Abstract
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are life-threatening conditions triggered by multiple intra- and extra-pulmonary injury factors, characterized by complicated molecular mechanisms and high mortality. Great strides have been made in the field of immunometabolism to clarify the interplay between intracellular metabolism and immune function in the past few years. Emerging evidence unveils the crucial roles of immunometabolism in inflammatory response and ALI. During ALI, both macrophages and lymphocytes undergo robust metabolic reprogramming and discrete epigenetic changes after activated. Apart from providing ATP and biosynthetic precursors, these metabolic cellular reactions and processes in lung also regulate inflammation and immunity.In fact, metabolic reprogramming involving glucose metabolism and fatty acidoxidation (FAO) acts as a double-edged sword in inflammatory response, which not only drives inflammasome activation but also elicits anti-inflammatory response. Additionally, the features and roles of metabolic reprogramming in different immune cells are not exactly the same. Here, we outline the evidence implicating how adverse factors shape immunometabolism in differentiation types of immune cells during ALI and summarize key proteins associated with energy expenditure and metabolic reprogramming. Finally, novel therapeutic targets in metabolic intermediates and enzymes together with current challenges in immunometabolism against ALI were discussed.
Keywords: ALI, Immunometabolism, Macrophages, Drugtarget, Inflammation
1. Introduction
Acute lung injury (ALI) is an acute hypoxic respiratory insufficiency caused by various intra- and extra-pulmonary injury factors including sepsis, trauma, ischemia-reperfusion, pneumonia,drug toxicity, and mechanical ventilation, giving rise to the destruction of the barrier of capillary endothelial cells and alveolar epithelial cells [1,2]. Pathologically, ALI is featured by alveolar edema, excessive infiltration of inflammatory cells, and diffuse pulmonary interstitial. In recent years, severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection has caused a large number of death cases resulting from ALI and acute respiratory distress syndrome (ARDS), incurring a tremendous economicburden [3]. However, there is no effective treatment for patients with ALI/ARDS, apart from mechanical ventilator and oxygen therapy [1,4].
Immunometabolism is an emerging field highlighting the effects of cellular metabolism on the maintenance and modulation of immune cells. Apart from discriminating self and nonself, immune system also regulates the resolution following injury involving exposure to toxins, hypoxia, mechanical damage, and infection [5,6]. Once injury occurs, immune cells could quickly sense the perturbation and relay local as well as systemic signals to cytokines or antibodies to trigger injury-adapted effector responses. Next, immune system will further regulate and monitor repair and resolution of the insult, eventually reestablishing immune homeostasis [7,8]. And both innate immunity and adaptive immunity participate in the different phases of ALI. In innate immunity, precise immunometabolism regulation of lung-resident macrophages and bone marrow-derived macrophagesis essential for the control of ALI and maintenance of lung tissue homeostasis. More importantly, metabolic reprogramming of macrophages exerts a crucial role inregulating their phenotype and plasticity [9,10]. As for adaptive immunity, metabolic programs of Naïve T and B cells are dynamically regulated upon injury occurs. And glucolipid metabolism is rapidly activated to produce lactate during theperiod of quiescence exit, accompanied by enhanced oxidative phosphorylation (OXPHOS) and mitochondrial metabolism in these lymphocytes [11,12]. Metabolic reprogramming of these immune cells can trigger both pro-inflammatory and anti-inflammatory responses, which is mainly determined by the phenotype of immunecells as well as the activities of specific enzymein metabolism [13]. While a great many studies have focused on metabolic characteristics and configuration of immune cells in homeostasis and inflamed lungs at present, the metabolic cross-talk between immune cellsand parenchymal cells in injured lung is still an important and challenging issue to address. Hence, better understanding of molecular mechanisms in ALI may result in the development of novel immune targeted therapies in the future.
Thepresent review will provide a framework for understanding the key concept of immunometabolism and immune cells in steady-state and ALI. We highlight the roles of innate immune and adaptive immune in immunometabolism during ALI. In addition, metabolic pathways and mechanisms contributing to metabolic reprogramming during ALI were also discussed.
2. The roles of innate immune and adaptive immune in immunometabolism during ALI
The relationship between metabolism and immunity is bidirectional. On the one hand, inflammation is activated in many metabolic disorders including obesity, metabolic syndrome, diabetes and hypertension [14,15]. The intertwined metabolic signaling pathways of the tricarboxylic acid (TCA) cycle, glycolysis, the pentose phosphate pathway (PPP), fatty acid synthesis, fatty acid oxidation (FAO), as well as amino acid metabolism are responsible for inflammatory reaction, aside from generating energy and producing building blocks for cellular survival and signaling transduction [16]. On the other hand, some metabolic factors such as glycolysis and mitochondrial dynamics within the immune cells also regulate immune cell functions [17,18]. Also, metabolic state of the environment also affects systemic inflammation by regulating substrates available and changing the gradients of chemokine as well as local cytokine [19]. For instance, in obesity andmetabolic syndrome, overabundant substrates display significant effects on the phenotype of resident and infiltratingimmune cells [20]. Accumulating evidence has demonstrated that the cells including macrophages, neutrophils, dendritic cells (DCs), myeloid-derived suppressor cells(MDSCs), T cells, B cells, and NK cellsengage in the development of ALI. Here, we summarized the metabolic disturbance of these innate and adaptive immune cells during ALI (Fig. 1 , Table 1 ).
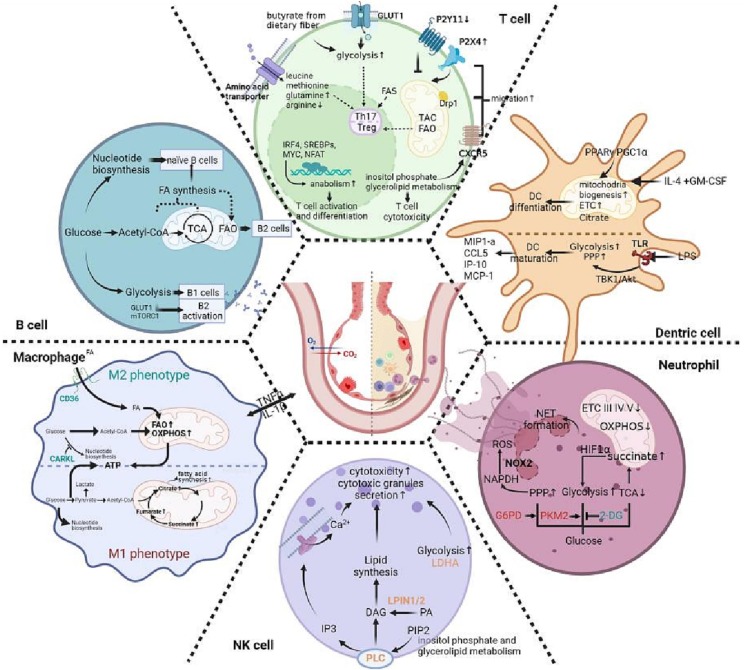
Fig. 1.
Schematic representation of immunometabolism and its change in immune cells during ALI. During ALI, innate immune cells including macrophage, NK cell, neutrophil, dentric cell and adaptive immune cells including T and B cell go through magnificent change, of which metabolism counts. This figure roughly illustrates immunometabolism change in each cells including glycolysis, TCA cycle, the pentose phosphate pathway(PPP), fatty acid synthesis and oxidation, OXPHOS, inositol phosphate and glycerolipid metabolism and so on. Different immune cells exhibit specific metabolism change for normal immune function and superlative immune response. See text for further explanations and references.
Table 1.
Immunometabolism and its change in immune cells during ALI.
| M1 Macrophage | M2 Macrophage | Neutrophils | DCs | NK cells | T cells | B cells | |
|---|---|---|---|---|---|---|---|
| Glycosis | ↑ exert pro-inflammatory properties | ↑derivation of most energy indispensible for NET formation or chromatin decondensation | ↑ initiate glycolysis by utilizing stored glycogen rather than FAO, which depends on PI3K/Akt signal and promotes maturation of DCs | ↑lactate dehydrogenase A (LDHA)-deficient NK cells fail to display optimal antiviral effects | ↑glucose transporter 1 (GLUT1) is important in differentiation of CD4+ T cell ↑glycosis is important for Th17 cells via epigenetic remodeling such as GLUT3-dependent acetyl-CoA generation |
glycolysis mainly offers energy in B1 B cells | |
| glycolysis promotes B cells to produce antibodies including IgG, IgM, and IgA | |||||||
| PPP | ↑ generate NADPH, which is essential for cholesterol metabolism and the synthesis of pro-inflammatory lipid mediators | The carbohydrate kinase-like protein CARKL shifts macrophage to M2 phenotype via PPP | activated glucose-6-phosphate dehydrogenase (G6PD) enhances NADPH production and NET formation | ↑ | glucose acts as the ingredient of ribonucleotide synthesis in naïve B cells | ||
| TCA | Fumarate accumulation, which induces monocyte epigenetic reprogramming by inhibiting KDM5 histone demethylases | Succinate accumulation, which stabilizes of HIF-1α and enhance glycolysis | TCA cycle produces main energy in naïve B cells | ||||
| Citrate accumulation, which generates fatty acids, serving as important ingredient for prostaglandin production membrane biogenesis | |||||||
| Succinate accumulation, which upregulates glycolytic enzymes and pro-inflammatory IL-1β | |||||||
| Lipid metabolism | ↓ Blocked triglyceride lipolysis Triglyceride accumulation |
↑FAO increase The scavenger receptor CD36-mediated uptake of triacylglycerol substrates and lysosomal lipolysis |
↑upregulated PPARγ and increased citrate synthase activity,which induced fatty acid synthesis and promote DC differentiation | ↑glycerolipid metabolism including LPIN1 and LPIN2 that increases the level of intracellular calcium and facilitates NK cell cytotoxicity | enhanced cytidine diphosphate (CDP) –ethanolamine pathway could promotes its migration via stabilizing the CXCR5 on the T cell surface | B2 B cells rely on FAO for their energy demand, which will shift to glycolysis by increasing GLUT1-mediated glucose uptake and mTORC1 and c-Myc expression upon activation | |
| short-chain fatty acids (SCFAs) like butyrate enhance mitochondrial and glycolytic metabolism, promoting anti-influenza immunity in CD8+ T; trigger T cell differentiation by inducing NETs during ALI. | |||||||
| Fatty acis synthesis are required for the function of Th17 cells and fatty acid oxidation for Treg | |||||||
| Amino acid metabolism | ↑Glutamine: a source of citrate for fatty acid synthesis or be used for ATP production, thus feeding TCA cycle glutamine is essential for IL-1β secretion | leucine, methionine, glutamine, together with their transporters could promote terminal effector (Teff) cells proliferation and survival,which expressed higher amount in activated CD8+ T cell than CD4+ T cells | glutaminolysis accompanied by glycosis through an elevation in mTORC1 and c-Myc expression | ||||
| Tryptophan: anabolic growth and cellular proliferation Arginine: also produces NO | |||||||
| ↓arginine as well as its metabolites display a pronounced reduction, which may possibly impede normal Tmem or Teff responses during COVID-19 | |||||||
| glutaminolysis is important for Th17 cells | |||||||
| Mitochondria metabolism | ↓inhibitory OXPHOS | very few functional mitochondria in mature neutrophils; lower levels of OXPHOS complexes and mitochondrial enzymes involving fumarase and glutamate dehydrogenase (GDH) | ↑upregulated PPARγ and PGC1α that is responsible for mitochondrial biogenesis during DCs differentiation | ↑ mitochondrial pro-fission protein Drp1 directs T cells trafficking to migration and expansion | |||
| Mitochondrial electron transport chain at Complex III functions in NET formation; Impaired complex III, IV and V are associated with dysfunction of LPS-driven neutrophil chemotaxis | ↑Inhibiting mitochondrial respiration suppresses DCs differentiation | ||||||
| redistrubution of P2Y11 receptor signaling which shuts down the activation of nearby mitochondria and P2X4 receptor where mitochondrial ATP production amplifies needed for pseudopod protrusion and T cell migration. | |||||||
| others | ↑ inositol phosphate metabolism including phospholipase C γ 2 (PLCG2) that mobilizes intracellular calcium and secretes cytotoxic granules |
Many selective transcription factors including IRF4, SREBPs, MYC, and NFAT are necessary for anabolism and T cell function | |||||
| inositol phosphate and glycerolipid metabolism enhance cytotoxicity of CD8+ T cells | |||||||
| enhanced glycosaminoglycan metabolism, which is associated with CD8+ Teff cell↑ and CD8+ Tmem↓ |
2.1. The innate immune cells during ALI
2.1.1. Macrophage
Macrophages in lung tissues involving lung-resident macrophages and bone marrow-derived macrophages are usually classified as classically activated (M1) macrophages as well as alternatively activated (M2) macrophages, displaying pro- and anti-inflammatory phenotypes respectively [21]. The balance between M1 and M2 macrophage phenotypes plays a vital role in the regulation of inflammatory response during ALI [22]. While M1 and M2 macrophage phenotypes represent two extremes, the mechanistic exploration of macrophage metabolic reprogramming provides a proof for understanding how macrophage metabolism and activation intersect to determine cellular fate during ALI.
