Abstract
Introduction
Corticosteroids affect variably survival in sepsis trials, suggesting heterogeneity in patients’ response to corticosteroids. The RECORDS (Rapid rEcognition of COrticosteRoiD resistant or sensitive Sepsis) trial aimed at defining endotypes associated with adults with sepsis responsiveness to corticosteroids.
Methods and analysis
RECORDS, a multicentre, placebo-controlled, biomarker-guided, adaptive Bayesian design basket trial, will randomly assign to a biomarker stratum 1800 adults with community-acquired pneumonia, vasopressor-dependent sepsis, septic shock or acute respiratory distress syndrome. In each stratum, patients will be randomly assigned to receive a 7-day course of hydrocortisone and fludrocortisone or their placebos. Patients with COVID-19 will be treated with a 10-day standard course of dexamethasone and randomised to fludrocortisone or its placebo. Primary outcome will be 90-day death or persistent organ dysfunction. Large simulation study will be performed across a range of plausible scenarios to foresee power to detect a 5%–10% absolute difference with corticosteroids. We will assess subset-by-treatment interaction by estimating in a Bayesian framework two quantities: (1) measure of influence, relying on the value of the estimation of corticosteroids’ effect in each subset, and (2) measure of interaction.
Ethics and dissemination
The protocol was approved by the Ethics Committee (Comité de Protection des Personnes, Dijon, France), on 6 April 2020. Trial results will be disseminated at scientific conferences and results will be published in peer-reviewed journals.
Trial registration number
ClinicalTrials.gov Registry (NCT04280497).
Keywords: adult intensive & critical care, clinical trials, respiratory infections
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Observational period providing preliminary clinical and biological data.
Ongoing basket adaptative trial integrating biomarkers and signatures, designed to personalise corticosteroid therapy in sepsis.
Patients free of COVID-19 and influenza are stratified by biomarker/signature and are randomised to receive either combined glucocorticoid (hydrocortisone, or if SARS-CoV-2 positive, open-label dexamethasone) and mineralocorticoid (fludrocortisone) or placebo.
New biomarkers or signatures are continuously included and assessed in the trial.
Sequential intermediate analyses using a Bayesian model aimed at identifying relevant predictive biomarkers.
Introduction
The WHO identified sepsis as a high priority condition due to its incidence (~49 million cases/year), mortality (~11 million fatalities/year) and morbidity (cognitive decline at 5 years in up to half of survivors).1 Dysregulated immune and endogenous cortisol responses to infection are hallmarks of sepsis, requiring prompt source control, anti-infective treatment and cardiorespiratory management. Both overwhelming systemic inflammation and impaired endogenous cortisol response to infection are improved by the administration of corticosteroids.2 Evidence from 61 trials accounting for 12 192 patients, including both children and adults with sepsis, suggested that corticosteroids saved 1 additional life every 33 treated patients.3 There was no evidence of any difference in corticosteroids’ effects on survival between children and adults (Χ2=0.29; df=1; p=0.62; I2=0%), between uncomplicated sepsis, septic shock, acute respiratory distress syndrome (ARDS) and community-acquired pneumonia (CAP) (Χ2=7.60; df=3; p=0.06; I2=60.5%). International guidelines (panel including consumers) recommended administrating corticosteroids in sepsis.4 Likewise, the WHO recommended treatment with corticosteroids for patients with COVID-19 requiring respiratory support, in keeping with results from the RECOVERY trial.5 However, survival benefits of corticosteroids varied across trials highlighting the need to identify optimal target populations for corticosteroids.6–9 The effects of corticosteroids on survival from sepsis are independent of age, gender, disease severity, type or source of infection, or type of pathogen.3 No reliable, routinely available diagnostic test predicts the response to corticosteroids in sepsis. Nevertheless, preliminary studies in adults10 and children with sepsis11 suggested that transcriptomic signatures may be associated with increased mortality in corticosteroid-treated patients. Additionally, machine learning derived for individual prediction outperformed one-size-fits-all decisions of hydrocortisone treatment in septic shock.12 Theoretically, survival benefits related to the administration of corticosteroids require (1) hyperinflammation in response to an infection and (2) intact cellular mechanisms enabling corticosteroid bioactivity.2 Both hyperinflammation and cellular mechanisms enabling corticosteroid bioactivity are potential biomarkers of corticosteroid sensibility or resistance.
The RECORDS (Rapid rEcognition of COrticosteRoiD resistant or sensitive Sepsis) trial aims at identifying biomarkers defining populations with sepsis who may either benefit or be harmed by corticotherapy. Biomarkers assessed in this trial are selected through analysis of biological and clinical data from a previous cohort,8 from the observational period of this current trial and from any relevant newly reported cohort.
