Abstract
Coronavirus disease 19 (COVID-19) may lead to post-COVID syndrome a few weeks to months after the infection with various symptoms. Post-COVID thromboembolic syndrome may be a result of coagulopathy that occurs in both the arterial and venous circulation. Apart from direct cellular infection, post-COVID syndrome may occur due to immune system dysregulation, endothelial injury, and hypercoagulability, leading to thrombosis. We present a 32-year-old man who was diagnosed with mild symptoms of COVID-19 infection 4 months before an acute ischemic stroke and an asymptomatic pulmonary embolism. A COVID-19 antigen test was negative. An analysis of prothrombotic factors was negative. He could not receive any therapeutic intervention before his demise. The extent of COVID-19 infection after the onset of symptoms is a mystery and poses a fatal concern due to the increasing number of complications. The long-term complications after COVID-19 infection are still not understood. Clinicians need to be aware of any signs and symptoms that may arise months after COVID-19 infection and its possible causal relationship.
Keywords: Ischemic stroke, Pulmonary embolism, Thromboembolism, COVID-19, SARS-CoV-2, Tanzania
Introduction
A coronavirus disease 2019 (COVID-19) infection may lead to post-COVID syndrome, also coined “Long COVID,” which describes various symptoms that may occur a few weeks to months after the infection, with either a continuous or relapsing and remitting nature [1]. The variety of signs and symptoms may be nonspecific, such as fatigue and body aches, up to thromboembolic phenomena. Post-COVID thromboembolic syndrome may be a result of coagulopathy that occurs in both arterial and venous circulation.
Diagnosing Long COVID poses many challenges since there can be a persistence of 1 or more symptoms or the development of a new symptom that is irrespective of the viral status [1]. The recovery time varies depending on the severity of the infection since complications may persist after the acute infection. A large proportion of infected patients may not have been tested, and many COVID-19 patients may have been asymptomatic. Since testing policies have been different in each country and over time, many patients have only been tested on the basis of the symptoms they presented with. In Tanzania, all patients who presented with fever and difficulty breathing were tested for COVID-19. However, most COVID-19 infections have gone unrecognized on the African continent, with an estimated community seroprevalence for COVID-19 infection of 14.2% [2]. This makes a diagnosis of Long COVID after emerging symptoms without serologic evidence of COVID-19 infection difficult.
Case presentation
A 32-year-old male presented with a sudden onset of right-sided hemiplegia 3 days before admission. Approximately 1 hour before the onset of right-sided weakness, he presented with a headache and blurring of vision, which followed his facial deviation and inability to speak. No loss of consciousness or trauma to the head or neck was reported. He was tested and diagnosed with COVID-19 four months before admission and was treated as an outpatient without oxygen and recovered within 2 weeks. He was a military officer with no identifiable comorbidity or significant illness in the past and no history of COVID-19 vaccination. A negative family history of thrombotic events was identified. There was a history of occasional alcohol consumption, and no history of smoking or medication use.
On examination, he had a Glasgow Coma Score of 11/15, was aphasic, afebrile, not pale, and not tachypneic. His vital signs were a blood pressure of 103/59 mmHg, a pulse rate of 59 beats per minute, a respiratory rate of 18 breaths per minute, and oxygen saturation of 96% on room air. His pupils were isocoric, constricted, and reactive to light. He had right-sided hemihyperhidrosis of the face. There was a right-sided hemiplegia grade of 0/5 in the arm and 1/5 in the leg, and global aphasia with preserved understanding of simple instructions only. Auscultation of the heart and lungs was normal. Other system examinations were not significant.