Previous studies have demonstrated that glucosemetabolism, lipid metabolism, and amino acid metabolism are all implicated with the immune state of M1macrophages. M1 macrophages mainly depends on glycolysis to exert pro-inflammatory properties [23]. To be more specific, the activity of the PPP in M1 macrophages was enhanced togenerate nicotinamide adenine dinucleotide phosphate (NADPH), which is essential for cholesterol metabolism and the synthesis of pro-inflammatory lipid mediators [24]. In lipopolysaccharide (LPS)-induced macrophages, pyruvate triggered by glycolytic pathway is metabolized to lactate, while the accumulated TCA cycle intermediates give rise to inhibitory OXPHOS [25,26]. Fumarate, one of TCA cycle intermediates, triggers macrophage “innate immune memory” through KDM5 demethylases epigenetic remodeling [27]. After shuttled from mitochondrial matrix to thecytosol, citrate could be utilized to generate fatty acids, serving as important ingredient for prostaglandin production membrane biogenesis. And these fatty acids are also metabolized to antimicrobial itaconic acid [28]. Additionally,stabilization of thehypoxia-inducible factor-1α (HIF-1α) caused succinate accumulation, thus upregulating glycolytic enzymes and pro-inflammatory IL-1β [25]. Lipid metabolism is another important pathway to modulate the immune function of macrophage. LPS-induced activation of macrophages results in the accumulation of triglyceride accumulation by enhancing fatty acid uptake, increasing glucose uptake and incorporation into lipid, increasing triglyceride synthesis, and blocking triglyceride lipolysis [29]. Last but not least, amino acid metabolism also receives more and more attentions in recent years, owing to its emerging roles in the activation of macrophages.Glutamine could either act as a source of citrate for fatty acid synthesis or be used for ATP production, thus feeding TCA cycle. In M1 macrophages, glutamine is essential for IL-1β secretion. By contrast, tryptophan and arginine are metabolized to support and anabolic growth and cellular proliferation physiologically [30]. Arginine is metabolized by iNOS to produce NO, exerting an anti-microbial property upon LPS stimulation [31].
The metabolic phenotype of M2 macrophage is not well unveiled compared with M1 macrophage. In terms of glucose metabolism in M2 macrophage, a precipitous increase of FAO and OXPHOS contributes to its anti-inflammatory phenotype [26]. The scavenger receptor CD36-mediated uptake of triacylglycerol substrates and lysosomal acid lipase-mediated lipolysis give rise to M2 phenotype by elevating OXPHOS and enhancing spare respiratory capacity [32]. The carbohydrate kinase-like protein CARKL could catalyze an orphan reaction in the PPP, shifting macrophage to M2 phenotype [33].
One previous study showed that the increase of proinflammatory cytokines including pro-IL-1β and TNF-α preceded the activationof glycolysis in macrophages during LPS-induced ALI, suggesting that glycolysis maybe induced by inflammation [34]. By contrast,targeting PFKFB3 in alveolar epithelial cells could decrease inflammation and inhibit LPS-induced ALI [35].These two studies hint that inflammation and glycolysis interact with each other in sepsis-induced ALI. In silica-induced ALI, activating pattern of macrophages differs from the LPS, evidenced by decreased mitochondrial complex II activity as well as tricarboxylic acid cycle remodeling [36]. During cadmium-induced ALI, macrophage glycolytic intermediates involving cysteine and glycine synthesis were significantly altered [37]. Elevated blood glucose could promote SARS-CoV2 virus replication by upregulating glycolysis-associated genes in macrophage. However, similar phenomenon was not observed in patients infected with respiratory syncytial virus (RSV) or human influenza A H1N1 virus (IAV) [38], indicating that metabolic reprogramming is different in macrophages stimulated with differentvirus. The above studies suggest the critical roles of macrophages metabolism in ALI.
2.1.2. Neutrophils
As terminally differentiated leukocytes, neutrophils are the most abundant and short lived cells in human, exerting immune effector functions by oxidative burst, phagocytosis, and neutrophil extracellular traps (NETs) [39,40]. To meet energyrequirements, neutrophils must utilize certain metabolic intermediates generated in mitochondria and cytosol.In mature neutrophils, very few functional mitochondria are observed under the electron microcopy and mitochondrialrespiration inhibitors displays no effects on ATP levels [41,42]. What is more, there are significantly lower levels of OXPHOS complexes and mitochondrial enzymes involving fumarase and glutamate dehydrogenase (GDH) in mitochondria from neutrophils, hinting that role of mitochondria for respiration in neutrophilsis not as important as expected. NOX-2 dependent superoxide generation is the main reason that neutrophils utilize oxygen, which makes it challenging to investigate the functions of mitochondrial in neutrophils [42].
Neutrophils release mitochondrial or nuclear DNA, proteinases, as well as antimicrobial granular contents through NETosis to entrap and diminish pathogens during infections. The formation of NETs has been regarded as a major risk factor for ALI and mortality in patients with COVID-19 [43,44]. In fact, inter-mixing of decondensed nuclear chromatin with cytoplasmic granules, and expulsion offilamentous traps in NETosis is also energy-driven catabolic process. NETs formation possesses two distinguishable metabolic phases, namely glycolysis (chromatin decondensation) and the second phase (NET release) strictly dependent on exogenous glucose [45].
And NETosis could be also inhibited by 2-DG, suggesting that glycolysis is an important driver of NETs formation. During NETosis, activated glucose-6-phosphatedehydrogenase (G6PD) promotes glycolytic intermediate glucose-6-phosphatase (G6P)to PPP [45,46]. G6PD inhibition could incompletely reduce DNA release and ROS production in polymorphonuclear neutrophils, suggesting that G6P serves as a fuel for NOX2 activation. Additionally, this also hints the possibility of concurrently active glycolytic pathway or additional NADPH source than PPP [47].
Some NET-associated indicators including citrullinated histone H3, cell-free DNA and MPO/NE-DNA complexes are significantly elevated in tracheal aspiratesor circulation of patients with COVID-19. Additionally, in vitro neutrophils isolated from COVID-19 patients displayed elevated spontaneous NET formation [48,49]. The dysregulated metabolic pathways involving TCA cycle, eicosanoid biosynthesis, tryptophan metabolism, and polyunsaturated fatty acid mobilizationin neutrophils have also been unveiled. Meanwhile, SARS-CoV2 virus itself can trigger metabolic reprograming in neutrophils [48]. Additionally, the key rate-limiting enzyme catalyzing glycolysis, pyruvate kinase M2 (PKM2), is also upregulated in patients with COVID-19 [50]. The level of glycolysis in COVID-19 neutrophils significantly increased. Accumulation of cytosolic succinate during TCA could give rise to the increased stabilization of HIF-1α, causing a higher concentration HIF-1α in COVID-19 neutrophils. Excessive HIF-1α in cytoplasm elicited the increased ratio of cytosolic lactate to pyruvate ratio, suggesting an enhanced glycolysis in neutrophils duringCOVID-19 [50]. Duringsepsis, excessive NETs formation could also give rise to sepsis-induced ALI by amplifying the inflammatory response. Impaired complex V and electron transport chain complex III and IV are associated with dysfunction of LPS-driven neutrophil chemotaxis, which could be prevented by GSK3β inhibition and AMPK activation in sepsis-induced lung injury [51]. Collectively, the metabolic status of neutrophils displays a profound effect on NETs and NETosis, which further mediate the development of ALI.
2.1.3. Dendritic cells(DCs)
The function of DCs isto process and present antigens to antigens-specific T cells to trigger immuneresponse mediated by T cells. DCs develop from monocytes, committed DC progenitors. These monocytes share a common origin with macrophage and DC progenitors.Interleukin-4 (IL-4) and granulocyte–macrophage colony-stimulating factor(GM-CSF) could stimulate the differentiation of monocytes into DCs [52]. During this process, a key transcription factor in lipid metabolism, peroxisome proliferator-activated receptor-γ (PPARγ), is significantly upregulated. Also, the level of PPARγ co-activator 1α (PGC1α) that is responsible for mitochondrial biogenesis is also increased [53]. Inhibiting mitochondrial respiration by the electron transport chain inhibitor rotenone in monocytes is able to suppress DCs differentiation [53,54]. However, increased citrate synthase activity could promote DC differentiation by inducingfatty acid synthesis [55]. DCs areactivated by Toll-like receptor (TLR) agonists, giving rise to a rapid increase in glycolytic flux and the associated PPP, followed by an increase in spare respiratory capacity as well as fatty acid synthesis [56].