Methods and analysis
Study design and oversight
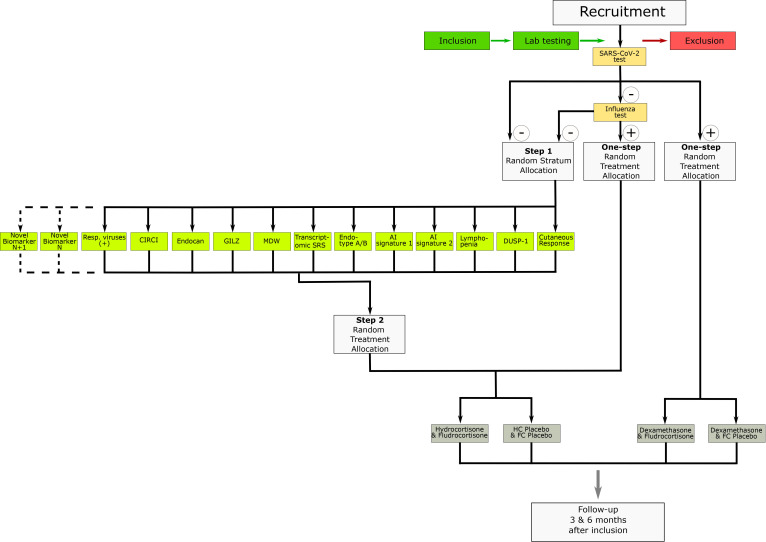
The study is divided into two distinctive stages, for example, a run-in prospective observational cohort followed by a biomarker-guided adaptive randomised controlled trial. As per request from the French National Agency for Drug Safety (ANSM, France), randomisation is first stratified by SARS-CoV-2 status (see figure 1). Patients tested positive for SARS-CoV-2 are then randomised with a 1:1 allocation ratio to receive either dexamethasone and fludrocortisone (FC) or dexamethasone and FC placebo. Subsequently, patients tested negative for SARS-CoV-2 are tested for influenza. Patients tested positive for influenza are then randomised for treatment (hydrocortisone (HC) and FC or their respective placebos). Patients negative for SARS-CoV-2 and influenza are first randomly assigned to a biomarker/signature stratum among all the biomarkers/signatures available at the time of randomisation for the patient; then, those patients are randomised with a 1:1 allocation ratio to either HC and FC or their respective placebos, using the randomisation list from this stratified biomarker/signature result. Patients positive to other respiratory viruses are not mandatorily assigned to the arms corresponding to their infections (other respiratory viruses (+)) as they are randomly assigned to any biomarker/signature arms of the two-step randomisation (see figure 1 and the Allocation of stratification section below).
Figure 1.
Study flow chart describing the process of recruitment and randomisation. AI, artificial intelligence; CIRCI, critical illness-related corticosteroid insufficiency; DUSP-1, dual-specificity phosphatase 1; FC, fludrocortisone; GILZ, glucocorticoid-induced leucine zipper; HC, hydrocortisone; MDW, monocyte distribution width; Resp., respiratory; SRS, sepsis response signature.
The protocol and qualification of all investigators were approved by the Ethics Committee (Comité de Protection des Personnes (CPP), Dijon, France), on 6 April 2020 and by the ANSM on 9 April 2020. Inclusion in the observational part of the trial started on 10 April 2020 in 19 centres in France and ended on 10 June 2021 with last patient follow-up on December 2021. The interventional part of the trial started on 10 June 2021, in 20 centres nationwide. Recruitment is expected to be completed by first trimester of 2025. Follow-up of the last patient is expected to be completed by last trimester of 2025.
Informed written consent of the patient is to be obtained prior to any act related to the study. Whenever the patients are unable to consent themselves, consent of a legally authorised representative is sought (France, art. L 1122-1 CSP). Whenever the patient is unable to consent and in the absence of a legally authorised representative, the ethics committee allowed for consent to be waived. Deferred consent or consent of a legally authorised representative is to be obtained for the continuation of the study procedures and utilisation of the patient’s data and biological samples.
A data safety and monitoring board (DSMB), including experts in intensive care and in infectious diseases, was set up before recruitment of the first patient. DSMB members have full access to the raw data of the trial and meet on a regular basis.
Data monitoring is performed by the sponsor (Assistance Publique-Hôpitaux de Paris (AP-HP), Paris Ile-de-France Ouest Clinical Trial Unit). The sponsor has full access to patients’ charts and checks for accuracy of all data recorded onto the electronic case report form (eCRF). Data management is performed by the sponsor.
The Biological Resource Centre of the Raymond Poincaré Hospital (AP-HP) provides centres with sample kits for biomarker measurements, collects and stores samples obtained from centres.
A central pharmacy (General Agency of Equipment and Health Products/Agence Générale des Equipements et Produits de Santé (AGEPS)) labels study drugs and ships them to participating centres. Unused drugs are destroyed by a local pharmacist.
The trial is registered at ClinicalTrials.gov under number NCT04280497.
Data access: the trial’s investigators will have access to all data and will vouch for the integrity of the data. Data will be shared within 6 months of the publication of the results of the primary analysis after trial completion. To access data, a third party will have to sign with the trial sponsor a data sharing agreement.
Study participants: inclusion and exclusion criteria, conduct of the trial
Inclusion criteria
Intensive care unit (ICU) patients aged ≥18 years will be eligible for inclusion into the trial if they meet all of the following criteria: (1) proven or suspected infection as the main diagnosis (Sepsis-3 definition)13; (2) CAP (as defined by the IDSA/ATS CAP severity criteria, table 1 of Metlay et al 14) or vasopressor-dependent sepsis (require vasopressor to maintain mean blood pressure of 65 mm Hg and lactate level of <2 mmol/L), or septic shock13 or infection-triggered ARDS (Berlin definition)15; (3) tested for one or more trial-specific biomarkers (see figure 1); (4) affiliated to the French social security or benefiting from universal health coverage. Patients under guardianship or curatorship may be included.
Table 1.
Data collection and conduct of the trial
| Variables/visits | Daily data until ICU discharge or 90 days (whichever occurs first) | Specific measures at days 1 and 7 | Last study visit (90-day and 180-day data) |
| Vital status | X | X | |
| Location (ICU/date of discharge), hospital ward/date of discharge, rehabilitation centre, long-term facility, home/date of discharge | X | ||
| Protocol adherence (receipt of every IMP dose until treatment completion) | X | X | |
| Cointerventions (mechanical ventilation, renal replacement therapy, vasopressors, unblinded corticosteroids, thiamine, vitamin C, other vitamins, nutrition, intravenous fluids, blood products, anticoagulants, sedatives, stress ulcer prophylaxis and antimicrobials) | X | X | |
| Core temperature (daily lowest and highest value) | X | X | |
| Vital signs (lowest and highest values for heart rate, systolic and diastolic blood pressure) | X | X | |
| Central haemodynamic data | X | ||
| Standard laboratory data (serum and urinary electrolytes, creatinine, urea, cholesterol, triglycerides and glucose levels, arterial lactate levels, arterial oxygen tension and haemoglobin oxygen saturation, arterial pH, white cell counts, haemoglobin and haematocrit levels, INR, platelet count, total bilirubin level) | X | ||
| Microbiological or virological samples | X | ||
| Other sampling left at the physicians’ discretion | X | ||
| Whole blood samples for measurements of biomarkers | X | ||
| Glasgow Coma Scale cognitive function | X | ||
| Muscular Disability Rating Scale score | X | ||
| Health-related quality of life (EQ-5D-5L)27 | X | ||
| PROMIS (fatigue 13a, ability to participate in social roles and activities 8a, physical function 8b, emotional distress-depression 8b, emotional distress-anxiety 8a, cognitive function 8a) | X |
ICU, intensive care unit; IMP, investigational medicinal product; INR, international normalised ratio.
