Abstract
Objective:
To investigate the extent to which self-reported menstrual cycle length during reproductive years is associated with menopausal symptoms and age at natural menopause at midlife.
Methods:
This analysis includes 634 women who enrolled in Project Viva during pregnancy (1999–2002) and completed the midlife visit approximately 18 years later. Women self-reported menstrual cycle length at enrollment (early pregnancy) and at midlife reported total and specific menopausal symptoms using the Menopause Rating Scale (MRS) as well as age at natural menopause. We used linear and regression models to evaluate associations of cycle length with total and specific menopausal symptoms. We also applied a time-to-event Cox proportional hazards model to investigate the relationship between menstrual cycle length and onset of natural menopause. We adjusted models for age at midlife visit, pre-pregnancy BMI, race/ethnicity, education and parity.
Results:
At enrollment (median age= 33.3 years), 90 (14%) women reported having short (<=25 days) and 39 (6%) long (>=35 days) menstrual cycles. Compared to women with a normal menstrual cycle length of 26–34 days, women whose cycles were short had a higher total MRS at midlife [β (95% CI)= 2.05 (0.73, 3.38)]. Specifically, women with short menstrual cycles during their reproductive years had higher odds of midlife sleep problems [OR (95% CI)= 1.92 (1.10, 3.37)], heart discomfort (OR (95% CI)=1.68 (1.03, 2.73)) and depressive symptoms [OR (95% CI)= 1.85 (1.16, 2.96)]. In addition, compared to women with a normal cycle length of 26–34 days, women reporting short cycles had an earlier onset of natural menopause [HR (95% CI)= 1.67 (1.11, 2.51).
Conclusions:
Compared to women with normal menstrual cycle length, those with short menstrual cycles during their reproductive years had a higher frequency of total and certain menopausal symptoms at midlife, and reached menopause earlier.
Keywords: Menstrual cycle length, Menopausal symptoms, Age at menopause
Introduction
The menopausal transition is a normal physiologic stage characterized by age-related decline in ovarian function.1,2 Perimenopause is usually marked by the onset of menstrual irregularities and it is followed by menopause, recognized retrospectively after 12 months of amenorrhea.3 Although variable, it has been calculated that perimenopause lasts a median of 4 years in women, and it is characterized by dramatic reproductive, neurophysiological, and metabolic changes that can extend years beyond the cessation of menses.1 Perimenopause represents an important reproductive transition in women, with common symptoms including vasomotor instability, depressive and anxiety symptoms, cognitive changes, and disturbances of sleep duration and quality.4 Hormone variability during the menopause transition likely contributes to these symptoms, which adversely affect quality of life.5 Therefore, identifying risk factors associated with menopausal symptoms in women is of clinical and public health importance. Traditional risk factors for menopausal symptoms include demographic (age, race, ethnicity) and several modifiable lifestyle factors like body mass index (BMI), smoking, and physical activity.6–9 Emerging epidemiologic data suggest that reproductive traits and conditions during reproductive age may be associated with menopausal symptoms during perimenopause.10–12 However, epidemiologic literature is still scarce and results contradictory. For example, compared to postmenopausal women with regular menstrual cycle length, history of irregular cycles were associated with reduced late-life depressive symptoms in Chinese13 and higher severe depressive symptoms among French postmenopausal women.14 Most importantly, it is understudied whether menstrual cycle characteristics during a woman’s reproductive years are related to other somatic, physiological, or urogenital menopausal symptoms. Therefore, the objective of this analysis is to investigate the extent to which self-reported menstrual cycle length during reproductive years is associated with menopausal symptoms at midlife in women.