In DCs infected with SARS-CoV2, incomplete viral replication is observed. These DCs release significantly higher levels of MIP1-a, CCL5, IP-10, and MCP-1,which subsequently give rise to the infiltration of neutrophil, monocyte/macrophage, and T cell [57]. Similarly with other myeloid immune cells, DCs mainly obtain their energy via OXPHOS. FAO serves as the fuel of OXPHOS for DCs derived from bone marrow. However, the fuel for plasmacytoid DCs and conventional DCs remains unclear [58]. Additionally, DCs derived from bone marrow initiate glycolysis by utilizing stored glycogen, the inhibition of which impairs maturation of DCs and their effector function [58]. During acute viral infection, DCs generate higher level of energy by shifting OXPHOS to glycolysis [59]. It is worthy that the glycolysis in DCs mainly depends on Akt signaling which requires TBK1 and IKK for activation but not mTORC1 as well as HIF-1α-dependent signaling. However, SARS-CoV2 infection inhibits TBK1 and IKK-mediated interferon-β generation and NF-κB-mediated inflammatory response in DCs [60].
2.1.4. NK cells
NK cells account for about 15% of the resident lung lymphocytes in humans, thus NK cells also named large granular lymphocytes [61,62]. As a subpopulation of lymphocytes,NK cells have no antigen specificity, which recognize virus-infected cells independent of major histocompatibility complexes (MHC) or antibodies. Hence, NK cells belong to the innate immune system. NK cells own a variety of functions including antibody-dependent cell-mediated cytotoxicity (ADCMC), cytolytic granule mediated cell apoptosis, cytotoxic T lymphocyte (CTL) activation, serine esterase, cytokine-induced NK cell and generation of memory NK cells [63].NK cells exert critical effects on viral clearance and ALI byexecuting immuno-modulatory activities. Accumulated evidence discloses the number ofNK cells significantly decreases in circulation, which plausibly give rise to the progression ofinflammation and ALI in patients with COVID-19 [64]. By contrast, the number of NK cells in lung tissue and the number of activated NK cells in human bronchoalveolar lavage from subjects with severe ischemia-reperfusion significantly increase. And suppressing NK cells could blunt ALI mediated by ischemia-reperfusion mice [65]. These results hint that NK cells may play different roles in ALI mediated by infection and hypoxia.
In patients with COVID-19,thescores of NK cell chemotaxis, activation, proliferation, and degranulation are significantly elevated. The level of inositol phosphate metabolism, glycolysis,and glycerolipid metabolism in NK cells are upregulated during COVID-19 [66]. During inflammation and viral infection,the glycolyticrate of NK cells obviously increases. However, lactate dehydrogenase A (LDHA)-deficient NKcells fail to display optimal antiviral effects [67]. Additionally, phospholipase C γ 2 (PLCG2)-deficient NK cells lose their ability to kill virally infected cells for the reason that theycould neither mobilize intracellular calcium nor secrete cytotoxic granules [68]. Additionally, during COVID-19, the expression level of PLCG2 in NK cells significantly increases, which boosts its cytotoxicity. And the two enzymes participating glycerolipid metabolism including LPIN1 and LPIN2 are also upregulated in NK cells upon SARS-CoV-2 infection, which are very important for the dephosphorylation of phosphatidic acid, thus increasing the level of intracellular calcium andfacilitating NK cell cytotoxicity [66].
2.2. The adaptive immune cells during ALI
2.2.1. T cells
The cellular immune response mediated by T cells could recognize andcontrol pathogens and is animportant componentof adaptive immune [69]. Productive T cell responses to infection depend on coordinated metabolic reprogramming among various immune cells [70]. Particularly, T cell effector and memory differentiation, exhaustion, as well as senescence are closely modulated by the metabolic reprogramming [71]. In quiescence exit, the levels of glucose metabolism, anabolism, nutrient uptake, mitochondrial metabolism and OXPHOS are enhanced in naïve T cells, leading to lactate production [72]. Many selectivetranscription factors inducing gene expression programs necessary for anabolism including IRF4 [73], SREBPs [74], MYC [75], andNFAT [76] coordinate the above events. Hence, blocking anabolic metabolismimpairs the activation and proliferation of T cells.These discrete metabolic events are mainly dependent upon certain transcription factors sensing metabolicsignals [77,78]. Briefly, anabolismis an important driver of the activation and differentiation of T cells.
At present, nutrients are regarded as the fourth signal to elicit T cell immunity by interacting with costimulatory, T cell receptor (TCR), and cytokine signals, thus it is critical for T cell activationand specialization [79].Indeed, differentiation as well asglucose uptake of effector CD4+ T cell subsets are driven by some signals including glucose transporter 1 (GLUT1) [80]. And some amino acids including leucine, methionine, glutamine, together with their transporters could promote terminaleffector (Teff) cells proliferation and survival [78,81]. In particular,activated CD8+ T cell subsets express higher amount of amino acid transporters than activated CD4+ T cells, which may be related with different compositions of protein translation complexes and ribosomes [82]. What is more, the metabolism of glutamine, methionine,and arginine also displays significantly differences among different CD4+ T cell subsets [82]. As for CD8+ T cells, its anti-influenza immunity is triggered by fermentation of dietary fiber, which is caused by enhanced mitochondrialand glycolytic metabolism owing to butyrate accumulation [83].These studies suggested the importance of nutrients and transporters modulate T cell responses.
During sepsis-induced ALI, activated CD4+ and CD8+ T cells candifferentiate into memory (Tmem) T cells or Teff T cells [69,84]. In the lung tissues from patients with COVID-19, there is a significant reduction of Tcells. Meanwhile, arginine as well as its metabolites display a pronounced reduction, which may possibly impede normal Tmem or Teff responses [85]. The cytotoxicity of CD8+ T cells is enhanced by inositol phosphate and glycerolipid metabolism in lung tissue from patients with COVID-19 [66].As one of the products of neutral fat metabolism, free fatty acids also trigger T cell differentiation by inducing NETs during ALI. But the above process is mediated by DCs but not the direct effect of NETs on T cells [86].Hospitalized COVID-19 patients with pulmonary disease displayed enhanced glycosaminoglycan metabolism, which is associated with an accumulation of effector-like CD8+ T cell populations and decreased CD8+ Tmem as well as memory B cells [87].During exudative phase of ALI, a large number of T cellsmigrate toward infectious foci ininjured lung, giving rise to the progression or resolution of ALI [88].Experimental and clinical studies have demonstrated a vital relationship between the infiltration of resident CD8+ T cells and ALI [88].Additionally, a two-photon study have also disclosed that lung-infiltrating T cells exhibit obvious interstitial migration during infection [89,90]. Metabolism affects T cells migration mainly through the following aspects. To begin with, mitochondria could direct T cells trafficking to facilitate chemokine-induced migration as well as extravasation in a Drp1-dependent manner [91].Secondly,purinergic receptors could sense local ATP to affect mitochondrial metabolism indifferent regions, which orchestrates T cells migration. In particular, in theuropod, purinergic receptor P2Y11 could block mitochondrial metabolism. By contrast, in the pseudopod, mitochondrialmetabolism is activated by purinergic receptor P2X4 [92]. Thirdly, de novo phosphatidylethanolamine synthesis via the enhanced cytidine diphosphate (CDP)–ethanolamine pathway could stabilize the follicular homing receptor CXCR5 on the T cell surface, thus promoting its migration [93].
Moreover, as subsets of CD4+ T cells, Th17 and Treg cells imbalance is an important index to surveil abnormal immunity. Upregulation of CD4 + IL-17 + Th17 cells and downregulated CD4 + CD25 + Foxp3+ Tregs were involved in the pathogenesis of various ALI, which demonstrates a pro-inflammatory phenotype [94]. In patients with severe COVID-19, high IL-17A and GM-CSF protein levels in serum were associated with a more severe clinical course and pulmonary tissue-resident memory-like Th17 cells (Trm17 cells) contributes to hyperinflammation by interacting with lung macrophages and cytotoxic CD8+T cells in severe COVID-19 [95]. However, increased Treg proportions, combined with the expression of proinflammatory mediatorsare generally associated with poor prognosis [96]. In terms of immunometabolism in both cellls, while glycolysis, glutaminolysis and fatty acis synthesis are required for the differentiation and function of Th17 cells, Treg cells, in contrast, perform a catabolism that enhanced fatty acid oxidation can induce mitochondrial oxidative metabolism [97]. The glucose transporter GLUT3-dependent acetyl-CoA generation controls T helper 17 cell responses through epigenetic regulation [98]. In turn, extracellular lactate which is end-product of glycosis, induces deregulation of the Th17-specific gene expression with increased levels of genome-wide histone H3K18 lactylation, which offers Tregs a metabolic advantage by Foxp3 [99].
Collectively, metabolic reprogramming has become a key driver of T cells function and fate during ALI, with Tcells exhibiting extensive adaptability and plasticity to metabolic perturbations including COVID-19 and sepsis.
2.2.2. B cells
B cells are classified into B1 cells, B2 cells including follicular cells and marginal zone cells, as well as regulatory B cells [100]. The development of B cells is a strictly regulated process. The naïve B cells obtain energy mainly by TCA cycle, OXPHOS, as well as nucleotide biosynthesis.Restricting glucose in naïve B cells hasnoeffectson their function for the reason that glucose acts as the ingredient of ribonucleotide synthesis but not TCA cycle [101].B1 B cellsmainly presenting in the peritoneal cavity rely on glycolysis while B2 B cells presenting in the spleen rely on FAO for their energy demand. However, for these B2 B cells, FAO will shift to glycolysis by increasing GLUT1-mediated glucose uptake upon activation [102]. B cells produce antibodies including IgG, IgM, and IgA in a glycolysis-dependent manner in patients with COVID-19 [103]. The glycolysis and glutaminolysis accompany an elevation in mTORC1 and c-Myc expression, increasing GLUT1 expression for increased glucose transport in B cells [104]. Inpatients with moderate and severe COVID-19, the number of exhausted memory B cells and neutralizing Abs significantly increases, demanding much more energy. However,there are no new memory B cells or long-lasting plasma cells in this process, which explains why patients with COVID-19 could not develop memory B cells and long-lasting Abs to protect combat SARS-CoV2 infection [105].
3. Thepotentialdrugtargetsassociatedwith immunometabolism during ALI
The interaction between immuneresponse and metabolism is bidirectional. On the one hand, cytokine receptors, toll-like receptors (TLRs), together with T and B cell receptors could intersect with certain downstream metabolic pathways. On the other hand, these changes of metabolic pathways in immune cells also regulate intracellular innate immune sensors/receptors. Hence, a better understanding of the roles of key players in immunometabolism can provide potential therapeutic targets to either harnessexcessive pathologic inflammation during ALI or trigger immune defenses to prevent pathogen infections. Here, we sum up the impacts of core players participating in immunometabolism during ALI.
