Abstract
Chronic kidney disease (CKD) is the most common cause of end-stage renal disease in patients with type 2 diabetes mellitus (T2DM). CKD increases the risk of cardiovascular diseases; therefore, its prevention and treatment are important. The prevention of diabetic kidney disease (DKD) can be achieved through intensive glycemic control and blood pressure management. Additionally, DKD treatment aims to reduce albuminuria and improve kidney function. In patients with T2DM, renin-angiotensin-aldosterone system inhibitors, sodium glucose cotransporter 2 inhibitors, and glucagon-like peptide-1 receptor agonists can delay the progression of DKD. Hence, there is a need for novel treatments that can effectively suppress DKD progression. Finerenone is a first-in-class nonsteroidal mineralocorticoid receptor antagonist with clinically proven efficacy in improving albuminuria, estimated glomerular filtration rate, and risk of cardiovascular events in early and advanced DKD. Therefore, finerenone is a promising treatment option to delay DKD progression. This article reviews the mechanism of renal effects and major clinical outcomes of finerenone in DKD.
Keywords: Renal insufficiency, chronic; Diabetic nephropathies; Finerenone; Mineralocorticoid receptor antagonists; Diabetes mellitus
INTRODUCTION
Diabetic kidney disease (DKD) is a major microvascular complication of diabetes that can progress to chronic kidney disease (CKD) and accounts for approximately 50% of cases of end-stage renal failure [1,2]. The incidence of DKD, including albuminuria or chronic renal disease (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2), is approximately 40% in patients with type 2 diabetes mellitus (T2DM) [3,4]. Currently, several drugs, such as renin-angiotensin-aldosterone system (RAAS) inhibitors and sodium glucose cotransporter 2 inhibitors (SGLT2i), can help delay the progression of DKD to end-stage renal disease [5,6]. However, as the number of patients with diabetes and their life expectancy increases, the incidence of DKD and end-stage renal disease is also on the increase. End-stage renal disease can also increase the risk of cardiovascular disease (CVD) and mortality [7,8]. Therefore, early diagnosis and prevention of DKD, in addition to preventing its progression to end-stage renal disease, are of utmost clinical importance.
DKD can be prevented through intensive glycemic control [5,6]. Furthermore, DKD treatment aims to reduce albuminuria and improve kidney function. In addition to strict glycemic control, lipid management, RAAS inhibitors, SGLT2i, and glucagon-like peptide-1 receptor agonists (GLP1-RA) are generally used in patients with T2DM [9]. Recently, aldosterone- and aldosterone-induced mineralocorticoid receptor (MR) activation, which is mechanistically independent of RAAS, have been reported as key mechanisms in CKD progression [10-12]. MR overactivation not only increases intraglomerular pressure, but also causes inflammation and fibrosis, ultimately leading to the progression of CKD [13].
The use of mineralocorticoid receptor antagonists (MRAs) alone or in combination with angiotensin-converting enzyme inhibitors (ACEi) is effective in reducing albuminuria and delaying a decline in kidney function in patients with T2DM [14-16]. However, despite these renoprotective effects, MRAs have not received attention as suitable agents for the treatment of DKD because of the risk of hyperkalemia and acute renal failure [17,18].
Finerenone is a potent, selective, nonsteroidal MRA (NS-MRA) that is effective in reducing albuminuria and in preventing the progression of acute kidney injury to CKD [19,20]. In addition, finerenone is effective in reducing albuminuria and delaying the decline in eGFR in large-scale clinical trials of patients with DKD [21,22].
The number of patients with DKD is increasing; although multiple drugs can delay the progression of DKD, there are insufficient treatment options that can be used to halt the progression of DKD. It is of interest to determine whether treatment with finerenone is effective in patients with DKD; therefore, this article aimed to review the roles of finerenone in the treatment of DKD.
THE ROLE OF ALDOSTERONE AND MINERALOCORTICOID RECEPTORS IN THE PATHOGENESIS OF DKD
Relative hyperaldosteronism is observed in patients with CKD, regardless of volume expansion. In approximately half of patients on long-term ACEi/angiotensin receptor blocker (ARB) therapy, high serum aldosterone levels, referred to as the “aldosterone breakthrough,” is observed [23]. Hyperaldosteronism is known to have proinflammatory effects. Activation of aldosterone and MRs induces proinflammatory and profibrotic pathways, which are associated not only with CKD but also with CVD, including atherosclerosis [10,24-29].
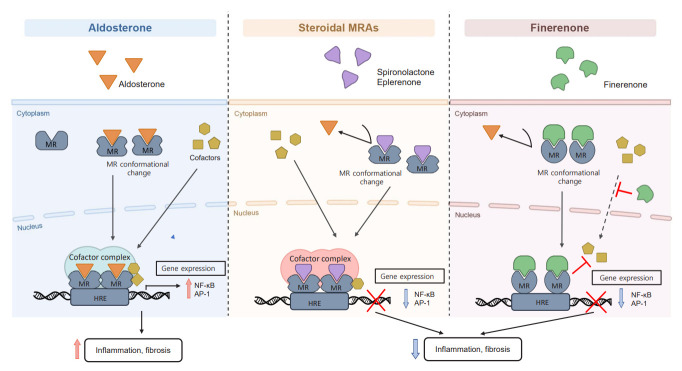
Aldosterone acts through MRs, which are distributed in multiple organs. MR is an intracellular steroid hormone receptor and ligand-induced transcription factor of the nuclear receptor superfamily. Aldosterone, the biological ligand, binds to MRs, and promotes a conformational change and translocation from the cell cytoplasm to the nucleus. The MR then binds to specific hormone response elements, recruits transcriptional cofactors, and induces the transcription or repression of target genes (Fig. 1) [30,31]. MRs can also be activated by non-MR hormones, such as cortisol and progesterone; however, the “aldosterone target cells” of the distal nephron contain 11-β hydroxysteroid dehydrogenase type 2 (11β-HSD2), which cleaves cortisol, allowing aldosterone to preferentially act on MRs [32], and maintain blood pressure and extracellular fluid volume. In addition to the distal nephrons, MRs are distributed around various nonepithelial tissues (podocytes, adipocytes, inflammatory cells, endothelial cells, vascular smooth muscle cells, and cardiomyocytes). Such MRs in nonepithelial tissues do not express 11β-HSD2 and are associated with cardiorenal inflammation and fibrosis [10,13,29,33]. In other words, cortisol-induced MR activation promotes proinflammatory and profibrotic gene expression, and produces reactive oxygen species, which lead to inflammation and fibrosis [34-37]. A cell-specific MR-knockout mouse model identified the roles of MR overactivation in podocytes, vascular cells, inflammatory cells, and fibroblasts in kidney injury. In vivo, spironolactone increases autophagy in podocytes and helps to restore autophagy under mechanical stress [38,39]. In vitro administration of aldosterone to podocytes induces downregulation of nephrin, podocin, podoplanin, and podocalyxin [40]. MR activation in renal fibroblasts contributes to kidney remodeling; moreover, aldosterone promotes fibronectin synthesis in renal fibroblasts [41], and culturing fibroblasts with MRA causes a decrease in extracellular matrix component production [42]. In endothelial cells, aldosterone enhances the expression of intercellular adhesion molecule 1, vascular cell adhesion molecule 1, E-selectin, and monocyte chemoattractant protein 1 [43-45]. Furthermore, aldosterone increases the expression of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 2 (Nox2), p47phox, and p22phox, which are NADPH oxidase subunits, and promotes oxidative injury by activating the Ras-related C3 botulinum toxin substrate (Rac) family of small guanosine triphosphatase 1 (Rac1) [46,47]. Lastly, aldosterone impairs endothelial function by suppressing endothelial nitric oxide synthase phosphorylation [48]. Increased levels of inflammation and fibrosis can result in poor cardiorenal outcomes [49-52]. Therefore, the potential anti-inflammatory and antifibrotic effects of MRAs are expected to have therapeutic effects in cardiorenal diseases.
