Abstract
Objective
To explore the pattern of health services utilisation of people who had had a documented SARS-Cov-2 infection.
Design
Retrospective cohort study.
Setting
The Italian province of Reggio Emilia.
Participants
36 036 subjects who recovered from SARS-CoV-2 infection during the period September 2020–May 2021. These were matched for age, sex and Charlson Index with an equal number of subjects never found positive at the SARS-Cov-2 swab test over the study period.
Main outcome measures
Hospital admissions for all medical conditions and for respiratory or cardiovascular conditions only; access to emergency room (for any cause); outpatient specialist visits (pneumology, cardiology, neurology, endocrinology, gastroenterology, rheumatology, dermatology, mental health) and overall cost of care.
Results
Within a median follow-up time of 152 days (range 1–180), previous exposure to SARS-Cov-2 infection was always associated with higher probability of needing access to hospital or ambulatory care, except for dermatology, mental health and gastroenterology specialist visits. Post-COVID subjects with Charlson Index≥1 were hospitalised more frequently for heart disease and for non-surgical reasons than subjects with Charlson index=0, whereas the opposite occurred for hospitalisations for respiratory diseases and pneumology visits. A previous SARS-CoV-2 infection was associated with 27% higher cost of care compared with people never infected. The difference in cost was more evident among those with Charlson Index>1. Subjects who had anti-SARS-CoV-2 vaccination had lower probability of falling in the highest cost quartile.
Conclusions
Our findings reflect the burden of post-COVID sequelae, providing some specific insight on their impact on the extra-use of health services according to patients’ characteristics and vaccination status. Vaccination is associated with lower cost of care following SARS-CoV-2 infection, highlighting the favourable impact of vaccines on the use of health services even when they do not prevent infection.
Keywords: Organisation of health services, Clinical governance, COVID-19, EPIDEMIOLOGY, PUBLIC HEALTH
Strengths and limitations of this study.
Our study provide insight on the extra-use of specific health services and related cost of care by COVID patients after they have recovered, compared with people never infected by SARS-Cov-2.
Subgroup and sensitivity analyses provide further insight on how demographic/clinical characteristics, vaccination status, time to recovery, recent hospital and emergency room admissions and subsequent admissions due to COVID are associated with use of health services.
Limits in the quality of administrative data cannot be excluded, as well as the possibility of residual confounding.
Further studies should provide longer follow-up data, also with higher numbers of vaccinated people to allow a comparison between those who developed COVID and those who did not, and to warrant the inclusion of boosted people who could not be included in our cohort yet.
Introduction
The SARS-Cov-2 pandemic forced radical changes in the organisation and delivery of care, requiring a rapid expansion of health services capacity in some sectors (ie, additional hospital beds in general and intensive care units in particular), the adoption and implementation of public health measures and interventions for efficient identification of new cases and contact tracing and the reorganisation of primary care services to allow COVID-19 patients to be cared for as much as possible at home. As it has been described, these changes had been at the expense of the management of other diseases, being the volume of procedures and interventions for conditions other than COVID-19 drastically reduced.1
However, the pandemic could have also long-term implications for healthcare systems, generating additional healthcare needs in individuals who have been diagnosed with SARS-CoV-2 infection. Indeed, after infection a variable proportion of individuals experience a condition defined as ‘post-COVID-19 syndrome’, or ‘long COVID-19’, with the persistence of signs and symptoms (or occurrence of new symptoms) after 1–3 months from the end of the acute phase.2 3 In particular, cohort studies and systematic reviews have described the persistence of several symptoms including neurologic disorders (ie, the so called brain fog, ageusia/anosmia), mental health disorders (eg, anxiety, depression and sleep problems), functional impairment, respiratory, cardiac, digestive and skin disorders, etc. Although their incidence is widely variable across studies and depends on the background health status and on the initial COVID symptomatology, some of these symptoms (in particular, respiratory and neurologic disorders) can affect up to half to three-quarters of recovered patients.4–8
In this study, conducted in the Italian province of Reggio Emilia (population 539 652), we assessed the additional burden (if any) to health services due to the management of those who had a documented SARS-Cov-2 infection, exploring their pattern of specific inpatient and outpatient health services utilisation after recovering from the infection.
Methods
Study design
We conducted a retrospective cohort study in which individuals from the resident population who had a negative PCR on naso and oropharyngeal swab test for SARS-Cov-2 after having been found positive at the same test, or who were asymptomatic after 21 days from the positive test, were followed up over time and their rates of health services utilisation assessed and compared with a matched cohort of residents never found positive at the SARS-Cov-2 test.
Study population and data sources
Since the inception of the pandemic in March 2020, a surveillance database has been implemented in the province, including all the citizens undergoing SARS-Cov-2 swab test and its result, as well as time of recovery.9 10 For this study, we identified from the SARS-Cov-2 surveillance database all those who, after being diagnosed with SARS-Cov-2 infection, recovered during the period September 2020–May 2021. These individuals (n=36 036) represented the cohort of those who previously had a documented SARS-Cov-2 infection.
Through record linkage procedures between the SARS-CoV-2 database and the administrative databases available to the Local Health Authority, we then assessed their rates of use of health services from the date of recovery indicated in the COVID database (index date) up to 30 June 2021.
