Abstract
Objective:
Sleep deprivation has been associated with risk of autoimmune diseases. We investigated whether it was associated with risk of developing SLE using the Nurses’ Health Study (NHS) (1986–2016) and NHSII (1989–2017) cohorts.
Methods:
Average sleep duration in a 24-hour period was reported in the NHS (1986–2014) and NHSII (1989–2009). Lifestyle, exposure, and medical information was collected on biennial questionnaires. Adjusted Cox regression analyses modeled associations between cumulative average sleep duration (categorical variables) and incident SLE. Interactions between sleep duration and shiftwork, bodily pain (Short-Form 36 questionnaire, SF-36) and depression were examined.
Results:
We included 186,072 women with 187 incident SLE cases during 4,246,094 person-years of follow-up. Chronic low sleep duration (≤5 hours/night vs reference >7–8 hours) was associated with increased SLE risk (adjusted HR 2.47, 95%CI:1.29–4.75), which persisted after the analysis was lagged (4 years, adjusted HR 3.14, 95%CI:1.57–6.29) and adjusted for shiftwork, bodily pain, and depression (adjusted HR 2.13, 95%CI:1.11–4.10). We detected additive interactions between low sleep duration and high bodily pain (SF-36 <75) with an attributable proportion (AP) of 64% (95%CI:40%-87%) and HR for SLE of 2.97 (95%CI:1.86–4.75) for those with both risk factors compared to those with neither. Similarly, there was an interaction between low sleep duration and depression with an AP of 68% (95%CI:49%-88%) and an HR for SLE of 2.82 (95%CI:1.64–4.85).
Conclusion:
Chronic low sleep duration was associated with higher SLE risk, with stronger effects among those with bodily pain and depression, highlighting the potential role of adequate sleep in disease prevention.
Keywords: systemic lupus erythematosus, epidemiology, risk factor, shiftwork, sleep
Introduction
Sleep deprivation is an underrecognized public health concern that is increasing in prevalence worldwide. Sleeping less than the recommended 7 hours a night has been linked to several adverse health outcomes and increased overall mortality (1). Sleep is also crucial to supporting the immune system as sleep deprivation and sleep disorders have been associated with increased risk of other autoimmune diseases, including inflammatory bowel disease (IBD), rheumatoid arthritis (RA), ankylosing spondylitis, and Sjögren’s syndrome in past studies (2, 3).
Several small case-control and cohort studies have provided conflicting results on the relationship of sleep duration and systemic lupus erythematosus (SLE) risk (3–6). A challenge in studying sleep duration and SLE is that there may be many potential confounders and effect modifiers, including pain, depression, shiftwork, and hormonal status, that need to be considered. Furthermore, sleep disturbances in established SLE are common (7), and active disease has been associated with sleep fragmentation, which in turn, may induce sleep deprivation (8). Therefore, reverse causation is also a concern, but have not been well studied in relation to SLE development.
The Nurses’ Health Study (NHS) consists of two large cohorts of women with detailed prospectively collected exposure and health information that can address some of the challenges in studying sleep. There is already evidence from the NHS cohorts that sleep deprivation may influence the immune system to increase the risk of IBD (2). The purpose of this study was to prospectively evaluate the association between sleep duration and risk of SLE in the NHS cohorts.
Methods
Study design and population
The NHS and NHSII cohorts were established in 1976 and 1989, respectively, with 121,700 married female registered nurses enrolled in NHS and 116,430 married female registered nurses enrolled in NHSII. Questionnaires including assessments of a range of lifestyle factors and the development of new diseases and other outcomes were mailed to and completed by participants at baseline and then biennially in follow-up. We excluded participants with prevalent SLE or other connective tissue disease (CTD) at baseline (n=7,177 in NHS and n=1,371 in NHSII). We included 96,240 women in the NHS (followed 1986–2016) and 105,460 women in the NHSII (followed 1991–2017). Follow-up rates in these longitudinal cohort studies have been high with only ~5% of person-time lost to follow-up. This study was approved by the Mass General Brigham Institutional Review Board.
Assessment of Sleep Duration
In the NHS and NHSII, self-reported sleep duration has been validated previously (9). In the NHS, participants were asked about “total hours of actual sleep in 24-hour period” and the available responses were ≤ 5, 6, 7, 8, 9, 10, or 11 hours or more. This was reported in the NHS in 1986, 2000, 2002, 2008, 2012, 2014; and 1989, 1991, 1993, 1995, 1997, 1999, 2003, 2005, and 2009 in the NHSII. The cumulative average of sleep duration was calculated by taking the sum of the sleep duration for an individual divided by the number of assessments over the assessment period. Figure 1 illustrates the study design and how the cumulative average of sleep duration was calculated, as an example for a NHSII participate, using 9 repeated assessments (in 1989, 1991, 1993, 1995, 1997, 1999, 2003, 2005, and 2009) to predict whether SLE occurred in the window between 2011 and 2016 (end of NHSII follow-up). The cumulative average of sleep duration was categorized as: <=5, >5–6, >6–7, >7–8, and >8 hours. There were small numbers of participants in the high categories so sleep duration beyond 8 hours was collapsed into one category as done in other NHS studies (2, 9). The category >7–8 hours was made the reference category to be consistent with the Institutes of Medicine’s recommendation of 7–8 hours of sleep per night (1).