Figure 1.
Mechanism of steroidal mineralocorticoid receptor antagonists (MRAs) and nonsteroidal MRA finerenone. Aldosterone binds to mineralocorticoid receptors (MRs) and promotes a conformational change after which it is translocated into the nucleus. The MR then binds to specific hormone response elements, recruits transcriptional cofactors, and then initiates the transcription of target genes. MR overactivation promotes the expression of proinflammatory and profibrotic genes and the activation of signaling pathways implicated in the progression of renal disease. Both steroidal and nonsteroidal MRAs bind to MRs, which causes a different conformational change that inhibits aldosterone from binding to MRs. This prevents the downstream transcription of proinflammatory and profibrotic factors. Steroidal MRAs can interact with cofactors that affect gene transcription; hence, they function as partial MR agonists. Finerenone inhibits cofactor recruitment to the MR from the cytoplasm to the nucleus. Inhibition of MR cofactor binding can occur even in the absence of aldosterone. Additionally, the gene regulation profile by finerenone differs from that for steroidal MRAs. The anti-inflammatory and antifibrotic activities of finerenone are more potent than those of steroidal MRAs. HRE, hormone response element; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cell; AP-1, activator protein-1.
MINERALOCORTICOID RECEPTOR ANTAGONISTS
Steroidal mineralocorticoid receptor antagonists
Studies using animal models have shown that selective inhibition of aldosterone reduces proteinuria and nephrosclerosis regardless of renin-angiotensin blockade, whereas aldosterone administration causes such damage to reoccur [53,54].
Spironolactone and eplerenone are steroidal MRAs that have shown therapeutic benefits in CVD (especially heart failure) and improved proteinuria in patients with DKD in a clinical cohort study [14-16]. The effect of eplerenone on albuminuria in patients with T2DM was first reported in 2006 [55]. A multicenter, randomized, double-blind, placebo-controlled, parallelgroup trial that evaluated the effect of eplerenone (50 and 100 mg) combined with an ACEi (enalapril) on albuminuria in 268 patients with albuminuric T2DM, showed that the combined treatment significantly decreased albuminuria. Despite this finding, the use of steroidal MRAs is limited in patients with advanced CKD owing to the risk of hyperkalemia [17,18,55-57].
Nonsteroidal MRAs
Owing to concerns regarding the safety profile of steroidal MRAs, several novel NS-MRAs have been developed, including esaxerenone, AZD9977, apararenone, KBP-5074, and finerenone, which are currently under development or have been approved for clinical use [12]. This review focuses specifically on finerenone, which has been recently approved by the U.S. Food and Drug Administration (FDA) [58] and the European Medicines Agency for adult patients with T2DM and CKD.
Finerenone acts as a bulky, passive NS-MRA and suppresses MR signaling at multiple levels [30,59]. When finerenone binds to MRs which causes a conformational change that inhibits aldosterone or other mineralocorticoids from binding to MRs. It also decreases the accumulation and turnover of MRs in the nucleus and suppresses the recruitment of transcriptional cofactors (Fig. 1) [30,31]. The renoprotective effects of finerenone in DKD are mediated via its anti-inflammatory, antifibrotic, and antioxidative actions that are associated with preservation of the renal structure (Fig. 2) [26,60]. Clinical trials of the beneficial renocardiovascular effects of finerenone are described in more detail in later sections.
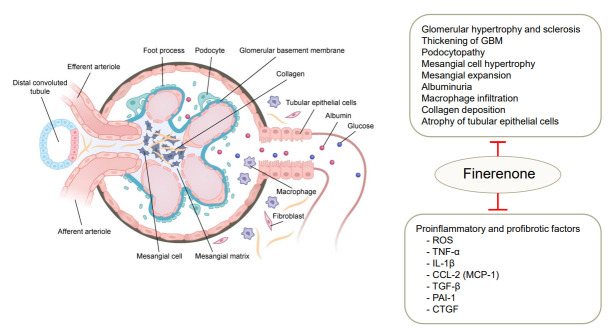
Figure 2.
Renoprotective effects of finerenone in diabetic kidney. In diabetic kidney, mineralocorticoid receptor (MR) activation induces deleterious glomerular alternation and tubulointerstitial fibrosis. Finerenone binds to MRs and prevents the transcription of proinflammatory and profibrotic factors in renal cells including podocytes, mesangial cells, macrophage, endothelial cells, and fibroblasts. GBM, glomerular basement membrane; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; CCL-2, C-C motifchemokine ligand 2; MCP-1, monocyte chemoattractant protein-1; TGF-β, transforming growth factor β; PAI-1, plasminogen activator inhibitor-1; CTGF, connective tissue growth factor.
Finerenone vs. steroidal MRAs
Finerenone has pharmacological properties that are different from those of steroidal MRAs. Nuclear receptors, such as MRs, bind to co-regulatory elements that determine cell-specific transcriptional responses [27]. Steroidal MRAs act as partial agonists in cofactor recruitment [13], and finerenone, a passive MRA, suppresses cofactor binding even in the absence of aldosterone (Fig. 1) [30]. In a study of the transcriptome of human renal cell lines, compared with spironolactone, finerenone was found to be more effective in inhibiting aldosterone regulatory genes [61]. Spironolactone, a first-generation MRA, is potent yet nonselective, whereas eplerenone, a second-generation MRA, is more selective but less potent. On the other hand, finerenone is potent and selective [13].
There are also differences in pharmacokinetic characteristics. Spironolactone produces several active metabolites with long half-lives that can accumulate in patients with impaired kidney function, whereas finerenone has a short half-life (2 to 3 hours) and produces no active metabolites. Spironolactone and eplerenone appear to accumulate to higher levels in the kidneys than in the heart. In contrast, finerenone has a more balanced heartkidney distribution, which reduces the risk of predominant renal distribution and results in less hyperkalemia [62]. Table 1 presents key pharmacodynamic and pharmacokinetic characteristics of steroidal and NS-MRAs.
Table 1.
Key Pharmacodynamic and Pharmacokinetic Characteristics of Mineralocorticoid Receptor Antagonists
| 1st generation |
2nd generation |
3rd generation |
|
|---|---|---|---|
| Spironolactone | Eplerenone | Finerenone | |
| Structural properties | Flat (steroidal) | Flat (steroidal) | Bulky (nonsteroidal) |
| Selectivity to MR | + | ++ | +++ |
| Potency to MR | +++ | + | +++ |
| Tissue distribution | Kidney > Heart | Kidney > Heart | Kidney = Heart |
| Active metabolites | ++ | - | - |
| Half-life | >20 hra | 4–6 hra | 2–3 hrb |
| Effect on BP | +++ | ++ | + |
| Affinity for progesterone and androgen receptors | +++ | ++ | + |
| Side effects | |||
| Gonadal axis-relatedc | +++ | ++ | + |
| Hyperkalemia | +++ | ++ | + |
MR mineralocorticoid receptor; BP blood pressure.
