Abstract
Objectives
This study aims to characterize the specific organ metastatic rates in lung adenocarcinoma (LUAD) patients and identify the prognosis‐associated factors.
Methods
Using the Surveillance, Epidemiology and End Results database, 40 117 patients diagnosed with positive histology as the only primary LUAD were included. We stratified patients by diagnosed year, age, sex, race/ethnicity, marital status, insurance, location, TNM stage, organ‐specific metastases, surgery, chemotherapy, and radiation therapy. We performed multivariable logistic and Cox regression to identify the factors associated with the presence of specific organ metastases and prognosis predictors.
Results
For the 40 117 LUAD patients, 43.69%, 26.25%, 19.66%, 10.60%, and 17.89% had specific organ, bone, brain, liver, and lung metastases, respectively. The average survival in patients with organ metastases was 12.19 months, compared to 36.40 months in patients without metastases. In different kinds of metastatic organ cohorts, the longest average survival was 12.60 months in the lung metastases cohort, and the shortest was 8.43 months in liver metastases cohort. In total, 571 patients with metastases received surgery, which was significantly associated with decreased mortality (hazard ratio 1.82, 95% confidence interval 1.65–2.01, p < 0.01). Patients received surgery of lobectomy or extended (251 of 571, 43.96%) displayed the longest average survival (35.16 months); patients (294 of 571, 51.49%) received sub‐lobar resection, had the average survival (19.90 months); patients received local tumor destruction (26 of 571, 4.55%) had the shortest average survival (13.73 months).
Conclusion
This study provides insights into the specific organ metastatic rates and prognosis in LUAD patients on a population level. These findings suggest that surgery resection should be taken into consideration in the treatment for these LUAD patients.
Keywords: lung adenocarcinoma, metastases, SEER database, specific organ, thoracic surgery
Overall survival estimates in LUAD specific organ metastases cohort were better in the group received surgery, stratified by whether received surgery and different kinds of resections categorized by the range of surgery (no surgery, local tumor destruction, sublobular resection, and lobectomy or extended). While no surgery and just local tumor destruction were associated with shorter overall survival time compared to patients with other more aggressive surgery.
INTRODUCTION
Lung adenocarcinoma (LUAD) is the dominant type of lung cancer, one of the most prevalent malignancies as well as the leading cancer related mortality worldwide. 1 , 2 Specific organ metastases, characteristics of late stage, represent a cause of treatment difficulty and high mortality in LUAD patients, significantly impacting on the clinical course. 3 , 4 , 5 Despite the profound impact of specific organ metastases in LUAD patients, information on the metastatic rates and prognosis estimates in newly diagnosed LUAD patients is lacking on a population level. Additionally, the demographic, social status, and clinical treatments factors associated with the overall survival outcomes have not been fully studied on a population level. 6
As an important treatment procedure for LUAD, surgery resection of the primary tumor is not recommended to those patients with specific organ metastases according to clinical practice guidelines, considering the limited efficacy and possible complications. 7 , 8 However, in recent years, it has been suggested by more and more evidence that surgery resection for primary tumors offers favorable overall survival in some late‐stage malignancies such as breast cancer and colorectal cancer. 9 , 10 , 11 , 12 There are therefore strong requirements to identify whether surgery is suitable and which treatment procedure for the primary tumor is the best in LUAD patients with specific organ metastases.
Using the large cancer statistics database, the Surveillance, Epidemiology and End Results (SEER) database, the presence or absence of specific organ metastases at diagnosis was available to researchers. Accordingly, the purpose of our study was to describe and characterize the specific organ metastatic rates in LUAD patients, using the database on a population level. We also aimed to show the overall survival time and to analyses the demographic, social status, and clinical treatments factors, like surgery resection, for survival outcomes in LUAD patients with specific organ metastases. The intention was to provide a credible explanation of routine screening of the specific organs metastatic status in newly diagnosed LUAD patients and to better optimize the treatment procedures, and thus improve the prognosis of this poor outcome cohort.
MATERIALS AND METHODS
The SEER database provides cancer statistics information, including cancer incidence, treatment, and survival covering approximately 48% of the US population. Using the software SEER*Stat 8.4.0, based on the November 2021 submission, we searched and collected data of patients diagnosed as having a primary LUAD between 2010 and 2015 within the SEER plus database. 13 As the data was anonymized and public, written informed consent and additional institutional review board approval were waived.
Patients reported from hospitals and clinics, diagnosed with positive histology as the only primary LUAD (ICD‐O‐3 histology code 8140), with a defined period of follow‐up and without cancer statistics information missing were included. We stratified patients by the interest variables of diagnosed year, age, sex, race/ethnicity, marital status, insurance status, primary site, TNM stage (7th edition), organ‐specific metastases, surgery, chemotherapy, and radiation therapy. Patients who had died of other causes or with any unknown variables information (demographic, clinical, and treatment) were excluded.
We calculated the absolute numbers for LUAD patients with specific organ metastases at diagnosis. Also, the specific organ metastases incidence rates were calculated by the number of metastatic LUAD patients divided by total number of LUAD patients. We also calculated the metastatic rates after interest variables stratification. According to the SEER database, surgery for the primary site was categorized as no surgery, local tumor destruction, sublobular resection, lobectomy or extended (including bilobectomy and pneumonectomy).
We performed multivariable logistic regression to determine if the interest demographic and clinical variables were associated with organ‐specific metastases combination at diagnosis. Kaplan–Meier analysis was performed to obtain the patients' prognostic overall survival estimates. Using the interest variables, univariate analysis and the multivariable Cox regression proportional hazards model were used to obtain the independent risk factors associated with increased LUAD mortality. Statistical calculations were performed using SPSS version 26.0 (IBM) and PRISM version 9.3 (GraphPad Software).
RESULTS
In total, 40 117 patients diagnosed as LUAD were included for specific organ metastatic rates analysis, with 43.69%, 26.25%, 19.66%, 10.60%, and 17.89% with specific organ metastases, bone metastases, brain metastases, liver metastases, and lung metastases, respectively. Stratified by metastatic organ number, 10 507 (26.19%) patients developed one metastatic organ, 4899 (12.21%) patients had two metastatic organs, 1745 (4.35%) patients had three metastatic organs, 378 (0.94%) patients had four metastatic organs, thus 7022 (17.50%) patients had two to four metastatic organs. In the LUAD metastatic cohort, metastatic rates were highest in patients presenting with bone metastases (53.85%), more than half of the entire group, while the liver metastatic rate was the lowest, 21.59%. The LUAD patient number and specific organ metastatic information at diagnosis are listed in Table 1.
