Abstract
Early life phenology is a crucial factor for population dynamics in a climate change scenario. As such, understanding how the early life cycle of marine fishes is influenced by key oceanic and climate drivers is of chief importance for sustainable fisheries. This study documents interannual changes in early life phenology of two commercial flatfishes: European flounder (Platichthys flesus) and common sole (Solea solea) from 2010 to 2015 based on otolith microstructure. Using GAMs, we looked for correlations of the North Atlantic Oscillation (NAO), Eastern Atlantic pattern (EA), sea surface temperature (SST), chlorophyl a concentration (Chla) and upwelling (Ui) variation with the onset of hatch, metamorphosis, and benthic settlement day. We concluded that higher SST, more intensive upwelling, and EA were coincident with a later the onset of each stage, while increasing NAO induces an earlier onset of each stage. Although similar to S. solea, P. flesus showed a more complex interaction with the environmental drivers, most possibly because it is at its southern limit of its distribution. Our results highlight the complexity of the relationship between climate conditions and fish early life history, particularly those with complex life cycles that include migrations between coastal areas and estuaries.
Subject terms: Ichthyology, Biooceanography, Marine biology
Introduction
Climate change is significantly affecting the distribution, population dynamics and phenology of marine fishes at a global scale1,2, with contrasting effects throughout their life cycle3,4. Indeed, fish larvae are in general more sensitive to environmental variability, being more susceptible to climate change5. Also, heavily exploited fish populations by fisheries have been recognized as less resilient to climate change6–8, particularly those with complex life cycles such as flatfishes, which usually includes temporal and spatial segregation among life stages9,10. Flatfish juveniles often use coastal areas and estuaries as nursery grounds11–14, which they reach after migration from offshore spawning grounds. These migrations are coincident with the transition from pelagic to benthic life, which occurs through metamorphosis. This represents a critical phase in flatfish life cycle that ought to take place as fast as possible, as long as suitable environmental conditions are met10,15. Connectivity between these habitats plays a key role in recruitment16,17, which is mainly regulated by varying oceanic conditions that drive survival, growth, life history, food abundance and transport18.
As ectotherms, fish are directly exposed to oceanic processes, which are known key drivers in life history traits like growth, metabolic activity, and phenology. Indeed, fish move in response to abiotic (e.g. temperature, salinity, light and turbulence) and biotic factors (variations in abundance of food and predators)19. For instance, large-scale climate drivers such as the North Atlantic Oscillation index (NAO) and the East Atlantic pattern (EA) have been described as major long-term ecological drivers of fish abundance and growth20,21. The NAO is described as the main pattern of atmospheric variability in the Euro Atlantic, with its spatial pattern characterized by a meridional dipole in the pressure field between Azores and Iceland. Its positive phase is characterized by warm and wet conditions and in negative phase, the opposite pattern is registered22. In turn, the EA, also a dipole in the pressure field, when compared with NAO, has its centers of variability shift southern wards and more zonally oriented, and is also related to environmental variability in southern Europe20. On the other hand, seawater temperature modulates fish metabolic activity and thus, early life development processes like spawning, hatching, metamorphosis and settlement14,23,24. Additional extrinsic constraints for fish early life stages include oceanic phenomena like the wind-driven rising of nutrient-rich colder waters (i.e., upwelling25) and food availability26.
Changes in the onset and duration of fish early life history events such as hatching, metamorphosis and benthic settlement, have been reported at various temporal and spatial scales12,14,27,28. These studies were based on extracting temporally resolved information from sagittae otoliths (ear stones), whose microstructural landmarks allow for a precise estimation of early life history traits with a daily resolution12,14. However, the relationship between interannual variability in early life history and environmental variability has received far less attention10,28,29, which nonetheless provides a long-term link between fish early life history and climate variations.
This study uses two flatfishes as model species—the European flounder (Platichthys flesus; Linnaeus 1758) and the common sole (Solea solea, Linnaeus 1758), which have a broad geographical distribution in the North Atlantic: from the White Sea to the Mediterranean and Black Sea (72°N–40°N) for flounder12,30, and from the North Sea to Senegal (67°N–17°N) for sole14. These species share a life cycle typical of flatfishes that includes offshore batch spawning, after which pelagic larvae develop and metamorphose into benthic juveniles. During this period, they are transported towards coastal areas by favorable currents and wind-driven circulation31,32, and settle near estuaries and shallow coastal areas, where juveniles usually concentrate. Despite being models for several studies involving larval transport, juvenile recruitment and connectivity12,14,33,34 there is a significant lack of knowledge on the interannual variability in early life history when facing changing oceanic and atmospheric processes, which will necessarily better inform management and protection measures. These species were chosen given their reported shifts in geographical distribution towards higher latitudes in response to the warming of the sea over the last decades11,35.
The aim of this study was to investigate the influence of climate and oceanic variability on early life history events of two highly commercially valued flatfishes (European flounder and common sole) on the Portuguese Atlantic coast over a 6-year period (2010–2015), to test the hypothesis that variability in extrinsic forcing resulted in changes in fish hatching, metamorphosis and settlement phenology.
Material and methods
Study area and fish sampling
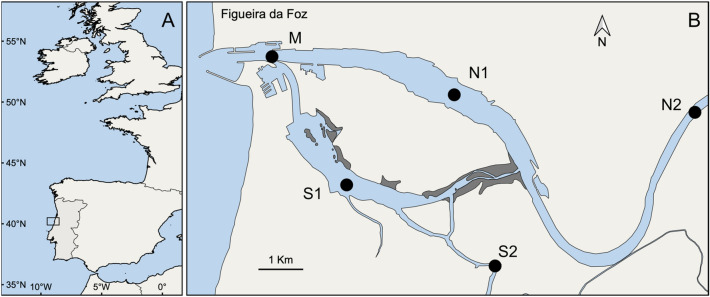
The Portuguese coast is located on the Iberian Peninsula and is the northern limit of the Canary Current Upwelling System (Fig. 1A). This area is characterized by seasonal variability in currents and water temperature, which include a summer wind-driven upwelling-type shelf circulation with associated equatorward surface currents36, a winter poleward surface circulation designated as Iberian Poleward Current (IPC) and a surface water mass fed by river runoff (Western Iberia Buoyant Plume; WIBP)25,37. Such highly dynamic oceanic conditions are known to influence the connectivity and survival of fish early life stages25,29.
Figure 1.
Geographical location of the Portuguese Atlantic coast (A) and the sampling stations in the Mondego estuary (B), denoted by the letters M, N1, N2, S1 and S2. Maps were created using QGIS 3.10.10 software (https://qgis.org).
Juvenile 0-group P. flesus and S. solea were obtained in the Mondego estuary, located in the western Atlantic coast of Portugal (40º 08’ N, 8º 50’ W) (Fig. 1B). This temperate estuary has an area of 8.6 Km2 with nearly 75% of intertidal mudflats, and is divided in two hydrologically distinct arms (northern and southern) in its terminal part, which join again near the mouth38. Previous work has established the estuary as a key nursery area for this and other marine fish species13,14,29,32,38,39. Sampling occurred during spring/summer from 2010 to 2015 in 5 stations (Fig. 1) to ensure that the whole estuary was represented. Fish samples were obtained at night with a 2-m beam trawl equipped with one tickler chain and 5 mm mesh size in the cod end. At each station, 3 hauls were towed at a speed of 2 knots, covering a minimum area of 500 m2, and the fish caught were immediately transferred to iceboxes.
Once in the laboratory, we registered fish total length (TL; mm) and wet weight (WW, g), and removed the respective sagittae otoliths, cleaned and stored them in eppendorfs until further analysis. This study was carried out following the recommendations of Directive 2010/63/EU, and fish handling protocols were approved by the Portuguese National Authority for Animal Health (DGAV; Ref 0421/000/000/2017). No live animals were used in this study, and both species are neither protected nor endangered. Reporting in the manuscript follows the recommendations in the ARRIVE guidelines40.