In heart failure patients;
In healthy volunteers;
Gynecomastia, impotence, breast tenderness, menstrual irregularities.
MAJOR STUDY OUTCOMES OF STEROIDAL MRAs AND FINERENONE
Research has shown that steroidal MRAs, spironolactone and eplerenone, have clinical benefits for hypertension, heart failure, and left ventricular dysfunction [15,16]. The beneficial effects of MRAs on RAAS inhibitors in patients with albuminuria were confirmed in a small-scale clinical study [60]. However, spironolactone and eplerenone also cause anti-adrenergic side effects, hyperkalemia, and impairment of kidney function in patients with CKD; therefore, their use is limited [15,16,63,64]. A phase II Mineralocorticoid Receptor Antagonist Tolerability Study (ARTS) program showed that finerenone is effective in reducing albuminuria in patients with DKD and has a lower risk of hyperkalemia compared with steroidal MRAs [19,20].
Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) and Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD) are independent, event-driven, randomized, double-blind, placebo-controlled, phase 3 trials with reciprocal primary and secondary outcomes [21,22]. FIDELIO-DKD assessed the efficacy of finerenone in reducing CKD progression in patients with advanced CKD, while FIGARO-DKD assessed the efficacy of finerenone in reducing cardiovascular morbidity and mortality in early-stage CKD. In 2021, The Finerenone in Chronic Kidney Disease and Type 2 Diabetes: Combined FIDELIO-DKD and FIGARO-DKD Trial Programme Analysis (FIDELITY), which analyzed cardiovascular and renal endpoints in patients who received finerenone based on combined data from both studies, was published (Table 2) [65].
Table 2.
Dedicated Kidney Disease-Focused Outcome Trials
| CREDENCE | DAPA-CKD | EMPA-KIDNEY | FIDELIO-DKD | FIDELITY | |
|---|---|---|---|---|---|
| Study drug | Canagliflozin vs. Placebo | Dapagliflozin vs. Placebo | Empagliflozin vs. Placebo | Finerenone vs. Placebo | Finerenone vs. Placebo |
| Study population | Adults, T2DM and CKD | Adults, CKD | Adults, CKD | Adults, T2DM and CKD | Adults, T2DM and CKD |
| Key inclusion criteria | eGFR 30–90 mL/min/1.73 m2 and UACR 300–5,000 mg/g | eGFR 25–75 mL/min/1.73 m2 and UACR 200–5,000 mg/g | 1) eGFR 20–45 mL/min/1.73 m2 regardless of albuminuria | 1) UACR 30–300 mg/g, eGFR 25–60 mL/min/1.73 m2, and diabetic | 1) FIDELIO-DKD |
| 2) eGFR 45–90 mL/min/1.73 m2 and UACR ≥ 200 mg/g retinopathy | 2) eGFR 25–75 mL/min/1.73 m2 and UACR 300–5,000 mg/g | 2) FIGARO-DKD: UACR 30–300 mg/g and eGFR 25–90 mL/min/1.73 m2; UACR 300–5,000 mg/g and eGFR ≥60 mL/min/1.73 m2 | |||
| Patient number | 4,401 | 4,304 | 6,609 | 5,734 | 13,026 |
| Background RAAS inhibitor use | Yes | Yes | Yes | Yes | Yes |
| Mean baseline eGFR, mL/min/1.73 m2 | 56.2±18.2 | 43.1±12.4 | 37.3±14.5 | 44.3±12.6 | 57.6±21.7 |
| Median baseline UACR, mg/g | 927 | 949 | 329 | 852 | 515 |
| Median follow-up duration, yr | 2.62 | 2.4 | 2.0 | 2.6 | 3.0 |
| Primary kidney endpoint | Composite of ESKD, a doubling of serum creatinine, or renal or CV death | Composite of sustained decline in the eGFR of ≥50%, ESKD, or renal or CV death | Composite of progression of kidney disease, or CV death | Composite of kidney failure, a sustained decrease in the eGFR ≥40%, or renal death | Composite of kidney failure, a sustained ≥57% decrease in eGFR (equivalent to doubling of serum creatinine), or renal death |
| Primary outcome | 11.1% vs. 15.5% (HR, 0.70; 95% CI 0.59–0.82; P=0.00001) | 9.2% vs. 14.5% (HR, 0.61; 95% CI, 0.51–0.72; P<0.001) | 13.1% vs. 16.9% (HR, 0.72; 95% CI 0.64–0.82; P<0.001) | 17.8% vs. 21.1% (HR, 0.82; 95% CI 0.73–0.93; P=0.001) | 5.5% vs. 7.1% (HR, 0.77; 95% CI 0.67–0.88; P=0.0002) |
Plus–minus values are mean±standard deviation. For CREDENCE and DAPA-CKD, ESKD was defined as dialysis, transplantation, or a sustained eGFR of <15 mL/min/1.73 m2; In EMPA-KIDNEY, progression of kidney disease was defined as ESKD (the initiation of maintenance dialysis or receipt of a kidney transplant), a sustained decrease in the eGFR to <10 mL/min/1.73 m2, a sustained decrease from baseline in the eGFR of ≥40%, or death from renal causes; In FIDELIO-DKD, kidney failure was defined as ESKD (defined as dialysis or kidney transplantation) or an eGFR <15 mL/min/1.73 m2.
CREDENCE, Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy; DAPA-CKD, A Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease; EMPAKIDNEY, The Study of Heart and Kidney Protection With Empagliflozin; FIDELIO-DKD, Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease; FIDELITY, The Finerenone in Chronic Kidney Disease and Type 2 Diabetes: Combined FIDELIO-DKD and FIGARO-DKD Trial Programme Analysis; T2DM, type 2 diabetes mellitus; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; UACR, urinary albumin-to-creatinine ratio; FIGARO-DKD, Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease; RAAS, renin-angiotensin-aldosterone system; ESKD, end-stage kidney disease; CV, cardiovascular; HR, hazard ratio; CI, confidence interval.
FIDELIO-DKD was conducted in patients with T2DM who met the CKD criteria: (1) urinary albumin-to-creatinine ratio (UACR) ≥30 mg/g (but <300 mg/g) and eGFR 25 to <60 mL/min/1.73 m2 and a history of diabetic retinopathy; or (2) UACR ≥300 mg/g (but ≤5,000 mg/g) and eGFR 25 to <75 mL/min/1.73 m2. A total of 5,734 participants were randomly allocated to the finerenone and placebo groups [21]. Finerenone was significantly more effective in reducing DKD progression over a median follow-up period of 2.6 years; finerenone reduced the primary composite outcome (renal failure, a sustained decrease of at least 40% in the eGFR from baseline, death from renal causes) by 18% (hazard ratio [HR], 0.82; 95% confidence interval [CI], 0.73 to 0.93; P=0.001). The key secondary composite outcomes, including cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for heart failure, also decreased by 14% (HR, 0.86; 95% CI, 0.75 to 0.99; P=0.03). These effects were observed from 1-month after administration and continued throughout the study period, indicating that finerenone has protective effects against adverse cardiovascular outcomes in patients with DKD. Finerenone also showed modest effects in reducing systolic blood pressure but did not affect glycated hemoglobin levels. Therefore, it can be assumed that the cardiorenal protective effects of finerenone are not the result of blood pressure-or blood glucose-mediated mechanisms.
