Abstract
The current study is designed to evaluate the antiemetic effect of the diterpenoid phytol (PHY) using in vivo and in silico studies. For this, emesis was induced in 4-day-old chicks by the oral administration of copper sulfate (CuSO4.5H2O) at 50 mg/kg. To see the possible antiemetic mechanism of PHY, we used a number of reference drugs such as domperidone (80 mg/kg), ondansetron (24 mg/kg) and hyoscine (100 mg/kg) as positive controls, while the vehicle served as a negative control group. PHY was administered orally at the doses of 50 and 75 mg/kg. Both PHY and reference drugs were given alone or in combined groups to evaluate their synergistic or antagonistic effects on the chicks. Molecular docking of PHY and reference drugs was carried out against 5HT3, D2, D3, H1, NK1, and mAChRs (M1–M5) receptors for estimating binding affinity to the receptors. Drug-receptor interactions and active sites of the receptors were observed with the aid of different computational tools. The drug-likeness and pharmacokinetics of all the drugs were predicted through the SwissADME online database. The results suggest that PHY reduces the mean number of retches and increases latency dose-dependently in the birds. In the combination groups, PHY75 showed better antiemetic effects with domperidone and ondansetron. In addition, PHY exhibited the highest binding affinity with the D2 receptor (6CM4) (− 7.3 kcal/mol). In conclusion, PHY showed an antiemetic activity in chicks, possibly through the D2 receptor interaction pathway.
Keywords: Nausea, Vomiting, Diterpenoid alcohol, Chicks, Molecular docking
Introduction
Emesis, also known as vomiting, is usually a distasteful condition that results in the forcible ejection of stomach objects through the mouth and is distinctly connected with gastrointestinal motor activity. As such, it could be interpreted as the body's reaction to particular medications, disease co-morbidities, and protective mechanisms against food poisoning (Hall and Driscoll 2005). While emesis can perform the operation of emptying noxious substances from the bowel, nausea plays the role of conditioned repercussion to desist from the ingestion of offending elements (Scorza et al. 2007). Emesis can occur for a variety of reasons, including illnesses such as food poisoning, motion sickness, gastroenteritis (diarrhea), intestinal obstruction, head injury, pregnancy, appendicitis, or hangover; or it can be a common side effect of certain diseases such as brain tumors, ionizing radiation overexposure, and elevated intracranial pressure (Grahame-Smith 1986). It is also the most common side effect of cancer chemotherapy and radiation therapy (Shankar et al. 2015).
The mechanisms are quite complex. The vomiting center (VC), known as the central emetic generator, hosted by the fourth ventricle of the brain and an area of that region called the chemoreceptor trigger zone (CTZ), mainly plays a crucial role in inducing emesis or nausea (Iqbal and Spencer 2012; MacDougall and Sharma 2021). Besides the CTZ, some other sites such as the GI tract, the higher centers in the cortex, the vestibular system, and the thalamus are also reliable for inducing emesis (Becker 2010). The VC in the reticular construction can be stimulated by either convergent afferent stimuli from the GIT or by the CTZ, and it also synchronizes the activities of smooth muscles and skeletal functions related to emesis (Khan et al. 2014; Navari 2013). Emesis is triggered by the CTZ when various receptors within the CTZ, such as dopamine receptors (D2, D3), serotonin receptor (5-HT3), muscarinic acetylcholine receptors (mAChRs), neurokinin 1 receptor (NK1) for substance P, histamine (H1), and opioid receptors, detect emetogenic toxins in the blood and CSF and transfer this message to the neighboring nucleus tractus solitarius (NTS) (Hornby 2001; Naylor and Inall 1994). The NTS is the origin of a final extensive pathway by which all the emetic inputs provoke vomiting (Miller and Leslie 1994). During emesis, the stomach muscle relaxes and secretion of HCl is inhibited. A backward extensive contraction of the small intestine appeals to the stomach to provoke retching and vomiting (Lang 1990). At present, a variety of antiemetic drugs are used to treat nausea and vomiting, which can be classified as serotonin antagonists, anti-dopaminergic drugs, antihistamines, anticholinergic drugs, NK1-receptor inhibitors, corticosteroids, cannabinoids, 5-HT1A, GABAB, and CB1 receptor agonists (Ahmed et al. 2013).
The search for novel antiemetic medicines derived from natural sources continues to focus mechanism-based methods that involve distinct cellular and molecular targets. Flavonoids, cannabinoids, chalcones, glucosides, hydroxycinnamic acids, diarylheptanoids, lignans, phenylpropanoids, saponins, polysaccharides, and terpenes are some of the bioactive chemicals that fall under this group for searching novel antiemetic drug candidates (Ahmed et al. 2014).
Phytol (PHY) is an organic phytochemical, an acyclic monounsaturated diterpene alcohol in nature, commonly obtainable in particular aromatic plants and having various pharmacological activities (McGinty et al. 2010; Islam et al. 2018). The compound is known to possess antioxidant properties as well as some other medicinal properties (Santos et al. 2013). Recent investigation revealed that PHY is a prominent immunostimulant and assists in activating both innate and acquired immunity (Lim et al. 2006). The compound also has antimicrobial, antidiarrheal (Pejin et al. 2014), antinociceptive (Santos et al. 2013), anti-inflammatory (Islam et al. 2020), antitumor, antifungal, antidiabetic, anticonvulsant, autophagy- and apoptosis-inducing, and hepatoprotective (Islam et al. 2018).
There are a variety of in vivo and in vitro models for evaluating the antiemetic activity of a compound or plant extract. The chick emesis model is one of them (Ahmed et al. 2013). In this model, copper sulfate induces emesis in young chicks (Gallus gallus domesticus) when administered orally. The test sample or standard is administered before 30 min of copper sulfate (CuSO4), either orally or peritoneally. The antiemetic activity of the test sample is evaluated by comparing the number of retches with control groups (Akita et al. 1998). On the other hand, computational approaches in drug discovery and development enable rapid screening of a vast compound library and estimation of potential binders via modeling/simulation and visualization techniques. It also helps to predict pharmacokinetics and binding sites, which is indispensable for determination of mechanistic steps and binding in identifying and generating promising drug candidates (Sliwoski et al. 2014; Palermo and De Vivo 2014). Therefore, the objective of this study is to evaluate the antiemetic activity of PHY and to predict the mechanism of action as well as to assess the pharmacokinetic properties of the drugs through computational methods.