Methods
Study Participants
From April 1999 to July 2002, we enrolled pregnant women into Project Viva, a longitudinal pre-birth cohort of mother-offspring pairs in eastern Massachusetts, USA.15 The study protocol was approved by the Institutional Review Board of Harvard Pilgrim Health Care and all participants provided written informed consent at enrollment and follow-up visits. Study population, enrollment and follow-up procedures, have been previously described in detail.16 Briefly, we recruited 2100 women attending their initial prenatal visit (median 9.9 weeks of gestation) at Atrius Harvard Vanguard Medical Associates, a multi-specialty group practice. Eligibility criteria included fluency in English, a singleton pregnancy, gestational age ≤22 weeks at recruitment and no plans to move away from the study area before delivery. We collected data from multiple sources, including interviews/surveys, medical records, examinations and biospecimens. From the 785 who remained under active follow-up and participated in the midlife visit (approximately 18 years after enrollment), we excluded 15 women who did not complete at least 10 items on the MRS, 79 women who were less 45 years old at this visit and did not reach menopause yet, and 57 women who at the time of study recruitment (first visit during index pregnancy) reported having no periods, periods that were too irregular to count or did not know about their menstrual cycles, leaving a final sample size of 634 women with complete data on self-reported menstrual cycle length and menopausal symptoms (Supplemental Figure 1). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Self-reported menstrual cycle length
On a questionnaire administered at study enrollment in early pregnancy, women were asked “When you are not on the pill, breastfeeding or pregnant, what is the typical length of your menstrual cycle? By this we mean the interval from the first day of your period to the first day of your next period”. Responses included: “less than 21 days, 21 to 25 days, 26 to 34 days, more than 35 days, too irregular to estimate, no periods, and don’t know”. Because only 6 women reported a typical cycle length of less than 21 days, we combined women in this group with women reporting a typical cycle length of 21 to 25 days (n=84) into a single category (n=90) and considered this group as short menstrual cycle length. As a result, for statistical analyses we used history of menstrual cycle length as three-category variable: <=25 days, 26–34 days and >=35 days. We excluded women who reported too irregular to estimate (n=31), no periods (n=1) or don’t know (n=25).
Menopausal symptoms and onset of menopause.
Approximately 18 years after enrollment (median age= 51.5 years), women attended a midlife visit and via written questionnaire self-reported the presence and severity of 11 menopausal symptoms over the past year using the Menopause Rating Scale (MRS).17 This scale evaluates three dimensions of menopausal symptoms/complaints including somatic (hot flashes/sweating, sleep problems, joint/muscular and heart discomfort), psychological (depressive mood, irritability, anxiety and physical/mental exhaustion), and urogenital (vaginal dryness, sexual and bladder problems) factors, with scores ranging from none (0 points) to very severe (4 points) for each item and a total score ranging from 0 to 44 points. We included women who completed at least 10 of the 11 items and used the MRS as a continuous outcome variable to evaluate the relationship of self-reported menstrual cycle length with total menopausal symptoms. We also evaluated each component of the MRS as a binary outcome as no symptoms vs. any symptom. At the midlife visit, we also collected information on menopausal status and, for women who had reached this milestone, age at menopause. Specifically, women were asked whether their menstrual periods had stopped for at least 12 months, and if so, the reason including natural or secondary (surgical, radiation or chemotherapy).
Covariate and depressive mood variables
We collected sociodemographic and medical data through in-person interviews at enrollment and subsequent follow-up visits. At enrollment, women reported their age, race/ethnicity, education, household income, marital status, parity, pre-pregnancy weight and height. We calculated pre-pregnancy BMI as kg/m2. History of depression was collected in the mid-pregnancy questionnaire. Women were asked whether they felt depressed or down, had lost interest in pleasurable activities, or had been diagnosed or treated for depression before pregnancy. We used history of depression as a binary variable and considered women to have a history of depression if they responded “yes” to feeling depressed/down and also if they were either diagnosed by a professional or treated for depression during pregnancy. Women reported age at menarche in the early teen visit. At the midlife visit, we collected information on age at first pregnancy. Women also completed the Patient Health Questionnaire-9 (PHQ-9), an instrument for screening, monitoring and measuring the severity of depression. The PHQ-9 scores 9 symptoms as “0” (not at all) to “3” (nearly every day) and it has been previously validated.18
Statistical analyses
We described demographic and reproductive characteristics at baseline/pregnancy and the midlife visit as well as the distribution of the total MRS and each menopausal factor by self-reported menstrual cycle length using median ± interquartile ranges (IQRs) or percentages. We used linear regression models to estimate the association between self-reported menstrual cycle length and continuous total menopausal symptoms and presented results as regression coefficients (β) and their 95% confidence intervals (CI). We also used logistic regression models to evaluate whether women with self-reported short or long menstrual cycles had higher odds of any specific menopausal symptoms (reference=none), compared to women whose usual menstrual cycle length ranged between 26 and 34 days. Results are reported as Odds Ratios (OR) with 95% CIs.
Confounding was assessed using both prior knowledge regarding biological relevance and descriptive statistics from our study population. Variables that were associated with both exposure and/or outcome were considered. We adjusted models for age at midlife visit (years), pre-pregnancy BMI (kg/m2), race/ethnicity [White vs. Black vs. other (Asian, Hispanic, American Indian/Alaska Native and more than one race)], education (graduate degree vs. other) and parity (nulliparous yes vs. no). We included race/ethnicity as a covariate in the models to account for observed differences in menstrual cycle length across women belonging to different races/ethnicities as shown in Table 1; we consider race/ethnicity to be a social rather than biological variable and do not think it is causally associated with menstrual cycle length differences. We conducted two sensitivity analyses to confirm the robustness of the main findings related to depressive mood symptoms. In the first one we additionally adjusted for pre-enrollment history of depression in the depressive mood symptom models, since depression in early years is a predictor for depression later in life, as explained in the discussion. In the second one, we used linear regression models to explore menstrual cycle length in relation to severity of depression measured using the PHQ-9, a validated instrument to diagnose and measure the severity of depression.