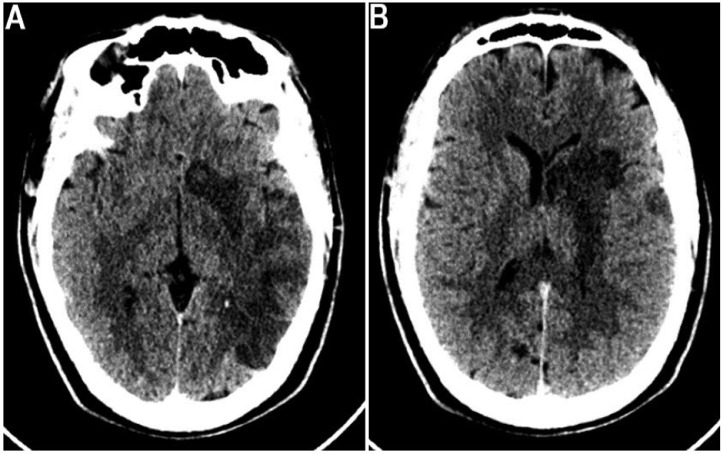
His initial laboratory investigations revealed a normal leukocyte count of 10.9 × 109/L, a hemoglobin of 16.6 g/dL, and a platelet count of 261 × 109/L. He had a normal creatinine of 110 mol/L with a good urine output, total cholesterol of 4.5 mmol/L, and an alanine transferase of 41.8 U/L. An electrocardiogram revealed sinus rhythm, and an echocardiogram identified no right to left shunt, cardiac thrombus or valvular pathology. Initial noncontrasted brain computed tomography image showed a large-wedge-shaped ischemic infarction in the left parietal lobe and left basal ganglia involving the proximal left middle cerebral artery territory (Figs. 1A and B), with significant edema and a midline shift of 0.5 cm. He was initiated on aspirin daily 75 mg once daily (qd), atorvastatin 20 mg qd, and subcutaneous heparin 5,000 units twice daily.
Fig. 1.
Axial brain CT shows (A) noncontrast image showing a large-wedge-shaped ischemic infarction in the left parietal lobe and (B) left basal ganglia with loss of gray and white matter differentiation involving the left middle cerebral artery territory.
Follow-up investigations revealed that C-reactive protein and troponin were elevated to 3795 ng/mL (normal 0-700) and 0.9 ng/mL (normal 0-0.1), respectively. A D-dimer done to assess for thromboembolism was elevated to 2.7 UgFEU/mL (normal 0-0.5). A normal partial thromboplastin time of 27.3 seconds (normal 25-35) and an international normalized ratio of 1.1 (normal 0.8-1.2) were identified. A rapid antigen test for COVID-19 was negative.
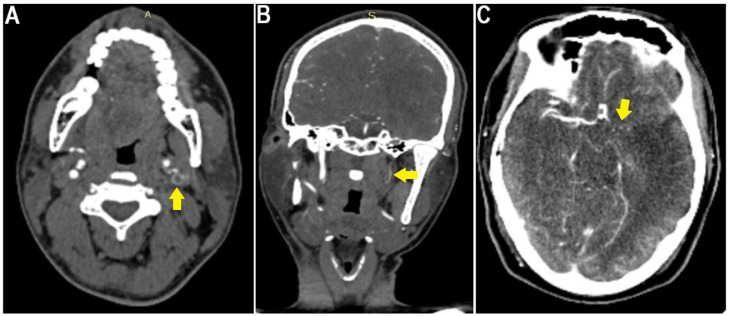
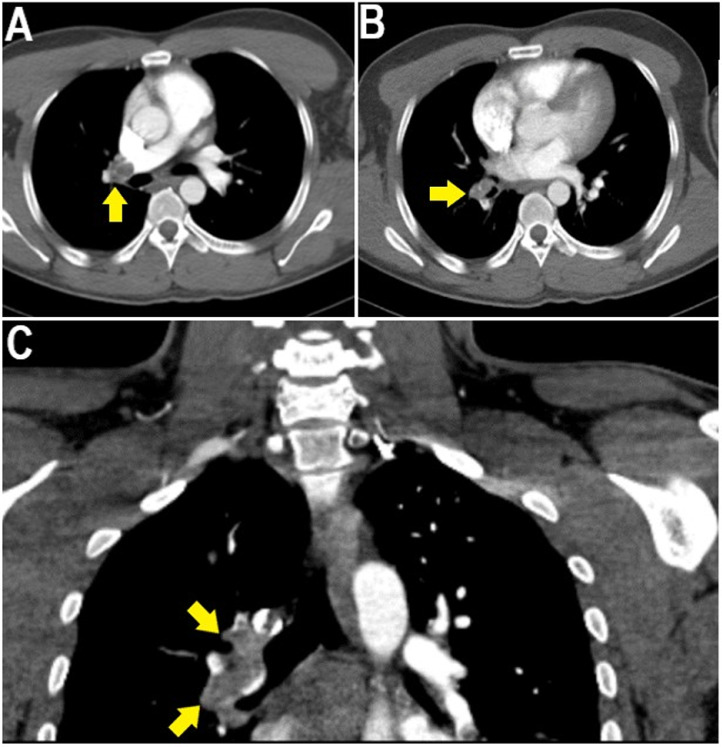
Because of the unknown etiology of the ischemic stroke, computed tomography angiography imaging, after prehydration, showed thromboembolic occlusion of the left middle cerebral artery (Fig. 1C), left internal carotid artery (Fig. 2), right main pulmonary artery, and segmental and subsegmental branches of the right upper, middle, and lower lobe pulmonary arteries (Fig. 3). However, shortly after the patient had been diagnosed with multiple thromboembolic occlusions, he developed sudden cardiac arrest whereby resuscitation was unsuccessful and death was certified before the initiation of any therapeutic intervention.