Figure 1. Study schematic illustrating the prospective cohort design for NHSII.
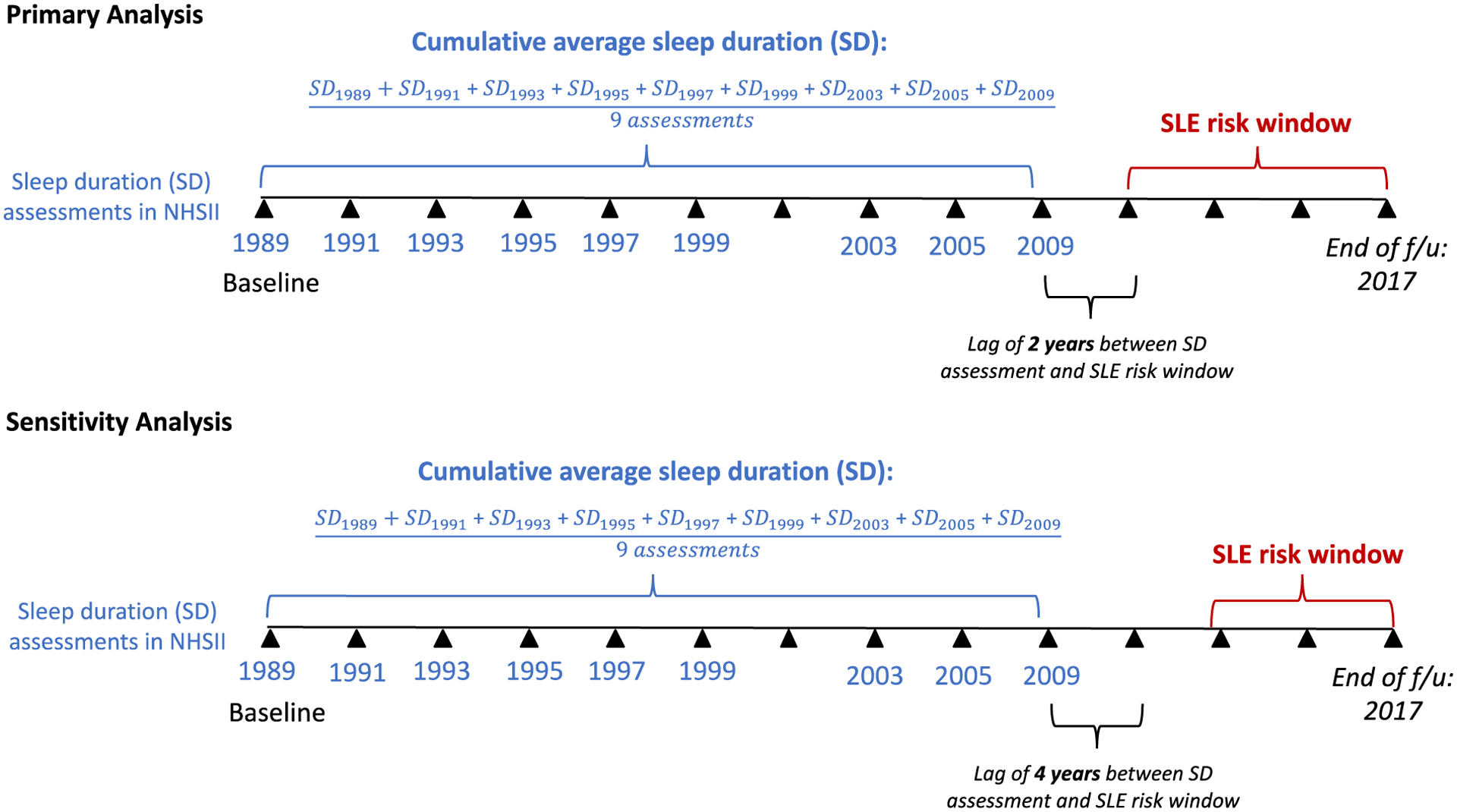
The primary exposure was cumulative average sleep duration and the outcome was SLE onset at least two years after last sleep duration assessment. The primary analysis was conducted so that there was always at least two years between the last sleep duration exposure assessment and the outcome date of SLE diagnosis that occurred in the SLE risk window. The analysis for NHS was similar except for the years of sleep duration assessment (1986–2014). The sensitivity analysis was lagged by another follow-up cycle of two years so that there was at least four years between the last sleep duration exposure assessment and the outcomes dates of SLE diagnosis. NHS, Nurses’ Health Study, NHSII, Nurses’ Health Study II, SD, sleep duration, SLE, systemic lupus erythematosus.
