Abstract
Objective:
To determine the ability of quantitative electroencephalography (QEEG) to improve the accuracy of predicting recovery of consciousness by post-cardiac arrest day 10.
Methods:
Unconscious survivors of cardiac arrest undergoing daily clinical and EEG assessments through post-cardiac arrest day 10 were studied in a prospective observational cohort study. Power spectral density, local coherence, and permutation entropy were calculated from daily EEG clips following a painful stimulus. Recovery of consciousness was defined as following at least simple commands by day 10. We determined the impact of EEG metrics to predict recovery when analyzed with established predictors of recovery using partial least squares regression models. Explained variance analysis identified which features contributed most to the predictive model.
Results:
367 EEG epochs from 98 subjects were analyzed in conjunction with clinical measures. Highest prediction accuracy was achieved when adding QEEG features from post-arrest days 4–6 to established predictors (area under the receiver operating curve improved from 0.81 ± 0.04 to 0.86 ± 0.05). Prediction accuracy decreased from 0.84 ± 0.04 to 0.79 ± 0.04 when adding QEEG features from post-arrest days 1–3. Patients with recovery of command-following by day 10 showed higher coherence across the frequency spectrum and higher centro-occipital delta-frequency spectral power by days 4–6, and globally-higher theta range permutation entropy by days 7–10.
Conclusions:
Adding quantitative EEG metrics to established predictors of recovery allows modest improvement of prediction accuracy for recovery of consciousness, when obtained within a week of cardiac arrest. Further research is needed to determine the best strategy for integration of QEEG data into prognostic models in this patient population.
Keywords: Death, EEG, Electrocerebral, Circulatory arrest, Coma, Consciousness
Introduction
Predictions regarding recovery of consciousness following cardiac arrest (CA) carry considerable weight for withdrawal of life-sustaining therapy (WLST). Recent guidelines recommend a multimodal assessment including a combination of clinical history and examination, laboratory tests, and supporting neuroimaging and electrophysiologic assessments to guide prognostication.1–3 Electroencephalography (EEG) in particular can be used to provide information regarding the integrity of functional brain networks in after acute brain injury.4,5 In spite of this, accuracy of prognosis remains variable. Increasingly sophisticated applications of EEG are used to contribute to this multimodal approach. Quantitative EEG analysis (QEEG) may have the potential to comprehensively capture the neuronal network integrity that is fundamental for neurologic recovery but is not currently utilized routinely in clinical practice.4,6 Predicting recovery of consciousness may be of particular interest, as it is a prerequisite for functional recovery and has major impact on decisions regarding WLST.7,8
The primary objective of this study was to determine if adding QEEG data to a multimodal prognostic model (including clinical exam components, laboratory and electrophysiologic markers of brain injury) improves the ability to predict early recovery of consciousness (designated by recovery of command-following by post-CA day 10). Secondarily, we describe patterns of changing QEEG metrics that may help identify patients who will go on to recover command-following by day 10. We chose to study short-term recovery (by post-CA day 10) as decisions regarding WLST are often made in this timeframe.
Methods
Inclusion criteria
Patients admitted to the neurological, medical, or cardiac intensive care units (ICUs) at a tertiary-care medical center from April 2013 through March 2016 were screened for enrollment in an observational cohort study. We enrolled all patients who suffered a cardiac arrest and were not following verbal commands by the time of enrollment. Our hospital policy dictates that all cardiac arrest patients that do not follow commands in the immediate post-resuscitation period are considered as candidates for TTM and are subject to a standardized approach obtaining neuroprognostication studies, if appropriate as outlined in prior publications.9–11 These neuroprognostic studies include daily neuronspecific enolase measurements on post arrest days 1–5, EEG assessing epileptiform activity and reactivity, somatosensory evoked potentials, and brain MRI. We did not exclude patients with neurological disease from this study.
Informed consent was obtained from family or a legal representative. The study was approved by the local Institutional Review Board. Patients were managed according to a standardized hospital-wide post-cardiac arrest treatment protocol.9–11 This protocol indicates targeted temperature management (TTM) to 33 °C for 24 h unless medically contraindicated. While undergoing TTM patients may be sedated with fentanyl or propofol as needed to prevent shivering, routine monitoring of electrolytes, and regular bedside glucose testing for tight glycemic control. Our institutional protocol does not specify standardized criteria for WLST. Continuous EEG monitoring is used during the hypothermia period and post-cooling period if the patient remains unconscious to rule out non-convulsive seizures. Patients transferred from outside hospitals were also enrolled; only clinical and EEG data obtained at our institution was included and was analyzed with regards to the date of arrest, not the date of transfer. All patients who were post-CA and had EEG data available were included in this study. STROBE guidelines were used to ensure standardized reporting of this observational study.12
Clinical data and outcomes
Demographic and baseline risk factor data were collected. In addition, the daily behavioral assessments based on the attending neuro-intensivist exam were used to create a “command score” following previously published methodology: this is a 6-point scale (range 0–5) in which elements of conscious behavior are quantified; 0—no response to any stimulus, 1—eye opening to verbal stimulus, 2—spontaneous eye opening, 3—tracking or attending to examiner, 4—following simple commands 5—following complex command.13 Command scores were documented daily for the first 10 days post-CA, and served as a clinical assessment of consciousness. We documented daily pupillary and corneal reflexes, neuron-specific enolase (NSE) levels daily on post-CA days 1–5, and one assessment of somatosensory evoked potentials (SSEP) between post-CA days 4–10, if available. Cerebral Performance Category (CPC) was obtained at hospital discharge, with the understanding that the majority of prognostication studies assess CPC at a longer timeframe due to the risk of short-term morbidity and mortality affecting final outcomes.14,15
EEG data acquisition and preparation
EEG recordings, were conducted using a digital bedside video monitoring system with 21 electrodes applied according to the international 10–20 system (XLTEK, Excel-Tech Corp., Natus Medical Incorporated, Oakville, Ontario, Canada; low-pass filter = 70 Hz, high-pass = 0.1 Hz, sampling rate = 256 Hz). 20-min EEG clips were obtained at the time of tactile stimulation consisting of puncturing the patients’ fingertip with a small lancet to obtain a blood drop for the bedside glucose measurement, which were performed as part of our glycemic control protocol. These blood draws were retrospectively chosen as ideal times to analyze EEG recordings, as they provided a minimal but standardized stimulation method already being performed as part of routine clinical care for all patients, and were precisely time-stamped by the automated glucometer. EEG clips were obtained until post-CA day 10. For analyses purposes, in an effort to match circadian cycles, we selected the blood draw closest to 10AM during which patient had EEG data available. (Fig. S1) EEG clips were exported in European Data Format (EDF) for quantitative analysis.
