Abstract
Background:
Electroencephalography (EEG) findings following cardiovascular collapse in death are uncertain. We aimed to characterize EEG changes immediately preceding and following cardiac death.
Methods:
We retrospectively analyzed EEGs of patients who died from cardiac arrest while undergoing standard EEG monitoring in an intensive care unit. Patients with brain death preceding cardiac death were excluded. Three events during fatal cardiovascular failure were investigated: (1) last recorded QRS complex on electrocardiogram (QRS0), (2) cessation of cerebral blood flow (CBF0) estimated as the time that blood pressure and heart rate dropped below set thresholds, and (3) electrocerebral silence on EEG (EEG0). We evaluated EEG spectral power, coherence, and permutation entropy at these time points.
Results:
Among 19 patients who died while undergoing EEG monitoring, seven (37%) had a comfort-measures-only status and 18 (95%) had a do-not-resuscitate status in place at the time of death. EEG0 occurred at the time of QRS0 in five patients and after QRS0 in two patients (cohort median − 2.0, interquartile range − 8.0 to 0.0), whereas EEG0 was seen at the time of CBF0 in six patients and following CBF0 in 11 patients (cohort median 2.0 min, interquartile range − 1.5 to 6.0). After CBF0, full-spectrum log power (p < 0.001) and coherence (p < 0.001) decreased on EEG, whereas delta (p = 0.007) and theta (p = 0.007) permutation entropy increased.
Conclusions:
Rarely may patients have transient electrocerebral activity following the last recorded QRS (less than 5 min) and estimated cessation of cerebral blood flow. These results may have implications for discussions around cardiopulmonary resuscitation and organ donation.
Keywords: Death, Encephalography, Consciousness, Cardiac arrest, Brain hypoxia, Hypotension
Introduction
Physicians report an exact time of death on death certificates, which models the prolonged process of fatal organ failure as a simple binary. However, organ failure in death is neither synchronized nor coordinated. Rather, organs cease to function at different rates [1, 2], and some cellular activity transiently increases [3, 4].
In animal studies of electroencephalographs (EEG) at the time of cardiac death, increased coherence in the gamma band frequency has been seen following the final heartbeat [5]. In patients, diffuse EEG attenuation after cardiac arrest [6] and cessation of arterial blood pressure [7] were seen, as well as an increased bispectral index for at least 18 min after the loss of blood pressure [8] and withdrawal of life-sustaining therapies (WLST) [9]. However, these studies did not deconstruct electrocerebral activity in ways that provide insight into the coordination of functions across the whole cortex during cardiac death.
Studies suggest that behavioral assessments alone may not reliably exclude the comprehension of verbal commands and conscious processing in patients with acute brain injury [10, 11]. More recently, brain activation to tone changes was observed in patients in end-of-life hospices who were unresponsive [2]. Further, highly attenuated EEG (less than 10 μV) in cortical neurons does not necessarily preclude their excitability [12]. Certainty about cerebral function at the time of cardiac arrest, which excludes the possibility of consciousness, may have implications for the management of patients during cardiopulmonary resuscitation and potentially for organ donation after cardiac death.
The aim of this study was to determine a time line of events associated with fatal organ failure and to identify EEG signatures associated with those events. We hypothesized that we would observe EEG slowing and attenuation [6, 7, 13–15], as well as transient surges in EEG spectral power and coherence [5, 7–9, 16, 17], as common signatures of cerebral activity following cardiovascular collapse in death.
Methods
Study Cohort
We included all adult patients who were admitted to our neurological intensive care unit and who had continuous EEG monitoring recorded at the time of cardiac death between February 2009 and January 2019. Patients underwent EEG monitoring for the purposes of detecting seizures or ischemia. None of the patients were placed on EEG for the purposes of recording EEG during the dying process. We excluded patients with the diagnosis of brain death and those who had no EEG recorded during the estimated cessation of cerebral blood flow, as defined below. Demographics and admission diagnoses were collected from the medical record. Data recorded prior to cardiac death included medication administration (such as sedatives and vasopressors), anticonvulsant medications, mechanical ventilation status, WLST, and administration of advanced cardiovascular life support (ACLS) measures. The time from hospitalizing injury to death and the cause of death were noted. The study was approved by the local institutional review board (IRB-AAAL4106), and the use of informed consent was waived per section IV of the local institutional review board’s Health Insurance Portability and Accountability Act policy, citing 45 CFR 164.512(i)(1)(iii).
Surrogate Events for Fatal Organ Failure
We studied three events as surrogates for organ failure associated with cardiac death: (1) cessation of cerebral blood flow, (2) electrocerebral silence on EEG, and (3) last recorded QRS complex on electrocardiogram (ECG). Each event was independently assessed by two board-certified critical care physicians (W-TC and AA), and a third expert (JC) was consulted for disagreements. EEG was assessed by two critical care EEG experts (AA and JC). Assessors were blinded to the rest of the physiologic data.
Events were defined as follows:
Cessation of cerebral blood flow (CBF0) was assumed to occur following a permanent (1) heart rate of less than 20 beats per minute and (2) blood pressure below a set threshold as measured by the arterial line laced into the radial artery. Because blood pressure measures varied in availability between patients, this second criterion was met if at least one of the following subcriteria, as well as all others available, was observed: (2a) mean arterial pressure of less than 20 mm Hg, (2b) systolic blood pressure of less than 40 mm Hg, and (2c) diastolic blood pressure of less than 20 mm Hg. These thresholds were chosen because ventricular asystole [18] and EEG slowing [14] have been observed just below these blood pressure levels.
Electrocerebral silence (EEG0) was defined as the time when the EEG amplitude permanently dropped below 2 μV, following standards for the diagnosis of brain death [19]. Notably, this EEG measure as defined above does not fulfill EEG criteria to declare brain death [19].
Last QRS complex (QRS0) was defined as the time of the final QRS complex with a clear R peak, as recorded on ECG, based on studies of QRS morphology as an indicator of cardiac dysfunction [20].