Ascertainment of cases
Methods for SLE case identification and validation have previously been reported (10). Briefly, participants who self-reported a diagnosis of SLE was asked to complete the validated CTD Screening Questionnaire, which included 13 questions concerning symptoms of SLE (10). For those who screened positive, medical records were requested and independently reviewed by two board-certified rheumatologists to confirm whether patients fulfilled the Updated American College of Rheumatology (ACR) 1997 SLE Classification Criteria (11). Participants were censored during follow-up upon self-report of a non-SLE CTD, or upon SLE self-report not confirmed by medical record review. Anti-dsDNA antibody status at SLE diagnosis was determined by medical record review. Laboratory tests including the antinuclear antibody and anti-dsDNA antibody tests were performed using standard assays.
Covariates
Demographic and clinical data were updated on biennial questionnaires. Race was treated as binary (White vs. non-White). Household median income for each U.S. Census-tract as a marker of socioeconomic status was categorized by quartiles. We also included potential reproductive covariates associated with incident SLE as confounders, including oral contraceptive use (never vs. ever), age at menarche (≤10 years vs. >10 years), and menopausal status (pre-menopausal, post-menopausal/never used post-menopausal hormones, and post-menopausal/ever used post-menopausal hormones).
We additionally adjusted for shiftwork given its relationship with sleep duration. In 1988, shiftwork was assessed in the NHS when participants were asked “What is the total number of years during which you worked rotating night shifts (at least 3 nights per month in addition to days or evenings in that month)?”. In 1989, this question was asked to participants of the NHSII. Response categories included never, 1–2, 3–5, 6–9, 10–14, 15–19, 20–29, and ≥ 30 years in the NHS, while the highest response category in NHSII was ≥ 20 years. For this analysis, we used none, >0–5.9 years vs. >6 years of shiftwork. We also adjusted for history of depression, which can influence sleep duration. We used a composite measure of depression, as in our past studies, in which women who reported antidepressant use, had a depression diagnosis, or had a mental health inventory (MHI-5) score (subscale of the general health measure Short Form-36 (SF-36) score) indicating probable depression, were classified as having a history of depression at the first year in which they reported one of these indicators and for all subsequent years. Self-reported pain was also measured using the bodily pain subscale of the Short Form-36, categorized in quartiles.
Statistical methods
Time-varying Cox regression models estimated the multivariable-adjusted hazard ratio (HRs [95% confidence intervals]) of incident SLE associated with sleep duration as cumulative average falling in the categories ≤ 5 hours, >5–6, >6–7, >7–8, and >8, adjusting for potential confounders. Confounders were chosen a priori based on existing literature. This includes age, White race, household median income, BMI, alcohol consumption, smoking status, physical activity, diet, shiftwork, depression, bodily pain, oral contraceptive use, age at menarche, and menopausal status (pre-menopausal, post-menopausal/never used post-menopausal hormones, and post-menopausal/ever used post-menopausal hormones) (refer to covariates section above for how these variables were defined). All variables were time-varying except for race.
We performed a sensitivity analysis by excluding incident cases occurring within four years of sleep assessment, i.e., “lagged” by two questionnaire cycles, to address potential reverse causation whereby underlying disease might affect sleep duration (Figure 1). Small sample size method (Breslow) was also used to account for low event rate, particularly in the <=5 hours category.
For missing variables, we used missing indicators and excluded those missing the main exposure. For the additive interaction with bodily pain, the pain missings were also excluded. Additive interactions were assessed between sleep duration (<=6 hours vs. >6 hours) and shiftwork (any shiftwork vs. none), depression (history of depression vs. none), and bodily pain (high bodily pain (SF-36 <75) vs. no high bodily pain (SF-36 >=75). We carried forward variables that were not asked every cycle. A two-sided p-value <0.05 indicated independent statistical significance. All analyses were performed using SAS software 9.4 (SAS Institute, Cary, NC).
Results
Characteristics of Participants
During 4,246,094 person-years of follow-up, there were 187 incident SLE cases out of 186,072 women, diagnosed at a mean duration of 51.58 (standard deviation 11.82) years (Supplemental Table 1). Table 1 summarizes the study baseline and disease characteristics of the participants. Women who reported shorter sleep duration consumed less alcohol and were more likely to exercise regularly, perform shiftwork >=6 years, have higher bodily pain (lower SF-36 score, worse pain), and report a younger age of menarche, compared to women with longer sleep duration.