Data preparation included careful visual screening for artifact, seizures, and high-frequency periodic discharges. EEG epochs that contained muscle artifact, seizures, or periodic discharges at frequencies >2.0 Hz were excluded from analysis. EEG clips following administration of high-dose sedative medications in the 3 h preceding the clip were excluded for potential confounding of QEEG properties. High-dose sedative medications were defined at or above the following thresholds: propofol 40 mcg/kg/min, midazolam 0.1 mg/kg/h, fentanyl 75 mcg/h (or other opioid equivalent), or dexmedetomidine 0.2 mcg/kg/h.
EEG measures
All EEG analyses were performed in MatLab (Mathworks, Natick, MA), using FieldTrip16 and Chronux toolbox.17 Unless noted, EEG data analysis was performed according to previously-described methods.13 Artifact cleaning involved the identification of poor quality EEG channels based on statistical comparison with neighboring channels with distance-weighted linear interpolation.18,19 Epochs were sub- The primary compa activity, and amplitude above threshold (110 μV) were excluded from each of these trials. Data analysis was performed on each individual electrode for each epoch and analyzed separately in five frequency bands Delta (1–4 Hz), Theta (4–8 Hz), Alpha (8–14 Hz), Beta (14–24 Hz), and Gamma (24–50 Hz). For each electrode the following three quantitative metrics were calculated:
Power spectral density (PSD) using multitaper method across all frequencies.21
Local coherence, a metric quantifying the degree of signal synchronization between immediately adjacent electrodes, using weighted pairwise phase consistency (WPPC) method.19
Permutation entropy, a metric quantifying the complexity/disorder of a time series signal which can be applied to EEG analysis.18,22
Statistical analysis
The primary comparison was between patients who would regain the ability to follow commands by post-CA day 10 (“recover”) and those who would not. To facilitate robust statistical comparisons, EEG clips were grouped into three time bins for analysis, acquired on post-CA days 1–3, 4–6, or 7–10. A maximum of one EEG clip per day per patient was used. The majority of patients had available EEG clips from days in each of the three time bins. Median values of each QEEG metric were used to generate topographic maps based on QEEG metrics from these three time periods comparing patients that would recover to those that would not. Statistically significant differences were determined using the Wilcoxon rank-sum method applying a threshold of p < 0.01 after correction for multiple comparisons.
Partial least squares regression was used to identify clinical and QEEG features that predicted recovery of command following by post-CA day 10. Model building followed a two-step approach: (1) We first built a model using 12 clinical features previously identified as recovery predictors in patients with cardiac arrest: age, gender, witnessed arrest, in-hospital arrest, initiation of bystander cardiopulmonary resuscitation, initiation of therapeutic hypothermia, shockable initial rhythm, premorbid CPC, bilateral absence of pupillary reflexes and corneal reflexes through post-CA day 5, peak NSE level, and bilateral absence of N20 response on SSEP evaluation.23 We limited the clinical features of our model to those recommended as part of a multimodal prognostication strategy or widely-established to be predictive of outcome.24 Next we determined which QEEG measures remained significant when analyzed together with the clinical model developed above. This approach generated different versions of the model to predict 10-day recovery at three different time points (post-CA days 1–3, 4–6, or 7–10). For each time point only clinical and QEEG data available at that time was entered into the model. Any data collected after a patient recovered command-following was censored from the analysis. 315 individual QEEG-based input features (21 electrodes × 3 QEEG metrics × 5 frequency bands) were utilized. The predictive model was generated using 50% of data for training and 50% for testing, with random assignment of data into each group and 1000-fold repetition of set allocation. Predictive accuracy was expressed as the area under the Receiver Operating Characteristics curve (AUC) averaged across all trials. Explained variance (R2) was calculated to determine which features of the model contributed the most to the predictive accuracy at each of the time periods.
Results
EEG recordings
We obtained a total of 494 EEG epochs from 102 subjects. Of these, 127 epochs were excluded from analysis due to the presence of seizures or high frequency periodic discharges (n = 28), high sedation levels (n = 98), or abundant muscle artifact (n = 9), leaving 367 epochs from 98 subjects for final analysis. 144 epochs were acquired from post-CA days 1–3, 131 epochs from days 4–6, and 92 epochs from days 7–10. Of the 28 clips excluded for presence of seizures or high-frequency periodic discharges, 4 were from two patients who would go on to recover command-following by day 10. One of these two patients had generalized convulsive seizures and the other generalized periodic discharges and electrographic seizures.