Exclusion criteria
Patients will not be eligible for the trial if they meet at least one of the following criteria: (1) expected death or withdrawal of life-sustaining treatments within 48 hours, (2) previously enrolled in the study, (3) formal indication for corticosteroids according to most recent international guidelines, (4) vaccination with live virus within the past 6 months, (5) hypersensitivity to HC or FC, or microsined betamethasone dipropionate, or any of their excipients (Summary of Product Characteristics (SPC)), (6) pregnancy, women of childbearing potential not using contraception, (7) nursing women.
Study measurements and procedures
Initial study visit
After obtaining consent, a study visit is performed in order to collect (1) patient demographics; (2) characteristics of infection; (3) severity of illness (Simplified Acute Physiology Score [SAPS] II and Sepsis-related Organ Failure Assessment [SOFA] score); (4) pre-existing comorbidities as defined by the Charlson Comorbidity Index, Clinical Frailty Scale; (5) core temperature and vital signs; (6) central haemodynamic data; (7) standard laboratory data including serum and urinary electrolytes, creatinine, urea, cholesterol, triglycerides and glucose levels, arterial lactate levels, arterial oxygen tension, haemoglobin oxygen saturation, arterial pH, white cell count, haemoglobin and haematocrit levels, international normalised ratio, platelet count, total bilirubin level, microbiology and virology, results of the adrenocorticotropic hormone test; (8) measurement of biomarkers (serum endocan, monocytes expression of glucocorticoid-induced leucine zipper (GILZ) and of dual-specificity protein phosphatase 1 (DUSP-1), monocyte distribution width (MDW), and blood genomic, transcriptomic and metabolomic measurements, exhaled air metabolomic); (9) interventions including mechanical ventilation, renal replacement therapy, vasopressors, administration of open-label corticosteroid, thiamine, vitamin C, other vitamins, nutrition, intravenous fluids, blood products, anticoagulants, sedatives, stress ulcer prophylaxis and antimicrobials; (10) results of the skin vasoconstrictor response to glucocorticoids. The screening visit ends with the randomisation of eligible patients within 24 hours of obtaining the results of intelligent algorithms and biomarkers.
Endotyping
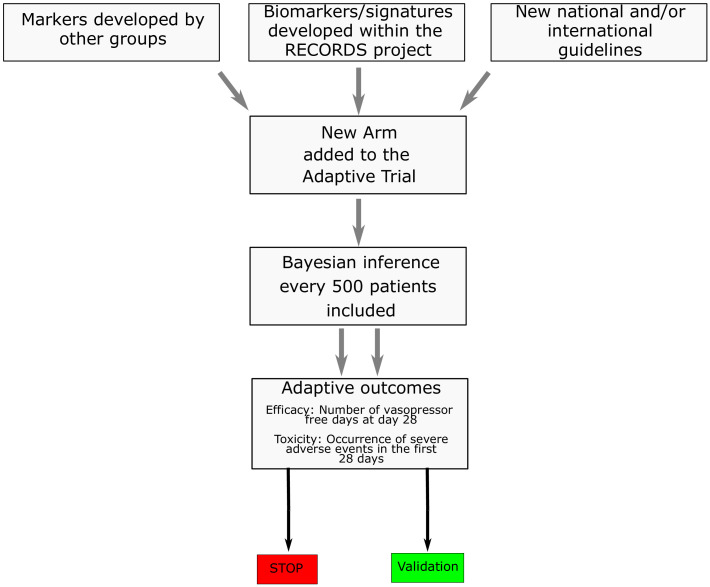
In the COVID-19 stratum, patients will be randomised in a single step (figure 1) to receive dexamethasone 6 mg per day for 10 days5 combined with FC or its placebo. The added value of a drug exhibiting a mineralocorticoid activity is worth evaluating in view of the role of the ACE2 receptor and endothelial dysfunction in the pathogenesis of COVID-19.16 The influenza virus stratum and non-influenza respiratory virus stratum aim at filling the evidence gap related to the lack of randomised trial having assessed corticotherapy in viral pneumonia-related sepsis.17 Otherwise, patients’ randomisation will be stratified according to candidate biomarkers, selected from: (1) signatures described in the literature, (2) biomarkers identified from RECORDS data obtained in the observational period of the study and (3) any national and/or international guidelines (figure 2). At the onset of the trial, 11 biomarkers/signatures had been identified (critical illness-related corticosteroid insufficiency (CIRCI),18 endocan,19 MDW,20 lymphocyte count,20 transcriptome sepsis response signature (SRS),10 21 adaptive immunity signature (endotype A/B),11 22 GILZ,23 DUSP-1, cutaneous vasoconstrictor to glucocorticoids,24 machine learning algorithm 1,12 machine learning algorithm 2), as described below. The CIRCI stratum is defined by baseline total cortisol of 10 µg/dL or less or a maximum increment in total cortisol of less than 9 µg/dL at 30 and 60 min following a 250 µg intravenous bolus of cosyntropin.18 Endocan is an endothelial peptidoglycan that contributes to regulate inflammation by counteracting leucodiapesis, a key target for corticosteroids.19 We identified serum levels of endocan below 12.8 ng/mL as a marker of hyperinflammation in the run-in period of RECORDS trial. The MDW stratum is defined by MDW >25.20 The lymphocyte count stratum is defined by lymphocyte count below 870×103/mL, according to a recent study suggesting that lymphopenia was associated with corticosteroid resistance.20 The transcriptomic SRS stratum is based on two distinct signatures suggesting immune suppression (Sepsis Response Signature [SRS]1) and relative immune competency (SRS2).21 Using generalised linear model based on a set of seven genes (DYRK2, CCNB1IP1, TDRD9, ZAP70, ARL14EP, MDC1 and ADGRE3), HC may be associated with increased mortality in SRS2 patients.10 The endotype A/B stratum is based on transcriptomic analysis of 100 genes reflecting the adaptive immunity and glucocorticoid receptor signalling. In children with sepsis, two distinct endotypes were identified, endotype A characterised by immune suppression and a higher risk of mortality when compared with endotype B.22 The GILZ stratum and DUSP-1 stratum are defined by reduced expression of GILZ and DUSP-1 by unstimulated isolated circulating monocytes. GILZ23 and DUSP-1 are key endogenous regulators of the immune response. Preliminary data from the observational period of RECORDS suggested an association between the spontaneous expression of GILZ and of DUSP-1 and increased mortality in serious COVID-19 (unpublished). The cutaneous vasoconstrictor response to glucocorticoids stratum is defined according to previous report in asthma.24 