EEG and Physiologic Data Acquisition
Electroencephalograph and ECG recordings were obtained using a digital bedside video monitoring system (XLTEK; Excel-Tech Corp, Natus Medical Incorporated; Oakville, Ontario, Canada; low-pass filter = 70 Hz, high-pass filter = 1 Hz, sampling rate = 200, 256, and 512 Hz) with 21 EEG electrodes placed according to the International 10–20 system. EEG data were evaluated using a 5-μV/mm sensitivity and 60-Hz notch filter. Blood pressure and heart rate data were acquired using a Phillips or GE bedside physiologic monitoring system with Bed-masterEX software (Excel Medical Electronics; Jupiter, FL, USA; sampling rate = 0.2 Hz). EEG, ECG, and other physiologic measurements were recorded as part of routine clinical care. Bedside monitoring systems were synchronized to an Internet clock.
Data Preprocessing
Signal preprocessing was done with Matlab (Matlab 2019a; The MathWorks, Inc; Natick, MA). Raw EEG data were preprocessed using the FieldTrip [21] and Chronux [22] toolboxes. Noisy EEG channels were identified upon visual inspection by EEG experts (AA and JC) and excluded from analyses. A Hjorth surface Laplacian filter was applied, which, from each channel, subtracts the spatially averaged signal of its closest neighbors. This approach dampens EEG signals common to spatially proximate electrodes (i.e., signals often seen in movement or muscle artifact) while amplifying signals localized to each channel. We calculated three quantitative EEG features: spectral power, coherence, and permutation entropy in 30-s epochs. Only frequencies between 1 and 50 Hz were used. Data were analyzed in five different frequency bands: delta (1–4 Hz), theta (4–8 Hz), alpha (8–14 Hz), beta (14–26 Hz), and gamma (26–50 Hz). Data from 5 min before to 5 min after each event were used. Each 10-min EEG recording was discretized into 20 epochs for analysis.
Spectral Power
Spectral power is a measure of the energy carried by a signal and defined as the squared EEG amplitude in a discrete frequency range. Power for each epoch was calculated using multitaper decomposition, as implemented in the mtspectrumc function of the Chronux toolbox. Analyses were performed after applying a natural logarithm.
Coherence
Coherence is a measure of phase synchrony between signals. Coherence was calculated for all combinations of channel pairs using weighted pairwise phase consistency as implemented in the ft_connectivityanalysis function of the FieldTrip toolbox.
Permutation Entropy
Permutation entropy is a measure of complexity in a time series based on the probability of ordinal sequences of different permutations in the time series [23]. Permutation entropy of permutation order n = 4 was calculated over a time lag vector τ = (2,4,8).
Statistical Tests
Event-related changes to EEG features were calculated for each frequency band as well as the full frequency spectrum. We conducted repeated measures of analysis of variance, comparing features across all epochs. Not all data were normally distributed and spherical, so pairs of epochs were compared using Wilcoxon signed-rank tests and corrected for multiple comparisons using the Benjamini–Hochberg false discovery rate method. Each pair contained epochs occurring 5 min apart, with one epoch before and one epoch after an event. With 10 epoch pairs and 19 patients, this resulted in 190 total comparisons.
Results
Study Cohort
We identified 19 patients who had continuous EEG recorded at the time of cardiovascular collapse in cardiac death. The median age at death was 57 years (interquartile range [IQR] 45–82), and the most common admission diagnoses were status epilepticus and cardiac arrest (Table 1). At the time of death, 14 patients had a status of comfort measures only or WLST with do not resuscitate, and 18 had a do-not-resuscitate status in place. Three patients received ACLS, including chest compressions and epinephrine, and two of these patients were placed on a do-not-resuscitate status after ACLS was performed. In the 24 h preceding death, 16 patients received anticonvulsants and/or sedatives and 10 received vasopressors. QRS0 was not available in two patients because of ECG artifacts following chest compressions and poorly connected ECG leads.
Table 1.
Patient cohort (n = 19)
| Age, median (IQR) (year) | 57.2 (45.5–82.0) |
| Sex, n (%) | |
| Female | 7 (37) |
| Admission etiology, n (%) | |
| Status epilepticus | 4 (21) |
| Cardiac arrest | 3 (16) |
| Stroke | 2 (11) |
| SDH | 2 (11) |
| ICH | 2 (11) |
| Other | 5 (26) |
| Days from hospitalizing injury to death, median (IQR) | 4 (2.5–7.5) |
| Mechanically ventilated, n (%) | 6 (32) |
| WLST and/or CMO, n (%) | 14 (74) |
| DNRa, n (%) | 18 (95) |
| ACLSa, n (%) | 3 (16) |
| Received sedatives/anticonvulsantsb, n (%) | 16 (84) |
| Received vasopressorsb, n (%) | 10 (53) |
Demographic breakdown of the cohort by age, sex, and admission etiology: WLST, CMO, mechanical ventilation status prior to WLST, and receipt of ACLS. Other: seizure (n = 1), brain tumor (n = 1), cardiomyopathy (n = 1), traumatic brain injury (n = 1), arteriovenous fistula (n = 1), and abdominal compartment syndrome (n = 1)
ACLS advanced cardiac life support, CMO comfort measures only, DNR do not resuscitate, ICH intracerebral hemorrhage, IQR interquartile range, SDH subdural hemorrhage, WLST withdrawal of life-sustaining therapies
Two patients were placed on DNR status after ACLS was performed
Received in the 24 h before death
Time Line of Events
The time line of CBF0, EEG0, and QRS0 varied between the 19 patients tremendously (Fig. 1). EEG0 occurred prior to QRS0 in ten patients, at the time of QRS0 in five, and following QRS0 in two. The range of EEG0 in relation to QRS0 was from − 80.0 to 2.0 min (median − 2.0, IQR − 8.0 to 0.0). EEG0 occurred prior to CBF0 in six patients, at the time of CBF0 in two, and following CBF0 in 11. The range of EEG0 in relation to CBF0 was from − 33.0 to 34.0 min (median 2.0, IQR − 1.5 to 6.0).
Fig. 1.