Material and methods
Chemicals and reagents
PHY (3,7,11,15-Tetramethyl-2-hexadecen-1-ol), 97%, mixture of isomers (CAS No. 7541-49-3) was purchased from Sigma-Aldrich (USA), while copper sulfate pentrahydrate (CuSO4.5H2O) and 1% tween 80 were purchased from Merck (India). Reference drugs, domperidone, ondansetron, and hyoscine butyl bromide were collected from Square Pharma Ltd., Healthcare Pharma Ltd., and Opsonin Pharma Ltd., Bangladesh, respectively.
Animals
Young chickens (Gallus gallus domesticus) of either sex, 2 days old, weighing about 55–65 gm (Grade-A) were collected from Nourish Grand Parent Ltd., Rangpur, Bangladesh. All chickens were housed for an additional 2 days prior to starting the experiment in stainless steel cages opened in the upper hood at room temperature with a twelve-hour light and dark cycle and were permitted to take standard food and water ad libitum. After 12 h of fasting, the antiemetic test was carried out. This study was granted by the Department of Pharmacy and acts of the Ethical Committee of Bangabadhu Sheikh Mujibur Rahman Science and Technology University [#BSMRSTU/R2022(1)1].
In vivo study
The study was carried out according to the protocols of Akita et al. (1998) with a slight modification. All the birds were divided into nine groups, containing five in each. Before being given the treatments, each chick was kept in a large transparent plastic container for 10 min. The two doses of PHY referred as PHY50 (50 mg/kg) and PHY75 (75 mg/kg) were prepared by dissolving them in a 0.9% NaCl solution containing a small amount of (1% tween 80) and administered orally. Domperidone (DPD), ondansetron (ODN) and hyoscine butyl bromide (HYS) were administered orally as positive controls at 80, 24 and 100 mg/kg (b.w.), respectively. Three combined doses of PHY (75 mg/kg) with the positive controls were also administered orally to animals. The vehicle was considered a negative control (NC). After 30 min of treatment, emesis was induced through CuSO4.5H2O at a dose of 50 mg/kg (b.w.) by administering it orally to every bird. Then, the latency (first retch after having CuSO4.5H2O treatment) and number of retches (within 10 min after having CuSO4.5H2O treatment) were recorded carefully. The percentage increase in latency and decrease in retches in comparison with the NC group were calculated according to the following equations:
where, A = Mean of latency in seconds in NC group, B = Mean of latency in seconds in standard and test groups, C = Mean of retches in NC group, D = Mean of retches in standard and test groups.
Statistical analysis
Values of antiemetic activity are presented as mean ± SEM (standard error of mean). The statistical significance of the difference is calculated by using Graph Pad Prism (version 6.0) considering a 95% confidence interval. P values of < 0.05 were considered significant, and p < 0.0001 was highly significant.
In silico studies
Selection and preparation of receptors
Based on the literature review, we have targeted 10 receptors responsible for inducing emesis. 3D structures in PDB format of the targeted receptors: 5HT3 (PDB ID: 6Y5B) (Gregory and Ettinger 1998), D2 (PDB ID: 6CM4) (Davis and Walsh 2000), D3 (PDB ID: 3PBL) (Darmani et al. 1999), H1 (PDB ID: 7DFL) (Doenicke et al. 2004), M1 (PDB ID: 6WJC) (Pleuvry et al. 2006), M2 (PDB ID: 5ZK8) (Pleuvry et al., 2006), M3 (PDB ID: 4U15) (Pleuvry et al. 2006), M4 (PDB ID: 7V6A) (Pleuvry et al. 2006), M5 (PDB ID: 6OL9) (Pleuvry et al. 2006) and NK1 (PDB ID: 6HLP) (Navari et al. 1999) were collected from the RCSB Protein Data Bank (https://www.rcsb.org/). After collection, the receptors were optimized to avoid docking interference by deleting all unnecessary molecules, e.g., lipids, water molecules, and heteroatoms from the sequence of proteins via the PyMol software package (v2.4.1). Finally, energy minimization and geometry optimization of the receptors were carried out through the SwissPDB Viewer software package by appealing to the GROMOS96 force field and saving the PDB file to perform molecular docking.
Collection and preparation of ligands
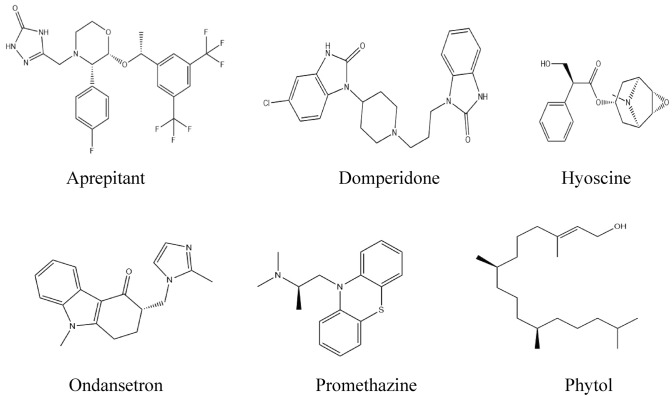
3D conformers of aprepitant (Compound CID: 135413536), domperidone (Compound CID: 3151), hyoscine (Compound CID: 3000322), ondansetron (Compound CID: 4595), promethazine (Compound CID: 4927), and phytol (Compound CID: 5280435) were collected in SDF format from the PubChem chemical database (https://pubchem.ncbi.nlm.nih.gov/). Then, the 3D conformers of the chemical agents were minimized and saved in SDF files and converted into MOL files through the Chem3D 16.0 program package for performing molecular docking and predicting pharmacokinetics, respectively. Finally, all the ligands were optimized utilizing Gaussian view software (v5.0). The two-dimensional images of the chemical agents are displayed in Fig. 1.
Fig. 1.
Structures of phytol and selected standards screened against the receptors
Molecular docking and prediction of active site of receptors
Molecular docking was performed by utilizing the PyRx software package to predict the active binding potential of the drugs against the active sites of receptors. For performing docking, the grid box dimensions were set as 76.37 × 55.95 × 83.32 Å along x-, y- and z-axes, respectively, and the calculation was run at 200 steps (Ibrahim et al. 2022). The result of the docking potential is saved in '.csv' format, and the complex of ligand–protein is collected in PDB format for collecting the ligand in PDBQT format. The interactions of ligand-receptors and the receptor’s active site were observed under the Discovery Studio Visualizer (v21.1.020298) and PyMol (v2.4.1) program packages, and amino acid residues or receptor (D2) that interacted with the drug are listed.
Prediction of drug-likeness and pharmacokinetics
Drug-likeness is a qualitative measurement employed in drug design and development to assess how the chemical compound acts like a drug with respect to factors like bioavailability, and it is also related to ADME (Bhadra 2020). Drug-likeness and pharmacokinetics of a chemical agent can be estimated through various online servers and software. In this study, we described various factors for assessing the selected molecule’s physicochemical properties important in drug development with the aid of SwissADME (http://www.swissadme.ch/index.php) (Daina et al. 2017).
