Abstract
Fibroblast growth factor receptor 2 (FGFR2) is almost exclusively expressed in glial cells in postnatal mouse brain, but its impact in glia for brain behavioral functioning is poorly understood. We compared behavioral effects from FGFR2 loss in both neurons and astroglial cells and from FGFR2 loss in astroglial cells by using either the pluripotent progenitor-driven hGFAP-cre or the tamoxifen-inducible astrocyte-driven GFAP-creERT2 in Fgfr2 floxed mice. When FGFR2 was eliminated in embryonic pluripotent precursors or in early postnatal astroglia, mice were hyperactive, and had small changes in working memory, sociability, and anxiety-like behavior. In contrast, FGFR2 loss in astrocytes starting at 8 weeks of age resulted only in reduced anxiety-like behavior. Therefore, early postnatal loss of FGFR2 in astroglia is critical for broad behavioral dysregulation. Neurobiological assessments demonstrated that astrocyte-neuron membrane contact was reduced and glial glutamine synthetase expression increased only by early postnatal FGFR2 loss. We conclude that altered astroglial cell function dependent on FGFR2 in the early postnatal period may result in impaired synaptic development and behavioral regulation, modeling childhood behavioral deficits like attention deficit hyperactivity disorder (ADHD).
Subject terms: Molecular neuroscience, ADHD
Introduction
Fibroblast growth factor (FGF) signaling has been characterized extensively for its role in the brain, particularly in neurons and their precursors [1–4]. We and others have determined the importance of FGF signaling for embryonic and postnatal neurogenesis and cortical expansion [5–8], dendrite and synapse development [9], and expression and responsiveness of neuronal components of the glucocorticoid-response system [10, 11]. Furthermore, changes in locomotor activity, anxiety-like behavior, altered stress response, and social interaction occur in animal models with disrupted FGF ligands or FGF receptor 1 (FGFR1) dependent signaling in the forebrain [3, 10, 12–16], implicating FGF signaling in basic brain development and functioning relevant to psychiatric illness. However, even in these studies, understanding the specific role of FGF receptors and ligands in different cell types for specific behavioral brain functions has been difficult. There are many different interactions of FGF ligands and FGFRs, such that specific roles for FGF ligands—such as FGF17 in social behavior, FGF22 in anhedonia-like behavior, or FGF2 or FGF8 in anxiety-like behavior—leaves undetermined what receptors and cells mediate these effects [10, 14, 17, 18]. This is an important challenge to overcome to understand how FGF signaling may contribute to neuropsychiatric risk [19–22] and potential for treatment [23].
FGF receptors, and in particular FGFR2, are almost exclusively expressed in non-neurons in the postnatal forebrain [24–29]. Although FGF signaling is known to promote astrocyte differentiation and astrocyte activation in the mature brain [30, 31], the role of specific FGFRs in astrocytes is still undefined, and unraveling their separate roles at different developmental stages has been challenging. Evidence has recently arisen through animal model systems that suggests the importance of FGF-signaling alterations in astrocytes for processes of brain development and behavior: specifically, FGFR1 expression in dorsal forebrain astroglia secondarily affects the early postnatal development of cortical interneurons, but impacts of astrocytic FGFR2 during early postnatal development is not known, despite its almost-exclusive presence in astroglia postnatally. In cultured human astrocytes, both FGFR1 and FGFR2 are needed for signal transduction effects of anti-depressants, but FGFR2, not FGFR1, is necessary for acidic-FGF (FGF1) to further stimulate activated astrocytes [24, 32]. Exogenous FGF2 reduces stress-induced changes in astrocytes which may be a pathway to regulation of anxiety-like behavior; [33–35] however, anti-anxiety-like effects of FGF2 in mice do not require FGFR1 or R2 [10]. Many gaps in knowledge about the behavioral impacts of astroglial FGF signaling exist. Specifically, how astrocyte-dependent signaling through FGFR2 affects behavioral regulation across different domains has not been determined, despite its importance in postnatal astroglia.
Astrocyte dysfunction in the forebrain results in mixed behavioral outcomes but suggest a role for astroglia in regulation of activity—specifically disrupted astroglial SynCAMI or adenosine kinase increases locomotor activity [36, 37]. Astrocytes have a critical role in regulating neuronal synaptogenesis and pruning, processes that underlie early learning and behavioral outcomes [38, 39], emphasizing the importance of examining not only behavioral outcomes from FGF effects on astrocyte function but how these relate to synaptic development. There have been calls for greater investigation of astrocytic mechanisms relevant to childhood neurodevelopmental disorders [40, 41].
Here, we specifically targeted FGF signaling in the brain at different times as important mechanisms underlying multiple behaviors by inducing the knockout of FGFR2 at different stages of development and in different cell types. We examined three different sets of mice that had a cell type-targeted knock-out of FGFR2: 1) knockout during embryogenesis, in radial glial cells and therefore both their neuronal and glial progeny 2) knockout during the rodent early postnatal period, largely in proliferating astroglial precursors and glial progeny and 3) knockout during adulthood, in largely post-mitotic astroglia during mature brain functioning. We hypothesized that early postnatal loss of FGFR2 in astrocytes would affect astrocyte biological function and impact the regulation of behaviors that are disrupted at early postnatal time points in psychiatric disorders.
Materials and method
Mice
All experimental procedures involving animals were performed in accordance with the Yale University and University of Iowa Animal Resources Center and Institutional Animal Care and Use Committee (IACUC) policies. Sufficient animals or samples were generated for each assessments using a power analysis based on previous studies and α = 0.05 and β = 0.2
Conditional hGFAP-cre fgfr2 knockout mice (referred to here as cKO) on a mixed background have been previously described [1, 8]. The conditional fgfr2 null allele harbors loxP recombination sites flanking regions encoding the Ig III binding and transmembrane domains of the fgfr2 gene (fgfr2f) [42]. Mice homozygous for fgfr2f alleles were crossed with mice expressing the Cre recombinase transgene under the control of the human GFAP promoter (hGFAP) [43]. The hGFAP-cre transgene targets Cre recombination to radial glia progenitors of the dorsal telencephalon starting at E13.5 [2]. Cre negative mice, littermates when possible, were used as control animals.
To assess the contribution of FGFR2 solely in the postnatal brain, mice homozygous for the fgfr2f alleles were crossed with GFAP-creERT2 (GCE) mice [44] also on a mixed background. The latter express a tamoxifen-inducible Cre recombinase-estrogen receptor fusion protein (CreERT2) [45] under the control of the GFAP promoter. Specifying gene knock outs to astroglia is challenging due to the overlapping nature of most genetic drivers in both postnatal neural stem cells and astroglia. Therefore, genetic approaches may have impacts on a small number of neural stem cells (which may impact neurons in the dentate gyrus and olfactory bulb) but will effect astroglia much more substantially; our approach must be considered with this caveat. Previous investigations of the GCE line demonstrate that it largely affects astrocytes; [44] in combination with the postnatal expression of FGFR2 in non-neuronal cells, postnatal induction with the GCE line principally targets fgfr2 loss in astrocytes. We used two different tamoxifen-induction protocols to target the knockout of fgfr2 at different time points. In the first neonatal protocol (referred to here as nKO), mother mice received intraperitoneal (IP) injections of 1 mg of tamoxifen dissolved in sunflower seed oil once daily for five consecutive days, beginning on postnatal day 1, 2, or 3 while Cre- and Cre+ experimental animals were nursing. In the second adult induction protocol (referred to here as iKO), Cre- and Cre+ adult mice received injections of 0.5 mg of tamoxifen dissolved in sunflower seed oil twice daily for five consecutive days at 2–4 months of age as previously [8]. Behavioral testing began at least 9 days after the time of the last tamoxifen injection. A few control animals with solely sunflower seed oil injection using the neonatal and adult protocols were created and tested on some behavioral assays
Behavior testing
All behavior assessments were performed during the light cycle in a dedicated testing room with only one behavior assessment performed per day in the order described below, allowing mice to habituate to the testing room for 60 min prior to testing. Unless otherwise noted, mice remained in their home cage with cage-mates immediately before and after assessments. Each of the three types of knockout mice were tested alongside their control littermates. Only male mice were tested due to limited resources; childhood psychiatric disorders have a higher prevalence in males which allows for these data only in males, while limited, to be translationally informative [46]. Behavioral testing in each cohort was performed with mice grouped together for testing across close birthdates, with testing beginning for some mice in each cohort at age 2.5 months and others at 4.5 months of age. All testing was completed when mice were between 5.5 and 7.5 months of age.
Open Field: In a square or rectangular plastic arena, at least 1500 cm2 in area, mice were tested for locomotor activity for at least 50 min. Test mice were placed in the corner of the arena and their movements recorded using an overhead camera (Anymaze software; Stoelting Co, Wood Dale, Illinois). The main measure of distance traveled was evaluated in 5 min epochs and repeated measures ANOVA was used to evaluate group differences over time.
Social Approach: In a three-chamber social approach apparatus [47], mice were tested for social recognition. Two male “stranger” mice unfamiliar to the test mouse but of the same strain and age were first habituated for five minutes to small cylinders in each side chamber. Then, test mice were habituated to the center chamber for five minutes. Subsequently, the test mouse underwent a first stage—a ten-minute trial in which only one stranger was present in one side chamber. This was followed by a second stage—a ten minute trial in which the previous stranger mouse and a new stranger mouse were present in the two side chambers. Movement was recorded using an overhead camera and evaluated for the amount of time the test mouse spent in each chamber and in a one-inch diameter around the cylinders on each side (Anymaze). Social approach was calculated from behavior during the first stage taking the quotient of the time spent with the first stranger divided by the time spent in both sides overall. Social recognition was calculated from the second stage, using the time spent with the second stranger divided by the time spent in both sides overall. Statistical difference was evaluated with ANOVA and Student’s t-test.