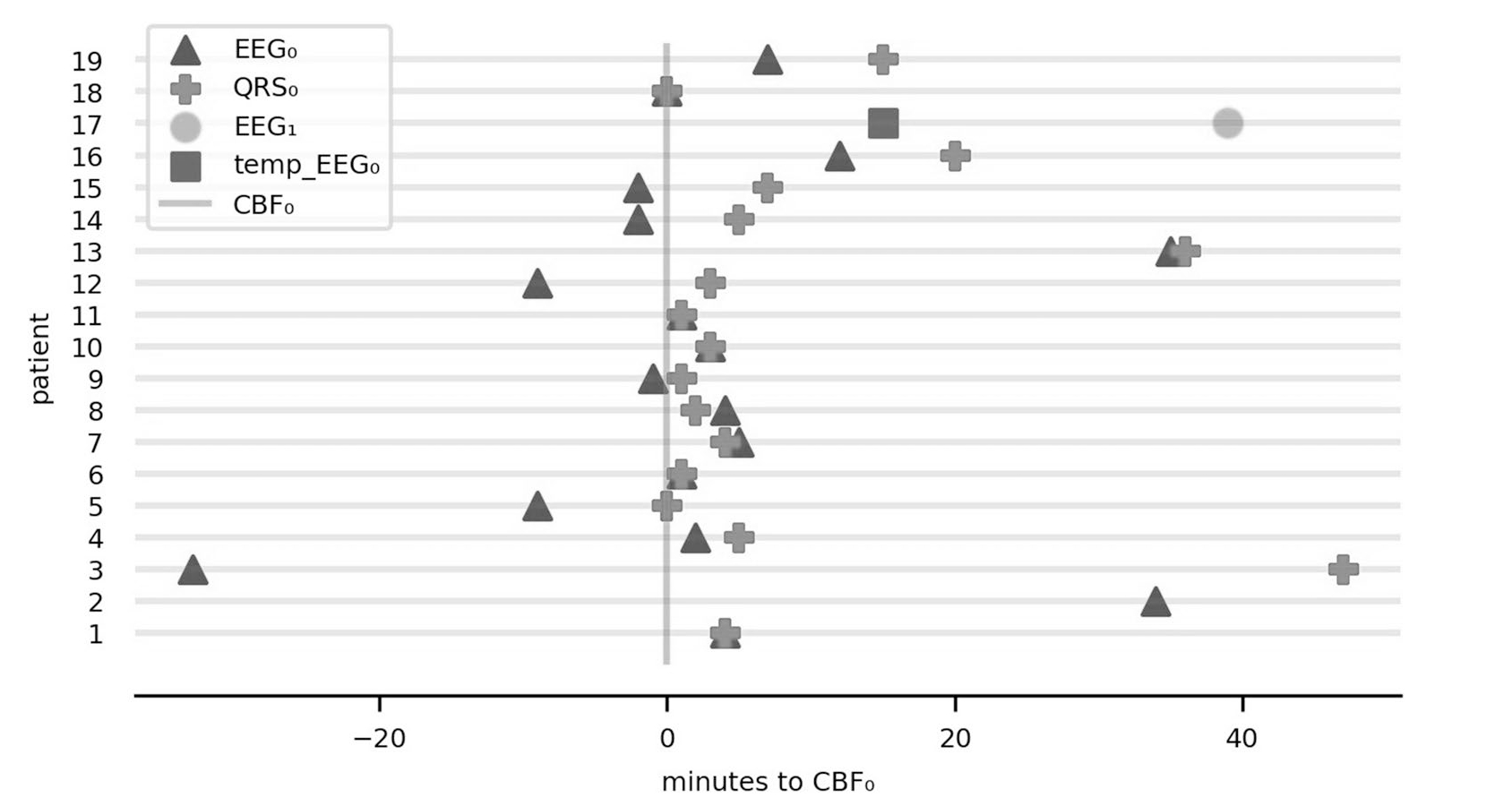
Timeline of three pathophysiologic events. Relationship of electrocerebral silence on EEG (EEG0) and last QRS complex on ECG (QRS0) in relation to cessation of cerebral blood flow (CBF0). The y axis represents the 19 patients in the study and the x axis represents time in minutes. Patients are ordered by magnitude of change in global power 5 min before vs. 5 min after CBF0. For patient 12, temp_EEG0 represents time of temporary electrocerebral silence before the return of cerebral activity (EEG1; see Fig. 3 for details). QRS0 was not available in two patients (no. 2 and no. 17) because of ECG artifacts following chest compressions and poorly connected ECG leads. ECG electrocardiogram, EEG electroencephalograph, EEG1 return of cerebral activity
Significant EEG Feature Changes
After CBF0, full-spectrum log power and full-spectrum coherence decreased (Fig. 2, Supplemental Fig. 1), largely driven by low frequencies. Concurrently, theta and delta permutation entropy increased. After EEG0, full-spectrum log power, delta coherence, and beta permutation entropy decreased, whereas delta and theta permutation entropy increased. Following QRS0, delta coherence decreased (Table 2). A case study of a patient with returning EEG activity following CBF0 and a brief period of isoelectric EEG is given by Fig. 3 and Supplemental Fig. 2. Statistical dispersion of EEG features at CBF0 is given by Table 3.
Fig. 2.
