Abstract
Dupilumab (DUP) is a monoclonal antibody that acts on the interleukin (IL)-4 receptor alpha, which inhibits IL-4 and IL-13 signalling and is approved for type 2 inflammatory diseases such as asthma, chronic rhinosinusitis with nasal polyposis and atopic dermatitis; however, the efficacy of DUP to IgG4-related disease (IgG4-RD) is under discussion due to the controversial outcomes based on the several case reports. Here, we reviewed the efficacy of DUP in four consecutive patients with IgG4-RD in our institute and the previous literature.
All patients administered DUP fulfilled the 2019 ACR/EULAR classification criteria for IgG4-RD complicated with severe asthma and chronic rhinosinusitis with nasal polyposis. Two cases were administered DUP without systemic glucocorticoids (GCs), and in 6 months, the volume of swollen submandibular glands (SMGs) was reduced by approximately 70%. Two cases receiving GCs successfully reduced their daily dose of GCs (10 and 50% reduction, respectively) with dupilumab in 6 months. In all four cases, serum IgG4 concentration and IgG4-RD responder index decreased in 6 months.
DUP reduced the volume of the swollen SMGs, serum IgG4 levels, responder index and the daily dose of GCs in patients with IgG4-RD with severe asthma or eosinophilic rhinosinusitis in 6 months.
The efficacy of DUP to IgG4-RD is under discussion due to the limited case reports with controversial outcomes. Here, we demonstrated that two patients with IgG4-RD treated by DUP without systemic GCs, showed volume reduction of swollen SMGs and two cases showed GC-sparing effects by DUP. DUP can ameliorate the disease activity and be a steroid-sparing agent in patients with IgG4-RD.
Keywords: therapeutics, immune system diseases, glucocorticoids, cytokines, biological therapy
WHAT IS ALREADY KNOWN ON THIS TOPIC
Dupilumab (DUP) is a monoclonal antibody, which inhibits interleukin (IL)-4 and IL-13 signalling, is approved for type 2 inflammatory diseases such as asthma, chronic rhinosinusitis with nasal polyposis and atopic dermatitis. The efficacy of DUP to IgG4-related disease (IgG4-RD) is under discussion due to the controversial outcomes based on the several case reports.
WHAT THIS STUDY ADDS
The efficacy of DUP was reviewed by a case series with IgG4-RD and the previous literature.
Two cases without systemic glucocorticoids (GCs) reduced the volume of swollen submandibular glands, and two patients receiving GCs successfully reduced their daily dose of GCs with DUP in 6 months.
Serum IgG4 concentration and IgG4-RD responder index can decrease in 6 months by DUP administration.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
DUP can be a potential biological treatment of patients with IgG4-RD with type 2 inflammatory diseases.
Introduction
IgG4-related disease (IgG4-RD) is a progressive fibrosing condition characterised that can affect various organs with relapsing-remitting progression, typically occurring in middle-aged to elderly. Glucocorticoids (GCs) are the first-line therapy for IgG4-RD and have promising effects; however, the relapse rates are relatively high, 34%–53% after discontinuation of GCs.1 Therefore, patients often need to be treated with the maintenance dose of GCs to reduce relapse. On the other hand, GC has numerous adverse effects1 and to reduce them, novel molecular targeted therapies, such as anti-CD20 antibodies,2 and anti-CD19 antibodies (NCT04540497), are under the investigation. Although, there are no established molecularly targeted therapies in IgG4-RD.
DUP inhibits IL-4 and IL-13 signalling, approved for type 2 inflammatory diseases such as asthma, chronic rhinosinusitis with nasal polyposis. A total of 19%–31% of patients with IgG4-RD have histories of allergic disorders3 4 and IL-4 plays a vital role in the production of IgG4 in IgG4-RD.5 In addition, there are several studies IL-13 relates to IgG4-related sialadenitis.6 7 Therefore, DUP seems adequate to IgG4-RD; however, the efficacy of DUP to IgG4-RD is under discussion.8–11 Here, we report four consecutive cases with IgG4-RD administered DUP for severe asthma or chronic rhinosinusitis with a review of the literatures.
