Abstract
Introduction
Radical cystectomy (RC) is the standard treatment for patients with non-metastatic muscle-invasive bladder cancer, as well as for patients with therapy refractory high-risk non-muscle invasive bladder cancer. However, 50–65% of patients undergoing RC experience perioperative complications. The risk, severity and impact of these complications is associated with a patient’s preoperative cardiorespiratory fitness, nutritional and smoking status and presence of anxiety and depression. There is emerging evidence supporting multimodal prehabilitation as a strategy to reduce the risk of complications and improve functional recovery after major cancer surgery. However, for bladder cancer the evidence is still limited. The aim of this study is to investigate the superiority of a multimodal prehabilitation programme versus standard-of-care in terms of reducing perioperative complications in patients with bladder cancer undergoing RC.
Methods and analysis
This multicentre, open label, prospective, randomised controlled trial, will include 154 patients with bladder cancer undergoing RC. Patients are recruited from eight hospitals in The Netherlands and will be randomly (1:1) allocated to the intervention group receiving a structured multimodal prehabilitation programme of approximately 3–6 weeks, or to the control group receiving standard-of-care. The primary outcome is the proportion of patients who develop one or more grade ≥2 complications (according to the Clavien-Dindo classification) within 90 days of surgery. Secondary outcomes include cardiorespiratory fitness, length of hospital stay, health-related quality of life, tumour tissue biomarkers of hypoxia, immune cell infiltration and cost-effectiveness. Data collection will take place at baseline, before surgery and 4 and 12 weeks after surgery.
Ethics and dissemination
Ethical approval for this study was granted by the Medical Ethics Committee NedMec (Amsterdam, The Netherlands) under reference number 22–595/NL78792.031.22. Results of the study will be published in international peer-reviewed journals.
Trial registration number
Keywords: Urological tumours, REHABILITATION MEDICINE, Nutritional support, SURGERY
Strengths and limitations of this study
This is a multicentre randomised controlled trial investigating the effects of multimodal prehabilitation in an understudied group of patients with bladder cancer undergoing radical cystectomy.
This data collected in this study enables exploration of the effects of prehabilitation on tumour hypoxia and immune cell infiltration.
The intervention accounts for heterogeneity in cancer treatment by offering additional support during neoadjuvant treatment when appropriate.
This study includes a cost-effectiveness analysis from a societal perspective.
Due to the two-group design and the multimodal intervention, it will not be possible to disentangle the independent effects of physical exercise training, nutritional support, psychological counselling and smoking cessation.
Introduction
Bladder cancer is the 10th most common diagnosed cancer worldwide, with over 573 000 new patients and 213 000 deaths each year.1 Radical cystectomy (RC) is the standard treatment for patients with non-metastatic muscle-invasive bladder cancer,2 3 as well as for patients with therapy refractory high-risk non-muscle invasive bladder cancer.4 RC is a challenging and costly surgical procedure with high morbidity and mortality rates5: 50–65% of the patients experience perioperative complications, of which 10–20% are high-grade.6–8 Low cardiorespiratory fitness,9 10 poor nutritional status,11 the presence of anxiety and depression12 and smoking13 increase the risk of perioperative complications, length of hospital stay and the associated medical costs.9 10 In daily clinical practice, 25% of patients with muscle-invasive bladder cancer receive neoadjuvant treatment, which might further impair preoperative cardiorespiratory fitness and nutritional status.14–17
Emerging evidence has identified the preoperative period as a window of opportunity to address lifestyle. Multimodal preoperative interventions, including physical exercise training, nutritional support, psychological counselling and smoking cessation, that aim to increase a patient’s tolerance to surgery, reduce the incidence, severity and impact of complications, accelerate and improve the quality of recovery and improve quality of life have been termed prehabilitation.
To date, no adequately powered trial has been performed to establish the effectiveness of a multimodal prehabilitation programme for reducing the incidence, severity and impact of complications and costs associated with RC for bladder cancer. A few studies investigated the impact of a single modal18 19 or multimodal20 21 prehabilitation programme on functional recovery following RC. A phase I/II trial with 54 patients showed improvement in patient-reported quality of life after 4 weeks of a supervised physical exercise training programme before surgery.19 A feasibility randomised controlled trial with 60 patients provided evidence that a 3–6 weeks supervised vigorous aerobic exercise training programme before surgery led to improvements in cardiorespiratory fitness parameters and possibly fewer surgical complications.18 A randomised controlled trial with 107 patients demonstrated improvement in walking distance during 7 days after surgery20 and improved muscle power.22 Finally, a randomised controlled trial with 70 patients showed significantly better functional capacity in the intervention group that followed a multimodal programme compared with the control group, at 4 weeks after surgery.21 However, the physical exercise intervention in these studies including patients with bladder cancer had limitations with regard to therapeutic validity23 due to a short duration of the intervention,20 22 relatively low exercise intensity20–22 or only patient-reported compliance with the exercise intervention.20–22
Preclinical studies indicate that exercise may directly affect tumour characteristics such as normalisation of tumour vasculature and immune cell infiltration, thereby enhancing tumour perfusion and reducing hypoxia.24 25 These factors are associated with treatment efficacy and survival.26–28 The current study provides a unique opportunity to explore the effects of prehabilitation on tumour inflammation and hypoxia markers, which has not yet been studied in human patients.
Study objectives
The ENHANCE study is designed to investigate the effectiveness of a multimodal prehabilitation programme versus standard-of-care in patients approaching RC. The primary aim is to investigate the superiority of a multimodal prehabilitation programme in terms of reducing one or more grade ≥2 perioperative complications within 90 days in patients with bladder cancer undergoing RC. Secondary outcomes include changes in preoperative cardiorespiratory fitness, length of hospital stay, health-related quality of life, tumour tissue biomarkers of hypoxia, immune cell infiltration and cost-effectiveness.
Methods and analysis
The ENHANCE study is a multicentre, open label, two-arm randomised controlled trial. Patient inclusion and data collection started in August 2022. Ethical approval, extending to all participating centres, was granted by the Medical Ethical Committee NedMec (Amsterdam, The Netherlands), under reference number 22–595/NL78792.031.22. The trial is prospectively registered in Clinical Trials on 29 July 2022.
Study population
The study aims to include 154 patients who meet the following inclusion criteria: aged ≥18 years, histologically confirmed, primary, bladder cancer (cTa-4N0-3M0) and planned to undergo RC. Surgery will not be delayed in favour of prehabilitation. Hence, patients who are scheduled for surgery within 3 weeks are not eligible for the trial. Patients who express the intention to follow a similar exercise training programme regardless of randomisation outcome, patients with severe cognitive or psychiatric disorders, patients with a contraindication to perform physical exercise training or a cardiopulmonary exercise test (CPET) and patients unable to read or understand the Dutch language will also be excluded.