The administrative databases include, for each resident in the province, demographic information, hospital discharge data (coded according to the International Classification of Diseases-9-CM (ICD-9-CM)) of diagnosis and procedures, admission and discharge dates, vital status at discharge and outpatient pharmacy data at the individual prescription level, as well as access to outpatient ambulatory care. Data were anonymised, and record linkage procedures were performed according to the unique identification number which is assigned to each resident. In addition, for each individual, we searched for information on previous hospitalisations (up to preceding 10 years), as registered in these local administrative databases, in order to assess the presence of specific comorbidities individually (chronic obstructive pulmonary disease, arrhythmia, diabetes, acute myocardial infraction, heart failure, vascular diseases and obesity), and to estimate individual patients’ overall degree of comorbidity (if any), according to the (not age-adjusted) Charlson Index.11
Relying on the same data sources, we identified individuals to be included in the control group among residents alive at 1 January 2020 and never found positive at the SARS-Cov-2 swab test over the study period (either with negative tests or with no test at all). Individuals in the control group were matched according to age, sex and Charlson Index (in four classes: 0, 1, 2 and ≥3). Each control had the same index date of the matched SARS-Cov-2 infected individual. Therefore, the matching procedure made available 36 036 persons for each cohort, with equal distribution as for sex (18 481 were female, 51.3%), age (mean 43, range 1–103), comorbidities (32 561 had Charlson Index 0, while 1391, 1146 and 938 had Charlson Index 1, 2 and >3, respectively).
People who died during the follow-up were censored at the time of death.
Outcome measures
The following items of care provided over the study period were considered, taking into account the most common symptoms persisting after the acute phase of COVID-19 disease:3
Hospital admissions for all medical conditions.
Hospital admissions for respiratory or cardiovascular conditions only.
Access to Emergency Room (for any cause).
Outpatient specialist visits (pneumology, cardiology, neurology, endocrinology, gastroenterology, dermatology, rheumatology, mental health).
The cost of individual procedures and services (according to official fees) and of drugs was taken as overall measure of the burden to the regional healthcare system of the care provided to individuals in both the cohorts. In cost analysis, in addition to the above reported items of care, we considered also all the outpatient diagnostic procedures and tests (ie, blood tests, chest X-ray, etc.) and drug prescriptions.
Statistical analyses
Descriptive analysis of the items of care provided to individuals in the two cohorts are reported, as well as Poisson rates with 95% CI. Rates have the total number of episodes of care observed as numerator, and person-months as denominator.
The strength of the association between previous SARS-Cov-2 infection and rates of use of the items of care considered was assessed through HR. In order to better disentangle the effect of the previous SARS-Cov-2 infection, rather than of coexisting diseases, we stratified the analysis by Charlson comorbidity index, thus estimating HRs separately for convalescent COVID-19 patients and control individuals with Charlson Index=0 and with Charlson Index≥1. We also calculated separate HRs considering COVID convalescents who were hospitalised and not hospitalised (vs controls), controls who either had negative test or no test at all (vs COVID convalescents), HRs according to quartiles of time to recovery and HRs for outpatient visits, hospital and emergency room admissions in the first 90 days and after 90 days from the index date, to assess their trend over time. A sensitivity analysis adjusting for imbalances in hospital and emergency room admissions in the year before the index date was also performed, to limit the possible influence of recent acute health problems on the risk of subsequent use of health services.
Mean case versus control cost differences (with 95% CI) are reported overall and according to age, sex and Charlson Index. Total cost of care was divided in quartiles and the association between the characteristics of those who had SARS-Cov-2 infection and higher costs was assessed through a logistic regression model, with the highest costs quartile as dependent variable, and age (in categorised in four classes: <30, 31–50, 51–70 and >71), sex, presence of symptoms at diagnosis and hospital admission for COVID-19 (both proxy indicators of COVID-19 severity) and pre-existence of specific comorbidities as covariates. As 986 (3%) of those who had SARS-CoV-2 infection had also received SARS-Cov-2 vaccination before testing positive at the swab test, we included SARS-Cov-2 vaccination status among the covariates.
Patient and public involvement
None.
Results
Seventy-seven per cent of people in the SARS-Cov-2 cohort recovered within 21 days from a positive test and had a negative exit test; the remaining 23% were asymptomatic (without an exit test) after 21 days. After a median follow-up of 152 days (range 1–180), 51 and 186 individuals died, in the control and the SARS-Cov-2 positive group, respectively. Among those who had been positive at the swab test, 16 286 (45%) individuals did not use hospital care and never accessed outpatient services, versus 18 055 (50%) in the control group (X21df = 174.65; p<0.001).
Both the proportion of individuals having at least one access at the items of care considered and the overall frequency of use was always higher in the SARS-Cov-2 positive cohort, especially for respiratory and cardiovascular hospital admissions and outpatients visits. Dermatology, mental health and gastroenterology specialist visits have a different pattern, being the difference between the two groups negligible, if any (table 1)
Table 1.