Patient cohort
Of 102 subjects initially included, 4 were excluded as their only available EEG data was not acceptable per the above criteria. We included 98 unconscious patients with CA of whom 40 regained command-following by post-CA day 10 (Table 1). Mean age was 57 ± 18 years, return of spontaneous circulation (ROSC) time 19 ± 19 min, and shockable initial rhythm present in 31%. 19 patients underwent WLST before post-CA day 10. 13 patients who recovered command-following by post-CA day 10 subsequently died before hospital discharge, while 7 patients who did not recover command-following by day 10 did so prior to hospital discharge. Median CPC at hospital discharge (including expired patients) was 5 (IQR 3, 5) (Table 2).
Table 1 –
Patient characteristics (n = 98).
| Demographics and comorbidities | |
|
| |
| Age in years | 58 ±18 |
| Male sex | 61 (60%) |
| Pre-arrest mRS | 2 [0, 3] |
| Pre-arrest CPC | 1 [1, 2] |
| CPC Distribution: 1 (n = 70), 2 (n = 15), 3 (n = 12), 4 (n = 1), 5 (n = 0) | |
| Past medical history | |
| Myocardial infarct | 19 (19%) |
| Heart failure | 37 (36%) |
| Arrhythmia | 17(17%) |
| Diabetes | 30 (30%) |
| Cerebrovascular disease | 18 (18%) |
| Peripheral vascular disease | 12 (12%) |
| Hypertension | 57 (56%) |
| Dementia | 11 (11%) |
|
| |
| Arrest characteristics | |
|
| |
| In-hospital arrest | 49 (48%) |
| Witnessed arrest | 82 (80%) |
| Bystander CPR (if witnessed) | |
| None | 26 (26%) |
| By lay bystander | 15 (15%) |
| By medical personnel | 57 (56%) |
| Shockable initial rhythm (VT or VF) | 32 (31%) |
| Targeted temperature management | 76 (75%) |
| Initial temperature in degrees C | 36.4 [35.9, 36.9]a |
| ROSC time (min) | 12 [8, 21]b |
Normally-distributed variables reported as mean ± SD. Non-normally-distributed data reported as median [interquartile range].
Abbreviations: CPC, Cerebral Performance Category; mRS, Modified Rankin scale; ROSC, return of spontaneous circulation; VF, ventricular fibrillation; VT, ventricular tachycardia.
Data available for 81 subjects only.
Data available for 91 subjects only.
Table 2 –
Outcomes and prognostic data.
| Outcomes | |
|
| |
| DNR order placed | 53 (52%) |
| Post-arrest day of DNR order | 5 [2, 8] |
| WLST performed (overall) | 35 (34%) |
| WLST performed (by post-CA day 10) | 19 (19%) |
| Median post-arrest day of WLST | 8 [5, 14] |
| Following commands at post-CA day 10 | 40 (39.2%) |
| Length of ICU stay (days) | 9 [6, 15] |
| CPC at hospital discharge | 5 [3, 5] |
| CPC Distribution: 1 (n = 11), 2 (n = 11), 3 (n = 11), 4 (n = 5), 5 (n = 60) | |
|
| |
| Prognostication characteristics | |
|
| |
| SSEPs performed | 25 (26%) |
| Bilaterally absent N20 response | 14 (56.0%) |
| SSEP performed (post-arrest day) | 6 [5, 8] |
| NSE levels collected | 77 (79%) |
| Peak NSE (ng/mL) | 72.8 ± 10.0 |
| Peak NSE (CPC 1–2 at hospital discharge) | 24.8 ± 3.4 |
| Peak NSE (CPC 3–5 at hospital discharge) | 83.4 ± 11.8 |
| Peak NSE (post-arrest day) | 3 [2, 4] |
| EEG reactivity identified by day 5 | 55 (56%) |
| Bilaterally absent pupils and corneal reflexes through day 5 | 11 (10.8%) |
Normally-distributed variables reported as mean ± SD. Non-normally-distributed data reported as median [interquartile range].
Abbreviations: CPC, Cerebral Performance Category; DNR, do not resuscitate, GCS, Glasgow Coma Scale; NSE, neuron specific enolase; SSEPs, somatosensory evoked potentials; WLST, withdrawal of life sustaining therapy.
Predictive model
Predictive ability of the different versions of our model expressed as mean AUC, utilizing clinical and/or QEEG features, is presented in Fig. 1. When QEEG metrics were added to clinical data (Supplemental Tables 1–3), predictive ability improved significantly for data from post-CA days 4–6 and 7–10 (p < 10e−10). The best predictive ability was an average AUC of 0.86 0.05, obtained from the model using all clinical and QEEG data from post-CA days 4–6. When looking at data from post-CA days 1–3, predictive ability worsened significantly with the addition of QEEG metrics, from 0.84 ± 0.04 to 0.79 ± 0.05 (p < 10e−10).