Results
In vivo antiemetic activity
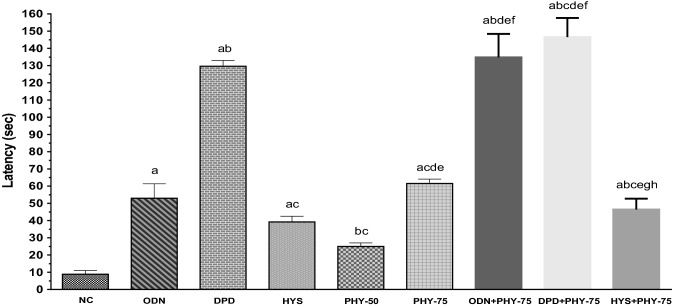
The administered doses of PHY remarkably decreased the number of retches and increased the latent period in chicks. The combined drug therapy (standard plus test sample) expressed higher latency, such as first retching was observed in the DPD + PHY-75 group at 146.60 s, on the other hand, at 8.80 s in the NC group (values are mean). The onset of retching in test groups was observed at 25.00 and 61.60 s for PHY-50 and PHY-75, respectively (Fig. 2).
Fig. 2.
Latency (sec) of retches observed in test sample, controls and combinations. [Values are mean ± S.E.M. (n = 5). acompared to the NC (vehicle), bcompared to the OND (positive control); ccompared to the DPD; dcompared to the HYS; ecompared to the PHY-50; fcompared to the PHY-75; gcompared to the ODN + PHY-75;.hcompared to the DPD + PHY-75; p < 0.05 (DPD vs. DPD + PHY-75); p < 0.01(HYS vs. PHY-75, PHY-50 vs. HYS + PHY-75); p < 0.001(ODN vs. PHY-50); p < 0.0001(NC vs. ODN, NC vs. DPD, NC vs. HYS, NC vs. PHY-75, NC vs. ODN + PHY-75, NC vs. DPD + PHY-75, NC vs. HYS + PHY-75, ODN vs. DPD, ODN vs. ODN + PHY-75, ODN vs. DPD + PHY-75, DPD vs. HYS, DPD vs. PHY-50, DPD vs. PHY-75, DPD vs. HYS + PHY-75, HYS vs. ODN + PHY-75, HYS vs. DPD + PHY-75, PHY-50 vs. PHY-75, PHY-50 vs. ODN + PHY-75, PHY-50 vs. DPD + PHY-75, PHY-75 vs. ODN + PHY-75, PHY-75 vs. DPD + PHY-75, ODN + PHY-75 vs. HYS + PHY-75, DPD + PHY-75 vs. HYS + PHY-75)]
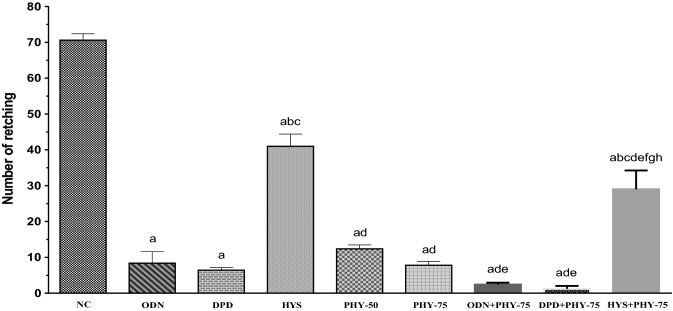
The highest number of retches was observed in the NC group (mean value: 70.60). The number of retches in the test groups reduced gradually with increasing dose, and the values were 12.40 and 7.80 for the PHY-50 and PHY-75 groups, respectively, which presented better antiemetic activity than the NC and HYS groups in the animals. The combined groups expressed a reduced number of retches. The lowest number of retches was observed in the DPD + PHY-75 group (Fig. 3). In comparison with the number of retches and latency of PHY with the number of retches and latency of control groups, PHY provided a mild antiemetic activity in this experiment.
Fig. 3.
Number of retches observed in test sample, controls and combinations. [Values are mean ± S.E.M. (n = 5). acompared to the NC (vehicle), bcompared to the OND (positive control); ccompared to the DPD; dcompared to the HYS; ecompared to the PHY-50; fcompared to the PHY-75; gcompared to the ODN + PHY-75;.hcompared to the DPD + PHY-75; p < 0.01 (PHY-50 vs. ODN + PHY-75); p < 0.001 (HYS vs. HYS + PHY-75, PHY-50 vs. DPD + PHY-75); p < 0.0001 (NC vs. ODN, NC vs. DPD, NC vs. HYS, NC vs. PHY-50, NC vs. PHY-75, NC vs. ODN + PHY-75, NC vs. DPD + PHY-75, NC vs. HYS + PHY-75, ODN vs. HYS, ODN vs. HYS + PHY-75, DPD vs. HYS, DPD vs. HYS + PHY-75, HYS vs. PHY-50, HYS vs. PHY-75, HYS vs. ODN + PHY-75, HYS vs. DPD + PHY-75, PHY-50 vs. HYS + PHY-75, PHY-75 vs. HYS + PHY-75, ODN + PHY-75 vs. HYS + PHY-75, DPD + PHY-75 vs. HYS + PHY-75)]
The percentage increase in latency compared to the NC group for the test groups was recorded as 64.80 and 85.71% for the PHY-50 and PHY-75 groups, respectively. The highest percentage increase in latency (94%) was observed in the DPD + PHY-75 group. On the other hand, the highest %decrease in retching in comparison with the NC group was also recorded in the same group. The value of %decrease in retches of test groups compared to the NC group is 82.44% and 88.95% for the PHY-50 and PHY-75 groups, respectively (Table 1). The result demonstrated that PHY provided protective and antiemetic activity against copper sulfate-induced emesis in chicks in a dose-dependent manner.
Table 1.