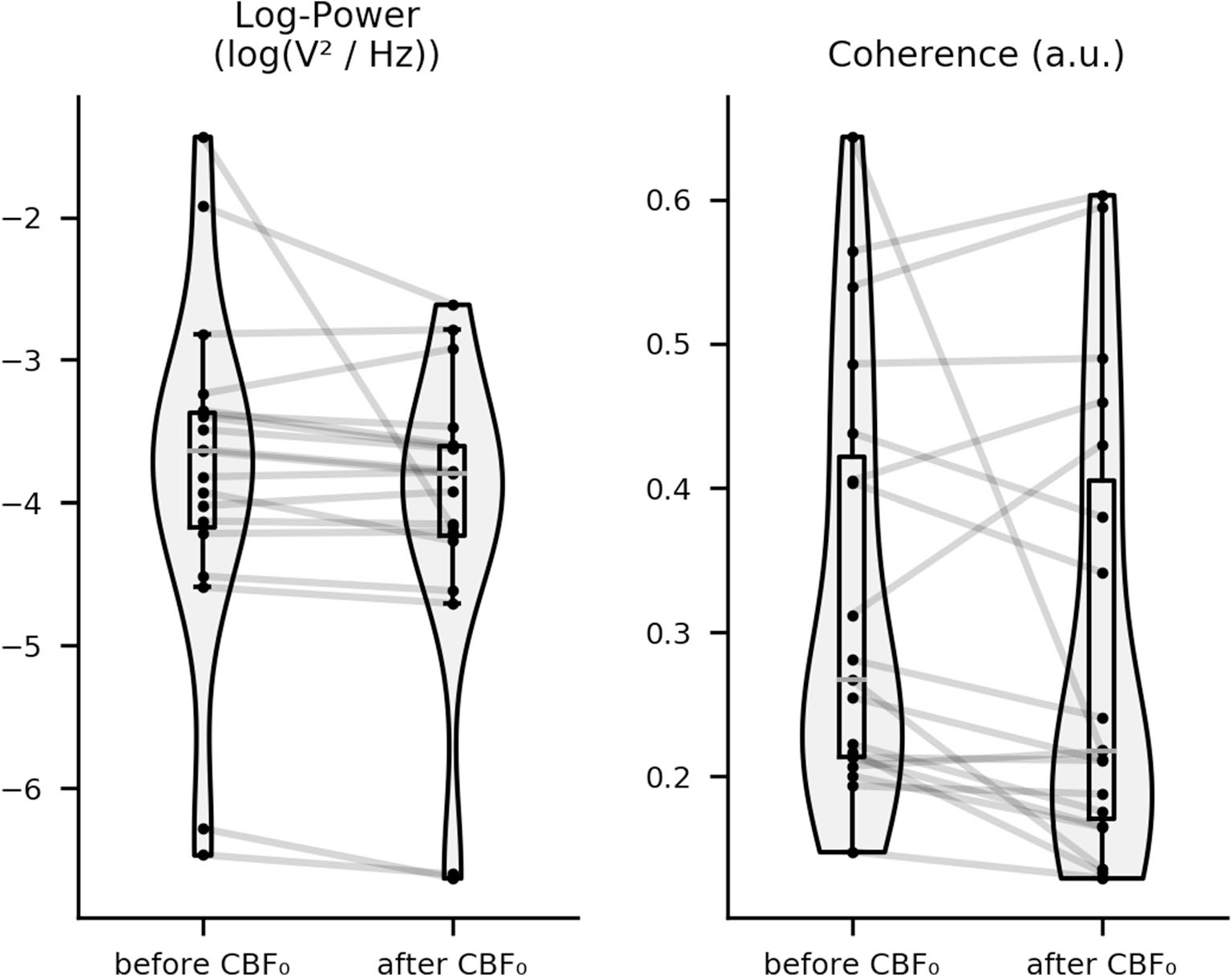
Change in full-spectrum EEG features after CBF0. Boxplot showing change in epoch-averaged and channel-averaged full-spectrum EEG features 5 min before vs. 5 min after CBF0 for all patients. Cohort-wide changes in log power and coherence are statistically significant (p < 0.028). CBF0 cessation of cerebral blood flow, EEG electroencephalograph
Table 2.
Statistical results
| EEG feature | Frequency | Event | ANOVA | Wilcoxon signed-rank test |
|---|---|---|---|---|
|
| ||||
| Log power | Full-spectrum | CBF0 | F18,324 = 2.87, p < 0.001 | W = 3163, p < 0.001, RBC = 0.57 |
| Log power | Full-spectrum | EEG0 | F18,324 = 4.44, p < 0.001 | W = 1973, p < 0.001, RBC = 0.73 |
| Coherence | Full-spectrum | CBF0 | F18,324 = 1.71, p = 0.037 | W = 4421, p < 0.001, RBC = 0.40 |
| Coherence | Delta | EEG0 | F18,324 = 2.48, p < 0.001 | W = 4795, p < 0.001, RBC = 0.35 |
| Coherence | Delta | QRS0 | F18,288 = 2.40, p = 0.001 | W = 3418, p < 0.001, RBC = 0.42 |
| Permutation entropy | Delta | CBF0 | F18,324 = 2.01, p = 0.009 | W = 5621, p = 0.007, RBC = −0.24 |
| Permutation entropy | Theta | CBF0 | F18,324 = 3.05, p < 0.001 | W = 4391, p < 0.001, RBC = −0.40 |
| Permutation entropy | Delta | EEG0 | F18,324 = 3.24, p < 0.001 | W = 4492, p < 0.001, RBC = −0.39 |
| Permutation entropy | Theta | EEG0 | F18,324 = 1.82, p = 0.022 | W = 5423, p = 0.003, RBC = −0.26 |
| Permutation entropy | Beta | EEGo | F18,324 = 1.73, p = 0.033 | W = 4915, p < 0.001 RBC = 0.33 |
Comprehensive statistical test results for all EEG features with statistically significant changes during a pathophysiologic event, stratified by frequency band. If full-spectrum changes were significant, frequency band-specific changes were not reported
ANOVA analysis of variance, CBF0 cessation of cerebral blood flow, EEG electroencephalograph, EEG0 electrocerebral silence on EEG, QRS0 last QRS complex on electrocardiogram, RBC rank-biserial correlation, W Wilcoxon signed-rank test statistic
Fig. 3.