Fig. 1 –
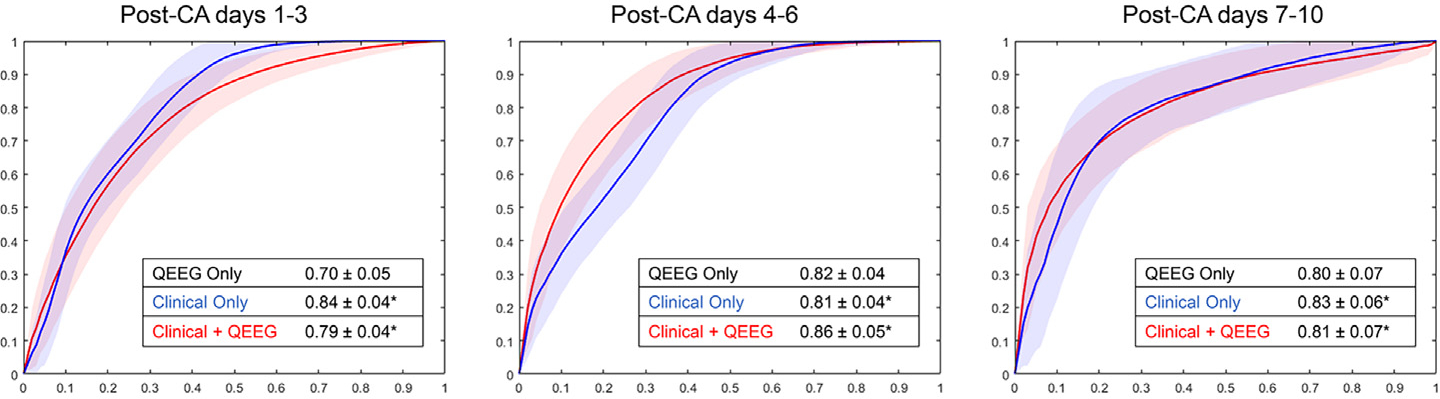
Mean area under the Receiver Operating Characteristics curve (AUC) averaged across 1000 trials of predictive model generation, for each of the three time periods under study. The most significant benefit of adding QEEG data to clinical data was seen on post-CA days 4–6. In each of the later two time bins, QEEG data alone was as effective as clinical data alone in predicting recovery of command-following by day 10. * = significance of p < 10e−10.
Explained variance analysis
Our predictive model was constructed with 12 clinical and 315 QEEG features. To better understand which features contributed most weight to the overall predictive accuracy, we calculated the explained variance (R2) of each feature in each of the three time bins. We found that individual clinical features carried the most weight in the analysis of data from post-CA days 1–3, but were significantly less influential compared to QEEG features in the later two time bins (Fig. 2). All QEEG features explained 11% of the model variance, clinical features explained 30%, and all features combined explained 35% of the model variance.
Fig. 2 –
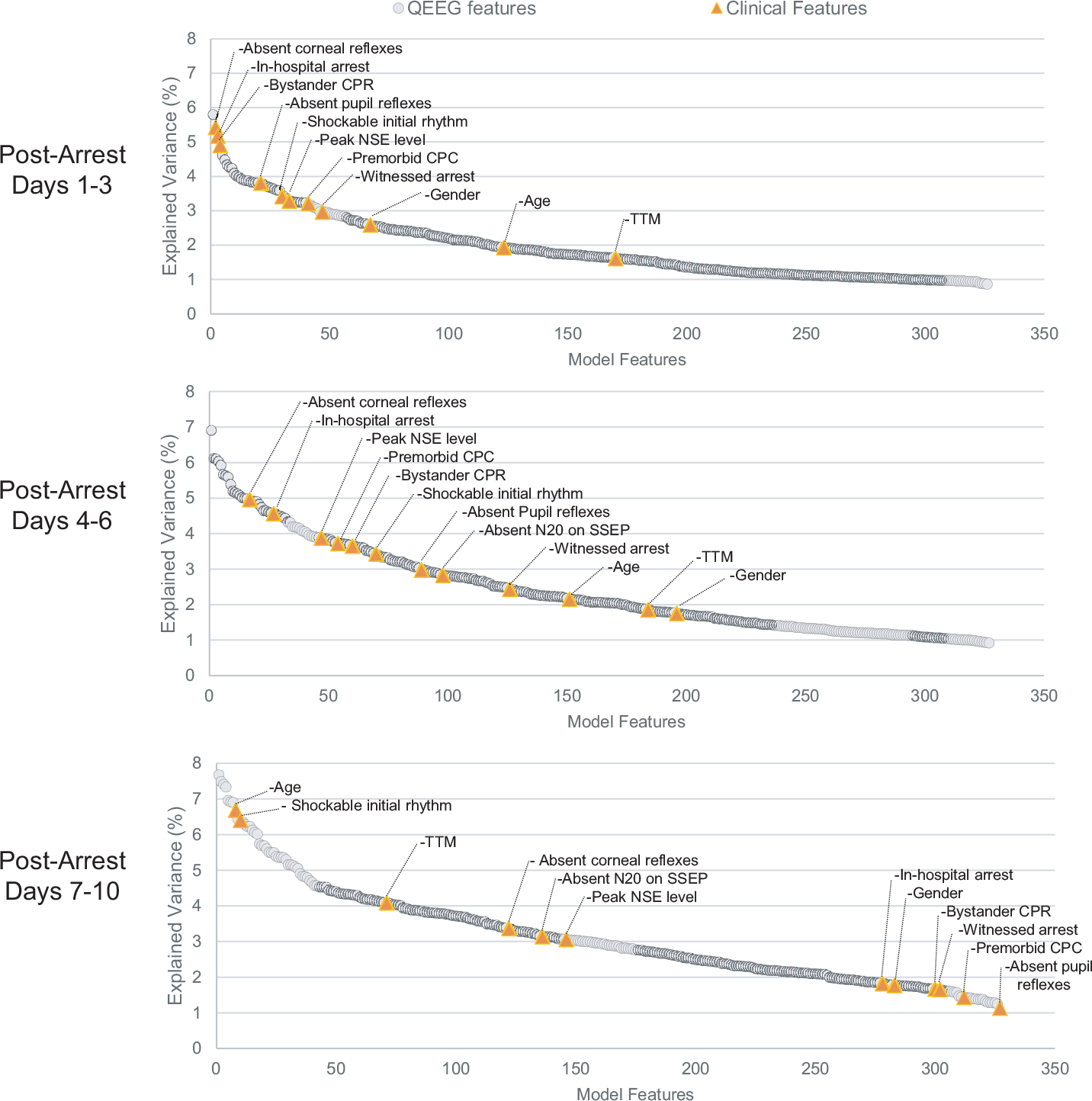
Explained variance (R2) for each model feature, presented for each time bin and ranked in order of highest R2 value. In post-CA days 1–3, clinical features showed a higher influence compared to QEEG features. In the later time periods this trend had reversed, with QEEG features playing a much larger relative role in the prognostic model. When combined, all QEEG features explained 10.9% of the model variance, while all clinical features combined explained 29.0%, and all features combined explained 34.9% of the model variance.