Recruitment and randomisation
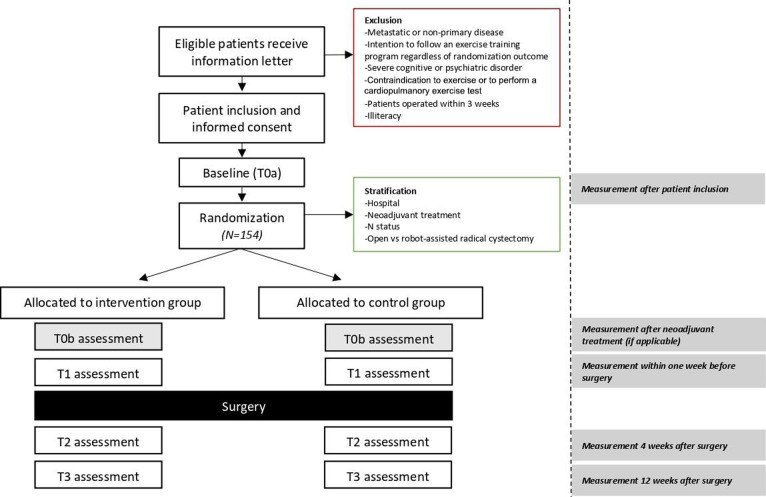
Patients are recruited from eight academic or teaching hospitals across different regions in The Netherlands (Catharina Hospital, Erasmus University Medical Center, Maastricht University Medical Center+, Noordwest Hospital Group, Radboud University Medical Center, Rijnstate Hospital, University Medical Center Groningen and University Medical Center Utrecht). The Netherlands Cancer Institute is the coordinating centre. After establishment of diagnosis and indication for surgery, the urologist or nurse specialist invites patients to participate in the study and provides the patient information letter. The study coordinator contacts these patients to provide further oral information and answer potential questions about the study. When a patient agrees to participate, written informed consent is obtained. After collection of baseline data, patients are randomised in a 1:1 ratio, using a minimisation algorithm29 aimed to achieve optimal balance between the two study arms with regard to the recruiting hospital, neoadjuvant treatment (yes/no), nodal status (N0/N1-3) and type of surgery (open/robot-assisted RC). Minimisation is done using the Minirand package in R V.4.0.4.30 31 The algorithm includes a random component to ensure blinding of treatment allocation. Due to the nature of the intervention, blinding of participants and research investigators is not possible. After randomisation, patients in both the intervention and control groups receive a leaflet with recommendations on physical activity, diet and smoking cessation, according to the latest guidelines for patients with cancer.32 33 These recommendations are not further individualised or actively supported.
Patients who do not wish to participate in the study are asked to participate in a one-time questionnaire as described in table 1. A participant flow diagram is shown in figure 1.
Table 1.
Outcome measures in the intervention and control group
| Description of outcome | Assessment | Description of measure | T0a | T0b | T1 | T2 | T3 |
| Primary outcome | |||||||
| Proportion of patients who develop one or more grade ≥2 perioperative complications within 90 days | Perioperative complications, grade ≥2 according to Clavien-Dindo classification | Number of grade ≥2 complication is abstracted from medical records | X | X | |||
| Secondary outcomes | |||||||
| Proportion of patients who develop one or more high-grade (≥3) complications, number of complications, length of hospital stay and readmissions within 90 days | Perioperative complications, grade ≥3 according to Clavien-Dindo classification, length of hospital stay, readmissions | Number of grade ≥3 complications, number of complications, length of hospital stay in days and readmissions abstracted from medical records | X | X | |||
| Cardiorespiratory fitness | Cardiopulmonary exercise test on a cycle ergometer using a ramp protocol40 | Peak oxygen uptake (VO2peak in mL/kg/min) | X | X | |||
| Physical functioning | Short physical performance battery84 | Five items organised into three subscales of balance, walking speed and lower extremity muscle strength | X | X | X | ||
| Upper extremity muscle strength | Handgrip strength using a handheld dynamometer85 | The maximum score of three attempts of both hands in kilogram (kg) | X | X | X | ||
| Lower extremity functional muscle strength | 30-s sit-to-stand test86 | The number of sit to stands within 30 s | X | X | X | ||
| Nutritional intake | 24-hour recall | Protein (total gram) and caloric intake (total kcal) | X | X | |||
| Nutritional status | Patient-generated subjective global assessment short form87 | Four items assessing weight (status), nutritional intake, symptoms and physical functioning | X | X | X | X | X |
| Nil per mouth consumption during hospitalisation | The number of days after RC abstracted from medical records | X | X | ||||
| Body composition | Bioelectrical impedance analysis | Body mass (kg), body height (cm), (subcutaneous) fat mass (%) and (upper limb) muscle mass (kg) are measured, and BMI (kg/m2) | X | X | X | ||
| Health-related quality of life | EORTC quality of life questionnaire core 3088 | Thirty items, organised into five functional scales (physical, role, emotional, cognitive and social), three symptom scales (pain, fatigue and emesis), six items (dyspnoea, sleep disturbance, appetite loss, constipation, diarrhoea and financial impact), and an overall quality of life scale | X | X | X | X | X |
| Bladder cancer-related quality of life | EORTC muscle-invasive bladder cancer specific module89 | Thirty items, assessing urinary symptoms, bowel symptoms, sexual functioning, urostomy problems, difficulties associated with the use of a catheter and body image | X | X | X | X | X |
| Anxiety and depression | Hospital Anxiety and Depression Scale37 | Seven items assessing anxiety and seven items assessing depression | X | X | X | X | X |
| Fatigue | Multidimensional fatigue inventory90 | Twenty items, categorised into five scales: general fatigue, physical fatigue, reduced activity, reduced motivation and mental fatigue | X | X | X | X | X |
| Physical activity | Short questionnaire to assess health-enhancing physical activity91 | Eleven items, organised into four different physical activities measuring frequency, duration and intensity (physical activity to and from home, household activities, activities at work and physical activities performed during leisure time) | X | X | X | X | X |
| Tumour hypoxia and immune cell infiltration | Two tumour biopsies: from routine diagnostic investigation and from surgical tumour excision | Immunohistochemistry analysis will be performed for hypoxia and immune cell infiltration biomarkers using the laboratory of the Karolinska Institutet (Sweden) and their according protocols | X | X | |||
| Cost-effectiveness | EuroQol 5-dimension-5 levels44 | Five items (dimensions) multiattribute utility questionnaire that measures mobility, self-care, usual activities, pain/discomfort and anxiety/depression in five levels | X | X | X | X | X |
| iMTA Medical Consumption Questionnaire42 and Productivity Costs Questionnaire43 | Patient-reported productivity losses and medical consumption | X | X | X | X | ||
| Healthcare costs | Medical activities abstracted from the management systems of the hospitals | X | |||||
| Other outcomes | |||||||
| Socio-demographic and clinical data | Socio-demographic data, disease and treatment characteristics will be abstracted from medical records or reported by the patient | Patient reported: place of birth, sex, marital status, living and work situation, education, lifestyle variables and the self-administrated comorbidity questionnaire92 Medical records: birth month and year, date of diagnosis, date and type of treatment, type of urinary diversion, tumour characteristics, ASA score, WHO score |
X | ||||
| Patient reported: smoking status | X | X | X | X | X | ||
| Coping mechanism | Sense of coherence questionnaire93 | Thirteen items, categorised into three scales: comprehensibility, manageability and meaningfulness | X | ||||
| Compliance to the intervention | Adherence rates | Patient reported: self-composed (activity) diary. Physical therapist: adherence of the physical exercise training intervention on standardised training session forms |
X | X | X | ||
| Patient evaluation | Self-composed questionnaire | Patients in the intervention group: satisfaction with the programme and willingness to participate in focus group Patients in the control group: evaluate contamination Both groups: evaluation of possible post-surgical intervention |
X | ||||
| Non-participation | Self-composed questionnaire | Patient-reported outcomes: socio-demographic, health-related and bladder cancer-related quality of life, anxiety and depression, fatigue, physical activity, coping mechanism and reason(s) for not participating. Medical records: birth month and year, date of diagnosis, date and type of treatment, tumour characteristics, ASA score, WHO score |
X |
ASA, American Society of Anesthesiologists; BMI, body mass index; EORTC, European Organisation for Research and Treatment of Cancer; RC, radical cystectomy; VO2peak, oxygen uptake at peak exercise.