Percentage increase in latency and decrease in retches in treatment groups
| Name of group | % increase in latency | % decrease in retches |
|---|---|---|
| NC | – | – |
| HYS | 77.55 | 41.93 |
| ODN | 83.40 | 88.10 |
| DPD | 93.21 | 90.93 |
| PHY-50 | 64.80 | 82.44 |
| PHY-75 | 85.71 | 88.95 |
| HYS + PHY-75 | 81.03 | 58.92 |
| ODN + PHY-75 | 93.47 | 96.60 |
| DPD + PHY-75 | 94.00 | 98.30 |
Values are percentage inspect of NC group (Negative control or vehicle) (n = 5); ODN = Ondansetron (Dose 24 mg/kg); DPD = Domperidone (Dose 80 mg/kg); HYS = Hyoscine (Dose 100 mg/kg); PHY-50 = Phytol (Dose 50 mg/kg); PHY-75 = Phytol (Dose 75 mg/kg); ODN + PHY-75 = Ondansetron + Phytol (Dose 24 mg/kg + 75 mg/kg; DPD + PHY-75 = Domperidone + Phytol (Dose 80 mg/kg + 75 mg/kg); HYS + PHY-75 = Hyoscine + Phytol (Dose 100 mg/kg + 75 mg/kg)
In silico analysis
Molecular docking study
Molecular docking is carried out to predict the probable binding affinity and interactions between drugs and receptors. In our investigation, aprepitant (APT) was screened against the NK1 receptor. And the binding energy against NK1 is − 11.3 and − 6.1 kcal/mol for APT and PHY, respectively. DPD is the antagonist of the dopamine receptors. DPD scored − 9.8 kcal/mol and − 9.7 kcal/mol against the D2 and D3 receptors, respectively, whereas PHY scored − 7.3 kcal/mol against D2, which is the highest score of binding affinity of PHY against an emesis producing receptor. HYS is the mAChRs antagonist. The docking scores against mAChRs such as M1, M2, M3, M4, and M5 are − 8.5, − 7.6, − 9.2, − 9, and − 6.3 kcal/mol, respectively. The binding energy of PHY against serotonin receptor (5HT3) is − 5.8 kcal/mol, whereas the standard ODN scored − 8 kcal/mol. The antihistamine PMN scored − 7 kcal/mol against the histaminic H1 receptor, where PHY exhibited − 5.6 kcal/mol. The binding affinity of all the drugs against the selected receptors is provided in Table 2.
Table 2.
Molecular docking scores (kcal/mol) of phytol and selected reference standards
| Ligands | Receptors | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Common Name | 5HT3 | D2 | D3 | H1 | M1 | M2 | M3 | M4 | M5 | NK1 | |
| PDB ID | 6Y5B | 6CM4 | 3PBL | 7DFL | 6WJC | 5ZK8 | 4U15 | 7V6A | 6OL9 | 6HLP | |
| Aprepitant | – | – | – | – | – | – | – | – | – | − 11.3 | |
| Domperidone | – | − 9.8 | − 9.7 | – | – | – | – | – | – | – | |
| Hyoscine | – | – | – | – | − 8.5 | − 7.6 | − 9.2 | − 9 | − 6.3 | – | |
| Ondansetron | − 8 | – | – | – | – | – | – | – | – | – | |
| Promethazine | – | – | – | – | – | – | – | – | – | – | |
| Phytol | − 5.8 | − 7.3 | − 6 | − 5.6 | − 5.1 | − 6.2 | − 6.9 | − 5.6 | − 6.2 | − 6.1 | |
Prediction of drug-likeness and pharmacokinetics
Drug-likeness, which describes the molecular properties of a drug candidate, is an important parameter for developing a chemical compound into a drug and evaluating pharmacokinetics. MW, Log P, HBA, HBD, and MR are the parameters by which drug likeness is assessed. In our findings, the molecular weight of all the drugs was retained under 500 Dalton except APT, the drug also having 12 HBA (Table 3). According to Lipinski's rule of five, except for APT, the values of HBA (≤ 10) and HBD (≤ 5) are within the limit. HYS and ODN are soluble in water, and others are moderately soluble. APT and PHY are slightly absorbable through the GI membrane, and others are highly absorbable. Values of the pharmacokinetic parameters such as BBB permeability, P-gp substrate, CYP2C19 inhibitor, bioavailability score, along with water solubility and GI absorption are also provided in Table 3.
Table 3.
Drug-likeness and pharmacokinetic properties of phytol predicted by SwissADME
| Parameters | APT | DPD | HYS | ODN | PMN | PHY |
|---|---|---|---|---|---|---|
| MF | C23H21F7N4O3 | C22H24ClN5O2 | C17H21NO4 | C18H19N3O | C17H20N2S | C20H40O |
| MW | 534.43 | 425.91 | 303.35 | 293.36 | 284.42 | 296.53 |
| Log P | 4.05 | 3.28 | 1.19 | 1.75 | 3.84 | 5.25 |
| HBA | 12 | 3 | 5 | 2 | 1 | 1 |
| HBD | 2 | 2 | 1 | 0 | 0 | 1 |
| MR | 118.82 | 124.08 | 83.48 | 87.39 | 90.07 | 98.94 |
| Solubility (water) | Moderately soluble | Moderately soluble | Soluble | Soluble | Moderately soluble | Moderately soluble |
| GI absorption | Low | High | High | High | High | Low |
| BBB permeant | No | Yes | No | Yes | Yes | No |
| P-gp substrate | Yes | Yes | No | Yes | No | Yes |
| CYP2C19 int | No | Yes | No | Yes | No | No |
| BIO Score | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
MF Molecular formula, MW Molecular weight (g/mol), LogP Log Po/w (MLOGP), HBA Hydrogen bond acceptor, HBD Hydrogen bond donor, MR Molar refractivity, APT Aprepitant, PMN Promethazine, CYP2C19 int CYP2C19 inhibitor, BIO Score Bioavailability Score
Estimation of non-bond interactions between drug-receptor complexes
