrast enhancement of the leptomeninges surrounding the conus was noted (figure 2). MRI brain showed diffuse leptomeningitis. On retrospective review, linear radial perivascular enhancement surrounding the lateral ventricles was noted (figure 3). Lumbar puncture demonstrated a normal opening pressure, elevated white blood cells (ranging from 85 to 501/mm3) with a lymphocytic predominance (87%), elevated red blood cells (ranging from 8 to 123/mm3), elevated protein (306 mg/dL), normal glucose and normal lactate dehydrogenase. Metabolic, neoplastic and preliminary autoimmune workup were negative, including oligoclonal bands, ACE, AQP4 and myelin oligodendrocyte glycoprotein (MOG). Extensive infectious workup was negative (cerebral spinal fluid, CSF and serum), including MTB-QuantiFERON, MTB PCR, AFB, HIV, syphilis, coccidioides, histoplasma, cryptococcus, brucella, West Nile, Lyme disease and a meningitis/encephalitis panel (including HSV, cytomegalovirus and enterovirus). Due to the rapidly progressive disease course despite broad antimicrobial coverage, he underwent biopsy of the meninges and brain, which showed no evidence of infectious organisms.
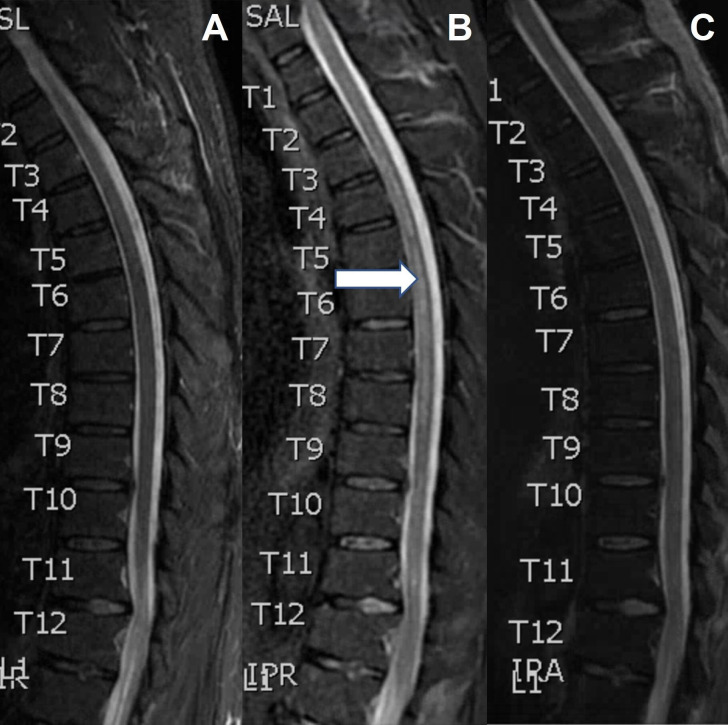
Figure 1.
Sagittal short tau inversion recovery (STIR) MRI spine at admission (A); 11 days later, showing longitudinal T2 hyperintensity (B, arrow); and 2 weeks after the second MRI, following steroids and plasmapheresis (C).
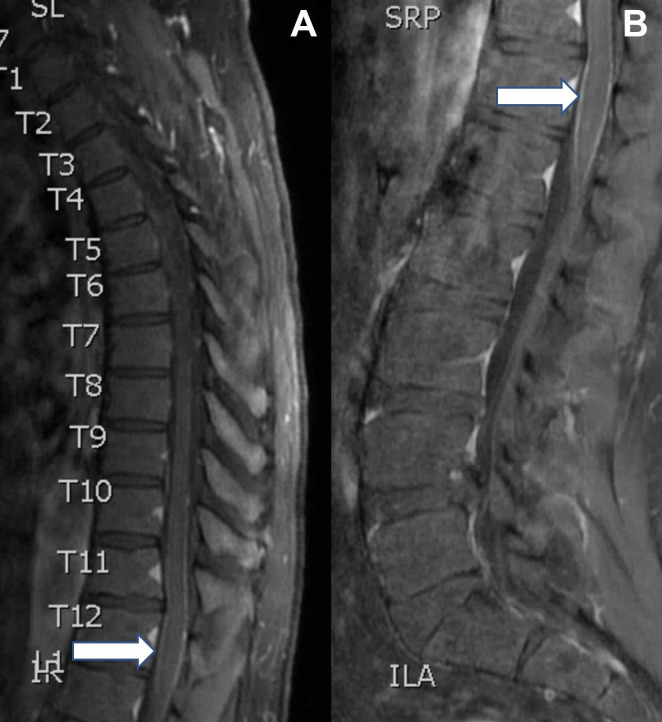
Figure 2.
Sagittal T1 MRI spine postcontrast showing leptomeningeal enhancement (A, arrow), detail (B, arrow).
Figure 3.
T1 postcontrast MRI brain showing linear, radial perivascular pattern of enhancement (arrow).