Figure 1.
Study flow chart.
Control group—standard-of-care
Patients randomised to the standard-of-care arm receive care as usual, which does not include a comprehensive multimodal prehabilitation programme. In all participating centres, enhanced recovery after surgery protocols are used to optimise medical conditions to enhance recovery.34 These protocols include advice on smoking cessation when a patient is smoking and a referral to a dietician when malnutrition is detected. Preoperatively, the urologist determines whether the patient is physically fit for surgery. Patients in the control group are not prohibited to be physically active or seek counselling for nutritional advice, psychological support or smoking cessation.
Intervention group—prehabilitation
Patients randomised to the intervention arm will participate in a multimodal prehabilitation programme including supervised physical exercise training, nutritional support and—when relevant—psychological counselling and professional support for smoking-cessation, to enhance their health status. The intervention starts as soon as possible after baseline measurements and randomisation, approximately 3–6 weeks before surgery and is continued until surgery. Patients who are included before undergoing neoadjuvant treatment (including chemotherapy and immunotherapy) will participate in a physical exercise training programme from inclusion until completion of neoadjuvant treatment to prevent the often observed decline in physical fitness throughout neoadjuvant therapy. Subsequently, these patients will participate in the full multimodal prehabilitation programme until the date of surgery. Adverse events related to the intervention are monitored.
Physical exercise training programme
The physical exercise training programme consists of three training sessions per week under the supervision of a physical therapist. An overview of the training programme and training progression is described in table 2. The programme will be delivered at a physical therapy practice near a patient’s home to minimise travel time, preferably supervised by a physical therapist affiliated with Onconet, a nationwide network of physical therapists with additional competencies in cancer care. The study coordinator provides the physical therapists instructions via a video call covering the specifics of the training programme for the current study.
Table 2.
Content of the supervised physical exercise training programme in the intervention group
| Frequency | Intensity | Time | Type |
| Three times a week | Aerobic interval training, consisting of four intervals of alternating effort performed on a cycle ergometer:
|
24–28 min | Aerobic interval training |
| Two times a week | During neoadjuvant treatment:
|
30 min | Aerobic training |
| Two times a week | Resistance training, consisting of training six large muscle groups (leg press, bench press or chest press, abdominal crunch, pull over, low row and step up)† in two sets:
|
~20 min | Resistance training |
| One time a week | Progressive muscle relaxation techniques.35 | ~20 min | Relaxation exercises |
*Further tailoring is done by the physical therapists: if a patient is not able to complete the high-intensity interval, the intensity will be reduced by 10%. The intensity can be reduced further in steps of 10% until the patient can complete all four high-intensity intervals. If a patient is able to complete all high-intensity intervals, moderate and high intensity will be increased by 10%.94 During neoadjuvant treatment95: if a patient scores below a Borg score of 12 intensity is increased, if a patient scores above a Borg score of 15 intensity is decreased.
†Physical therapists can offer alternative resistance exercises targeting the same muscle group to accommodate a patient’s abilities and preferences.
‡If the patient is able to do two repetitions more than planned, the load will be increased by 10%. The load will be decreased by 10% if the number of repetitions the patient achieves is two less than the planned number of repetitions.
1RM, one-repetition maximum; CPET, cardiopulmonary exercise test; VO2peak, oxygen uptake at peak exercise.
Training sessions last 1 hour and consist of individualised aerobic interval training (three times a week), resistance training (two times a week) and relaxation exercises (one time a week). Results of the CPET will be used to establish the individual training intensity for aerobic interval training. For the resistance training, six large muscle groups will be targeted, in two sets of a defined maximum number of repetitions. The aim of these training sessions is to improve cardiorespiratory fitness, and muscle strength and mass. Relaxation exercises consist of guided breathing exercises and progressive muscle relaxation.35 The aim of these exercises is to reduce possible anxiety and stress. In addition to the supervised sessions, patients are encouraged to be moderately active on at least two additional days per week for 30 min.
The supervised sessions during neoadjuvant treatment consist of twice-weekly moderate-intensity aerobic and resistance training. Patients receive the physical exercise training programme as described above for the remaining 3–6 weeks before surgery.
Nutritional support
Patients receive tailored advice from a registered dietician at the participating hospitals aiming at a total protein intake of 1.9–2.3 g/per kg of fat-free mass as estimated with bioelectrical impedance analysis, to promote an anabolic state. Dietary advice will emphasise the benefit of spreading protein consumption over three meals, with a goal of 25–30 g protein per meal and includes advice for optimal energy intake. To achieve this, and to increase adaptive responses of the skeletal muscle, participants will receive high-quality protein supplements containing 30 g of whey protein, 20 µg of vitamin D and 250 mg of calcium36 in standardised supplements produced for the purpose of the study (FrieslandCampina, The Netherlands). These supplements are prescribed after each physical exercise training session and daily at least 1 hour before sleep or in the morning (depending on the patient’s preference). The dietician provides intake consultation and one or two follow-up sessions to evaluate nutritional intake. Protein intake will be restricted according to the severity of renal impairment. Depending on the patient’s protein intake, patients will be advised to either reduce or drop the intake of protein supplements. If necessary, additional dietary advice will be provided to reduce nutritional protein intake. Nutritional support starts at 3–6 weeks before surgery for all patients.
Psychological counselling
Patients are screened for anxiety and depression using the Hospital Anxiety and Depression Scale questionnaire at baseline.37 If a patient’s score falls between 11 and 18, the option of psychological counselling is discussed and referral is arranged for patients indicating a need for such counselling. Patients who score ≥19 are directly referred to psychological counselling. Referred patients receive an initial counselling session of 1.5 hours, and additional sessions at the discretion of the psychological counsellor and the patient.
Smoking cessation
Intensive counselling and nicotine replacement therapy is offered to all smoking patients in the 3–6 weeks before surgery. Counselling includes at least one in-person session and one or more telephone or in-person follow-up sessions by trained counsellors. If the patient indicates to be smoking and is willing to quit, the treating physician will refer the patient.
Study outcomes
Data are collected before randomisation (T0a), before surgery (T1), and 4 (T2), and 12 (T3) weeks after surgery. If applicable, additional data will be collected after neoadjuvant treatment (T0b). At T0a, clinical data (eg, treatment, clinical stage) will be abstracted from the medical records and socio-demographic characteristics (eg, age, sex and education) will be obtained via a questionnaire. In addition, coping mechanisms38 will be assessed via a questionnaire as this can help understand participation, attendance and dropouts. Data is collected in Castor EDC and access is restricted to the investigator team. Due to the low risk of the intervention a data safety monitoring board is not instated.