TABLE 1.
Specific organ metastatic rates and average survivals in LUAD patients
| Patients | LUAD patients | Organ metastatic cohort | Average survival (months) | 95% CI | |
|---|---|---|---|---|---|
| No. | Percentage | Percentage | |||
| Total | 40 117 | 100% | NA | 25.82 | 25.56–26.09 |
| Without any organ metastasis | 22 588 | 56.31% | NA | 36.40 | 36.03–36.78 |
| With specific organ metastasis | 17 529 | 43.69% | 100% | 12.19 | 11.95–12.42 |
| With bone metastasis | 9440 | 23.53% | 53.85% | 10.81 | 10.52–11.11 |
| With brain metastasis | 7167 | 17.87% | 40.89% | 11.94 | 11.58–12.31 |
| With liver metastasis | 3784 | 9.43% | 21.59% | 8.43 | 8.03–8.82 |
| With lung metastasis | 6661 | 16.60% | 38.00% | 12.60 | 12.21–12.99 |
| Metastatic organ no. | |||||
| 1 | 10 507 | 26.19% | 59.94% | 13.81 | 13.48–14.15 |
| 2 | 4899 | 12.21% | 27.95% | 10.23 | 9.84–10.62 |
| 3 | 1745 | 4.35% | 9.95% | 8.70 | 8.12–9.27 |
| 4 | 378 | 0.94% | 2.16% | 8.31 | 7.20–9.42 |
| 2–4 | 7022 | 17.50% | 40.06% | 9.75 | 9.43–10.06 |
Abbreviations: CI, confidence interval; LUAD, lung adenocarcinoma; NA, not applicable.
For multivariable analysis logistic regression in LUAD patients with specific organ metastases, age older than 70 years (odds ratio [OR] = 1.68, 95% confidence interval [CI] = 1.46–1.93, p < 0.01) and age 46–70 years (OR = 1.43, 95% CI = 1.25–1.65, p < 0.01) referred to age 18–45 years, without insurance (no Purchased/Referred Care Delivery Area [PRCDA]), (OR = 1.06, 95% CI = 1.01–1.12, p = 0.02) referred to insured status, female (OR = 1.17, 95% CI = 1.12–1.22, p < 0.01) referred to male, black race (OR = 1.10, 95% CI = 1.03–1.17, p = 0.01) referred to white, and right side of primary tumors (OR = 1.13, 95% CI = 1.08–1.18, p < 0.01) referred to left were significantly associated with bigger ORs of LUAD having specific organ metastases at diagnosis. In addition, 2013–2015 diagnosed years (OR = 0.93, 95% CI = 0.89–0.97, p < 0.01) referred to 2010–2012, unmarried patients (OR = 0.92, 95% CI = 0.86–0.97, p < 0.01) referred to married, Asian or Pacific Islander race (OR = 0.90, 95% CI = 0.84–0.97, p = 0.01) referred to white, and lower lobe location of primary tumors (OR = 0.90, 95% CI = 0.86–0.94, p < 0.01) and main bronchus location (OR = 0.76, 95% CI = 0.67–0.87, p < 0.01) referred to upper lobe were significantly associated with smaller ORs of LUAD having specific organ metastases. However, diagnosed years and age in LUAD patients in the liver or lung metastases, sex in brain or lung metastases, marital status in bone, liver or lung metastases, insurance status in bone, brain or liver metastases, and laterality in brain or liver metastases cohorts were not associated with a risk of each organ metastases in the multivariable logistic regression model. Table 2 shows all the detailed multivariable logistic regression analysis results, categorized by the different kinds of LUAD metastatic organ.
TABLE 2.
Multivariable analysis logistic regression for LUAD patients with specific organ metastases
| Variables | LUAD patients | Specific organ metastatic cohort | P | Bone metastatic cohort | P | ||
|---|---|---|---|---|---|---|---|
| N = 40 117 | N = 17 529 | Odds ratio | N = 9440 | Odds ratio | |||
| N (%) | N (%) | OR (95% CI) | N (%) | OR (95% CI) | |||
| Diagnosed years | |||||||
| 2010–2012 | 18 116 (45.16%) | 7835 (44.70%) | 1 | NA | 4101 (43.44%) | 1 | NA |
| 2013–2015 | 22 001 (54.84%) | 9694 (55.30%) | 0.93 (0.89–0.97) | <0.01 | 5339 (56.56%) | 0.88 (0.84–0.93) | <0.01 |
| Sex | |||||||
| Male | 19 109 (47.63%) | 8936 (50.98%) | 1 | NA | 5059 (53.59%) | 1 | NA |
| Female | 21 008 (52.37%) | 8593 (49.02%) | 1.17 (1.12–1.22) | <0.01 | 4381 (46.41%) | 1.29 (1.23–1.36) | <0.01 |
| Diagnosed age | |||||||
| 18–45 | 1001 (2.50%) | 583 (3.33%) | 1 | NA | 325 (3.44%) | 1 | NA |
| 46–70 | 23 938 (59.67%) | 10 908 (62.23%) | 1.43 (1.25–1.65) | <0.01 | 5889 (62.38%) | 1.30 (1.12–1.49) | <0.01 |
| >70 | 15 178 (37.83%) | 6038 (34.45%) | 1.68 (1.46–1.93) | <0.01 | 3226 (34.17%) | 1.47 (1.27–1.70) | <0.01 |
| Marital status | |||||||
| Married | 33 857 (84.40%) | 14 576 (83.15%) | 1 | NA | 7937 (84.08%) | 1 | NA |
| Unmarried | 6260 (15.60%) | 2953 (16.85%) | 0.92 (0.86–0.97) | <0.01 | 1503 (15.92%) | 1.03 (0.96–1.10) | 0.37 |