Briefly, the test is performed by applying 30 µg/mL betamethasone to the skin of the forearm. The degree of skin blanching is assessed after 12 hours of exposure with a score ranging from 0 to 4: 0 no skin blanching, 1 skin blanching is less than 50% of the area of application, 2 skin blanching is more than 50% of the area of application, 3 if blanching recovers the whole area of application and 4 if blanching expands beyond the area of application. A score of 2 or less indicates resistance to corticosteroids. A photography of the forearm restricted to the tested skin area will be recorded. The intelligent algorithms strata include two algorithms aiming at predicting the response to corticosteroids, one developed through machine learning12 and another one using other machine learning modelling approaches (unpublished), both being embedded into the eCRF.
Figure 2.
Flow diagram describing the potential addition and subsequent validation of biomarkers during the trial. RECORDS, Rapid rEcognition of COrticosteRoiD resistant or sensitive Sepsis.
Follow-up
All patients are followed up at 3 and 6 months from randomisation. The details of follow-up are given in table 1.
Vital status by day 90 after randomisation will be collected from the medical file and/or through phone calls with patients or their legal representative.
Outcomes
The primary outcome for the sequential analyses will be the number of vasopressor-free days at day 28 as the measure of efficacy, and the occurrence of severe adverse events within the first 28 days as the measure of toxicity.
The primary outcome of the terminal analysis, assessed at 90 days, is a composite of death or persistent organ dysfunction—defined as SOFA score >6 or a continued dependency on either mechanical ventilation, new renal replacement therapy or vasopressors.8 25 The primary endpoint is a binary composite variable. Patients will be classified as a success if alive on day 90 and free of vasopressor therapy, mechanical ventilation, renal replacement therapy and organ dysfunction. Patients will be classified as a failure if (1) death occurred within the 90 days following randomisation, (2) dependent on either vasopressor, mechanical ventilation, renal replacement therapy or (3) exhibited a SOFA score >6.
Secondary outcomes include: (1) mortality at 7, 14 and 28 days and 6 months; (2) vasopressor-free days: defined as the number of days with permanent haemodynamic stability in the absence of any vasopressor agent including norepinephrine, phenylephrine, epinephrine, dopamine, vasopressin or its analogues. When a patient dies while receiving vasopressors, the number of vasopressor-free days is arbitrarily set at 0; (3) mechanical ventilation-free days: defined as the number of days with permanent appropriate oxygenation while the patient is extubated and breathing spontaneously, that is, no need for non-invasive ventilation, high-flow oxygen or continuous positive airway pressure. Other uses of non-invasive ventilation (eg, chronic night-time use for chronic obstructive pulmonary disease) are not compatibilised. When a patient dies while receiving mechanical ventilation or is discharged home while receiving mechanical ventilation, the number of mechanical ventilation-free days is arbitrarily set at 0; (4) organ dysfunction-free days: organ function (including renal function) will be assessed using the SOFA score.26 Organ dysfunction will be defined by a SOFA score >6.8 Organ dysfunction-free days is defined by the number of days with a total SOFA score of 6 or less. When a patient dies before the SOFA decreased to 6 or less, the number of organ dysfunction-free days is arbitrarily set at 0; (5) 6-month health-related quality of life (HRQoL) in survivors assessed using the EQ-5D-5L.27 This standardised questionnaire was developed to provide a simple, generic measure of health for clinical and economic appraisal. It is made up of two components: health status description and health status self-assessment. Health status is assessed using five dimensions; mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Health status self-assessment reports on patients’ overall health status using a Visual Analogue Scale; (6) fatigue, ability to partake in social activities, physical function, emotional distress, depression, anxiety and cognitive function are assessed using the PROMIS short-form questionnaire; (7) proportion of patients with a decision to withhold and/or withdraw active treatments; (8) ICU and hospital length of stay; (9) rate of ICU readmission up to 180 days after randomisation.
Safety endpoints include: proportion of patients affected by any serious adverse event associated with corticosteroids, (1) hospital-acquired infection (Center for Diseases Control [CDC], Healthcare-Associated Infections progress report, 2020) within 90 days of randomisation; (2) hyperglycaemia (blood glucose level >150 mg/dL) and hypernatraemia (serum sodium >145 mmol/L) during the ICU stay (or up to day 90, whichever occurs first); (3) gastroduodenal bleeding requiring transfusion or haemostatic treatment during the ICU stay (or up to day 90, whichever occurs first); (4) neurological disorder (coma, stroke or muscle weakness, as defined below) up to 180 days from randomisation. Coma is defined by a Glasgow Coma Score <8 in the absence of sedation. Neuromuscular sequelae are assessed using the Muscular Disability Rating Scale, with a score of 1 indicating no deficit, 2 minor deficit with no functional disability, 3 distal motor deficit, 4 mild-to-moderate proximal motor deficit and 5 severe proximal motor deficit.