Primary outcome
The primary outcome is the proportion of patients who develop one or more grade ≥2 perioperative complications within 90 days after surgery. Complications are graded according to the Clavien-Dindo classification system39 as described in table 3.
Table 3.
The Clavien-Dindo classification of surgical complications
| Grade | Definition |
| Grade I | Any deviation from the normal postoperative course without the need for pharmacological treatment or surgical, endoscopic and radiological interventions. Allowed therapeutic regimens are: drugs as antiemetics, antipyretics, analgetics, diuretics and electrolytes and physical therapy. This grade also includes wound infections opened at the bedside. |
| Grade II | Requiring pharmacological treatment with drugs other than such allowed for grade I complications. Blood transfusions and total parenteral nutrition are also included. |
| Grade III | Requiring surgical, endoscopic or radiological intervention. |
| Grade-IIIa | Intervention not under general anaesthesia. |
| Grade-IIIb | Intervention under general anaesthesia. |
| Grade IV | Life-threatening complication (including CNS complications)* requiring IC/ICU-management. |
| Grade-IVa | Single organ dysfunction (including dialysis). |
| Grade-IVb | Multiorgan dysfunction. |
| Grade V | Death of a patient. |
Adapted from the study by Dindo et al.39
*Brain haemorrhage, ischaemic stroke, subarachnoidal bleeding, but excluding transient ischaemic attacks.
CNS, central nervous system; IC, intermediate care; ICU, intensive care unit.
Secondary outcomes
Secondary outcomes are the proportion of patients who develop one or more high-grade (grade ≥3) complications, total number of complications, length of hospital stay, number of readmissions, disease status (progression/recurrence) and additional treatment within 90 days. Secondary outcomes also include the intermediate outcomes of the intervention: cardiorespiratory fitness (measured using the standardised CPET)40, physical functioning, upper and lower extremity (functional) muscle strength, nutritional intake, nutritional status, body composition, health-related quality of life, bladder cancer-related quality of life, anxiety and depression, fatigue, physical activity and adherence to the intervention. Direct and indirect costs are collected for the cost-effectiveness analysis. Tumour hypoxia, immune cell infiltration and pathological response are assessed as explorative outcomes. Participants in the control group will be asked whether they participated in any (structured) lifestyle programme during the preoperative period, to monitor contamination. In both groups, participants will be asked whether they received any postoperative intervention. The timing and type of outcome measures are presented in table 1.
Costs
For direct costs in both groups, medical records will be used to gather data on treatment, complications, length of hospital stay and number of readmissions, follow-up and diagnostics. For the intervention group, the costs for the prehabilitation programme, including physical exercise training, nutritional support, psychological counselling and smoking cessation, will be determined by means of the activity-based costing41 method. Costs for neoadjuvant treatment are expected to be equal for both arms and will therefore not be included in the calculation. For indirect costs, the iMTA Medical Consumption Questionnaire (iMCQ)42 will be used to gather data on medical consumption outside the hospital. The iMCQ includes questions related to frequently occurring contacts with healthcare providers. For patients who currently have remunerative employment, productivity losses will be obtained through the iMTA Productivity Costs Questionnaire.43 To calculate quality-adjusted life years, utilities will be derived from the EuroQol 5-dimension-5 levels (EQ-5D-5L) questionnaire.44
Compliance
To monitor therapeutic validity, compliance with the physical exercise training programme is assessed by attendance rates and compliance to all parts of the training programme as scored by the physical therapist on standardised training session forms, as well as by patient self-report via an activity diary. Compliance to nutritional supplement intake is assessed via a diary. Whether smoking cessation has been successful is assessed through questionnaires.
Satisfaction
Patients in the intervention group are asked to complete a short questionnaire at the end of the study about the perceived effectiveness of and satisfaction with the programme, whether they would suggest any changes and whether they would recommend it to other patients with bladder cancer. In addition, patients will be asked if they are willing to be contacted for participation in a focus group where the intervention programme and changes that may positively affect implementation will be further discussed.
Sample size calculation
Previous studies in diverse types of cancer reported a reduction of postoperative complications in the intervention group compared with the control group, with ORs ranging from 0.11 to 0.88.45–51 This study aims to reduce the number of patients with any grade ≥2 perioperative complication within 90 days from 60%52 53 to 35% (relative risk 0.58, OR 0.36).54 55 Assuming a two-sided Fisher’s exact test with a power of 80% and an alpha of 0.05, in total 140 patients will be needed. To account for 10% dropout, including dropout due to cancelling of the planned surgery, 154 patients will be included. Approximately 380 patients with bladder cancer undergo RC in the eight participating hospitals annually. This implies that it is feasible to complete inclusion within two and a half years, if recruitment rate is at least 17%.
Statistical analysis
Primary outcome
All analyses will be performed on an intention-to-treat basis. Descriptive statistics will be calculated to describe and evaluate the comparability of the two groups at baseline on socio-demographic and clinical variables, and to assess the adequacy of the randomisation. Patients who do not receive the planned surgery or have an open-closed procedure, independent of group allocation, will be excluded from the primary analysis. The proportion of patients who develop any grade ≥2 complications will be compared in the two study arms by using Fisher’s exact test and a Poisson regression model with a log link, adjusted for the stratification factors and relevant baseline imbalances. The relative risk will be reported with a 95% CI based on robust SEs.56
Secondary study outcomes
Between-group differences over time will be evaluated in measures of physical functioning and patient-reported outcomes using linear mixed effects regression analysis. For high-grade complications, length of hospital stay and number of readmissions, Poisson regression models with an appropriate link function will be used. For continuous outcomes, differences in mean change scores between the two study arms will be accompanied by effect sizes. Standardised effect sizes will be calculated by subtracting the mean change scores of the control group from those of the intervention group, and subsequently dividing this by the pooled SD. Effect sizes of 0.2 are considered small, 0.5 moderate and 0.8 large.57 A p value<0.05 will be considered statistically significant.
Intervention fidelity
Descriptive statistics will be used to summarise compliance rates of the supervised exercise sessions as well as home-based physical activity, supplement consumption and smoking cessation. Compliance rates are based on number of completed training sessions, supplementation consumption and number of patients who stop smoking in the study intervention. Whether the level of compliance is associated with changes over time in primary and secondary study outcomes will be evaluated using generalised linear mixed effects models.
Non-participants
Baseline data of participants will be compared with those of non-participants using χ2 statistics for categorical variables and analysis of variance for continuous variables.
Exploratory analysis
Exploratory analyses will be performed to explore the moderating effect of intervention duration, and differences in effectiveness of the physical exercise training programme between those who received neoadjuvant chemotherapy and those who did not. This will be done by adding interaction terms to the model and by performing stratified analyses if the interaction term is statistically significant at p<0.10. Exploratory analyses will also be executed to study the relationship of post-intervention/preoperative physical fitness parameters (ie, oxygen uptake at peak exercise) and nutritional status, with perioperative outcomes (grade ≥2 complications yes/no and number of days in the hospital). For this analysis, univariable and multivariable Poisson regression analyses will be used.