Table 1.
Demographic and reproductive characteristics [median (IQR) or N (%)] by menstrual cycle length during reproductive years among 634 women participating in Project Viva.
| Overall | Cycles ≤ 25 days | Cycles 26 – 34 days | Cycles ≥ 35 days | |
|---|---|---|---|---|
| N=634 | N=90 (14%) | N=505 (80%) | N=39 (6%) | |
| At enrollment/pregnancy | ||||
| Age, years | 33.3 (30.8, 36.4) | 33.2 (30.8, 36.4) | 33.4 (31.1, 36.5) | 31.5 (29.8, 34.5) |
| Race, N (%) | ||||
| White | 458 (72) | 51 (57) | 376 (75) | 31 (79) |
| Black | 76 (12) | 25 (28) | 50 (10) | 1 (3) |
| Othera | 99 (16) | 14 (16) | 78 (15) | 7 (18) |
| College degree, N (%) | 504 (80) | 61 (68) | 408 (81) | 35 (90) |
| Married/cohabiting, N (%) | 605 (96) | 83 (92) | 483 (96) | 39 (100) |
| Household income >$70,000/year, N (%) | 406 (68) | 41 (51) | 336 (70) | 29 (81) |
| Pre-pregnancy BMI, kg/m2 | 23.3 (20.9, 26.2) | 25.0 (21.9, 28.9) | 23.2 (20.9, 25.9) | 22.9 (20.6, 25.8) |
| Age at first menstrual period, years | 13.0 (12.0, 14.0) | 13.0 (12.0, 13.0) | 13.0 (12.0, 14.0) | 13.0 (12.0, 14.0) |
| Age at first pregnancy, years | ||||
| Nulliparous, N (%) | 293 (46) | 44 (49) | 229 (45) | 20 (51) |
| History of depression, N (%) | 63 (11) | 11 (15) | 49 (11) | 3 (8) |
| At midlife | ||||
| Age, years | 51.5 (49.1, 54.8) | 51.6 (49.0, 55.1) | 51.7 (49.3, 54.8) | 49.9 (48.0, 53.5) |
| Onset of natural menopause, N (%) | 229 (37) | 36 (40) | 187 (38) | 6 (15) |
| Age at natural menopause, yearsb | 50.0 (48.0, 53.0) | 49.0 (45.0, 51.0) | 51.0 (49.0, 53.0) | 52.0 (45.0, 54.0) |
Other included Asian, Hispanic, American Indian/Alaska Native and more than one race.
Among 223 women who had reached menopause naturally and reported their age.
We also applied a time-to-event Cox proportional hazards model using age (in years) as the time scale to investigate the relationship between menstrual cycle length and onset of natural menopause adjusting for age at midlife visit (years), pre-pregnancy BMI (kg/m2), race/ethnicity (White vs. Black vs. other), education (graduate degree vs. other) and parity (nulliparous yes vs. no). Natural menopause was the event in these models. Women who had not reached menopause by the midlife visit or who experienced menopause secondary to surgery, radiation therapy or chemotherapy were censored at the age of the follow-up visit or age at secondary menopause, respectively. Sensitivity analyses excluding 6 women who reported having menstrual cycles < 21 days were conducted to evaluate the robustness of the findings. Results of this survival analysis are presented as hazard ratios (HR) and 95% CI. Statistical analyses were performed using SAS (version 9.4; SAS Institute Inc., Cary, NC, USA).
Results
The 634 women included in this analysis had a median (IQR) age at enrollment of 33.3 (30.8, 36.4) years and pre-pregnancy BMI of 23.3 (20.9, 26.2) kg/m2, were predominantly White (72%), had a college degree (80%) and were married or cohabiting (96%). At enrollment, 90 (14%) women reported having short (<=25 days) and 39 (6%) long (>=35 days) menstrual cycles. Median (IQR) age at the midlife visit was 51.5 (49.1, 54.8) years. Compared to women with a normal menstrual cycle length of 26–34 days, women who reported short cycles were less likely to be White (57% vs. 75%), to have a college degree (68% vs. 81%) or a household income >$70,000/year (51% vs. 70%) (Table 1). Also, women with short menstrual cycles during reproductive years had higher pre-pregnancy BMI compared to women with normal menstrual cycles (median 25.0 vs 23.2 kg/m2). Age at menarche, enrollment, and midlife did not differ by menstrual cycle length.