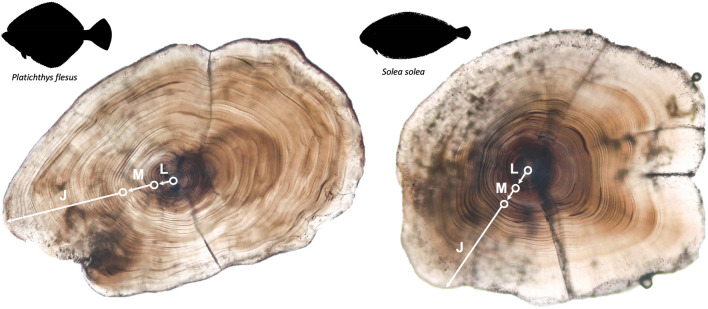
From the selected specimens, otoliths were mounted sulcus up on microscope slides with Crystalbond 509 and polished on the sagittal plane using P4000 (5 µm) Buehler silicon carbide grinding paper until the daily rings were clearly visible from the core to the edge. All daily increment counts were made using a light microscope at 100× and 400× magnifications for the peripheral areas, and at 1000× magnifications for the core. Otolith microstructure analysis was used to determine fish age, the main early life history events: hatching, metamorphosis and benthic settlement day, and the duration of the larval and metamorphosis stages, using the back-calculation method12,14,27 (Fig. 2). Briefly, the pelagic larval stage (L) corresponds to the otolith core and the metamorphosis stage to the daily rings between the innermost and outermost accessory growth centers (accessory primordia) (M). The benthic juvenile stage (J) corresponds to the number of daily rings between the first uninterrupted ring after the outermost accessory primordia and the otolith edge. Hatch day was determined as the first ring in the otolith core, day at metamorphosis the first day when accessory primordia are accounted for, and settlement day the first ring of the juvenile benthic stage (Fig. 2).
Figure 2.
0-group European flounder (Platichthys flesus) and common sole (Solea solea) sagittae otoliths at 50× magnifications, highlighting the daily growth increments and the early ontogenic development stages: L—larval (pelagic), M—metamorphosis, J—juvenile (benthic). Circles indicate the respective onset day for each stage.
Environmental data
For the 6-year study period (2010–2015), a set of environmental variables, were collected with different temporal resolutions and spatial scale of operation: Sea surface temperature (SST; °C), Chlorophyll a (Chl a; mg L−1), North Atlantic index (NAO), East Atlantic pattern (EA) and Upwelling index (Ui; m3 s−1 km−1). These variables were selected based on their role as key drivers of marine life history traits like growth, metabolic activity and phenology25,29. SST and Chl a data consisted of 8 day means obtained within a radius of 20 km near the Mondego estuary. Data was obtained from US NASA Oceancolor Web (https://oceancolor.gsfc.nasa.gov). NAO and EA data consisted of monthly means and were acquired from the US NOAA National Weather Service Climate Prediction Centre (https://www.cpc.ncep.noaa.gov). Lastly, monthly means of the Upwelling index were obtained for the Figueira da Foz FNMOC station from the Spanish Oceanographic Institute (IOE; http://www.indicedeafloramiento.ieo.es).
Data analysis
A total of 219 flounder and 204 sole 0-group juveniles were used in this study (Table 1). Previous work had determined the linear relationship between fish total length and otolith length in both species28,41. Hence, a linear regression model between total length (TL) and the estimated Age (days) in both species was performed, considering the year of capture (Year) as a fixed factor, and including the interaction between Age and Year to investigate possible year-dependent growth patterns. Then, Kruskal–Wallis ANOVAs followed by pairwise Wilcoxon tests with a Bonferroni correction were performed to investigate interannual differences in hatch, metamorphosis, and settlement dates for both species.
Table 1.
Summary of the number of Platichthys flesus and Solea solea specimens per year (n), with the respective range in total length (TL, cm), wet weight (WW, g) and age (days).
| Year | Platichthys flesus | Solea solea | ||||||
|---|---|---|---|---|---|---|---|---|
| n | TL | WW | Age | n | TL | WW | Age | |
| 2010 | 10 | 4.7–7.5 | 0.8–4.2 | 86–107 | 29 | 5.0–9.8 | 0.7–5.3 | 66–120 |
| 2011 | 34 | 5.1–9.3 | 0.8–8.3 | 88–146 | 34 | 2.8–10.2 | 0.2–7.9 | 68–89 |
| 2012 | 49 | 4.9–9.9 | 0.8–7.5 | 88–153 | 44 | 5.0–9.9 | 0.3–4.7 | 61–94 |
| 2013 | 50 | 1.9–9.1 | 0.3–7.1 | 49–142 | 32 | 2.5–7.7 | 0.1–8.3 | 45–87 |
| 2014 | 28 | 3.0–9.8 | 0.6–9.1 | 70–150 | 26 | 3.7–9.5 | 0.3–6.3 | 54–82 |
| 2015 | 47 | 3.1–8.1 | 0.9–2.6 | 69–126 | 39 | 3.2–9.8 | 0.83–5.2 | 51–87 |
The relationship between the onset of each stage and the environmental factors (SST, Chla, NAO, EA and Ui) was investigated with generalized additive models (GAM; Gaussian family, Identity link function), as preliminary analyses determined a non-linear relationship between the dependent and independent variables38,42. Chla data was log-transformed to reduce the skewness of the original data. Prior to further analyses, the collinearity between the environmental predictors was assessed with a Pearson correlation coefficient. The only case when two variables had a correlation coefficient above 0.4 occurred in flounder hatch day—NAO and Ui, which led us to not include the latter. All environmental variables were included in the subsequent analyses. Considering the different temporal resolutions of the environmental data, all hatch, metamorphosis, and settlement dates were assigned to the corresponding 8-day (SST, Chla) and monthly (NAO, EA, Ui) values.
GAM development included testing the correlation between all possible combinations of the environmental factors (NAO, EA, Ui, SST, Chla) and year of capture on the early life history of flounder and sole, and the models with lower AIC were selected. These analyses were performed in R software (R development Core Team, 2018) using the mgcv package43, considering a 5% significance level.
Results
Environmental conditions
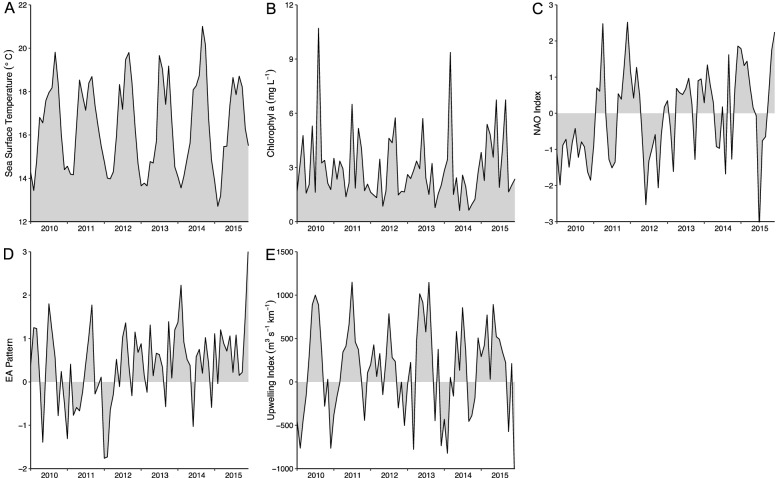
From 2010 to 2015, the annual mean of SST was higher in 2014 (16.70 °C) and lower in 2013 (15.90 °C) (Fig. 3A). For Chlorophyll a, the highest value was registered in 2015 (3.79 mg m−3) and the lowest in 2012 (2.52 mg m−3) (Fig. 3B). Coastal upwelling ranged between 198.4 m3 s−1 km−1 (2015) and the particularly low values in 2010 and 2014 (50.3 m3 s−1 km−1 and 2.8 m3 s−1 km−1, respectively; Fig. 3C). Both the NAO and EA showed a general increasing trend in mean annual values towards the end of our study period (Fig. 3D, E). The mean NAO index varied between 0.43 in 2015 and − 1.15 in 2010 (Fig. 3D), while EA ranged between 0.57 in 2014 − 0.02 in 2011 (Fig. 3E).
Figure 3.
Monthly values for the selected environmental factors in the Portuguese Atlantic coast (2010–2015): (A) Sea Surface Temperature (SST; °C); (B) Chlorophyll a (Chla; mg L−1); (C) North Atlantic Oscillation index (NAO); (D) East Atlantic Pattern (EA); (E) Upwelling index (Ui; m3 s−1 km−1).