Spontaneous Alternation: In a plastic Y maze with three 14 inch x 3 inch arms, mice were tested for working memory for 5 min. Mouse movement throughout the maze was monitored from live video recorded from above, noting arm entry order with experimenter blinded to group. For each set of three entries, the spontaneous alternation of those entries through all three arms was counted, and alternation percent calculated. Differences were evaluated with Student’s t-test.
Radial Arm Water Maze: Due to lack of resources, this task was not assessed in FGFR2 cKO mice. A 6-foot maze was filled with room temperature water and fitted with 6 dividers to create 6 radial arms., Mice were tested for spatial memory with distant visual spatial cues across two days with experimenter blinded to group [48]. Briefly, using visible and hidden platforms, mice were evaluated for working memory of platform location across 15 trials on day one. Day two of testing assessed consolidation of short-term memory using only the hidden platform across 15 trials. Working memory was calculated via arm entry errors and time to reach platform in three-trial blocks, with repeated measures ANOVA to evaluate group differences over time.
Elevated Plus Maze: In a Stoelting (Wood Dale, Illinois) Elevated Plus Maze, mice were tested for anxiety-like behavior for 5 min. Mouse movement throughout the maze was recorded using an overhead camera. The amount of time spent in each arm and the center of the maze was assessed (Anymaze). Differences were evaluated with repeated measures ANOVA across zones and with a t-test of the ratio of time spent in the open to closed zones.
Gene expression
To evaluate the penetrance of Cre-mediated deletion of the fgfr2 gene and other genes related to glial regulation of brain function, dorsal forebrain, hippocampus, or medial frontal cortex was dissected out from brains of animals which were neonatally exposed to tamoxifen through maternal injection. RNA was isolated using standard Trizol methods and concentration assessed (Nanodrop Spectrophotometer, Thermo Scientific). cDNA (Superscript III First Strand Synthesis Kit, Invitrogen) was used to evaluate relative gene expression to β-actin using TaqMan primers (B-actin: predeveloped; vGat: Mm00494138_m1; vGlut1: Mm00812886_m1; Fgfr2: Mm1269938_m1) and GeneAmp PCR Mastermix in a StepOne™ Instrument (Applied Biosystems).
Immunocytochemistry
At least 10 days after completion of behavioral testing, animals were anesthetized and perfused (phosphate buffered saline (1X PBS), then 4% paraformaldehyde), and brain tissue was post-fixed, cryoprotected with a sucrose solution in 1X PBS and embedded in OCT compound, and cryostat (Leica, CM1900, Bannockburn, Illinois) sectioned at 50 µm thickness. Standard immunostaining methods were then used on free floating brain sections: blocking with 10% goat serum in 1XPBS, TritonX-100 and Tween20, incubation with primary antibodies GFAP (DAKO Z0334, rabbit, 1:500), VGLUT1 (EMD Millipore AB5905, guinea pig, 1:4000), PSD95 (Abcam Ab12093, goat, 1:500), gephyrin (SySy 147021, mouse, 1:500), VGAT (SySy 131002, rabbit, 1:1000), glutamine synthetase (EDM Millipore MAB302, mouse, 1:500) washing the primary antibodies 3x in 1XPBS, incubation with Alexa dye-conjugated secondary antibodies, anti guinea-pig or anti mouse Alexa 594, anti-rabbit or anti-goat Alexa 488 (1:500; Molecular Probes), and coverslipping using mounting medium with DAPI (Vector Laboratories, #H-1200).
Stereology
Glutamine synthetase+ cells in coronal tissue sections were measured using fluorescent microscopy with a Zeiss Axiolmager M2 microscope. Stereological estimates of hippocampus and medial frontal cortex (mFC) cell densities were calculated using optical fractionator approach and unbiased counting rules with 3-dimensional 150 × 100 × 10 μm counting frames, on a 450 × 450 μm grid for mFC and 600 × 600 μm grid for hippocampal CA, using a 40× objective lens with experimenter blinded to group (Stereoinvestigator; MBF Biosciences). Stereological counting to determine cell density, displayed as means and standard errors of the mean, was performed in 3–8 serial coronal sections (every 10th section) of the adult mFC and the hippocampus as previously described [49, 50].
Astrocyte morphology
Astrocytes, GFAP + cells with well-delineated cell bodies and branches, in the mFC and hippocampus were randomly selected for morphology reconstruction. Ten z-stacks per cell were acquired at 100x and traced in each experimental group (Control and FGFR2 nKO) by using Neurolucida 11.03 (MBF Bioscience, Williston, VT USA) The coordinate files obtained by the 3D reconstruction were analysed in Neuroexplorer [51].
Punctal assessment
VGLUT1, PSD95, and colocalized puncta as well as VGAT, Gephyrin, and colocalized puncta were assessed by imaging the adult mFC of the FGFR2 nKO with a Zeiss Axioimager M2 microscope equipped with ApoTome2. Four mice per group were examined. Eight z-stacks spanning the entire cortical layers were imaged at 40x with experimenter blinded to group. The ImageJ software (National Institute of Health) Puncta Analyzer plugin for the estimation of these puncta was used as previously described [52].
Electron microscopy
We performed assessments of glial and synaptic structure as previously [53]. Electron microscopy photographs (16,300×) were used to first measure the perimeter of each neuronal profile analyzed, followed by determination of the amount of membrane covered by astrocytes with experimenter blinded to group. The results are reported as percentage of astrocyte coverage neuronal cell membrane. Synaptic boutons in direct contacts with the same neuronal profiles analyzed for glial coverage was determined as described in our earlier studies [53–55]. Synapse number is reported per 100 μm perikaryal membrane.
Statistical methods
Data normality and variance was assessed with GraphPad Prism8 to select appropriate statistical tests. Graphs were made and two-tailed Student’s t-tests were performed with Microsoft Excel. ANOVA for repeated measures outcomes were performed with GraphPad Prism8. Outliers were excluded if they were >2 standard deviations from the mean.
Results
Mice with conditional loss of FGFR2 starting in embryonic radial glia (cKO) have been previously described [1, 8]. In brief, the FGFR2 cKO resulted from recombination of the conditional fgfr2f alleles with the hGFAP-cre transgene, where Cre is expressed in radial glia beginning at embryonic day 13.5, therefore affecting all their neuronal and glial progeny in regions where the hGFAP-cre transgene is expressed, primarily the forebrain and cerebellum. An 80–91% loss of fgfr2 gene expression was previously found and a substantial reduction of FGFR2 protein level [1]. The adult astrocyte FGFR2 knock out (FGFR2 iKO) has also been previously described, with an 80% reduction in fgfr2 gene expression, and was accomplished by recombination of the same fgfr2f alleles with the hGFAP-CreERT2 transgene, where Cre was expressed in postnatal GFAP+ glial cells after tamoxifen injection in adulthood [8].
To knock out FGFR2 in astroglial cells in the neonatal period (FGFR2 nKO), the same mice carrying fgfr2f alleles and the hGFAP-CreERT2 transgene received tamoxifen via the milk in the neonatal period by injecting the Cre negative dam with 1 mg tamoxifen once daily for 5 days. The reduction of fgfr2 assessed by qRT-PCR in juvenile or adult cortex or hippocampus varied between 29% and 43% (Supplementary Table 1). Control animals used for the FGFR2 nKO and FGFR2 iKO lines were also injected with tamoxifen to control for the potential impact of this manipulation.
Embryonic knock-out of FGFR2: Behavioral changes
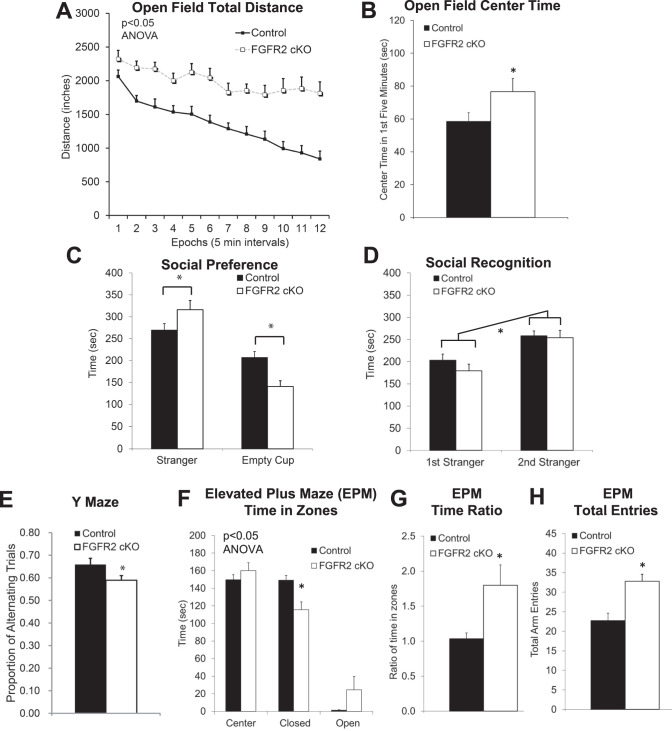
In FGFR2 cKO mice, multiple behavioral abnormalities were identified in addition to learning and memory deficits demonstrated previously [8]. Locomotor hyperactivity was found to be similar to that previously characterized in FGFR1 cKO mice (Fig. 1A; 55% greater; rmANOVA: F(1,26) = 20.17, p = 0.0001) [12]. Compared to their control littermates, FGFR2 cKO mice were more active in an open field, also spending more time in the center of the open field (Fig. 1B; p = 0.04)), a phenotype suggesting reduced anxiety-like behavior.
Fig. 1. Adult male mice embryonically lacking FGFR2 driven by hGFAP-Cre (beginning by at least E13.5) showed locomotor hyperactivity, reduced anxiety-like behavior, increased sociability, and reduced working memory.