At admission, there was concern for an infective meningoencephalitis given the preponderance of constitutional symptoms. The patient was empirically started on broad antimicrobial coverage with vancomycin, ceftriaxone, metronidazole, acyclovir, fluconazole, RIPE (rifampin, isoniazid, pyrazinamide and ethambutol) and steroids (dexamethasone 8 mg every 6 hours as part of RIPE). RIPE and antifungal treatments were chosen due to concern for an atypical bacterial or fungal infection given the subacute presentation and CSF profile. The patient’s fever and confusion improved with RIPE and dexamethasone, which led to a working diagnosis of MTB encephalomyelitis (despite negative MTB studies). However, his weakness continued to progress and began to involve the upper extremities. His lower extremities further declined to 2–3/5 strength, and he developed significant sensory loss throughout. Repeat spine imaging (eleven days after initiation of RIPE and dexamethasone) showed longitudinally extensive patchy T2 hyperintense signal throughout the central grey and lateral columns of the cervical and thoracic spine (figure 1). In the absence of an identifiable infection, he was started on pulse dose steroids with methylprednisolone 1000 mg intravenously daily for 5 days, followed by solumedrol 60 mg intravenously daily (in place of the RIPE associated dexamethasone). With this treatment he had no further clinical decline, however, his lower extremity weakness remained unchanged. Several days later, the Mayo Clinic CSF autoimmune encephalitis panel returned positive for GFAP 1:128 (ref <1:2) confirming a diagnosis of meningomyeloencephalitis due to GFAP astrocytopathy. At this point, antimicrobials were stopped, and he underwent five sessions of plasmapheresis. Two weeks after the second MRI, he had clinical and radiologic improvement (figure 1).
Outcome and follow-up
Prior to discharge patient’s strength improved to 4/5 strength in the lower extremities. Given the association between GFAP astrocytopathy and malignancy, he was screened with CT chest-abdomen-pelvis and testicular ultrasound, which found no evidence of occult malignancy. He was discharged to a rehab facility on a steroid taper and mycophenolate mofetil. A steroid-sparing agent was chosen based on the severity of his disease and reported relapse rates on steroids from prior case series. On follow-up 3 months later, he was ambulating with a cane with full strength throughout aside from 4+/5 strength in the hip flexors bilaterally.
Discussion
GFAP astrocytopathy is an autoimmune neuroinflammatory disorder that can affect the entire neuroaxis and present with a wide range of symptoms. Patients typically present with meningoencephalitis, but can also manifest with myelitis, optic disc oedema, tremor, ataxia, psychiatric symptoms and/or autonomic dysfunction.1 2 Typical radiographic findings include linear, radial perivascular enhancement surrounding the lateral ventricles on MRI brain and longitudinally extensive T2 hyperintensities on MRI spine.6 Most patients respond to steroids, but some cases are refractory and require second-line treatments.5 6 We present a case of GFAP astrocytopathy that illustrates the radiographic evolution of myelitis from symptom onset to recovery. Initial MRI of the spine did not show myelitis, but radiographic myelitis eventually developed days later despite high-dose steroids (dexamethasone 8 mg every 6 hours). Repeat imaging following treatment with pulse dose steroids and plasmapheresis demonstrated radiographic resolution of myelitis.
Myelitis is a common feature of GFAP astrocytopathy with one cohort of 71 patients reporting myelitis in 43% of cases.4 Our patient presented with 48 hours of symptoms localising to the spinal cord despite normal parenchymal signal on MRI C and T spine (figure 1). It was only on retrospective review that subtle contrast enhancement was noted in the leptomeninges surrounding the conus (figure 2). While the lack of overt myelitis on imaging was surprising, there is at least one other documented case of GFAP astrocytopathy associated myelitis presenting with normal spine imaging.4 Additionally, MRI-negative myelitis has been described in a variety of other neuroinflammatory disorders. In one cohort of 73 patients with myelitis due to MOG associated disorder (MOGAD), MRI was normal in 10% of cases.7 In two cohorts, including a total of 26 patients with systemic lupus erythematosus-associated myelitis, MRI was normal in 8%–30% of cases.8 9 The imaging findings in our case demonstrate that myelitis may not appear on MRI early in the disease course of GFAP astrocytopathy. A high index of suspicion should be maintained for spinal cord involvement regardless of negative imaging since this may cause more disability and require more aggressive treatment. The lumbar leptomeningeal enhancement observed initially in our case may represent an early sign of ongoing or impending spinal cord involvement.