3.1. NLRP3 inflammasome
The NLR familyis a group of evolutionarily conserved receptorswhich could sense pathogens as well as homeostatic alterations. The members in NLR family own a leucine-rich repeat domain and a nucleotide-binding domain [106].The inflammasome serves as a large multimeric protein complex composed of the adapter apoptosis-associated speck-likeprotein containing a C-terminal caspase recruitment domain (ASC), cytosolicsensor NLR, and procaspase-1. The activation of inflammasome could promote maturation of the proinflammatory cytokines IL-18 and IL-1β by promoting innate andadaptive immune responses, as well as induce pyroptotic cell death in a caspase-1-dependent manner during ALI [107,108]. NLRP3 inflammasome could be activated by various endogenous signals involving intracellular calcium mobilization, ROS, the efflux of chloride or potassium ions, endoplasmic reticulum (ER) stress, mitochondrial DNA (mtDNA), lipid uptake and accumulation, and multiple intracellular kinase signaling [109,110].
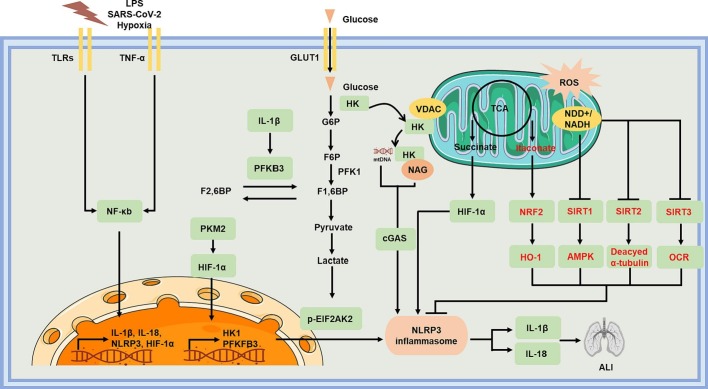
In immune myeloid cells, the activation of NLRP3 is tightly modulated by altered intracellular metabolic pathways (Fig. 2 ). Forexample, HK1-dependent glycolysis could directly trigger the activation of NLRP3 in LPS-treated macrophages. By contrast, glycolysis inhibition, silencing HK1 or glucose deprivation could block caspase-1 activation as well as IL-1β secretion [111,112].Pharmacologic inhibition of PKM2-dependent glycolysis prevented LPS-induced inflammatory responses of macrophages and alleviated ALI by hampering NLRP3 inflammasome activation [112]. Additionally, peptidoglycan also activates NLRP3 inflammasome by releasing N-acetylglucosamine via inactivating the glycolytic enzyme hexokinase, thus triggering the dissociation of hexokinase from the mitochondrial membrane into the cytosol. N-acetylglucosamine can activate NLRP3 inflammasome by giving rise to cytosolic translocation of mtDNA, which acts as a very important inducer of NLRP3 inflammasome [113,114]. Glycolytic disruption in macrophages could also result in NLRP3 inflammasome formation, IL-1β secretion, as well as pyroptosis by producing mtROS and impairing NADH formation [115]. Hence, promoting the TCA cycle by increasingthe glycolytic end-product pyruvate abolished NLRP3 inflammasomeactivation. ALI favors aerobic glycolysis via mitochondrial OXPHOS, which gives rise to the accumulation of succinate. Subsequently, succinate dehydrogenase further oxidizes succinate to fumarate in mitochondria, producing excessive mtROS [116,117]. As an important immunometabolite, succinate could drive NLRP3 activation and IL-1β upregulation via activating HIF-1α in LPS-treated macrophages [25]. Itaconate isa TCA-derived immune metabolite which could suppress IL-1β by blocking NLRP3 activation specifically [118]. Additionally, other derivatives of TCA cycle metabolites including dimethylfumarate and ethyl pyruvate also hamper the activation of NLRP3 in macrophages. For instance, ethyl pyruvate relieves mitochondrial damage, thus inhibiting mtDNA release into thecytoplasm [119]. As for dimethylfumarate, it could mediate the activation of Nrf2, which subsequently blocking the production of mitochondrial ROS and the cytoplasmic translocation of mtDNA [113,120].Mitochondria damage and disturbances in OXPHOS are also related with the reduction in NAD+ levels, giving rise to the suppression of NAD+-dependent enzymes, such as SIRT1, SIRT2, and SIRT3. The activation of NLRP3 was also restrained by SIRT1 through enhancing mitochondrial biogenesis and OXPHOS in a LKB1 (liver kinase B1)/AMPK-dependent manner [121,122]. What is more, the activity of NAD+-dependent SIRT2 could also affect the activation of NLRP3. ROS disrupted mitochondrial homeostasis and reduce intracellular NAD+, consequently blocking SIRT2-mediated deacetylation of α-tubulin. The accumulation of acetylated α-tubulin facilitated dynein-dependent transport to the endoplasmic reticulum, causing the accumulation of mitochondrial ASC with NLRP3 on the endoplasmic reticulum [123].During ALI, LPS could induce the inactivation of SIRT3 in lung and lead to impaired oxygen consumption rate (OCR) and redox dysfunction of mitochondria in macrophage, giving rise to ALI [124]. In turn, the activation of NLRP3 inflammasome also regulates metabolic status in macrophages. For instance, the expression and activity of SIRT1 could be inhibited by inflammasome-activated caspase-1 [125]. And NLRP3 inflammasome in macrophages could also further enhance glycolysis by increasing PFKFB3 in an IL-1β-dependent manner [126]. These results implicate NLRP3 may be a hub regulating metabolism to inflammation, which can be potential target for metabolism maintenance in ALI/ARDS.
Fig. 2.
Mechanisms of immunometabolism during ALI. Undesirable stimulus such as LPS, SARS-CoV-2, and hypoxia could activate toll-like and TNF-α receptors, subsequently transcriptionally activating IL-1β, IL-18, NLRP3, and HIF-1α in a NF-κb dependent manner. PKM2-dependent glycolysis triggers LPS-induced inflammatory responses of macrophages and aggravated ALI by activating NLRP3 inflammasome. Dimeric PKM2 could interact with HIF-1α, thus activating glycolysis. Bacterial NAG could bind to and inhibit HK activity, giving rise to the dissociation of HK from VDACs and in turn release mitochondrial DNA to activate NLRP3 inflammasome. Aberrantmitochondrial homeostasis also gives rise to the accumulation of the TCA, which could derivative both succinate and itaconate. Succinate and itaconate exert opposite effects on NLRP3 inflammasome activation. Reduced NAD+ availability could inhibit SIRT1/2/3, thus activating NLRP3 inflammasome.
3.2. AIM2
AIM2 serves as a non-NLR molecule in AIM2-like receptor (ALR) family, which could induce the activation of inflammasome by monitoring and removing cellular damaged DNA [127]. AIM2 recognizes dsDNA for inflammasome activation in a length-dependent manner, requiring a minimum of 80 base pairs [128]. During infection and ALI,dsDNA can bind to the C-terminal HIN-200 of AIM2 to recruit ASCand interact with its free N-terminal pyrin domain, initiating the oligomerization and activation of inflammasome [129]. In sepsis, both NLRP3 inflammasome and AIM2 inflammasome are promoted by PKM2-mediated glycolysis, which is associated the phosphorylation of EIF2AK2 in macrophage. Hence, inhibiting PKM2-EIF2AK2 pathway significantly blocked the activation of AIM2 inflammasome, thereby exerting protective roles in endotoxemia and sepsis [112]. In bleomycin-induced mice infected with Streptococcus pneumoniae, the AIM2 acts as a danger signal in regulating GLUT1-mediated lung fibrosis. GLUT1 knockout significantly decreased the expression and activation of the AIM2 inflammasome [130]. In adaptive immune response, AIM2 deficiency in Treg cells promoted glycolysis as well as lipid OXPHOS, and enhanced AKT/mTOR signaling, indicating that AIM2 is essential in the regulation of Treg cellmetabolism. In terms of mechanism, AIM2 could form a complex with AKT and PP2A, further dephosphorylating AKT and blocking AKT/mTOR signaling [131]. Taken together, these studies disclosed that AIM2 plays important roles in immunometabolism of adaptive immune cells by interacting with the AKT/mTOR metabolic pathway.
3.3. cGAS-STING axis
Double-stranded DNA (dsDNA) sensor cyclic-GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway is an essential immune-surveillance mediator in the settings of cellular stress, infection, and tissue damage.The catalytic activity of cGAS in mammals is usually triggered by cytoplasmic double strand DNA (dsDNA), giving rise to 2′,3′-cGAMP synthesis. Subsequently, cGAMP binds to STING dimers localized at the endoplasmic reticulum membrane, which results in profound conformational alterations triggering STING oligomerization. As a consequence, STING could activate the expression of interferon-stimulated genes (ISGs), and certain inflammatory genes by recruiting TANK-binding kinase 1 (TBK1) [132,133]. In different immune cells, STING is regulated by distinct mechanisms. For instance, activated STING could repolarize macrophages from M2 to proinflammatory M1 phenotype by inducing metabolic reprogramming. During this process, an impaired TCA cycle in M1 macrophages promoted the accumulation of succinate, further suppressing prolyl hydroxylase (PHD) activity and stabilizing HIF-1α. STING activation in M1 cells also stabilizes HIF-1α by increasing mitochondrial ROS, giving rise to increasing glycolysis and reducing OXPHOS [134]. Additionally, DNA virus infection also promoted the interactio nbetween the signaling adaptor STING and the metabolic checkpoint kinase S6K1 inacGAS-dependentmanner.The kinase domain of S6K1 was critical for the formation of the tripartite S6K1-STING-TBK1 complex and the activation of IRF3 during T cell response [135]. STING could also inhibit mTORC1 in T cells and T cell proliferation by activating IRF3/7 pathway.In turn, the activation of mTORC1 gave rise to STING-induced type I interferonproduction. Blocking the activation of mTORC1 activatedCD4+ T cells offset cGAMP-induced IFN-I production [136]. Hence, targeting T cell proliferation and differentiation through STING-mediated metabolic reprogramming could be a novel strategy to combat ALI.
3.4. AMPK and mTOR
AMP-activated protein kinase (AMPK) plays crucial roles in the attenuation of various inflammation- and oxidative stress-induced diseases including ALI, one mechanism of which is AMPK/GSK3β-Nrf2 signal axis [137]. AMPK impacts immunometabolism of ALI in many facets. AMPK enhances glucose uptake and increases glycolysis while decreasing gluconeogenesis and glycogen synthesis, which can be important in most immune cell like neutrophils and macrophages. AMPK also promotes fatty acid oxidation and mitochondria biogenesis. Smiglaside A can promote macrophage polarization to M2 phenotype via stimulating the AMPK-PPARγ signaling pathway [138]. AMPK/SIRT1 pathway can facilitate the differentiation of Tregs from naïve CD4+ T cells in ALI under impact of dexmedetomidine [139]. On the other hand, another target tightly related to metabolism is mammalian target of rapamycin (mTOR), which can be negative regulated by AMPK. mTOR is important in protein synthesis and anabolism through transcription factor eukaryotic initiation factor 4E binding protein 1 (4EBP1) and ribosomal protein S6 kinase beta-1 (P70(S6K)), the activation of which is usually associated with high metabolic activity, whereas low mTOR activation is characteristic of a quiescent state. mTOR has a pleiotropic role in immune responses. mTOR/HIF-1α mediates aerobic glycolysis in TREM-1-activated macrophages during ALI [140]. The activation of the PI3K/AKT/mTOR signaling pathway also regulates the function and maturation of DCs and neutrophil upon ALI [141,142]. During SARS-CoV-2 related ALI, PI3K/AKT/mTOR signaling is suppressed by ROS accumulation and promotes inflammation and apoptosis through elevated autophagy [143]. Dysregulated mTOR is associated with reduced glycolytic activity, enhanced OXPHOS demands and CD8+ T-cell exhaustion in HIV, which indicated poor prognosis [144]. mTOR signal also relates to Th17 and Treg differentiation and actvation in HIV infection [145]. In summary, AMPK and mTOR stand in center of reaction to metabolism change, which put more emphasize on immunometabolisim in ALI.Relevant inhibitors and agonists has been developed to control abnormal inflammation.