A Persistently increased distance traveled in the open field in FGFR2 cKO mice. B Reduced anxiety-like behavior with open field increased time in the center in FGFR2 cKO mice. C Three chamber social task with social side compared with non-social side showed increased social preference in FGFR2 cKO mice. D Three chamber social task with familiar social side compared with novel social side showed no differences in social recognition. E Reduced working memory with less Y maze spontaneous alternation in FGFR2 cKO mice. F Reduced anxiety-like behavior with altered time spent in the closed and open arms of the EPM. G Reduced anxiety-like behavior with altered ratio of time in zones of the EPM. H Increase locomotor activity with increased overall entries into all arms of the EPM. N = 14,14; *p < 0.05 two-tailed Student’s t-tests or ANOVA. Means and SEM shown.
FGFR2 cKO mice also showed alterations in social approach. When tested on their social preference, they demonstrated a small but significantly higher preference than controls for interacting socially with a novel stranger versus spending time with a novel object (Fig. 1C; ANOVA interaction: F(1,55) = 12.24, p = 0.0009). This higher sociability was true in approach behavior in close proximity to the novel mouse or object (sociability index 32% increase: cKO: 0.77 vs controls: 0.58, p = 0.0003), as well as in larger chambers (sociability index 26% increase: cKO: 0.68 vs controls: 0.54, p = 0.002). This effect was not attributable to altered social recognition, as both cKO and control mice had similar interaction with a familiar versus a stranger mouse target (Fig. 1D; recognition indices: large chamber cKO: 0.60 vs controls: 0.53, p = 0.19; close proximity cKO: 0.64 vs controls: 0.61, p = 0.46).
Working memory was impaired in FGFR2 cKO mice (Fig. 1E, p = 0.048), as shown by a small but significant 10% lower spontaneous alternation in a Y maze.
Behavior on the elevated plus maze was also significantly different from control littermates overall (Fig. 1F, ANOVA interaction: F (2,78) = 7.991, p = 0.0007). Mice demonstrated a small effect on anxiety-like behavior, with less time spent in the closed arms of the maze (p = 0.02) and a lower ratio of time across the elevated plus maze zones (Fig. 1G; p = 0.03), but no difference in the ratio of entries across zones (data not show; p = 0.32). Performance on the elevated plus maze, a different environment than an open field, also confirmed the higher activity level of these mice regardless of context (Fig. 1H; p = 0.001).
Neonatal knock-out of FGFR2: Behavior changes
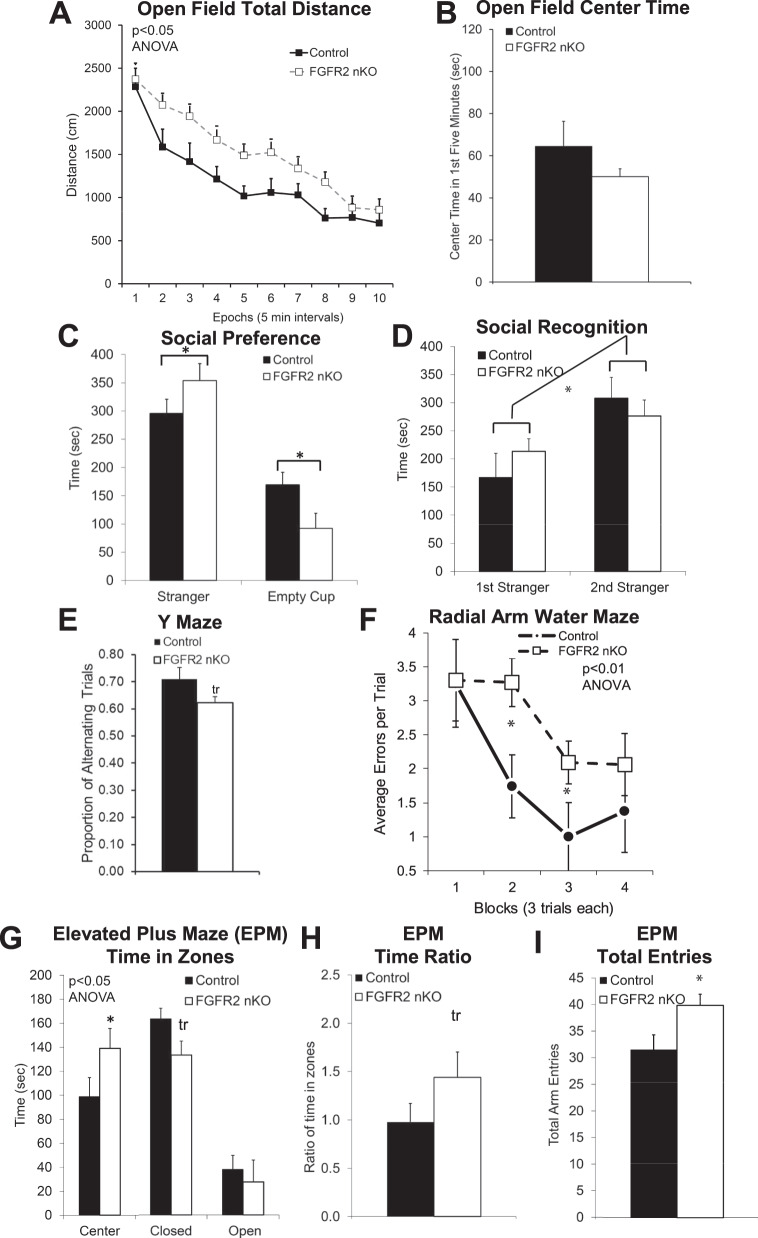
The behavior of FGFR2 nKO mice, lacking FGFR2 signaling only in GFAP+ astroglial cells beginning in the neonatal period, was characterized on the same tasks described above (Fig. 2A–H) as well as further characterization of working memory with a radial arm water maze. Compared to Cre- negative littermates with the same tamoxifen exposure, FGFR2 nKO animals showed a small but significant 32% increase in locomotor activity in an open field but no increase in time in the center (Fig. 2A, B; rmANOVA: F (1,15) = 5.542 p = 0.03; center time p = 0.97). We also examined activity level of FGFR2 nKO mice compared to an additional control group—a small sample of vehicle/oil injected Cre+ controls. Activity of FGFR2 nKO mice also trended increased by this comparison (rmANOVA n = 3 oil inj Cre+ vs n = 10 tam inj Cre + , rmANOVA: F (1, 11) = 4.574, p = 0.056, Supplementary Fig. 1A), and tamoxifen injection itself compared to oil in Cre- mice did not alter open field behavior.
Fig. 2. Adult male mice early postnatally lacking FGFR2 driven by GFAP- CreERT2 (induced with neonatal tamoxifen injections P1-7) showed locomotor hyperactivity, reduced anxiety-like behavior, increased sociability, and reduced working memory.
A Persistently increased distance traveled in the open field in FGFR2 nKO mice. B No differences in anxiety-like behavior with open field time in the center. C Three chamber social task with social side compared with non-social side showed increased social preference in FGFR2 nKO mice. D Three chamber social task with familiar social side compared with novel social side showed no differences in social recognition. E Reduced working memory with less Y maze spontaneous alternation in FGFR2 nKO mice. F Reduced working memory with increased errors in the Radial Arm Water Maze training trials in FGFR2 nKO mice. G Reduced anxiety-like behavior with altered time spent in the closed and center arms of the EPM. H Reduced anxiety-like behavior with altered ratio of time in zones of the EPM. I Increased locomotor activity with increased overall entries into all arms of the EPM. N = 10,10; *p < 0.05 two-tailed Student’s t-tests or ANOVA. Means and SEM shown.
FGFR2 nKO mice also showed the same small increased preference for social interaction indicating higher sociability (Fig. 2C: ANOVA interaction: F (2,28) = 3.343, p = 0.04; sociability index: large chamber 31% increase: nKO: 0.76 vs controls: 0.58, p = 0.02; close proximity 21% increase: nKO: 0.75 vs controls: 0.62, p = 0.11) without a deficit in social recognition (social recognition index: large chamber: nKO: 0.64 vs controls: 0.57, p = 0.49; close proximity: nKO: 0.70 vs controls: 0.66, p = 0.64) (Fig. 2C, D).
Working memory on the Y maze was trend deficient in FGFR2 nKO mice to the same small extent (12%) as shown for FGFR2 cKO mice (Fig. 2E, p = 0.07). Working memory was further measured by performance on the radial arm water maze (Fig. 2F). This task also showed that working memory, as measured by errors during the training phase when animals must keep location information in working memory, was significantly impaired with small effect size in FGFR2 nKO mice (rmANOVA: F (2.725, 49.06) = 5.363, p = 0.0037).
Measures of anxiety-like behavior on the elevated plus maze was also shown to be altered in FGFR2 nKO mice in a comparable fashion to the FGFR2 cKO mice, with a small reduction in anxiety-like behavior: less time in the closed arms of the maze and more time in the center (Fig. 2G, ANOVA interaction: F (2,48) = 3.414, p = 0.04; p = 0.08, p = 0.048). FGFR2 nKO mice showed a trend higher ratio of time in the open to closed zones (Fig. 2H, p = 0.08), but no difference in the ratio of entries in the zones (data not shown; p = 0.34). We also examined these differences in the vehicle/oil injected animals and found that tamoxifen injection which induced the FGFR2 nKO was needed to see this effect (Supplementary Fig. 1B). Just as seen in FGFR2 cKO mice, levels of activity in FGFR2 nKO mice were confirmed to be increased to a small extent on the EPM compared to controls (Fig. 2I, p = 0.01).