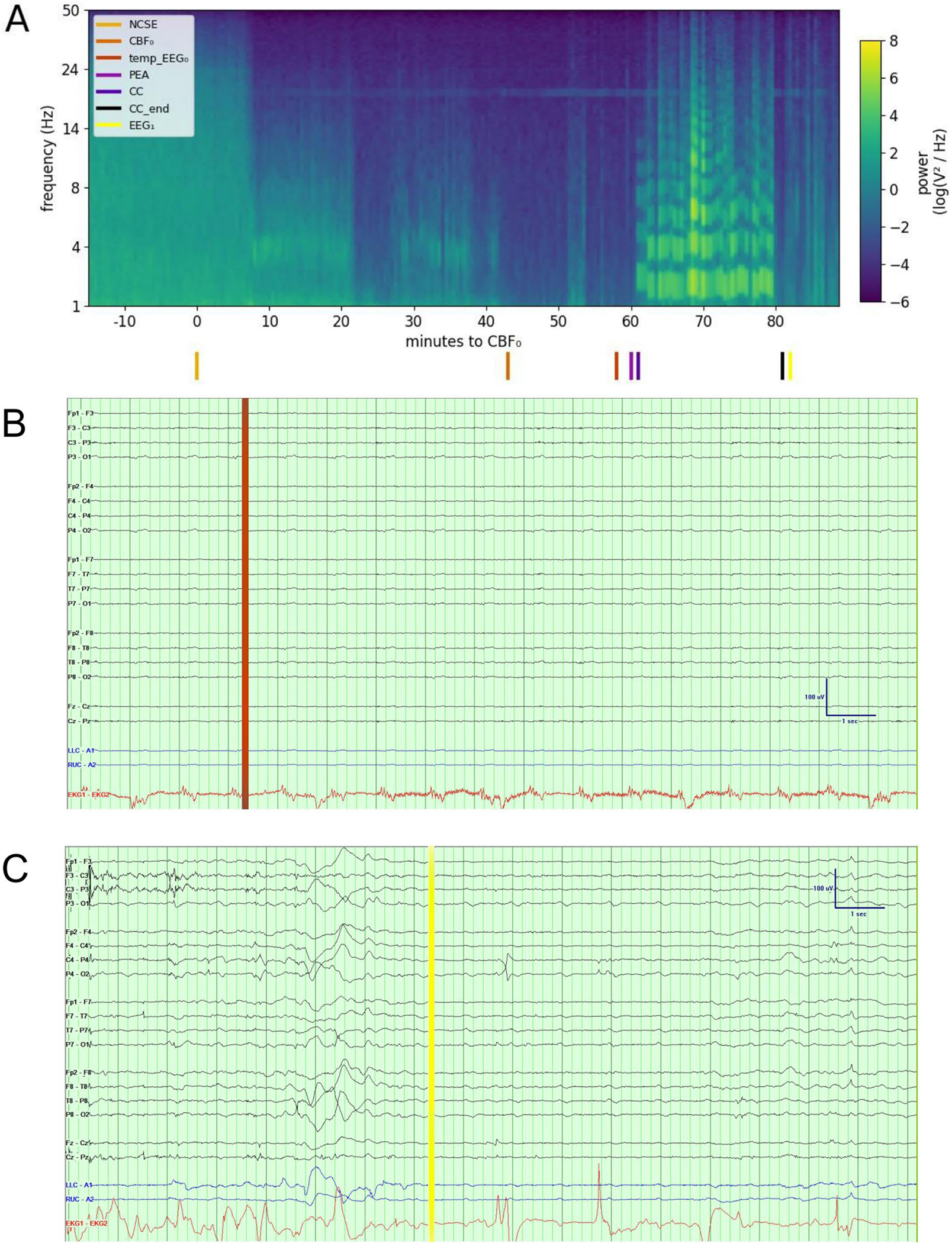
Raw EEG data and power spectral density in a patient with electrocerebral activity on EEG after CBF0 and a brief period of isoelectric EEG. Sixty-five-year-old woman who was hospitalized for refractory status epilepticus in the setting of anoxic brain injury. On hospital day 10, the course was complicated by cardiac arrest with PEA. After chest compressions were performed for 20 min, the patient underwent withdrawal of life-sustaining therapies at the request of the family. Power spectral density of her EEG is displayed (a), calculated from channels Fz, Cz, and Pz because these are less frequently affected by muscle and ECG artifact. Colored lines at the bottom indicate nonconvulsive status epilepticus (NCSE), CBF0, EEG0, PEA, start of chest compressions (CC), end of chest compressions (CC_end), and return of cerebral activity ( EEG1). EEG (bipolar montage, sensitivity of 5 μV/mm, 60-Hz notch filter) at the time (temp_EEG0) demonstrated no clear electrocerebral activity (b). Following the activity after CBF0 and a brief period of isoelectric EEG, electrocerebral activity returned ( EEG1) (c) (see Supplemental Fig. 2 for raw EEG data at the remaining events). The power spectral density plot revealed decreased power 36 min before CBF0. Artifacts are seen throughout in the 20-Hz range, and prominent artifacts due to chest compressions are seen between 18 and 38 min following CBF0. At CBF0, median log power was − 4.10 (IQR − 12.08 to − 2.69, range − 16.61 to 2.96), median coherence was 0.23 (IQR 0.01–0.94, range − 0.50 to 0.99), and median permutation entropy was 3.31 (IQR 2.60–3.87, range 1.85–4.45). CBF0 cessation of cerebral blood flow, ECG electrocardiogram, EEG electroencephalograph, EEG0 electrocerebral silence on EEG, IQR interquartile range, PEA pulseless electrical activity
Table 3.
Dispersion of EEG features around CBF0
| EEG feature (units) | Median | IQR | Range |
|---|---|---|---|
|
| |||
| Log power (log[V2/Hz]) | −3.42 | −4.11 to 2.40 | −16.83 to 7.83 |
| Coherence | 0.09 | −0.05 to 0.48 | −0.97 to 0.99 |
| Permutation entropy | 3.03 | 2.27 to 3.76 | 1.22 to 4.47 |
Median, IQR, and range of cohort’s EEG features around CBF0
CBF0 cessation of cerebral blood flow, EEG electroencephalograph, IQR interquartile range
Discussion
In this case series, we observed that the timing of electrocerebral silence following cardiovascular collapse in death was highly variable in relation to the permanent cessation of the electrical and pump function of the heart. Across all patients, decreases in full-spectrum coherence and log power, with increases in low-frequency permutation entropy, were seen at the time of estimated cessation of cerebral blood flow. To our knowledge, this study is the first to look at the order and timing of these three pathophysiologic events. None of these observations allow any conclusion on the possibility of awareness following cardiac death but, instead, support the notion of a more complex and poorly synchronized process of dying [24].
In humans, voltage suppression [6, 7] and isoelectric EEG within 30 s of asystole [13, 19, 25, 26] have been reported. In rare occasions, prolonged EEG activity minutes after cessation of blood flow has been observed [8, 9]. Rhythmic [6, 13], bursting [7], and background [6] neural activity in the delta band frequency range have been reported following cardiac arrest. Sustained background neural activity [6] and increased amplitude [15] in the theta band have been found, as well. A recent large-scale study found a transient return of cardiac activity (arterial pressure of 5 mm Hg or more with a QRS complex) in 14% of patients after cessation of cardiac activity during WLST [27].
In our case series, we saw persistent EEG activity in some patients minutes after CBF0 and QRS0. Our results support the notion that, in rare cases, electrical brain activity can briefly continue in the absence of cardiac function supporting cerebral perfusion. The excitability and potential preservation of cortical neurons several minutes after cardiac arrest, suggested by our case study (see Fig. 3), contributes to evidence that electrocerebral silence during the dying process is not always permanent [12, 28–30].