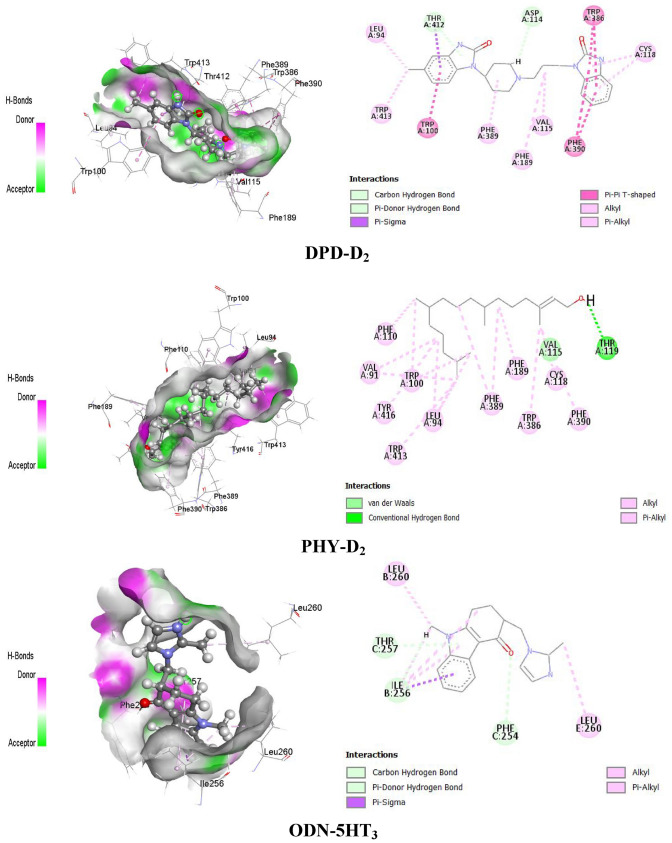
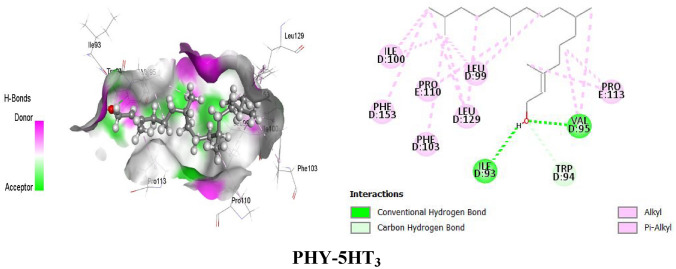
PHY forms different types of bonds with the D2 receptor, such as hydrogen bonds (HB) and hydrophobic bonds (alkyl, pi-alkyl bonds), to provide the best interactions with the D2 receptor. PHY formed HB with the amino acid residues of Thr119, Val115 and hydrophobic bonds (alkyl and pi-alkyl) with Val91, Leu94, Cys118, Trp100, Phe110, Phe189, Trp386, Phe389, Phe390, Trp413 and Tyr416 of D2 receptor. On the other hand, DPD formed HB with the amino acid residue of Ser409, an electrostatic bond with Asp114, and hydrophobic bonds (alkyl, pi-alkyl, pi-pi stacked) with Tyr408, Ile184, Val91, Phe189, His393, Tyr408, Trp413, and Leu94 of the D2 receptor. Besides D2, PHY also interacted with 5HT3 receptors to provide an antiemetic effect by forming HB and hydrophobic bonds (alkyl and pi-alkyl) with the amino acid residues of that receptor. PHY formed HB with Ile93, Val95, and Trp94 amino acid residues, as well as hydrophobic bonds with Leu99, Leu129, Val95, Ile100, Pro110, Pro113 (alkyl), Phe103, and Phe153 (pi-alkyl). In contrast, reference drug ODN interacted with the respective receptor by forming HB with Ile256, Phe254, Thr257 amino acid residues and hydrophobic bonds (pi-sigma, pi-alkyl, alkyl) with Ile256, Leu260 amino acid residues. The drug-receptor interactions (PHY, DPD with D2 and PHY, ODN with 5HT3) and active sites of the receptors (D2 and 5HT3) are presented in Fig. 4 and Table 4.
Fig. 4.
Active sites and drug-receptor interactions among drugs (PHY and DPD) and receptors (D2 and 5HT3)
Table 4.
Amino acid residues of non-bond interactions between the ligands and receptors
| Drug-receptor complexes | Non-bond interactions | ||
|---|---|---|---|
| Amino acid residues | Bond type | ||
| HB | Others | ||
| DPD-D2 | Ser409 | CHB | – |
| Asp114: | – | Pi-anion | |
| Tyr408 | – | Pi-Pi Stacked | |
| Ile184, Val91, Leu94 | – | Alkyl | |
| Phe189, His393, Tyr408, Trp41, Leu94 | – | Pi-alkyl | |
| PHY-D2 | Thr119 | CHB | – |
| Val115 | CaHB | – | |
| Val91, Leu94, Cys118 | – | Alkyl | |
| Trp100, Phe110, He189, Trp386, Phe389, Phe390, Trp413, Tyr416 | – | Pi-alkyl | |
| ODN-5HT3 | Ile256, Phe254 | CaHB | – |
| Thr257 | Pi-DHB | – | |
| Ile256 | – | Pi-sigma | |
| Ile256, Leu260, | – | Alkyl | |
| Ile256 | – | Pi-alkyl | |
| PHY-5HT3 | Ile93, Val95 | CHB | – |
| Trp94 | CaHB | – | |
| Leu99, Leu129, Val95, Pro113, Ile100, Leu129, Pro110, | – | Alky | |
| Phe103, Phe153 | – | Pi-alkyl | |
HB Hydrogen bond, CHB Conventional hydrogen bond, CaHB Carbon hydrogen bond, Pi-DHB Pi-donor hydrogen bond
Discussion
Ingestion of toxic CuSO4 potentially provides a specific vagal emetic stimulus because it is an oxidizing agent and corrosive to the mucous membranes of GIT (Horn et al. 2014). Emesis is persuaded by peripheral functions through the excitation of visceral afferent nerve fibers of the GIT by way of transmitting the stimuli to the vomiting center (Hossein et al. 2005; Bowman and Rand 1980). It has also been confirmed that the peripheral serotonin receptors (5-HT3, 5-HT4) (Fukui et al. 1993, 1994), NK1 receptor (Ariumi et al. 2000) and H1-histamine receptors (Katzung 2007) are engaged in emesis. And some other types of receptors, such as dopamine-type 2 (D2) (Becker 2010), muscarinic acetylcholine receptors (M1, M2, M3, M4) within the CTZ are also stimulated at their own receptor sites and induce emesis (Kudlak and Tadi 2021; Hornby 2001). Our selected standard drug (DPD) functioned as a peripherally selective antagonist of dopamine receptors, especially D2 and D3 receptors, and ensured redemption by antagonizing or inhibiting the activity of the receptors at CTZ in the brain (Jacoby 2018). In our investigation, the DPD ingested group exhibited 6.40 (mean) retches in chicks and the mean of retches in the NC group was 70.60. On the other hand, 5HT3 receptors play a role in inducing emesis by transforming information in the GTI, and in the enteric nervous system they regulate peristalsis and bowel motility (Galligan 2002). And the 5HT3 antagonists such as ODN block the function of the receptor and provide relief from vomiting.
In this experiment, ODN and HYS (mAChRs antagonist) also reduced the number of retches in the chick group compared to the vehicle group. On the basis of experimental results, it can be hypothesized that PHY exerts a protective effect against toxicity by reducing or preventing nerve stimuli that are liable to induce emesis. Because both the groups of PHY remarkably diminished the number of retches in comparison with the NC group, the value is near to the value of the standard groups. But the potency of DPD is greater than the others in this experiment, and it is related to the D2 receptor.