Unlike FIDELIO-DKD, FIGARO-DKD assessed cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for heart failure as the primary cardiovascular composite outcome, and renal failure, sustained eGFR decrease ≥40%, and death from renal causes as key secondary outcomes. Compared to FIDELIO-DKD, the FIGARO-DKD study included patients with T2DM with a wide range of kidney function levels. The CKD criteria for eligible participants were as follows: (1) UACR ≥30 mg/g (but <300 mg/g) and eGFR, 25 to 90 mL/min/1.73 m2; or (2) UACR ≥300 mg/g (but ≤5,000 mg/g) and eGFR ≥60 mL/min/1.73 m2. A total of 7,437 patients were randomized. During the median follow-up period of 3.4 years, the primary cardiovascular composite outcome in the finerenone group decreased by 13% (HR, 0.87; 95% CI, 0.76 to 0.98; P=0.03), mostly due to a reduction in hospitalization due to heart failure. The secondary renal composite outcome did not show a statistically significant difference, but was decreased by 13% in the finerenone group compared to that in the placebo group (HR, 0.87; 95% CI, 0.76 to 1.01). Furthermore, UACR showed a significant reduction in the first 4 months of finerenone administration [22].
FIDELITY combined the outcomes from FIDELIO-DKD and FIGARO-DKD and analyzed the effects of finerenone on the kidney and heart. In the FIDELITY pooled analysis, the composite renal outcome (a sustained ≥57% decrease in eGFR from baseline over ≥4 weeks or renal death) and composite cardiovascular outcome (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure) of 13,026 participants during a median period of 3 years (interquartile range, 2.3 to 3.8) were analyzed. Finerenone showed a significant risk reduction compared with placebo in all composite cardiovascular and renal outcomes. Composite cardiovascular events showed a 14% risk reduction (HR, 0.86; 95% CI, 0.78 to 0.95; P=0.0018), and the composite renal outcome showed a 23% risk reduction (HR, 0.77; 95% CI, 0.67 to 0.88; P=0.0002). In particular, the risk of end-stage renal disease, which requires dialysis, showed a 20% reduction (HR, 0.80; 95% CI, 0.64 to 0.99; P=0.040). The blood pressure changes in the participants in this study were modest, which indicates that the cardiovascular and renoprotective effects of finerenone cannot be fully explained by changes in blood pressure [65].
In summary, the FIDELIO-DKD and FIGARO-DKD studies demonstrated the cardiovascular and renal benefits of finerenone in patients with T2DM and albuminuria who were on standardized treatment (maximum tolerated dose of RAAS inhibitor, glycemic control, and blood pressure management). However, as these studies only included patients with CKD with albuminuria, future studies on patients with CKD without albuminuria are necessary. In 2021, finerenone received FDA approval to reduce the risk of decreased renal function, renal failure, cardiovascular death, nonfatal heart attack, and hospitalization for heart failure in patients with T2DM based on the FIDELIO-DKD and FIGARO-DKD studies [58]. In addition, the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO) consensus report recommended finerenone administration in patients with T2DM with persistent albuminuria, despite the maximum tolerated dose of a RAAS inhibitor [5].
SAFETY OF FINERENONE
The use of MRAs is associated with the safety concerns of increased serum potassium concentration, especially when used in hyperkalemia-prone patients, such as patients with CKD. Compared with steroidal MRAs, NS-MRAs, such as finerenone, have been shown to have a more balanced heart-kidney distribution and a lower risk of hyperkalemia [66]. However, hyperkalemia remains an important issue that must be considered when using either steroid MRAs or finerenone. Elevated baseline serum potassium level, low baseline eGFR, and high baseline UACR are known risk factors for hyperkalemia when using finerenone [67].
The total incidence of adverse events in the placebo and finerenone groups during the FIDELIO-DKD and FIGARO-DKD studies was similar. Hyperkalemia-related adverse events were two-fold higher in the finerenone group than in the placebo group (FIDELIO-DKD 18.3% vs. 9.0%; FIGARO-DKD 10.8% vs. 5.3%) [21,22]. However, the finerenone dose during FIDELIO-DKD was adjusted based on the serum potassium level; thus, there was a limit in accurately predicting the effects of the drug. In the FIDELITY pooled analysis, there were more cases of treatment discontinuation due to hyperkalemia in the finerenone group than in the placebo group (1.7% vs. 0.6%). In addition to hyperkalemia, no clinically significant adverse events, including gynecomastia, impotence, or menstrual irregularities, which have been reported with steroidal MRAs, were observed in the FIDELIO-DKD and FIGARO-DKD trials.
One study investigated the incidence of hyperkalemia in patients with CKD and heart failure who were administered finerenone or a steroidal MRA (spironolactone); the mean potassium increment (0.04–0.30 mmol/L vs. 0.45 mmol/L, P<0.01) and rate of hyperkalemia (5.3% vs. 12.7%, P=0.048) in the finerenone group were significantly lower than those in the spironolactone group [68]. In the Mineralocorticoid Receptor Antagonist Tolerability Study, Heart Failure (ARTS-HF), eplerenone increased the mean serum potassium by 0.262 mmol/L from baseline, which was higher than the increase in potassium levels shown in the five groups of different doses of finerenone (baseline to up-titration based on the study protocol: 2.5→5, 5→10, 7.5→15, 10→20, and 15→20 mg) (0.119 to 0.202 mmol/L) [20].
Recently, potassium binders, such as patiromer and sodium zirconium cyclosilicate (SZC) have been reported to control hyperkalemia, and it is expected that when used in combination with ACEi/ARBs and MRAs in patients with CKD, they could reduce the risk of hyperkalemia and prevent discontinuation of MRA and may delay the progression of renal function [69-74]. Patiromer resulted in reduced serum potassium levels and lower risk of recurrence of hyperkalemia compared to placebo in patients with CKD and hyperkalemia who were prescribed RAAS inhibitors [73]. In the Spironolactone with Patiromer in the Treatment of Resistant Hypertension in Chronic Kidney Disease (AMBER) study conducted on 295 patients with resistant hypertension and an eGFR of 25 to 45 mL/min/1.73 m2, patiromer, a sodium-free potassium binder, showed a reduced risk of hyperkalemia and improved drug compliance compared to placebo. MRA treatment was continued in 86% of the patients in the patiromer group (vs. 66% of the placebo group), and a similar outcome was observed in a subgroup analysis of patients with diabetes [70].