Otolith microstructure analysis
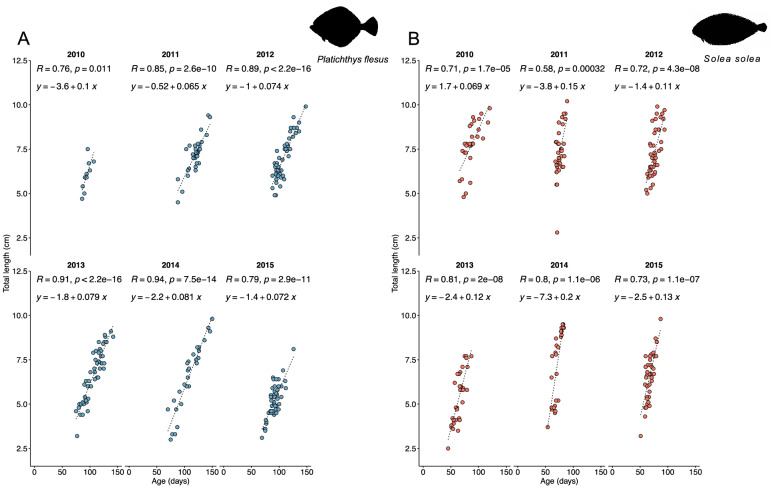
The linear regression between total length and age showed that for P. flesus, both age and year of capture were significant (p = 2.2e−16 and p = 1.447e−06, respectively). However, their interaction was not significant (p = 0.3481), indicating that the slopes were similar between years (Fig. 4A). In S. solea, a significant interaction for age and year was found: 2010 was different from all years, 2011 and 2012 were different from 2015 (2015p = 2.2e−16, p = 2.395e−06 and p = 0.0003305, respectively) (Fig. 4B).
Figure 4.
Linear regression between total length (cm) and age (days) for 0-group juvenile P. flesus (A) and S. solea (B) between 2010 and 2015.
Early life history
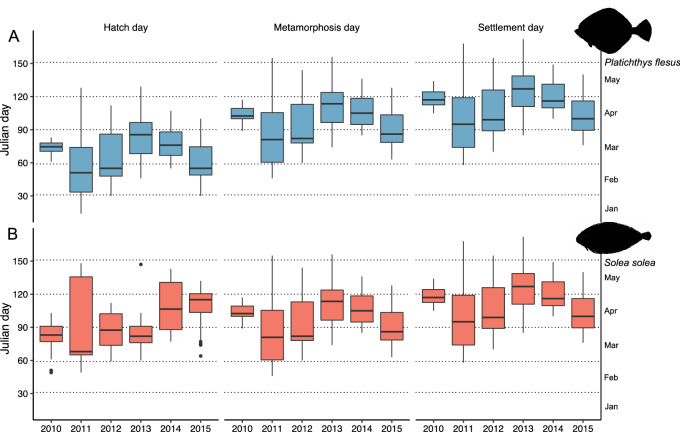
Flounder hatch onset day varied between years (H = 38.561; p = 2.91e−07) (Fig. 5). The earliest and latest hatch day were registered in 2011 (22nd January and 24th April). In more detail, hatch day distributions were significantly different between 2011 and 2013 (p = 0.0003), 2012 and 2103 (p = 0.001), 2011 and 2014 (p = 0.028), 2013 and 2015 (p = 5.7e−05), and 2014 and 2015 (p = 0.009) (Table S1). Significant differences were also found between years on the onset of metamorphosis in P. flesus (H = 36.864; p = 6.377e−07) (Fig. 5). In line with the previous stage, the onset period of metamorphosis was also shorter in 2010. The earliest and latest days at metamorphosis also occurred in 2011, at 28 of January and 10 of April, respectively. Finally, differences between years for the onset of settlement (H = 39.602; p = 1.796e−07) were also found (Fig. 5). The widest onset period of settlement was in 2011 (27 of February to 17 June), and the shortest in 2010 (15 of April to 14 of May). Significant differences between years for metamorphosis and settlement were also observed for the same years as in hatch day (Table S1).
Figure 5.
Hatch, metamorphosis, and settlement day distribution boxplots for (A) Platichthys flesus and (B) Solea solea from 2010 to 2015 (in Julian days). The secondary Y-axis shows the corresponding month. The boxplot horizontal thick lines represent the median value and each box the interquartile range from 25 to 75%. The bars represent the largest value within 1.5 times the interquartile range above the 75th percentile and the smallest below the 25th percentile respectively, and the outliers are represented by the black dots. The secondary Y-axis represent the respective month.
Solea solea also presented different hatch day periods between years (H = 39.768; p = 1.663e−07) (Fig. 5), which was smaller in 2013, with a duration from the 1st of March to the 27th of May, and longer in 2011, from the 18th of February to 29th of May. The onset of metamorphosis was also significantly different between years (H = 37.403; p = 4.972e−07): the onset period of this stage was longer in 2014 (11th of April to 27th of September) and shorter in 2012 (21st of March to 20th of May). Finally, the day at settlement was also different between years (H = 38.359; p = 3.195e−07), with the cohort of 2011 presenting the widest period (16th of March to 4th of June), and the 2013 cohort the shortest one (9th of April to 27th of May). Significant differences were found for the three stages between 2014 and 2014, 2013 and 2013, and between 2015 and 2010, 2012 and 2013 (Table S2).
Response to the main environmental drivers
The relationship between several environmental factors (SST, Chla, Ui, NAO and EA) was investigated for all P. flesus and S. solea early life stages along the period between 2010 and 2015, using GAMs.
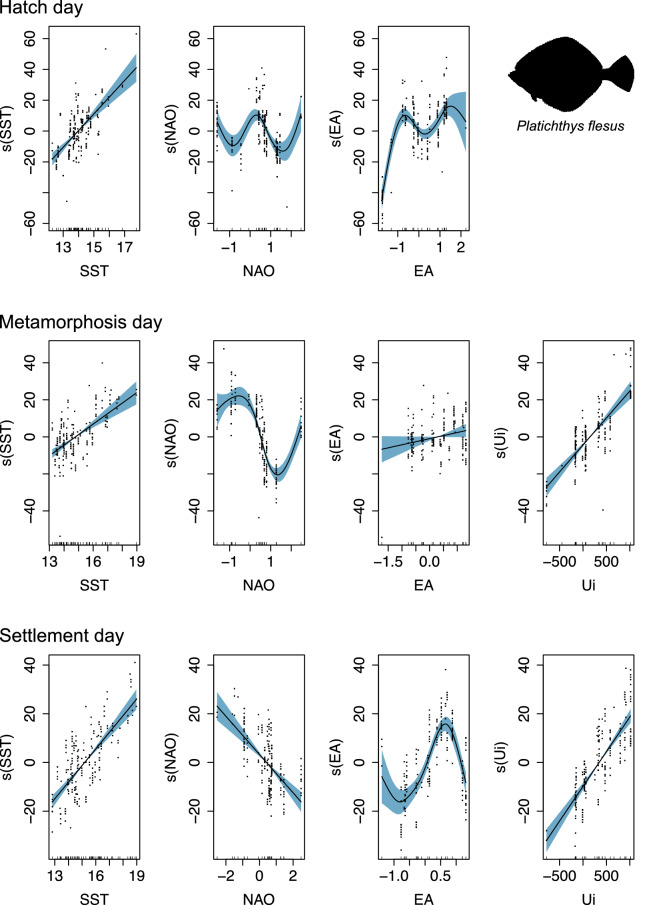
For flounder, the hatch, metamorphosis and settlement dates increased when higher seawater temperatures, more intense upwelling events and positive EA occurred, leading to a later development (Fig. 6). In contrast, flounder hatch, metamorphosis and settlement phenology decreased when increasing NAO was registered. Adjusted R2 and deviance explained for the three models regarding each life stage were always superior to 0.77 and 78.10%, respectively (Table 2).
Figure 6.
Generalized Additive Models (GAM) smoother response curves of the environmental drivers explaining the variation in Platichthys flesus hatch, metamorphosis, and settlement day for the period between 2010 and 2015. SST—Sea Surface Temperature (°C); Ui—Upwelling index (m3 s−1 km−1); NAO—North Atlantic Oscillation; EA—Eastern Atlantic Pattern. Black dots depict the partial residuals for each term. The uncertainty bands denote the 95% confidence intervals for each term.
Table 2.