Frequency of use of hospital and ambulatory care by a cohort of 36 036 convalescent COVID-19 patients vs a matched control cohort, in the Province of Reggio Emilia (Italy)
| Convalescent COVID-19 | Matched control | %Difference | ||||||
| In H for respiratory disease | Total N admissions | 126 | 45 | +180% | ||||
| In H for heart disease | Total N admissions | 143 | 76 | +88% | ||||
| In H for any medical reason | At least one, N (%) | 724 (2.0) | 538 (1.5) | |||||
| Total N admissions | 916 | 675 | +36% | |||||
| Access to emergency room | At least one, N (%) | 3383 (9.4) | 2491 (6.9) | |||||
| Total N accesses | 4299 | 3186 | +35% | |||||
| Death | 186 | 51 | +264% | |||||
| Outpatient specialist visits | ||||||||
| Pneumology | At least one, N (%) | 766 (2.0) | 310 (0.9) | |||||
| Total N | First visits (%) | 924 | 572 (61.9) | 380 | 154 (40.5) | +143% | +271% | |
| Cardiology | At least one, N (%) | 1588 (4.4) | 1119 (3.0) | |||||
| Total N | First visits | 1708 | 1173 (68.7) | 1198 | 716 (59.7) | +43% | +64% | |
| Neurology | At least one, N (%) | 758 (2.1) | 625 (1.7) | |||||
| Total N | First visits | 939 | 568 (60.5) | 756 | 424 (56.1) | +24% | +34% | |
| Rheumatology | At least one, N (%) | 533 (1.5) | 461 (1.3) | |||||
| Total N | First visits | 670 | 254 (37.9) | 571 | 181 (31.6) | +17% | +40% | |
| Gastroenterology | At least one, N (%) | 268 (0.7) | 258 (0.7) | |||||
| Total N | First visits | 317 | 181 (57.0) | 304 | 157 (51.6) | +4% | +15% | |
| Endocrinology | At least one, N (%) | 1397 (3.9) | 1116 (3.1) | |||||
| Total N | First visits | 1942 | 458 (23.6) | 1500 | 320 (21.3) | +29% | +43% | |
| Dermatology | At least one, N (%) | 1470 (4.1) | 1407 (3.9) | |||||
| Total N | First visits | 1670 | 501 (30.0) | 1574 | 481 (30.5) | +6% | +4% | |
| Mental Health | At least one, N (%) | 174 (0.5) | 175 (0.5) | |||||
| Total N | First visits | 190 | 117 (61.6) | 209 | 104 (49.5) | −9% | +12% | |
HRs, overall, by Charlson Index and by COVID-related hospitalisation are outlined in table 2.
Table 2.
Rates (x1000 x month) and HRs of access to hospital care, outpatient specialist visits and death for convalescent COVID-19 patients and the control group, overall and according to Charlson Index and hospitalisation for COVID-19
| Rates x 1000 (95% CI) |
HR (95% CI) |
HR in Charlson Index=0 (95%CI) | HR in Charlson Index≥1 (95%CI) | HR hospitalised COVID-19 vs control (95% CI) | HR non-hospitalised COVID-19 vs control (95% CI) | ||
| Non-surgical h admissions | Control | 3.8 (3.5 to 4.1) | 1 | 1 | 1 | 1 | 1 |
| COVID-19 | 5.2 (4.9 to 5.5) | 1.38 (1.25 to 1.52) | 1.11 (0.97 to 1.27) | 1.83 (1.73 to 2.14) | 3.16 (2.53 to 3.95) | 1.12 (1.0 to 1.29) | |
| H admissions for respiratory disease | Control | 0.3 (0.2 to 0.4) | 1 | 1 | 1 | 1 | 1 |
| COVID-19 | 0.7 (0.6 to 0.9) | 2.09 (2.07 to 4.09) | 6.69 (2.84 to 15.70) | 2.30 (1.57 to 3.36) | 4.19 (2.41 to 7.30) | 1.91 (1.27 to 2.89) | |
| H admissions for heart disease | Control | 0.4 (0.3 to 0.5) | 1 | 1 | 1 | 1 | 1 |
| COVID-19 | 0.8 (0.7 to 1.0) | 1.93 (1.46 to 2.55) | 1.49 (1.37 to 1.62) | 2.38 (1.62 to 3.5) | 3.56 (2.02 to 6.25) | 1.44 (1.04 to 1.97) | |
| Accesses to emergency room |
Control |
7.4 (7.1 to 7.7) | 1 | 1 | 1 | 1 | 1 |
| COVID-19 | 9.5 (9.2 to 9.8) | 1.36 (1.29 to 1.42) | 1.32 (1.25 to 1.39) | 1.51 (1.36 to 1.66) | 2.05 (1.79 to 2.35) | 1.23 (1.18 to 1.29) | |
| Death | Control | 0.19 (0.16 to 0.22) | 1 | 1 | 1 | 1 | 1 |
| COVID-19 | 0.41 (0.35 to 0.47) | 3.69 (2.73 to 4.98) | 3.07 (1.73 to 5.47) | 3.68 (2.59 to 5.23) | 6.20 (3.50 to 10.10) | 2.87 (2.01 to 4.11) | |