At midlife, median (IQR) total MRS was 7 (4, 11) points and ranged from 0 to 33 points. Women whose menstrual cycles were short during reproductive age had higher sleep problems (79% vs. 69%) depressive mood (57% vs 44%) and physical/mental exhaustion (69% vs. 59%) at midlife, compared to women with normal cycle length (Table 2). No other symptoms differed by menstrual cycle length.
Table 2.
Menopausal symptoms (%) by menstrual cycle length during reproductive years among 634 women participating in Project Viva.
| Overall | Cycles ≤ 25 days | Cycles 26 – 34 days | Cycles ≥ 35 days | |
|---|---|---|---|---|
| N=634 | N=90 (14%) | N=505 (80%) | N=39 (6%) | |
| Somatic symptoms | ||||
| Any hot flashes/sweating symptoms | 61% | 66% | 60% | 64% |
| Any sleep problem symptoms | 70% | 79% | 69% | 67% |
| Any joint and muscular discomfort symptoms | 52% | 58% | 52% | 41% |
| Any heart discomfort symptoms | 29% | 38% | 28% | 26% |
| Psychological symptoms | ||||
| Any depressive mood symptoms | 47% | 57% | 44% | 54% |
| Any irritability symptoms | 51% | 57% | 50% | 54% |
| Any anxiety symptoms | 52% | 56% | 51% | 56% |
| Any physical/mental exhaustion symptoms | 61% | 69% | 59% | 69% |
| Urogenital symptoms | ||||
| Any sexual problem symptoms | 43% | 44% | 44% | 31% |
| Any bladder problem symptoms | 37% | 33% | 39% | 31% |
| Any vaginal dryness symptoms | 33% | 31% | 33% | 34% |
Cycle length was associated with menopausal symptoms after adjustment for age, pre-pregnancy BMI, race/ethnicity, education and parity. Compared to women with a normal menstrual cycle length of 26–34 days, women with short cycles had a significantly higher total MRS score at midlife [β (95% CI)=2.05 (0.73, 3.38] (Figure 1). This association was driven by somatic and psychological symptoms (Table 3). Specifically, women with short menstrual cycles had higher odds of reporting any sleep problem [OR (95% CI)= 1.92 (1.10, 3.37)], heart discomfort [OR (95% CI)= 1.68 (1.03, 2.73)] and any depressive mood symptoms [OR (95% CI)= 1.85 (1.16, 2.96)] at mid-life than women with a normal menstrual cycle length of 26–34 days. Results for depressive mood symptoms were similar after further adjustment for