Randomisation
The period from inclusion to randomisation, including the period of laboratory testing for biomarkers, should not exceed 24 hours.
A computer process is used to generate allocation sequences in a 1:1 ratio, independently from healthcare staff, research personnel, ICU personnel, participants, members of the Executive and Steering Committees, or the data analyst.
Randomisation will be concealed by being centralised and performed with an internet-centralised service running 24/7 imbedded in the eCRF. Healthcare staff, research personnel, ICU personnel, participants, members of the Executive and Steering Committees, and the data analyst will be blinded to treatment allocation. Only the central pharmacist, from the AGEPS–AP-HP will be unblinded.
Allocation of stratification
Stratification of randomisation between the two trial arms first concerns the SARS-CoV-2 results, generating a separate 1:1 randomisation list for patients with COVID-19 between FC and its placebo, given all treated with dexamethasone. The second list concerns the influenza-positive patients, between HC and FC versus their respective placebos. SARS-CoV-2-negative patients with influenza are then randomly allocated to a randomisation list among all assessed biomarkers for each patient based on data and strata cut-offs, and then randomised to HC and FC or their respective placebos according to the list of the biomarker/signature result. This will ensure the balance of treatment groups among each biomarker/signature stratum.
Centres are not required to assess all biomarkers for a given patient. The randomisation algorithm will run on all the available biomarkers (a single biomarker is sufficient). The randomisation algorithm runs separately while communicating with the eCRF. Data related to biomarker results, virology results and input required for determining intelligent algorithms are sent to the randomisation algorithm.
Interventions
Experimental treatments
Investigational medicinal products are presented in numbered boxes, labelled for this study according to the Good Manufacturing Practices under the responsibility of AGEPS. Each numbered box contains enough corticosteroids or placebo to fully treat one patient. A drug box contains 30 vials of HC 100 mg or placebo and 1 blister of 10 tablets of FC 50 µg or placebo.
HC hemisuccinate (SERB, Paris) or its placebo is administered as a 50 mg intravenous bolus every 6 hours for 7 days. FC or its placebo (HAC Pharma, Caen, France) is administered as a 50 µg tablet via a nasogastric tube once a day in the morning, for 7 days. Patients with COVID-19 are to be treated with open-label dexamethasone (6 mg) once a day over 10 days and 50 µg FC or its placebo via a nasogastric tube or orally for patients not requiring a nasogastric tube, once a day in the morning, for 7 days.
Allowed cointerventions
Treatments are allowed according to best practice guidelines.28 These treatments may include mechanical ventilation, renal replacement therapy, vasopressor therapy, thiamine, nutrition therapies including multivitamins, intravenous fluids, blood products, sedatives and antimicrobials. We will carefully record the use of these treatments. All recommended treatments (including interleukin-6 inhibitors and JAK inhibitors) in severe COVID-19 are authorised to be used in this trial.
Forbidden interventions
HC and other corticosteroids, whatever the dose and the route (except local administration, nebulisation not being considered as a local administration), are not allowed, except dexamethasone 6 mg per day for 10 days in patients with COVID-19. If an unavoidable indication occurs after randomisation (eg, autoimmune disease), the patient should be treated. In this case, study treatments are to be suspended to avoid the administration of unnecessarily high dose of HC. However, short-duration administration of open-labelled corticosteroids is allowed for prevention of post-extubation laryngeal oedema.
Continuous infusion of neuromuscular blocking agents is to be avoided. Whenever neuromuscular blocking drugs are mandatory (eg, for severe ARDS), it should be interrupted every 12 hours to avoid prolonging the treatment longer than necessary.
Statistical analysis
A full statistical analysis plan will be reported in a separate paper.
Sample size
Sequential analyses will use the number of vasopressor-free days at day 28 as the measure of efficacy, and the occurrence of severe adverse events within the first 28 days as the measure of toxicity. In a recent study,8 the number of vasopressor-free days at day 28 with HC+FC was 17.1±10.8 vs 15.0±11.1 in placebos. Thus, a sample of 176×2=352 patients achieves 80.04% power to reject the null hypothesis of equal means when the mean difference between arms is 3 days with SD of 10 days, and with a significant level (alpha) of 0.05 using a two-sample t-test. For interactions to be detected with the same power as the overall effect, sample sizes should be inflated,29 estimated here at a multiple of fourfold; therefore, to handle potential dropouts, a sample was estimated at 1800 patients. Bayesian inference will be used for sequential analyses.
To detect a 10% absolute risk reduction from 45% to 35% in 90-day all-cause mortality,8 a sample size of 373 evaluable patients per arm (thus a total of 746 patients) is required to reach 80% power. The planned sample of 1800 patients will achieve a 99.16% power to reject the null hypothesis of equal mortality when the difference between arms is 10% overall, and with a 5% alpha level. Sample sizes were computed using PASS V.15 software (2017).
The power to detect this difference within each group of analysis will depend on the prevalence of each biomarker of interest.
Performance of the Bayesian design
To restrict inclusion to patients most likely to benefit from corticosteroids during trial accrual, an enrichment design will be used, based on treatment-by-subset interaction. Two main objectives will be considered: (1) to estimate corticosteroid marginal effect; and (2) to test for heterogeneity across subsets. We previously reported that this strategy successfully selected patients sensitive to treatment and discarded the less sensitive ones.30 Performance of design will be challenged using a large simulation study performed across a range of plausible scenarios to foresee power to detect a 5%–10% absolute difference with corticosteroids, considering a fixed sample size of 1800 patients, several sizes of each subset, under several scenarios of treatment effect and interaction.
Data analysis
All patients, whatever their biomarker status, will be randomised across the two treatment groups. Statistical analysis will be conducted using ‘biomarker-by-treatment interaction’ with separate tests, given the high number of potential biomarkers of interest. This is also referred to as ‘separate randomisation designs’ and ‘separate-by-treatment interaction designs’.