| Insurance, PRCDA | |||||||
| Yes | 10 826 (26.99%) | 4816 (27.47%) | 1 | NA | 2580 (27.33%) | 1 | NA |
| No | 29 291 (73.01%) | 12 713 (72.53%) | 1.06 (1.01–1.12) | 0.02 | 6860 (72.67%) | 1.03 (0.97–1.09) | 0.32 |
| Race, ethnicity | |||||||
| White | 31 135 (77.61%) | 13 450 (76.73%) | 1 | NA | 7290 (77.22%) | 1 | NA |
| American Indian | 188 (0.47%) | 94 (0.54%) | 0.90 (0.66–1.24) | 0.53 | 48 (0.51%) | 1.01 (0.72–1.43) | 0.94 |
| Asian or PI | 3776 (9.41%) | 1758 (10.03%) | 0.90 (0.84–0.97) | 0.01 | 989 (10.48%) | 0.90 (0.83–0.97) | 0.01 |
| Black | 5018 (12.51%) | 2227 (12.70%) | 1.10 (1.03–1.17) | 0.01 | 1113 (11.79%) | 1.17 (1.08–1.26) | <0.01 |
| Laterality | |||||||
| Left | 16 017 (39.93%) | 7169 (40.90%) | 1 | NA | 3951 (41.85%) | 1 | NA |
| Right | 24 100 (60.07%) | 10 360 (59.10%) | 1.13 (1.08–1.18) | <0.01 | 5489 (58.15%) | 1.15 (1.09–1.20) | <0.01 |
| Primary site | |||||||
| Upper lobe | 24 825 (61.88%) | 10 632 (60.65%) | 1 | NA | 5623 (59.57%) | 1 | NA |
| Middle lobe | 2091 (5.21%) | 910 (5.19%) | 0.92 (0.83–1.02) | 0.10 | 481 (5.10%) | 0.92 (0.82–1.03) | 0.14 |
| Lower lobe | 11 596 (28.91%) | 5076 (28.96%) | 0.90 (0.86–0.94) | <0.01 | 2849 (30.18%) | 0.86 (0.81–0.91) | <0.01 |
| Overlapping | 440 (1.10%) | 200 (1.14%) | 1.19 (0.98–1.46) | 0.09 | 92 (0.97%) | 1.28 (1.01–1.63) | 0.04 |
| Main bronchus | 1165 (2.90%) | 711 (4.06%) | 0.76 (0.67–0.87) | <0.01 | 395 (4.18%) | 0.79 (0.69–0.89) | <0.01 |
| Brain metastatic cohort | P | Liver metastatic cohort | P | Lung metastatic cohort | P | |||
|---|---|---|---|---|---|---|---|---|
| N = 7167 | Odds ratio | N = 3784 | Odds ratio | N = 6661 | Odds ratio | |||
| N (%) | OR (95% CI) | N (%) | OR (95% CI) | N (%) | OR (95% CI) | |||
| 3180 (44.37%) | 1 | NA | 1689 (44.64%) | 1 | NA | 2978 (44.71%) | 1 | NA |
| 3987 (55.63%) | 0.94 (0.89–0.99) | 0.02 | 2095 (55.36%) | 0.96 (0.89–1.02) | 0.20 | 3683 (55.29%) | 0.96 (0.90–1.01) | 0.14 |
| 3467 (48.37%) | 1 | NA | 1956 (51.69%) | 1 | NA | 3315 (49.77%) | 1 | NA |
| 3700 (51.63%) | 0.95 (0.90–1.00) | 0.07 | 1828 (48.31%) | 1.12 (1.04–1.20) | <0.01 | 3346 (50.23%) | 0.98 (0.93–1.04) | 0.58 |
| 285 (3.98%) | 1 | NA | 115 (3.04%) | 1 | NA | 218 (3.27%) | 1 | NA |
| 4983 (69.53%) | 1.33 (1.15–1.53) | <0.01 | 2361 (62.39%) | 1.02 (0.84–1.25) | 0.82 | 3918 (58.82%) | 1.14 (0.96–1.35) | 0.13 |
| 1899 (26.50%) | 2.34 (2.01–2.72) | <0.01 | 1308 (34.57%) | 1.10 (0.90–1.36) | 0.35 | 2525 (37.91%) | 0.99 (0.83–1.18) | 0.91 |
| 5872 (81.93%) | 1 | NA | 3164 (83.62%) | 1 | NA | 5550 (83.32%) | 1 | NA |
| 1295 (18.07%) | 0.90 (0.83–0.96) | <0.01 | 620 (16.38%) | 0.97 (0.89–1.07) | 0.60 | 1111 (16.68%) | 0.96 (0.89–1.04) | 0.36 |
| 1936 (27.01%) | 1 | NA | 1026 (27.11%) | 1 | NA | 1978 (29.70%) | 1 | NA |
| 5231 (72.99%) | 1.01 (0.95–1.07) | 0.74 | 2758 (72.89%) | 1.00 (0.92–1.08) | 0.97 | 4683 (70.30%) | 1.21 (1.13–1.29) | <0.01 |
| 5494 (76.66%) | 1 | NA | 2953 (78.04%) | 1 | NA | 4944 (74.22%) | 1 | NA |
| 41 (0.57%) | 0.89 (0.62–1.27) | 0.51 | 20 (0.53%) | 0.99 (0.61–1.58) | 0.95 | 44 (0.66%) | 0.76 (0.52–1.11) | 0.16 |
| 766 (10.69%) | 0.86 (0.79–0.94) | <0.01 | 369 (9.75%) | 1.01 (0.90–1.13) | 0.92 | 820 (12.31%) | 0.67 (0.62–0.74) | <0.01 |
| 866 (12.08%) | 1.20 (1.11–1.31) | <0.01 | 442 (11.68%) | 1.18 (1.06–1.32) | <0.01 | 853 (12.81%) | 1.02 (0.93–1.11) | 0.68 |
| 2922 (40.77%) | 1 | NA | 1547 (40.88%) | 2766 (41.53%) | 1 | NA | ||
| 4245 (59.23%) | 1.07 (1.01–1.13) | 0.02 | 2237 (59.12%) | 1.07 (1.00–1.15) | 0.06 | 3895 (58.47%) | 1.15 (1.08–1.22) | <0.01 |
| 4473 (62.41%) | 1 | NA | 2188 (57.82%) | 1 | NA | 3944 (59.21%) | 1 | NA |
| 370 (5.16%) | 1.01 (0.89–1.14) | 0.89 | 183 (4.84%) | 0.97 (0.83–1.15) | 0.74 | 366 (5.49%) | 0.81 (0.71–0.92) | <0.01 |
| 1973 (27.53%) | 1.04 (0.98–1.1) | 0.24 | 1172 (30.97%) | 0.83 (0.77–0.89) | <0.01 | 1966 (29.52%) | 0.83 (0.78–0.89) | <0.01 |
| 63 (0.88%) | 1.55 (1.18–2.04) | <0.01 | 49 (1.29%) | 0.89 (0.65–1.20) | 0.44 | 101 (1.52%) | 0.90 (0.70–1.14) | 0.38 |
| 288 (4.02%) | 0.89 (0.77–1.03) | 0.11 | 192 (5.07%) | 0.67 (0.57–0.79) | <0.01 | 284 (4.26%) | 0.98 (0.84–1.13) | 0.75 |
Abbreviations: CI, confidence interval; LUAD, lung adenocarcinoma; OR, odds ratio; NA, not applicable; PI, pacific islander; PRCDA, purchased/referred care delivery area.