Topographic plots of quantitative metrics
Review of topographic maps for each metric revealed patterns of electrode clusters with significant differences (p < 0.01) between those from patients who would or would not recover consciousness by post-CA day 10. Patients with recovery by day 10 compared to those without had early in their post arrest course (at days 4–6) higher broad-spectrum coherence and higher centro-occipital slow frequency spectral power (Delta and Theta range) Later in the course (days 7–10) these patients demonstrated globally higher permutation entropy in theta frequency range (Fig. 3). All plots in which clusters of 3 or more statistically-significant adjacent electrodes were identified are presented in Supplemental materials (Fig. S2).
Fig. 3 –
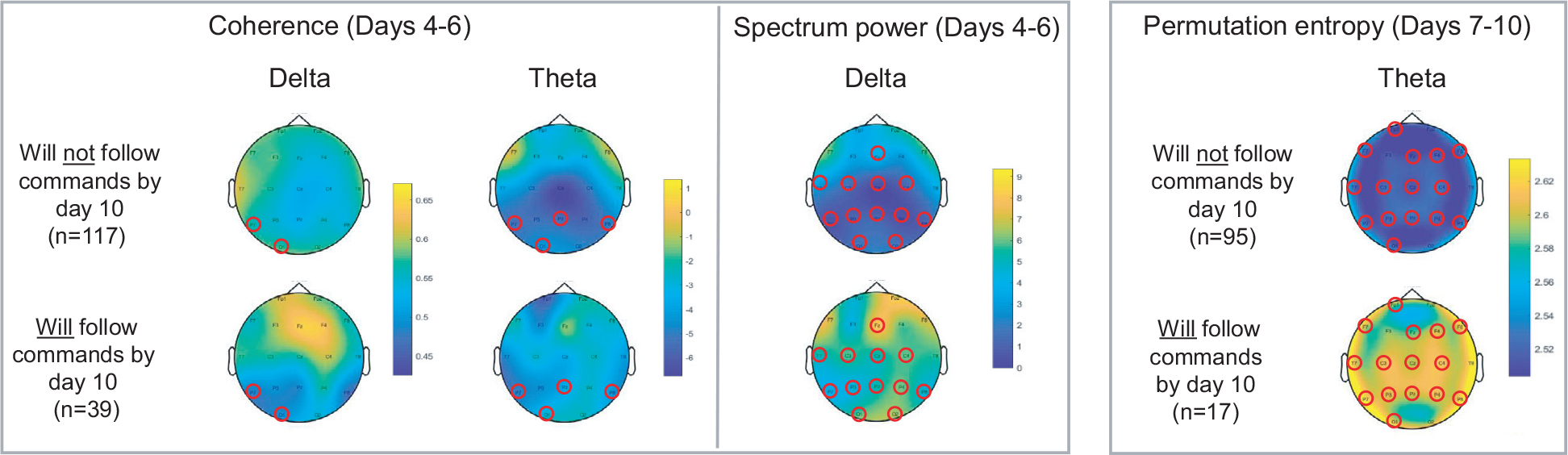
Red circles designate electrodes which showed a significant difference (p < 0.01) in mean QEEG values between patients who would or would not go on to recover command following by day 10. Patients with recovery by day 10 had higher broad-spectrum coherence and higher centro-occipital slow frequency spectral power (Delta and Theta range) early in their post-arrest course (at days 4–6). Later in the course (days 7–10) these patents demonstrated globally higher permutation entropy in theta frequency range.
Discussion
In this study, we found that the addition of QEEG data from post-CA days 4–6 improved prediction of early recovery of command-following 10 days after CA, compared to a model using previously-identified clinical predictors alone. The degree to which prognostic accuracy improved adding QEEG measures was modest; further work will be needed to identify the ideal metrics and strategy to yield the greatest improvement. Much of the focus has been on identifying predictors of long-term recovery. This is problematic as WLST is common and predictors of long-term outcomes are difficult to disentangle from the self-fulfilling prophecy of early WLST in clinical studies. As early recovery of consciousness after cessation of sedation may reduce the likelihood of WLST, we chose to focus on identifying clinical and electrophysiological predictors of short term recovery of command following. Additionally, short-term recovery of consciousness is valuable as it predicts long-term functional recovery.7 Timeframes were chosen based on clinical practice, selecting times when goals of care discussions with families typically occur. While this timing is not uniform, often prognostication and goals-of-care discussions are initiated following rewarming (day 3) and within 7 days of CA.