Table 1.
Age-standardized baseline characteristics in the Nurses’ Health Study (NHS) in 1986 and the NHSII in 1989 by sleep duration (n=186,072)
| Characteristic | <=5 hours (n=9609) | >5–6 hours (n=47131) | >6–7 hours (n=76202) | >7–8 hours (n=45269) | >8 hours (n=7861) |
|---|---|---|---|---|---|
| Sociodemographic | |||||
| Mean age, years (SD) | 46.4 (12.4) | 45.6 (11.8) | 45.5 (11.7) | 46.1 (12.3) | 49.8 (13) |
| White, % | 88.5 | 91.7 | 94.1 | 94.0 | 92.7 |
| Census-tract median household income by zip code <$60,000, % | 25.2 | 23.6 | 22.7 | 23.3 | 23.9 |
| Mean BMI, kg/m2 (SD) | 26.3 (6.1) | 25.4 (5.4) | 24.8 (5.0) | 24.8 (5.0) | 25.8 (5.8) |
| BMI <25 kg/m2, % | 43.4 | 43.6 | 43.5 | 41.6 | 41.5 |
| Mean alcohol consumption, g/day (SD) | 3.7 (7.2) | 4.3 (7.6) | 4.7 (7.8) | 5.0 (8.6) | 5.9 (10.3) |
| Alcohol >= 5 g/day, % | 20.7 | 25.3 | 27.6 | 28.5 | 28.5 |
| Never or past smokers (quit >4 years), % | 77.1 | 77.5 | 79.7 | 80.7 | 78.7 |
| Regular exercise1 (>= 19 MET-hrs/week), % | 36.1 | 33.5 | 33.4 | 33.8 | 31.4 |
| Highest 40 percentile of the AHEI, % | 36.0 | 36.4 | 36.8 | 37.5 | 33.8 |
| Shiftwork2 >0–5.9 yrs’, % | 38.4 | 40.8 | 41.5 | 40.5 | 39.3 |
| Shiftwork2 >=6 yrs’, % | 23.7 | 17.6 | 13.3 | 12.2 | 13.5 |
| History of Depression3 | 30.9 | 24.9 | 22.5 | 23.8 | 31.6 |
| SF-36 Bodily Pain4 ~0–60 (most pain)’, % | 24.9 | 20.3 | 17.5 | 17.4 | 21.9 |
| SF-36 Bodily Pain4 ~60–75’, % | 15.6 | 17.6 | 18.0 | 17.4 | 15.8 |
| SF-36 Bodily Pain4 ~75–85’, % | 17.8 | 22.1 | 24.5 | 23.1 | 20.2 |
| SF-36 Bodily Pain4 ~85–100 (least pain)’, % | 14.1 | 16.3 | 18.5 | 20.3 | 18.7 |
| Medications and Reproductive Factors | |||||
| Oral contraceptive use, % | 66.7 | 66.5 | 66.5 | 66.0 | 66.2 |
| Age at menarche <=10 years, % | 9.9 | 7.7 | 6.3 | 6.1 | 6.1 |
| Pre-menopausal, % | 57.9 | 59.1 | 60.2 | 60.9 | 60.3 |
| Post-menopausal, never used post-menopausal hormones, % | 16.6 | 16.7 | 15.3 | 15.0 | 14.0 |
| Post-menopausal, ever used post-menopausal hormones, % | 21.5 | 21.0 | 21.5 | 21.2 | 22.8 |
AHEI, Alternative Healthy Eating Index; BMI, body mass index; MET, metabolic equivalent; SD, standard deviation; SF-36, Short Form-36; yrs, years.
Regular exercise defined as at least 19 metabolic equivalent (MET) hours per week, corresponding to at least 30 minutes of brisk walking every day
Shiftwork questionnaire started in 1988/1989
History of depression questionnaire started in 1996/1997
SF-36 Bodily Pain Questionnaire started in 1992/1993, the cut-offs for each quartile are approximated
Sleep duration and risk of SLE
Chronic sleep duration of <=5 hours (vs reference >7–8 hours) per night was associated with increased SLE risk (adjusted HR 2.47, 95%CI 1.29–4.75), which persisted after further adjustment for shiftwork, bodily pain, and depression (adjusted HR 2.13, 95%CI 1.11–4.10) (Table 2).
Table 2.