After interpreting our EEG features, we assume that power is a function of aggregate neuronal electrical activity, that coherence may be proportional to functional connectivity, and that permutation entropy is theoretically proportional to the amount of information communicated by a signal. A prior study reported increased power and coherence in the lower gamma band (25–55 Hz) after circulatory death in rats [5]. Translating these findings to humans is challenging because normal and abnormal frequency spectra may differ [31–33]; however, in contrast to the animal work, we found decreases in full-spectrum EEG log power and coherence following the estimated circulatory cessation. In accordance with attenuation seen in previous human studies within seconds of cardiac arrest [6, 7, 15], we found lower full-spectrum log power after CBF0.
Concurrent increases in permutation entropy and decreases in coherence in lower frequencies after CBF0 may represent a swift loss of both global and local synchronous neuronal activity following the loss of blood flow. This observed increase in signal complexity may also reflect abnormal information exchanges between neurons, implying that arrhythmic, local neural signaling is another possible signature of a blood-flow-deprived brain [6, 7, 15]. Given that permutation entropy is associated with changes in consciousness [34–36], these results suggest that perceptual changes, subsequent to abnormal perfusion of the brain, may be at least mediated by disintegrated long-distance cortical processing and elevations in locally complex processing.
Status epilepticus may predispose patients to transient electrocerebral activity in the absence of oxygenation and blood flow necessary to sustain life. In patients with status epilepticus, noise-driven perturbations are sufficient to trigger oscillations in pathologically hyperexcitable cortical neurons [37–39]. The effects of seizure-induced disruption of the blood–brain barrier [40–44] and pharmacological agents on hyperexcitable neurons may influence mechanisms that underlie the recurrence of EEG activity following electrocerebral silence during cardiac death. Intramuscular epinephrine administered to patient 17 during pulseless electrical activity and shortly before the recurrence of EEG activity, which may have mimicked an endogenous stress response, induced cerebral activity [45–47]. The underlying mechanisms of a brief return of EEG activity following a brief period of isoelectric EEG are uncertain and require further study.
Our case series contributes to a small corpus of literature that has evaluated the time course of electrocerebral function following cardiac arrest [5, 7–9, 13, 16, 17, 48]. Relevant to organ donation after cardiac death, a return of EEG activity after cardiopulmonary resuscitation was observed in pigs who underwent no more than 4.5 min of ventricular-fibrillation-induced cardiac arrest [48]. The authors suggest a 10-min “no-touch” period before organ retrieval to ensure brain death in all human donors. Our data support that a 5-min wait time may be sufficient, as EEG0 after the last QRS occurred for no more than 2 min in two patients. Further evidence is required to better determine the minimum time that needs to pass prior to proceeding with organ donation after cardiac death, as the quality of donor organs deteriorates rapidly following cardiac death.
Of 19 patients who died a cardiac death, 11 had EEG activity following permanent cessation of the electrical or pump function of the heart. The persistence of cortical activity and the reversibility of electrocerebral silence in patients who are critically ill after physician-determined death has implications for continued discussions surrounding cardiopulmonary resuscitation [6, 28, 49, 50], EEG criteria for brain death [19, 51–54], and organ donation after cardiac death [7, 26, 27, 53, 55]. The use of EEG to support outcome prediction during resuscitation [6, 48] and to determine the nociceptive capacity of the brain under life-threatening conditions, such as cardiovascular failure [56–60], may be of interest for future research.
Our study has multiple limitations. First, although times for both QRS0 and EEG0 were assessed from the same EEG monitor clock, a clock from another bedside monitor was used to assess blood pressure criteria for CBF0, which limits the precision of timing between events. Second, the study is a retrospective case series with a small number of patients collected over many years. This prevents us from making generalizable inferences. Third, although we took artifacts into consideration during the quantitative EEG analysis, the intensive care unit is an artifact-rich environment, thus making artifact-free analysis challenging. Fourth, we did not analyze cortical spreading depolarization because patients only underwent surface EEG [28, 61]. Fifth, the EEG in this series was obtained using standard clinical EEG criteria and not those recommended for brain death determination, which is pertinent to our assessment of electrocerebral silence. Finally, our cohort comprised a group of patients with different etiologies and preexisting neurological diagnoses. Our observations of post-QRS0 EEG activity in patients receiving epinephrine and patients with status epilepticus immediately preceding cardiac arrest may be insufficient to make generalizable inferences about all patients. Although challenging, a larger prospectively collected multicenter cohort study may better elucidate electrocerebral patterns during the process of cardiac death.
Supplementary Material
Acknowledgments
Thank you to the nurses, attending physicians, fellows, and residents of the Neurological ICU of Columbia University Irving Medical Center for their overall support of this project. We would like to thank Professor Laura Lennihan for her insightful comments regarding the ethical implications of our work and for providing helpful critiques for our paper.
Source of support
JC is supported by grant funding from the National Institutes of Health (R01 NS106014 and R03 NS112760), the James McDonnell Foundation, and the Dana Foundation. AA is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under the Miami Clinical and Translational Science Institute KL2 Career Development Award (UL1TR002736). SP is supported by the National Institutes of Health (K01 ES026833).
Footnotes
Conflicts of Interest
JC reports grants from the National Institute of Neurological Disorders and Stroke and the Dana Foundation. He is a minority shareholder at iCE Neuro-systems. None of these constitute a conflict of interest to the work presented here. The remaining authors do not have any conflicts of interest.
Ethical approval/informed consent
We confirm adherence to ethical guidelines and indicate ethical approvals (local institutional review board, IRB-AAAL4106) and the use of informed consent, as appropriate. Because this was a retrospective study approved by the local institutional review board at Columbia University, informed consent was waived per section IV of the local institutional review board’s Health Insurance Portability and Accountability Act policy, citing 45 CFR 164.512(i)(1)(iii).
Supplementary Information
The online version contains supplementary material available at https://doi.org/10.1007/s12028-021-01233-0.