Generalized additive model (GAM) outputs for parametric coefficients and smooth terms testing the interaction between environmental variables and Platichthys flesus hatch, metamorphosis, and settlement days between 2010 and 2015.
| Life Stage | Parametric coefficients | Smooth terms | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Estimate | Std. Error | t value | Pr( >|t|) | Covariate | edf | Ref.df | F | p value | R2 (adj) | Deviance explained (%) | |
| Hatch | 2010 | 58.909 | 6.033 | 9.764 | < 2E−16* | SST | 1.000 | 1.000 | 89.430 | < 2E−16* | 0.77 | 78.10 |
| 2011 | − 8.749 | 6.918 | − 1.265 | 2.07E−01 | NAO | 3.508 | 4.111 | 12.260 | 2.58E−09* | |||
| 2012 | 22.076 | 7.982 | 2.766 | 6.20E−03* | EA | 3.585 | 4.186 | 46.430 | < 2E−16* | |||
| 2013 | 11.375 | 5.280 | 2.154 | 3.24E−02* | ||||||||
| 2014 | 9.568 | 6.336 | 1.510 | 1.33E−01 | ||||||||
| 2015 | 16.164 | 7.042 | 2.295 | 2.27E−02* | ||||||||
| Metamorphosis | 2010 | 79.914 | 4.198 | 19.038 | < 2E−16* | SST | 1.000 | 1.000 | 52.915 | 5.90E−12* | 0.84 | 84.60 |
| 2011 | 4.367 | 4.418 | 0.988 | 3.24E−01 | NAO | 3.160 | 3.768 | 62.262 | < 2E−16* | |||
| 2012 | 28.931 | 4.432 | 6.527 | 5.72E−10* | EA | 1.000 | 1.000 | 3.254 | 7.28E−02* | |||
| 2013 | 25.535 | 5.510 | 4.634 | 6.57E−06* | Ui | 1.000 | 1.000 | 107.682 | < 2E−16* | |||
| 2014 | 23.537 | 4.522 | 5.205 | 4.93E−07* | ||||||||
| 2015 | 15.638 | 4.823 | 3.242 | 1.40E−03* | ||||||||
| Settlement | 2010 | 107.498 | 3.883 | 27.687 | < 2E−16* | SST | 1.000 | 1.000 | 155.010 | < 2E−16* | 0.85 | 85.80 |
| 2011 | 10.303 | 4.261 | 2.418 | 1.65E−02* | NAO | 1.000 | 1.000 | 63.760 | 5.34E−14* | |||
| 2012 | 11.281 | 3.996 | 2.823 | 5.23E−03* | EA | 3.209 | 3.897 | 32.410 | < 2E−16* | |||
| 2013 | 10.236 | 4.560 | 2.245 | 2.59E−02* | Ui | 1.000 | 1.000 | 187.370 | < 2E−16* | |||
| 2014 | − 1.581 | 4.333 | − 0.365 | 7.16E−01 | ||||||||
| 2015 | − 8.700 | 5.133 | − 1.695 | 9.16E−02* | ||||||||
Significant terms are highlighted with *.
In sole, there was also a consistent concordance among life stages of SST and the large-scale climate index NAO (Fig. 7). Like flounder, higher water temperatures were coincident with later hatch, metamorphosis and settlement, while increasing NAO index values were found with earlier life stages. Adjusted R2 and deviance explained for the three models regarding each life stage were always superior to 0.63 and 64.40%, respectively (Table 3).
Figure 7.
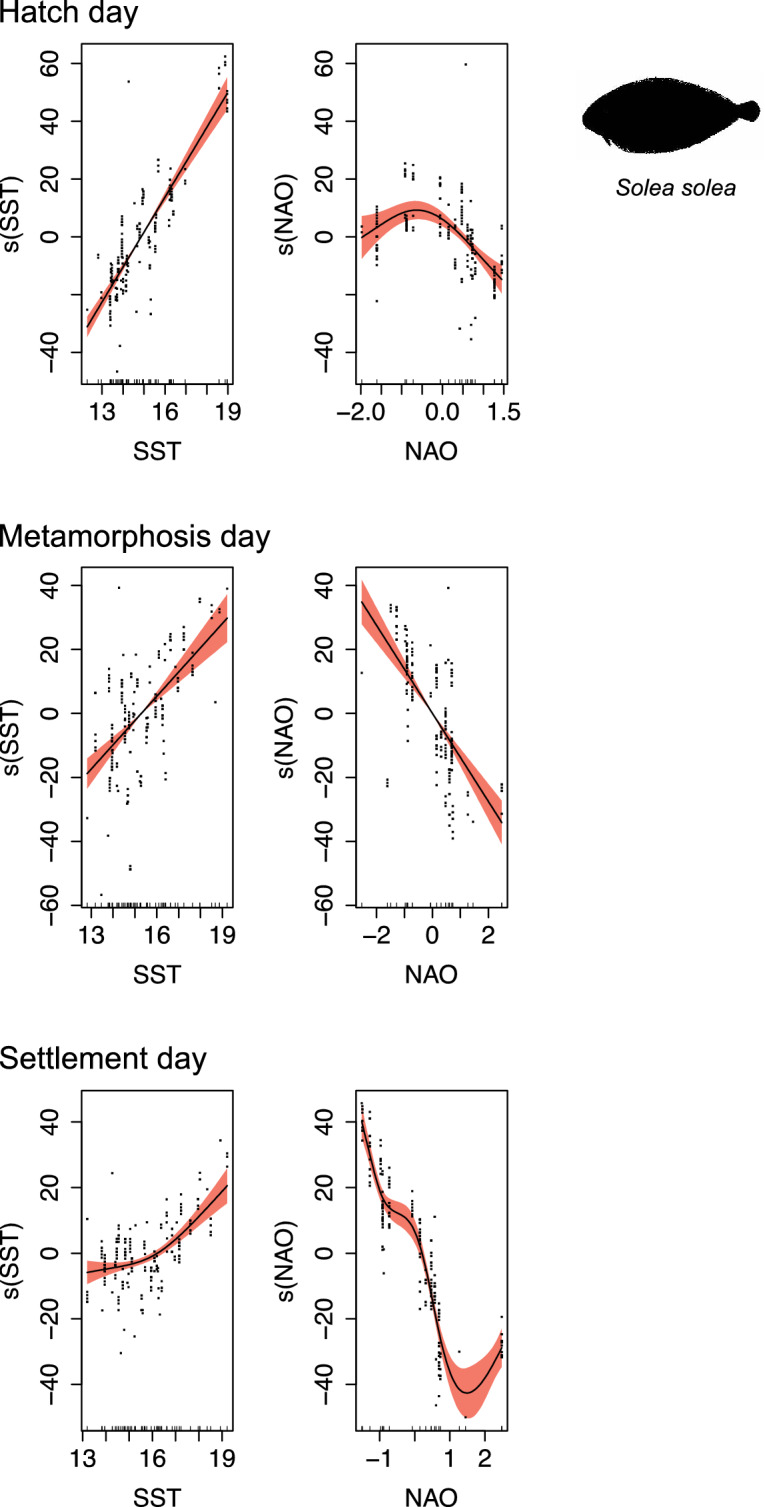
Generalized Additive Models (GAM) smoother response curves of the environmental drivers explaining the variation in Solea solea hatch, metamorphosis, and settlement day for the period between 2010 and 2015. SST—Sea Surface Temperature (°C); NAO—North Atlantic Oscillation. Black dots depict the partial residuals for each term. The uncertainty bands denote the 95% confidence intervals for each term.
Table 3.
Generalized additive model (GAM) outputs for parametric coefficients and smooth terms testing the interaction between environmental variables and Solea solea hatch, metamorphosis, and settlement days between 2010 and 2015.
| Life stage | Parametric coefficients | Smooth terms | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Estimate | Std. Error | t value | Pr( >|t|) | Covariate | edf | Ref.df | F | p value | R2 (adj) | Deviance explained (%) | |
| Hatch | 2010 | 70.354 | 2.465 | 28.543 | < 2E−16* | SST | 1.000 | 1.000 | 318.880 | < 2E−16* | 0.81 | 81.70 |
| 2011 | 8.599 | 3.371 | 2.551 | 1.15E−02* | NAO | 1.930 | 2.419 | 18.330 | 7.43E−09* | |||
| 2012 | 34.296 | 3.476 | 9.866 | < 2E−16* | ||||||||
| 2013 | 24.135 | 3.067 | 7.869 | 2.37E−13* | ||||||||
| 2014 | 37.394 | 3.105 | 12.044 | < 2E−16* | ||||||||
| 2015 | 33.574 | 3.549 | 9.461 | < 2E−16* | ||||||||
| Metamorphosis | 2010 | 83.097 | 2.924 | 28.416 | < 2E−16* | SST | 1.000 | 1.000 | 63.710 | 6.53E−14* | 0.63 | 64.40 |
| 2011 | 33.462 | 3.781 | 8.850 | 5.46E−16* | NAO | 1.000 | 1.000 | 100.970 | < 2E−16* | |||
| 2012 | 37.981 | 3.991 | 9.517 | < 2E−16* | ||||||||
| 2013 | 32.446 | 4.041 | 8.028 | 9.29E−14* | ||||||||
| 2014 | 33.557 | 3.952 | 8.491 | 5.29E−15* | ||||||||
| 2015 | 52.541 | 3.802 | 13.820 | < 2E−16* | ||||||||
| Settlement | 2010 | 88.190 | 1.869 | 47.180 | < 2E−16* | SST | 1.613 | 2.017 | 32.110 | 3.79E−13* | 0.88 | 88.80 |
| 2011 | 37.184 | 2.889 | 12.870 | < 2E−16* | NAO | 3.922 | 4.581 | 171.690 | < 2E−16* | |||
| 2012 | 34.789 | 2.432 | 14.310 | < 2E−16* | ||||||||
| 2013 | 52.199 | 3.031 | 17.220 | < 2E−16* | ||||||||
| 2014 | 37.049 | 2.460 | 15.060 | < 2E−16* | ||||||||
| 2015 | 56.298 | 2.742 | 20.530 | < 2E−16* | ||||||||
Significant terms are highlighted with *.