Case reports
Case 1
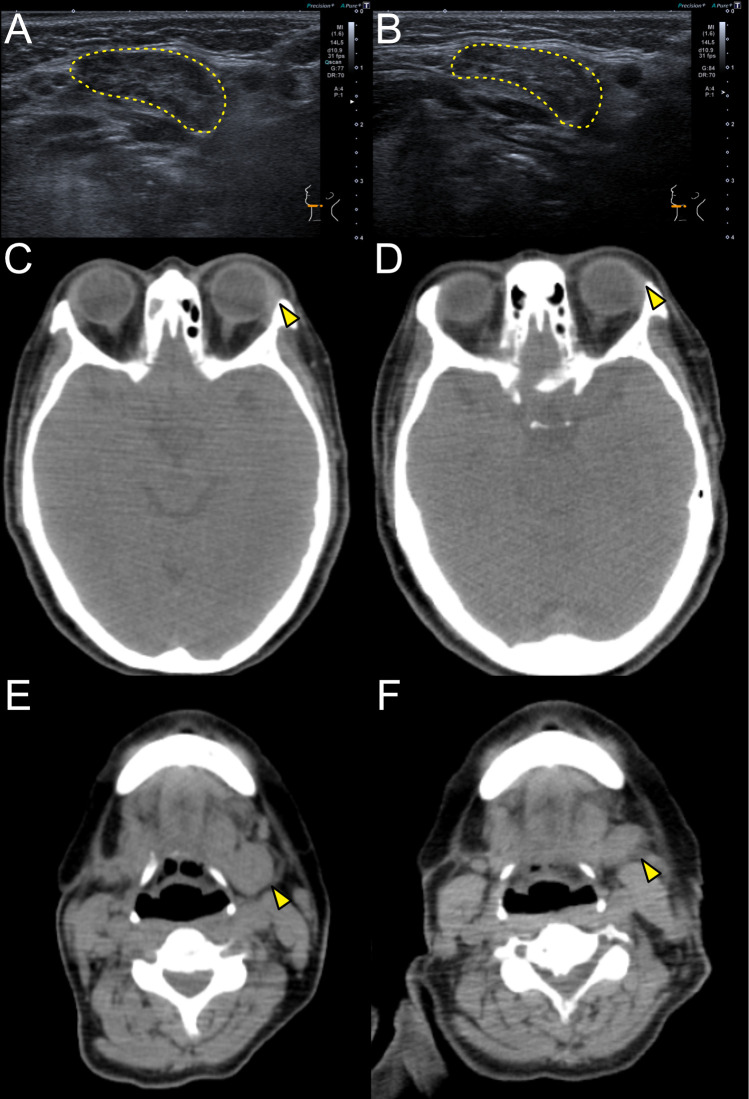
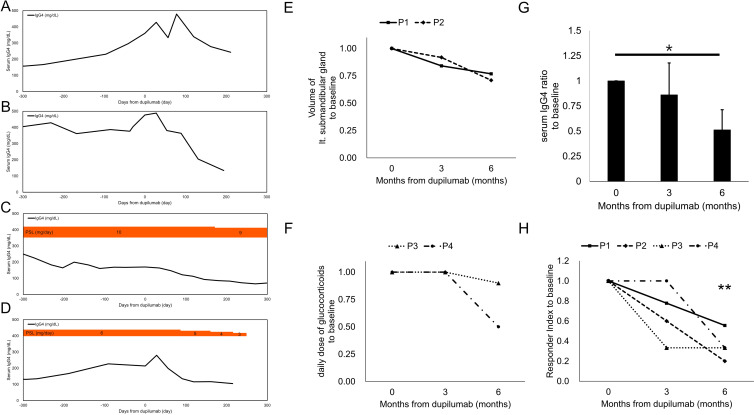
A 53-year-old woman presented with recurrent nasal closure with high serum IgG4 levels on January 2021. A nasal polyp biopsy indicated eosinophilic chronic rhinosinusitis (ECRS). The physical examination revealed swollen SMGs, and CT showed swollen SMGs with enlarged submandibular lymph nodes. A right SMG biopsy revealed storiform fibrosis, IgG4+/IgG+ ratio >0.4 and IgG4+ cells >10/HPF. Laboratory tests revealed high serum IgG4 levels (360 mg/dL) with eosinophilia (671/µL). She was diagnosed as IgG4-RD according to the 2019 ACR/EULAR classification criteria12 (table 1). A 300 mg of DUP was administered every 2 weeks without systemic GCs for ECRS. Nasal closure disappeared in several months. The volume of left SMG decreased to 77% (from 9.658 to 7.418 mL, using the volume analyser, SYNAPSE VINCENT, Fujifilm, Tokyo, Japan), and multiple hypoechoic foci and heterogeneous echotexture disappeared in 6 months (figures 1A, B and 2E). Serum IgG4 levels changed by 133% and 77% (479, 278 mg/dL) (figure 2A) and IgG4-RD responder index (RI)13 declined from 9 to 7 and 5 (78, 56%) in 3 and 6 months, respectively (figure 2G, H).