ADDITIVE RENAL EFFECT OF FINERENONE
ACEi/ARB
RAAS inhibitors ACEi/ARB, are recommended as first-line drugs for the management of CKD, regardless of the presence of diabetes. RAAS inhibitors are recommended for patients with hypertension, proteinuric CKD, or reduced eGFR <60 mL/min/1.73 m2 and UACR ≥300 mg/g. Combination therapy with an MRA and RAAS inhibitor can reduce albuminuria and CKD progression in patients with T2DM and CKD, independent of blood pressure control. In the Mineralocorticoid Receptor Antagonist Tolerability Study, the Diabetic Nephropathy trial (ARTS-DN), when finerenone was added to RAAS inhibitor treatment in patients with diabetes and proteinuric CKD, UACR decreased in a dose-dependent manner with a low incidence of hyperkalemia [19]. Furthermore, in the FIDELIO-DKD and FIGARO-DKD trials, finerenone reduced DKD progression in patients with background of maximal RAAS blockade therapy [21,22]. In case of hyperkalemia during combinatorial treatment with an MRA and RAAS inhibitor, dietary potassium restrictions, acidosis correction, and the use of potassium binders, such as patiromer or SZC, can be considered.
SGLT2i
SGLT2i are known to improve cardiorenal outcomes in patients with and without diabetes [75] and are widely used as the standard of care for the management of DKD. A recently published study on patients with CKD (the Study of Heart and Kidney Protection With Empagliflozin [EMPA-KIDNEY]) reported that empagliflozin reduced the risk of the primary composite outcome (end-stage kidney disease, a sustained decrease in eGFR <10 mL/min/1.73 m2, a sustained decrease in eGFR ≥ 40% from baseline, death from renal causes, or death from cardiovascular causes) by 28% (HR, 0.72; 95% CI, 0.64 to 0.82; P<0.001) in patients with CKD; the results were consistent among subgroups based on baseline diabetes and eGFR range (Table 2) [76]. The major renal protection mechanism of SGLT2i is blocking glucose reabsorption, inducing glycosuria and natriuresis, which ultimately leads to the normalization of altered tubuloglomerular feedback [77].
Co-administration of finerenone and empagliflozin in an animal model of hypertension-induced end-organ damage showed improvements in proteinuria, plasma creatinine and uric acid levels, blood pressure, renal and cardiac fibrotic lesions, and mortality [78]. In a post hoc analysis conducted on similar patient groups from Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) and FIDELIO-DKD, canagliflozin and finerenone showed similar results regarding the cardiorenal endpoint compared to the placebo group (canagliflozin vs. placebo: HR, 0.70; 95% CI, 0.59 to 0.82; P<0.0001; finerenone vs. placebo: HR, 0.74; 95% CI, 0.63 to 0.87; P=0.0003) [79]. In addition, in the FIDELITY analysis, 6.7% of the patients were already being administered SGLT2i, and there was no difference in the outcomes of these patients compared with those of participants who were not treated with SGLT2i. This result implies that the co-administration of finerenone and SGLT2i may have additive renoprotective effects.
The occurrence of hyperkalemia during DKD treatment is a common reason for discontinuation of specific medications. SGLT2i are known to reduce the risk of hyperkalemia with a modest potassium-lowering effect. The hypotheses for the mechanism of such effects are as follows: (1) SGLT2i induce osmotic diuresis and natriuresis, which cause high urine flow and kaliuresis; and (2) SGLT2i preserve renal function more than placebo. In the Dapagliflozin in Patients with Heart Failure trial (DAPA-HF), 70% of the participants were administered MRAs; post hoc analysis of this subgroup showed that the risk of mild (potassium level >5.5 mmol/L) and moderate-severe hyperkalemia (potassium level >6.0 mmol/L) were reduced (HR, 0.86 and 0.5, respectively) [80]. According to the post hoc analysis of the CREDENCE study, canagliflozin decreased the risk of hyperkalemia by 22% compared with placebo [81]. These findings suggest that the risk of hyperkalemia associated with finerenone can be reduced by the co-administration of SGLT2i. In fact, post hoc analysis of FIDELIO-DKD found that the risk of hyperkalemia in the combined finerenone and SGLT2i group was reduced by 55% compared to that in the group without SGLT2i (HR, 0.45; 95% CI, 0.66 to 0.87; P<0.0001). However, since only 4.6% of the participants in this study were administered SGLT2i, the results should be interpreted cautiously [67]. In a small-scale clinical study conducted on 20 patients with T2DM and chronic, stable heart failure, there was no change in the 6-hour urine potassium excretion after 2 weeks of empagliflozin treatment, with no difference in the serum potassium level observed compared to those of patients who received placebo [82].
GLP1-RA
GLP1-RA exhibit a renoprotective effect by suppressing proteinuria in patients with T2DM with cardiovascular risk factors and albuminuria [83,84]. Currently, no clinical study has investigated the co-administration of GLP1-RA and finerenone as a study protocol. However, the effects of the co-administration of these two drugs can be predicted indirectly by examining previous studies with GLP1-RA as a subgroup. Among the 5,674 patients included in the FIDELIO-DKD study, 394 (6.9%) received GLP1-RA as the baseline treatment. Reduced albuminuria was observed in the finerenone group independent of GLP1-RA treatment (P for interaction=0.20). In addition, the primary kidney outcome and key secondary cardiovascular outcomes did not show significant differences from those of the placebo group regardless of GLP1-RA treatment (P for interaction=0.15 and 0.51, respectively) [85]. This suggests that the combined use of these two drugs may have an additive or synergistic effect in delaying DKD progression.
CONCLUSIONS
Many studies have been conducted on the prevention and treatment of DKD. The KDIGO guidelines recommend blockade of the RAAS and SGLT2i in patients with DKD [5]. Aldosterone overactivation causes inflammation and fibrosis, which play a notable role in the progression of DKD, and suppression of aldosterone can reduce proteinuria and the risk of CVD [10,29]. However, MRA has received little attention as a treatment option because of the risk of hyperkalemia in patients with decreased renal function. Finerenone is an NS-MRA that selectively binds to MR and can effectively inhibit aldosterone activity, with a lower risk of hyperkalemia [66]. In clinical trials, finerenone reduced eGFR decline and the incidence of end-stage renal disease and cardiac death in patients with DKD with advanced renal disease [21,22]. Accordingly, the ADA recommends the use of finerenone to reduce the progression of renal disease in patients with CKD associated with T2DM, and persistent albuminuria despite standard of care treatment [5]. In addition, co-administration of finerenone and SLGT2i in patients with DKD may be considered with the expectation of additive effects [79]. In particular, SGLT2i have both renoprotective and potassium-lowering effects; therefore, a reduced risk of hyperkalemia may also be expected. Moreover, if finerenone is administered with new potassium binders, the incidence of treatment discontinuation owing to hyperkalemia can be lowered, thereby delaying the progression of DKD.
An ongoing study is investigating whether the co-administration of finerenone, RAAS inhibitors, and SGLT2i could reduce the progression of CKD in patients with T2DM (Table 3). In addition, we believe that future studies on the renoprotective effects of combination therapy with GLP1-RA and finerenone, and triple therapy consisting of GLP1-RA, finerenone, and SGLT2i are required in patients with DKD.
Table 3.