Discussion
This study documented how key climate and oceanic factors may be linked to early life phenology of two commercially important flatfishes—P. flesus and S. solea in the Portuguese Atlantic coast. Although previous work addressed the onset of early life stages at larger spatial scales12,14, very few have attempted to understand how environmental drivers are correlated with the early life cycle of S. solea and P. flesus10,44. As such, this work provides a stepping stone for the comparison between two flatfishes with distinct biogeographical affinity (P. flesus—temperate; S. solea—sub-tropical), disentangling the relationship between climate and oceanic conditions on fish early life history, which coincides with their migration from the spawning grounds towards estuarine nurseries. Studies of this nature are important in the view of climate change, contributing to a better understanding of how species early life history and phenology are shaped by climate variability, as well as to predicting possible consequences to species recruitment13,28,29 and stock structure in the long term. Unravelling the impacts of climate variability on the early life history of marine fishes will also contribute to the development of more sustainable management and exploitation strategies10.
Our results showed that the early life cycle of P. flesus (i.e. hatching, metamorphosis, and settlement) suffered changes when different SST and upwelling conditions were recorded, as well as with the large climate drivers NAO and EA pattern. For S. solea, SST and the NAO where the only relevant environmental drivers across early life stages. Interestingly, SST and the NAO had a consistent relationship among species and throughout their early life history.
Water temperature plays a key role in the life cycle of marine fishes, being responsible for most metabolic processes involved in vital processes, somatic growth, movements, and survival12,14. For P. flesus our results showed that hatching occurred at sea surface temperatures between 13.9 °C (2nd of March 2010) and 15.3 °C (14th of January 2011), and in S. solea between 14.6 and 15.3 °C (18th March 2014 and 18th February 2011, respectively). In both species, hatching occurred near winter’s end and early spring, which corroborates previous studies, corresponding to the reproductive period of these species14. In fact, it has been demonstrated that reproductive migrations and spawning on several species, including those in the current study, are influenced by water temperature14,45,46. A synchronous effect of SST on P. flesus and on S. solea early life history was observed, with a positive linear relationship between SST and the onset of each stage, indicating a correlation between warmer water temperatures and a later onset hatch, metamorphosis, and settlement days. Indeed, both species displayed inter-annual variability in hatch dates, and the later hatching in both species took place in 2011, which corresponded to the warmest water temperatures experienced (P. flesus, 17.8 °C; S. solea, 19.0 °C).
One of the key aspects of climate change on marine ectotherms concerns the effect of warming water temperatures on early life stages. Indeed, studies have demonstrated the potential of global warming to reduce population connectivity and dispersal in several marine fishes4,23, by reducing the pelagic larval duration (PLD) and increasing growth rates24. Such changes in development, as well starvation, may induce a lower swimming capacity47 which can end up affecting the dispersal and recruitment48. Our study provides additional insights on the expected impacts of global warming, which include the delaying of hatch phenology in these two commercially important species. Such temporal displacement might, for instance, lead to the decoupling of predator–prey interactions (i.e., flatfish larvae and zooplankton abundance49,50), as well as to higher energy expenditure to overcome the energetic costs of survival in a warmer ocean51. Still, the effect of ocean warming will vary according to the position within a species’ geographical distribution gradient, as populations show distinct phenotypical responses in relation to the latitudinal temperature cline. For instance, several authors showed that the timing and duration of early life history stages (as well as growth) of these species and those with similar life cycle vary with the prevailing temperature regimes12,14,29,33, showing a negative relationship between water temperature and hatch day across species geographical distribution ranges12,14.
There is a consensus that spawning and early life stages have the narrowest thermal window within a fish life cycle52. As such, relatively small variations in water temperature as those reported here are likely to contribute to a temporal shift in spawning, and consequently, in larval hatching, metamorphosis, and settlement. Previous work has indicated that earlier spawning in warmer waters occurs mainly through faster gonadal development in adults53. However, our results point to the opposite direction, in which warmer waters were coincident with latter hatching in flounder and sole, which can be related to (but not exclusively) reduced fertility and developmental success, egg and spermatozoa morphology and composition54. Still, fish spawning and hatching can also be modulated by the timing and duration of exposure to unexpected water temperatures in a fish life cycle54, as well as other important factors such as day length during pre- and spawning stages55. A similar relationship with water temperature was recently demonstrated for the European seabass Dicentrarchus labrax in the NE Atlantic29.
For P. flesus, earlier spawning migrations in the UK were associated with colder periods46, by avoiding lower than average water temperatures at the estuaries where they reside, and thus maximizing their gonadal growth rates before spawning. Delaying of spawning has also been described in freshwater fishes56, which can be a consequence of diverting energy from gonadal development to cope with increased metabolic costs of a warmer ocean5. Solea solea North Sea populations spawn at water temperature higher than 7 °C, and they are expected to spawn earlier in face of a warming ocean, which leads to an earlier arrival of larvae in nurseries, but at the cost of higher mortality due to slow growth early in spring56. As such, we hypothesize that warming may benefit populations living presently in colder conditions, such as the North Sea (i.e., faster growth rates, earlier maturation), while those living at lower latitudes (i.e., warmer) such as the Portuguese coast might face additional challenges that can ultimately delay their reproductive and early life phenology. A previous study highlighted that S. solea recruitment in the Portuguese coast was higher in “warm” years, while nil recruitment was observed in P. flesus, particularly in the southern Iberian coast, most probably because this species is at its southern limit of distribution, and thus, faces additional thermal stress that hampers their ability to cope with increasing water temperatures57.
The two main large scale atmospheric circulation patterns in the Northeast Atlantic—North Atlantic Oscillation (NAO) and the East Atlantic pattern (EA), were correlated with a early life phenology of both flounder and sole. Indeed, they are both known to induce variability in oceanic and atmospheric circulation20, inter-annual variations in SST, precipitation, wind speed and direction58,59, and associated biological responses in marine and estuarine areas44. Comparatively to the NAO, the EA is a dipole with a more zonally orientation, with higher influence over southern Europe20. However, the NAO and the EA had a contrasting relationship with flounder and sole early life phenology: while increasing NAO was related with earlier hatching, metamorphosis, and settlement in flounder and sole, increasing EA values were synchronous with later hatching and subsequent stages, but only for flounder.
Analyzing the variation of these two climate patterns in the present study, 2011 was the year in which the NAO reached the highest values (2.52) and EA the lowest (− 0.015), which coincided with the earliest start of all life stages in both species: between January–April for P. flesus, and between January and May for S. solea. From 2010 to 2015, the response to NAO was also similar in both species and life stages. The onset of hatching had a bimodal response, with near zero NAO with later hatches. We also found a negative linear response with metamorphosis and settlement day, indicating that higher NAO is associated with an earlier onset of these stages. Our results match with those recently reported for the European seabass (Dicentrarchus labrax)29, demonstrating a consistent NAO effect on marine fish species with complex life cycles that involve larval migration from coastal spawning sites into inshore nurseries. At higher latitudes, positive NAO phases during winter are associated to warmer weather60, which contribute with more favorable conditions to fish development and growth61. However, its direct influence at more southern areas like the Portuguese coast is comparably lower22, given its combined effect on ocean temperature62, wind and water circulation patterns63. Still, the shortest hatch, metamorphosis, and settlement periods for both species occurred in 2010, which was characterized by a strong negative NAO. This situation is consistent with generalized unfavorable weather conditions, cold water and low primary production, promoting small windows of opportunity for fish individual growth and development64,65.