history of depression prior to study enrollment [OR (95% CI)= 2.03 (1.18, 3.49)]. Although not statistically significant, short menstrual cycles were related to a higher frequency of vasomotor and all other psychological symptoms included in the MRS (Table 3). Short cycles were unrelated to urogenital symptoms. Long menstrual cycles were not associated with any of the menopausal symptoms examined (Figure 1, Table 3).
Figure 1.
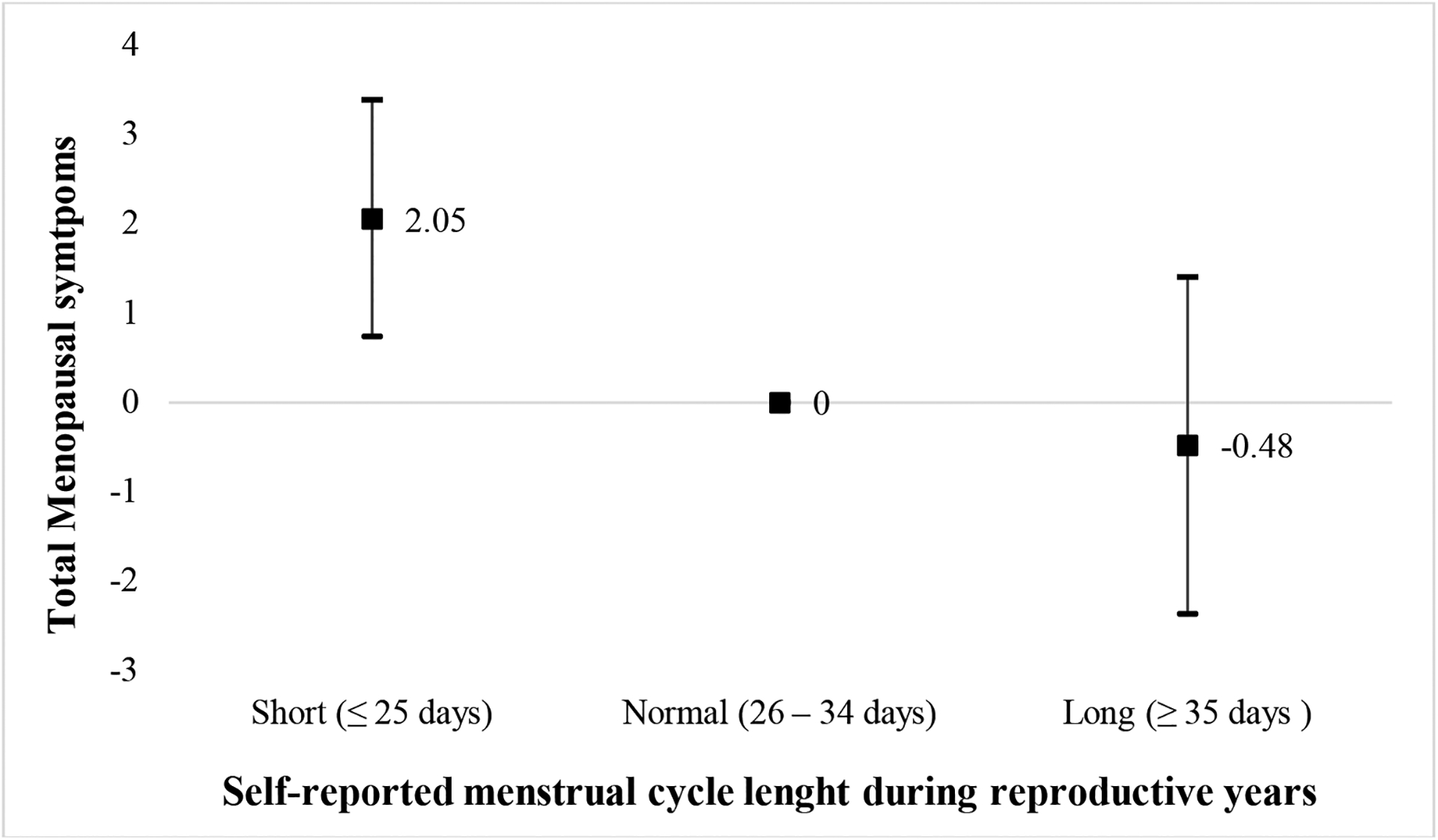
Adjusteda difference in total menopausal symptoms (β, 95% CI) using the Menopause Rating Scale (MRS) by menstrual cycle length during reproductive years among 634 women participating in Project Viva.
aAdjusted for age at midlife, pre-pregnancy BMI, race/ethnicity, education and parity.
Table 3.
Adjusteda odds of specific menopausal symptoms (any vs. none) by menstrual cycle length during reproductive years among 634 women participating in Project Viva.
| Cycles ≤ 25 days | Cycles 26 – 34 days | Cycles ≥ 35 days | |
|---|---|---|---|
| N=90 (14%) | N=505 (80%) | N=39 (6%) | |
| Odds ratio (95% CI) | |||
| Somatic symptoms | |||
| Any hot flashes/sweating symptoms | 1.38 (0.84, 2.26) | 1.0 (ref) | 1.31 (0.66, 2.60) |
| Any sleep problem symptoms | 1.92 (1.10, 3.37) | 1.0 (ref) | 0.87 (0.43, 1.76) |
| Any joint and muscular discomfort symptoms | 1.14 (0.71, 1.83) | 1.0 (ref) | 0.76 (0.39, 1.50) |
| Any heart discomfort symptoms | 1.68 (1.03, 2.73) | 1.0 (ref) | 0.86 (0.41, 1.82) |
| Psychological symptoms | |||
| Any depressive mood symptoms | 1.85 (1.16, 2.96) | 1.0 (ref) | 1.38 (0.71, 2.66) |
| Any irritability symptoms | 1.48 (0.92, 2.36) | 1.0 (ref) | 1.05 (0.54, 2.03) |
| Any anxiety symptoms | 1.30 (0.82, 2.08) | 1.0 (ref) | 1.21 (0.62, 2.34) |
| Any physical/mental exhaustion symptoms | 1.52 (0.92, 2.48) | 1.0 (ref) | 1.55 (0.77, 3.16) |
| Urogenital symptoms | |||
| Any sexual problem symptoms | 1.11 (0.70, 1.78) | 1.0 (ref) | 0.56 (0.28, 1.14) |
| Any bladder problem symptoms | 0.82 (0.50, 1.35) | 1.0 (ref) | 0.73 (0.36, 1.50) |
| Any vaginal dryness symptoms | 0.93 (0.56, 1.54) | 1.0 (ref) | 1.24 (0.61, 2.51) |
Adjusted for age at midlife, pre-pregnancy BMI, race/ethnicity, education and parity.