3.5. IRG1
Immune responsive gene 1(IRG) serves as a mitochondrial metabolic enzyme aconitate decarboxylase 1 (ACOD1), catalyzing decarboxylation of cis-aconitate to produce itaconate in activated macrophages [146]. In LPS-treated macrophages, IRG1 could be induced in a TRIF-dependent manner [147].Itaconate could further result in succinate-dehydrogenase inhibition, succinate accumulation, alkylation of protein cysteine residues, apart from inducing the electrophilic stress response and impairing aerobic glycolysis by regulating NRF2 and IκB. Inactivated macrophages, itaconate could modulate macrophage metabolism as well as effector functions by suppressing succinate dehydrogenase-mediated oxidation of succinate, thus exerting anti-inflammatory effects [148]. Also, itaconate together with its derivative, 4-octyl itaconate (4-OI), blocked NLRP3 inflammasome activation in LPS-treated macrophageby inhibiting the interaction between NLRP3 and NEK7, likely through modifying C548 on NLRP3 [149]. In addition, other enzymes including glycolytic enzymes aldolase A (ALDOA), lactate dehydrogenase A (LDHA), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were also identified as potential targets of itaconate. By modulating and inhibiting these key enzymes catalyzing glycolysis, itaconate could block the LPS-induced macrophage activation [[150], [151], [152]]. Recent studies have unveiled that itaconate could protect against ALI by multiple mechanisms. For instance, itaconate could bind to the same site on TET2 as the co-substrate α-ketoglutarate, suppressing TET2 catalytic activity. Then genes associated with NF-κB and STAT signaling pathways could be further downregulated in LPS-treated macrophages [153]. In addition, in endotoxemia-induced ALI, IRG1 or itaconate also alleviated pathological injury by decreasing inflammatory effect and ferroptosis through activating NRF2 and enhancing autophagy [154,155]. Taken together, itaconate serves as a key immunometabolite with various roles in inflammation and immunity, which may be an endogenous drug against ALI.
3.6. Beta-hydroxybutyrate (BHB)
Ketone bodies are endogenous metabolites which could not only maintain cellular energy but also featuredrug-like signaling activities, thus regulating immune activity and metabolism. Previousstudieshaveunveiledthat BHB was implicated with SARS-CoV-2 and ALI mainly bythefollowingmechanisms. To begin with,BHB could block proinflammatory NLRP3 activation directly, without undergoing oxidation in the TCA cycle, and independently of SIRT2, UCP2, the G protein-coupled receptor GPR109A or HCAR2 [156]. As reported,both influenza and SARS-CoVare sensed by the NLRP3 inflammasome directly, mediating strong inflammatory response.And SARS-CoV proteins E, 3a, and8b could also activate NLRP3 inflammasome. E is a viroporin that encodes a cation channel which can increaseintracellular sodium, calcium, as well as potassium flux, with the latter two being critical inducers of NLRP3 inflammasome activation. One recent study reported SARS-CoV-2-induced immune dysregulation was correlated with an attenuated increase in BHB in the serum, indicating that infection-induced ketogenesis is impaired in COVID-19.In terms of mechanism,BHB substantially enhanced the antiviral immune response by boosting the production of IFNγ and the survival of CD4+ T cells through rewiring T cells to OXPHOS by fuelling the TCA cycle and increasing the synthesis of amino acids [157]. In addition, BHB could also strongly decrease NF-kB-mediated inflammation by binding to HCAR2 in macrophages in nervous system [158]. However,the role of BHB in NF-kB in lung-resident macrophages has not yetbeen investigated. Therefore, more investigation on the way that BHB influence immune metabolism in ALI should be attempted, since its biocompatible feature.
4. Expert opinion and future prospects
Given the multiple impacts of targets above, more and more potential drugs targeting immunometabolism can be developed to surveil abnomal immunity and inflammation, which can be a treatment for ALI/ARDS (Table 2 ). Classical drugs on market for AMPK and mTOR pathway like metformin [159], rapamycin [160] and everolimus [161] has reveals a role of metabolisim regulator in animal studies, but more clinical trials are needed for further provement. Some novel drugs including NLRP3 inhibitor [[162], [163], [164], [165]] and AIM2 inhibitor [166] can attenuate pro-inflammatory immune environment mainly on macrophage, while AIM2 also functions in Treg cell. Drugs targeting cGAS-STING axis [167] can act as two contradictory role, inhibition of which not only can restrain overt inflammation but decrease anti-viral response via IFN-I pathway. For their biocompatible feature as endogenous molecule, itaconate derivatives including 4-octyl itaconate (OI) [168]and dimethyl itaconate (DI) [169]which derived from macrophage can demonstrate anti-inflammatory macrophage in innate immunity. In addition, Beta-hydroxybutyrate can both function in innate and adaptive immunity, which emphasize on a ketone-based metabolic therapy in ALI/ARDS. Other metabolic intermediates like glutamine [170] and alpha-ketoglutarate [171] also possess potential for treatment. In conclusion, more investigation on metabolism targets in various immune cell and immune environment should be continued.
Table 2.
The potential immunometabolic targets of ALI.
| Potential drug targets | Molecular Effectors | Function in Immunity | Metabolic Profile | Potential Drugs |
|---|---|---|---|---|
| NLRP3 inflammasome | glycolytic enzyme HK1, PKM, PFKFB3 NAG-related mtDNA release; TCA cycle metabolites accumulation: succinate fumarate; mitochondrial damage:mtROS mtDNA; NAD + -dependent enzymes SIRT1, 2, 3 |
promote pyroptosis in macrophage; pro-inflammation phenotype |
glycolysis↑ mitochondria damage↑ mtROS↑ NAD+/NADH↓ mtDNA release↑ |
NLRP3 inhibitor:MCC950 [165], INF39 [172],Glyburide [163],RRx-001[162] |
| AIM2 | glycolysis pathway:GLUT1, PKM2, EIF2AK2 AKT and PP2A AKT/mTOR signaling |
pro-inflammatoy macrophage a T cell-intrinsic and inflammasome-independent role in the function of Treg cells |
glycolysis ↑/↓ lipid OXPHOS↓ |
oligodeoxynucleotides (ODNs): ODN-A151 [166] |
| cGAS-STING axis | HIF-1α S6K1-STING-TBK1 complex mTORC1 |
repolarize macrophages from M2 to M1; T cell response↑ T-cell proliferation ↓ |
glycolysis↑ TCA cycle↓ OXPHOS↓ mtROS↑ |
STING inhibitor: H-151,C-176[167] STING agonist: diABZI [173], DMXAA, cGAMP [174], STING-LNP [175] |
| AMPK | AMPK/GSK3β-Nrf2 signal AMPK-PPARγ AMPK/SIRT1 |
repolarize macrophages to M2; neutrophils ↓ Differentiation of Tregs↑ |
glycolysis↑ gluconeogenesis and glycogen synthesis↓ fatty acid oxidation↑ OXPHOS↑ mitochondria biogenesis↑ |
AMPK agonist: metformin [159] |
| mTOR | negative regulated by AMPK; suppressed by ROS accumulation 4EBP1 and P70(S6K) mTOR/HIF-1α autophagy |
macrophage activation↑ DCs maturation↑ neutrophil function↑ CD8+ T-cell ↑ Th17 cell differentiation↑ Treg activation↑ |
protein synthesis and anabolism↑ aerobic glycolysis ↑ autophagy↓ |
mTOR inhibitors: everolimus [161],Rapamycin [160] |
| IRG1 | itaconate NRF2 and IκB NLRP3 inflammasome glycolytic enzymes:ALDOA, LDHA, GAPDH TET2 catalytic activity |
anti-inflammatory macrophage | succinate accumulation↑ alkylation of protein cysteine residues↑ aerobic glycolysis↓ ferroptosis↓ autophagy↑ |
itaconate derivatives: 4-octyl itaconate (OI) [169] dimethyl itaconate (DI) [168] |
| Beta-hydroxybutyrate (BHB) | NLRP3 inflammasome NF-kB-mediated inflammation by binding to HCAR2 |
IFNγ production↑ CD4+ T cells survival↑ anti-inflammatory macrophage |
OXPHOS↑ TCA cycle↑ synthesis of amino acids↑ |
Beta-hydroxybutyrate (BHB) |
5. Conclusion
ARDS is a complex syndrome of ALI triggering noncardiogenic pulmonary edema from multiple causes which is associated with a very high mortality rate. Animal studies and clinical data have clearly demonstrated that metabolism and immunity are also inextricably linked during the development of ALI. Glucose metabolism could modulate metabolic reprogramming of macrophages, thus affecting inflammation. In turn, immune state of various innate immune cells and adaptive immune cells could also alter the pathway of energy utilization in immune cells. Therefore, understanding immunometabolismas well as its reprogramming mechanisms during ALI will help us to understand immunopathogenesis and open the door to combat ALI through immune cells and their potential targets. Future work focusing on immunometabolism against ALI is keenly awaited.
Author contributions
Li Ning, and Wang Bo consulted a large number of literatures. Li Ning and Zou Shishi designed and drawn the figures in the manuscript. Wang Bo drafted the manuscript. Lin Huiqing reviewed and modified this manuscript. Zou Shishi revised the work. All authors read and approved the final manuscript.
Funds
This work was supported by the National Natural ScienceFoundation of China (82170106).