In summary, the induced loss of FGFR2 in neonatal astrocytes resulted in many alterations on the same behaviors as those noted here in the FGFR2 cKO mice in which the FGFR loss starts in radial glial cells in the embryonic period—social preference behavior, spontaneous alternation rate, and elevated plus maze closed arm time and total entries were changed to similar extents in both types of FGFR2 deficit mice. In FGFR2 nKO mice, open field activity and elevated plus maze time ratio were increased as in FGFR2 cKO animals but not to the same extent; additionally, open field center time was not increased in FGFR2 nKO mice unlike FGFR2 cKO animals.
Adult Knock-Out of FGFR2: Behavior Changes
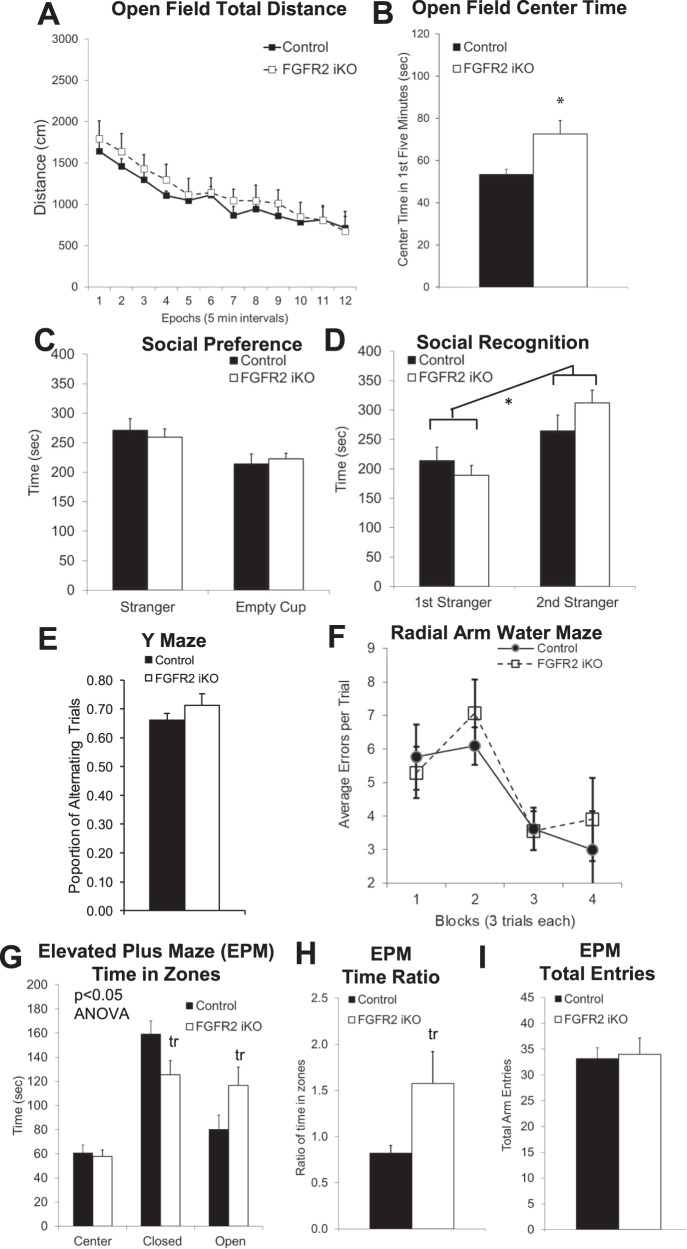
In contrast to the comparable behavioral alterations of FGFR2 cKO and nKO mouse models, FGFR2 iKO mice, lacking FGFR2 signaling only in GFAP+ glial cells beginning in adulthood, showed no alterations of locomotor activity on the open field, working memory on the Y maze or the radial arm water maze, or social preference (Fig. 3A, C, D, E, F). The similar behavior of Cre- control and Cre+ FGFR2 iKO on many behaviors validated that the Cre transgene was not, itself, a source of behavioral differences. We verified that Cre- control mice injected with tamoxifen in adulthood did not differ from Cre- control mice injected with tamoxifen in the neonatal period when considering locomotor activity on the open field, working memory on the Y maze, or social preference (Supplementary Fig. 2A–C).
Fig. 3. Adult male mice lacking FGFR2 in adulthood driven by GFAP- CreERT2 (induced with adult tamoxifen injections P56-60) showed only reduced anxiety-like behavior.
A No difference in distance traveled in the open field. B Reduced anxiety-like behavior with open field increased time in the center in FGFR2 iKO mice. C, D Three chamber social task showed no difference in social preference or social recognition. E No difference in working memory with Y maze spontaneous alternation. F No difference in working memory with errors on Radial Arm Water Maze training trials. G Reduced anxiety-like behavior with altered time spent in the closed and open arms of the EPM. H Reduced anxiety-like behavior with altered ratio of time in zones of the EPM. I No difference in locomotor activity with overall entries into all arms of the EPM. N = 7,10; *p < 0.05 two-tailed Student’s t-tests or ANOVA. Means and SEM shown.
Differences of FGFR2 iKO mice from wild type littermates were found only for anxiety-like behavior. On the elevated plus maze (Fig. 3G, ANOVA interaction: F (2, 36) = 5.260, p = 0.0099) these FGFR2 iKO mice demonstrated trend less time spent in the closed arms (p = 0.05), more time in the open arms (p = 0.08), and higher ratio of time in the open to closed zones (Fig. 3H; p = 0.07), indicative of less anxiety-like behavior, but no differences in overall activity level (Fig. 3I) or in the ratio of entries in the zones (data not shown; p = 0.23). There was no effect of adult oil injection itself on elevated plus maze performance (Supplementary Fig. 1C), confirming that the adult FGFR2 iKO with tamoxifen was needed for this effect. This effect was clear despite the increased open arm time in Cre- control mice injected with tamoxifen in adulthood (Supplementary Fig. 2D). Decreased anxiety-like behavior was also demonstrated by increased time spent in the center of the open field (Fig. 3B, p = 0.02).
Neurobiological findings
Given the multiple behavioral abnormalities induced when FGFR2 was lacking only beginning in neonatal life in primarily astrocytes, we performed pilot investigations of their neurobiology focused mainly on hippocampus as a major region implicated in regulation of the behaviors assessed here. We first assessed GFAP + astrocyte density in the hippocampus of FGFR2 nKO mice, finding no differences (n = 3,3; p = 0.36, control = 8.45 ± 0.97 × 10−6, FGFR2 nKO=10.14 ± 1.37 × 10−6 cells/µm3). The volume of the hippocampus was also unchanged (n = 3,3, p = 0.86, control = 3.1 ± 0.6 mm3, FGFR2 nKO = 3.2 ± 0.6 mm3). In addition, the most broadly affected model used here, FGFR2 cKO mice, showed no deficit in GFAP + cell density either (n = 3,3; p = 0.32, control = 9.26 ± 1.05 × 10−6, FGFR2 cKO = 8.44 ± 1.69 × 10−6 cells/µm3). This suggested that astrocyte numbers themselves were intact regardless of early loss of FGFR2.
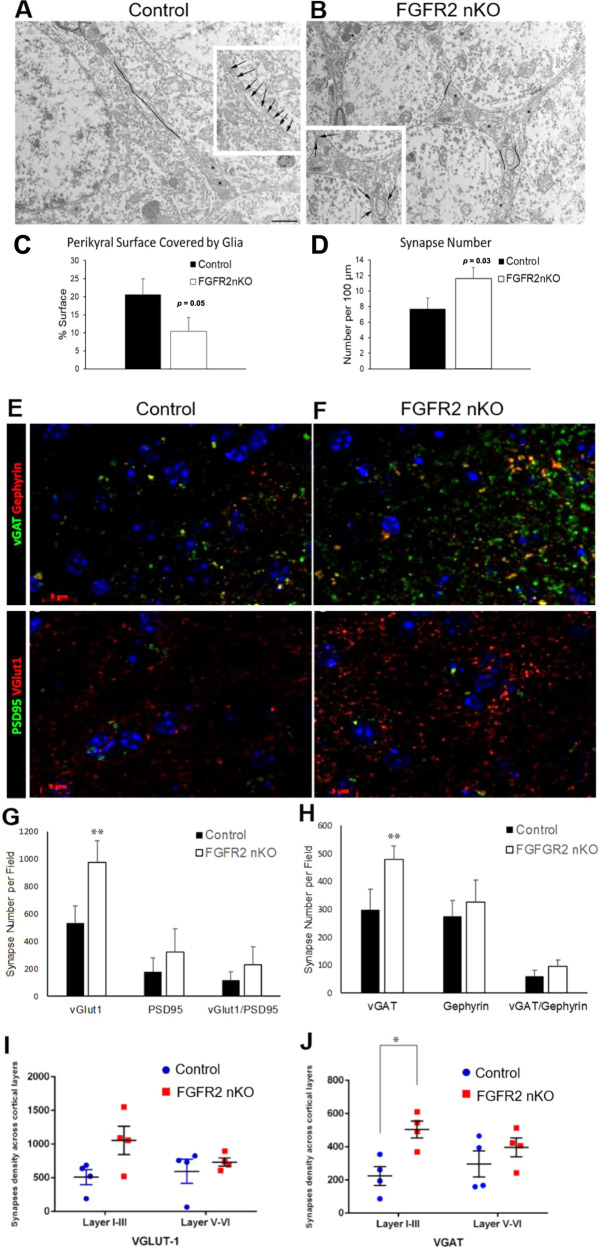
To gain insights into astrocyte morphology after early postnatal loss of FGFR2 in astroglia, we performed electron microscopy (EM) analyses of the hippocampus in FGFR2 nKO mice. The analysis of the astrocytic coverage of the cell membrane of neurons in the principal cell layer of the CA3 region of the hippocampus showed reduced coverage by almost half (Fig. 4A–C). Of note, this measure of astrocyte-neuron contact was coupled to increased number of synapses on the same cells (Fig. 4D).