The East Atlantic pattern is known as a key driver in inter-annual variations of air and ocean temperature, precipitation, and wind, with ecological consequences at local and regional scales58. In our study, the EA pattern had a contrasting trend with the NAO, showing a positive (albeit non-linear) relationship with the onset of hatch, metamorphosis and settlement only on P. flesus, indicating that positive EA values influenced their early life phenology. In southern Europe, positive EA values are associated with warmer temperatures, which match the effect of water temperature, at least for flounder. Thus, the influence of the EA pattern can be felt not only on fish physiology and energy allocation, but also on ecosystem productivity and changes in prey-predator interactions. A recent study highlighted that positive EA values led to decreased population growth in deep-sea scorpaenid fishes (Helicolenus dactylopterus and Pontinus kuhlii), which was mainly due to increased summer temperatures and associated declines in oceanic productivity21. Previous studies have also indicated that both the EA and the NAO have the strongest impacts on atmospheric and oceanic processes in the winter41, which is the reproductive period of both species12,14, and thus, are key drivers for changes in reproductive and early life phenology in marine fishes. However, the EA usually interacts with the NAO, which reflect mainly the latitudinal position and speed of the Atlantic jet stream during the winter66. In this case, a composite negative NAO and positive EA lead to increased storminess in the Iberian Peninsula20, which based on our results, is linked to a delayed hatching, metamorphosis, and settlement in flounder.
In P. flesus, upwelling showed a similar linear positive trend as SST, where more intense upwelling occurred in parallel with a later hatching, metamorphosis, and benthic settlement. This oceanic phenomenon is characterized by the rising of colder and nutrient-rich waters mainly during spring and summer67, promoting phytoplankton blooms that increase the zooplankton production68, enduring higher food availability for the newly hatched larvae. There has been a constant increase in upwelling in the main upwelling systems worldwide, among which the Iberian coast is included68, induced by increasing northerly winds in spring and summer months. This will have a major impact on many biological processes with a seasonal frequency, such as reproduction, early life ontogeny and growth. This is the case of the present work, where more intense upwelling events were related with a later onset of metamorphosis and settlement stages in flounder, which might also cause larvae to be more susceptible to offshore advection, having a direct impact on ocean-estuary connectivity and recruitment.
Connectivity between spawning grounds and estuaries is a key feature in marine fishes, as it shapes population structure, dynamics, and habitat colonization patterns69. In this work, we demonstrated that climate and oceanic variability directly impacted on marine flatfishes’ life cycle, particularly during their early life stages and in the transition between the continental shelf and the estuarine nurseries. This was more evident in P. flesus, which demonstrated a tighter environmental control, most probably because the Portuguese coast is its southernmost limit of distribution. Further investigations on this topic are encouraged, namely broadening the spatial and temporal coverage, given the links between larval and juveniles stages, recruitment, and fish stocks. Our work contributed to the increasing body of evidence that climate and oceanic variability is directly linked to early ontogenic development and connectivity of marine fishes with complex life cycles.
Supplementary Information
Acknowledgements
We would like to thank the colleagues and students who have participated in the fieldwork in the Mondego estuary. Ana Vaz, Filipe Martinho and Ana Lígia Primo are funded by national funds (OE), through Fundação para a Ciência e Tecnologia, I.P. (FCT), in the scope of a PhD Grant (AV; SFRH/BD/137862/2018) and Decree-Law 57/2016 (FM, ALP). This work was supported by the FCT in the scope of the research project “Mytag—Integrating natural and artificial tags to reconstruct fish migrations and ontogenetic niche shifts” (PTDC/MAR-EST/2098/2014), under the Project 9471—Reforçar a Investigação, o Desenvolvimento Tecnológico e a Inovação (Projeto 9471-RIDTI) and subsidized by the European Regional Development Fund (FEDER, POCI-01-0145-FEDER-016787), and by the strategic plan of the Centre for Functional Ecology—Science for People and the Planet (CFE; UIDB/04004/2020), financed by FCT/MCTES through national funds (PIDDAC). Additional support was also obtained via project “RENATURE—Valorization of the Natural Endogenous Resources of the Centro Region” (CENTRO-01-0145-FEDER-000007), funded by the Comissão de Coordenação da Região Centro.
Author contributions
A.V. and F.M. conceptualised the research, performed data analyses and wrote the manuscript text. A.L.P. and D.C. provided assistance with fieldwork, laboratory and statistical analyses. M.P. and F.M. acquired funding. All authors reviewed the manuscript and approved the final version.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ana Vaz and Filipe Martinho.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-30384-7.
References
- 1.Cheung WWL, Sarmiento JL, Dunne J, Frölicher TL, Lam VWY, Palomares ML, Watson RA, Pauly D. Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nat. Clim. Change. 2012;3:1–5. [Google Scholar]
- 2.Pilotto F, Kühn I, Adrian R, Alber R, Alignier A, Andrews C, Bäck J, et al. Meta-analysis of multidecadal biodiversity trends in Europe. Nat. Commun. 2010;11:3486. doi: 10.1038/s41467-020-17171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ong JJL, Rountrey AN, Meeuwig JJ, Newman SJ, Zinke J, Meekan MG. Contrasting environmental drivers of adult and Juvenile growth in a marine fish: Implications for the effects of climate change. Sci. Rep. 2015;5:10859. doi: 10.1038/srep10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rijnsdorp AD, Peck MA, Engelhard GH, Moellmann C, Pinnegar JK. Resolving the effect of climate change on fish populations. ICES J. Mar. Sci. 2009;66(7):1570–1583. doi: 10.1093/icesjms/fsp056. [DOI] [Google Scholar]
- 5.Pankhurst NW, Munday PL. Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 2011;62(9):1015. doi: 10.1071/MF10269. [DOI] [Google Scholar]
- 6.Ainsworth CH, Samhouri JF, Busch DS, Cheung WWL, Dunne J, Okey TA. Potential impacts of climate change on Northeast Pacific marine foodwebs and fisheries. ICES J. Mar. Sci. 2011;68:1217–1229. doi: 10.1093/icesjms/fsr043. [DOI] [Google Scholar]
- 7.Morrongiello JR, Horn PL, Ó Maolagáin C, Sutton PJH. Synergistic effects of harvest and climate drive synchronous somatic growth within key New Zealand fisheries. Glob. Change Biol. 2021;27(7):1470–1484. doi: 10.1111/gcb.15490. [DOI] [PubMed] [Google Scholar]
- 8.Ottersen G, Hjermann DO, Stensenth NC. Changes in spawning stocks structure strengthen the link between climate and recruitment in a heavily fished cod (Gadus morhua) stock. Fish. Oceanogr. 2006;15(3):230–243. doi: 10.1111/j.1365-2419.2006.00404.x. [DOI] [Google Scholar]
- 9.Cheung WWL, Oyinlola MA. Vulnerability of flatfish and their fisheries to climate change. J. Sea Res. 2018;140:1–10. doi: 10.1016/j.seares.2018.06.006. [DOI] [Google Scholar]
- 10.Fedewa EJ, Miller JA, Hurst TP. Pre-settlement process of northern rock sole (Lepidopsetta polyxystra) in relation to interannual variability in the Gulf of Alaska. J. Sea Res. 2016;111:25–36. doi: 10.1016/j.seares.2015.11.008. [DOI] [Google Scholar]
- 11.Cabral HN, Vasconcelos R, Vinagre C, França S, Fonseca V, Maia A, Reis-Santos P, Lopes M, Ruano M, Campos J, Freitas V, Santos PT, Costa MJ. Relative importance of estuarine flatfish nurseries along the Portuguese coast. J. Sea Res. 2007;57:209–217. doi: 10.1016/j.seares.2006.08.007. [DOI] [Google Scholar]
- 12.Martinho F, van der Veer HW, Cabral HN, Pardal MA. Juvenile nursery colonization patterns for the European flounder (Platichthys flesus): A latitudinal approach. J. Sea Res. 2013;84:61–69. doi: 10.1016/j.seares.2013.07.014. [DOI] [Google Scholar]
- 13.Primo AL, Vaz AC, Crespo D, Costa F, Pardal MA, Martinho F. Contrasting links between growth and survival in the early life stages of two flatfish species. Estuar. Coast. Shelf Sci. 2021;254:107314. doi: 10.1016/j.ecss.2021.107314. [DOI] [Google Scholar]
- 14.Vaz, A., Scarcella, G., Pardal, M. A. & Martinho, F. Water temperature gradients drive early life-history patterns of the common sole (Solea solea L.) in the Northeast Atlantic and Mediterranean. Aquat. Ecol.53(5) (2019).