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Swenson K.E., Swenson E.R. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit. Care Clin. 2021;37(4):749–776. doi: 10.1016/j.ccc.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He Y.Q., et al. Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol. Res. 2021;163 doi: 10.1016/j.phrs.2020.105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson C.B., et al. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022;23(1):3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camporota L., et al. Pathophysiology of coronavirus-19 disease acute lung injury. Curr. Opin. Crit. Care. 2022;28(1):9–16. doi: 10.1097/MCC.0000000000000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pålsson-McDermott E.M., O’Neill L.A.J. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res. 2020;30(4):300–314. doi: 10.1038/s41422-020-0291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishra P.K., et al. Diabetic cardiomyopathy: an Immunometabolic perspective. Front Endocrinol (Lausanne) 2017;8:72. doi: 10.3389/fendo.2017.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mouton A.J., et al. Obesity, hypertension, and cardiac dysfunction: novel roles of Immunometabolism in macrophage activation and inflammation. Circ. Res. 2020;126(6):789–806. doi: 10.1161/CIRCRESAHA.119.312321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao N., et al. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nat. Commun. 2021;12(1):1618. doi: 10.1038/s41467-021-21907-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar V. Pulmonary innate immune response determines the outcome of inflammation during pneumonia and Sepsis-associated acute lung injury. Front. Immunol. 2020;11:1722. doi: 10.3389/fimmu.2020.01722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y., et al. B7H3 ameliorates LPS-induced acute lung injury via attenuation of neutrophil migration and infiltration. Sci. Rep. 2016;6:31284. doi: 10.1038/srep31284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aziz M., et al. B-1a cells protect mice from sepsis-induced acute lung injury. Mol. Med. 2018;24(1):26. doi: 10.1186/s10020-018-0029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chai Y.S., et al. Curcumin regulates the differentiation of naïve CD4+T cells and activates IL-10 immune modulation against acute lung injury in mice. Biomed. Pharmacother. 2020;125 doi: 10.1016/j.biopha.2020.109946. [DOI] [PubMed] [Google Scholar]
- 13.Kelly B., O’Neill L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25(7):771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira N.S., et al. Aldosterone, inflammation, immune system, and hypertension. Am. J. Hypertens. 2021;34(1):15–27. doi: 10.1093/ajh/hpaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox A.J., West N.P., Cripps A.W. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015;3(3):207–215. doi: 10.1016/S2213-8587(14)70134-2. [DOI] [PubMed] [Google Scholar]
- 16.O’Neill L.A., Kishton R.J., Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16(9):553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raghuraman S., et al. The emerging role of epigenetics in inflammation and Immunometabolism. Trends Endocrinol. Metab. 2016;27(11):782–795. doi: 10.1016/j.tem.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Norata G.D., et al. The cellular and molecular basis of translational Immunometabolism. Immunity. 2015;43(3):421–434. doi: 10.1016/j.immuni.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Barrat F., et al. Sex and parity modulate cytokine production during murine ageing. Clin. Exp. Immunol. 1997;109(3):562–568. doi: 10.1046/j.1365-2249.1997.4851387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sell H., Habich C., Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8(12):709–716. doi: 10.1038/nrendo.2012.114. [DOI] [PubMed] [Google Scholar]
- 21.Aegerter H., Lambrecht B.N., Jakubzick C.V. Biology of lung macrophages in health and disease. Immunity. 2022;55(9):1564–1580. doi: 10.1016/j.immuni.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morales-Nebreda L., et al. The heterogeneity of lung macrophages in the susceptibility to disease. Eur. Respir. Rev. 2015;24(137):505–509. doi: 10.1183/16000617.0031-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S., et al. Metabolic reprogramming of macrophages during infections and cancer. Cancer Lett. 2019;452:14–22. doi: 10.1016/j.canlet.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S., et al. Immunometabolism of phagocytes and relationships to cardiac repair. Front Cardiovasc Med. 2019;6:42. doi: 10.3389/fcvm.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tannahill G.M., et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496(7444):238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jha A.K., et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42(3):419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Arts R.J., et al. Glutaminolysis and fumarate accumulation integrate Immunometabolic and epigenetic programs in trained immunity. Cell Metab. 2016;24(6):807–819. doi: 10.1016/j.cmet.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Infantino V., et al. The mitochondrial citrate carrier: a new player in inflammation. Biochem. J. 2011;438(3):433–436. doi: 10.1042/BJ20111275. [DOI] [PubMed] [Google Scholar]
- 29.Feingold K.R., et al. Mechanisms of triglyceride accumulation in activated macrophages. J. Leukoc. Biol. 2012;92(4):829–839. doi: 10.1189/jlb.1111537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van den Bossche J., O’Neill L.A., Menon D. Macrophage Immunometabolism: where are we (going)? Trends Immunol. 2017;38(6):395–406. doi: 10.1016/j.it.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Wallace C., Keast D. Glutamine and macrophage function. Metabolism. 1992;41(9):1016–1020. doi: 10.1016/0026-0495(92)90130-3. [DOI] [PubMed] [Google Scholar]
- 32.Huang S.C., et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 2014;15(9):846–855. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haschemi A., et al. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 2012;15(6):813–826. doi: 10.1016/j.cmet.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong W.J., et al. Inhibition of glycolysis alleviates lipopolysaccharide-induced acute lung injury in a mouse model. J. Cell. Physiol. 2019;234(4):4641–4654. doi: 10.1002/jcp.27261. [DOI] [PubMed] [Google Scholar]
- 35.Gong Y., et al. Blockage of glycolysis by targeting PFKFB3 alleviates sepsis-related acute lung injury via suppressing inflammation and apoptosis of alveolar epithelial cells. Biochem. Biophys. Res. Commun. 2017;491(2):522–529. doi: 10.1016/j.bbrc.2017.05.173. [DOI] [PubMed] [Google Scholar]
- 36.Marrocco A., et al. Metabolic adaptation of macrophages as mechanism of defense against crystalline silica. J. Immunol. 2021;207(6):1627–1640. doi: 10.4049/jimmunol.2000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larson-Casey J.L., et al. Cadmium-mediated lung injury is exacerbated by the persistence of classically activated macrophages. J. Biol. Chem. 2020;295(46):15754–15766. doi: 10.1074/jbc.RA120.013632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Codo A.C., et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent Axis. Cell Metab. 2020;32(3):498–499. doi: 10.1016/j.cmet.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stojkov D., et al. Physiological and pathophysiological roles of metabolic pathways for NET formation and other neutrophil functions. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.826515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar S., Dikshit M. Metabolic insight of neutrophils in health and disease. Front. Immunol. 2019;10:2099. doi: 10.3389/fimmu.2019.02099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaplan M.J. Mitochondria shape neutrophils during hypoxia. Blood. 2022;139(2):159–160. doi: 10.1182/blood.2021013440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunham-Snary K.J., et al. Mitochondria in human neutrophils mediate killing of Staphylococcus aureus. Redox Biol. 2022;49 doi: 10.1016/j.redox.2021.102225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhan Y., et al. HMGB1-mediated neutrophil extracellular trap formation exacerbates intestinal ischemia/reperfusion-induced acute lung injury. J. Immunol. 2022;208(4):968–978. doi: 10.4049/jimmunol.2100593. [DOI] [PubMed] [Google Scholar]
- 44.Garnier Y., et al. Plasma microparticles of intubated COVID-19 patients cause endothelial cell death, neutrophil adhesion and netosis, in a phosphatidylserine-dependent manner. Br. J. Haematol. 2022;196(5):1159–1169. doi: 10.1111/bjh.18019. [DOI] [PubMed] [Google Scholar]
- 45.Rodríguez-Espinosa O., et al. Metabolic requirements for neutrophil extracellular traps formation. Immunology. 2015;145(2):213–224. doi: 10.1111/imm.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amini P., et al. Neutrophil extracellular trap formation requires OPA1-dependent glycolytic ATP production. Nat. Commun. 2018;9(1):2958. doi: 10.1038/s41467-018-05387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azevedo E.P., et al. A metabolic shift toward pentose phosphate pathway is necessary for amyloid fibril- and Phorbol 12-Myristate 13-acetate-induced neutrophil extracellular trap (NET) formation. J. Biol. Chem. 2015;290(36):22174–22183. doi: 10.1074/jbc.M115.640094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gardinassi L.G., et al. Immune and metabolic signatures of COVID-19 revealed by transcriptomics data reuse. Front. Immunol. 2020;11:1636. doi: 10.3389/fimmu.2020.01636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adrover J.M., et al. Disulfiram inhibits neutrophil extracellular trap formation protecting rodents from acute lung injury and SARS-CoV-2 infection. JCI Insight. 2022;7(5):e157342. doi: 10.1172/jci.insight.157342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McElvaney O.J., et al. Characterization of the inflammatory response to severe COVID-19 illness. Am. J. Respir. Crit. Care Med. 2020;202(6):812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Z., et al. AMP-activated protein kinase and glycogen synthase kinase 3β modulate the severity of Sepsis-induced lung injury. Mol. Med. 2016;21(1):937–950. doi: 10.2119/molmed.2015.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hussain A., et al. Dendritic cell-targeted therapies to treat neurological disorders. Mol. Neurobiol. 2022;59(1):603–619. doi: 10.1007/s12035-021-02622-4. [DOI] [PubMed] [Google Scholar]
- 53.Zaccagnino P., et al. An active mitochondrial biogenesis occurs during dendritic cell differentiation. Int. J. Biochem. Cell Biol. 2012;44(11):1962–1969. doi: 10.1016/j.biocel.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 54.Del Prete A., et al. Role of mitochondria and reactive oxygen species in dendritic cell differentiation and functions. Free Radic. Biol. Med. 2008;44(7):1443–1451. doi: 10.1016/j.freeradbiomed.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 55.Rehman A., et al. Role of fatty-acid synthesis in dendritic cell generation and function. J. Immunol. 2013;190(9):4640–4649. doi: 10.4049/jimmunol.1202312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pearce E.J., Everts B. Dendritic cell metabolism. Nat Rev Immunol. 2015;15(1):18–29. doi: 10.1038/nri3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang D., et al. Attenuated interferon and Proinflammatory response in SARS-CoV-2-infected human dendritic cells is associated with viral antagonism of STAT1 phosphorylation. J. Infect. Dis. 2020;222(5):734–745. doi: 10.1093/infdis/jiaa356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krawczyk C.M., et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115(23):4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thwe P.M., et al. Cell-intrinsic glycogen metabolism supports early glycolytic reprogramming required for dendritic cell immune responses. Cell Metab. 2017;26(3):558–567.e5. doi: 10.1016/j.cmet.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Everts B., et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation. Nat. Immunol. 2014;15(4):323–332. doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langers I., et al. Natural killer cells: role in local tumor growth and metastasis. Biologics. 2012;6:73–82. doi: 10.2147/BTT.S23976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cooper G.E., et al. Human CD49a(+) lung natural killer cell cytotoxicity in response to influenza a virus. Front. Immunol. 2018;9:1671. doi: 10.3389/fimmu.2018.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Subedi N., et al. Understanding natural killer cell biology from a single cell perspective. Cell. Immunol. 2022;373 doi: 10.1016/j.cellimm.2022.104497. [DOI] [PubMed] [Google Scholar]