Fig. 2.
(A) Axial and (B) coronal neck angiography showing thromboembolic occlusion of the left internal carotid artery (yellow arrows), and (C) angiography showing thromboembolic occlusion of the left middle cerebral artery (yellow arrow) leading to the left middle cerebral artery territory infarction.
Fig. 3.
Contrasted pulmonary angiography showing thromboembolism in the right main pulmonary artery and segmental and subsegmental branches of the right upper, middle, and lower lobe pulmonary arteries (yellow arrows).
Discussion
Acute COVID-19 infection is associated with a prothrombotic state driven by immune cell activation, leading to platelet activation and endothelial damage. However, the extent of the prothrombotic state in post-COVID syndrome is not known, but can be fatal [3]. The possible mechanism of developing a stroke in acute COVID-19 infection starts with the binding of COVID-19 to angiotensin-converting enzyme 2, which allows the virus to enter the host cell and start the recruitment of macrophages, neutrophils, and monocytes. Excessive activation of the innate immune system disrupts the blood–brain barrier throughout endothelial dysfunction, allowing inflammation in the brain to cause thromboembolism formation and acute stroke [4].
Apart from direct cellular infection, post-COVID syndrome may occur due to immune system dysregulation, endothelial injury, and hypercoagulability, leading to thrombosis [5]. We demonstrate a healthy young man who suffered thrombosis in 2 arterial locations (left middle cerebral artery and left internal carotid artery) and 1 venous location (right main pulmonary artery).
Elevated D-dimer levels and cryptogenic strokes suggest an increased risk of thromboembolism in COVID-19 infection, which is associated with high mortality. A significant increase in D-dimer in acute ischemic stroke suggests that COVID-19 triggers a hypercoagulable state, causing thromboembolism in organs other than the brain [6].
In this case, post-COVID thromboembolic syndrome occurred in a young man without preexisting cardiovascular risk factors. Considering the age of the patient and the absence of accompanying cardiovascular risk factors, one may conclude that COVID-19 is an independent risk factor for acute ischemic stroke [7]. Inherited thrombophilia was considered in this patient since it might increase the risk of stroke in young adults. However, this may be limited to patients with a concomitant patent foramen ovale which was not observed in this patient. In contrast, antiphospholipid syndrome, an acquired thrombophilia. is associated with an increased risk of arterial ischemic events, such as cryptogenic ischemic stroke, among younger people [8].
Thirty days after COVID-19, patients had an elevated risk of 1.5 for Long COVID with the highest risk for pulmonary embolism [9]. Ischemic stroke has been identified in patients who had survived the first 30 days of COVID-19 with a burden of 3.4 per 1000 persons at 12 months [10].
An autopsy was not possible, in our case due to cultural issues, to rule out cause of thromboembolism or Long COVID. In literature, ≥50% of late cases had persistent viral RNA in the myocardium, lymph nodes, and central nervous system, due to less efficient clearance of viral RNA in nonrespiratory tissues [11].
The mortality rate of stroke patients is higher among those with acute COVID-19 infection [4]. The baseline mortality rate for stroke in Africa is also higher compared to other regions [12]. A temporary American Heart Association/American Stroke Association guideline states that, first, when a stroke patient presents to the hospital, a rapid and laboratory assessment should be made to identify the patient as a suspect or confirmed COVID-19 case [13]. However, many stroke patients present with a neurologic deficit, which makes it difficult to triage, and therefore safer to maintain a high index of suspicion and to use precaution even if the patient is asymptomatic [14]. After rapid assessment, brain imaging is preferred within 20 minutes of arrival, and management should be directed toward thrombolysis, mechanical thrombectomy, and other supportive care [13].