Fig. 4. Adult male mice early postnatally lacking FGFR driven by GFAP- CreERT2 (induced with neonatal tamoxifen injections P1-7) show reduced astrocyte processes in neuropil by electron microscopy.
A–D Less glial coverage of perikaryal membrane (black lines) and more synapse number on the same membrane (asterisks) in FGFR2 nKO compared to control littermate mice in the hippocampus CA3 region (30 cells per genotype). N = 3,3. These mice also show increased density of pre-synaptic proteins assessed by immunofluorescence. E–H Greater number of puncta of vGLUT1 and vGAT per field of analysis in lateral cortex. I, J Greater number of vGAT puncta in cortical layers I-III when evaluated separately from cortical layers IV-VI. N = 4,4; *p < 0.05, **p < 0.01 two-tailed Student’s t-tests or ANOVA. Means and SEM shown.
The loss of FGFR2 signaling did influence the expression of glutamine synthetase (GS), a metabolic component of glial cells which is regulated by glutamate transport and synaptic activity. In FGFR2 nKO, the density of glutamine synthetase positive cells, as measured by stereological counts in adult brains, trended increased in hippocampus and was also increased to a greater extent in cerebral cortex of FGFR2 nKO mice (Table 1). Regardless of these changes, gross astrocyte morphology in hippocampus was qualitatively normal (Supplementary Fig. 3A). Increased glutamine synthetase expression was also found in the hippocampus after embryonic knock out of FGFR2 (FGFR2 cKO) which had similar behavioral alterations to nKO mice and also lacked FGFR2 in astrocytes from early developmental stages. However, glutamine synthetase change was not seen in FGFR2 iKO mice (Table 1).
Table 1.
Glutamine Synthetase+ cell density differences with reduction of FGFR2 signaling.
| Control Mean ± SEM cells x 10−5/μm3 | Experimental Mean ± SEM cells x 10−5/μm3 | Difference | p-value | |
|---|---|---|---|---|
| FGFR2 nKO Medial Frontal Cortex | 0.23 ± 0.01 (n = 3) | 0.47 ± 0.05 (n = 3) | ↑104% | 0.01* |
| FGFR2 nKO Hippocampus | 1.20 ± 0.15 (n = 3) | 1.99 ± 0.43 (n = 3) | ↑68% | 0.07¥ |
| FGFR2 cKO Hippocampus | 1.55 ± 0.06 (n = 4) | 2.05 ± 0.13 (n = 6) | ↑30% | 0.02* |
| FGFR iKO Hippocampus | 1.77 ± 0.19 (n = 3) | 2.09 ± 0.40 (n = 3) | ↑18% | 0.55 |
*p < 0.05, ¥-trending significance, 0.05 ≤ p < 0.10.
With the greater change in astrocyte glutamine synthetase expression in the cortex of the FGFR2 nKO mice, we examined cortical density of synaptic proteins in adult FGFR2 nKO mice, as assessed by immunohistochemical puncta analysis. FGFR2 nKO mice were found to have increased puncta density after immunostaining for both the GABA neurotransmitter release protein, vGAT, and the glutamate neurotransmitter release protein, vGLUT1 (Fig. 4E–J). In contrast, the density of post-synaptic protein puncta (gephyrin and PSD95) or co-localized pre- and post-synaptic protein densities were unchanged (data not shown). The vGAT and vGLUT1 punctal increases were localized to upper cortical layers I-III (Fig. 4I, J).
Lastly, we examined gene expression of these same synaptic proteins in the hippocampus of another cohort of FGFR2 nKO mice and controls (n = 8,6). GABA transporter vGat (by RT-qPCR) was increased and synaptic glutamate transporter vGlut1 trended increased in the juvenile hippocampus (n = 8,5; vGat: p < 0.005, control = 1.00 ± 0.07, FGFR2 nKO = 3.62 ± 0.91; vGlut1: p = 0.09 control = 1.00 ± 0.12, FGFR2 nKO = 3.70 ± 1.86). These findings suggest that early postnatal glial FGFR2 loss in the dorsal forebrain induces a decrease in astrocyte-neuron contacts and potentially an increase in neuronal synaptic contacts, as shown by EM and level of presynaptic marker expression, particularly in hippocampus.
Discussion
We have demonstrated a distinct behavioral triad of hyperactivity, working memory deficits, and increased sociability in mice lacking FGFR2 in astroglial cells, only when that loss begins by at least the neonatal period of development. In concomitance with this behavior, the loss of FGFR2 in neonatal astroglial cells results in data suggesting decreased astroglia-neuron membrane appositions as well as increased neuronal synapses and their signaling proteins, as shown by EM and puncta density of presynaptic vesicular proteins. We further show that expression of GS, a critical astrocytic protein for both glutamatergic and GABAergic synaptic function [56, 57], is increased in astroglia. We hypothesize that the increase in GS immunostaining likely represents a functional aftereffect of increased neuronal signaling, informed by others’ findings that GS expression is influenced by neuronal activity [56, 58]. Distinct from these phenotypes, we found that a small decrease in anxiety-like behavior was present in animals with induced loss of FGFR2 in GFAP + cells at all developmental time periods, even when induced only in adulthood. This suggests that hyperactivity, working memory deficits, and increased sociability might be related to the developmental roles of FGFR2 in astrocytes in the neonatal period, whereas the modest decrease in anxiety-like behavior might reflect a continuous, ongoing role of FGFR2 in the functioning of astroglial cells as we previously demonstrated for short term memory [8].
Fibroblast growth factor signaling in the brain has previously been investigated for its role in embryonic patterning and regulation of neurogenesis [19] or, in adulthood, for its implications in behavioral alterations, such as anxiety-like behavior and learning [8, 10, 13]. Because hyperactivity, working memory deficits, and increased sociability were present here when FGFR2 was lacking from the neonatal period onward but not when the loss was induced in adulthood, these results support a new line of thinking, implicating fibroblast growth factor signaling in the early postnatal brain [59] and further suggest that these processes may affect the risk for behavioral disorders [38]. Convergent data on knock out of early postnatal FGF22, a ligand partner of FGFR2, affecting anhedonia supports this idea [18]. These findings together support the notion of “sensitive periods” in development, implying a crucial role of FGFR2 signaling in the perinatal/juvenile period.
The three cohorts here with FGFR2 loss at different time points, as well as in different subsets of cells, showed some similarity and some difference. All three FGFR2 KO lines which had in common their lack of FGFR2 in astroglial cells in adulthood showed small reductions in anxiety-like behavior in the elevated plus maze, as assessed by small shifts in time from closed to open zones; open arm time, the metric most robustly associated with anxiety-like constructs in the literature, was not always increased and EPM findings were only trending at times. These findings of small effect may reflect FGFR2’s mixed roles with multiple ligands (FGFs and other molecules which bind to FGFR2) [60, 61] in regulating anxiety-like behavior [10, 62, 63]. The clearest findings were in the adult-induced FGFR2 knock-out mice (FGFR2 iKO); this suggests that the reduced anxiety-like behavior found in embryonically-induced FGFR2 knock-out (FGFR2 cKO) and FGFR2 nKO mice may also be attenuated by the other roles FGFR2 plays in early development potentially underlying their other behavioral deficits. For example, greater locomotor hyperactivity in the FGFR2 cKO mice compared to FGFR2 nKO and FGFR2 iKO may interact with their anxiety-like behavior regulation in complex ways.
More distinct differences between these three FGFR2 KO lines included the presence of the deficits in hyperactivity, sociability, and working memory in only FGR2 cKO and FGFR2 nKO mice. Analysis of results from vehicle/oil injected mice and comparing mice tamoxifen-injected at different ages provided reassurance that differences were due to the knock out of fgfr. The 25–30% increase in the social preference index was similar in both these mouse lines and involved increased time spent with a novel stranger mouse relative to time spent with an empty social interaction cup. This may reflect a deficit in typical down-regulation of social approach over time or an increased drive for social reward. While models for the study of neuropsychiatric disorders commonly demonstrate decreased sociability [64], increased rodent social interaction on a variety of tasks has also been demonstrated in a number of studies with different genetic backgrounds relevant to autism spectrum disorder (ASD), schizophrenia, intellectual disability, mood disorders, and attention deficit hyperactivity disorder (ADHD) [64–71]. Other studies have demonstrated heritable patterns of increased social interaction in monkeys which has also been described as social impulsivity [72]. Directionality of behavior on non-human animal social tests cannot be directly compared to human social interactions, although may generally inform the understanding of the development of neural systems underlying social behavior.
Further behavior similarities arose when FGFR2 loss began early in development. While working memory in FGFR2 cKO mice was only tested with the Y maze, this Y maze deficit was very similar to that in the FGFR2 nKO mice (small 10% and 12% deficits respectively) which also showed a radial arm water maze working memory deficit. These deficits were distinct from the short and long term memory deficits previously identified with FGFR2 iKO and cKO mice respectively [8], but findings here were similar to other working memory deficits found with targeted astrocyte dysfunction [73, 74] and models for the study of ADHD [75]. Lastly, hyperactivity levels were much greater in FGFR2 cKO compared to FGFR2 nKO mice (55% vs 32%), suggesting that the earlier and greater loss of FGFR2 expression including in all dorsal forebrain neurons and glia may underlie this outcome.