Average overall survival in LUAD patients in different kinds of LUAD metastatic organ cohorts is showed in Table 1. The average survival in LUAD patients with specific organ metastases cohort was 12.19 months, compared to 36.40 months in patients without any organ metastases cohort. In the different kinds of LUAD metastatic organ cohorts, the longest average overall survival was 12.60 months in the lung metastases cohort, and the shortest average overall survival was 8.43 months in the liver metastases cohort.
For multivariable analysis Cox regression in LUAD patients with specific organ metastases, age older than 70 years (hazard ratio [HR] = 1.63, 95% CI = 1.48–1.79, p < 0.01) and age 46 to 70 years (HR = 1.39, 95% CI = 1.27–1.52, p < 0.01) referred to age 18–45 years, unmarried patients (HR = 1.05, 95% CI = 1.01–1.10, p = 0.02) referred to married, middle lobe location of primary tumors (HR = 1.20, 95% CI = 1.11–1.30, p < 0.01) referred to upper lobe, T‐stage (T2, HR = 1.17, 95% CI = 1.11–1.23, p < 0.01; T3, HR = 1.19, 95% CI = 1.11–1.24, p < 0.01; T4, HR = 1.17, 95% CI = 1.11–1.23, p < 0.01; referred to T1), N‐stage (N1, HR = 1.13, 95% CI = 1.06–1.20, p < 0.01; N2, HR = 1.27, 95% CI = 1.22–1.32, p < 0.01; N3, HR = 1.35, 95% CI = 1.28–1.41, p < 0.01; referred to N0), metastatic organ number (two metastatic organs, HR = 1.28, 95% CI = 1.24–1.33, p < 0.01; three metastatic organs, HR = 1.51, 95% CI = 1.43–1.59, p < 0.01; four metastatic organs, HR = 1.65, 95% CI = 1.48–1.83, p < 0.01; referred to one metastatic organ), without surgery (HR = 1.82, 95% CI = 1.65–2.01, p < 0.01) referred to with surgery, without chemotherapy (HR = 2.69, 95% CI = 2.60–2.79, p < 0.01) referred to with chemotherapy, and without radiation and surgery (HR = 1.17, 95% CI = 1.09–1.25, p < 0.01) were significantly associated with bigger HRs of LUAD having specific organ metastases at diagnosis. In addition, 2013–2015 diagnosed years (HR = 0.91, 95% CI = 0.89–0.94, p < 0.01) referred to 2010–2012, female (HR = 0.81, 95% CI = 0.78–0.83, p < 0.01) referred to male, Asian or Pacific Islander race (HR = 0.68, 95% CI = 0.64–0.71, p < 0.01) referred to white, and lower lobe location of primary tumors (HR = 0.93, 95% CI = 0.86–1.00, p = 0.04) referred to upper lobe, and without radiation (HR = 0.97, 95% CI = 0.93–1.00, p = 0.04) referred to radiation were significantly associated with decreased cancer‐cause mortality in LUAD patients having specific organ metastases. Insurance (PRCDA), laterality or therapy with both chemotherapy and surgery were not associated with cancer‐cause mortality in the multivariable Cox regression model. The cancer‐specific mortality in different kinds of LUAD metastatic organ patients is showed in Table 3 and Table S1.
TABLE 3.
Multivariable analysis Cox regression for cancer‐specific survival in patients with LUAD specific organ metastases
| Variables | LUAD patients with specific organ metastases | Variable | LUAD patients with specific organ metastases | ||||
|---|---|---|---|---|---|---|---|
| N = 17 529 | Hazard ratio (95% CI) | P | n = 17 529 | Hazard ratio (95% CI) | P | ||
| N (%) | No. | ||||||
| Diagnosed years | T stage | ||||||
| 2010–2012 | 7835 (44.70%) | 1 | NA | T1 | 2343 (13.37%) | 1 | NA |
| 2013–2015 | 9694 (55.30%) | 0.91 (0.89–0.94) | <0.01 | T2 | 4798 (27.37%) | 1.17 (1.11–1.23) | <0.01 |
| Sex | T3 | 4472 (25.51%) | 1.18 (1.11–1.24) | <0.01 | |||
| Male | 8936 (50.98%) | 1 | NA | T4 | 5916 (33.75%) | 1.17 (1.11–1.23) | <0.01 |
| Female | 8593 (49.02%) | 0.81 (0.78–0.83) | <0.01 | N‐stage | |||
| Diagnosed age | N0 | 4040 (23.05%) | 1 | NA | |||
| 18–45 | 583 (3.33%) | 1 | NA | N1 | 1471 (8.39%) | 1.13 (1.06–1.20) | <0.01 |
| 46–70 | 10 908 (62.23%) | 1.39 (1.27–1.52) | <0.01 | N2 | 8086 (46.13%) | 1.27 (1.22–1.32) | <0.01 |
| >70 | 6038 (34.45%) | 1.63 (1.48–1.79) | <0.01 | N3 | 3932 (22.43%) | 1.35 (1.28–1.41) | <0.01 |
| Marital status | Metastatic organ no. | ||||||
| Married | 14 576 (83.15%) | 1 | NA | 1 | 10 507 (59.94%) | 1 | NA |
| Unmarried | 2953 (16.85%) | 1.05 (1.01–1.10) | 0.02 | 2 | 4899 (27.95%) | 1.28 (1.24–1.33) | <0.01 |
| Insurance, PRCDA | 3 | 1745 (9.95%) | 1.51 (1.43–1.59) | <0.01 | |||
| Yes | 4816 (27.47%) | 1 | NA | 4 | 378 (2.16%) | 1.65 (1.48–1.83) | <0.01 |
| No | 12 713 (72.53%) | 1.01 (0.97–1.04) | 0.70 | Surgery | |||
| Race, ethnicity | Yes | 571 (3.26%) | 1 | NA | |||
| White | 13 450 (76.73%) | 1 | NA | No | 16 958 (96.74%) | 1.82 (1.65–2.01) | <0.01 |
| American Indian | 94 (0.54%) | 0.89 (0.72–1.10) | 0.29 | Chemotherapy | |||
| Asian or PI | 1758 (10.03%) | 0.68 (0.64–0.71) | <0.01 | Yes | 10 600 (60.47%) | 1 | NA |
| Black | 2227 (12.70%) | 0.99 (0.95–1.04) | 0.72 | No | 6929 (39.53%) | 2.69 (2.60–2.79) | <0.01 |
| Laterality | Radiation | ||||||
| Left | 7169 (40.90%) | 1 | NA | Yes | 9794 (55.87%) | 1 | NA |
| Right | 10 360 (59.10%) | 1.03 (1.00–1.06) | 0.08 | No | 7735 (44.13%) | 0.97 (0.93–1.00) | 0.04 |
| Primary site | Chemotherapy and surgery | ||||||
| Upper lobe | 10 632 (60.65%) | 1 | NA | Yes | 2348 (13.39%) | 1 | NA |
| Middle lobe | 910 (5.19%) | 1.20 (1.11–1.30) | <0.01 | No | 15 181 (86.61%) | 1.01 (0.95–1.08) | 0.76 |
| Lower lobe | 5076 (28.96%) | 0.93 (0.86–1.00) | 0.04 | Radiation and surgery | |||
| Overlapping | 200 (1.14%) | 1.03 (0.99–1.06) | 0.13 | Yes | 2246 (12.81%) | 1 | NA |
| Main bronchus | 711 (4.06%) | 1.08 (0.93–1.24) | 0.32 | No | 15 283 (87.19%) | 1.17 (1.09–1.25) | <0.01 |
Abbreviations: CI, confidence interval; LUAD, lung adenocarcinoma; NA, not applicable; PI, pacific islander; PRCDA, purchased/referred care delivery area.