Earlier efforts to integrate EEG data into post-CA prognostication have used relatively simple qualitative EEG features, such as generalized suppression, burst suppression, or specific patterns of generalized discharges.25,26 Visual assessment of EEG reactivity has specifically been studied, and one multicenter cohort study showing that this metric alone was not able to predict outcome, however when added to a multimodal predictive model, it did significantly increase predictive ability.27 Other simple QEEG metrics such as amplitude-adjusted EEG have also been studied to a limited extent.28 The approach used here of combining data from multiple QEEG metrics with other clinical data into a single predictive model is a potentially more sensitive application of QEEG in this patient population. One study using machine-learning-based analysis of QEEG data has successfully demonstrated improved prognostication for long term outcomes, however the optimal method for analyzing QEEG data and integrating it with established predictors is not yet known.29 Resting state EEG can detect neuronal activity, however there are large variations in level of arousal in patients in the ICU setting; we chose to use EEG epochs specifically from moments of known standardized physical stimulation (blood draws) to reduce this variability as much as possible.
Therapeutic hypothermia may impact QEEG measures. Both human and animal studies have shown that quantitative metrics including frequency band amplitude, approximate entropy, wavelet subband entropy, bispectral index, and burst suppression ratio are significantly impacted by hypothermia in the range of 32–34 °C.6 At least one study concluded that hypothermia does not impact the prognostic ability of a QEEG-based model, however this is controversial.30–32 In our study, the predictive ability of our model was moderately improved by the addition of QEEG data during all later time points, however was impaired by adding this data during the first 3 days. As hypothermia was the major clinical variable present during days 1–3 only, one interpretation is that hypothermia may negatively impact the prognostic value of QEEG in this setting. This is further supported by the analysis of explained variance of individual model features, showing a relatively lower influence of QEEG metrics compared to clinical features in the first 1–3 days. This observation supports the view that early prognostication before clinical re-warming may be premature due to multiple potential confounding factors.33
The impact of sedatives on prognostic tests, including QEEG metrics, is not well established. One study found that propofol infusion does not impact reliability of a multimodality prognostic model utilizing QEEG.31 Other studies have also suggested that sedation does not impact prognostic utility of QEEG, however it does alter QEEG values (specifically causing a higher suppression ratio, decrease in amplitude-adjusted EEG power, but no impact on alpha-delta ratio).34 We reduced the impact of this confounder in our study by excluding all subjects who were receiving high doses of sedatives. While this approach could potentially introduce other exclusion bias, we felt this was a lesser concern than the possible direct impact of sedatives themselves in altering QEEG values.
Several QEEG patterns identified here are consistent with prior observations. Higher total spectral power in the first 6 days after arrest was seen in delta and theta frequencies only in those subjects who would go on to recover, consistent with prior observations describing reafferentation of thalamo-cortical and cortico-cortical networks during acute recovery of consciousness.4 Global increase in permutation entropy was seen in theta frequency only in the few days immediately preceding return of command-following in our data set. A similar finding has been reported in studies of chronic disorders of consciousness, in which higher values for theta-band permutation entropy are specifically associated with subjects in higher states of consciousness.35 Finally, we identified local coherence to be higher in a cluster of posterior region electrodes in post-CA days 4–6 in subjects who would recover, across multiple frequency bands. This result is somewhat different than prior observations in a propofol-induced loss of consciousness model, in which return of consciousness was associated with increased frontal coherence and decrease in posterior coherence in the alpha band.36 This may be explained by the fact that our data was from the period immediately preceding recovery of consciousness and differences in the disease model.
There are several weaknesses of this study. Results may be less generalizable to all post-CA populations, as our cohort was from a single center and included a high number of in-hospital arrests with high degree of medical comorbidity, compared to most large studies of post-CA outcomes. Verbal command-following is only one manifestation of consciousness, and our clinical assessment scale would not detect some features of minimally-conscious states, such as visual tracking and object manipulation, or patients with cognitive-motor dissociation. Our results are impacted by survival and treatment biases due to WLST, which affects most studies of this nature. Median day of WLST in our study however was post-CA day 8 (IQR 5.5–14), leaving a relatively narrow timeframe for a small number of subjects who might have otherwise recovered command-following to do so, such that we feel this bias had a relatively small impact on our conclusions. Additionally, not all patients had all prognostic markers available, and we did not have long-term outcome data available for this cohort. Importantly, only qEEG measures obtained in the post arrest day 4–6 improved, whereas qEEG measures obtained prior to 4 days or after 6 days actually worsened the prediction accuracy of the model. This raises questions as to the consistency of this measure which will need to be demonstrated in follow-up studies replicating these findings. We want to submit however, that qEEG measures obtained within the first 3 days after arrest would have had the highest risk of being heavily affected by sedation and hypothermia, resulting in qEEG measures adding noise to the model. Beyond one week outcomes will more likely be confounded by WLST and qEEG measures would likely add little. As anticipated, qEEG measures obtained 4–6 days after the arrest are most informative adding modestly to the outcome prediction model. The strengths of this study include (a) blinding features of the prognostic model which would not be known to a clinician at that time, simulating real-world application of this data, (b) stringent exclusion of epochs with potential confounders such as sedation and seizure activity from analysis, (c) standardized stimulation method at the time of EEG collection, and (d) care guided by standardized institutional protocol.
Conclusions
Integration of quantitative EEG metrics into a prognostic model for post-CA patients modestly improves discriminatory ability to predict those who will recover command-following by post-arrest day 10. In clinical practice, this approach may be more practical than integration of EEG data based on visual review of the raw EEG such as reactivity. We found that the predictive value from QEEG in our model was more significant than that from SSEP. As there is often a broader role for EEG for seizure detection in this population, QEEG may be a more cost-effective prognostic tool than SSEP or other supplementary prognostic measures in this population. Data from post-CA days 4–6 had the highest predictive ability in our model, and integration of QEEG data from days 1–3 paradoxically decreased prognostic ability of our model. QEEG data appears to assist in neuro-prognostication, however the optimal strategy for integration into prognostic models has yet to be determined.