Hazard ratios (95% confidence intervals) for risk of incident SLE in Nurses’ Health Study NHS (1986–2016) and NHSII (1989–2017) by sleep duration (n=186,072)
| Sleep Duration | <=5 hours | >5–6 hours | 6–7 hours | >7–8 hours | >8 hours |
|---|---|---|---|---|---|
| No. of cases/person-years | 12/119522 | 38/792679 | 83/1798274 | 45/1299088 | 9/236532 |
| Multivariable modela | 2.47 (1.29–4.75) | 1.22 (0.78–1.89) | 1.22 (0.84–1.75) | Ref | 1.20 (0.58–2.45) |
| Multivariable modela + shiftwork, depression, and pain | 2.13 (1.11–4.10) | 1.14 (0.73–1.77) | 1.23 (0.85–1.78) | Ref | 1.08 (0.52–2.21) |
CI, confidence interval
Adjusted for age, race, smoking, body mass index, census tract household income, alcohol, exercise, oral conceptive use, menopausal status, and hormone use, and diet (Alternative Healthy Eating Index)
Sensitivity Analysis
In analyses updating the exposure assessment only until > 4 years before outcome window (lagged by >4 years, which excluded 33 SLE cases over 518,396 person years), we demonstrated that the risk of SLE with short sleep duration remained elevated (adjusted HR 3.14, 95%CI 1.57–6.29) (Table 3). Using small sample size methods to account for low event rate, the results of the primary analysis were unchanged.
Table 3.
Hazard ratios (95% confidence intervals) for risk of incident SLE in Nurses’ Health Study and NHSII by sleep duration (n=180,359) with lag (at least four years between sleep measurement and SLE risk window)
| Sleep Duration | <=5 hours | >5–6 hours | 6–7 hours | >7–8 hours | >8 hours |
|---|---|---|---|---|---|
| No. of cases/person-years | 11/110155 | 29/719055 | 73/1592723 | 34/1113861 | 7/191905 |
| Multivariable modela | 3.14 (1.57–6.29) | 1.22 (0.74–2.01) | 1.39 (0.92–2.10) | Ref | 1.26 (0.56–2.87 |
| Multivariable modela + shiftwork, depression, and pain | 2.43 (1.21–4.90) | 1.09 (0.66–1.81) | 1.38 (0.92–2.08) | Ref | 1.05 (0.46–2.39) |
CI, confidence interval
Adjusted for cohort, questionnaire cycle, age, race, smoking, body mass index, census tract household income, alcohol, exercise, oral conceptive use, menopausal status, and hormone use, and diet (Alternative Healthy Eating Index)
We detected additive interactions between low sleep duration and high bodily pain (SF-36 <75) and found an attributable proportion (AP) of 64% (95%CI 40%-87%, p<0.0001). The HR for SLE between those with low sleep duration and high bodily pain compared to those with neither risk factor was 2.97 (95%CI 1.86–4.75). There was also an interaction between low sleep duration and depression with an AP of 68% (95%CI 49%-88%), p<0.0001. The HR for SLE was 2.82 (95%CI 1.64–4.85) comparing those with low sleep duration and depression to those with neither risk factor. There was a trend towards an interaction between sleep duration and shiftwork (AP 36%, 95%CI −3%–76%, p=0.07) with a HR of 1.55 (95%CI 1.00–2.41) for SLE risk.
Discussion
In this large well-characterized prospective cohort study, we demonstrated that lack of sleep was associated with increased SLE risk (3, 5). Our study adjusted for and investigated interactions with other factors, such as shiftwork, depression, and bodily pain, potentially either confounders or on a causal pathway. Although based on a small number of incident SLE cases, we found an almost three-fold elevated risk of SLE among women with concomitant chronic low sleep and depression and bodily pain compared to women with none of these risk factors. This may suggest that depression (AP for interaction 68%, p< 0.0001) and pain (AP for interaction 64%, p< 0.0001) act synergistically with low sleep duration to increase risk of SLE development.
Several mechanisms have been proposed to explain the relationship between sleep deprivation and autoimmune disease. In experimentally sleep-deprived healthy humans, increased genetic expression and production of pro-inflammatory cytokines (interleukin (IL)-6, TNF-α), C-reactive protein (CRP), and impaired function of T cells and CD4 regulatory T cells important in self-tolerance, have been detected (12–16). In rats subjected to 72-hour rapid eye movement (REM) sleep deprivation, increased levels of IL-6, TNF-α, IL-1α, IL-β, IL-17α, and other markers of inflammation such as corticosterone have been reported, while anti-inflammatory cytokine, IL-10, did not change (17). After a week of sleep restoration, the levels of IL-17α, TNF-α, and cortisone remained high. Reduced natural killer (NK) cell activity and suppressed IL-2 production, both of which have been implicated in the pathogenesis of SLE (18, 19), have been demonstrated in healthy human volunteers after a night of partial night sleep deprivation (20). After a night of sleep recovery, NK cell activity returned to baseline, while IL-2 production remained decreased. The role of sleep deprivation and SLE risk is further supported by a murine study that demonstrated that in mice genetically susceptible to SLE, sleep deprivation was associated with an earlier onset of disease as suggested by an increased frequency of antinuclear antibody positivity compared to non-sleep deprived control mice (21). These studies suggest that sleep loss may have both immediate and long-lasting effects on the processes that drive immune hyperactivation or dysfunction resulting in autoantibody production and systemic inflammation, thereby predisposing to autoimmune disease development. Whether or not these changes to the immune system can be reversed or halted with improvement in sleep requires further study.