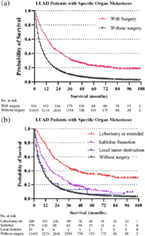
Surgery was significantly associated with decreased cancer‐cause mortality in LUAD patients with all kinds of specific metastatic organs. The surgery HR (1.51) was almost the same as that for chemotherapy HR (1.72) in LUAD patients with bone metastases. Average overall survival in LUAD patients with specific organ metastases was categorized by the type of surgery (no surgery, local tumor destruction, sub‐lobar resection, and lobectomy or extended). In total, 571 LUAD patients with specific organ metastases received surgery management. Sublobar resection was the most common surgery type (294 of 571,51.49%), followed by lobectomy or extended surgey (251 of 571, 43.96%), and local tumor destruction (26 of 571,4.55%). Overall survival estimates in the specific organ metastases cohort (Figure 1) and each specific organ metastases cohort (Figure 2, bone and brain metastases cohort; Figure 3, liver and lung metastases cohort), stratified by whether received surgery and different kinds of resections categorized by the range of surgery resection (no surgery, local tumor destruction, sublobular resection, and lobectomy or extended), are shown in the figures 1–3. In general, survival was better in patients who received surgery therapy with more extensive resection, even the ratios of patients who received surgery were small in each cohort. No surgery and local tumor destruction only were associated with shorter overall survival time compared to patients with other more aggressive surgery (Table 4).
FIGURE 1.
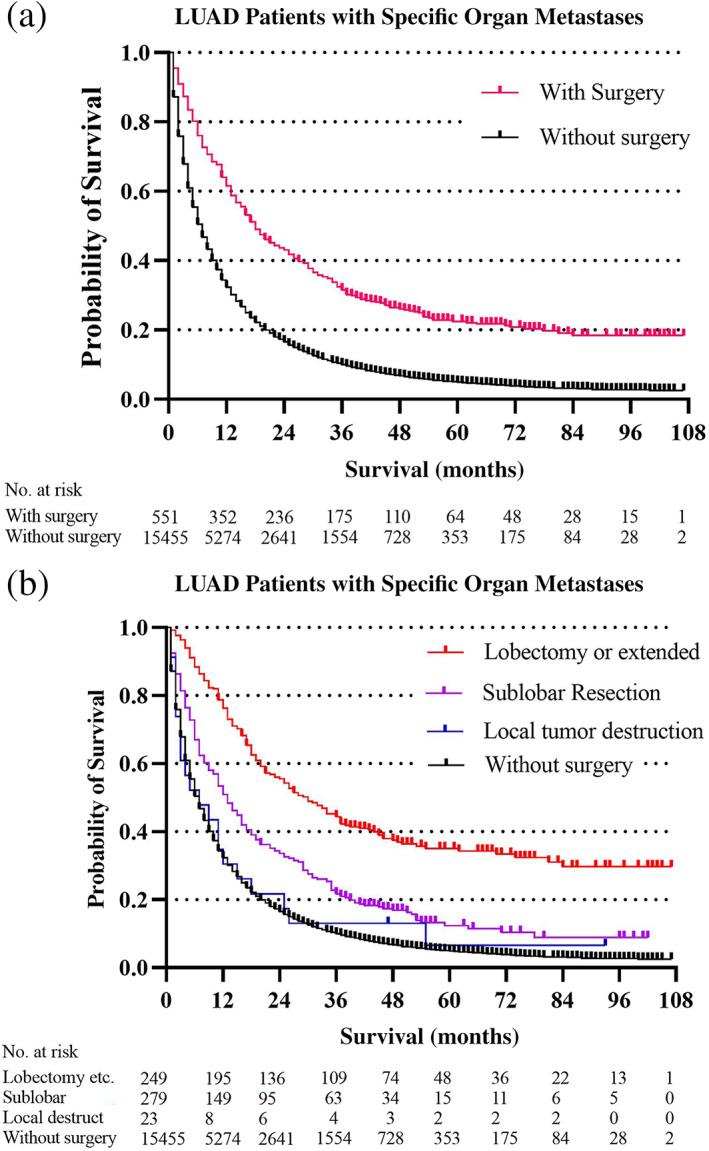
Overall survival outcomes in LUAD patients with specific organ metastases stratified by surgery (a) and different surgery procedures (b)
FIGURE 2.
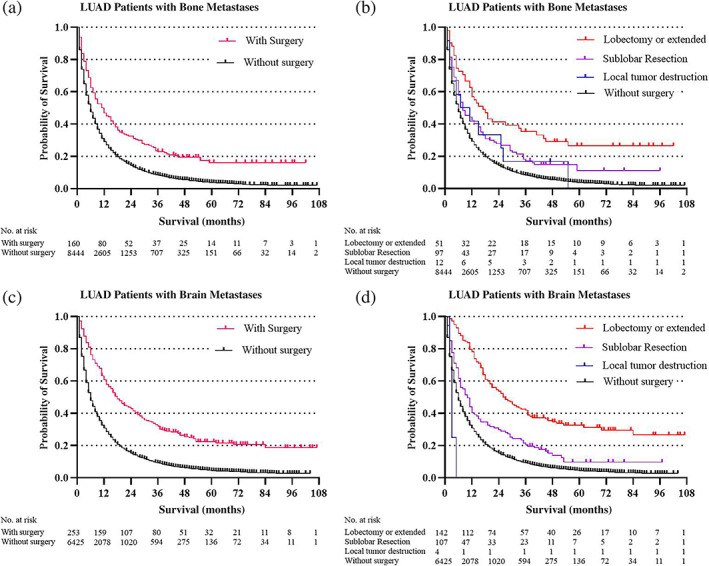
Overall survival outcomes in LUAD patients with different organ metastases stratified by surgery and different surgery procedures: (a, b) Bone metastases cohort; (c, d) brain metastases cohort
FIGURE 3.