To confirm the associations with menopausal depressive mood symptoms, we then examined the relationship between menstrual cycle length in reproductive years and severity of depressive symptoms using the PHQ-9 completed at the midlife visit [median (IQR)=1.0 (0, 3.0)]. In agreement with our MRS findings, we found that women with short menstrual cycles had higher PHQ-9 scores in midlife compared to women with normal menstrual cycles [β (95% CI)=1.62 (0.86, 2.38)], and the association was maintained when further adjusting for history of depression [β (95% CI)=1.90 (1.09, 2.71)]. No association was evident for women with long menstrual cycles [β (95% CI)=−0.47 (−1.52, 0.57)], even when additionally adjusting for history of depression [β (95% CI)=−0.28 (−1.33, 0.77)].
Among women who had reached menopause by the mid-life visit, those with short cycles had an earlier age at natural menopause than women with either normal or long cycles (Table 1). Survival analysis adjusted for age at midlife visit, pre-pregnancy BMI, race/ethnicity, education and parity, revealed that women with short menstrual cycles had significantly higher risk of earlier onset of natural menopause than women with normal cycle length [HR (95% CI)=1.67 (1.11, 2.51)]. No association was found between having long menstrual cycles and onset of natural menopause [HR (95% CI)=0.56 (0.28, 1.11)], however, this analysis was conducted including 39 women with long cycle and only 6 of them having reached menopause naturally. Associations of short cycles with total and specific menopausal symptoms as well as onset of natural menopause remained after excluding 6 women who reported having cycles < 21 days, except for sleep problems which became attenuated and no longer significant (data not shown).
Discussion
In this longitudinal study of 634 women participating in Project Viva, we investigated the association between menstrual cycle length during reproductive years and menopausal symptoms as well as timing of natural menopause at midlife (approximately 18 years later). We observed that women with self-reported menstrual cycles ≤25 days had more somatic and psychological menopausal symptoms as well as reached menopause earlier than women with menstrual cycles between 26 and 34 days. History of menstrual cycle length was not associated with urogenital menopausal symptoms assessed in the MRS. These results, if confirmed, add to the existing epidemiologic literature on menstrual cycle length and women’s health.
Alterations in cycle length are the hallmark of perimenopause, with shortening of cycles due to early follicular recruitment often followed by lengthening of cycle intervals due to anovulation19. Menopausal symptoms typically are frequent and bothersome during this important lifestage.4 Among 5,537 postmenopausal women in China, researchers found that history of irregular (including long and short cycles and compared to regular) menstrual cycles was significantly associated with reduced late-life depressive symptoms.13 They also found a negative association between lifetime menstrual cycle length and postmenopausal depressive symptoms. However, results from this Chinese study are difficult to compare with ours since authors included only postmenopausal women and used menstrual cycle length as a continuous variable (ranging from 14 to 90 days), which is not ideal given the very different pathophysiology of short vs. long menstrual cycles. In a study conducted among more than 51,000 postmenopausal women in France, those with long (>34 days) or irregular menstrual cycles during peri-menopause had higher severe depressive symptoms, compared to those women with normal menstrual cycle length (25–31 days).14 However, authors did not find any association with short menstrual cycles (<24 days). In contrast, we found that short menstrual intervals during reproductive years predicted a more symptomatic menopause, especially higher sleep problems, heart discomfort and depressive symptoms, as well as an early natural menopause. Epidemiologic studies have demonstrated that short menstrual cycles are a marker of diminished ovarian reserve.20–22 Diminished ovarian reserve, as reflected by decreased circulating AMH levels, is characterized by a decrease in the number or quality of oocytes, a common infertility diagnosis in women with anovulatory cycles23 and a predictor of early menopause.24 This may explain why women with short cycle intervals reached menopause at an earlier age. The observed increase in depressive symptoms may be due to the increased risk of being menopausal or further in the menopause transition, with more menopausal symptoms, such as sleep disruption contributing and heart discomfort to worsening of mood. Another explanation may be related to alterations in the hypothalamic-pituitary-ovarian axis, since irregular or abnormal menstrual cycles are normally attributed to functional disruption of the hypothalamic-pituitary-ovarian axis25 and hypothalamic-pituitary-ovarian axis dysregulations have been found in depressed women.26–28 Another hypothesis is related to changes in circulating levels of reproductive hormones, specifically estradiol. Estradiol is the principal estrogen and modulates the synthesis, availability, and metabolism of serotonin, which is a key neurotransmitter in depression.29 In a systematic review and meta-analysis, older age at menopause and longer reproductive period, both reflecting longer exposure to endogenous estrogens, were associated with a lower risk of depression in later life.30 We also did not find any associations of either short or long menstrual cycles with urogenital symptoms. We hypothesize that this may be due to the fact that only approximately half of women included in this analysis reached menopause by the time we collected information on menopausal symptoms, and the genitourinary syndrome of menopause (GSM) is commonly observed among postmenopausal women .31 Further studies to confirm these hypotheses are warranted.