In pharmacology, when the combined effect of two or more medications is greater than the effects observed in the drug administered alone, it is called a synergistic effect, and this term is called synergism (Garcia-Fuente et al. 2018). The combined drug therapy in this study exhibited a lower number of retches and an elevated latency period in chicks, resulting in a synergistic effect. The study says that antiemetic drug therapy delayed nausea or vomiting against the emesis stimuli created by cancer chemotherapy or acute toxicity (Perwitasari et al. 2011). In our investigation, the latency of retching in seconds of the test groups was higher than the NC group, and the highest latency (sec) was observed in the combined group (DPD + THY-75).
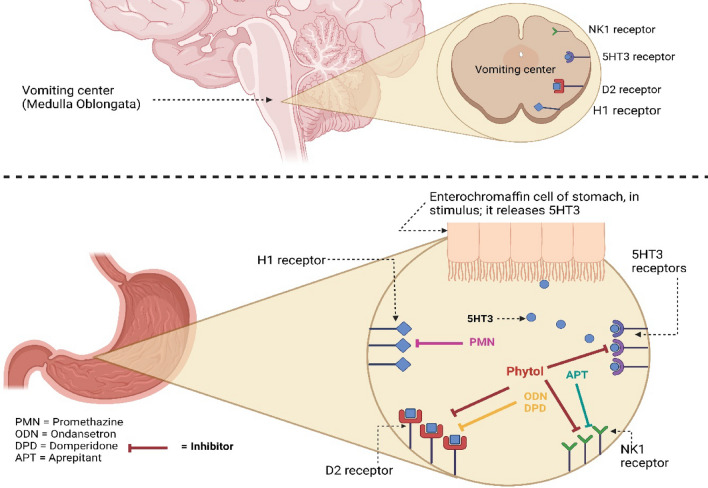
Copper sulfate does not follow the vagal nerve stimulation; in a study, it was observed that vagotomy (cutting the end of the vagus nerve in GIT) could not stop emesis (Wang and Borison 1951; Niijima et al. 1987), so there could be involvement of chemoreceptor signaling like the PHY follows in Fig. 5.
Fig. 5.
Proposed anti-emetic mechanism of the test sample and reference drugs [This figure represents possible anti-emetic mechanisms of PHY, ODN, PMN, DPD and APT based on the binding affinity of these ligands with the H1, 5HT3, NK1, and D2 receptors. Here, PHY act as inhibitor of 5HT3, NK1 and D2 receptors, whether ODN and DPD inhibited D2 receptor, APT blocked NK1 receptor and PMN acted on H1 receptor. Antagonizing of these stomach receptors leads to no stimulation of the vomiting center (medulla oblongata), which results in no GIT contraction, no muscle contraction, and finally no emesis]
Molecular docking is a computational technique for exploring a suitable ligand that fits the receptor's binding site both geometrically and energetically (Kumar Bhardwaj et al. 2022; Sing et al. 2022a). Computational studies have recently opened up a new avenue for screening, designing, and developing drug candidates. It also reduces total evaluation time and animal and laboratory costs (Kumar et al. 2022; Sing et al. 2022b). The level of interaction between ligand and receptor is estimated through binding affinity (Azam and Abbasi 2013). In this experiment, PHY expressed more elevated binding interactions with the D2 receptor (PDB ID: 6CM4) than the other receptors responsible for inducing emesis. The binding energy of PHY required for interacting with D2 is − 7.3 kcal/mol, where the standard DPD expressed the value of − 9.8 kcal/mol. As a result, its our view that PHY is more potent for dopaminergic receptors than the other receptors liable for emesis as the docking scores of PHY for D2 receptor are higher than the other receptors as well as in vivo combined therapy with DPD demonstrated more activity than other combination.
Drug-likeness is an important parameter in the case of drug discovery and development, and it predicts qualitatively the possibility of a chemical compound becoming an oral medication with respect to bioavailability. It is estimated through the physicochemical properties of the drug, indicating drug nature related to pharmacokinetics (Daina et al. 2017). Lipinski’s rule of five is widely used to predict drug-likeness and pharmacokinetics. According to Lipinski’s rule of five, a drug candidate should have a molecular weight of not more than 500 g/mol, not more than 5 hydrogen bond donors, not more than 10 hydrogen bond acceptors, and lipophilicity (LogPo/w) within 5, and the acceptable range of violation of the rule is 0–1 (Lipinski 2004). According to Lipinski’s rule, all the ligands are within the limits of becoming drugs though except PHY all are established drugs. According to our in silico investigation, the absorption properties of PHY are lower through GI than the standard except for APT, but it can be overcome through parental administration. The visualization of drug-receptor interaction estimates that the binding sites of PHY and DPD are more closely related to D2 than the interaction of PHY and ODN with 5HT3 because some of the amino acid residues of D2 are identical to those that form hydrophobic bonds with the drugs, such as Val91, Leu94, Phe189, and Trp413. Phe110, Phe390, Met117, Phe164, Cys118, Phe189, Trp386, Val190, and His394 residues of the D2 receptor form a mostly hydrophobic pocket for dopamine (Kalani et al. 2004). Whereas, we found that PHY interacts with Cys118, Phr110 and Phe390 residues of D2 which formed the same hydrophobic pocket for dopamine. Therefore, we predict that Cys118, Phe110 and Phe390 are the key residues involved in the antagonizing activity of PHY against D2.
So, the result of this investigation revealed that the antiemetic activity of PHY against copper sulfate-induced emesis is due to its D2 receptor antagonizing capacity, and the response is dose-dependent.
Conclusion
The results of this investigation demonstrate that PHY has significant antiemetic activity and the compound protects against CuSO4.5H2O-induced retching in chicks, perhaps by peripheral action. The molecular docking study confirmed that PHY has a higher affinity for dopamine receptors, especially D2, than the other receptors liable for inducing emesis. The compound also has synergistic effects when combined with the established antiemetic drugs targeting different receptors. Based on the in silico ADMET analysis, it was also thought that the compound has good pharmacokinetics and drug-like properties. Taken together, PHY reduced CuSO4.5H2O-induced emesis in chicks in combination with DPD, suggesting its antiemetic potential, possibly through interacting with the D2 receptor. PHY may be one of the plant-derived antiemetic agents. More research is needed to determine the optimal dose and exact mechanism in emesis caused by other causes.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This article is according with to the international, national and institutional rules considering biodiversity rights.