Economic evaluation
A trial-based and model-based economic evaluation will be performed, based on the intention-to-treat analysis. The model-based evaluation will use literature for the potential long-term consequences and parametric survival methods to extrapolate the trial data beyond the included follow-up. The analysis will be approached from a societal perspective of The Netherlands and a lifelong time horizon. A Markov decision model will be built, with relevant health states derived from the EQ-5D-5L questionnaire. Outcomes are (1) the incremental costs per reduced proportion of patients who develop one or more grade ≥2 perioperative complications within 90 days (trial-based), and (2) incremental costs, incremental quality-adjusted life years and the incremental cost-effectiveness ratio (model-based). An estimation of the degree of uncertainty around each input parameter will be included with the use of probabilistic sensitivity analyses. Parameter values will be drawn randomly from the assigned distributions, using Monte Carlo simulations.58 To capture necessary support regarding adoption and further research, value of information analyses will be performed.59 Where appropriate, Dutch guidelines for costing studies will be used in applying tariffs to units of resource use.60 Finally, a budget impact analysis will be performed according to the International Society for Pharmacoeconomics and Outcomes Research guidelines.61
Patient and public involvement
Patients or the public were not involved in the development of the study design. A patient representative is currently involved in the study and input will be obtained whenever relevant during the trial. Annual consortium meetings with the urologists are organised.
Discussion
A considerable proportion of patients with bladder cancer scheduled for RC has a poor cardiorespiratory fitness,9 10 is malnourished11 62 and/or is an active smoker at diagnosis.63 This implies that there is a substantial potential for improving cardiorespiratory fitness and nutritional status in this patient population. Here, the rationale and design of a multimodal prehabilitation programme for patients with bladder cancer who are scheduled for RC is presented. It is hypothesised that the programme will be effective in reducing the number and severity of perioperative complications.
This study has several strengths, including its multicentre, randomised design, the use of an intention-to-treat basis for the data analysis and a minimisation technique to ensure blinded treatment allocation and comparable groups. Most importantly, the study intervention consists of a tailored programme for physical exercise training and nutritional support following current best-practice for prehabilitation. Moreover, a cost-effectiveness analysis will be performed to anticipate smooth implementation and reimbursement, and tumour hypoxia and immune cell infiltration analysis will be performed exploratively. Intervention fidelity will be monitored in detail, as recommended previously,64 as will adverse events related to the intervention. Another notable strength is the additional analysis in non-participants. Selective non-participation is a serious risk for the generalisability of physical exercise training studies. Previous physical exercise training studies in other cancer populations have shown relevant differences between those who participate and those who do not.65 66 It has previously been described that patients who were eligible for prehabilitation programmes for colon cancer surgery expressed several reservations.67 It is vital to understand the characteristics of non-participants and reasons for non-participation in bladder cancer prehabilitation. This will not only help judge the generalisability of the results but will also support implementation in a way that will maximise the potential value of the prehabilitation programme and achieve equitable health outcomes. Finally, the exploratory subgroup analysis in patients who receive neoadjuvant treatment might be relevant to inform future studies on risk stratification.
To limit barriers to participation and adherence, the exercise programme will be delivered as near to the patients’ homes as possible, to minimise travel time. This is a very important factor for patients with cancer according to previous studies.55 68–70 The availability of and close collaboration with the nationwide Onconet network of physical therapists, who are specifically educated to supervise patients with cancer, is an important advantage. An additional benefit of this approach is that it will facilitate implementation if the intervention proves to be effective.
This study also has some limitations. Patients with bladder cancer will be followed for a period of 90 days after surgery, meaning that longer-term evaluation of outcomes will not be possible. Because up to 60% of patients-report complications within 90 days after surgery,71 it is expected that the time frame will be adequate for our primary outcome. A possible limitation is the risk of contamination in the control group. An evaluation questionnaire will be used in the control group to determine whether patients were physically active or received a (structured) lifestyle intervention preoperatively and postoperatively. Although objective measurements of habitual physical activity may provide a more detailed insight into physical activity levels not prone to recall bias, the collection of physical activity levels will be restricted to using questionnaires for feasibility reasons. This programme is designed to maximally improve a patient’s health status by including physical exercise training, nutritional support, psychological counselling and smoking cessation. It is not likely that patients who are randomised to the control group would initiate a programme consisting of all these components on their own. The multimodal approach prohibits disentangling of the individual effects of each lifestyle component in the prehabilitation programme. Considering the number of intervention components and the prevalence of bladder cancer, a larger study using a full factorial design is unlikely to be feasible in this population. Moreover, the current best-practice for other types of cancer supports the use of multimodal interventions over unimodal approaches.72 73 Higher levels of physical exercise training have been demonstrated to be beneficial for both cancer prevention and, in some solid tumours, progression of disease and cancer-related mortality.74 75 However, the underlying biological mechanism has yet to be demonstrated. It is expected that the prehabilitation programme has positive effects on the tumour microenvironment. The hypoxic tumour microenvironment is a common characteristic of a solid tumour when oxygen levels become low, as a result of the rapid proliferation of tumour cells76 and is linked to poor prognosis in bladder cancer.77 Physical exercise training has regulatory effects on the angiogenesis of skeletal muscles, which has raised interest in whether these effects might translate to solid tumours.78 Preclinical research has shown that training may acutely reduce tumour hypoxia through vascular normalisation and thereby improve the perfusion of tumour tissue.79–82 In addition, exercise training has been suggested to alter immune cell infiltration in solid tumours and thereby contribute to enhanced immune surveillance and improved vascular function.25 However, current evidence is inconsistent and inconclusive.83 Clinical trials are very limited and this preoperative setting provides an excellent opportunity to investigate the potential role of prehabilitation on tumour hypoxia and immune cell infiltration.
To summarise, this study will provide empirical evidence on the benefits of multimodal prehabilitation for patients with bladder cancer planned for RC who are at high risk of perioperative complications and a long recovery period. When proven (cost-)effective, the study results will support implementation of a multimodal prehabilitation programme for patients with bladder cancer in daily clinical practice.
Supplementary Material
Acknowledgments
Research at the Netherlands Cancer Institute is supported by institutional grants of the Dutch Cancer Society and of the Dutch Ministry of Health, Welfare and Sport. The protein supplements in the clinical study will be kindly provided by FrieslandCampina.
Footnotes
Contributors: AV, AML-K, AGH, ELK, VPR, WHVH, AMM, WGG and MMS conceived the study. AV, WHVH, AMM, WGG, MMS, MGS and EA contributed to the design of the study protocol. RPM, AML-K, AGH, VCR, ELK, SDB, CJW, TATM and BCB reviewed the study protocol with a clinical perspective. VPR wrote the statistical approach of the economic evaluation. HR composed the protocol of tumour hypoxia and immune cell infiltration analysis. AV, WHVH, AMM, WGG, MMS, MGS, and EA wrote the manuscript. All authors read, commented on and approved the manuscript.
Funding: This work was supported by the Dutch Cancer Society (KWF, Delflandlaan 17, 1062 EA, Amsterdam, The Netherlands), grant number NKI 2020-1/12877. The funder has no role in the design, data collection and analysis of the study.