In a prospective study of 436 initially unaffected first degree relatives of patients with SLE, participants were more likely to transition to SLE if they reported sleeping less than seven hours a day after adjusting for age, sex, and race (odds ratio [OR] 2.8, 95%CI:1.6–5.1) (5). The association between less sleep and SLE transition remained after adjusting for additional factors and symptoms that could affect sleep including prednisone use (22), vitamin D deficiency (23), and number of ACR criteria. Although we did not account for these in our study, we adjusted for other important factors associated with sleep duration and/or SLE risk, i.e., depression, bodily pain, and shiftwork, and considered their interactions with low sleep duration.
Depression, pain, and sleep have been described as a triad that often co-occur, each with the potential to moderate and/or mediate the expression of the other (24). Majority of patients with depression (~75%) report symptoms of disturbed sleep (25). There is a higher risk of depression among people with insomnia (26) and objective evidence of abnormal sleep architecture among patients with depression (27). Insomnia complaints are also common among patients with chronic pain and related conditions (28). Compared to patients with chronic pain but normal sleep, those who also have insomnia report more severe pain, longer pain duration, and greater depression (29). As all three are regulated by the central nervous system, it has been proposed that common neurobiological mechanisms, such as the mesolimbic dopamine system and other neurotransmitter systems, may underlie the shared pathophysiology and interplay of these disorders (24). Perhaps then, these overlapping pathways may explain why depression and pain may act synergistically with sleep deprivation to accentuate SLE risk as in our study. Indeed, in established SLE, these disorders are commonly reported by patients and are correlated with one another (30, 31). Therefore, a better understanding of these mechanisms will not only enable us to identify effective therapeutic and prevention strategies for these disorders, but also the subsequent risk of autoimmune disease development.
Stress has been identified as a common factor that may account for the clustering of depression, pain, and sleep disorders (32, 33). By itself, occupational stress, such as working nights or rotating shifts, has been associated with the development of autoimmunity in past studies including RA, thyroid disorders, and multiple sclerosis (3). In a cohort study of 265 SLE patients, a weak association between SLE and shiftwork (OR 1.6, 95%CI: 0.99–2.7) was detected (34). Stress hormones can promote inflammation through induction of IL-1, IL-6, IL-8, IL-18, TNF-α, and CRP and imbalances between Th1 and Th2 cytokine production, a key player in the induction of autoimmunity (35). Interestingly, activation of nitric oxide (NO), an important neurotransmitter in stress and sleep regulation, is lower in regions of the brain involved in sleep regulation in females than males (36). SLE is predominantly seen in females, therefore further studies examining NO as a potential mechanism in sleep-deprived individuals who develop SLE are needed. Disrupted melatonin production in nightshift workers has been proposed as an important mechanism of autoimmune diseases including SLE. Abnormal circadian rhythm of melatonin levels in response to light/dark cycle has been found in lupus-prone mice and administration of melatonin to these mice has been shown to decrease levels of autoantibodies, inflammatory cytokines, reduce renal injury, and increase levels of anti-inflammatory cytokine IL-10 (37), particularly for females. Studies in humans are needed to study the mechanism of melatonin and SLE risk and whether it could be a potential therapeutic strategy.
Lifestyle risk factors are increasingly being recognized as important triggers for the development of SLE, in addition to genetic susceptibility. Recently, it was demonstrated in the NHS cohorts that a greater than expected proportion of SLE risk (almost 50%) was attributed to a combination of lifestyle factors including diet, exercise, smoking, alcohol consumption, and body weight (38). The results of this study provide further evidence that primary prevention through lifestyle interventions, perhaps also including adequate sleep duration, is important and should be promoted.
An important strength of our study is that the NHS cohorts are well-described cohorts with up to 5.8 million person-years of prospective follow-up. Detailed data on potential time-varying confounders reduces the within-subject variation, inaccuracy of exposure data, and the potential for reverse causation and recall biases. To further address potential reverse causation as there may be a long prodromal period in the development SLE, an extra lag period between the exposure window and the outcome window was added. Important confounders such as shiftwork, depression, and pain were also adjusted for in the analysis. This study was limited by the small number of cases of SLE in some sleep categories and restricted generalizability, as all participants were female nurses and predominantly White background. More diverse SLE cohorts are needed to validate the findings of this study given a higher prevalence of SLE in non-White races/ethnicities. It will also be important to re-examine in a cohort of younger patients and those with more severe SLE disease. Finally, self-reported questionnaires were used, therefore, there may be unknown confounders not accounted for in the analysis.