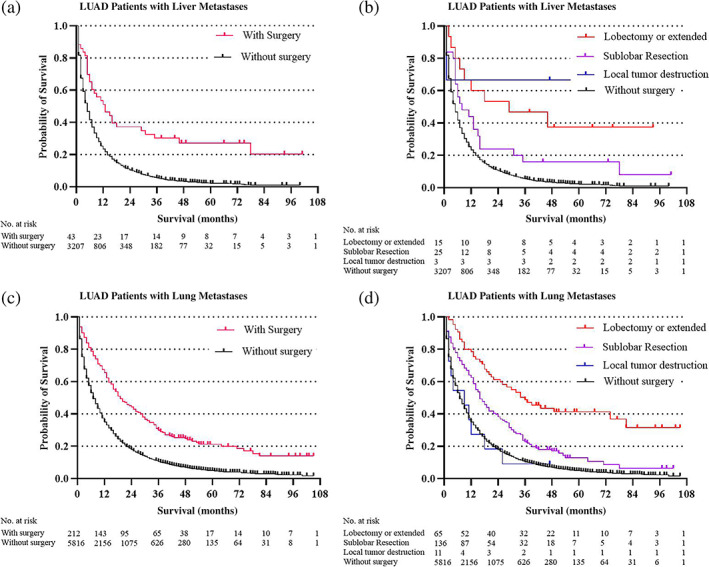
Overall survival outcomes in LUAD patients with different organ metastases stratified by surgery and different surgery procedures: (a, b) liver metastases cohort; (c, d) lung metastases cohort
TABLE 4.
Average survival categorized by surgery type in LUAD patients with specific organ metastases
| Patients | LUAD patients | Without surgery | Average survival (95% CI) | Local tumor destruction | |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | Average survival (95% CI) | ||
| With specific organ metastases | 17 529 (43.69%) | 16 958 (96.74%) | 11.71 (11.48–11.94) | 26 (0.15%) | 13.73 (5.09–22.37) |
| With bone metastases | 9440 (26.25%) | 9273 (98.23%) | 10.63 (10.34–10.93) | 12 (0.13%) | 16.58 (4.97–28.19) |
| With brain metastases | 7167 (19.66%) | 6910 (96.41%) | 11.39 (11.04–11.75) | 5 (0.07%) | 2.60 (0.34–4.86) |
| With liver metastases | 3784 (10.60%) | 3734 (98.68%) | 8.24 (7.85–8.62) | 4 (0.11%) | NA |
| With lung metastases | 6661 (17.89%) | 6443 (96.73%) | 12.15 (11.77–12.53) | 12 (0.18%) | 11.25 (2.53–19.97) |
| Metastatic organ no. | |||||
| 1 | 10 507 (26.19%) | 10 043 (95.58%) | 13.14 (12.82–13.47) | 20 (0.19%) | 13.65 (3.08–24.22) |
| 2 | 4899 (12.21%) | 4806 (98.10%) | 10.08 (9.69–10.46) | 5 (0.10%) | NA |
| 3 | 1745 (4.35%) | 1731 (99.20%) | 8.66 (8.08–9.23) | 1 (0.06%) | NA |
| 4 | 378 (0.94%) | 378 (100.00%) | 8.31 (7.20–9.42) | 0 (0.00%) | NA |
| 2–4 | 7022 (17.50%) | 6915 (98.48%) | 9.63 (9.32–9.94) | 6 (0.09%) | NA |
| Patients | Sublobar resection | Lobectomy or extended | ||
|---|---|---|---|---|
| N (%) | Average survival (95% CI) | N (%) | Average survival (95% CI) | |
| With specific organ metastases | 294 (1.68%) | 19.90 (17.46–22.33) | 251 (1.43%) | 35.16 (31.69–38.63) |
| With bone metastases | 104 (1.10%) | 16.36 (12.57–20.14) | 51 (0.54%) | 30.69 (22.11–39.26) |
| With brain metastases | 110 (1.53%) | 18.54 (14.62–22.45) | 142 (1.98%) | 33.89 (29.44–38.35) |
| With liver metastases | 31 (0.82%) | 16.16 (6.90–25.43) | 15 (0.40%) | 32.67 (16.79–48.54) |
| With lung metastases | 139 (2.09%) | 22.47 (18.97–25.98) | 67 (1.01%) | 35.52 (28.86–42.19) |
| Metastatic organ no. | ||||
| 1 | 214 (2.04%) | 20.88 (18.00–23.76) | 230 (2.19%) | 36.58 (32.91–40.24) |
| 2 | 70 (1.43%) | 17.84 (12.69–22.99) | 18 (0.37%) | 22.28 (12.63–31.92) |
| 3 | 10 (0.57%) | 13.3 (4.21–22.39) | 3 (0.17%) | NA |
| 4 | 0 (0.00%) | NA | 0 (0.00%) | NA |
| 2–4 | 80 (1.14%) | 17.28 (12.67–21.88) | 21 (0.30%) | 19.62 (10.93–28.31) |
Abbreviations: CI, confidence interval; LUAD, lung adenocarcinoma; NA, not applicable.