| Outpatient specialist visits | |||||||
| Pneumology | Control | 2.1 (1.9 to 2.4) | 1 | 1 | 1 | 1 | 1 |
| COVID-19 | 5.2 (4.9 to 5.6) | 2,45 (2.17 to 2.76) | 3.35 (3.09 to 3.65) | 1.53 (1.36 to 1.71) | 4.44 (3.36 to 5.86) | 1.83 (1.61 to 2.09) | |
| Cardiology | Control | 6.8 (6.4 to 7.2) | 1 | 1 | 1 | 1 | 1 |
| COVID-19 | 9.7 (9.2 to 10.2) | 1.44 (1.33 to 1.55) | 1.53 (1.36 to 1.71) | 1.23 (1.07 to 1.41) | 1.72 (1.43 to 2.06) | 1.28 (1.18 to 1.39) | |
| Neurology | Control | 4.8 (4.5 to 5.2) | 1 | 1 | 1 | 1 | 1 |
| COVID-19 | 6.0 (5.7 to 6.4) | 1,25 (1.14 to 1.36) | 1.22 (1.1 to 1.35) | 1.37 (1.12 to 1.68) | 1.46 (1.10 to 1.92) | 1.17 (1.06 to 1.28) | |
| Rheumatology | Control | 3.2 (3.0 to 3.5) | 1 | 1 | 1 | 1 | 1 |
| COVID-19 | 3.8 (3.5 to 4.1) | 1,17 (1.05 to 1.31) | 1.19 (1.04 to 1.36) | 1.15 (0.93 to 1.42) | 1.09 (0.78 to 1.51) | 1.23 (1.09 to 1.38) | |
| Gastroenterology | Control | 1.7 (1.5 to 1.9) | 1 | 1 | 1 | 1 | 1 |
| COVID-19 | 1.8 (1.6 to 2.0) | 1.04 (0.89 to 1.22) | 1.0 (0.84 to 1.20) | 1.21 (0.85 to 1.73) | 1.25 (0.68 to 2.32) | 0.97 (0.82 to 1.14) | |
| Mental health | Control | 1.2 (1.0 to 1.4) | 1 | 1 | 1 | 1 | 1 |
| COVID-19 | 1.1 (0.9 to 1.2) | 0.91 (0.75 to 1.10) | 0.90 (0.73 to 1.11) | 0.97 (0.54 to 1.74) | 1.38 (0.59 to 3.24) | 0.93 (0.76 to 1.14) | |
| Dermatology | Control | 8.9 (8.5 to 9.4) | 1 | 1 | 1 | 1 | 1 |
| COVID-19 | 9.5 (9.0 to 9.9) | 1.06 (0.99 to 1.14) | 1.06 (0.98 to 1.14) | 1.10 (0.92 to 1.31) | 0.95 (0.72 to 1.26) | 1.07 (1.00 to 1.15) | |
| Endocrinology | Control | 8.5 (8.0 to 8.9) | 1 | 1 | 1 | 1 | 1 |
| COVID-19 | 11.0 (10.5 to 11.5) | 1,30 (1.21 to 1.39) | 1.28 (1.18 to 1.40) | 1.34 (1.19 to 1.50) | 2.05 (1.74 to 2.41) | 1.11 (1.03 to 1.20) |
As shown, previous exposure to SARS-Cov-2 infection was always associated with a higher probability of needing access to hospital care which was more evident among those with Charlson Index≥1. The only exception were hospitalisations for respiratory diseases, whose HR associated with previous SARS-Cov-2 infection was higher among individuals without relevant comorbidities (HR: 6.69%–95% CI 2.84 to 15.70 vs HR: 2.30–95% 1.57 to 3.36 for those with Charlson Index≥1), and access to emergency room, whose HRs of the two subgroups were overlapping.
The same pattern held true for outpatient services, for whom previous exposure to SARS-Cov-2 infection was always associated with higher probability of use but for gastrointestinal and mental health specialist visits.
Subsequent use of these services was higher for people who had been hospitalised for COVID-19, except for rheumatology and dermatology visits (higher risk of subsequent visits for people not hospitalised for COVID-19).
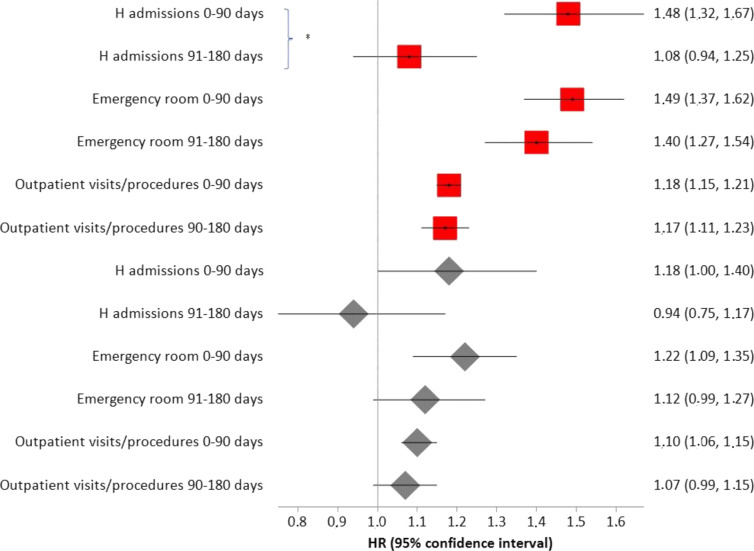
Risk of hospital and emergency room accesses and outpatient visits were highest for SARS-CoV-2 positive subjects compared with controls who did not have swab tests (figure 1). Looking more closely to each specific outcome, this specific pattern was significantly shown only for accesses to emergency room and for pneumology and gastroenterology visits (table 3). A decline in HRs after 90 days from the index date was observed, although this decline was statistically significant only for hospital admissions when SARS-CoV-2 positive subjects were compared with controls who did not have swab tests (figure 1). Subjects with higher Charlson index were more likely to have been tested. At the same time, younger people were more likely to have been tested (table 4).