- 64.Jeyaraman M., et al. Bracing NK cell based therapy to relegate pulmonary inflammation in COVID-19. Heliyon. 2021;7(7) doi: 10.1016/j.heliyon.2021.e07635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calabrese D.R., et al. Natural killer cells activated through NKG2D mediate lung ischemia-reperfusion injury. J. Clin. Invest. 2021;131(3) doi: 10.1172/JCI137047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li S., et al. Cellular metabolic basis of altered immunity in the lungs of patients with COVID-19. Med. Microbiol. Immunol. 2022;211(1):49–69. doi: 10.1007/s00430-021-00727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sheppard S., et al. Lactate dehydrogenase A-dependent aerobic glycolysis promotes natural killer cell anti-viral and anti-tumor function. Cell Rep. 2021;35(9) doi: 10.1016/j.celrep.2021.109210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caraux A., et al. Phospholipase C-gamma2 is essential for NK cell cytotoxicity and innate immunity to malignant and virally infected cells. Blood. 2006;107(3):994–1002. doi: 10.1182/blood-2005-06-2428. [DOI] [PubMed] [Google Scholar]
- 69.Bevilacqua A., Li Z., Ho P.C. Metabolic dynamics instructs CD8(+) T cell differentiation and functions. Eur. J. Immunol. 2022;52(4):541–549. doi: 10.1002/eji.202149486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Møller S.H., et al. Metabolic programs tailor T cell immunity in viral infection, cancer, and aging. Cell Metab. 2022;34(3):378–395. doi: 10.1016/j.cmet.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 71.Han Z., et al. A deep insight into regulatory T cell metabolism in renal disease: facts and perspectives. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.826732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reina-Campos M., Scharping N.E., Goldrath A.W. CD8(+) T cell metabolism in infection and cancer. Nat Rev Immunol. 2021;21(11):718–738. doi: 10.1038/s41577-021-00537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Man K., et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat. Immunol. 2013;14(11):1155–1165. doi: 10.1038/ni.2710. [DOI] [PubMed] [Google Scholar]
- 74.Kidani Y., et al. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat. Immunol. 2013;14(5):489–499. doi: 10.1038/ni.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang R., et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35(6):871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klein-Hessling S., et al. NFATc1 controls the cytotoxicity of CD8(+) T cells. Nat. Commun. 2017;8(1):511. doi: 10.1038/s41467-017-00612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clever D., et al. Oxygen sensing by T cells establishes an immunologically tolerant metastatic niche. Cell. 2016;166(5):1117–1131.e14. doi: 10.1016/j.cell.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chapman N.M., Chi H. Metabolic adaptation of lymphocytes in immunity and disease. Immunity. 2022;55(1):14–30. doi: 10.1016/j.immuni.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Long L., et al. CRISPR screens unveil signal hubs for nutrient licensing of T cell immunity. Nature. 2021;600(7888):308–313. doi: 10.1038/s41586-021-04109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zeng H., et al. Discrete roles and bifurcation of PTEN signaling and mTORC1-mediated anabolic metabolism underlie IL-7-driven B lymphopoiesis. Sci. Adv. 2018;4(1):eaar5701. doi: 10.1126/sciadv.aar5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu B., et al. Succinyl-CoA ligase deficiency in pro-inflammatory and tissue-invasive T cells. Cell Metab. 2020;32(6):967–980.e5. doi: 10.1016/j.cmet.2020.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Howden A.J.M., et al. Quantitative analysis of T cell proteomes and environmental sensors during T cell differentiation. Nat. Immunol. 2019;20(11):1542–1554. doi: 10.1038/s41590-019-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trompette A., et al. Dietary Fiber confers protection against flu by shaping Ly6c(−) patrolling monocyte hematopoiesis and CD8(+) T cell metabolism. Immunity. 2018;48(5):992–1005.e8. doi: 10.1016/j.immuni.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 84.Peñaloza H.F., Lee J.S., Ray P. Neutrophils and lymphopenia, an unknown axis in severe COVID-19 disease. PLoS Pathog. 2021;17(9) doi: 10.1371/journal.ppat.1009850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nie X., et al. Multi-organ proteomic landscape of COVID-19 autopsies. Cell. 2021;184(3):775–791.e14. doi: 10.1016/j.cell.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen W., et al. Free fatty acids-induced neutrophil extracellular traps lead to dendritic cells activation and T cell differentiation in acute lung injury. Aging (Albany NY) 2021;13(24):26148–26160. doi: 10.18632/aging.203802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Andonegui-Elguera S., et al. Molecular alterations prompted by SARS-CoV-2 infection: induction of Hyaluronan, glycosaminoglycan and Mucopolysaccharide metabolism. Arch. Med. Res. 2020;51(7):645–653. doi: 10.1016/j.arcmed.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mrass P., et al. ROCK regulates the intermittent mode of interstitial T cell migration in inflamed lungs. Nat. Commun. 2017;8(1):1010. doi: 10.1038/s41467-017-01032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Connors T.J., et al. Airway CD8(+) T cells are associated with lung injury during infant viral respiratory tract infection. Am. J. Respir. Cell Mol. Biol. 2016;54(6):822–830. doi: 10.1165/rcmb.2015-0297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maeno T., et al. CD8+ T cells are required for inflammation and destruction in cigarette smoke-induced emphysema in mice. J. Immunol. 2007;178(12):8090–8096. doi: 10.4049/jimmunol.178.12.8090. [DOI] [PubMed] [Google Scholar]
- 91.Simula L., et al. Drp1 controls effective T cell immune-surveillance by regulating T cell migration, proliferation, and cMyc-dependent metabolic reprogramming. Cell Rep. 2018;25(11):3059–3073.e10. doi: 10.1016/j.celrep.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ledderose C., et al. The purinergic receptor P2Y11 choreographs the polarization, mitochondrial metabolism, and migration of T lymphocytes. Sci. Signal. 2020;13(651) doi: 10.1126/scisignal.aba3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fu G., et al. Metabolic control of T(FH) cells and humoral immunity by phosphatidylethanolamine. Nature. 2021;595(7869):724–729. doi: 10.1038/s41586-021-03692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muyayalo K.P., et al. COVID-19 and Treg/Th17 imbalance: potential relationship to pregnancy outcomes. Am. J. Reprod. Immunol. 2020;84(5) doi: 10.1111/aji.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao Y., et al. Clonal expansion and activation of tissue-resident memory-like Th17 cells expressing GM-CSF in the lungs of severe COVID-19 patients. Sci Immunol. 2021;6(56) doi: 10.1126/sciimmunol.abf6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Galván-Peña S., et al. Profound Treg perturbations correlate with COVID-19 severity. Proc. Natl. Acad. Sci. U. S. A. 2021;118(37) doi: 10.1073/pnas.2111315118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saravia J., Chapman N.M., Chi H. Helper T cell differentiation. Cell Mol Immunol. 2019;16(7):634–643. doi: 10.1038/s41423-019-0220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hochrein S.M., et al. The glucose transporter GLUT3 controls T helper 17 cell responses through glycolytic-epigenetic reprogramming. Cell Metab. 2022;34(4):516–532.e11. doi: 10.1016/j.cmet.2022.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Angelin A., et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017;25(6):1282–1293.e7. doi: 10.1016/j.cmet.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Y., et al. B cell development and maturation. Adv. Exp. Med. Biol. 2020;1254:1–22. doi: 10.1007/978-981-15-3532-1_1. [DOI] [PubMed] [Google Scholar]
- 101.Robinson G.A., Wilkinson M.G.L., Wincup C. The role of Immunometabolism in the pathogenesis of systemic lupus erythematosus. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.806560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shiraz A.K., Panther E.J., Reilly C.M. Altered germinal-center metabolism in B cells in autoimmunity. Metabolites. 2022;12(1) doi: 10.3390/metabo12010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cantor J., et al. CD98hc facilitates B cell proliferation and adaptive humoral immunity. Nat. Immunol. 2009;10(4):412–419. doi: 10.1038/ni.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Donnelly R.P., Finlay D.K. Glucose, glycolysis and lymphocyte responses. Mol. Immunol. 2015;68(2 Pt C):513–519. doi: 10.1016/j.molimm.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 105.Kumar V. How could we forget immunometabolism in SARS-CoV2 infection or COVID-19? Int. Rev. Immunol. 2021;40(1–2):72–107. doi: 10.1080/08830185.2020.1840567. [DOI] [PubMed] [Google Scholar]
- 106.Shim D.W., Lee K.H. Posttranslational regulation of the NLR family pyrin domain-containing 3 Inflammasome. Front. Immunol. 2018;9:1054. doi: 10.3389/fimmu.2018.01054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang P., Ma J., Zhang R. Inflammasomes: potential therapeutic targets in atherosclerotic cardiovascular disease. Endocr Metab immune Disord drug, Targets. 2022;22(14):1378–1389. doi: 10.2174/1871530322666220407090916. [DOI] [PubMed] [Google Scholar]
- 108.Hsu C.G., et al. The lipid peroxidation product 4-hydroxynonenal inhibits NLRP3 inflammasome activation and macrophage pyroptosis. Cell Death Differ. 2022;29(9):1790–1803. doi: 10.1038/s41418-022-00966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu B., et al. Inflammatory caspases drive Pyroptosis in acute lung injury. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.631256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu B., et al. Buformin alleviates sepsis-induced acute lung injury via inhibiting NLRP3-mediated pyroptosis through an AMPK-dependent pathway. Clin Sci (Lond) 2022;136(4):273–289. doi: 10.1042/CS20211156. [DOI] [PubMed] [Google Scholar]