The analysis plan will be based on separate estimation of corticosteroid effect in each biomarker-defined stratum to detect treatment by subgroup interactions: in other words, it aims at determining whether the corticosteroid effect may differ according to each biomarker/signature result, assessed through an interaction measure.
We will assess subset-by-treatment interaction by estimating two quantities30: (1) measure of influence (efficacy), relying on the value of the estimation of corticosteroid effect in each subset, and (2) measure of interaction, either based on the Gail and Simon statistics or derived from the Millen et al’s criteria based on the ratio of the estimated effect in both subsets.31 In a Bayesian setting, the two criteria can be expressed as posterior probabilities related to the comparison of outcomes across the arms and/or the subsets. Decision thresholds will be defined based on a grid search to optimise the rate of false positive and false negative findings based on large simulated trials.
This analysis will be performed only for biomarkers with at least 100 available measures. Main interaction measures will consider only patients randomly allocated to the specific strata under study. As sensitivity analyses, we will also consider all the patients with the available biomarker, possibly handling imbalances across treatment arms using propensity score-based approaches.
Analysis of secondary outcomes will be performed at the time of terminal analysis. Mortality will be estimated by the Kaplan-Meier method, compared by the log-rank test across groups. Vasopressor-free days, mechanical ventilation-free days and organ dysfunction-free days will be compared by the non-parametric Wilcoxon rank-sum test. HRQoL will be analysed by a joint mixed model for longitudinal and survival data, that is, a shared parameter model where the HRQoL and survival models share common random effect(s).
Details of statistical analysis will be reported on a statistical analysis plan.
Role of funding source
The sponsor has no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Ethics and dissemination
RECORDS is funded by public grants ‘Programme d’Investissements d’Avenir’, part of ‘France 2030’ (reference ANR-18-RHUS-0004) and Programme Hospitalier de Recherche Clinique (reference PHRC-20-0778). The protocol was approved by the Ethics Committee (CPP, Dijon, France), on 6 April 2020 and by the ANSM on 9 April 2020. Trial results will be disseminated at relevant clinical conferences and societies, published in peer-reviewed journals.
Patient and public involvement
The trial protocol will be discussed with France Sepsis Association (an association of patients who recover from sepsis and families of patients who had sepsis) and trial findings will be shared with this entity.
Discussion
RECORDS is the first multicentre, placebo-controlled, biomarker-guided, adaptive Bayesian design basket trial aimed at discovering signatures enabling administration of HC plus FC in patients with sepsis and determining the best chance for improved outcome with minimal risk of harm. RECORDS trial relies on the concept that benefit-to-risk balance of corticosteroids is greater when given to patients with sepsis and evidence of hyperinflammation and intact corticosteroid-related intracellular signalling. The RECORDS trial also hypothesises that different clinical or biological phenotypes of sepsis, that is, CAP-related sepsis, septic shock, or sepsis-related ARDS, bacterial or viral sepsis, may share common signatures relevant to corticosteroid responsiveness. The study design is highly innovative, integrating a broad range of candidate biomarkers from multiomics signatures to intelligent algorithms. Candidate biomarkers are to be selected from various sources including peer-reviewed literature and from our own consortium, thanks to the translational research programme embedded into the RECORDS trial. The adaptive design of the study is a clear strength insomuch as unsatisfying biomarkers may rapidly be phased out and new biomarkers may be phased in. All assessed biomarkers must provide readily available results within 24 hours, meaning that the findings of the current trial will be easily translated into clinical practice. We must acknowledge some limits to the current trial including the fact that some complex biomarkers may not be available at all participating centres.
RECORDS trial is a major step in the implementation of precision medicine in sepsis. Indeed, personalised corticosteroid treatments for sepsis may decrease the burden related to corticosteroid complications by avoiding exposure of patients unlikely to respond to this treatment.
Supplementary Material
Footnotes
Twitter: @a6tole, @Quenot
Correction notice: The article was corrected since it was first published. The Author list is updated with new author
Lamiae Grimaldi.
Collaborators: RECORDS consortium: Alexandrou Antigoni, Annane Djillali, Arlt Birte, Badie Julio, Benghanem Sarah, Berdaguer Ferrari Fernando, Cerf Charles, Chelly Dagdia Zaineb, Chevret Sylvie, Colin Gwenhaël, Daniel Christel, Declercq Pierre-Louis, Delbove Agathe, Devillier Philippe, Fleuriet Jérome, François Bruno, Garchon Henri-Jean, Godot Véronique, Grassin-Delyle Stanislas, Grisolia Mathieu, Guitton Christophe, Helms Julie, Heming Nicholas, Herzog Marielle, Kamel Toufik, Kedad Zoubida, Lassalle Philippe, Lhermite Guillaume, Megarbane Bruno, Mekontso Dessap Armand, Mercier Emmanuelle, Meziani Ferhat, Mira Jean-Paul, Monchi Mehran, Monnet Xavier, Muller Grégoire, Plantefève Gaëtan, Quenot Jean-Pierre, Reignier Jean, Robine Adrien, Rottman Martin, Roux Anne-Laure, Schneider Francis, Siami Shidasp, Tissieres Pierre, Troché Gilles, Uhel Fabrice, Zeitouni Karine. CRICS TRIGGERSEP network: Laetitia Bodet-Contentin, Walid Darwiche, Stephan Ehrmann, Denis Garot, Antoine Guillon, Youenn Jouan, Annick Legras, Julie Mankikian, Emmanuelle Mercier, Marlene Morisseau, Yonatan Perez, Emmanuelle Rouve, Charlotte Salmon-Gandonniere, Julie Helms, Hassene Rahmani, Alexandra Monnier, Hamid Merdji, Raphael Clere-Jehl, Laure Stiel, Antoine Studer, Pascal Andreu, Jean-Baptiste Roudaut, Marie Labruyere, Marine Jacquier, Francois Barbier, Dalila Benzekri, Thierry Boulain, Sophie Jacquier, Armelle Mathonnet, Gregoire Muller, Mai-Anh Nai, Isabelle Runge, Sophie Tollec, Damien Roux, Jonathan Messika, Constance Vuillard, Louis-Marie Dumont, Laura Federici, Noemie Zucman, Marc Amouretti, Djillali Annane, Pierre Moine, Paris Meng, Rania Bounab, Muriel-Sarah Fartoukh, Michel Djibre, Alexandre Elabbadi, Marie-Ange Azais, Konstantinos Bachoumas, Arthur Bailly, Remi Bernardon, Gauthier Blonz, Luc Desmedt, Brian Emonet, Maud Fiancette, Matthieu Henry, Jean-Claude Lacherade, Jean-Baptiste Lascarrou, Christine Lebert, Julien Lorber, Laurent Martin-Lefevre, Caroline Pouplet, Isabelle Vinatier, Aihem Yehia, Sarah Benghanem, Julien Charpentier, Clara Vigneron, Nicolas Pichon, Anne-Laure Fedou, Claire Mancia, Emmanuelle Begot, Thomas Daix, Philippe Vignon, Antoine Galy, Celine Gonzalez, Marine Goudelin, Bruno Evrard, Arnaud Desachy, Julien Vaidie, Guillaume Gilbert, Cedric Darreau, Benoit Derrien, Marjorie Saint-Martin, Patrice Tirot, Mickael Landais, Nicolas Chudeau, Jean Christophe Callahan, Dominique Vivier, Charlene Le Moal, Pierre-Yves Olivier, Remy Marnai, Francis Schneider, Nicolas Sedillot, Xavier Tchenio, Adrien Robine, Yves Poncelin, Remi Bruyere.