The importance of glial cells during early developmental time periods has been suggested by other lines of research. Glial proliferation occurs predominantly in the last few embryonic days and first few postnatal weeks of mouse development which also may be a critical time period for establishing these cells’ own later functioning. Although FGF signaling regulates glial proliferation and fate [31, 76], astrocyte density was not reduced by the early loss of FGFR2 in this study. This suggests that this process is redundant between different FGFRs and/or is regulated by non-FGFR2 mechanisms. In the neonatally-induced loss of FGFR2 in GFAP expressing cells, impacts on behavior may be from alterations in more differentiated glial cells. We cannot exclude that loss of FGFR2 in a small number of neural stem cells and their neuronal progeny targeted by GFAP-creERT2 also plays a role in behavioral impacts in the tamoxifen-induced lines. However, regardless of possible inclusion of neuronal FGFR2 signaling in these mechanisms, there were definitive impacts on astrocytes. Our neurobiological data suggest that the deficiency of FGFR2 has an impact on astrocyte differentiation and maturation, processes that occur in the early postnatal period in mouse brain, and which may result in abnormal astrocyte ultrastructure. Astrocyte maturation is regulated by multiple FGF ligands, including changes in protein levels that occur as astrocytes mature: for example, upregulation of the glutamate transporter, GLT-1, and downregulation of GFAP [31]. Our data suggests that FGFR2 may be an important signaling partner for these FGF ligands in these processes.
Early postnatal astrocytes are in a distinct phase of differentiation; [77] as astrocytes develop, cellular extensions are made to promote astroglia-neuron interactions [78], the extent of which we found to be deficient at the ultrastructural level with FGFR2 knockout, similar to findings in drosophila lacking the FGF receptor, Heartless [79]. Decreased FGF signaling reduced astrocytic coverage of neuronal membranes, with synaptic processes increased in these same glial-neuronal couplets. Astrocyte-neuron contacts may in turn regulate the number of neuronal synapses during the early postnatal period by competitive processes or by affecting pruning of synapses, a process that is time-dependent [80, 81] and regulated by astrocytes [80, 82]. Synaptic pruning during these sensitive periods of development allows for experience to shape brain development [38, 39]. In sum, the present study suggests a possible competitive antagonism between astrocyte/neurons at synapses which may be important in regulation of neuronal signaling and synaptic pruning in early postnatal development. These data suggest intriguing mechanisms that should be assessed in future studies.
Given that punctal density of vesicular proteins was increased in neonatally-induced FGFR2 knockout mice when assessed by immunocytochemistry, a disruption of astrocyte regulation of neuronal signaling is likely. Increased glial metabolism, as shown through increased glutamine synthetase positive cells, may reflect a response to a higher level of neuronal signaling from increased vesicular proteins. A similar reduction in FGFR2 signaling in glial cells just a few days later in juvenile brain (postnatal day 8) decreased vesicular proteins [9] suggesting a dynamic role of this receptor with shifting impacts on neuronal structure and functioning as synapse formation, elimination, and maintenance occurs in the developing dorsal forebrain.
Astrocyte functioning has also been implicated in psychiatric disorders [83–85], with a main focus in adult psychopathology. The role of astrocytes in common psychiatric disorders of childhood, in which clinical impairment and pathophysiological mechanisms clearly begin earlyis potentially important given the early timing of astrocyte maturation [41, 86, 87]. Glial functioning may be altered in patients with ASD [88–90] and ADHD [91, 92]. Clinical investigations in ADHD show decreased cortical surface area during childhood [93], which has implications for many aspects of neurobiology beyond dopamine and norepinephrine signaling that are targeted by current treatments. Proton magnetic resonance spectroscopy studies have implicated glutamate-glutamine metabolism in ADHD, which is highly dependent on astrocyte functioning [94]. Especially intriguing, as shown by the results here, is the potential role in childhood psychopathology of astrocytes in their early postnatal phases of differentiation. Astrocyte differentiation could be affected by early postnatal developmental insults [58, 95] but could also be a mechanism by which inherited risk for ADHD and other disorders is manifested.
The range of behavioral alterations manifested by the glial-targeted FGFR2 mice resembles that which individuals with ADHD-combined type display; the diagnostic criteria include increased locomotor activity, poor attention—relevant to working memory deficits— and impulsivity which leads to dysregulated increased social interaction. Some other models for the study of ADHD consistently show all of these impacts, although social approach is not a task typically assessed [75]. Because of the importance of social functioning for children’s success, we suggest this may be an important consideration for future studies. This and other studies suggest the possibility that diagnostic and treatment options for ADHD could be developed to incorporate glial functioning as a target generally or specifically with nanomedicine technological advances [96]. Treatments with great efficacy for ADHD exist. However, because of the relatively common occurrence of the disorder [97], significant numbers of children, adolescents, and adults are not successfully treated and experience great impairments in their functioning. Advancements in understanding the neurobiology of ADHD has the potential to benefit many.
Supplementary information
Acknowledgements
This work was supported by the Roy J. Carver Charitable Trust (HES), National Institute of Mental Health R01 MH067715 (FMV), International PhD program in Neuropharmacology at the University of Catania Medical School, Italy (SS), and Ida P. Haller Chair in Child and Adolescent Psychiatry at the University of Iowa (HES). We thank Robert J. Taylor for mouse management and testing.
Author contributions
HES and FMV designed the study. HES, SS, SCC, ST, and TLH collected and analyzed data. HES, SS, TLH, and FMV prepared the manuscript including figures. All authors reviewed and edited the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-023-02372-y.
References
- 1.Stevens HE, Smith KM, Maragnoli ME, Fagel D, Borok E, Shanabrough M, et al. Fgfr2 is required for the development of the medial prefrontal cortex and its connections with limbic circuits. J Neurosci. 2010;30:5590–602. doi: 10.1523/JNEUROSCI.5837-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohkubo Y, Uchida AO, Shin D, Partanen J, Vaccarino FM. Fibroblast growth factor receptor 1 is required for the proliferation of hippocampal progenitor cells and for hippocampal growth in mouse. J Neurosci. 2004;24:6057–69. doi: 10.1523/JNEUROSCI.1140-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner CA, Clinton SM, Thompson RC, Watson SJ, Jr, Akil H. Fibroblast growth factor-2 (FGF2) augmentation early in life alters hippocampal development and rescues the anxiety phenotype in vulnerable animals. Proc Natl Acad Sci USA. 2011;108:8021–5. doi: 10.1073/pnas.1103732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norton WH, Stumpenhorst K, Faus-Kessler T, Folchert A, Rohner N, Harris MP, et al. Modulation of Fgfr1a signaling in zebrafish reveals a genetic basis for the aggression-boldness syndrome. J Neurosci. 2011;31:13796–807. doi: 10.1523/JNEUROSCI.2892-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen K, Ohkubo Y, Shin D, Doetschman T, Sanford LP, Li H, et al. Decrease in excitatory neurons, astrocytes and proliferating progenitors in the cerebral cortex of mice lacking exon 3 from the Fgf2 gene. BMC Neurosci. 2008;9:94. doi: 10.1186/1471-2202-9-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rash BG, Lim HD, Breunig JJ, Vaccarino FM. FGF signaling expands embryonic cortical surface area by regulating Notch-dependent neurogenesis. J Neurosci. 2011;31:15604–17. doi: 10.1523/JNEUROSCI.4439-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rash BG, Tomasi S, Lim HD, Suh CY, Vaccarino FM. Cortical gyrification induced by fibroblast growth factor 2 in the mouse brain. J Neurosci. 2013;33:10802–14. doi: 10.1523/JNEUROSCI.3621-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens HE, Jiang GY, Schwartz ML, Vaccarino FM. Learning and memory depend on fibroblast growth factor receptor 2 functioning in hippocampus. Biol Psychiatry. 2012;71:1090–8. doi: 10.1016/j.biopsych.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terauchi A, Johnson-Venkatesh EM, Toth AB, Javed D, Sutton MA, Umemori H. Distinct FGFs promote differentiation of excitatory and inhibitory synapses. Nature. 2010;465:783–7. doi: 10.1038/nature09041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salmaso N, Stevens HE, McNeill J, ElSayed M, Ren Q, Maragnoli ME, et al. Fibroblast growth factor 2 Modulates hypothalamic pituitary axis activity and anxiety behavior through glucocorticoid receptors. Biol Psychiatry. 2016;80:479–89. doi: 10.1016/j.biopsych.2016.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simard S, Shail P, MacGregor J, EL Sayed M, Duman RS, Vaccarino FM, et al. Fibroblast growth factor 2 is necessary for the antidepressant effects of fluoxetine. PLoS One. 2018;13:e0204980. doi: 10.1371/journal.pone.0204980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller Smith K, Fagel DM, Stevens HE, Rabenstein RL, Maragnoli ME, Ohkubo Y, et al. Deficiency in inhibitory cortical interneurons associates with hyperactivity in fibroblast growth factor receptor 1 mutant mice. Biol Psychiatry. 2008;63:953–62. doi: 10.1016/j.biopsych.