Competing interests: CJW received a research grant from ZonMw and gives courses on robot-assisted radical cystectomy during conferences entitled with EAU. TATM received consulting fees as member of the advisory board prostate cancer of Janssen-Cilag B.V. BCB participates on a Data Safety Monitoring Board of an international prehabilitation study, is board member of Netherlands Society of Human Movement sciences, member of Exercise is Medicine of Vereniging voor Sportgeneeskunde and member of the Scientific Committee of Fit4Surgery. RPM received research grants from Janssen, Roche and Astellas, an educational grant from Merck, received consulting fees from Merck, MSD, Janssen, Bristol-Myers Squibb Company, received support for attending the 2022 Global Congress on Bladder Cancer, and is EAU Panel member of the Muscle Invasive Bladder Cancer Guideline. MMS is member of the Scientific Committee of Fit4Surgery and board member of Onconet.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Stein JP, Skinner DG. Radical cystectomy for invasive bladder cancer: long-term results of a standard procedure. World J Urol 2006;24:296–304. 10.1007/s00345-006-0061-7 [DOI] [PubMed] [Google Scholar]
- 3.Alfred Witjes J, Lebret T, Compérat EM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol 2017;71:462–75. 10.1016/j.eururo.2016.06.020 [DOI] [PubMed] [Google Scholar]
- 4.Babjuk M, Burger M, Capoun O, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (TA, T1, and carcinoma in situ). Eur Urol 2022;81:75–94. 10.1016/j.eururo.2021.08.010 [DOI] [PubMed] [Google Scholar]
- 5.Maibom SL, Joensen UN, Poulsen AM, et al. Short-Term morbidity and mortality following radical cystectomy: a systematic review. BMJ Open 2021;11:e043266. 10.1136/bmjopen-2020-043266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol 2009;55:164–74. 10.1016/j.eururo.2008.07.031 [DOI] [PubMed] [Google Scholar]
- 7.Novara G, De Marco V, Aragona M, et al. Complications and mortality after radical cystectomy for bladder transitional cell cancer. J Urol 2009;182:914–21. 10.1016/j.juro.2009.05.032 [DOI] [PubMed] [Google Scholar]
- 8.Bochner BH, Sjoberg DD, Laudone VP, et al. A randomized trial of robot-assisted laparoscopic radical cystectomy. N Engl J Med 2014;371:389–90. 10.1056/NEJMc1405213 [DOI] [PubMed] [Google Scholar]
- 9.Prentis JM, Trenell MI, Vasdev N, et al. Impaired cardiopulmonary reserve in an elderly population is related to postoperative morbidity and length of hospital stay after radical cystectomy. BJU Int 2013;112:E13–9. 10.1111/bju.12219 [DOI] [PubMed] [Google Scholar]
- 10.Tolchard S, Angell J, Pyke M, et al. Cardiopulmonary reserve as determined by cardiopulmonary exercise testing correlates with length of stay and predicts complications after radical cystectomy. BJU Int 2015;115:554–61. 10.1111/bju.12895 [DOI] [PubMed] [Google Scholar]
- 11.Arora K, Hanson KT, Habermann EB, et al. Early complications and mortality following radical cystectomy: associations with malnutrition and obesity. Bladder Cancer 2018;4:377–88. 10.3233/BLC-180173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One 2011;6:e20306. 10.1371/journal.pone.0020306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sathianathen NJ, Weight CJ, Jarosek SL, et al. Increased surgical complications in smokers undergoing radical cystectomy. Bladder Cancer 2018;4:403–9. 10.3233/BLC-180185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jack S, West MA, Raw D, et al. The effect of neoadjuvant chemotherapy on physical fitness and survival in patients undergoing oesophagogastric cancer surgery. Eur J Surg Oncol 2014;40:1313–20. 10.1016/j.ejso.2014.03.010 [DOI] [PubMed] [Google Scholar]
- 15.West MA, Parry MG, Lythgoe D, et al. Cardiopulmonary exercise testing for the prediction of morbidity risk after rectal cancer surgery. Br J Surg 2014;101:1166–72. 10.1002/bjs.9551 [DOI] [PubMed] [Google Scholar]
- 16.Sinclair R, Navidi M, Griffin SM, et al. The impact of neoadjuvant chemotherapy on cardiopulmonary physical fitness in gastro-oesophageal adenocarcinoma. Ann R Coll Surg Engl 2016;98:396–400. 10.1308/rcsann.2016.0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomson IG, Wallen MP, Hall A, et al. Neoadjuvant therapy reduces cardiopulmunary function in patients undegoing oesophagectomy. Int J Surg 2018;53:86–92. 10.1016/j.ijsu.2018.03.030 [DOI] [PubMed] [Google Scholar]
- 18.Banerjee S, Manley K, Shaw B, et al. Vigorous intensity aerobic interval exercise in bladder cancer patients prior to radical cystectomy: a feasibility randomised controlled trial. Support Care Cancer 2018;26:1515–23. 10.1007/s00520-017-3991-2 [DOI] [PubMed] [Google Scholar]
- 19.Kaye DR, Schafer C, Thelen-Perry S, et al. The feasibility and impact of a presurgical exercise intervention program (prehabilitation) for patients undergoing cystectomy for bladder cancer. Urology 2020;145:106–12. 10.1016/j.urology.2020.05.104 [DOI] [PubMed] [Google Scholar]
- 20.Jensen BT, Petersen AK, Jensen JB, et al. Efficacy of a multiprofessional rehabilitation programme in radical cystectomy pathways: a prospective randomized controlled trial. Scand J Urol 2015;49:133–41. 10.3109/21681805.2014.967810 [DOI] [PubMed] [Google Scholar]
- 21.Minnella EM, Awasthi R, Bousquet-Dion G, et al. Multimodal prehabilitation to enhance functional capacity following radical cystectomy: a randomized controlled trial. Eur Urol Focus 2021;7:132–8. 10.1016/j.euf.2019.05.016 [DOI] [PubMed] [Google Scholar]
- 22.Jensen BT, Laustsen S, Jensen JB, et al. Exercise-based pre-habilitation is feasible and effective in radical cystectomy pathways-secondary results from a randomized controlled trial. Support Care Cancer 2016;24:3325–31. 10.1007/s00520-016-3140-3 [DOI] [PubMed] [Google Scholar]
- 23.Thomas G, Tahir MR, Bongers BC, et al. Prehabilitation before major intra-abdominal cancer surgery: a systematic review of randomised controlled trials. Eur J Anaesthesiol 2019;36:933–45. 10.1097/EJA.0000000000001030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiggins JM, Opoku-Acheampong AB, Baumfalk DR, et al. Exercise and the tumor microenvironment: potential therapeutic implications. Exerc Sport Sci Rev 2018;46:56–64. 10.1249/JES.0000000000000137 [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Ashcraft KA, Betof Warner A, et al. Can exercise-induced modulation of the tumor physiologic microenvironment improve antitumor immunity? Cancer Res 2019;79:2447–56. 10.1158/0008-5472.CAN-18-2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shannon AM, Bouchier-Hayes DJ, Condron CM, et al. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev 2003;29:297–307. 10.1016/s0305-7372(03)00003-3 [DOI] [PubMed] [Google Scholar]
- 27.Peixoto A, Fernandes E, Gaiteiro C, et al. Hypoxia enhances the malignant nature of bladder cancer cells and concomitantly antagonizes protein O-glycosylation extension. Oncotarget 2016;7:63138–57. 10.18632/oncotarget.11257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boström PJ, Thoms J, Sykes J, et al. Hypoxia marker GLUT-1 (glucose transporter 1) is an independent prognostic factor for survival in bladder cancer patients treated with radical cystectomy. Bladder Cancer 2016;2:101–9. 10.3233/BLC-150033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975;31:103–15. [PubMed] [Google Scholar]
- 30.Jin M, Polis A, Hartzel J. R package “minirand”. 0.1.3. ed. CRAN; 2020. Available: https://cran.r-project.org/web/packages/Minirand/Minirand.pdf [Google Scholar]
- 31.Jin M, Polis A, Hartzel J. Algorithms for minimization randomization and the implementation with an R package. Communications in Statistics - Simulation and Computation 2021;50:3077–87. 10.1080/03610918.2019.1619765 [DOI] [Google Scholar]
- 32.Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc 2019;51:2375–90. 10.1249/MSS.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rock CL, Thomson CA, Sullivan KR, et al. American cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin 2022;72:230–62. 10.3322/caac.21719 [DOI] [PubMed] [Google Scholar]
- 34.Cerantola Y, Valerio M, Persson B, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: enhanced recovery after surgery (ERAS(®)) society recommendations. Clin Nutr 2013;32:879–87. 10.1016/j.clnu.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 35.Jacobson E. You must relax. 1934.