Contributors: DA, SC, JF and NH have designed the study. SC is responsible for statistical analysis plan. DA has obtained funding for the study. DA, SC, JF and NH have drafted this manuscript. FM, JR, P-LD, EM, GM, GC, XM, AR, SS, FU, J-PQ, GP, JB, FS, CC, GT, MM, J-PM and BF contributed to critical evaluation of the study design, and of the manuscript of which they approved the final version and its submission for publication. All authors gave their agreement to be accountable for all aspects of the work, and ensure the accuracy and integrity of any part of it.
Funding: RECORDS is funded by public grants 'Programme d’Investissements d’Avenir' (PIA), part of 'France 2030' (reference ANR-18-RHUS-0004) and French Ministry of Health Programme Hospitalier de Recherche Clinique (PHRC) (contract number PHRC-20-0778).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
RECORDS consortium:
Alexandrou Antigoni, Annane Djillali, Arlt Birte, Badie Julio, Benghanem Sarah, Berdaguer Ferrari Fernando, Cerf Charles, Chelly Dagdia Zaineb, Chevret Sylvie, Colin Gwenhaël, Daniel Christel, Declercq Pierre-Louis, Delbove Agathe, Devillier Philippe, Fleuriet Jérome, François Bruno, Garchon Henri-Jean, Godot Véronique, Grassin-Delyle Stanislas, Grisolia Mathieu, Guitton Christophe, Helms Julie, Heming Nicholas, Herzog Marielle, Kamel Toufik, Kedad Zoubida, Lassalle Philippe, Lhermite Guillaume, Megarbane Bruno, Mekontso Dessap Armand, Mercier Emmanuelle, Meziani Ferhat, Mira Jean-Paul, Monchi Mehran, Monnet Xavier, Muller Grégoire, Quenot Jean-Pierre, Reignier Jean, Robine Adrien, Rottman Martin, Roux Anne-Laure, Schneider Francis, Siami Shidasp, Tissieres Pierre, Troché Gilles, Uhel Fabrice, Zeitouni Karine, Plantefève Gaëtan, Derridj Nawal, Grimaldi Lamiae, CRICS TRIGGERSEP network, Laetitia Bodet-Contentin, Walid Darwiche, Stephan Ehrmann, Denis Garot, Antoine Guillon, Youenn Jouan, Annick Legras, Julie Mankikian, Emmanuelle Mercier, Marlene Morisseau, Yonatan Perez, Emmanuelle Rouve, Charlotte Salmon-Gandonniere, Julie Helms, Hassene Rahmani, Alexandra Monnier, Hamid Merdji, Raphael Clere-Jehl, Laure Stiel, Antoine Studer, Pascal Andreu, Jean-Baptiste Roudaut, Marie Labruyere, Marine Jacquier, Francois Barbier, Dalila Benzekri, Thierry Boulain, Sophie Jacquier, Armelle Mathonnet, Gregoire Muller, Mai-Anh Nai, Isabelle Runge, Sophie Tollec, Damien Roux, Jonathan Messika, Constance Vuillard, Louis-Marie Dumont, Laura Federici, Noemie Zucman, Marc Amouretti, Djillali Annane, Pierre Moine, Paris Meng, Rania Bounab, Muriel-Sarah Fartoukh, Michel Djibre, Alexandre Elabbadi, Marie-Ange Azais, Konstantinos Bachoumas, Arthur Bailly, Remi Bernardon, Gauthier Blonz, Luc Desmedt, Brian Emonet, Maud Fiancette, Matthieu Henry, Jean-Claude Lacherade, Jean-Baptiste Lascarrou, Christine Lebert, Julien Lorber, Laurent Martin-Lefevre, Caroline Pouplet, Isabelle Vinatier, Aihem Yehia, Sarah Benghanem, Julien Charpentier, Clara Vigneron, Nicolas Pichon, Anne-Laure Fedou, Claire Mancia, Emmanuelle Begot, Thomas Daix, Philippe Vignon, Antoine Galy, Celine Gonzalez, Marine Goudelin, Bruno Evrard, Arnaud Desachy, Julien Vaidie, Guillaume Gilbert, Cedric Darreau, Benoit Derrien, Marjorie Saint-Martin, Patrice Tirot, Mickael Landais, Nicolas Chudeau, Jean Christophe Callahan, Dominique Vivier, Charlene Le Moal, Pierre-Yves Olivier, Remy Marnai, Francis Schneider, Nicolas Sedillot, Xavier Tchenio, Adrien Robine, Yves Poncelin, and Remi Bruyere
Ethics statements
Patient consent for publication
Not required.