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Eren-Kocak E, Turner CA, Watson SJ, Akil H. Short-hairpin RNA silencing of endogenous fibroblast growth factor 2 in rat hippocampus increases anxiety behavior. Biol Psychiatry. 2011;69:534–40. doi: 10.1016/j.biopsych.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scearce-Levie K, Roberson ED, Gerstein H, Cholfin JA, Mandiyan VS, Shah NM, et al. Abnormal social behaviors in mice lacking Fgf17. Genes Brain Behav. 2008;7:344–54. doi: 10.1111/j.1601-183X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- 15.Fagel DM, Ganat Y, Cheng E, Silbereis J, Ohkubo Y, Ment LR, et al. Fgfr1 is required for cortical regeneration and repair after perinatal hypoxia. J Neurosci. 2009;29:1202–11. doi: 10.1523/JNEUROSCI.4516-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner CA, Calvo N, Frost DO, Akil H, Watson SJ. The fibroblast growth factor system is downregulated following social defeat. Neurosci Lett. 2008;430:147–50. doi: 10.1016/j.neulet.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks LR, Enix CL, Rich SC, Magno JA, Lowry CA, Tsai PS. Fibroblast growth factor deficiencies impact anxiety-like behavior and the serotonergic system. Behav Brain Res. 2014;264:74–81. doi: 10.1016/j.bbr.2014.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terauchi A, Durlacher E, Pitino J, Umemori H. Neuronal fibroblast growth factor 22 signaling during development, but not in adults, is involved in anhedonia. Neuroreport. 2020;31:125–30. doi: 10.1097/WNR.0000000000001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens HE, Smith KM, Rash BG, Vaccarino FM. Neural stem cell regulation, fibroblast growth factors, and the developmental origins of neuropsychiatric disorders. Front Neurosci. 2010;4:59. doi: 10.3389/fnins.2010.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terwisscha van Scheltinga AF, Bakker SC, Kahn RS, Kas MJ. Fibroblast growth factors in neurodevelopment and psychopathology. Neuroscientist. 2013;19:479–94. doi: 10.1177/1073858412472399. [DOI] [PubMed] [Google Scholar]
- 21.Turner CA, Akil H, Watson SJ, Evans SJ. The fibroblast growth factor system and mood disorders. Biol Psychiatry. 2006;59:1128–35. doi: 10.1016/j.biopsych.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 22.Wang T, Zeng Z, Hu Z, Zheng L, Li T, Li Y, et al. FGFR2 is associated with bipolar disorder: a large-scale case-control study of three psychiatric disorders in the Chinese Han population. World J Biol Psychiatry. 2012;13:599–604. doi: 10.3109/15622975.2011.650203. [DOI] [PubMed] [Google Scholar]
- 23.Kato M, Okugawa G, Wakeno M, Takekita Y, Nonen S, Tetsuo S, et al. Effect of basic fibroblast growth factor (FGF2) gene polymorphisms on SSRIs treatment response and side effects. Eur Neuropsychopharmacol. 2009;19:718–25. doi: 10.1016/j.euroneuro.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Lee M, Kang Y, Suk K, Schwab C, Yu S, McGeer PL. Acidic fibroblast growth factor (FGF) potentiates glial-mediated neurotoxicity by activating FGFR2 IIIb protein. J Biol Chem. 2011;286:41230–45. doi: 10.1074/jbc.M111.270470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azim K, Raineteau O, Butt AM. Intraventricular injection of FGF-2 promotes generation of oligodendrocyte-lineage cells in the postnatal and adult forebrain. Glia. 2012;60:1977–90. doi: 10.1002/glia.22413. [DOI] [PubMed] [Google Scholar]
- 26.Chadashvili T, Peterson DA. Cytoarchitecture of fibroblast growth factor receptor 2 (FGFR-2) immunoreactivity in astrocytes of neurogenic and non-neurogenic regions of the young adult and aged rat brain. J Comp Neurol. 2006;498:1–15. doi: 10.1002/cne.21009. [DOI] [PubMed] [Google Scholar]
- 27.Zheng W, Nowakowski RS, Vaccarino FM. Fibroblast growth factor 2 is required for maintaining the neural stem cell pool in the mouse brain subventricular zone. Dev Neurosci. 2004;26:181–96. doi: 10.1159/000082136. [DOI] [PubMed] [Google Scholar]
- 28.Bansal R, Magge S, Winkler S. Specific inhibitor of FGF receptor signaling: FGF-2-mediated effects on proliferation, differentiation, and MAPK activation are inhibited by PD173074 in oligodendrocyte-lineage cells. J Neurosci Res. 2003;74:486–93. doi: 10.1002/jnr.10773. [DOI] [PubMed] [Google Scholar]
- 29.Collette JC, Choubey L, Smith KM. -Glial and stem cell expression of murine Fibroblast Growth Factor Receptor 1 in the embryonic and perinatal nervous system. PeerJ. 2017;5:e3519. doi: 10.7717/peerj.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang W, Balordi F, Su N, Chen L, Fishell G, Hebert JM. Astrocyte activation is suppressed in both normal and injured brain by FGF signaling. Proc Natl Acad Sci USA. 2014;111:E2987–2995. doi: 10.1073/pnas.1320401111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savchenko E, Teku GN, Boza-Serrano A, Russ K, Berns M, Deierborg T, et al. FGF family members differentially regulate maturation and proliferation of stem cell-derived astrocytes. Sci Rep. 2019;9:9610. doi: 10.1038/s41598-019-46110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hisaoka K, Tsuchioka M, Yano R, Maeda N, Kajitani N, Morioka N, et al. Tricyclic antidepressant amitriptyline activates fibroblast growth factor receptor signaling in glial cells: involvement in glial cell line-derived neurotrophic factor production. J Biol Chem. 2011;286:21118–28. doi: 10.1074/jbc.M111.224683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith KM, Maragnoli ME, Phull PM, Tran KM, Choubey L, Vaccarino FM. Fgfr1 inactivation in the mouse telencephalon results in impaired maturation of interneurons expressing parvalbumin. PLoS One. 2014;9:e103696. doi: 10.1371/journal.pone.0103696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia L, Zhai M, Wang L, Miao D, Zhu X, Wang W. FGF2 blocks PTSD symptoms via an astrocyte-based mechanism. Behav Brain Res. 2013;256:472–80. doi: 10.1016/j.bbr.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 35.Feng D, Guo B, Liu G, Wang B, Wang W, Gao G, et al. FGF2 alleviates PTSD symptoms in rats by restoring GLAST function in astrocytes via the JAK/STAT pathway. Eur Neuropsychopharmacol. 2015;25:1287–99. doi: 10.1016/j.euroneuro.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 36.Sandau US, Alderman Z, Corfas G, Ojeda SR, Raber J. Astrocyte-specific disruption of SynCAM1 signaling results in ADHD-like behavioral manifestations. PLoS One. 2012;7:e36424. doi: 10.1371/journal.pone.0036424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osborne DM, Sandau US, Jones AT, Vander Velden JW, Weingarten AM, Etesami N, et al. Developmental role of adenosine kinase for the expression of sex-dependent neuropsychiatric behavior. Neuropharmacology. 2018;141:89–97. doi: 10.1016/j.neuropharm.2018.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neniskyte U, Gross CT. Errant gardeners: glial-cell-dependent synaptic pruning and neurodevelopmental disorders. Nat Rev Neurosci. 2017;18:658–70. doi: 10.1038/nrn.2017.110. [DOI] [PubMed] [Google Scholar]
- 39.Stogsdill JA, Eroglu C. The interplay between neurons and glia in synapse development and plasticity. Curr Opin Neurobiol. 2017;42:1–8. doi: 10.1016/j.conb.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Todd RD, Botteron KN. Is attention-deficit/hyperactivity disorder an energy deficiency syndrome? Biol Psychiatry. 2001;50:151–8. doi: 10.1016/S0006-3223(01)01173-8. [DOI] [PubMed] [Google Scholar]
- 41.Stevens HE. In this issue/abstract thinking: glial contributions to childhood psychiatric disorders, here and there, September 2009. J Am Acad Child Adolesc Psychiatry. 2009;48:871–2. doi: 10.1097/CHI.0b013e3181ae0a1b. [DOI] [PubMed] [Google Scholar]
- 42.Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, et al. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–74. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- 43.Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
- 44.Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, et al. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006;26:8609–21. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, Chambon P. Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci USA. 1996;93:10887–90. doi: 10.1073/pnas.93.20.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis LK. Bridging molecular genetics and epidemiology to better understand sex differences in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2018;83:e55–e57. doi: 10.1016/j.biopsych.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–14. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 48.Alamed J, Wilcock DM, Diamond DM, Gordon MN, Morgan D. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat Protoc. 2006;1:1671–9. doi: 10.1038/nprot.2006.275. [DOI] [PubMed] [Google Scholar]
- 49.Gumusoglu SB, Hing BWQ, Chilukuri ASS, Dewitt JJ, Scroggins SM, Stevens HE. Chronic maternal interleukin-17 and autism-related cortical gene expression, neurobiology, and behavior. Neuropsychopharmacology. 2020;45:1008–17. doi: 10.1038/s41386-020-0640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lussier SJ, Stevens HE. Delays in GABAergic interneuron development and behavioral inhibition after prenatal stress. Dev Neurobiol. 2016;76:1078–91. doi: 10.1002/dneu.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arisi GM, Ruch M, Foresti ML, Mukherjee S, Ribak CE, Shapiro LA. Astrocyte alterations in the hippocampus following pilocarpine-induced seizures in aged rats. Aging Dis. 2011;2:294–300. [PMC free article] [PubMed] [Google Scholar]