Repeat imaging 11 days after initial imaging demonstrated longitudinally extensive, patchy T2 hyperintense signal throughout the central grey and lateral columns of the cervical and thoracic spine (figure 1). This correlated with our patient’s clinical symptoms of upper and lower extremity weakness, numbness, paresthesias and bowel and bladder dysfunction. Longitudinally extensive myelitis is the most common pattern seen in GFAP astrocytopathy-associated myelitis. While there is variation in the literature, some studies report longitudinally extensive lesions in over 80% of cases of myelitis due to GFAP astrocytopathy.4 Other characteristic features include involvement of the central grey matter with subtle, poorly defined margins, which was also demonstrated in our case.4
While GFAP astrocytopathy is generally considered to be steroid responsive, our patient developed radiographic myelitis while on high-dose steroids (dexamethasone 8 mg every 6 hours).2 There may have been some response to pulse dose steroids since disease progression plateaued after administration. However, it was not until our patient underwent plasmapheresis that he improved clinically and radiographically. Two weeks after the second MRI, there was near complete resolution of his imaging abnormalities (figure 1). This degree of radiographic resolution is similar to what is observed in MOGAD patients, where individuals can have complete resolution of imaging abnormalities.10 Other authors have also reported incomplete responses to steroids in GFAP astrocytopathy. Shan et al found a general trend towards worse outcomes in severe cases of GFAP astrocytopathy treated solely with steroids or intravenously Ig and favoured plasmapheresis or alternative immunosuppressants.4 Yang et al reported poor outcomes with severe disability and even death in a cohort of seven patients treated with steroids and intravenously Ig during their initial attack.5 Furthermore, Yang et al describes relapse in four of their seven patients which necessitated long-term steroid therapy and the initiation of a steroid sparing agent (azathioprine or mycophenolate mofetil).5 Our findings support early implementation of alternative therapies such as plasmapheresis in severe cases of GFAP astrocytopathy.
Learning points.
Glial fibrillar acidic protein (GFAP) astrocytopathy is a rare neuroinflammatory disorder that can present with meningoencephalomyelitis.
GFAP astrocytopathy can be mistaken for an infectious meningoencephalitis given the prominent constitutional and nonspecific symptoms that many patients present with.
MRI of the spine may be normal early on despite clinical evidence of spinal cord involvement.
Leptomeningeal enhancement surrounding the spinal cord may precede the radiographic appearance of myelitis.
While steroids are first line for GFAP astrocytopathy, not all patients respond and alternative treatments should not be delayed.
Acknowledgments
Dr. Max Kazer for edits and obtaining consent.
Footnotes
Contributors: CEG drafted the main body of the article, generated the images/figures and performed research of the topic. DP edited the article and oversaw the drafting and submission of the article. JH provided radiographic consultation, edited figures and performed research on the topic. AR provided infectious disease consultation and edited the article. Patient consent for this case report was obtained by Max Kazer.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Fang B, McKeon A, Hinson SR, et al. Autoimmune glial fibrillary acidic protein astrocytopathy: a novel meningoencephalomyelitis. JAMA Neurol 2016;73:1297–307. 10.1001/jamaneurol.2016.2549 [DOI] [PubMed] [Google Scholar]
- 2.Flanagan EP, Hinson SR, Lennon VA, et al. Glial fibrillary acidic protein immunoglobulin G as biomarker of autoimmune astrocytopathy: analysis of 102 patients. Ann Neurol 2017;81:298–309. 10.1002/ana.24881 [DOI] [PubMed] [Google Scholar]
- 3.Yang X, Xu H, Ding M, et al. Overlapping autoimmune syndromes in patients with glial fibrillary acidic protein antibodies. Front Neurol 2018;9:251. 10.3389/fneur.2018.00251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shan F, Long Y, Qiu W. Autoimmune glial fibrillary acidic protein astrocytopathy: a review of the literature. Front Immunol 2018;9:2802. 10.3389/fimmu.2018.02802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X, Liang J, Huang Q, et al. Treatment of autoimmune glial fibrillary acidic protein astrocytopathy: follow-up in 7 cases. Neuroimmunomodulation 2017;24:113–9. 10.1159/000479948 [DOI] [PubMed] [Google Scholar]
- 6.Kunchok A, Zekeridou A, McKeon A. Autoimmune GFAP astrocytopathy. Curr Opin Neurol 2019;32:452–8. 10.1097/WCO.0000000000000676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sechi E, Krecke KN, Pittock SJ, et al. Frequency and characteristics of MRI-negative myelitis associated with MOG autoantibodies. Mult Scler 2021;27:303–8. 10.1177/1352458520907900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovacs B, Lafferty TL, Brent LH, et al. Transverse myelopathy in systemic lupus erythematosus: an analysis of 14 cases and review of the literature. Ann Rheum Dis 2000;59:120–4. 10.1136/ard.59.2.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu X, Gu Y, Wang Y, et al. Prognostic factors of lupus myelopathy. Lupus 2008;17:323–8. 10.1177/0961203307088005 [DOI] [PubMed] [Google Scholar]
- 10.Sechi E, Krecke KN, Messina SA, et al. Comparison of MRI lesion evolution in different central nervous system demyelinating disorders. Neurology 2021;97:109. 10.1212/WNL.0000000000012467 [DOI] [PMC free article] [PubMed] [Google Scholar]