- 111.Moon J.S., et al. mTORC1-induced HK1-dependent glycolysis regulates NLRP3 Inflammasome activation. Cell Rep. 2015;12(1):102–115. doi: 10.1016/j.celrep.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 112.Xie M., et al. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat. Commun. 2016;7:13280. doi: 10.1038/ncomms13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wolf A.J., et al. Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell. 2016;166(3):624–636. doi: 10.1016/j.cell.2016.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin H.C., et al. Lactic acid fermentation is required for NLRP3 Inflammasome activation. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.630380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sanman L.E., et al. Disruption of glycolytic flux is a signal for inflammasome signaling and pyroptotic cell death. Elife. 2016;5 doi: 10.7554/eLife.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nunns G.R., et al. Succinate activation of SUCNR1 predisposes severely injured patients to neutrophil-mediated ARDS. Ann. Surg. 2022;276(6):e944–e954. doi: 10.1097/SLA.0000000000004644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Loftus R.M., Finlay D.K. Immunometabolism: cellular metabolism turns immune regulator. J. Biol. Chem. 2016;291(1):1–10. doi: 10.1074/jbc.R115.693903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hoyle C., et al. Itaconate and fumarate derivatives inhibit priming and activation of the canonical NLRP3 inflammasome in macrophages. Immunology. 2022;165(4):460–480. doi: 10.1111/imm.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhong X., et al. Ethyl pyruvate protects against sepsis-associated encephalopathy through inhibiting the NLRP3 inflammasome. Mol. Med. 2020;26(1):55. doi: 10.1186/s10020-020-00181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tastan B., et al. Dimethyl fumarate alleviates NLRP3 Inflammasome activation in microglia and sickness behavior in LPS-challenged mice. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.737065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li X., Kazgan N. Mammalian sirtuins and energy metabolism. Int. J. Biol. Sci. 2011;7(5):575–587. doi: 10.7150/ijbs.7.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ganesan R., et al. Salmonella typhimurium disrupts Sirt1/AMPK checkpoint control of mTOR to impair autophagy. PLoS Pathog. 2017;13(2) doi: 10.1371/journal.ppat.1006227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Misawa T., et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 2013;14(5):454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 124.Kurundkar D., et al. SIRT3 diminishes inflammation and mitigates endotoxin-induced acute lung injury. JCI. Insight. 2019;4(1) doi: 10.1172/jci.insight.120722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chalkiadaki A., Guarente L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 2012;16(2):180–188. doi: 10.1016/j.cmet.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Finucane O.M., et al. The NLRP3 inflammasome modulates glycolysis by increasing PFKFB3 in an IL-1β-dependent manner in macrophages. Sci. Rep. 2019;9(1):4034. doi: 10.1038/s41598-019-40619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang L., et al. AIM2 Inflammasome’s first decade of discovery: focus on Oral diseases. Front. Immunol. 2020;11:1487. doi: 10.3389/fimmu.2020.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jin T., et al. Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity. 2012;36(4):561–571. doi: 10.1016/j.immuni.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang J., et al. HMGB1 participates in LPS-induced acute lung injury by activating the AIM2 inflammasome in macrophages and inducing polarization of M1 macrophages via TLR2, TLR4, and RAGE/NF-κB signaling pathways. Int. J. Mol. Med. 2020;45(1):61–80. doi: 10.3892/ijmm.2019.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cho S.J., et al. GLUT1-dependent glycolysis regulates exacerbation of fibrosis via AIM2 inflammasome activation. Thorax. 2020;75(3):227–236. doi: 10.1136/thoraxjnl-2019-213571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chou W.C., et al. AIM2 in regulatory T cells restrains autoimmune diseases. Nature. 2021;591(7849):300–305. doi: 10.1038/s41586-021-03231-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Decout A., et al. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021;21(9):548–569. doi: 10.1038/s41577-021-00524-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wan D., Jiang W., Hao J. Research advances in how the cGAS-STING pathway controls the cellular inflammatory response. Front. Immunol. 2020;11:615. doi: 10.3389/fimmu.2020.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Olson G.S., et al. Type I interferon decreases macrophage energy metabolism during mycobacterial infection. Cell Rep. 2021;35(9) doi: 10.1016/j.celrep.2021.109195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang F., et al. S6K-STING interaction regulates cytosolic DNA-mediated activation of the transcription factor IRF3. Nat. Immunol. 2016;17(5):514–522. doi: 10.1038/ni.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Imanishi T., et al. Reciprocal regulation of STING and TCR signaling by mTORC1 for T-cell activation and function. Life Sci Alliance. 2019;2(1) doi: 10.26508/lsa.201800282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lv H., et al. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017;12:311–324. doi: 10.1016/j.redox.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Y., et al. Smiglaside a ameliorates LPS-induced acute lung injury by modulating macrophage polarization via AMPK-PPARγ pathway. Biochem. Pharmacol. 2018;156:385–395. doi: 10.1016/j.bcp.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 139.Zhang Z.T., et al. Dexmedetomidine alleviates acute lung injury by promoting Tregs differentiation via activation of AMPK/SIRT1 pathway. Inflammopharmacology. 2023;31(1):423–438. doi: 10.1007/s10787-022-01117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhong W.J., et al. TREM-1 governs NLRP3 inflammasome activation of macrophages by firing up glycolysis in acute lung injury. Int. J. Biol. Sci. 2023;19(1):242–257. doi: 10.7150/ijbs.77304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lorne E., et al. Participation of mammalian target of rapamycin complex 1 in toll-like receptor 2- and 4-induced neutrophil activation and acute lung injury. Am. J. Respir. Cell Mol. Biol. 2009;41(2):237–245. doi: 10.1165/rcmb.2008-0290OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li R., et al. HMGB1/PI3K/Akt/mTOR signaling participates in the pathological process of acute lung injury by regulating the maturation and function of dendritic cells. Front. Immunol. 2020;11:1104. doi: 10.3389/fimmu.2020.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Basile M.S., et al. The PI3K/Akt/mTOR pathway: a potential pharmacological target in COVID-19. Drug Discov. Today. 2022;27(3):848–856. doi: 10.1016/j.drudis.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rahman A.N., et al. Elevated glycolysis imparts functional ability to CD8(+) T cells in HIV infection. Life Sci Alliance. 2021;4(11) doi: 10.26508/lsa.202101081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yero A., et al. Immuno-metabolic control of the balance between Th17-polarized and regulatory T-cells during HIV infection. Cytokine Growth Factor Rev. 2023;69:1–13. doi: 10.1016/j.cytogfr.2023.01.001. [DOI] [PubMed] [Google Scholar]
- 146.Li Y., et al. The IRG1-Itaconate axis: a regulatory hub for immunity and metabolism in macrophages. Int. Rev. Immunol. 2022:1–15. doi: 10.1080/08830185.2022.2067153. [DOI] [PubMed] [Google Scholar]
- 147.Peace C.G., O’Neill L.A. The role of itaconate in host defense and inflammation. J. Clin. Invest. 2022;132(2) doi: 10.1172/JCI148548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lampropoulou V., et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 2016;24(1):158–166. doi: 10.1016/j.cmet.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hooftman A., et al. The immunomodulatory metabolite Itaconate modifies NLRP3 and inhibits Inflammasome activation. Cell Metab. 2020;32(3):468–478.e7. doi: 10.1016/j.cmet.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liao S.T., et al. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to exert anti-inflammatory effects. Nat. Commun. 2019;10(1):5091. doi: 10.1038/s41467-019-13078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Qin W., et al. S-glycosylation-based cysteine profiling reveals regulation of glycolysis by itaconate. Nat. Chem. Biol. 2019;15(10):983–991. doi: 10.1038/s41589-019-0323-5. [DOI] [PubMed] [Google Scholar]
- 152.Mills E.L., et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556(7699):113–117. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen L.L., et al. Itaconate inhibits TET DNA dioxygenases to dampen inflammatory responses. Nat. Cell Biol. 2022;24(3):353–363. doi: 10.1038/s41556-022-00853-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Qiu J.H., et al. Deficiency of IRG1/itaconate aggravates endotoxemia-induced acute lung injury by inhibiting autophagy in mice. Exp. Anim. 2022 doi: 10.1538/expanim.22-0104. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.He R., et al. Itaconate inhibits ferroptosis of macrophage via Nrf2 pathways against sepsis-induced acute lung injury. Cell Death Discov. 2022;8(1):43. doi: 10.1038/s41420-021-00807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Youm Y.H., et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015;21(3):263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Karagiannis F., et al. Impaired ketogenesis ties metabolism to T cell dysfunction in COVID-19. Nature. 2022;609(7928):801–807. doi: 10.1038/s41586-022-05128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rahman M., et al. The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat. Commun. 2014;5:3944. doi: 10.1038/ncomms4944. [DOI] [PubMed] [Google Scholar]
- 159.Zhang Y., et al. Metformin alleviates LPS-induced acute lung injury by regulating the SIRT1/NF-κB/NLRP3 pathway and inhibiting endothelial cell Pyroptosis. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.801337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yan Z., et al. Rapamycin attenuates acute lung injury induced by LPS through inhibition of Th17 cell proliferation in mice. Sci. Rep. 2016;6:20156. doi: 10.1038/srep20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Üstün S., et al. Effects of the mTOR inhibitor everolimus and the PI3K/mTOR inhibitor NVP-BEZ235 in murine acute lung injury models. Transpl. Immunol. 2015;33(1):45–50. doi: 10.1016/j.trim.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen Y., et al. RRx-001 ameliorates inflammatory diseases by acting as a potent covalent NLRP3 inhibitor. Cell Mol Immunol. 2021;18(6):1425–1436. doi: 10.1038/s41423-021-00683-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hou L., et al. NLRP3/ASC-mediated alveolar macrophage pyroptosis enhances HMGB1 secretion in acute lung injury induced by cardiopulmonary bypass. Lab. Investig. 2018;98(8):1052–1064. doi: 10.1038/s41374-018-0073-0. [DOI] [PubMed] [Google Scholar]
- 164.Xiang Y., et al. USP9X promotes lipopolysaccharide-stimulated acute lung injury by deubiquitination of NLRP3. Cell Biol. Int. 2023;47(2):394–405. doi: 10.1002/cbin.11932. [DOI] [PubMed] [Google Scholar]
- 165.Cao Z., et al. Crosstalk of pyroptosis, ferroptosis, and mitochondrial aldehyde dehydrogenase 2-related mechanisms in sepsis-induced lung injury in a mouse model. Bioengineered. 2022;13(3):4810–4820. doi: 10.1080/21655979.2022.2033381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kaminski J.J., et al. Synthetic oligodeoxynucleotides containing suppressive TTAGGG motifs inhibit AIM2 inflammasome activation. J. Immunol. 2013;191(7):3876–3883. doi: 10.4049/jimmunol.1300530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu B., et al. STING inhibitor ameliorates LPS-induced ALI by preventing vascular endothelial cells-mediated immune cells chemotaxis and adhesion. Acta Pharmacol. Sin. 2022;43(8):2055–2066. doi: 10.1038/s41401-021-00813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Runtsch M.C., et al. Itaconate and itaconate derivatives target JAK1 to suppress alternative activation of macrophages. Cell Metab. 2022;34(3):487–501.e8. doi: 10.1016/j.cmet.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 169.Bambouskova M., et al. Electrophilic properties of itaconate and derivatives regulate the IκBζ-ATF3 inflammatory axis. Nature. 2018;556(7702):501–504. doi: 10.1038/s41586-018-0052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.de Oliveira G.P., et al. Glutamine therapy reduces inflammation and extracellular trap release in experimental acute respiratory distress syndrome of pulmonary origin. Nutrients. 2019;11(4) doi: 10.3390/nu11040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu M., et al. α-Ketoglutarate modulates macrophage polarization through regulation of PPARγ transcription and mTORC1/p70S6K pathway to ameliorate ALI/ARDS. Shock. 2020;53(1):103–113. doi: 10.1097/SHK.0000000000001333. [DOI] [PubMed] [Google Scholar]
- 172.Fu Q., et al. NLRP3 deficiency alleviates severe acute pancreatitis and pancreatitis-associated lung injury in a mouse model. Biomed. Res. Int. 2018;2018:1294951. doi: 10.1155/2018/1294951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhu Q., et al. Inhibition of coronavirus infection by a synthetic STING agonist in primary human airway system. Antivir. Res. 2021;187 doi: 10.1016/j.antiviral.2021.105015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xu N., et al. STING agonist promotes CAR T cell trafficking and persistence in breast cancer. J. Exp. Med. 2021;218(2) doi: 10.1084/jem.20200844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nakamura T., et al. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation. J Immunother Cancer. 2021;9(7) doi: 10.1136/jitc-2021-002852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.