- 15.Geffen A, van der Veer HW, Nash R. The cost of metamorphosis in flatfishes. J. Sea Res. 2007;58(1):35–45. doi: 10.1016/j.seares.2007.02.004. [DOI] [Google Scholar]
- 16.Cowen RK, Lwiza KMM, Sponaugle S, Paris CB, Olson DB. Connectivity in marine populations: Open or closed? Science. 2000;287:857–859. doi: 10.1126/science.287.5454.857. [DOI] [PubMed] [Google Scholar]
- 17.Gillanders BM, Black BA, Meekan MG, Morrison MA. Climatic effects on the growth of a temperate reef fish from the Southern Hemisphere: a biochronological approach. Mar. Biol. 2012;159:1327–1333. doi: 10.1007/s00227-012-1913-x. [DOI] [Google Scholar]
- 18.Treml EA, Ford JR, Black KP, Swearer SE. Identifying the key biophysical drivers, connectivity outcomes, and metapopulation consequences of larval dispersal in the sea. Mov. Ecol. 2015;3(1):345. doi: 10.1186/s40462-015-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson RN. Behaviour and the distribution of flatfishes. J. Sea Res. 1997;37(1997):241–256. doi: 10.1016/S1385-1101(97)00019-1. [DOI] [Google Scholar]
- 20.Mellado-Cano J, Barriopedro D, García-Herrera R, Trigo RM, Hernández A. Examining the North Atlantic Oscillation, East Atlantic Pattern, and jet variability since 1685. J. Clim. 2019;32:6285–6298. doi: 10.1175/JCLI-D-19-0135.1. [DOI] [Google Scholar]
- 21.Tanner SE, Giacomello E, Menezes GM, Mirasole A, Neves J, Sequeira V, Vasconcelos R, Vieira AR, Morrongiello JR. Marine regime shifts impact synchrony of deep-sea fish growth in the northeast Atlantic. Oikos. 2020;129(12):1781–1794. doi: 10.1111/oik.07332. [DOI] [Google Scholar]
- 22.Trigo RM, Osborn TJ, Corte-Real JM. The North Atlantic Oscillation influence on Europe: Climate impacts and associated physical mechanisms. Clim. Res. 2002;20:9–17. doi: 10.3354/cr020009. [DOI] [Google Scholar]
- 23.Leis JM, Caselle JE, Bradbury IR, Kristiansen T, Llopiz JK, Miller MJ, O'Connor MI, Paris CB, Shanks AL, Sogard SM, Swearer SE, Treml EA, Vetter RD, Warmer RR. Does fish larval dispersal differ between high and low latitudes? Proc. R. Soc. B Biol. Sci. 2013;280(1759):20130327. doi: 10.1098/rspb.2013.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raventos N, Torrado H, Arthur R, Alcoverro T, Macpherson E. Temperature reduces fish dispersal as larvae grow faster to their settlement size. J. Anim. Ecol. 2021;90(6):1419–1432. doi: 10.1111/1365-2656.13435. [DOI] [PubMed] [Google Scholar]
- 25.Santos AMP, Chicharo A, Santos A, Moita T, Oliveira PB, Peliz A, Re P. Physical-biological interactions in the life history of small Pelagic Fish in the Western Iberia upwelling ecosystem. Prog. Oceanogr. 2007;74(2):192–209. doi: 10.1016/j.pocean.2007.04.008. [DOI] [Google Scholar]
- 26.Le Pape O, Bonhommeau S. The food limitation hypothesis for juvenile marine fish. Fish Fish. 2015;16(3):373–398. doi: 10.1111/faf.12063. [DOI] [Google Scholar]
- 27.Fox C, Geffen A, Taylor N, Davison P, Rossetti H, Nash R. Birth-date selection in early life stage of plaice Pleuronectes platessa in the eastern Irish Sea (British Isles) Mar. Ecol. Prog. Ser. 2007;345:255–269. doi: 10.3354/meps06967. [DOI] [Google Scholar]
- 28.Joh M, Wada A. Inter-annual and spatial difference in hatch date and settlement date distribution and planktonic larval duration in yellow striped flounder Pseudopleuronectes Herzensteini. J. Sea Res. 2018;137:26–34. doi: 10.1016/j.seares.2018.04.002. [DOI] [Google Scholar]
- 29.Pinto M, Monteiro JN, Crespo D, Costa F, Rosa J, Primo AL, Pardal MA, Martinho F. Influence of oceanic and climate conditions on the early life history of European seabass Dicentrarchus labrax. Mar. Environ. Res. 2021;169:105362. doi: 10.1016/j.marenvres.2021.105362. [DOI] [PubMed] [Google Scholar]
- 30.Morais P, Dias E, Babaluk J, Antunes C. The migration patterns of the European flounder Platichthys flesus (Linnaeus, 1758) (Pleuronectidae, Pisces) at the southern limit of its distribution range: Ecological implications and fishery management. J. Sea Res. 2011;65:235–246. doi: 10.1016/j.seares.2010.11.001. [DOI] [Google Scholar]
- 31.Lacroix G, Maes GE, Bolle LJ, Volckaert F. Modelling dispersal dynamics of the early life stages of a marine flatfish (Solea Solea L.) J. Sea Res. 2013;84(C):13–25. doi: 10.1016/j.seares.2012.07.010. [DOI] [Google Scholar]
- 32.Tanner SE, Teles-Machado A, Martinho F, Peliz A, Cabral HN. Modelling larval dispersal Dynamics of common sole (Solea solea) along the western Iberian coast. Prog. Oceanogr. 2017;156:78–90. doi: 10.1016/j.pocean.2017.06.005. [DOI] [Google Scholar]
- 33.Amorim E, Ramos S, Elliott M, Bordalo AA. Immigration and early life stages recruitment of the European flounder (Platichthys flesus) to an estuarine nursery: The influence of environmental factors. J. Sea Res. 2016;107(Part 1):56–66. doi: 10.1016/j.seares.2015.07.005. [DOI] [Google Scholar]
- 34.Vasconcelos RP, Reis-Santos P, Costa MJ, Cabral HN. Connectivity between estuaries and marine environment: Integrating metrics to assess estuarine nursery function. Ecol. Indic. 2011;11(5):1123–1133. doi: 10.1016/j.ecolind.2010.12.012. [DOI] [Google Scholar]
- 35.Orio A, Bersgstrom U, Florin AB, Lehmann A, Sics I, Casini M. Spatial contraction of demersal fish populations in a large marine ecosystem. J. Biogeogr. 2019;46(3):633–645. doi: 10.1111/jbi.13510. [DOI] [Google Scholar]
- 36.Peliz A, Rosa TL, Santos AMP, Pissarra JL. Fronts, jets, and counter-flows in the Western Iberian upwelling system. J. Mar. Syst. 2002;35:61–77. doi: 10.1016/S0924-7963(02)00076-3. [DOI] [Google Scholar]
- 37.Teles-Machado A, Peliz A, McWilliams JC, Dubert J, Le Cann B. Circulation on the Northwestern Iberian Margin: Swoddies. Prog. Oceanogr. 2016;140:116–133. doi: 10.1016/j.pocean.2015.09.011. [DOI] [Google Scholar]
- 38.Primo AL, Azeiteiro UM, Marques SC, Martinho F, Baptista J, Pardal MA. Colonization and nursery habitat use patterns of larval and juvenile flatfish species in a small temperate estuary. J. Sea. Res. 2013;76(C):126–134. doi: 10.1016/j.seares.2012.08.002. [DOI] [Google Scholar]
- 39.Vasconcelos RP, Reis-Santos P, Tanner S, Maia A, Latkoczy C, Günther D, Costa MJ, Cabral H. Evidence of estuarine nursery origin of five coastal fish species along the Portuguese coast through otolith elemental fingerprints. Estuar. Coast. Shelf Sci. 2008;79:317–327. doi: 10.1016/j.ecss.2008.04.006. [DOI] [Google Scholar]
- 40.du Sert NP, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Peterson OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Wurbel H. The ARRIVAGE guidelines 2.0: updated guidelines for reporting animal research. J. Physiol. Lond. 2020;598(18):3793–3801. doi: 10.1113/JP280389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trigo RM, Valente MA, Trigo IF, Miranda PMA, Ramos AM, Paredes D, García-Herrera R. The impact of north atlantic wind and cyclone trends on European precipitation and significant wave height in the Atlantic. Ann. N. Y. Acad. Sci. 2008;1146(1):212–234. doi: 10.1196/annals.1446.014. [DOI] [PubMed] [Google Scholar]