- 36.Cermak NM, Res PT, de Groot LCPGM, et al. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 2012;96:1454–64. 10.3945/ajcn.112.037556 [DOI] [PubMed] [Google Scholar]
- 37.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 38.Antonovsky H, Sagy S. The development of a sense of coherence and its impact on responses to stress situations. J Soc Psychol 1986;126:213–25. [PubMed] [Google Scholar]
- 39.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berkel AEM, Bongers BC, van Kamp M-JS, et al. The effects of prehabilitation versus usual care to reduce postoperative complications in high-risk patients with colorectal cancer or dysplasia scheduled for elective colorectal resection: study protocol of a randomized controlled trial. BMC Gastroenterol 2018;18:29. 10.1186/s12876-018-0754-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooper R, Kaplan RS. Profit priorities from activity-based costing. Harv Bus Rev 1991;69:130–5. [Google Scholar]
- 42.Bouwmans C, et al. Medical cost questionnaire (imcq). Rotterdam: iMTA, Erasmus Universiteit Rotterdam, 2013. [Google Scholar]
- 43.Bouwmans C, et al. Productivity cost questionnaire. Erasmus University Rotterdam: Institute for Medical Technology Assesment, 2013. [Google Scholar]
- 44.Uyl-de Groot CA, Rutten FF, Bonsel GJ. Measurement and valuation of quality of life in economic appraisal of cancer treatment. Eur J Cancer 1994;30A:111–7. 10.1016/s0959-8049(05)80030-9 [DOI] [PubMed] [Google Scholar]
- 45.Dronkers J, Veldman A, Hoberg E, et al. Prevention of pulmonary complications after upper abdominal surgery by preoperative intensive inspiratory muscle training: a randomized controlled pilot study. Clin Rehabil 2008;22:134–42. 10.1177/0269215507081574 [DOI] [PubMed] [Google Scholar]
- 46.Dronkers JJ, Lamberts H, Reutelingsperger IMMD, et al. Preoperative therapeutic programme for elderly patients scheduled for elective abdominal oncological surgery: a randomized controlled pilot study. Clin Rehabil 2010;24:614–22. 10.1177/0269215509358941 [DOI] [PubMed] [Google Scholar]
- 47.Kulkarni SR, Fletcher E, McConnell AK, et al. Pre-operative inspiratory muscle training preserves postoperative inspiratory muscle strength following major abdominal surgery - a randomised pilot study. Ann R Coll Surg Engl 2010;92:700–7. 10.1308/003588410X12771863936648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barbalho-Moulim MC, Miguel GPS, Forti EMP, et al. Effects of preoperative inspiratory muscle training in obese women undergoing open bariatric surgery: respiratory muscle strength, lung volumes, and diaphragmatic excursion. Clinics (Sao Paulo) 2011;66:1721–7. 10.1590/s1807-59322011001000009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soares SM de TP, Nucci LB, da Silva MM de C, et al. Pulmonary function and physical performance outcomes with preoperative physical therapy in upper abdominal surgery: a randomized controlled trial. Clin Rehabil 2013;27:616–27. 10.1177/0269215512471063 [DOI] [PubMed] [Google Scholar]
- 50.van Adrichem EJ, Meulenbroek RL, Plukker JTM, et al. Comparison of two preoperative inspiratory muscle training programs to prevent pulmonary complications in patients undergoing esophagectomy: a randomized controlled pilot study. Ann Surg Oncol 2014;21:2353–60. 10.1245/s10434-014-3612-y [DOI] [PubMed] [Google Scholar]
- 51.Halliday LJ, Doganay E, Wynter-Blyth VA, et al. The impact of prehabilitation on post-operative outcomes in oesophageal cancer surgery: a propensity score matched comparison. J Gastrointest Surg 2021;25:2733–41. 10.1007/s11605-020-04881-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking complications associated with esophagectomy. Ann Surg 2019;269:291–8. 10.1097/SLA.0000000000002611 [DOI] [PubMed] [Google Scholar]
- 53.Wijburg CJ, Michels CTJ, Hannink G, et al. Robot-assisted radical cystectomy versus open radical cystectomy in bladder cancer patients: a multicentre comparative effectiveness study. Eur Urol 2021;79:609–18. 10.1016/j.eururo.2020.12.023 [DOI] [PubMed] [Google Scholar]
- 54.Barberan-Garcia A, Ubré M, Roca J, et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg 2018;267:50–6. 10.1097/SLA.0000000000002293 [DOI] [PubMed] [Google Scholar]
- 55.Berkel AEM, Bongers BC, Kotte H, et al. Effects of community-based exercise prehabilitation for patients scheduled for colorectal surgery with high risk for postoperative complications: results of a randomized clinical trial. Ann Surg 2022;275:e299–306. 10.1097/SLA.0000000000004702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6. 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 57.Cohen J. Statistical power analysis for the behavioral sciences. Routledge, 2013. 10.4324/9780203771587 [DOI] [Google Scholar]
- 58.Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oup Oxford, 2006. [Google Scholar]
- 59.Vallejo-Torres L, Steuten LMG, Buxton MJ, et al. Integrating health economics modeling in the product development cycle of medical devices: a Bayesian approach. Int J Technol Assess Health Care 2008;24:459–64. 10.1017/S0266462308080604 [DOI] [PubMed] [Google Scholar]
- 60.Hakkaart-van Roijen L, Tan S, Bouwmans C. Manual for cost research: methods and standard cost prices for economic evaluations in health care. Diemen: Health Care Insurance Board, 2010. [Google Scholar]
- 61.Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR Task force on good research practices -- budget impact analysis. Value Health 2007;10:336–47. 10.1111/j.1524-4733.2007.00187.x [DOI] [PubMed] [Google Scholar]
- 62.Gregg JR, Cookson MS, Phillips S, et al. Effect of preoperative nutritional deficiency on mortality after radical cystectomy for bladder cancer. J Urol 2011;185:90–6. 10.1016/j.juro.2010.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barbosa ALA, Vermeulen SHHM, Aben KK, et al. Smoking intensity and bladder cancer aggressiveness at diagnosis. PLoS One 2018;13:e0194039. 10.1371/journal.pone.0194039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH behavior change Consortium. Health Psychol 2004;23:443–51. 10.1037/0278-6133.23.5.443 [DOI] [PubMed] [Google Scholar]
- 65.van Waart H, van Harten WH, Buffart LM, et al. Why do patients choose (not) to participate in an exercise trial during adjuvant chemotherapy for breast cancer? Psychooncology 2016;25:964–70. 10.1002/pon.3936 [DOI] [PubMed] [Google Scholar]