Supplementary Material
Acknowledgements
We would like to thank the nurses, attending physicians, fellows, and neurology residents of the Neurological ICU of Columbia University Medical Center for their overall support of this project.
Financial support and sponsorship
JC is supported by grant funding from the NIH R01 NS106014 and R03 NS112760, and the DANA Foundation. SP is supported by grant funding from the NIH K01 ES026833.
Footnotes
CRediT authorship contribution statement
Andrew Bauerschmidt: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft. Andrey Eliseyev: Methodology, Software, Formal analysis, Investigation. Kevin W. Doyle: Methodology, Formal analysis, Investigation. Angela Velasquez: Data curation, Writing - review & editing. Jennifer Egbebike: Investigation, Data curation, Writing - review & editing. Wendy Chiu: Investigation, Data curation, Writing - review & editing. Vedika Kumar: Data curation, Writing - review & editing. Ayham Alkhachroum: Writing - review & editing. Caroline Der Nigoghossian: Writing - review & editing. Fawaz Al-Mufti: Writing - review & editing. LeRoy Rabbani: Writing - review & editing. Daniel Brodie: Writing - review & editing. Clio Rubinos: Writing - review & editing. Soojin Park: Writing - review & editing. David Roh: Writing - review & editing. Sachin Agarwal: Writing - review & editing. Jan Claassen: Conceptualization, Methodology, Investigation, Writing - original draft, Supervision, Funding acquisition.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.resuscitation.2021.06.008.
REFERENCES
- 1.Berg KM, Soar J, Andersen LW, et al. Adult advanced life support: 2020 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 2020;142:S92–S139, doi: 10.1161/CIR.0000000000000893 [published Online First: 2020/10/22]. [DOI] [PubMed] [Google Scholar]
- 2.Merchant RM, Topjian AA, Panchal AR, et al. Part 1: executive summary: 2020 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2020;142:S337–57, doi: 10.1161/CIR.0000000000000918 [published Online First: 2020/10/22]. [DOI] [PubMed] [Google Scholar]
- 3.Nolan JP, Sandroni C, Bottiger BW, et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: post-resuscitation care. Resuscitation 2021;161:220–69, doi: 10.1016/j.resuscitation.2021.02.012 [published Online First: 2021/03/29]. [DOI] [PubMed] [Google Scholar]
- 4.Forgacs PB, Frey HP, Velazquez A, et al. Dynamic regimes of neocortical activity linked to corticothalamic integrity correlate with outcomes in acute anoxic brain injury after cardiac arrest. Ann Clin Transl Neurol 2017;4:119–29, doi: 10.1002/acn3.385 [published Online First: 2017/02/09]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azabou E, Navarro V, Kubis N, et al. Value and mechanisms of EEG reactivity in the prognosis of patients with impaired consciousness: a systematic review. Crit Care 2018;22:184, doi: 10.1186/s13054-018-2104-z [published Online First: 2018/08/04]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asgari S, Moshirvaziri H, Scalzo F, et al. Quantitative measures of EEG for prediction of outcome in cardiac arrest subjects treated with hypothermia: a literature review. J Clin Monit Comput 2018;32:977–92, doi: 10.1007/s10877-018-0118-3 [published Online First: 2018/02/27]. [DOI] [PubMed] [Google Scholar]
- 7.Claassen J, Doyle K, Matory A, et al. Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med 2019;380:2497–505, doi: 10.1056/NEJMoa1812757 [published Online First: 2019/06/27]. [DOI] [PubMed] [Google Scholar]
- 8.Faugeras F, Rohaut B, Valente M, et al. Survival and consciousness recovery are better in the minimally conscious state than in the vegetative state. Brain Inj 2018;32:72–7, doi: 10.1080/02699052.2017.1364421 [published Online First: 2017/11/22]. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal S, Morris N, Der-Nigoghossian C, et al. The influence of therapeutics on prognostication after cardiac arrest. Curr Treat Options Neurol 2019;21:60, doi: 10.1007/s11940-019-0602-1 [published Online First: 2019/11/27]. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal S, Presciutti A, Roth W, et al. Determinants of long-term neurological recovery patterns relative to hospital discharge among cardiac arrest survivors. Crit Care Med 2018;46:e141–50, doi: 10.1097/CCM.0000000000002846 [published Online First: 2017/11/15]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds AS, Guo X, Matthews E, et al. Post-anoxic quantitative MRI changes may predict emergence from coma and functional outcomes at discharge. Resuscitation 2017;117:87–90, doi: 10.1016/j.resuscitation.2017.06.010 [published Online First: 2017/06/19]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–9, doi: 10.1016/j.jclinepi.2007.11.008 [published Online First: 2008/03/04]. [DOI] [PubMed] [Google Scholar]
- 13.Claassen J, Velazquez A, Meyers E, et al. Bedside quantitative electroencephalography improves assessment of consciousness in comatose subarachnoid hemorrhage patients. Ann Neurol 2016;80:541–53, doi: 10.1002/ana.24752 [published Online First: 2016/07/30]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet 1975;1:480–4, doi: 10.1016/s0140-6736(75)92830-5 [published Online First: 1975/03/01]. [DOI] [PubMed] [Google Scholar]