- 52.Ippolito DM, Eroglu C. Quantifying synapses: an immunocytochemistry-based assay to quantify synapse number. J Vis Exp. 2010;45:e2270. doi: 10.3791/2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M, et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci USA. 2010;107:14875–80. doi: 10.1073/pnas.1004282107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–5. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 55.Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, et al. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- 56.Tani H, Dulla CG, Farzampour Z, Taylor-Weiner A, Huguenard JR, Reimer RJ. A local glutamate-glutamine cycle sustains synaptic excitatory transmitter release. Neuron. 2014;81:888–900. doi: 10.1016/j.neuron.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liang SL, Carlson GC, Coulter DA. Dynamic regulation of synaptic GABA release by the glutamate-glutamine cycle in hippocampal area CA1. J Neurosci. 2006;26:8537–48. doi: 10.1523/JNEUROSCI.0329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schousboe A, Scafidi S, Bak LK, Waagepetersen HS, McKenna MC. Glutamate metabolism in the brain focusing on astrocytes. Adv Neurobiol. 2014;11:13–30. doi: 10.1007/978-3-319-08894-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scholze AR, Foo LC, Mulinyawe S, Barres BA. BMP signaling in astrocytes downregulates EGFR to modulate survival and maturation. PLoS One. 2014;9:e110668. doi: 10.1371/journal.pone.0110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Christensen C, Lauridsen JB, Berezin V, Bock E, Kiselyov VV. The neural cell adhesion molecule binds to fibroblast growth factor receptor 2. FEBS Lett. 2006;580:3386–90. doi: 10.1016/j.febslet.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 61.Li S, Christensen C, Kohler LB, Kiselyov VV, Berezin V, Bock E. Agonists of fibroblast growth factor receptor induce neurite outgrowth and survival of cerebellar granule neurons. Dev Neurobiol. 2009;69:837–54. doi: 10.1002/dneu.20740. [DOI] [PubMed] [Google Scholar]
- 62.Chiavaroli A, Recinella L, Ferrante C, Martinotti S, Vacca M, Brunetti L, et al. Effects of central fibroblast growth factor 21 and irisin in anxiety-like behavior. J Biol Regul Homeost Agents. 2017;31:797–802. [PubMed] [Google Scholar]
- 63.Turner CA, Lyons DM, Buckmaster CL, Aurbach EL, Watson SJ, Schatzberg AF, et al. Neural cell adhesion molecule peptide mimetics modulate emotionality: pharmacokinetic and behavioral studies in rats and non-human primates. Neuropsychopharmacology. 2019;44:356–63. doi: 10.1038/s41386-018-0052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kazdoba TM, Leach PT, Crawley JN. Behavioral phenotypes of genetic mouse models of autism. Genes Brain Behav. 2016;15:7–26. doi: 10.1111/gbb.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berton O, Ramos A, Chaouloff F, Mormde P. Behavioral reactivity to social and nonsocial stimulations: A multivariate analysis of six inbred rat strains. Behav Genet. 1997;27:155–66. doi: 10.1023/A:1025641509809. [DOI] [PubMed] [Google Scholar]
- 66.Maeta K, Hattori S, Ikutomo J, Edamatsu H, Bilasy SE, Miyakawa T, et al. Comprehensive behavioral analysis of mice deficient in Rapgef2 and Rapgef6, a subfamily of guanine nucleotide exchange factors for Rap small GTPases possessing the Ras/Rap-associating domain. Mol Brain. 2018;11:27. doi: 10.1186/s13041-018-0370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsuzaki T, Yoshihara T, Ohtsuka T, Kageyama R. Hes1 expression in mature neurons in the adult mouse brain is required for normal behaviors. Sci Rep. 2019;9:8251. doi: 10.1038/s41598-019-44698-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suliman-Lavie R, Title B, Cohen Y, Hamada N, Tal M, Tal N, et al. Pogz deficiency leads to transcription dysregulation and impaired cerebellar activity underlying autism-like behavior in mice. Nat Commun. 2020;11:5836. doi: 10.1038/s41467-020-19577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomson DM, Mitchell EJ, Openshaw RL, Pratt JA, Morris BJ. Mice lacking melatonin MT2 receptors exhibit attentional deficits, anxiety and enhanced social interaction. J Psychopharmacol. 2021;35:1265–76. doi: 10.1177/02698811211032439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wachi T, Cornell B, Toyo-Oka K. Complete ablation of the 14-3-3epsilon protein results in multiple defects in neuropsychiatric behaviors. Behav Brain Res. 2017;319:31–36. doi: 10.1016/j.bbr.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshida T, Yamagata A, Imai A, Kim J, Izumi H, Nakashima S, et al. Canonical versus non-canonical transsynaptic signaling of neuroligin 3 tunes development of sociality in mice. Nat Commun. 2021;12:1848. doi: 10.1038/s41467-021-22059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fairbanks LA, Newman TK, Bailey JN, Jorgensen MJ, Breidenthal SE, Ophoff RA, et al. Genetic contributions to social impulsivity and aggressiveness in vervet monkeys. Biol Psychiatry. 2004;55:642–7. doi: 10.1016/j.biopsych.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 73.Kambe Y, Thi TN, Hashiguchi K, Sameshima Y, Yamashita A, Kurihara T, et al. The dorsal hippocampal protein targeting to glycogen maintains ionotropic glutamate receptor subunits expression and contributes to working and short-term memories in mice. J Pharm Sci. 2022;148:108–15. doi: 10.1016/j.jphs.2021.10.008. [DOI] [PubMed] [Google Scholar]
- 74.Mederos S, Sanchez-Puelles C, Esparza J, Valero M, Ponomarenko A, Perea G. GABAergic signaling to astrocytes in the prefrontal cortex sustains goal-directed behaviors. Nat Neurosci. 2021;24:82–92. doi: 10.1038/s41593-020-00752-x. [DOI] [PubMed] [Google Scholar]
- 75.Regan SL, Williams MT, Vorhees CV. Review of rodent models of attention deficit hyperactivity disorder. Neurosci Biobehav Rev. 2022;132:621–37. doi: 10.1016/j.neubiorev.2021.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dinh Duong TA, Hoshiba Y, Saito K, Kawasaki K, Ichikawa Y, Matsumoto N, et al. FGF Signaling Directs the Cell Fate Switch from Neurons to Astrocytes in the Developing Mouse Cerebral Cortex. J Neurosci. 2019;39:6081–94. doi: 10.1523/JNEUROSCI.2195-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stanimirovic DB, Ball R, Small DL, Muruganandam A. Developmental regulation of glutamate transporters and glutamine synthetase activity in astrocyte cultures differentiated in vitro. Int J Dev Neurosci. 1999;17:173–84. doi: 10.1016/S0736-5748(99)00028-3. [DOI] [PubMed] [Google Scholar]
- 78.Bernardinelli Y, Muller D, Nikonenko I. Astrocyte-synapse structural plasticity. Neural Plast. 2014;2014:232105. doi: 10.1155/2014/232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stork T, Sheehan A, Tasdemir-Yilmaz OE, Freeman MR. Neuron-glia interactions through the Heartless FGF receptor signaling pathway mediate morphogenesis of Drosophila astrocytes. Neuron. 2014;83:388–403. doi: 10.1016/j.neuron.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Farhy-Tselnicker I, van Casteren ACM, Lee A, Chang VT, Aricescu AR, Allen NJ. Astrocyte-Secreted Glypican 4 Regulates Release of Neuronal Pentraxin 1 from Axons to Induce Functional Synapse Formation. Neuron. 2017;96:428–45.e413. doi: 10.1016/j.neuron.2017.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, et al. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci USA. 2011;108:E440–449. doi: 10.1073/pnas.1104977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valentine GW, Sanacora G. Targeting glial physiology and glutamate cycling in the treatment of depression. Biochem Pharm. 2009;78:431–9. doi: 10.1016/j.bcp.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Woodbury-Farina MA. The importance of glia in dealing with stress. Psychiatr Clin North Am. 2014;37:679–705. doi: 10.1016/j.psc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 85.Sanacora G, Banasr M. From pathophysiology to novel antidepressant drugs: Glial contributions to the pathology and treatment of mood disorders. Biol Psychiatry. 2013;73:1172–9. doi: 10.1016/j.biopsych.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guizzetti M, Zhang X, Goeke C, Gavin DP. Glia and neurodevelopment: Focus on fetal alcohol spectrum disorders. Front Pediatr. 2014;2:123. doi: 10.3389/fped.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zeidan-Chulia F, Salmina AB, Malinovskaya NA, Noda M, Verkhratsky A, Moreira JC. The glial perspective of autism spectrum disorders. Neurosci Biobehav Rev. 2014;38:160–72. doi: 10.1016/j.neubiorev.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 88.Crawford JD, Chandley MJ, Szebeni K, Szebeni A, Waters B, Ordway GA. Elevated GFAP protein in anterior cingulate cortical white matter in males with autism spectrum disorder. Autism Res. 2015;8:649–57. doi: 10.1002/aur.1480. [DOI] [PubMed] [Google Scholar]
- 89.Laurence JA, Fatemi SH. Glial fibrillary acidic protein is elevated in superior frontal, parietal and cerebellar cortices of autistic subjects. Cerebellum. 2005;4:206–10. doi: 10.1080/14734220500208846. [DOI] [PubMed] [Google Scholar]
- 90.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 91.Tafazoli S, O’Neill J, Bejjani A, Ly R, Salamon N, McCracken JT, et al. 1H MRSI of middle frontal gyrus in pediatric ADHD. J Psychiatr Res. 2013;47:505–12. doi: 10.1016/j.jpsychires.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oades RD, Myint AM, Dauvermann MR, Schimmelmann BG, Schwarz MJ. Attention-deficit hyperactivity disorder (ADHD) and glial integrity: An exploration of associations of cytokines and kynurenine metabolites with symptoms and attention. Behav Brain Funct. 2010;6:32. doi: 10.1186/1744-9081-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hoogman M, Muetzel R, Guimaraes JP, Shumskaya E, Mennes M, Zwiers MP, et al. Brain Imaging of the Cortex in ADHD: A Coordinated analysis of large-scale clinical and population-based samples. Am J Psychiatry. 2019;176:531–42. doi: 10.1176/appi.ajp.2019.18091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Naaijen J, Lythgoe DJ, Amiri H, Buitelaar JK, Glennon JC. Fronto-striatal glutamatergic compounds in compulsive and impulsive syndromes: a review of magnetic resonance spectroscopy studies. Neurosci Biobehav Rev. 2015;52:74–88. doi: 10.1016/j.neubiorev.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 95.Abbink MR, van Deijk AF, Heine VM, Verheijen MH, Korosi A. The involvement of astrocytes in early-life adversity induced programming of the brain. Glia. 2019;67:1637–53. doi: 10.1002/glia.23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang F, Lin YA, Kannan S, Kannan RM. Targeting specific cells in the brain with nanomedicines for CNS therapies. J Control Release. 2016;240:212–26. doi: 10.1016/j.jconrel.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hamed AM, Kauer AJ, Stevens HE. Why the diagnosis of attention deficit hyperactivity disorder matters. Front Psychiatry. 2015;6:168. doi: 10.3389/fpsyt.2015.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.