References
- Akita Y, Yang Y, Kawai T, Kinoshita K, Koyama K, Takahashi K, Watanabe K. New assay method for surveying anti-emetic compounds from natural sources. Nat Prod Sci. 1998;4(2):72–77. [Google Scholar]
- Ahmed S, Hasan MM, Ahmed SW. Natural antiemetics: an overview. Pakistan J Pharm Sci. 2014;27:1583–1598. [PubMed] [Google Scholar]
- Ahmed S, Hasan MM, Ahmed SW, Mahmood ZA, Azhar I, Habtemariam S. Anti-emetic effects of bioactive natural products. Phytopharmacology. 2013;4(2):390–433. [Google Scholar]
- Ariumi H, Saito R, Nago S, Hyakusoku M, Takano Y, Kamiya HO. The role of tachykinin NK-1 receptors in the area postrema of ferrets in emesis. Neurosci Lett. 2000;286(2):123–126. doi: 10.1016/s0304-3940(00)01113-7. [DOI] [PubMed] [Google Scholar]
- Azam SS, Abbasi SW. Molecular docking studies for the identification of novel melatoninergic inhibitors for acetylserotonin-O-methyltransferase using different docking routines. Theor Biol Med Model. 2013;10(1):1–16. doi: 10.1186/1742-4682-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DE. Nausea, vomiting, and hiccups: a review of mechanisms and treatment. Anesth Prog. 2010;57(4):150–157. doi: 10.2344/0003-3006-57.4.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman WC, Rand MJ. Textbook of pharmacology. 2. Blackwell Scientific Publications; 1980. [Google Scholar]
- Bhadra P. Green Chiretta (Andrographispaniculata): in silico analysis of therapy for breast cancer. Indian J Nat Sci. 2020;10(60):20645–20652. [Google Scholar]
- Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7(1):1–13. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MP, Walsh D. Treatment of nausea and vomiting in advanced cancer. Support Care Cancer. 2000;8(6):444–452. doi: 10.1007/s005200000151. [DOI] [PubMed] [Google Scholar]
- Doenicke AW, Hoernecke R, Celik I. Premedication with H1 and H2 blocking agents reduces the incidence of postoperative nausea and vomiting. Inflamm Res. 2004;53(2):S154–S158. doi: 10.1007/s00011-004-0367-0. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Zhao W, Ahmad B. The role of D2 and D3 dopamine receptors in the mediation of emesis in Cryptotis parva (the least shrew) J Neural Transm. 1999;106(11):1045–1061. doi: 10.1007/s007020050222. [DOI] [PubMed] [Google Scholar]
- Fukui H, Yamamoto M, Sasaki S, Sato S. Involvement of 5-HT3 receptors and vagal afferents in copper sulfate-and cisplatin-induced emesis in monkeys. Eur J Pharmacol. 1993;249(1–2):13–18. doi: 10.1016/0014-2999(93)90656-3. [DOI] [PubMed] [Google Scholar]
- Fukui H, Yamamoto M, Sasaki S, Sato S. Possible involvement of peripheral 5-HT4 receptors in copper sulfate-induced vomiting in dogs. Eur J Pharmacol. 1994;257(1–2):47–52. doi: 10.1016/0014-2999(94)90692-0. [DOI] [PubMed] [Google Scholar]
- Grahame-Smith DG. Nausea and vomiting: mechanisms and treatment. Berlin, Heidelberg: Springer; 1986. The multiple causes of vomiting: is there a common mechanism; pp. 1–8. [Google Scholar]
- Garcia-Fuente A, Vazquez F, Vieitez JM, Garcia Alonso FJ, Martín JI, Ferrer J. CISNE: an accurate description of dose-effect and synergism in combination therapies. Sci Rep. 2018;8(1):1–9. doi: 10.1038/s41598-018-23321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory RE, Ettinger DS. 5-HT3 receptor antagonists for the prevention of chemotherapy-induced nausea and vomiting. Drugs. 1998;55(2):173–189. doi: 10.2165/00003495-199855020-00002. [DOI] [PubMed] [Google Scholar]
- Galligan JJ. Ligand-gated ion channels in the enteric nervous system. Neurogastroenterol Motil. 2002;14(6):611–623. doi: 10.1046/j.1365-2982.2002.00363.x. [DOI] [PubMed] [Google Scholar]
- Hall J, Driscoll P. 10 Nausea, vomiting and fever. Emerg Med J. 2005;22(3):200–204. doi: 10.1136/emj.2004.022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornby PJ. Central neurocircuitry associated with emesis. Am J Med. 2001;111(8):106–112. doi: 10.1016/s0002-9343(01)00849-x. [DOI] [PubMed] [Google Scholar]
- Horn CC, Meyers K, Lim A, Dye M, Pak D, Rinaman L, Yates BJ. Delineation of vagal emetic pathways: intragastric copper sulfate-induced emesis and viral tract tracing in musk shrews. Am J Physiol Regul Integr Compar Physiol. 2014;306(5):R341–R351. doi: 10.1152/ajpregu.00413.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossein H, Mashallah M, Akbar G. Antiemetic effect of Mentha xpiperita aerial parts extracts in young chickens. Iranian J Pharmaceut Sci. 2005;1(1):21–24. [Google Scholar]
- Iqbal IM, Spencer R. Postoperative nausea and vomiting. Anaesth Intensive Care Med. 2012;13(12):613–616. [Google Scholar]
- Islam MT, Ali ES, Uddin SJ, Shaw S, Islam MA, Ahmed MI, Chandra SM, Karmakar UK, Yarla NS, Khan IN, Billah MM, Pieczynska MD, Zengin G, Malainer C, Nicoletti F, Gulei D, Berindan-Neagoe I, Apostolov A, Banach M, Yeung AWK, El-Demerdash A, Xiao J, Dey P, Yele S, Jóźwik A, Strzałkowska N, Marchewka J, Rengasamy KRR, Horbańczuk J, Kamal MA, Mubarak MS, Mishra SK, Shilpi JA, Atanasov AG. Phytol: a review of biomedical activities. Food Chem Toxicol. 2018;121:82–94. doi: 10.1016/j.fct.2018.08.032. [DOI] [PubMed] [Google Scholar]
- Islam MT, Ayatollahi SA, Zihad SNK, Sifat N, Khan MR, Paul A, Salehi B, Islam T, Mubarak MS, Martins N, Sharifi-Rad J. Phytol anti-inflammatory activity: Pre-clinical assessment and possible mechanism of action elucidation. Cell Mol Biol. 2020;66(4):264–269. [PubMed] [Google Scholar]
- Ibrahim MA, Abdelrahman AH, Badr EA, Almansour NM, Alzahrani OR, Ahmed MN, Soliman MES, Naeem MA, Shawky AM, Sidhom PA, Mekhemer GAH, Atia MA. Naturally occurring plant-based anticancerous candidates as prospective ABCG2 inhibitors: an in silico drug discovery study. Mol Diversity. 2022;26(6):3255–3277. doi: 10.1007/s11030-022-10389-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby HI. Toxicology of the gastrointestinal Tract. CRC Press; 2018. Safety pharmacology and the GI tract; pp. 53–81. [Google Scholar]