- 15.Taccone FS, Horn J, Storm C, et al. Death after awakening from post-anoxic coma: the “Best CPC” project. Crit Care 2019;23:107, doi: 10.1186/s13054-019-2405-x [published Online First: 2019/04/05]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oostenveld R, Fries P, Maris E, et al. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011;2011:156869, doi: 10.1155/2011/156869 [published Online First: 2011/01/22]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitra P, Bokil H. Observed brain dynamics. Oxford, New York: Oxford University Press; 2008. [Google Scholar]
- 18.Bandt C, Pompe B. Permutation entropy: a natural complexity measure for time series. Phys Rev Lett 2002;88:174102, doi: 10.1103/PhysRevLett.88.174102 [published Online First: 2002/05/15]. [DOI] [PubMed] [Google Scholar]
- 19.Vinck M, van Wingerden M, Womelsdorf T, et al. The pairwise phase consistency: a bias-free measure of rhythmic neuronal synchronization. Neuroimage 2010;51:112–22, doi: 10.1016/j.neuroimage.2010.01.073 [published Online First: 2010/02/02]. [DOI] [PubMed] [Google Scholar]
- 20.Hjorth B Source derivation simplifies topographical EEG interpretation. Am J EEG Technol 1980;20:121–32, doi: 10.1080/00029238.1980.11080015. [DOI] [Google Scholar]
- 21.Bokil H, Andrews P, Kulkarni JE, et al. Chronux: a platform for analyzing neural signals. J Neurosci Methods 2010;192:146–51, doi: 10.1016/j.jneumeth.2010.06.020 [published Online First: 2010/07/20]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wielek T, Lechinger J, Wislowska M, et al. Sleep in patients with disorders of consciousness characterized by means of machine learning. PLoS One 2018;13:e0190458, doi: 10.1371/journal.pone.0190458 [published Online First: 2018/01/03]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dumas F, Rea TD. Long-term prognosis following resuscitation from out-of-hospital cardiac arrest: role of aetiology and presenting arrest rhythm. Resuscitation 2012;83:1001–5, doi: 10.1016/j.resuscitation.2012.01.029 [published Online First: 2012/02/07]. [DOI] [PubMed] [Google Scholar]
- 24.Geocadin RG, Callaway CW, Fink EL, et al. Standards for studies of neurological prognostication in comatose survivors of cardiac arrest: a scientific statement from the American Heart Association. Circulation 2019;140:e517–42, doi: 10.1161/CIR.0000000000000702 [published Online First: 2019/07/12]. [DOI] [PubMed] [Google Scholar]
- 25.Muhlhofer W, Szaflarski JP. Prognostic value of EEG in patients after cardiac arrest—an updated review. Curr Neurol Neurosci Rep 2018;18:16, doi: 10.1007/s11910-018-0826-6 [published Online First: 2018/03/12]. [DOI] [PubMed] [Google Scholar]
- 26.Westhall E, Rossetti AO, van Rootselaar AF, et al. Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology 2016;86:1482–90, doi: 10.1212/WNL.0000000000002462 [published Online First: 2016/02/13]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Admiraal MM, van Rootselaar AF, Hofmeijer J, et al. Electroencephalographic reactivity as predictor of neurological outcome in postanoxic coma: a multicenter prospective cohort study. Ann Neurol 2019;86:17–27, doi: 10.1002/ana.25507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugiyama K, Miyazaki K, Ishida T, et al. Categorization of post-cardiac arrest patients according to the pattern of amplitude-integrated electroencephalography after return of spontaneous circulation. Crit Care 2018;22(1)226, doi: 10.1186/s13054-018-2138-2 [published Online First: 2018/09/22]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang Z, Wang Y, Sun X, et al. EEG entropy measures in anesthesia. Front Comput Neurosci 2015;9:16, doi: 10.3389/fncom.2015.00016 [published Online First: 2015/03/06]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofmeijer J, Ruijter BJ, van Putten M. Reply to “early electroencephalogram for neurologic prognostication: a self-fulfilling prophecy?”. Ann Neurol 2019;86:474, doi: 10.1002/ana.25538 [published Online First: 2019/06/30]. [DOI] [PubMed] [Google Scholar]
- 31.Ruijter BJ, van Putten MJAM, van den Bergh WM, et al. Propofol does not affect the reliability of early EEG for outcome prediction of comatose patients after cardiac arrest. Clin Neurophysiol 2019;130:1263–70, doi: 10.1016/j.clinph.2019.04.707. [DOI] [PubMed] [Google Scholar]
- 32.Cho SM, Mulder M, Geocadin RG. Early electroencephalogram for neurologic prognostication: a self-fulfilling prophecy? Ann Neurol 2019;86:473–4, doi: 10.1002/ana.25539 [published Online First: 2019/06/30]. [DOI] [PubMed] [Google Scholar]
- 33.Cronberg T Assessing brain injury after cardiac arrest, towards a quantitative approach. Curr Opin Crit Care 2019;25:211–7, doi: 10.1097/MCC.0000000000000611 [published Online First: 2019/04/26]. [DOI] [PubMed] [Google Scholar]
- 34.Drohan CM, Cardi AI, Rittenberger JC, et al. Effect of sedation on quantitative electroencephalography after cardiac arrest. Resuscitation 2018;124:132–7, doi: 10.1016/j.resuscitation.2017.11.068 [published Online First: 2017/12/05]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sitt JD, King JR, El Karoui I, et al. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain 2014;137:2258–70, doi: 10.1093/brain/awu141 [published Online First: 2014/06/13]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purdon PL, Pierce ET, Mukamel EA, et al. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci U S A 2013;110:E1142–51, doi: 10.1073/pnas.1221180110 [published Online First: 2013/03/15]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