References
- 1.Salehi S, Tran K, Grayson WL. Advances in perfusion systems for solid organ preservation. Yale J Biol Med. 2018;91(3):301–12. [PMC free article] [PubMed] [Google Scholar]
- 2.Blundon EG, Gallagher RE, Ward LM. Electrophysiological evidence of preserved hearing at the end of life. Sci Rep. 2020;10(1):10336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pozhitkov AE, Neme R, Domazet-Lošo T, et al. Tracing the dynamics of gene transcripts after organismal death. Open Biol. 2017;7(1):160267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira PG, Muñoz-Aguirre M, Reverter F, et al. The effects of death and post-mortem cold ischemia on human tissue transcriptomes. Nat Commun. 2018;9(1):490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borjigin J, Lee U, Liu T, et al. Surge of neurophysiological coherence and connectivity in the dying brain. Proc Natl Acad Sci. 2013;110(35):14432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reagan EM, Nguyen RT, Ravishankar ST, et al. Monitoring the relationship between changes in cerebral oxygenation and electroencephalography patterns during cardiopulmonary resuscitation. Crit Care Med. 2018;46(5):757–63. [DOI] [PubMed] [Google Scholar]
- 7.Norton L, Gibson RM, Gofton T, et al. Electroencephalographic recordings during withdrawal of life-sustaining therapy until 30 minutes after declaration of death. Can J Neurol Sci. 2017;44(2):139–45. [DOI] [PubMed] [Google Scholar]
- 8.Chawla LS, Akst S, Junker C, Jacobs B, Seneff MG. Surges of electroencephalogram activity at the time of death: a case series. J Palliat Med. 2009;12(12):1095–100. [DOI] [PubMed] [Google Scholar]
- 9.Auyong DB, Klein SM, Gan TJ, Roche AM, Olson D, Habib AS. Processed electroencephalogram during donation after cardiac death. Anesth Analg. 2010;110(5):1428–32. [DOI] [PubMed] [Google Scholar]
- 10.Edlow BL, Fins JJ. Assessment of covert consciousness in the intensive care unit: clinical and ethical considerations. J Head Trauma Rehabil. 2018;33(6):424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claassen J, Doyle K, Matory A, et al. Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med. 2019;380(26):2497–505. [DOI] [PubMed] [Google Scholar]
- 12.Altwegg-Boussac T, Schramm AE, Ballestero J, et al. Cortical neurons and networks are dormant but fully responsive during isoelectric brain state. Brain. 2017;140(9):2381–98. [DOI] [PubMed] [Google Scholar]
- 13.Levin P, Kinnel J. Successful cardiac resuscitation despite prolonged silence of EEG. Arch Intern Med. 1966;117(4):557. [PubMed] [Google Scholar]
- 14.Trojaborg W, Boysen G. Relation between EEG, regional cerebral blood flow and internal carotid artery pressure during carotid endarterectomy. Electroencephalogr Clin Neurophysiol. 1973;34(1):61–9. [DOI] [PubMed] [Google Scholar]
- 15.Clute HL, Levy WJ. Electroencephalographic changes during brief cardiac arrest in humans. Anesthesiology. 1990;73(5):821–5. [DOI] [PubMed] [Google Scholar]
- 16.Chawla LS, Terek M, Junker C, et al. Characterization of end-of-life electroencephalographic surges in critically ill patients. Death Stud. 2017;41(6):385–92. [DOI] [PubMed] [Google Scholar]
- 17.Lee DE, Lee LG, Siu D, et al. Neural correlates of consciousness at near-electrocerebral silence in an asphyxial cardiac arrest model. Brain Connect. 2017;7(3):172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moss J, Rockoff M. EEG monitoring during cardiac arrest and resuscitation. JAMA. 1980;244(24):2750–1. [PubMed] [Google Scholar]
- 19.Nichols GS. Guideline 3: minimum technical standards for EEG recording in suspected cerebral death. J Clin Neurophysiol. 2012;29(2):109. [DOI] [PubMed] [Google Scholar]
- 20.Das MK, Zipes DP. Fragmented QRS: a predictor of mortality and sudden cardiac death. Hear Rhythm. 2009;6(3):S8–14. [DOI] [PubMed] [Google Scholar]
- 21.Oostenveld R, Fries P, Maris E, Schoffelen J-M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bokil H, Andrews P, Kulkarni JE, Mehta S, Mitra PP. Chronux: a platform for analyzing neural signals. J Neurosci Methods. 2010;192(1):146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bandt C, Pompe B. Permutation entropy: a natural complexity measure for time series. Phys Rev Lett. 2002;88(17):174102. [DOI] [PubMed] [Google Scholar]
- 24.Azevedo MA, Bitencourt Othero JC. Human death as a triptych process. Mortality. 2020;25(4):490–504. 10.1080/13576275.2020.1756765. [DOI] [Google Scholar]
- 25.de Vries JW, Bakker PFA, Visser GH, Diephuis JC, van Huffelen AC. Changes in cerebral oxygen uptake and cerebral electrical activity during defibrillation threshold testing. Anesth Analg. 1998;87(1):16–20. [DOI] [PubMed] [Google Scholar]
- 26.Pana R, Hornby L, Shemie SD, Dhanani S, Teitelbaum J. Time to loss of brain function and activity during circulatory arrest. J Crit Care. 2016;34:77–83. [DOI] [PubMed] [Google Scholar]
- 27.Dhanani S, Hornby L, van Beinum A, et al. Resumption of cardiac activity after withdrawal of life-sustaining measures. N Engl J Med. 2021;384(4):345–52. [DOI] [PubMed] [Google Scholar]
- 28.Schramm AE, Carton-Leclercq A, Diallo S, et al. Identifying neuronal correlates of dying and resuscitation in a model of reversible brain anoxia. Prog Neurobiol. 2020;185:101733. [DOI] [PubMed] [Google Scholar]
- 29.Vrselja Z, Daniele SG, Silbereis J, et al. Restoration of brain circulation and cellular functions hours post-mortem. Nature. 2019;568(7752):336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hossmann K-A, Zimmermann V Resuscitation of the monkey brain after 1 H complete ischemia: I: physiological and morphological observations. Brain Res. 1974;81(1):59–74. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs J Hippocampal theta oscillations are slower in humans than in rodents: implications for models of spatial navigation and memory. Philos Trans R Soc Lond B Biol Sci. 2014;369(1635):20130304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watrous AJ, Lee DJ, Izadi A, Gurkoff GG, Shahlaie K, Ekstrom AD. A comparative study of human and rat hippocampal low-frequency oscillations during spatial navigation. Hippocampus. 2013;23(8):656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Li D, Li X, et al. Phase–amplitude coupling between theta and gamma oscillations during nociception in rat electroencephalography. Neurosci Lett. 2011;499(2):84–7. [DOI] [PubMed] [Google Scholar]