Table 1.
Features of cases with IgG4-RD administerd dupilumab
| Author | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Simpson et al11 | Ebbo et al8 | Otani et al10 | Nakajima et al9 | Our case 1 | Our case 2 | Our case 3 | Our case 4 | |
| Year | 2020 | 2020 | 2021 | 2021 | ||||
| Age | 67 | 51 | 57 | 51 | 53 | 56 | 67 | 50 |
| Gender | Male | Male | Male | Female | Female | Female | Male | Female |
| 2019 ACR/EULAR classification score | n/a | 29 | n/a | n/a | 32 | 47 | 38 | 28 |
| Organ involvement at diagnosis | Skin parotid glands paranasal sinus retropritoneal lung | Lacrimal glands parotid glands sublingual glands submandibular glands | Lacrimal glands parotid glands lung | Lacrimal glands paranasal sinus | Submandibular glands paranasal sinus | Lacrimal glands submandibular glands paranasal sinus lung | Pancreas submandibular glands paranasal sinus | Lacrimal glands |
| Biopsy proven (lesion of biopsy) |
Yes (prostate) |
Yes (lymph node) |
Yes (parotid gland) |
Yes (lacrimal gland) |
Yes (submandibular gland) |
Yes (submandibular gland) |
Yes (submandibular gland) |
Yes (lacrimal gland) |
| Serum IgG4 levels at diagnosis* | 20.60 g/L | 17.7 g/L | 270 mg/dL | 768 mg/dL | 360 mg/dL | 477 mg/dL | 716 mg/dL | 163 mg/dL |
| Previous immune therapy | None | GCs rituximab | GCs azathioprine | None | None | None | GCs mepolizumab | GCs |
| Indication of dupilumab | Atopic dermatitis asthma | Chronic rhinosinusitis with nasal polyps asthma | Asthma | Eosinophilic sinusitis | Eosinophilic sinusitis | Eosinophilic sinusitis | Asthma | Asthma |
| Dose of dupilumab | 600 mg for the first dose 300 mg every 2 weeks |
600 mg for the first dose 300 mg every 2 weeks |
600 mg for the first dose 300 mg every 2 weeks |
600 mg for the first dose 300 mg every 2 weeks |
300 mg every 2 weeks | 300 mg every 2 weeks | 300 mg every 4 weeks | 300 mg every 4 weeks |
| Glucocorticoids Immunosupressants |
None | None | 10 mg predonisolone 25 mg azathioprine |
None | None | None | 10 mg predonisolone | 6 mg predonisolone |
| IgG4-RD responder index at dupilumab administration | n/a | n/a | n/a | n/a | 9 | 15 | 3 | 3 |
| Organ involvement at dupilumab administration | Skin parotid glands paranasal sinus retropritoneal lung | Salivary glands paranasal sinus lympho nodes | Lacrimal glands parotid glands lung | Lacrimal glands paranasal sinus | Submandibular glands paranasal sinus | Lacrimal glands submandibular glands paranasal sinus lung | Lung | Lung |
| Organ involvements not responded to dupilumab | n/a for parotid glands paranasal sinus | Salivary glands lympho nodes | None | None | None | None | None | None |
| Outcome | Effective to organ involvement | Ineffective to organ involvement | Effective to GC sparing | Effective to organ involvement | Effective to organ involvement | Effective to organ involvement | Effective to GC sparing | Effective to GC sparing |
Summary table of the cases with IgG4-RD administered dupilumab.
*The originally reported units for serum IgG4 levels were written in the table because the methods of the measurement were estimated to be different.
GC, glucocorticoid; IgG4-RD, IgG4-related disease; n/a, not available.
Figure 1.
Changes in CT or sonography during dupilumab administration (A, B) Images of sonography around the left submandibular glands in case 1 at the baseline (A) and 6 months after the first administration of dupilumab (B). The yellow dashed lines show left submandibular glands. (C, D) Horizontal images of CT around the lacrimal glands in case 2 at the baseline (C) and 6 months after the first administration of dupilumab (D). The yellow arrowheads show left lacrimal glands. (E, F) Horizontal images of CT around the submandibular glands in case 2 at the baseline (E) and 6 months after the first administration of dupilumab (E). The yellow arrowheads show left submandibular glands.