In conclusion, we found an association between sleep deprivation and SLE risk, potentially exacerbated in those with a history of depression and bodily pain. A better understanding of the mechanisms involving the nervous system and immune system that may be underlying the complex interaction between depression, pain, sleep, shiftwork, and autoimmune disease development is needed. Our findings have implications for SLE prevention and the promotion of adequate sleep duration.
Supplementary Material
Significance and Innovations.
In this large well-characterized prospective cohort study, lack of sleep was associated with increased SLE risk after adjusting for and investigated interactions with other factors, such as shiftwork, depression, and bodily pain.
There was almost three-fold elevated risk of SLE among women with concomitant chronic low sleep and depression and bodily pain compared to women with adequate sleep duration and no high bodily pain or depression.
ACKNOWLEDGMENTS
We would like to acknowledge the Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital as home of the Nurses’ Health Studies. We would also like to acknowledge Emily Oakes for her careful technical review.
FUNDING
This work was supported by the Lupus Foundation of America, NIH [grant numbers R01 AR049880, K24 AR066109, K23 AR069688, R01 AR071326, R03 AR075886, L30 AR066953, K23 AR076453], UM1 CA186107, U01 CA176726, and U01 HL145386
COMPETING INTERESTS
The authors declare no competing interests. Dr. Choi has consulted for Janssen, AstraZeneca, Mallinckrodt Canada Inc., MitogenDx, and Glaxo Smith Kline. Dr. Costenbader has consulted for or collaborated on research projects with Janssen, Glaxo Smith Kline, Exagen Diagnostics, Eli Lilly, Merck, Astra Zeneca and Neutrolis (less than $10,000 each). Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant numbers R01 AR077607, P30 AR070253, and P30 AR072577), the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Sparks has received research support from Bristol Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer unrelated to this work. The funders had no role in the decision to publish or preparation of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University, its affiliated academic health care centers, or the National Institutes of Health.
Footnotes
ETHICS APPROVAL
The study protocol was approved by the institutional review board at Mass General Brigham HealthCare System.
PATIENT AND PUBLIC INVOLVEMENT
Patients and the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
OTHER DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DATA SHARING
Data from this project can be considered for release if the appropriate IRB and publication clearances have been made, and a project is in keeping with and has undergone the Nurses’ Health Study Cohorts review and approval process.
References
- 1.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, et al. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. J Clin Sleep Med. 2015;11(8):931–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Fuchs CS, et al. Sleep duration affects risk for ulcerative colitis: a prospective cohort study. Clin Gastroenterol Hepatol. 2014;12(11):1879–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsiao YH, Chen YT, Tseng CM, Wu LA, Lin WC, Su VY, et al. Sleep disorders and increased risk of autoimmune diseases in individuals without sleep apnea. Sleep. 2015;38(4):581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung WS, Lin CL, Kao CH. Association of systemic lupus erythematosus and sleep disorders: a nationwide population-based cohort study. Lupus. 2016;25(4):382–8. [DOI] [PubMed] [Google Scholar]
- 5.Young KA, Munroe ME, Harley JB, Guthridge JM, Kamen DL, Gilkensen GS, et al. Less than 7 hours of sleep per night is associated with transitioning to systemic lupus erythematosus. Lupus. 2018;27(9):1524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang JH, Lin HC. Obstructive sleep apnea and the risk of autoimmune diseases: a longitudinal population-based study. Sleep Med. 2012;13(6):583–8. [DOI] [PubMed] [Google Scholar]
- 7.Palagini L, Tani C, Mauri M, Carli L, Vagnani S, Bombardieri S, et al. Sleep disorders and systemic lupus erythematosus. Lupus. 2014;23(2):115–23. [DOI] [PubMed] [Google Scholar]
- 8.Valencia-Flores M, Resendiz M, Castano VA, Santiago V, Campos RM, Sandino S, et al. Objective and subjective sleep disturbances in patients with systemic lupus erythematosus. Arthritis Rheum. 1999;42(10):2189–93. [DOI] [PubMed] [Google Scholar]
- 9.Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27(3):440–4. [DOI] [PubMed] [Google Scholar]
- 10.Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995;5(4):297–302. [DOI] [PubMed] [Google Scholar]
- 11.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology. 1997;40(9):1725.–. [DOI] [PubMed] [Google Scholar]
- 12.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166(16):1756–62. [DOI] [PubMed] [Google Scholar]