Approximately 35% of patients still have residual symptoms after treatment for COVID-19 on an outpatient basis [1]. With a weekly turnover of more than 300 patients in our medical outpatient services in an estimated catchment area size of over 15 million people in Northern Tanzania [15], few patients have been observed after treatment for COVID-19 due to significant loss to follow up after an expensive hospital admission. Some presented with fatigue, dyspnea, and chest pain. Insomnia, dizziness, memory problems, depression, palpitations, and coughing were reported by fewer patients. Rarely have they complained of headaches. It may be ideal to include having a history of COVID-19 to rule out causes of unexplained symptoms.
Conclusion
The extent of a COVID-19 infection after symptom relief is a mystery and poses a fatal concern as the pandemic slows down. The persistence of symptoms or new onset of symptoms in people who have recovered from COVID-19 infection is becoming a major concern. This case highlights the prompt recognition of chronic symptoms and complications that may arise in all patients with a prior history of COVID-19 infection, regardless of the severity. Therefore, serologic testing for antibodies in the absence of other risk factors as a routine investigation in stroke patients may be a take home message from the COVID era.
Patient consent
Informed written consent was obtained from the patient for publication of this case report and all imaging studies.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Raveendran A.V., Jayadevan R., Sashidharan S. Long COVID: an overview. Diabetes Metab Syndr. 2021;15:869–875. doi: 10.1016/j.dsx.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO (World Health Organization) WHO; Africa: 2021. Six in seven COVID-19 infections go undetected in Africa.https://www.afro.who.int/news/six-seven-covid-19-infections-go-undetected-africa [Google Scholar]
- 3.Mehandru S., Merad M. Pathological sequelae of long-haul COVID. Nat Immunol. 2022;23:194–202. doi: 10.1038/s41590-021-01104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wijeratne T., Gillard Crewther S., Sales C., Karimi L. COVID-19 pathophysiology predicts that ischemic stroke occurrence is an expectation, not an exception—a systematic review. Front Neurol. 2021;11:1–17. doi: 10.3389/fneur.2020.607221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinzon R.T., Kumalasari M.D., Kristina H. Ischemic stroke following COVID-19 in a patient without comorbidities. Case Rep Med. 2021;2021:1–3. doi: 10.1155/2021/8178529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tu T.M., Seet C.Y.H., Koh J.S., Tham C.H., Chiew H.J., De Leon J.A., et al. Acute ischemic stroke during the convalescent phase of asymptomatic COVID-2019 infection in men. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salehi Omran S., Hartman A., Zakai N.A., Navi B.B. Thrombophilia testing after ischemic stroke. Stroke. 2021;52:1874–1884. doi: 10.1161/STROKEAHA.120.032360. [DOI] [PubMed] [Google Scholar]
- 9.Al-Aly Z., Bowe B., Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461–1467. doi: 10.1038/s41591-022-01840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu E., Xie Y., Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med. 2022;28:2406–2415. doi: 10.1038/s41591-022-02001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein S.R., Ramelli S.C., Grazioli A., Chung J.-Y., Singh M., Yinda C.K., et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022;612:758–763. doi: 10.1038/s41586-022-05542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akinyemi R.O., Ovbiagele B., Adeniji O.A., Sarfo F.S., Abd-Allah F., Adoukonou T., et al. Stroke in Africa: profile, progress, prospects and priorities. Nat Rev Neurol. 2021;17:634–656. doi: 10.1038/s41582-021-00542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AHA/ASA Stroke Council Leadership Temporary emergency guidance to US Stroke Centers during the coronavirus disease 2019 (COVID-19) pandemic: on behalf of the American Heart Association/American Stroke Association Stroke Council Leadership. Stroke. 2020;51:1910–1912. doi: 10.1161/STROKEAHA.120.030023. [DOI] [PubMed] [Google Scholar]
- 14.Alzahrani A.A., Al Abdulsalam H., Al-Sakkaf H., Yousef A., Albadr F.B. Arterial thrombosis in an asymptomatic COVID-19 complicated by malignant middle cerebral artery syndrome: a case report and literature review. Int Med Case Rep J. 2021;14:401–405. doi: 10.2147/IMCRJ.S306830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadiq A.M., Njau R.E., Kilonzo K.G. Disease patterns and clinical outcomes of medical admissions at a tertiary hospital in Northern Tanzania: a retrospective observational study. Heal Sci Rep. 2023;6 doi: 10.1002/hsr2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]