Currently Ongoing Dedicated Kidney Disease-Focused Outcome Trials with Finerenone
| CONFIDENCE | FIND-CKD | FIONA | |
|---|---|---|---|
| ClinicalTrials.gov identifier | NCT05254002 | NCT05047263 | NCT05196035 |
| Study drug | Finerenone+Empagliflozin vs. Finerenone vs. Empagliflozin | Finerenone vs. Placebo | Finerenone vs. Placebo |
| Study population | Adults, T2DM and CKD | Adults, nondiabetic CKD | Children, CKD and proteinuria |
| Background RAAS inhibitor use | Yes | Yes | Yes |
| Primary endpoint | Relative change from baseline in UACR at 180 days | Mean rate of change as measured by the total slope of eGFR from baseline to month 32 | UPCR reduction of ≥30% from baseline to day 180±7; Proportion of participants at day 180±7 with a ≥30% reduction in UPCR compared to baseline |
CONFIDENCE, A Study to Learn How Well the Treatment Combination of Finerenone and Empagliflozin Works and How Safe it is Compared to Each Treatment Alone in Adult Participants With Long-term Kidney Disease (Chronic Kidney Disease) and Type 2 Diabetes; FIND-CKD, A Trial to Learn How Well Finerenone Works and How Safe it is in Adult Participants With Non-diabetic Chronic Kidney Disease; FIONA, A Study to Learn More About How Well the Study Treatment Finerenone Works, How Safe it is, How it Moves Into, Through, and Out of the Body, and the Effects it Has on the Body When Taken With an ACE Inhibitor or Angiotensin Receptor Blocker in Children With Chronic Kidney Disease and Proteinuria; T2DM, type 2 diabetes mellitus; CKD, chronic kidney disease; RAAS, renin-angiotensin-aldosterone system; UACR, urinary albumin-to-creatinine ratio; eGFR, estimated glomerular filtration rate; UPCR, urinary protein-to-creatinine ratio.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.The Korean Society of Nephrology . Seoul: The Korean Society of Nephrology; 2022. Trends in epidemiologic characteristics of end-stage renal disease from 2020 KORDS (Korean Renal Data System) [Internet] [cited 2023 Jan 31]. Available from: https://ksn.or.kr. [Google Scholar]
- 2.Atkins RC. The epidemiology of chronic kidney disease. Kidney Int Suppl. 2005;94:S14–8. doi: 10.1111/j.1523-1755.2005.09403.x. [DOI] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98(4S):S1–115. doi: 10.1016/j.kint.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Fried LF, Folkerts K, Smeta B, Bowrin KD, Mernagh P, Millier A, et al. Targeted literature review of the burden of illness in patients with chronic kidney disease and type 2 diabetes. Am J Manag Care. 2021;27(8 Suppl):S168–77. doi: 10.37765/ajmc.2021.88660. [DOI] [PubMed] [Google Scholar]
- 5.de Boer IH, Khunti K, Sadusky T, Tuttle KR, Neumiller JJ, Rhee CM, et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Care. 2022;45:3075–90. doi: 10.2337/dci22-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SS, Kim JH, Kim IJ. Current challenges in diabetic nephropathy: early diagnosis and ways to improve outcomes. Endocrinol Metab (Seoul) 2016;31:245–53. doi: 10.3803/EnM.2016.31.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amin AP, Whaley-Connell AT, Li S, Chen SC, McCullough PA, Kosiborod MN, et al. The synergistic relationship between estimated GFR and microalbuminuria in predicting long-term progression to ESRD or death in patients with diabetes: results from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2013;61(4 Suppl 2):S12–23. doi: 10.1053/j.ajkd.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jankowski J, Floege J, Fliser D, Bohm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143:1157–72. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JF, Berzan E, Sridhar VS, Odutayo A, Cherney DZI. Cardiorenal protection in diabetic kidney disease. Endocrinol Metab (Seoul) 2021;36:256–69. doi: 10.3803/EnM.2021.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein M. Aldosterone and mineralocorticoid receptor signaling as determinants of cardiovascular and renal injury: from Hans Selye to the present. Am J Nephrol. 2021;52:209–16. doi: 10.1159/000515622. [DOI] [PubMed] [Google Scholar]
- 11.Jaisser F, Tan X, Chi S, Liu J, Wang P, Bush M, et al. The non-steroidal mineralocorticoid receptor antagonist KBP-5074 limits albuminuria and has improved therapeutic index compared with eplerenone in a rat model with mineralocorticoid-induced renal injury. Front Pharmacol. 2021;12:604928. doi: 10.3389/fphar.2021.604928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kintscher U, Bakris GL, Kolkhof P. Novel non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease. Br J Pharmacol. 2022;179:3220–34. doi: 10.1111/bph.15747. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal R, Kolkhof P, Bakris G, Bauersachs J, Haller H, Wada T, et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42:152–61. doi: 10.1093/eurheartj/ehaa736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol. 2017;13:11–26. doi: 10.1038/nrneph.2016.170. [DOI] [PubMed] [Google Scholar]
- 15.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 16.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 17.Chrysostomou A, Pedagogos E, MacGregor L, Becker GJ. Double-blind, placebo-controlled study on the effect of the aldosterone receptor antagonist spironolactone in patients who have persistent proteinuria and are on long-term angiotensin-converting enzyme inhibitor therapy, with or without an angiotensin II receptor blocker. Clin J Am Soc Nephrol. 2006;1:256–62. doi: 10.2215/CJN.01040905. [DOI] [PubMed] [Google Scholar]
- 18.El Mokadem M, Abd El Hady Y, Aziz A. A prospective single-blind randomized trial of ramipril, eplerenone and their combination in type 2 diabetic nephropathy. Cardiorenal Med. 2020;10:392–401. doi: 10.1159/000508670. [DOI] [PubMed] [Google Scholar]
- 19.Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314:884–94. doi: 10.1001/jama.2015.10081. [DOI] [PubMed] [Google Scholar]
- 20.Filippatos G, Anker SD, Bohm M, Gheorghiade M, Kober L, Krum H, et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37:2105–14. doi: 10.1093/eurheartj/ehw132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–29. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 22.Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385:2252–63. doi: 10.1056/NEJMoa2110956. [DOI] [PubMed] [Google Scholar]
- 23.Bomback AS. Mineralocorticoid receptor antagonists in end-stage renal disease: efficacy and safety. Blood Purif. 2016;41:166–70. doi: 10.1159/000441262. [DOI] [PubMed] [Google Scholar]
- 24.Ruggenenti P, Cravedi P, Remuzzi G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol. 2010;6:319–30. doi: 10.1038/nrneph.2010.58. [DOI] [PubMed] [Google Scholar]
- 25.Rossi GP, Belfiore A, Bernini G, Fabris B, Caridi G, Ferri C, et al. Body mass index predicts plasma aldosterone concentrations in overweight-obese primary hypertensive patients. J Clin Endocrinol Metab. 2008;93:2566–71. doi: 10.1210/jc.2008-0251. [DOI] [PubMed] [Google Scholar]