- 13.Bollinger T, Bollinger A, Skrum L, Dimitrov S, Lange T, Solbach W. Sleep-dependent activity of T cells and regulatory T cells. Clin Exp Immunol. 2009;155(2):231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43(4):678–83. [DOI] [PubMed] [Google Scholar]
- 15.Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107(1):165–70. [DOI] [PubMed] [Google Scholar]
- 16.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89(5):2119–26. [DOI] [PubMed] [Google Scholar]
- 17.Yehuda S, Sredni B, Carasso RL, Kenigsbuch-Sredni D. REM sleep deprivation in rats results in inflammation and interleukin-17 elevation. J Interferon Cytokine Res. 2009;29(7):393–8. [DOI] [PubMed] [Google Scholar]
- 18.Takeda K, Dennert G. The development of autoimmunity in C57BL/6 lpr mice correlates with the disappearance of natural killer type 1-positive cells: evidence for their suppressive action on bone marrow stem cell proliferation, B cell immunoglobulin secretion, and autoimmune symptoms. J Exp Med. 1993;177(1):155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieberman LA, Tsokos GC. The IL-2 defect in systemic lupus erythematosus disease has an expansive effect on host immunity. J Biomed Biotechnol. 2010;2010:740619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin JC. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996;10(5):643–53. [DOI] [PubMed] [Google Scholar]
- 21.Palma BD, Gabriel A Jr., Colugnati FA, Tufik S. Effects of sleep deprivation on the development of autoimmune disease in an experimental model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1527–32. [DOI] [PubMed] [Google Scholar]
- 22.Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am. 2002;31(1):15–36. [DOI] [PubMed] [Google Scholar]
- 23.Bertisch SM, Sillau S, de Boer IH, Szklo M, Redline S. 25-Hydroxyvitamin D Concentration and Sleep Duration and Continuity: Multi-Ethnic Study of Atherosclerosis. Sleep. 2015;38(8):1305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finan PH, Smith MT. The comorbidity of insomnia, chronic pain, and depression: dopamine as a putative mechanism. Sleep Med Rev. 2013;17(3):173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. 2008;10(4):473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–84. [DOI] [PubMed] [Google Scholar]
- 27.Nutt D, Wilson S, Paterson L. Sleep disorders as core symptoms of depression. Dialogues Clin Neurosci. 2008;10(3):329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith MT, Perlis ML, Smith MS, Giles DE, Carmody TP. Sleep quality and presleep arousal in chronic pain. J Behav Med. 2000;23(1):1–13. [DOI] [PubMed] [Google Scholar]
- 29.Tang NK. Insomnia Co-Occurring with Chronic Pain: Clinical Features, Interaction, Assessments and Possible Interventions. Rev Pain. 2008;2(1):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin R, Li L, Xu L, Sui W, Niu M, Xu R, et al. Association between depression and sleep quality in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Sleep Breath. 2022;26(1):429–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lillis TA, Tirone V, Gandhi N, Weinberg S, Nika A, Sequeira W, et al. Sleep Disturbance and Depression Symptoms Mediate Relationship Between Pain and Cognitive Dysfunction in Lupus. Arthritis Care Res (Hoboken). 2019;71(3):406–12. [DOI] [PubMed] [Google Scholar]
- 32.Senba E A key to dissect the triad of insomnia, chronic pain, and depression. Neurosci Lett. 2015;589:197–9. [DOI] [PubMed] [Google Scholar]
- 33.Healey ES, Kales A, Monroe LJ, Bixler EO, Chamberlin K, Soldatos CR. Onset of insomnia: role of life-stress events. Psychosom Med. 1981;43(5):439–51. [DOI] [PubMed] [Google Scholar]
- 34.Cooper GS, Parks CG, Treadwell EL, St Clair EW, Gilkeson GS, Dooley MA. Occupational risk factors for the development of systemic lupus erythematosus. J Rheumatol. 2004;31(10):1928–33. [PubMed] [Google Scholar]
- 35.Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann N Y Acad Sci. 2006;1069:62–76. [DOI] [PubMed] [Google Scholar]
- 36.Chiem E, Nichols I, Van C, Kori S, Paul K. Sleep loss mediates the effect of stress on nitrergic signaling in female mice. Neurosci Lett. 2021;740:135362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou LL, Wei W, Si JF, Yuan DP. Regulatory effect of melatonin on cytokine disturbances in the pristane-induced lupus mice. Mediators Inflamm. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi MY, Hahn J, Malspeis S, Stevens EF, Karlson EW, Sparks JA, et al. Association of a Combination of Healthy Lifestyle Behaviors With Reduced Risk of Incident Systemic Lupus Erythematosus. Arthritis Rheumatol. 2022;74(2):274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this project can be considered for release if the appropriate IRB and publication clearances have been made, and a project is in keeping with and has undergone the Nurses’ Health Study Cohorts review and approval process.