Figure 1.
HRs (from Poisson regression) comparing rates of occurrence of the events considered between COVID-19 convalescents and matched controls who had no swab test (red squares) and who had at least one (negative) swab test (grey diamonds). *P<0.05.
Table 3.
HRs (from Poisson regression) comparing rates of occurrence of the events considered between COVID-19 convalescents and matched controls who had no swab test (n=22 820) and who had at least one (negative) swab test (n=13 216)
| COVID-19 convalescents vs controls who never had swab test | COVID-19 convalescents vs controls who had (negative) swab test | |||
| HR | 95% CI | HR | 95% CI | |
| Non-surgical h admissions | ||||
| Control | 1 | 1 | ||
| COVID-19 convalescents | 1.46 | 1.30 to 1.64 | 1.21 | 1.06 to 1.40 |
| H admissions for respiratory disease | ||||
| Control | 1 | 1 | ||
| COVID-19 convalescents | 2.66 | 1.82 to 3.89 | 2.15 | 1.31 to 3.53 |
| H admissions for heart disease | ||||
| Control | 1 | 1 | ||
| COVID-19 convalescents | 1.76 | 1.30 to 2.39 | 1.78 | 1.15 to 2.75 |
| Accesses to emergency room | ||||
| Control | 1 | 1 | ||
| COVID-19 convalescents | 1.39 | 1.32 to 1.47 | 1.14 | 1.06 to 1.21 |
| Outpatient specialist visits | ||||
| Pneumology | ||||
| Control | 1 | 1 | ||
| COVID-19 convalescents | 2.50 | 2.17 to 2.88 | 1.71 | 1.45 to 2.02 |
| Cardiology | ||||
| Control | 1 | 1 | ||
| COVID-19 convalescents | 1.32 | 1.21 to 1.43 | 1.33 | 1.19 to 1.49 |
| Neurology | ||||
| Control | 1 | 1 | ||
| COVID-19 convalescents | 1.22 | 1.10 to 1.35 | 1.09 | 0.96 to 1.24 |
| Rheumatology | ||||
| Control | 1 | 1 | ||
| COVID-19 convalescents | 1.16 | 1.03 to 1.32 | 1.25 | 1.05 to 1.47 |
| Gastroenterology | ||||
| Control | 1 | 1 | ||
| COVID-19 convalescents | 0.83 | 0.70 to 0.99 | 1.39 | 1.08 to 1.78 |
| Mental health | ||||
| Control | 1 | 1 | ||
| COVID-19 convalescents | 0.88 | 0.71 to 1.10 | 1.01 | 0.77 to 1.33 |
| Dermatology | ||||
| Control | 1 | 1 | ||
| COVID-19 convalescents | 1.04 | 0.96 to 1.12 | 1.09 | 0.99 to 1.21 |
| Endocrinology | ||||
| Control | 1 | 1 | ||
| COVID-19 convalescents | 1.19 | 1.11 to 1.29 | 1.18 | 1.07 to 1.30 |
Table 4.
ORs of being tested for SARS-CoV-2 infection within the control cohort (logistic model)
| Covariates | OR | (95% CI) |
| Age<30 | 1 | |
| 31–50 | 0.61 | (0.58 to 0.64) |
| 51–70 | 0.43 | (0.40 to 0.45) |
| >71 | 0.36 | (0.33 to 0.40) |
| Charlson Index 0 | 1 | |
| 1 | 1.32 | (1.17 to 1.49) |
| 2 | 1.22 | (1.07 to 1.39) |
| 3 | 1.37 | (1.17 to 1.59) |
| Sex female | 1 | |
| Male | 0.93 | (0.89 to 0.97) |
We checked patterns of access to health services in the year before the index date as well, to verify the comparability of the two cohorts in terms of health-seeking behaviour. Compared with control patients, COVID convalescent patients had had more emergency room accesses (24% vs 14%), non-surgical hospital admissions (9% vs 3%) and admissions for respiratory problems (included in the former: 5% vs 0.2%) in the 365 days before the index date. This was somehow expected, considering that respiratory patients may be at higher risk of getting covid.12 Results of a sensitivity analysis adjusting for age, sex and occurrence of hospital admissions and/or accesses to emergency room over the 365 days before the index date are presented in table 5, where results are also stratified for time to recovery. The risk of hospital admissions for respiratory disease and heart disease, accesses to emergency room, pneumology, cardiology, rheumatology and endocrinology visits and the risk of death remained higher for COVID convalescent patients than controls, in particular for those with longer time to recovery, except for endocrinology visits (in the latter case, the higher risk does not seem to be related to time to recovery).
Table 5.