- 26.Barrera-Chimal J, Girerd S, Jaisser F. Mineralocorticoid receptor antagonists and kidney diseases: pathophysiological basis. Kidney Int. 2019;96:302–19. doi: 10.1016/j.kint.2019.02.030. [DOI] [PubMed] [Google Scholar]
- 27.Fuller PJ, Yang J, Young MJ. 30 Years of the mineralocorticoid receptor: coregulators as mediators of mineralocorticoid receptor signalling diversity. J Endocrinol. 2017;234:T23–34. doi: 10.1530/JOE-17-0060. [DOI] [PubMed] [Google Scholar]
- 28.Funder JW. Minireview: Aldosterone and mineralocorticoid receptors: past, present, and future. Endocrinology. 2010;151:5098–102. doi: 10.1210/en.2010-0465. [DOI] [PubMed] [Google Scholar]
- 29.Buonafine M, Bonnard B, Jaisser F. Mineralocorticoid receptor and cardiovascular disease. Am J Hypertens. 2018;31:1165–74. doi: 10.1093/ajh/hpy120. [DOI] [PubMed] [Google Scholar]
- 30.Amazit L, Le Billan F, Kolkhof P, Lamribet K, Viengchareun S, Fay MR, et al. Finerenone impedes aldosterone-dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor coactivator-1. J Biol Chem. 2015;290:21876–89. doi: 10.1074/jbc.M115.657957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauersachs J, Jaisser F, Toto R. Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension. 2015;65:257–63. doi: 10.1161/HYPERTENSIONAHA.114.04488. [DOI] [PubMed] [Google Scholar]
- 32.Vodosek Hojs N, Bevc S, Ekart R, Piko N, Petreski T, Hojs R. Mineralocorticoid receptor antagonists in diabetic kidney disease. Pharmaceuticals (Basel) 2021;14:561. doi: 10.3390/ph14060561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tesch GH, Young MJ. Mineralocorticoid receptor signaling as a therapeutic target for renal and cardiac fibrosis. Front Pharmacol. 2017;8:313. doi: 10.3389/fphar.2017.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiebeler A, Luft FC. The mineralocorticoid receptor and oxidative stress. Heart Fail Rev. 2005;10:47–52. doi: 10.1007/s10741-005-2348-y. [DOI] [PubMed] [Google Scholar]
- 35.Kiyomoto H, Rafiq K, Mostofa M, Nishiyama A. Possible underlying mechanisms responsible for aldosterone and mineralocorticoid receptor-dependent renal injury. J Pharmacol Sci. 2008;108:399–405. doi: 10.1254/jphs.08r02cr. [DOI] [PubMed] [Google Scholar]
- 36.Miyata K, Rahman M, Shokoji T, Nagai Y, Zhang GX, Sun GP, et al. Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. J Am Soc Nephrol. 2005;16:2906–12. doi: 10.1681/ASN.2005040390. [DOI] [PubMed] [Google Scholar]
- 37.Munoz-Durango N, Vecchiola A, Gonzalez-Gomez LM, Simon F, Riedel CA, Fardella CE, et al. Modulation of immunity and inflammation by the mineralocorticoid receptor and aldosterone. Biomed Res Int. 2015;2015:652738. doi: 10.1155/2015/652738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong D, Fan TT, Ji YS, Yu JY, Wu S, Zhang L. Spironolactone alleviates diabetic nephropathy through promoting autophagy in podocytes. Int Urol Nephrol. 2019;51:755–64. doi: 10.1007/s11255-019-02074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li D, Lu Z, Xu Z, Ji J, Zheng Z, Lin S, et al. Spironolactone promotes autophagy via inhibiting PI3K/AKT/mTOR signalling pathway and reduce adhesive capacity damage in podocytes under mechanical stress. Biosci Rep. 2016;36:e00355. doi: 10.1042/BSR20160086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu JJ, Chen YP, Yang M, Liu BL, Dong J, Dong HR, et al. Aldosterone is involved in the pathogenesis of obesity-related glomerulopathy through activation of Wnt/β-catenin signaling in podocytes. Mol Med Rep. 2018;17:4589–98. doi: 10.3892/mmr.2018.8386. [DOI] [PubMed] [Google Scholar]
- 41.Chen D, Chen Z, Park C, Centrella M, McCarthy T, Chen L, et al. Aldosterone stimulates fibronectin synthesis in renal fibroblasts through mineralocorticoid receptor-dependent and independent mechanisms. Gene. 2013;531:23–30. doi: 10.1016/j.gene.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 42.Koszegi S, Molnar A, Lenart L, Hodrea J, Balogh DB, Lakat T, et al. RAAS inhibitors directly reduce diabetes-induced renal fibrosis via growth factor inhibition. J Physiol. 2019;597:193–209. doi: 10.1113/JP277002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Callera GE, Yogi A, Briones AM, Montezano AC, He Y, Tostes RC, et al. Vascular proinflammatory responses by aldosterone are mediated via c-Src trafficking to cholesterolrich microdomains: role of PDGFR. Cardiovasc Res. 2011;91:720–31. doi: 10.1093/cvr/cvr131. [DOI] [PubMed] [Google Scholar]
- 44.Hashikabe Y, Suzuki K, Jojima T, Uchida K, Hattori Y. Aldosterone impairs vascular endothelial cell function. J Cardiovasc Pharmacol. 2006;47:609–13. doi: 10.1097/01.fjc.0000211738.63207.c3. [DOI] [PubMed] [Google Scholar]
- 45.Han SY, Kim CH, Kim HS, Jee YH, Song HK, Lee MH, et al. Spironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. J Am Soc Nephrol. 2006;17:1362–72. doi: 10.1681/ASN.2005111196. [DOI] [PubMed] [Google Scholar]
- 46.Chrissobolis S, Drummond GR, Faraci FM, Sobey CG. Chronic aldosterone administration causes Nox2-mediated increases in reactive oxygen species production and endothelial dysfunction in the cerebral circulation. J Hypertens. 2014;32:1815–21. doi: 10.1097/HJH.0000000000000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirohama D, Nishimoto M, Ayuzawa N, Kawarazaki W, Fujii W, Oba S, et al. Activation of Rac1-mineralocorticoid receptor pathway contributes to renal injury in salt-loaded db/db mice. Hypertension. 2021;78:82–93. doi: 10.1161/HYPERTENSIONAHA.121.17263. [DOI] [PubMed] [Google Scholar]
- 48.Nagata D, Takahashi M, Sawai K, Tagami T, Usui T, Shimatsu A, et al. Molecular mechanism of the inhibitory effect of aldosterone on endothelial NO synthase activity. Hypertension. 2006;48:165–71. doi: 10.1161/01.HYP.0000226054.53527.bb. [DOI] [PubMed] [Google Scholar]
- 49.Belden Z, Deiuliis JA, Dobre M, Rajagopalan S. The role of the mineralocorticoid receptor in inflammation: focus on kidney and vasculature. Am J Nephrol. 2017;46:298–314. doi: 10.1159/000480652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown NJ. Aldosterone and end-organ damage. Curr Opin Nephrol Hypertens. 2005;14:235–41. doi: 10.1097/01.mnh.0000165889.60254.98. [DOI] [PubMed] [Google Scholar]
- 51.Hollenberg NK. Aldosterone in the development and progression of renal injury. Kidney Int. 2004;66:1–9. doi: 10.1111/j.1523-1755.2004.00701.x. [DOI] [PubMed] [Google Scholar]
- 52.Jaisser F, Farman N. Emerging roles of the mineralocorticoid receptor in pathology: toward new paradigms in clinical pharmacology. Pharmacol Rev. 2016;68:49–75. doi: 10.1124/pr.115.011106. [DOI] [PubMed] [Google Scholar]
- 53.Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest. 1996;98:1063–8. doi: 10.1172/JCI118867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rocha R, Chander PN, Zuckerman A, Stier CT., Jr Role of aldosterone in renal vascular injury in stroke-prone hypertensive rats. Hypertension. 1999;33(1 Pt 2):232–7. doi: 10.1161/01.hyp.33.1.232. [DOI] [PubMed] [Google Scholar]
- 55.Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2006;1:940–51. doi: 10.2215/CJN.00240106. [DOI] [PubMed] [Google Scholar]