This study has some limitations. First, only half of the women who remained in this study by the midlife visit had reached menopause. Among all women, we assessed menopausal symptoms at only one time point, which likely did not reflect all symptoms throughout the menopausal transition. Second, the small number of women with abnormal menstrual cycle length, particularly with long menstrual cycles, may result in lack of study power to find other associations of cycle length with menopausal timing and symptoms. Third, women in this study were recruited in eastern Massachusetts and all had health care and insurance at study enrollment in pregnancy. In addition, these women were mostly White, married, well-educated, and with a normal BMI. This may limit our ability to extrapolate the observed results to women in the general population or to nulliparous women. Furthermore, selection bias remains a concern as less than half of the women who enrolled in Project Viva participated in the midlife visit. Fourth, as is the case for all studies based on self-reported questionnaires, measurement error and misclassification of the exposure and other covariates is a concern. Fifth, as all the observational studies, unmeasured confounding by other related factors (e.g. comorbidities, medications, etc.) is still possible. The major strength of the study is its prospective design with almost two decades of follow-up which minimizes the risk of reverse causation. Other strengths include the use of a well-established instrument to assess menopausal symptoms and a rich database of covariates collected both at study enrollment as well as the midlife visit.
Conclusions
In summary, we observed that women with short (<25 days) menstrual cycles during reproductive years had a higher frequency of total menopausal, primarily somatic and psychological symptoms, and an earlier age of natural menopause at midlife approximately 18 years later. Menstrual cycle length was not associated with any other examined psychological, somatic or urogenital symptoms. Using the menstrual cycle as an additional vital sign adds a powerful tool to the assessment of physical and mental health. Due to the limited epidemiologic literature on this topic, further studies are warranted, and in particular large studies that can evaluate separately women with usual cycle length <21 days, to confirm these results and further explore potential underlying mechanisms.
Supplementary Material
Funding and acknowledgments:
The project was funded by grants NIH grants R01 HD096032, R01HD034568, U54 AG062322. The authors gratefully acknowledge all members of the Project Viva study team and all the study participants.
Footnotes
Competing financial interests: None of the authors has any conflicts of interest to declare.
References
- 1.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. The Journal of clinical endocrinology and metabolism. 2012;97(4):1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E. Perimenopause as a neurological transition state. Nature reviews Endocrinology. 2015;11(7):393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shifren JL, Gass ML. The North American Menopause Society recommendations for clinical care of midlife women. Menopause. 2014;21(10):1038–1062. [DOI] [PubMed] [Google Scholar]
- 4.Woods NF, Mitchell ES. Symptoms during the perimenopause: prevalence, severity, trajectory, and significance in women’s lives. Am J Med. 2005;118 Suppl 12B:14–24. [DOI] [PubMed] [Google Scholar]
- 5.Allshouse A, Pavlovic J, Santoro N. Menstrual Cycle Hormone Changes Associated with Reproductive Aging and How They May Relate to Symptoms. Obstetrics and gynecology clinics of North America. 2018;45(4):613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lan Y, Huang Y, Song Y, et al. Prevalence, severity, and associated factors of menopausal symptoms in middle-aged Chinese women: a community-based cross-sectional study in southeast China. Menopause. 2017;24(10):1200–1207. [DOI] [PubMed] [Google Scholar]
- 7.Nelson HD, Haney EM, Humphrey L, et al. Management of menopause-related symptoms. 2005. [PMC free article] [PubMed]
- 8.Dennerstein L, Alexander JL, Kotz K. The menopause and sexual functioning: a review of the population-based studies. Annu Rev Sex Res. 2003;14:64–82. [PubMed] [Google Scholar]
- 9.Santoro N, Lasley B, McConnell D, et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women’s Health across the Nation (SWAN) Daily Hormone Study. The Journal of clinical endocrinology and metabolism. 2004;89(6):2622–2631. [DOI] [PubMed] [Google Scholar]