Figure 2.
Changes in serum IgG4 concentration, glucocorticoids (GC) dosage and volume of submandibular glands around the dupilumab administration serum IgG4 concentration (mg/dL) and GC dosage in case 1 (A), case 2 (B), case 3 (C) and case 4 (D). (E) Volume changes of the left submandibular glands from the baseline measured by the volume analyser, SYNAPSE VINSCENT. P1 is case 1 and P2 is case 2 in the main text; (F) Changes of the daily dose of glucocorticoids from the baseline. P3 is case 3 and P4 is case 4 in the main text. (G) Changes of serum IgG4 levels from the baseline of the four cases. The bar indicates SD. Significance levels are indicated as *p≤0.05 by paired t-test. (H) Changes of IgG4-RD responder index from the baseline of the four cases. Significance levels are indicated as **p≤0.01 by paired t-test.
Case 2
A 56-year-old woman presented to our department with recurrent nasal closure with swollen eyelids and SMGs on March 2021. The nasal closure was started in 2010, and systemic GCs were often prescribed. A nasal polyp biopsy indicated ECRS. CT showed swollen lacrimal glands and SMGs with enlarged submandibular and neck lymph nodes. She suffered from dry coughs and lung CT revealed peribronchovascular and septal thickening in the lung. A right SMG biopsy revealed IgG4+/IgG+ ratio >0.5 and IgG4+ cells >100/HPF. Laboratory tests revealed high serum IgG4 levels (477 mg/dL) without eosinophilia. A 300 mg of DUP was administered every 2 weeks without systemic GCs for ECRS. Nasal closure and dry coughs were disappeared in several months. Swollen eyelids were shrunken (figure 1C, D), and the volume of left SMGs decreased to 71% (from 9.71 to 6.925 mL) in 6 months (figures 1E, F and 2E). Serum IgG4 levels were reduced by 76 and 28% (364, 134 mg/dL) (figure 2B) and IgG4-RD RI declined from 15 to 9 and 3 (60, 20%) in 3 and 6 months, respectively (figure 2G, H).
Case 3
A 67-year-old man presented with swollen SMGs on February 2014. He had an asthma history. He was a rice farmer. CT revealed diffuse enlargement of the pancreas and capsule-like rim with decreased enhancement, fluid retention in the maxillary sinus and SMGs’ enlargement. Laboratory tests revealed high serum IgG4 levels (716 mg/dL) without eosinophilia. A right SMG biopsy showed IgG4+/IgG+ ratio >0.5 and IgG4+ cells >100/HPF. He was treated with prednisolone (PSL, 40 mg/day) from July 2014, showing a favourable response. PSL was tapered to 7 mg/day on March 2015. Afterwards, serum IgG4 level increased up to 528 mg/dL until September 2017 with the enlargement of para-aortic arch lymph nodes and fluid retention in the maxillary sinus. Dry coughs with wheeze started in December 2017, and the symptom worsened when he started to harvest rice. Combination therapy of inhaled GCs with long-acting beta-agonists, theophylline and anti-allergic drugs were not effective enough. A 100 mg of mepolizumab was administered from November 2018. On November 2019, he became organising pneumonia, and mepolizumab was changed to DUP on January 2020. A 300 mg of DUP was administered every 4 weeks for severe asthma and it controlled the asthma attacks. His serum IgG4 level kept decreasing and the swollen para-aortic arch lymph nodes and organised pneumonia were resolved on February 2021. PSL was reduced gradually to 9 mg/day 6 months after the administration of DUP and to 3 mg/day on January 2023 (figure 2C, F). IgG4-RD RI declined from 3 to 1 and 1 (33, 33%) in 3 and 6 months, respectively (figure 2H).