HR representing the risk of death and of requiring hospital care and outpatients specialist visits for COVID-19 convalescent patients vs controls, adjusting for age, sex and occurrence of hospital admissions and/or accesses to emergency room over the 365 days before the index date
| HR (overall) | HR (according to time to recovery) | ||||
| 1–14 days (n=10 469) |
15–19 days (n=10 022) |
20–21 days (n=7235) |
>21 days (n=8310) |
||
| Non-surgical h admissions | 1.04 (0.94–1.15) | 0.93 (0.78–1.10) | 0.85 (0.72–1.01) | 0.88 (0.74–1.06) | 1.06 (0.92–1.21) |
| H admissions for respiratory disease | 1.42 (1.02–2.00) | 1.41 (0.78–2.53) | 0.74 (0.37–1.48) | 0.94 (0.51–1.76) | 1.98 (1.32–2.95) |
| H admissions for heart disease | 1.43 (1.07–1.88) | 1.43 (0.92–2.25) | 1.11 (0.69–1.78) | 1.45 (0.93–2.28) | 1.62 (1.13–2.33) |
| Accesses to emergency room | 1.13 (1.08–1.19) | 1.05 (0.98–1.13) | 1.16 (1.08–1.24) | 1.12 (1.03–1.21) | 1.27 (1.19–1.36) |
| Death | 2.50 (1.83–3.42) | 1.45 (0.81–2.58) | 0.88 (0.45–1.69) | 2.03 (1.25–3.30) | 3.89 (2.73–5.55) |
| Outpatient specialist visits | |||||
| Pneumology | 1.79 (1.59–2.01) | 1.44 (1.20–1.73) | 1.77 (1.49–2,10) | 1.58 (1.30–1.93) | 2.90 (2.49–3.37) |
| Cardiology | 1.19 (1.11–1.29) | 1.22 (1.09–1.37) | 1.23 (1.08–1.39) | 1.18 (1.03–1.34) | 1.39 (1.25–1.54) |
| Neurology | 1.07 (0.97–1.17) | 1.00 (0.87–1.15) | 1.04 (0.90–1.19) | 1.14 (0.98–1.33) | 1.23 (1.09–1.41) |
| Reumathology | 1.16 (1.03–1.30) | 1.09 (0.92–1.30) | 1.11 (0.93–1.31) | 1.09 (0.90–1.33) | 1.16 (1.00–1.38) |
| Gastroenterology | 0.88 (0.76–1.04) | 0.75 (0.58–0.98) | 0.96 (0.75–1.22) | 1.06 (0.82–1.38) | 0.97 (0.76–1.25) |
| Mental health | 0.87 (0.72–1.07) | 0.77 (0.57–1.04) | 0.86 (0.63–1.16) | 0.86 (0.61–1.21) | 0.81 (0.58–1.14) |
| Dermatology | 1.06 (0.99–1.13) | 1.04 (0.94–1.16) | 1.07 (0.97–1.19) | 1.07 (0.95–1.21) | 1.01 (0.90–1.13) |
| Endocrinology | 1.09 (1.02–1.17) | 1.11 (1.00–1.24) | 1.17 (1.05–1.29) | 1.07 (0.95–1.21) | 1.12 (1.01–1.23) |
HRs are reported also according to time to swab test negativity (or otherwise certified end of disease).
Costs
Overall, the cost of care provided to those who had a previous SARS-CoV-2 infection was 27% higher (€10 357 221, mean: 287.41, range 0–1 14 610 vs 8 149 196, mean 226.14, range 0–69 143). The difference in cost between the two groups was more evident (+46%) among those with relevant comorbidities (ie, Charlson Index>1) than among those with Charlson Index=0 (+17%) (table 6).
Table 6.
Cost of care (in Euro) for convalescent COVID-19 convalescents and control cohort
| Convalescent COVID-19 | Control cohort | |||||||
| Total | Mean | Median | Range | Total | Mean | Median | Range | |
| Overall | 10 357 221 | 287.41 | 23.0 | 0–114 610 | 8 149 196 | 226.14 | 14.0 | 0–69 143 |
| Charlson Index=0 | 6 318 301 | 194.0 | 18.0 | 0–114 610 | 5 380 207 | 165.23 | 4.0 | 0–45 510 |
| Charlson Index≥1 | 4 038 919 | 1162.3 | 159.0 | 0–74 440 | 2 768 989 | 796.83 | 137.0 | 0–69 143 |
Among those in the highest quartile of total costs, 9670 (54%) had previous SARS-CoV-2 infection. The relationship between their individual characteristics and the likelihood of being in the highest quartile of costs is outlined in table 7, according to the logistic regression model employed. Factors representing degree of severity of SARS-CoV-2 infection were associated with higher cost of care in the following months, in particular ageing and degree of comorbidity. As for COVID-related factors, subjects with hospital admission for COVID-19 and presence of symptoms at diagnosis had 66% and 32% higher probability of higher cost of care. On the contrary, those who had anti-SARS-CoV-2 vaccination had 64% lower probability of falling in the highest costs quartile.
Table 7.