- 10.Nelson DB, Sammel MD, Patterson F, Lin H, Gracia CR, Freeman EW. Effects of reproductive history on symptoms of menopause: a brief report. Menopause. 2011;18(10):1143–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Boer EJ, den Tonkelaar I, te Velde ER, Burger CW, Klip H, van Leeuwen FE. A low number of retrieved oocytes at in vitro fertilization treatment is predictive of early menopause. Fertility and sterility. 2002;77(5):978–985. [DOI] [PubMed] [Google Scholar]
- 12.Hess R, Olshansky E, Ness R, et al. Pregnancy and birth history influence women’s experience of menopause. Menopause. 2008;15(3):435–441. [DOI] [PubMed] [Google Scholar]
- 13.Li F, He F, Sun Q, et al. Reproductive history and risk of depressive symptoms in postmenopausal women: A cross-sectional study in eastern China. J Affect Disord. 2019;246:174–181. [DOI] [PubMed] [Google Scholar]
- 14.Perquier F, Ryan J, Ancelin ML, Mesrine S, Clavel-Chapelon F. Lifetime endogenous reproductive factors and severe depressive symptoms in postmenopausal women: findings from the E3N cohort. Menopause. 2013;20(11):1154–1163. [DOI] [PubMed] [Google Scholar]
- 15.Oken E, Baccarelli AA, Gold DR, et al. Cohort profile: project viva. International journal of epidemiology. 2015;44(1):37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. The Journal of pediatrics. 2004;144(2):240–245. [DOI] [PubMed] [Google Scholar]
- 17.Heinemann K, Ruebig A, Potthoff P, et al. The Menopause Rating Scale (MRS) scale: a methodological review. Health Qual Life Outcomes. 2004;2:45–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of general internal medicine. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harlow SD. Menstrual Cycle Changes as Women Approach the Final Menses: What Matters? Obstetrics and gynecology clinics of North America. 2018;45(4):599–611. [DOI] [PubMed] [Google Scholar]
- 20.Kristensen SL, Ramlau-Hansen CH, Andersen CY, et al. The association between circulating levels of antimüllerian hormone and follicle number, androgens, and menstrual cycle characteristics in young women. Fertility and sterility. 2012;97(3):779–785. [DOI] [PubMed] [Google Scholar]
- 21.Small CM, Manatunga AK, Klein M, et al. Menstrual cycle characteristics: associations with fertility and spontaneous abortion. Epidemiology (Cambridge, Mass). 2006;17(1):52–60. [DOI] [PubMed] [Google Scholar]
- 22.Kolstad HA, Bonde JP, Hjøllund NH, et al. Menstrual cycle pattern and fertility: a prospective follow-up study of pregnancy and early embryonal loss in 295 couples who were planning their first pregnancy. Fertility and sterility. 1999;71(3):490–496. [DOI] [PubMed] [Google Scholar]
- 23.Broekmans FJ, Knauff EA, te Velde ER, Macklon NS, Fauser BC. Female reproductive ageing: current knowledge and future trends. Trends Endocrinol Metab. 2007;18(2):58–65. [DOI] [PubMed] [Google Scholar]
- 24.Ghezelayagh Z, Totonchi M, Zarei-Moradi S, et al. The Impact of Genetic Variation and Gene Expression Level of The Follicle-Stimulating Hormone Receptor on Ovarian Reserve. Cell J. 2018;19(4):620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diaz A, Laufer MR, Breech LL. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Pediatrics. 2006;118(5):2245–2250. [DOI] [PubMed] [Google Scholar]
- 26.Baischer W, Koinig G, Hartmann B, Huber J, Langer G. Hypothalamic-pituitary-gonadal axis in depressed premenopausal women: elevated blood testosterone concentrations compared to normal controls. Psychoneuroendocrinology. 1995;20(5):553–559. [DOI] [PubMed] [Google Scholar]
- 27.Meller WH, Grambsch PL, Bingham C, Tagatz GE. Hypothalamic pituitary gonadal axis dysregulation in depressed women. Psychoneuroendocrinology. 2001;26(3):253–259. [DOI] [PubMed] [Google Scholar]
- 28.Young EA, Midgley AR, Carlson NE, Brown MB. Alteration in the hypothalamic-pituitary-ovarian axis in depressed women. Arch Gen Psychiatry. 2000;57(12):1157–1162. [DOI] [PubMed] [Google Scholar]
- 29.Lokuge S, Frey BN, Foster JA, Soares CN, Steiner M. Depression in women: windows of vulnerability and new insights into the link between estrogen and serotonin. J Clin Psychiatry. 2011;72(11):e1563–1569. [DOI] [PubMed] [Google Scholar]
- 30.Georgakis MK, Thomopoulos TP, Diamantaras AA, et al. Association of Age at Menopause and Duration of Reproductive Period With Depression After Menopause: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2016;73(2):139–149. [DOI] [PubMed] [Google Scholar]
- 31.Phillips NA, Bachmann GA. The genitourinary syndrome of menopause. Menopause. 2021;28(5):579–588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