DISCUSSION
In this study, we described and characterized the absolute number, metastatic rates, and subsequent prognosis in primarily diagnosed LUAD patients with specific organ metastases. As the SEER database encompasses approximately half of the USA population, the LUAD metastatic rates and average patient survival results are highly generalizable. Moreover, this huge LUAD patients' number and the adequate follow‐up information enhance the power of our study and improve the ability to characterize reliable key factors associated with specific organ metastases and prognosis. 14
We stratified our estimates relating to the metastatic rates and survival outcomes of patients by diagnosed years, genders, age groups, insured status, ethnicities, and metastatic organs. We found almost half of LUAD patients were diagnosed with specific organ metastases. Among the LUAD patients with specific organ metastases, the metastatic rate of bone metastases was highest. The incidence proportion was near 10% in the liver metastases cohort with the lowest metastatic rate. Compared with LUAD patients without specific organ metastases, the average overall survival was significantly shorter in those with specific organ metastases. Also, the average overall survival varied in different types of metastatic organ cohorts. As an important cause of morbidity and mortality of LUAD‐specific organ metastases, it is necessary for recommendation of specific organ (bone, brain, liver, lung etc.) image screening among newly diagnosed LUAD patients. 15 , 16
Many variables were noted to be significantly associated with the ORs of the presence of specific organ metastases. Factors such as age and uninsured status have been shown to be associated with poorer outcomes in patients with cancer, so it is reasonable to assume that they have an increased risk of specific organ metastases at diagnosis. 17 , 18 The factor of more recently diagnosed year is associated with a decreased risk of specific organ metastases at diagnosis and favorable outcomes, which might be logical because the screening, diagnosis, and treatment technology are more advanced in more recent years. 19 , 20 This is also consistent with other malignances, such as breast cancer, where patients diagnosed as late stage today have a lower disease burden than those from earlier eras. 20 For race, a smaller proportion developing specific organ metastases and better outcomes among patients of Asian or Pacific Islander versus White race were noted, while larger specific organ metastases incidence rates among Black versus White race. 21 , 22 Previous studies have reported that race might independently predict the gene epidermal growth factor receptor (EGFR) mutation, which was showed to be an independent predictor and prognostic factor for brain metastases in LUAD patients. 23 Further research is warranted to find out whether environmental or biologic factors are responsible for these associations. 24 , 25
We also found that the primary site of the upper lobe was associated with shorter survival time compared with the lower lobe, consistently with the lung cancer. 26 , 27 The same was found for right side tumors versus left side. Other factors, such as unmarried status, increasing tumor nodal stage, and metastatic organ number, are significantly associated with gradually less favorable outcomes. As an important personal social environmental factor, marriage could be the psychological reflection of LUAD patients' overall survivals, consistent with previous studies which reported that marital status was a prognostic factor for distant metastases of non‐small‐cell lung cancer. 28 For different types of therapy, surgical resection, as well as chemotherapy and radiation, could effectively prolong the overall survival time of LUAD patients with specific organ metastases. 29 Of note, patients who received surgical resection had more favorable outcomes in each LUAD metastatic organ cohort. In addition, with more lung resection and more lymph node dissection, survival time gradually became longer, which is in line with the general clinical experience for earlier stage LUAD. Notably, for the different types of surgery management, lobectomy or extended resection had a survival benefit over sublobar resection, while local tumor destruction had almost the same survival as no surgery. Although our results show that surgery resection could prolong the overall survival time of LUAD patients with specific organ metastases, the patients who could be operated on were few. Because most patients were without surgical indications by the treatment guidelines.
In clinical practice, almost no patients with specific organ metastases were recommended to receive surgery resection. For the patients who did receive surgery, sublobar resection was the most common type as it reduces operation time, preserves pulmonary function, and reduces the incidence of postoperative complications. 30 , 31 This was consistent with the results of our study. We also found that lobectomy or extended surgery conferred a better survival outcome compared with sublobar resection for LUAD with specific organ metastases, while there was a poor long‐term survival for local tumor destruction. In LUAD patients in the brain metastases cohort, the most common type of surgery procedure was lobectomy or extended surgery, which also had the best overall survival. 32 To our knowledge, this is the first population‐based survival outcome study in newly diagnosed LUAD patients with specific organ metastases. As the number of patietns who received surgery was small, further randomized controlled trails are needed to confirm the surgery benefits for survival and to determine the best surgical procedure for LUAD patients with specific organ metastases.
Despite the sufficient evidences, reasonable arguments, and innovative viewpoints, there are some limitations to this study. First, the SEER database does not provide information on other risk factors influencing the prognosis, such as smoking history, family history, pulmonary function, gene mutations (proved to be oncogenic drivers, such as EGFR mutation), protein expression, comorbidities, chemotherapy regimens or reception of targeted therapy, so those variables were not used in our analyses, which might result in bias. 33 , 34 Regretfully, since it is an important treatment for LUAD, targeted therapy was not evaluated in this study. Importantly, the pathological subtypes information of LUAD could not be obtained when using the LUAD selection criteria, as the subtypes were labeled with different histology codes, for example 8550 for acinar cell carcinoma. Thus, the relationship between the subtypes and prognosis should be further analyzed in the future. Also, recurrence follow‐up information was not available, so our study was limited to the presence or absence of identified specific organ metastases at diagnosis. In addition, patients treated in hospitals or institutes outside the SEER network were not included or their clinical information was incomplete. Different levels of medical skills should be considered in future studies. Finally, our study was a retrospective cohort analysis, with retrospective research limitations. A prospective cohort validation study is warranted to obtain more evidence.
CONCLUSION
This study provides insights into the metastatic rates and prognosis of specific organ metastases in LUAD patients. Data for the incidence proportions of specific organ metastases, survival outcomes, and the impact of different surgical procedures on patients with specific organ metastases are of broad clinical interest and will continue to optimize diagnosis and treatment plans, and help improve the prognosis of these patients. According to our SEER database analysis results, surgery resection might improve overall survival outcomes in LUAD patients with specific organ metastases, and lobectomy or extended surgery might be the best surgery procedures. These findings warrant further investigation.
AUTHOR CONTRIBUTIONS
Conception and design: Z.Z.R., G.S.G., and H.J. Collection and assembly of data: Z.Z.R. and G.Y.B. Data analysis and interpretation: all authors. Manuscript writing: Z.Z.R., T.F.W., and X.Q. Final approval of manuscript: all authors. Accountable for all aspects of the work: all authors.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supporting information
TABLE S1. Multivariable analysis Cox regression for cancer‐specific survival in patients with lung adenocarcinoma‐specific organ metastases
ACKNOWLEDGMENTS
We thank all individuals who took part in this research. This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Zhao Z, Gao Y, Tan F, Xue Q, Gao S, He J. Specific organ metastases and prognosis in lung adenocarcinoma. Thorac Cancer. 2023;14(8):736–745. 10.1111/1759-7714.14801
DATA AVAILABILITY STATEMENT
The datasets presented in this study can be found in online repositories.