References
- 1. Sepsis. n.d. Available: https//www.who.int/health-topics/sepsistabtab1
- 2. Heming N, Sivanandamoorthy S, Meng P, et al. Immune effects of corticosteroids in sepsis. Front Immunol 2018;9:1736. 10.3389/fimmu.2018.01736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids for treating sepsis in children and adults. Cochrane Database Syst Rev 2019;12:CD002243. 10.1002/14651858.CD002243.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lamontagne F, Rochwerg B, Lytvyn L, et al. Corticosteroid therapy for sepsis: a clinical practice guideline. BMJ 2018;362:k3284. 10.1136/bmj.k3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dexamethsone in hospitalized patients with COVID-19. NEJM 2021:384. 10.1056/NEJMc2035374 [DOI] [Google Scholar]
- 6. Annane D, Sébille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002;288:862–71. 10.1001/jama.288.7.862 [DOI] [PubMed] [Google Scholar]
- 7. Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med 2008;358:111–24. 10.1056/NEJMoa071366 [DOI] [PubMed] [Google Scholar]
- 8. Annane D, Renault A, Brun-Buisson C, et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med 2018;378:809–18. 10.1056/NEJMoa1705716 [DOI] [PubMed] [Google Scholar]
- 9. Venkatesh B, Finfer S, Cohen J, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med 2018;378:797–808. 10.1056/NEJMoa1705835 [DOI] [PubMed] [Google Scholar]
- 10. Antcliffe DB, Burnham KL, Al-Beidh F, et al. Transcriptomic signatures in sepsis and a differential response to steroids. from the VANISH randomized trial. Am J Respir Crit Care Med 2019;199:980–6. 10.1164/rccm.201807-1419OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong HR, Cvijanovich NZ, Anas N, et al. Developing a clinically feasible personalized medicine approach to pediatric septic shock. Am J Respir Crit Care Med 2015;191:309–15. 10.1164/rccm.201410-1864OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pirracchio R, Hubbard A, Sprung CL, et al. Assessment of machine learning to estimate the individual treatment effect of corticosteroids in septic shock. JAMA Netw Open 2020;3:e2029050. 10.1001/jamanetworkopen.2020.29050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singer M, Deutschman CS, Seymour CW, et al. The third International consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315:801–10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American thoracic Society and infectious diseases Society of America. Am J Respir Crit Care Med 2019;200:e45–67. 10.1164/rccm.201908-1581ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:2526–33. 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 16. Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020;26:1017–32. 10.1038/s41591-020-0968-3 [DOI] [PubMed] [Google Scholar]
- 17. Lansbury L, Rodrigo C, Leonardi-Bee J, et al. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev 2019;2:CD010406. 10.1002/14651858.CD010406.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Annane D, Pastores SM, Rochwerg B, et al. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of critical care medicine (SCCM) and European Society of intensive care medicine (esicm) 2017. Intensive Care Med 2017;43:1751–63. 10.1007/s00134-017-4919-5 [DOI] [PubMed] [Google Scholar]
- 19. Gaudet A, Parmentier E, Dubucquoi S, et al. Low endocan levels are predictive of acute respiratory distress syndrome in severe sepsis and septic shock. J Crit Care 2018;47:121–6. 10.1016/j.jcrc.2018.06.018 [DOI] [PubMed] [Google Scholar]
- 20. MOCORSEP Study Group . Monocyte distribution width as a biomarker of resistance to corticosteroids in patients with sepsis: the MOCORSEP observational study. Intensive Care Med 2021;47:1161–4. 10.1007/s00134-021-06478-z [DOI] [PubMed] [Google Scholar]
- 21. Davenport EE, Burnham KL, Radhakrishnan J, et al. Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Respir Med 2016;4:259–71. 10.1016/S2213-2600(16)00046-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong HR, Cvijanovich NZ, Anas N, et al. Endotype transitions during the acute phase of pediatric septic shock reflect changing risk and treatment response. Crit Care Med 2018;46:e242–9. 10.1097/CCM.0000000000002932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ellouze M, Vigouroux L, Tcherakian C, et al. Overexpression of GILZ in macrophages limits systemic inflammation while increasing bacterial clearance in sepsis in mice. Eur J Immunol 2020;50:589–602. 10.1002/eji.201948278 [DOI] [PubMed] [Google Scholar]
- 24. Brown PH, Teelucksingh S, Matusiewicz SP, et al. Cutaneous vasoconstrictor response to glucocorticoids in asthma. Lancet 1991;337:576–80. 10.1016/0140-6736(91)91639-c [DOI] [PubMed] [Google Scholar]
- 25. Laterre P-F, Berry SM, Blemings A, et al. Effect of selepressin vs placebo on ventilator- and vasopressor-free days in patients with septic shock: the SEPSIS-ACT randomized clinical trial. JAMA 2019;322:1476–85. 10.1001/jama.2019.14607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vincent JL, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. on behalf of the Working group on sepsis-related problems of the European Society of intensive care medicine. Intensive Care Med 1996;22:707–10. 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 27. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021;47:1181–247. 10.1007/s00134-021-06506-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brookes ST, Whitely E, Egger M, et al. Subgroup analyses in randomized trials: risks of subgroup-specific analyses; power and sample size for the interaction test. J Clin Epidemiol 2004;57:229–36. 10.1016/j.jclinepi.2003.08.009 [DOI] [PubMed] [Google Scholar]
- 30. Vinnat V, Chevret S. Enrichment bayesian design for randomized clinical trials using categorical biomarkers and a binary outcome. BMC Med Res Methodol 2022;22:54. 10.1186/s12874-022-01513-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Millen BA, Dmitrienko A, Song G. Bayesian assessment of the influence and interaction conditions in multipopulation tailoring clinical trials. J Biopharm Stat 2014;24:94–109. 10.1080/10543406.2013.856025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.