- 34.Olofsen E, Sleigh JW, Dahan A. Permutation entropy of the electroencephalogram: a measure of anaesthetic drug effect. Br J Anaesth. 2008;101(6):810–21. [DOI] [PubMed] [Google Scholar]
- 35.Kreuzer M, Kochs EF, Schneider G, Jordan D. Non-stationarity of EEG during wakefulness and anaesthesia: advantages of EEG permutation entropy monitoring. J Clin Monit Comput. 2014;28(6):573–80. [DOI] [PubMed] [Google Scholar]
- 36.Sitt JD, King J-R, El Karoui I, et al. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain. 2014;137(8):2258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suffczynski P, Kalitzin S, Lopes Da Silva FH. Dynamics of non-convulsive epileptic phenomena modeled by a bistable neuronal network. Neuroscience. 2004;126(2):467–84. [DOI] [PubMed] [Google Scholar]
- 38.Ferlazzo E, Zifkin BG, Andermann E, Andermann F. Cortical triggers in generalized reflex seizures and epilepsies. Brain. 2005;128(4):700–10. [DOI] [PubMed] [Google Scholar]
- 39.Takeshita D, Sato YD, Bahar S. Transitions between multistable states as a model of epileptic seizure dynamics. Phys Rev E. 2007;75(5):051925. [DOI] [PubMed] [Google Scholar]
- 40.Nitsch C, Klatzo I. Regional patterns of blood-brain barrier breakdown during epileptiform seizures induced by various convulsive agents. J Neurol Sci. 1983;59(3):305–22. [DOI] [PubMed] [Google Scholar]
- 41.Clarke HB, Gabrielsen TO. Seizure induced disruption of blood-brain barrier demonstrated by CT. J Comput Assist Tomogr. 1989;13(5):889–92. [DOI] [PubMed] [Google Scholar]
- 42.Seiffert E, Dreier JP, Ivens S, et al. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24(36):7829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Vliet EA, Otte WM, Gorter JA, Dijkhuizen RM, Wadman WJ. Longitudinal assessment of blood-brain barrier leakage during epileptogenesis in rats: A quantitative MRI study. Neurobiol Dis. 2014;63:74–84. [DOI] [PubMed] [Google Scholar]
- 44.Löscher W Epilepsy and alterations of the blood–brain barrier: cause or consequence of epileptic seizures or both? Handb Exp Pharmacol. 2020. 10.1007/164_2020_406. [DOI] [PubMed] [Google Scholar]
- 45.Mueller AL, Dunwiddie TV. Anticonvulsant and proconvulsant actions of alpha- and beta-noradrenergic agonists on epileptiform activity in rat hippocampus in vitro. Epilepsia. 1983;24(1):57–64. [DOI] [PubMed] [Google Scholar]
- 46.Wortsman J Role of epinephrine in acute stress. Endocrinol Metab Clin North Am. 2002;31(1):79–106. [DOI] [PubMed] [Google Scholar]
- 47.Haut SR, Hall CB, Masur J, Lipton RB. Seizure occurrence: precipitants and prediction. Neurology. 2007;69(20):1905–10. [DOI] [PubMed] [Google Scholar]
- 48.Stiegler P, Sereinigg M, Puntschart A, et al. A 10 min “no-touch” time—is it enough in DCD? A DCD animal study Transpl Int. 2012;25(4):481–92. [DOI] [PubMed] [Google Scholar]
- 49.Nagao K, Nonogi H, Yonemoto N, et al. Duration of prehospital resuscitation efforts after out-of-hospital cardiac arrest. Circulation. 2016;133(14):1386–96. [DOI] [PubMed] [Google Scholar]
- 50.Morley PT, Lang E, Aickin R, et al. Part 2: evidence evaluation and management of conflicts of interest. Circulation. 2015;132(16 suppl 1):S40–50. [DOI] [PubMed] [Google Scholar]
- 51.Nakagawa TA, Ashwal S, Mathur M, Mysore M. Guidelines for the determination of brain death in infants and children: an update of the 1987 task force recommendations-executive summary. Ann Neurol. 2012;71(4):573–85. [DOI] [PubMed] [Google Scholar]
- 52.Greer DM, Shemie SD, Lewis A, et al. Determination of brain death/death by neurologic criteria: the world brain death project. JAMA. 2020;324(11):1078–97. [DOI] [PubMed] [Google Scholar]
- 53.Fernández-Torre JL, Hernández-Hernández MA, Muñoz-Esteban C. Non confirmatory electroencephalography in patients meeting clinical criteria for brain death: scenario and impact on organ donation. Clin Neurophysiol. 2013;124(12):2362–7. [DOI] [PubMed] [Google Scholar]
- 54.Truog RD. Defining death-making sense of the case of Jahi McMath. JAMA. 2018;319(18):1859–60. [DOI] [PubMed] [Google Scholar]
- 55.Dhanani S, Hornby L, Ward R, et al. Vital signs after cardiac arrest following withdrawal of life-sustaining therapy. Crit Care Med. 2014;42(11):2358–69. [DOI] [PubMed] [Google Scholar]
- 56.Greyson B, Evans BN. Distressing near-death experiences. Psychiatry. 1992;55(1):95–110. [DOI] [PubMed] [Google Scholar]
- 57.Bonenfant RJ. A child’s encounter with the devil: an unusual near-death experience with both blissful and frightening elements. JNDS. 2001;20(2):87–100. [Google Scholar]
- 58.French CC. Dying to know the truth: visions of a dying brain, or false memories? Lancet. 2001;358(9298):2010–1. [DOI] [PubMed] [Google Scholar]
- 59.Palmieri A, Calvo V, Kleinbub JR, et al. “Reality” of near-death-experience memories: evidence from a psychodynamic and electrophysiological integrated study. Front Hum Neurosci. 2014;8(429):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.KeshavarzShirazi T, Gonzales AM, Dickinson A, Parnia S. Abstract 314: cardiac arrest related cognitive activity. Circulation. 2020;142(suppl 4):314. [Google Scholar]
- 61.Dreier JP, Major S, Foreman B, et al. Terminal spreading depolarization and electrical silence in death of human cerebral cortex. Ann Neurol. 2018;83(2):295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