- Khan IA, Aziz A, Sarwar HS, Munawar SH, Manzoor Z, Anwar H. Evaluation of antiemetic potential of aqueous bark extract of Cinnamon loureiroi. Canad J Appl Sci. 2014;4:26–32. [Google Scholar]
- Kudlak M, Tadi P. StatPearls [Internet] StatPearls Publishing; 2021. Physiology, muscarinic receptor. [PubMed] [Google Scholar]
- Kalani MYS, Vaidehi N, Hall SE, Trabanino RJ, Freddolino PL, Kalani MA, Floriano WB, Kam VW, Goddard WA., III The predicted 3D structure of the human D2 dopamine receptor and the binding site and binding affinities for agonists and antagonists. Proc Natl Acad Sci. 2004;101(11):3815–3820. doi: 10.1073/pnas.0400100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzung BG. Basic and clinical pharmacology. 11. Lange Medical Publications; 2007. pp. 1084–1093. [Google Scholar]
- Kumar S, Bhardwaj VK, Singh R, Das P, Purohit R. Evaluation of plant-derived semi-synthetic molecules against BRD3-BD2 protein: a computational strategy to combat breast cancer. Mol Syst Design Eng. 2022;7(4):381–391. [Google Scholar]
- Kumar Bhardwaj V, Das P, Purohit R. Identification and comparison of plant-derived scaffolds as selective CDK5 inhibitors against standard molecules: Insights from umbrella sampling simulations. J Mol Liq. 2022;348:118015. [Google Scholar]
- Lang IM. Digestive tract motor correlates of vomiting and nausea. Can J Physiol Pharmacol. 1990;68(2):242–253. doi: 10.1139/y90-038. [DOI] [PubMed] [Google Scholar]
- Lim SY, Meyer M, Kjonaas RA, Ghosh SK. Phytol-based novel adjuvants in vaccine formulation: 1. Assessment of safety and efficacy during stimulation of humoral and cell-mediated immune responses. J Immune Based Therapies Vaccines. 2006;4(1):1–11. doi: 10.1186/1476-8518-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski CA. Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1(4):337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- MacDougall MR, Sharma S. StatPearls [Internet] StatPearls Publishing; 2021. Physiology, chemoreceptor trigger zone. [PubMed] [Google Scholar]
- Miller AD, Leslie RA. The area postrema and vomiting. Front Neuroendocrinol. 1994;15(4):301–320. doi: 10.1006/frne.1994.1012. [DOI] [PubMed] [Google Scholar]
- McGinty D, Letizia CS, Api AM. Fragrance material review on phytol. Food Chem Toxicol. 2010;48:S59–S63. doi: 10.1016/j.fct.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Navari RM. Management of chemotherapy-induced nausea and vomiting. Drugs. 2013;73(3):249–262. doi: 10.1007/s40265-013-0019-1. [DOI] [PubMed] [Google Scholar]
- Naylor RJ, Inall FC. The physiology and pharmacology of postoperative nausea and vomiting. Anaesthesia. 1994;49:2–5. doi: 10.1111/j.1365-2044.1994.tb03575.x. [DOI] [PubMed] [Google Scholar]
- Niijima A, Jiang ZY, Daunton NG, Fox RA. Effect of copper sulphate on the rate of afferent discharge in the gastric branch of the vagus nerve in the rat. Neurosci Lett. 1987;80(1):71–74. doi: 10.1016/0304-3940(87)90497-6. [DOI] [PubMed] [Google Scholar]
- Navari RM, Reinhardt RR, Gralla RJ, Kris MG, Hesketh PJ, Khojasteh A, Kindler H, Grote TH, Pendergrass K, Grunberg SM, Carides AD, Gertz BJ. Reduction of cisplatin-induced emesis by a selective neurokinin-1–receptor antagonist. N Engl J Med. 1999;340(3):190–195. doi: 10.1056/NEJM199901213400304. [DOI] [PubMed] [Google Scholar]
- Pejin B, Savic A, Sokovic M, Glamoclija J, Ciric A, Nikolic M, Radotic K, Mojovic M. Further in vitro evaluation of antiradical and antimicrobial activities of phytol. Nat Prod Res. 2014;28(6):372–376. doi: 10.1080/14786419.2013.869692. [DOI] [PubMed] [Google Scholar]
- Palermo G, De Vivo M. Encyclopedia of Nanotechnology. Springer; 2014. Computational chemistry for drug discovery; pp. 1–15. [Google Scholar]
- Perwitasari DA, Gelderblom H, Atthobari J, Mustofa M, Dwiprahasto I, Nortier JW, Guchelaar HJ. Anti-emetic drugs in oncology: pharmacology and individualization by pharmacogenetics. Int J Clin Pharm. 2011;33(1):33–43. doi: 10.1007/s11096-010-9454-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleuvry BJ. Physiology and pharmacology of nausea and vomiting. Anaesth Intensive Care Med. 2006;7(12):473–477. [Google Scholar]
- Santos CCDMP, Salvadori MS, Mota VG, Costa LM, de Almeida AAC, de Oliveira GAL, Costa JP, de Sousa DP, de Freitas RM, de Almeida RN. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci J. 2013 doi: 10.1155/2013/949452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorza KA, Williams A, Phillips JD, Shaw J. Evaluation of nausea and vomiting. Am Fam Physician. 2007;76(1):76–84. [PubMed] [Google Scholar]
- Singh R, Bhardwaj VK, Purohit R. Inhibition of nonstructural protein 15 of SARS-CoV-2 by golden spice: a computational insight. Cell Biochem Funct. 2022 doi: 10.1002/cbf.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Kumar S, Bhardwaj VK, Purohit R. Screening and reckoning of potential therapeutic agents against DprE1 protein of Mycobacterium tuberculosis. J Mol Liq. 2022;358:119101. [Google Scholar]
- Shankar A, Roy S, Malik A, Julka PK, Rath GK (2015) Prevention of Chemotherapy-Induced Nausea and Vomiting in Cancer Patients. Asian Pac J Cancer Prev 16(15):6207–6213 [DOI] [PubMed]
- Sliwoski G, Kothiwale S, Meiler J, Lowe EW. Computational methods in drug discovery. Pharmacol Rev. 2014;66(1):334–395. doi: 10.1124/pr.112.007336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SC, Borison HL. Copper sulphate emesis: A study of afferent pathways from the gastrointestinal tract. Am J Physiol-Legacy Content. 1951;164(2):520–526. doi: 10.1152/ajplegacy.1951.164.2.520. [DOI] [PubMed] [Google Scholar]