- 56.Hou J, Xiong W, Cao L, Wen X, Li A. Spironolactone addon for preventing or slowing the progression of diabetic nephropathy: a meta-analysis. Clin Ther. 2015;37:2086–103. doi: 10.1016/j.clinthera.2015.05.508. [DOI] [PubMed] [Google Scholar]
- 57.Mavrakanas TA, Gariani K, Martin PY. Mineralocorticoid receptor blockade in addition to angiotensin converting enzyme inhibitor or angiotensin II receptor blocker treatment: an emerging paradigm in diabetic nephropathy: a systematic review. Eur J Intern Med. 2014;25:173–6. doi: 10.1016/j.ejim.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 58.U.S. Food and Drug Administration . Silver Spring: FDA; 2021. FDA approves drug to reduce risk of serious kidney and heart complications in adults with chronic kidney disease associated with type 2 diabetes [Internet] [cited 2023 Jan 31]. Available from: https://www.fda.gov/drugs/news-eventshuman-drugs/fda-approves-drug-reduce-risk-serious-kidneyand-heart-complications-adults-chronic-kidney-disease. [Google Scholar]
- 59.Fagart J, Hillisch A, Huyet J, Barfacker L, Fay M, Pleiss U, et al. A new mode of mineralocorticoid receptor antagonism by a potent and selective nonsteroidal molecule. J Biol Chem. 2010;285:29932–40. doi: 10.1074/jbc.M110.131342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barrera-Chimal J, Bonnard B, Jaisser F. Roles of mineralocorticoid receptors in cardiovascular and cardiorenal diseases. Annu Rev Physiol. 2022;84:585–610. doi: 10.1146/annurev-physiol-060821-013950. [DOI] [PubMed] [Google Scholar]
- 61.Le Billan F, Perrot J, Carceller E, Travers S, Viengchareun S, Kolkhof P, et al. Antagonistic effects of finerenone and spironolactone on the aldosterone-regulated transcriptome of human kidney cells. FASEB J. 2021;35:e21314. doi: 10.1096/fj.202002043RR. [DOI] [PubMed] [Google Scholar]
- 62.Kolkhof P, Delbeck M, Kretschmer A, Steinke W, Hartmann E, Barfacker L, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64:69–78. doi: 10.1097/FJC.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 63.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–51. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 64.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 65.Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022;43:474–84. doi: 10.1093/eurheartj/ehab777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen KT, Kang YN, Lin YC, Tsai IL, Chang WC, Fang TC, et al. Efficacy and safety of mineralocorticoid receptor antagonists in kidney failure patients treated with dialysis: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2021;16:916–25. doi: 10.2215/CJN.15841020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agarwal R, Joseph A, Anker SD, Filippatos G, Rossing P, Ruilope LM, et al. Hyperkalemia risk with finerenone: results from the FIDELIO-DKD Trial. J Am Soc Nephrol. 2022;33:225–37. doi: 10.1681/ASN.2021070942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, et al. Safety and tolerability of the novel nonsteroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34:2453–63. doi: 10.1093/eurheartj/eht187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agarwal R, Rossignol P, Romero A, Garza D, Mayo MR, Warren S, et al. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2019;394:1540–50. doi: 10.1016/S0140-6736(19)32135-X. [DOI] [PubMed] [Google Scholar]
- 70.Agarwal R, Rossignol P, Mayo MR, Conrad A, Arthur S, Williams B, et al. Patiromer to enable spironolactone in patients with resistant hypertension and CKD (AMBER): results in the Prespecified Subgroup with Diabetes. Clin J Am Soc Nephrol. 2021;16:1407–9. doi: 10.2215/CJN.02890221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ali W, Bakris G. Evolution of patiromer use: a review. Curr Cardiol Rep. 2020;22:94. doi: 10.1007/s11886-020-01342-w. [DOI] [PubMed] [Google Scholar]
- 72.Borghi C, Ferri C, Pontremoli R, Sechi L, Grassi G. Possible advantages deriving from patiromer use in hypertensive patients made hyperkalemic by renin-angiotensin-aldosterone blocking agents. High Blood Press Cardiovasc Prev. 2021;28:555–9. doi: 10.1007/s40292-021-00478-2. [DOI] [PubMed] [Google Scholar]
- 73.Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211–21. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 74.Packham DK, Rasmussen HS, Lavin PT, El-Shahawy MA, Roger SD, Block G, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372:222–31. doi: 10.1056/NEJMoa1411487. [DOI] [PubMed] [Google Scholar]
- 75.Heerspink HJ, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–46. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 76.The EMPA-KIDNEY Collaborative Group. Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388:117–27. doi: 10.1056/NEJMoa2204233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeFronzo RA, Reeves WB, Awad AS. Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat Rev Nephrol. 2021;17:319–34. doi: 10.1038/s41581-021-00393-8. [DOI] [PubMed] [Google Scholar]
- 78.Kolkhof P, Hartmann E, Freyberger A, Pavkovic M, Mathar I, Sandner P, et al. Effects of finerenone combined with empagliflozin in a model of hypertension-induced end-organ damage. Am J Nephrol. 2021;52:642–52. doi: 10.1159/000516213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Agarwal R, Anker SD, Filippatos G, Pitt B, Rossing P, Ruilope LM, et al. Effects of canagliflozin versus finerenone on cardiorenal outcomes: exploratory post hoc analyses from FIDELIO-DKD compared to reported CREDENCE results. Nephrol Dial Transplant. 2022;37:1261–9. doi: 10.1093/ndt/gfab336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kristensen SL, Docherty KF, Jhund PS, Bengtsson O, Demets DL, Inzucchi SE, et al. Dapagliflozin reduces the risk of hyperkalaemia in patients with heart failure and reduced ejection fraction: a secondary analysis DAPA-HF. Eur Heart J. 2020;41(Supplement_2):ehaa946.0939. [Google Scholar]
- 81.Neuen BL, Oshima M, Perkovic V, Agarwal R, Arnott C, Bakris G, et al. Effects of canagliflozin on serum potassium in people with diabetes and chronic kidney disease: the CREDENCE trial. Eur Heart J. 2021;42:4891–901. doi: 10.1093/eurheartj/ehab497. [DOI] [PubMed] [Google Scholar]
- 82.Griffin M, Rao VS, Ivey-Miranda J, Fleming J, Mahoney D, Maulion C, et al. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation. 2020;142:1028–39. doi: 10.1161/CIRCULATIONAHA.120.045691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394:131–8. doi: 10.1016/S0140-6736(19)31150-X. [DOI] [PubMed] [Google Scholar]
- 84.Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–85. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 85.Rossing P, Agarwal R, Anker SD, Filippatos G, Pitt B, Ruilope LM, et al. Efficacy and safety of finerenone in patients with chronic kidney disease and type 2 diabetes by GLP-1RA treatment: a subgroup analysis from the FIDELIO-DKD trial. Diabetes Obes Metab. 2022;24:125–34. doi: 10.1111/dom.14558. [DOI] [PMC free article] [PubMed] [Google Scholar]