- 42.Murase H, Nagashima H, Yonezaki S, Matsukura R, Kitakado T. Application of a generalized additive model (GAM) to reveal relationships between environmental factors and distributions of Pelagic Fish and Krill: a Case Study in Senday Bay, Japan. ICES J. Mar. Sci. 2009;66(6):1417–1424. doi: 10.1093/icesjms/fsp105. [DOI] [Google Scholar]
- 43.Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2011;73(1):3–36. doi: 10.1111/j.1467-9868.2010.00749.x. [DOI] [Google Scholar]
- 44.Tanner SE, Vieira AR, Vasconcelos RP, Dores S, Azevedo M, Cabral HN, Morrongiello JR. Regional climate, primary productivity and fish biomass drive growth cariation and population resilience in a small pelagic fish. Ecol. Indic. 2019;103:530–541. doi: 10.1016/j.ecolind.2019.04.056. [DOI] [Google Scholar]
- 45.Almeida JR, Gravato C, Guilermino L. Effects of temperature in juvenile Seabass (Dicentrarchus labrax L.) biomarker responses and behaviour: implications for environmental monitoring. Estuaries Coasts. 2015;38:45–55. doi: 10.1007/s12237-014-9792-7. [DOI] [Google Scholar]
- 46.Sims DW, Wearmouth VJ, Genner MJ, Southward AJ, Hawkins SJ. Low-temperature-driven early spawning migration of a temperate marine fish. J. Anim. Ecol. 2004;73(2):333–341. doi: 10.1111/j.0021-8790.2004.00810.x. [DOI] [Google Scholar]
- 47.Faria AM, Muha T, Morote R, Chicharro MA. Influence of starvation on the critical swimming behaviour of the Senegalensis sole (Solea senegalensis) and its relationship with RNA/DNA ratios during ontogeny. Sci. Mar. 2011;75(1):87–94. doi: 10.3989/scimar.2011.75n1087. [DOI] [Google Scholar]
- 48.Downie AT, Illing B, Faria AM, Rummer JL. Swimming performance of marine fish larvae: review of a universal trait under ecological and environmental pressure. Rev. Fish Biol. Fish. 2020;30:93–108. doi: 10.1007/s11160-019-09592-w. [DOI] [Google Scholar]
- 49.Durant JM, Molinero JC, Ottersen G, Reygondeau G, Stige LC, Langangen Ø. Contrasting effects of rising temperatures on trophic interactions in marine ecosystems. Na. Sci. Rep. 2019;9(1):15213. doi: 10.1038/s41598-019-51607-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stenseth NC, Mysterud A, Ottersen G, Hurrell JW, Chan KS, Lima M. Ecological effects of climate fluctuations. Science. 2002;297(5585):1292–1296. doi: 10.1126/science.1071281. [DOI] [PubMed] [Google Scholar]
- 51.Harrington AM, Clark KF, Hamlin HJ. Expected ocean warming conditions significantly alter the transcriptone of developing postlarval American lobsters (Homarus americanus): Implications for energetic trade-offs. Comp. Biochem. Physiol. D Genom. Proteom. 2020;36:100716. doi: 10.1016/j.cbd.2020.100716. [DOI] [PubMed] [Google Scholar]
- 52.Pörtner HO, Farrell AP. Ecology. Physiol. Clim. Change. Sci. 2008;322(5902):690–692. doi: 10.1126/science.1163156. [DOI] [PubMed] [Google Scholar]
- 53.Drinkwater KF, Beaugrand G, Kaeriyama M, Kim S, Ottersen G, Perry RI, Portner HO, Polovina JJ, Takasuka A. On the processes linking climate to ecosystem changes. J. Mar. Syst. 2010;79:374–388. doi: 10.1016/j.jmarsys.2008.12.014. [DOI] [Google Scholar]
- 54.Alix M, Kjesbu OS, Anderson KC. From Gametogenesis to spawning: How climate-driven warming affects teleost reproductive biology. J. Fish Biol. 2020;97(3):607–632. doi: 10.1111/jfb.14439. [DOI] [PubMed] [Google Scholar]
- 55.Conover DO, Present TMC. Countergradient variation in growth rate: compensation for length of the growing season among Atlantic silversides from different latitudes. Oceanologia. 1990;83:316–324. doi: 10.1007/BF00317554. [DOI] [PubMed] [Google Scholar]
- 56.van de Wolfshaar, K. E., Barbut, L. & Lacroix, G. From spawning to first-year recruitment: the fate of Juvenile Sole Growth and survival under future climate conditions in the North Sea. ICES J. Mar. Sci. (2021).
- 57.Cabral H, Drouineau H, Teles-Machado A, Pierre M, Lepage M, Lobry J, Reis-Santos P, Tanner S. Contrasting impacts of climate change on connectivity and larval recruitment to estuarine nursery areas. Prog. Oceanogr. 2011;196:102608. doi: 10.1016/j.pocean.2021.102608. [DOI] [Google Scholar]
- 58.Iglesias I, Lorenzo MN, Taboada JJ. Seasonal predictability of the East Atlantic Pattern from sea surface temperatures. PLoS ONE. 2014;9(1):86439–86448. doi: 10.1371/journal.pone.0086439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodríguez-Puebla C, Encinas AH, García-Casado LA, Nieto S. Trends in warm days and cold nights over the Iberian Peninsula: relationships to large-scale variables. Clim. Change. 2010;100(3):667–684. doi: 10.1007/s10584-009-9721-0. [DOI] [Google Scholar]
- 60.Hurrell JW, Van Loon H. Decadal variations in climate associated with the North Atlantic oscillation. Clim. Change. 1997;36:301–326. doi: 10.1023/A:1005314315270. [DOI] [Google Scholar]
- 61.Henderson PA, Seaby RM. The role of climate in determining the temporal variation in abundance, recruitment and growth of sole Solea solea in the Bristol Channel. JMBA. 2005;85:197–204. [Google Scholar]
- 62.Rodwell MJ, Rowell DP, Folland CK. Oceanic forcing of the wintertime North Atlantic Oscillation and European Climate. Letters to Nature. 1999;398:320–323. doi: 10.1038/18648. [DOI] [Google Scholar]
- 63.Hurrell JW. Decadal trends in the North Atlantic oscillation: Regional temperatures and precipitation. Sci. 1995;269:676–679. doi: 10.1126/science.269.5224.676. [DOI] [PubMed] [Google Scholar]
- 64.Avalos MR, Ramos JA, Soares M, Ceia FR, Fagundes AI, Gouveia C, Menezes D, Paiva VH. Comparing the foraging strategies of a seabird predator when recovering from drastic climatic event. Mar. Biol. 2017;164:48. doi: 10.1007/s00227-017-3082-4. [DOI] [Google Scholar]
- 65.Wang C, Liu H, Lee SK. The record-breaking cold temperatures during the winter of 2009/2010 in the Northern Hemisphere. Atmos. Sci. Lett. 2010;11(3):161–168. doi: 10.1002/asl.278. [DOI] [Google Scholar]
- 66.Rodrigo FS. Exploring combined influences of Seasonal East Atlantic (EA) and North Atlantic Oscillation (NAO) on the temperature-precipitation relationship in the Iberian Peninsula. Geosciences. 2021;11(5):211. doi: 10.3390/geosciences11050211. [DOI] [Google Scholar]
- 67.Alvarez I, Gommez-Gesteira M, Decastro M, Dias JM. Spatiotemporal evolution of upwelling regime along the western coast of the Iberian Peninsula. J. Geophys. Res. Oceans. 2008;113(C7):C07020. doi: 10.1029/2008JC004744. [DOI] [Google Scholar]
- 68.Demarcq H. Trends in primary production, Sea surface temperature and wind in upwelling systems (1998–2007) Prog. Oceanogr. 2009;83(1):376–385. doi: 10.1016/j.pocean.2009.07.022. [DOI] [Google Scholar]
- 69.Thorrold SR, Latkoczy C, Swart PK, Jones CM. Natal homing in a marine fish metapopulation. Science. 2001;291:297–299. doi: 10.1126/science.291.5502.297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.