- 66.Vd Wiel HJ, Stuiver MM, May AM, et al. Characteristics of participants and nonparticipants in a blended internet-based physical activity trial for breast and prostate cancer survivors: cross-sectional study. JMIR Cancer 2021;7:e25464. 10.2196/25464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agasi-Idenburg CS, Zuilen MK, Westerman MJ, et al. “I am busy surviving” - views about physical exercise in older adults scheduled for colorectal cancer surgery. J Geriatr Oncol 2020;11:444–50. 10.1016/j.jgo.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 68.Ormel HL, van der Schoot GGF, Sluiter WJ, et al. Predictors of adherence to exercise interventions during and after cancer treatment: a systematic review. Psychooncology 2018;27:713–24. 10.1002/pon.4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Banerjee S, Semper K, Skarparis K, et al. Patient perspectives of vigorous intensity aerobic interval exercise prehabilitation prior to radical cystectomy: a qualitative focus group study. Disabil Rehabil 2021;43:1084–91. 10.1080/09638288.2019.1651907 [DOI] [PubMed] [Google Scholar]
- 70.van Wijk L, Bongers BC, Berkel AEM, et al. Improved preoperative aerobic fitness following a home-based bimodal prehabilitation programme in high-risk patients scheduled for liver or pancreatic resection. Br J Surg 2022;109:1036–9. 10.1093/bjs/znac230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krajewski W, Zdrojowy R, Tupikowski K, et al. How to lower postoperative complications after radical cystectomy-a review. Cent European J Urol 2016;69:370–6. 10.5173/ceju.2016.880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mewes JC, Steuten LMG, Ijzerman MJ, et al. Effectiveness of multidimensional cancer Survivor rehabilitation and cost-effectiveness of cancer rehabilitation in general: a systematic review. Oncologist 2012;17:1581–93. 10.1634/theoncologist.2012-0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lukez A, Baima J. The role and scope of prehabilitation in cancer care. Semin Oncol Nurs 2020;36:150976. 10.1016/j.soncn.2019.150976 [DOI] [PubMed] [Google Scholar]
- 74.Moore SC, Lee I-M, Weiderpass E, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med 2016;176:816–25. 10.1001/jamainternmed.2016.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Friedenreich CM, et al. Physical activity and cancer outcomes: A precision medicine approachphysical activity and cancer: A precision medicine approach. Clin Cancer Res 2016;22:4766–75. [DOI] [PubMed] [Google Scholar]
- 76.Petrova V, Annicchiarico-Petruzzelli M, Melino G, et al. The hypoxic tumour microenvironment. Oncogenesis 2018;7:1–13. 10.1038/s41389-017-0011-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Theodoropoulos VE, Lazaris AC, Sofras F, et al. Hypoxia-Inducible factor 1 alpha expression correlates with angiogenesis and unfavorable prognosis in bladder cancer. Eur Urol 2004;46:200–8. 10.1016/j.eururo.2004.04.008 [DOI] [PubMed] [Google Scholar]
- 78.Koelwyn GJ, Quail DF, Zhang X, et al. Exercise-dependent regulation of the tumour microenvironment. Nat Rev Cancer 2017;17:620–32. 10.1038/nrc.2017.78 [DOI] [PubMed] [Google Scholar]
- 79.Jones LW, Viglianti BL, Tashjian JA, et al. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J Appl Physiol (1985) 2010;108:343–8. 10.1152/japplphysiol.00424.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCullough DJ, Nguyen LM-D, Siemann DW, et al. Effects of exercise training on tumor hypoxia and vascular function in the rodent preclinical orthotopic prostate cancer model. J Appl Physiol (1985) 2013;115:1846–54. 10.1152/japplphysiol.00949.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Betof AS, Lascola CD, Weitzel D, et al. Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. J Natl Cancer Inst 2015;107:djv040. 10.1093/jnci/djv040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schumacher O, Galvão DA, Taaffe DR, et al. Exercise modulation of tumour perfusion and hypoxia to improve radiotherapy response in prostate cancer. Prostate Cancer Prostatic Dis 2021;24:1–14. 10.1038/s41391-020-0245-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seet-Lee C, Yee J, Morahan H, et al. The effect of aerobic exercise on tumour blood delivery: a systematic review and meta-analysis. Support Care Cancer 2022;30:8637–53. 10.1007/s00520-022-07132-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94. 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 85.Langius J, et al. Meetprotocol handknijpkracht mbv hand dynamometer. Nutritional Assessment Platform, 2016. [Google Scholar]
- 86.McAllister LS, Palombaro KM. Modified 30-second sit-to-stand test: reliability and validity in older adults unable to complete traditional sit-to-stand testing. J Geriatr Phys Ther 2020;43:153–8. 10.1519/JPT.0000000000000227 [DOI] [PubMed] [Google Scholar]
- 87.Bauer J, Capra S, Ferguson M. Use of the scored patient-generated subjective global assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr 2002;56:779–85. 10.1038/sj.ejcn.1601412 [DOI] [PubMed] [Google Scholar]
- 88.Aaronson NK, Ahmedzai S, Bergman B, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76. 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 89.Danna BJ, Metcalfe MJ, Wood EL, et al. Assessing symptom burden in bladder cancer: an overview of bladder cancer specific health-related quality of life instruments. Bladder Cancer 2016;2:329–40. 10.3233/BLC-160057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smets EM, Garssen B, Bonke B, et al. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res 1995;39:315–25. 10.1016/0022-3999(94)00125-o [DOI] [PubMed] [Google Scholar]
- 91.Wendel-Vos GCW, Schuit AJ, Saris WHM, et al. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol 2003;56:1163–9. 10.1016/s0895-4356(03)00220-8 [DOI] [PubMed] [Google Scholar]
- 92.Sangha O, Stucki G, Liang MH, et al. The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum 2003;49:156–63. 10.1002/art.10993 [DOI] [PubMed] [Google Scholar]
- 93.Antonovsky A. The structure and properties of the sense of coherence scale. Soc Sci Med 1993;36:725–33. 10.1016/0277-9536(93)90033-z [DOI] [PubMed] [Google Scholar]
- 94.van Rooijen S, Carli F, Dalton S, et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation. BMC Cancer 2019;19:98. 10.1186/s12885-018-5232-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Swain DP, Brawner CA, A.C.o.S. Medicine . ACSM’s resource manual for guidelines for exercise testing and prescription. Wolters Kluwer Health/Lippincott Williams & Wilkins, 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.