Characteristics of individuals who had SARS-CoV-2 infection associated with higher costs of care in the following months
| Covariates | OR | 95% CI |
| Sex | ||
| Female | 1 | |
| Male | 0.67 | (0.63 to 0.71) |
| Age | ||
| <30 | 1 | |
| 31–50 | 2.17 | (2.01 to 2.34) |
| 51–70 | 3.71 | (3.44 to 4.00) |
| >71 | 4.75 | (4.32 to 5.23) |
| Charlson Index | ||
| 0 | 1 | |
| 1 | 2.09 | (1.86 to 2.36) |
| 2 | 2.96 | (2.60 to 3.39) |
| >3 | 4.00 | (3.42 to 4.78) |
| Had anti-SARS-CoV-2 vaccine | ||
| No | 1 | |
| Yes | 0.36 | (0.30 to 0.43) |
| Had symptomatic infection | ||
| No | 1 | |
| Yes | 1.32 | (1.23 to 1.41) |
| Had hospital admission for COVID-19 | ||
| No | 1 | |
| Yes | 1.66 | (1.50 to 1.83) |
Discussion
This study provides data to investigate the possible impact of SARS-Cov-2 infection on the use of specific specialist care (hospital admissions and outpatient specialist visits) and on related extra costs within 6 months from recovery on a large cohort from an Italian province. Unfortunately, we could not include data on primary care encounters since in Italy they are not traceable.
Our data show that in the 6 months after recovery, out of 1000 individuals, a previous SARS-CoV-2 infection was associated with 139 additional accesses in emergency room, eight additional non-surgical hospital admission and two hospitalisations for respiratory disease and for heart disease. As for outpatient visits, there were 19 additional pneumology visits as well as 17 cardiology, 15 endocrinology, seven neurology and four more rheumatology visits. This is highly consistent with the higher incidence of related symptoms in post-COVID patients described in several studies. On the contrary, no increase was shown in rates of mental health, gastroenterology and dermatology visits, despite related symptoms have been frequently reported among long-COVID patterns. The latter findings may be unexpected, especially regarding mental health services, although also studies carried out in Norway did not find a higher use of these services in the post-COVID period.13 14 Further qualitative analyses are warranted to explore possible determinants of the observed pattern, also considering accessibility to services.
The sensitivity analysis performed adjusting also for the occurrence of hospital admissions and/or accesses to emergency room over the 365 days before the index date provided lower HRs but confirmed the statistical significance of these results (except for subsequent non-surgical admissions and neurological visits), mostly led by COVID convalescents who had longer time to recovery.
Subgroup analyses indicate that increase in rates of non-surgical hospital admissions, hospitalisations for heart disease and accesses to emergency room is more pronounced in people with comorbidities (Charlson Index>1), whereas an opposite pattern is observed for rate of hospitalisations for respiratory disease. This counterintuitive finding may be due to the fact that such admissions are rarer in people without comorbidities, so that their relative increase in post-COVID patients is more evident compared with people who, among their comorbidities, may have a higher background rate of respiratory problems. The same reason may hold for the less pronounced increase in rates of pneumology and cardiology outpatient visits among post-COVID patients with comorbidities. Further studies may help clarify these points.
As expected, subsequent use of health services was higher for people who had been hospitalised for COVID-19, except for rheumatology and dermatology visits (higher risk of subsequent visits for people not hospitalised for COVID). The latter findings are unexpected and difficult to explain, although their relevance may be limited since the corresponding CIs related to hospitalised and non-hospitalised patients are widely overlapping.
HRs of emergency room accesses, pneumology and gastroenterology visits comparing COVID convalescents to controls who had never tested were significantly higher than HRs versus controls with at least one negative test. The latter may be more likely to be tested for having higher health risks (this would explain why the corresponding HRs are lower). This hypothesis is also supported by a logistic model using the subjects in the control cohort: those with higher Charlson Index are more likely to have been tested. At the same time, younger people are more likely to have been tested (they are more likely to be socially involved), while older people are less (they are more likely to have been kept isolated in those months). For SARS-CoV-2-infected subjects, risk of outpatient visits and of hospital or emergency room accesses was (or tended to be) lower after 90 days from the negative test. Overall, in 6 months, in our province, there have been extra costs for more than 2.2 million euros (about four euros per capita) associated with post-COVID sequelae.
Factors representing degree of severity of SARS-CoV-2 infection (presence of symptoms at diagnosis, hospital admission for COVID-19) were all associated with higher cost of care in the following months, as well as age and degree of comorbidity. On the contrary, those who had anti-SARS-CoV-2 vaccination were associated with lower cost of care.
Our hypothesis is that COVID-19 brought the positives to a subsequent greater use of services, but we cannot assume (only through various adjustments and stratifications) that they were comparable in this regard also before: those who do not have a positive test may be more careful in lifestyles (and more likely to be able to avoid SARS-CoV-2 infection, as well as be more likely to be visited) or vice versa avoid tampons and visits. Residual confounding cannot be excluded.
We acknowledge potential risks of misclassification, although the risk of having ‘non-recovered’ positives in the COVID database is extremely low: three-quarters of former SARS-CoV-2-infected subjects had a negative exit test within 21 days from a positive test, and one quarter of them were asymptomatic (after 21 days from a positive test). As for the latter, a surveillance system with daily phone calls and interviews with all cases cared for in outpatient settings was into place. In addition, we did not use a time window but started the follow-up from the date of recovery. The assumption is that those who have been confirmed as recovered cannot be in an acute phase. This may bring a risk of misclassification too, although we consider it very small and not higher than the risk of missing cases which could occur within a time window.