Case 4
A 50-year-old woman presented with swollen eyelids on May 2011. Seven months before, she was diagnosed as dacryoadenitis and was treated by GCs; however, a month after the withdrawal of GCs the swollen eyelids recurred. A right lacrimal gland biopsy revealed IgG4+/IgG+ ratio >0.5. Laboratory tests revealed high serum IgG4 levels (163 mg/dL), without eosinophilia. There was no other interorgan involvement by IgG4-RD. She was treated with PSL (30 mg/day) on June 2012 showing a favourable response and PSL was tapered to 6 mg/day on January 2017. In 2020, she started to suffer from coughs with wheezing and CT revealed a thickening of bronchial walls, and fractional exhaled nitric oxide was 60 ppm. She was diagnosed with asthma and then inhaled GCs with long-acting beta-agonists were started; however, it cannot control the attacks completely. A 300 mg of DUP was administered every 4 weeks on January 2022 for severe asthma. It controlled asthma attacks and her serum IgG4 level kept decreasing. PSL was reduced gradually to 3 mg/day in 6 months (figure 2D, F). IgG4-RD RI changed from 3 to 3 and 1 (100, 33%) in 3 and 6 months, respectively (figure 2H).
Discussion
The efficacy of DUP to IgG4-RD is under discussion with the controversial outcomes.8–11 In this study, we demonstrated four patients with IgG4-RD administered DUP for coincident severe asthma or chronic rhinosinusitis. Two cases without systemic GCs reduced the volume of swollen SMGs and two cases receiving GCs successfully reduced their daily dose of GCs by DUP.
To better understand the efficacy of DUP to IgG4-RD, we collected the features of the reported cases8–11 and our cases in table 1. It is crucial to consider the reporting bias to interpret the reported cases; however, the majority of the patients showed favourable outcomes in both IgG4-RD and asthma or chronic rhinosinusitis by DUP. GC therapy in IgG4-RD starts to be effective within a month, typically. Compared with GC therapy, the response of DUP monotherapy in IgG4-RD is relatively slow, and it took 3–6 months to get the apparent effects.9 In our four cases, serum IgG4 concentration and IgG4-RD RI was reduced in 6 months (figure 2G, H). Considering of the relatively slow effects of DUP, it should not be considered in patients with the manifestations (eg, ureteral obstruction, proximal biliary strictures) in which urgent treatment is recommended.14 The reported cases might have a selection bias in patients of IgG4-RD because all cases were administered DUP for severe asthma or chronic rhinosinusitis. It should be carefully examined if DUP is effective and safe for IgG4-RD, especially in cases with interorgan involvement (eg, pancreas, kidney, aortitis), which were not yet evaluated (table 1). Long-term use of DUP for patients with asthma or atopic dermatitis seems safe15 16 so that it can be safer than the long-term use of GCs in patients with IgG4-RD, but it is still unclear if the efficacy of DUP will prolong or not. At least, in case 3, DUP kept administered for 3 years without adverse events with a favourable outcome.
The immunopathogenesis of IgG4-RD is not fully uncovered; however, at least, IL-4 is closely related to IgG4 production in IgG4-RD, inducing IgG4 class-switch mediated by T follicular helper (Tfh) cells.5 17 18 In addition, DUP can reduce the number of circulating Tfh cells.8 These can explain why DUP suppressed the serum IgG4 levels and IgG4-mediated inflammation. IL-13 also relates to IgG4-related sialadenitis6 by promoting cellular senescence through inducing mitochondrial dysfunction.7 It is unclear if IL-13 relates to fibrosis in IgG4-RD or not, but IL-13 is a fibrosis related cytokine19 20 so that blocking IL-13 signalling might be effective in the fibrotic condition of IgG4-RD.
This is the first case series and a literature review of patients with IgG4-RD who were administered DUP. There are still limited in case reports, but DUP can ameliorate the disease activity and be a steroid-sparing agent in patients with IgG4-RD.
Acknowledgments
The authors wish to thank the patients who participated in this study and the physicians who treated our patients.
Footnotes
Contributors: Design of the study: MK; acquisition of data: MK, RK, MS, KN, CS and HT; interpretation of data: MK, RK, MS, KN, CS, KT and HT; manuscript draft preparation: MK. All authors agreed to the final version of the manuscript.