REFERENCES
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 3. Martin AM, Cagney DN, Catalano PJ, Warren LE, Bellon JR, Punglia RS, et al. Brain metastases in newly diagnosed breast cancer: a population‐based study. JAMA Oncol. 2017;3(8):1069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cagney DN, Martin AM, Catalano PJ, Redig AJ, Lin NU, Lee EQ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population‐based study. Neuro Oncol. 2017;19(11):1511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Altorki NK, Markowitz GJ, Gao D, Port JL, Saxena A, Stiles B, et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat Rev Cancer. 2019;19(1):9–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deb D, Moore AC, Roy UB. 2021 global lung cancer therapy landscape. J Thorac Oncol. 2022;17:931–6. [DOI] [PubMed] [Google Scholar]
- 7. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non‐small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(5):497–530. [DOI] [PubMed] [Google Scholar]
- 8. Zhao Z, Gao Y, Xue Q, Gao S, He J. Safety and efficacy of neoadjuvant immune checkpoint inhibitor therapy in patients with Resectable non‐small‐cell lung cancer: a systematic review. Target Oncol. 2021;16(4):425–34. [DOI] [PubMed] [Google Scholar]
- 9. Thomas A, Khan SA, Chrischilles EA, Schroeder MC. Initial surgery and survival in stage IV breast cancer in the United States, 1988‐2011. JAMA Surg. 2016;151(5):424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tarantino I, Warschkow R, Worni M, Cerny T, Ulrich A, Schmied BM, et al. Prognostic relevance of palliative primary tumor removal in 37,793 metastatic colorectal cancer patients: a population‐based, propensity score‐adjusted trend analysis. Ann Surg. 2015;262(1):112–20. [DOI] [PubMed] [Google Scholar]
- 11. Wang K, Shi Y, Li ZY, Xiao YL, Li J, Zhang X, et al. Metastatic pattern discriminates survival benefit of primary surgery for de novo stage IV breast cancer: a real‐world observational study. Eur J Surg Oncol. 2019;45(8):1364–72. [DOI] [PubMed] [Google Scholar]
- 12. Wu W, Zhang H, Fang Z, Li F. Primary tumor surgery improves survival of cancer patients with synchronous solitary bone metastasis: a large population‐based study. Ann Transl Med. 2021;9(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. American Joint Committee on Cancer staging manual, 7th edition [online]. Available at: http://www.cancerstaging.org. Accessed April 18.
- 14. Xu S, Li X, Ren F, He J, Zhao S, Wang Y, et al. Sublobar resection versus lobectomy for early‐stage pulmonary carcinoid tumors ≤3 cm in size: a SEER population‐based study. Ann Surg. 2020;276:e991–9. [DOI] [PubMed] [Google Scholar]
- 15. Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311. [DOI] [PubMed] [Google Scholar]
- 16. Jemal A, Miller KD, Ma J, Siegel RL, Fedewa SA, Islami F, et al. Higher lung cancer incidence in young women than young men in the United States. N Engl J Med. 2018;378(21):1999–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aizer AA, Falit B, Mendu ML, Chen MH, Choueiri TK, Hoffman KE, et al. Cancer‐specific outcomes among young adults without health insurance. J Clin Oncol. 2014;32(19):2025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sung H, Siegel RL, Rosenberg PS, Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population‐based cancer registry. Lancet Public Health. 2019;4(3):e137–e47. [DOI] [PubMed] [Google Scholar]
- 19. Huang J, Deng Y, Tin MS, Lok V, Ngai CH, Zhang L, et al. Distribution, risk factors, and temporal trends for lung cancer incidence and mortality: a global analysis. Chest. 2022;161(4):1101–11. [DOI] [PubMed] [Google Scholar]
- 20. Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The effect of advances in lung‐cancer treatment on population mortality. N Engl J Med. 2020;383(7):640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sosa E, D'Souza G, Akhtar A, Sur M, Love K, Duffels J, et al. Racial and socioeconomic disparities in lung cancer screening in the United States: a systematic review. CA Cancer J Clin. 2021;71(4):299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shusted CS, Evans NR, Kane GC, Juon HS, Barta JA. Analysis of lung cancer screening by race after USPSTF expansion of screening eligibility in 2021. JAMA Netw Open. 2022;5(6):e2217578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Waarts MR, Stonestrom AJ, Park YC, Levine RL. Targeting mutations in cancer. J Clin Invest. 2022;132(8):e154943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sepesi B, Gold KA, Correa AM, Heymach JV, Vaporciyan AA, Roszik J, et al. The influence of body mass index on overall survival following surgical resection of non‐small cell lung cancer. J Thorac Oncol. 2017;12(8):1280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Slatore CG, Chien JW, Au DH, Satia JA, White E. Lung cancer and hormone replacement therapy: association in the vitamins and lifestyle study. J Clin Oncol. 2010;28(9):1540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ge C, Peters S, Olsson A, Portengen L, Schuz J, Almansa J, et al. Respirable crystalline silica exposure, smoking, and lung cancer subtype risks. A pooled analysis of case‐control studies. Am J Respir Crit Care Med. 2020;202(3):412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chai X, Yinwang E, Wang Z, Wang Z, Xue Y, Li B, et al. Predictive and prognostic biomarkers for lung cancer bone metastasis and their therapeutic value. Front Oncol. 2021;11:692788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abdel‐Rahman O. Outcomes of surgery as part of the management of metastatic non‐small‐cell lung cancer: a surveillance, epidemiology and end results database analysis. Cancer Invest. 2018;36(4):238–45. [DOI] [PubMed] [Google Scholar]
- 30. Sihoe AD, Van Schil P. Non‐small cell lung cancer: when to offer sublobar resection. Lung Cancer. 2014;86(2):115–20. [DOI] [PubMed] [Google Scholar]
- 31. Tong KM, Laskin J, Ho C. Maintenance chemotherapy in advanced NSCLC: a population‐based assessment of eligibility. Lung Cancer. 2015;87(3):296–302. [DOI] [PubMed] [Google Scholar]
- 32. Vernon J, Andruszkiewicz N, Schneider L, Schieman C, Finley CJ, Shargall Y, et al. Comprehensive clinical staging for resectable lung cancer: clinicopathological correlations and the role of brain MRI. J Thorac Oncol. 2016;11(11):1970–5. [DOI] [PubMed] [Google Scholar]
- 33. Lee DW, Shin DY, Kim JW, Keam B, Kim TM, Kim HJ, et al. Additional prognostic role of EGFR activating mutations in lung adenocarcinoma patients with brain metastasis: integrating with lung specific GPA score. Lung Cancer. 2014;86(3):363–8. [DOI] [PubMed] [Google Scholar]
- 34. Ou SH, Ahn JS, De Petris L, Govindan R, Yang JC, Hughes B, et al. Alectinib in crizotinib‐refractory ALK‐rearranged non‐small‐cell lung cancer: a phase II global study. J Clin Oncol. 2016;34(7):661–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1. Multivariable analysis Cox regression for cancer‐specific survival in patients with lung adenocarcinoma‐specific organ metastases
Data Availability Statement
The datasets presented in this study can be found in online repositories.