Our observational data have been collected as part of patients’ care: reimbursements to health services depend on completeness of these data, which can be assumed, and which should exclude the possibility of major biases. However, limits in the quality of administrative data cannot be excluded. The adjustment for imbalances in hospital and emergency room admissions in the year before the index date, performed as sensitivity analysis and stratification for Charlson Index to better disentangle the effect of the previous SARS-Cov-2 infection, rather than of coexisting diseases may limit, but of course not eliminate, the possibility of residual confounding. No scientific validation of the databases used and of the record-linkage procedures is available, although the unique patient identification number present in all the databases should ensure that no data are lost. As for generalisability, incidence of SARS-CoV-2 infection in our province since the start of the pandemic is similar to that in other areas of Northern Italy, although higher than the mean Italian incidence (about 5000 cases more out of 100 000 inhabitants).15
In conclusion, many studies reported the frequency of post-COVID symptoms that recovered patients suffered from. Our findings also suggest an extra burden for patients and health services due to post-COVID sequelae, providing some specific insight on association of SARS-CoV-2 infection with extra-use of health services after the acute phase, according to patients’ characteristics and vaccination status. Vaccination is associated with lower cost of care following SARS-CoV-2 infection, highlighting the possibly favourable impact of vaccines on the use of health services even when they do not prevent infection, in keeping with their capacity to reduce the clinical burden associated to SARS-CoV-2 infection. We plan to expand these data in a further paper using longer follow-up periods, also with higher numbers of vaccinated people to allow a comparison between those who developed COVID and those who do not, and to warrant the inclusion of boosted people who could not be included in our cohort yet.
Supplementary Material
Footnotes
Contributors: RG, GF and MM conceived the study; MM and DF extracted the data; RG and MM analysed the data; GF and RG interpreted its results and drafted the paper; GF revised the final version. All the authors revised the article critically for important intellectual content. All authors approved the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The lead author, GF, affirms that this manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.GF is the manuscript’s guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. Data will be made available on request to researchers who meet the criteria for access to confidential data. Requests should outline the objectives of the research and a protocol for the analyses. In order to obtain data, approval must be obtained from the Area Vasta Emilia Nord (AVEN) Ethics Committee, who would then authorise us to provide aggregated or anonymised data. Data access requests should be addressed to the Ethics Committee at CEReggioemilia@ausl.re.it as well as to the corresponding author.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study has been approved by the Area Vasta Emilia Nord Ethics Committee on 13/01/2022 n° 2022/0004443.
References
- 1.Moynihan R, Sanders S, Michaleff ZA, et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open 2021;11:e045343. 10.1136/bmjopen-2020-045343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . A clinical case definition of post COVID-19 condition by A delphi consensus. 2021. Available: www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1
- 3.National Institute for Health and Care Excellence (NICE), Scottish Intercollegiate Guidelines Network (SIGN), Royal College of General Practitioners (RCGP) . COVID-19 rapid guideline: managing the long-term effects of COVID-19. n.d. Available: www.nice.org.uk/guidance/ng188/resources/covid19-rapid-guideline-managing-the-longterm-effects-of-covid19-pdf-51035515742
- 4.Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of postacute sequelae of SARS-cov-2 infection: a systematic review. JAMA Netw Open 2021;4:e2128568. 10.1001/jamanetworkopen.2021.28568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 2021;398:747–58. 10.1016/S0140-6736(21)01755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021;38:101019. 10.1016/j.eclinm.2021.101019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nehme M, Braillard O, Chappuis F, et al. Prevalence of symptoms more than seven months after diagnosis of symptomatic COVID-19 in an outpatient setting. Ann Intern Med 2021;174:1252–60. 10.7326/M21-0878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heesakkers H, van der Hoeven JG, Corsten S, et al. Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. JAMA 2022;327:559–65. 10.1001/jama.2022.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giorgi Rossi P, Marino M, Formisano D, et al. Characteristics and outcomes of a cohort of COVID-19 patients in the province of reggio emilia, Italy. PLoS One 2020;15:e0238281. 10.1371/journal.pone.0238281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ragni P, Marino M, Formisano D, et al. Association between exposure to influenza vaccination and COVID-19 diagnosis and outcomes. Vaccines (Basel) 2020;8:675. 10.3390/vaccines8040675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 12.Johansen MD, Mahbub RM, Idrees S, et al. Increased SARS-cov-2 infection, protease, and inflammatory responses in chronic obstructive pulmonary disease primary bronchial epithelial cells defined with single-cell RNA sequencing. Am J Respir Crit Care Med 2022;206:712–29. 10.1164/rccm.202108-1901OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skyrud KD, Hernæs KH, Telle KE, et al. Impacts of mild COVID-19 on elevated use of primary and specialist health care services: a nationwide register study from norway. PLoS ONE 2021;16:e0257926. 10.1371/journal.pone.0257926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magnusson K, Skyrud KD, Suren P, et al. Healthcare use in 700 000 children and adolescents for six months after covid-19: before and after register based cohort study. BMJ 2022;376:e066809. 10.1136/bmj-2021-066809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The New York Times . Available: https://www.nytimes.com/interactive/2021/world/italy-covid-cases.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. Data will be made available on request to researchers who meet the criteria for access to confidential data. Requests should outline the objectives of the research and a protocol for the analyses. In order to obtain data, approval must be obtained from the Area Vasta Emilia Nord (AVEN) Ethics Committee, who would then authorise us to provide aggregated or anonymised data. Data access requests should be addressed to the Ethics Committee at CEReggioemilia@ausl.re.it as well as to the corresponding author.