Funding: This work was supported by the MHLW Research Programme on Rare and Intractable Diseases, Grant No. JPMH20FC1040.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Yoshifuji H, Umehara H. Glucocorticoids in the treatment of IgG4-related disease-prospects for new international treatment guidelines. Mod Rheumatol 2022:roac097. 10.1093/mr/roac097 [DOI] [PubMed] [Google Scholar]
- 2.Carruthers MN, Topazian MD, Khosroshahi A, et al. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis 2015;74:1171–7. 10.1136/annrheumdis-2014-206605 [DOI] [PubMed] [Google Scholar]
- 3.Zen Y, Nakanuma Y. Igg4-Related disease: a cross-sectional study of 114 cases. Am J Surg Pathol 2010;34:1812–9. 10.1097/PAS.0b013e3181f7266b [DOI] [PubMed] [Google Scholar]
- 4.Della Torre E, Mattoo H, Mahajan VS, et al. Prevalence of atopy, eosinophilia, and IgE elevation in IgG4-related disease. Allergy 2014;69:269–72. 10.1111/all.12320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perugino CA, Stone JH. Igg4-Related disease: an update on pathophysiology and implications for clinical care. Nat Rev Rheumatol 2020;16:702–14. 10.1038/s41584-020-0500-7 [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi M, Ohno K, Takata K. Interleukin 13-positive mast cells are increased in immunoglobulin G4-related sialadenitis. Sci Rep 2015;5:7696. 10.1038/srep07696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu M, Min S, Mao X, et al. Interleukin-13 promotes cellular senescence through inducing mitochondrial dysfunction in IgG4-related sialadenitis. Int J Oral Sci 2022;14:29. 10.1038/s41368-022-00180-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebbo M, De Sainte-Marie B, Muller R, et al. Correspondence on: “dupilumab as a novel steroid-sparing treatment for igg4-related disease” by simpson et al. Ann Rheum Dis 2022;81:e26. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima I, Taniguchi Y, Tsuji H, et al. Therapeutic potential of the interleukin-4/interleukin-13 inhibitor dupilumab for treating IgG4-related disease. Rheumatology (Oxford) 2022;61:e151–3. 10.1093/rheumatology/keab950 [DOI] [PubMed] [Google Scholar]
- 10.Otani, T, Iwamoto, H, Yoshida, Y, et al. Dupilumab as an adjunct treatment for a patient with steroid-dependent immunoglobulin G4-related disease complicated by asthma: a case report. J Asthma 2022;59:2395–401. 10.1080/02770903.2021.2022158 [DOI] [PubMed] [Google Scholar]
- 11.Simpson RS, Lau SKC, Lee JK. Dupilumab as a novel steroid-sparing treatment for IgG4-related disease. Ann Rheum Dis 2020;79:549–50. 10.1136/annrheumdis-2019-216368 [DOI] [PubMed] [Google Scholar]
- 12.Wallace ZS, Naden RP, Chari S, et al. The 2019 American College of rheumatology/european League against rheumatism classification criteria for IgG4-related disease. Ann Rheum Dis 2020;79:77–87. 10.1136/annrheumdis-2019-216561 [DOI] [PubMed] [Google Scholar]
- 13.Wallace ZS, Khosroshahi A, Carruthers MD, et al. An international Multispecialty validation study of the IgG4-related disease Responder index. Arthritis Care Res (Hoboken) 2018;70:1671–8. 10.1002/acr.23543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khosroshahi A, Wallace ZS, Crowe JL, et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol 2015;67:1688–99. 10.1002/art.39132 [DOI] [PubMed] [Google Scholar]
- 15.Deleuran M, Thaçi D, Beck LA. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol 2020;82:377–88. 10.1016/j.jaad.2019.07.074 [DOI] [PubMed] [Google Scholar]
- 16.Wechsler ME, Ford LB, Maspero JF. Long-term safety and efficacy of dupilumab in patients with moderate-to-severe asthma (traverse): an open-label extension study. Lancet Respir Med 2022;10:11–25. 10.1016/S2213-2600(21)00322-2 [DOI] [PubMed] [Google Scholar]
- 17.Jeannin P, Lecoanet S, Delneste Y, et al. Ige versus IgG4 production can be differentially regulated by IL-10. J Immunol 1998;160:3555–61. 10.4049/jimmunol.160.7.3555 [DOI] [PubMed] [Google Scholar]
- 18.Maehara T, Mattoo H, Mahajan VS, et al. The expansion in lymphoid organs of IL-4+ BATF+ T follicular helper cells is linked to igg4 class switching in vivo. Life Sci Alliance 2018;1:e201800050. 10.26508/lsa.201800050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CG, Homer RJ, Zhu Z, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta (1). J Exp Med 2001;194:809–21. 10.1084/jem.194.6.809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gieseck RL, Ramalingam TR, Hart KM. Interleukin-13 activates distinct cellular pathways leading to ductular reaction, steatosis, and fibrosis. Immunity 2016;45:145–58. 10.1016/j.immuni.2016.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]