Abstract
To establish the role of B cells and antibodies in destroying filariae, mice lacking mature B cells and therefore unable to produce antibodies were used. Litomosoides sigmodontis offers a good opportunity for this study because it is the only filarial species that completes its life cycle in mice. Its development was compared in B-cell-deficient mice (BALB/c μMT mice) and wild-type BALB/c mice in two different in vivo situations, vaccination with irradiated larvae and primary infection. In all cases, mice were challenged with subcutaneous inoculation of 40 infective larvae. Vaccine-induced protection was suppressed in B-cell-deficient mice. In these mice, eosinophils infiltrated the subcutaneous tissue normally during immunization; however, their morphological state did not change following challenge inoculation, whereas in wild-type mice the percentage of degranulated eosinophils was markedly increased. From this, it may be deduced that the eosinophil–antibody–B-cell complex is the effector mechanism of protection in vaccinated mice and that its action is fast and takes place in the subcutaneous tissue. In primary infection, the filarial survival and growth was not modified by the absence of B cells. However, no female worm had uterine microfilariae, nor did any mice develop a patent infection. In these mice, concentrations of type 1 (gamma interferon) and type 2 (interleukin-4 [IL-4], IL-5 and IL-10) cytokines in serum were lower and pleural neutrophils were more numerous. The effects of the μMT mutation therefore differ from those in B1-cell-deficient mice described on the same BALB/c background, which reveal a higher filarial recovery rate and microfilaremia. This outlines B2-cell-dependent mechanisms as favorable to the late maturation of L. sigmodontis.
Despite new chemotherapy protocols (30), filariae are still a major cause of serious tropical diseases (9, 35); therefore, it is worthwhile to consider a vaccination approach as a complementary measure. We now have a particularly relevant experimental model at our disposal with the rodent filaria Litomosoides sigmodontis. It is the only filarial species able to regularly develop from the infective larvae to the patent phase in the BALB/c mice (18, 32). Therefore, the strength of this model is that is allows us to study and modulate the immune reaction during vaccination as well as during primary infection and thus to describe the immune mechanism critical for parasite control.
The kinetics of the recovery rate have been well defined in the L. sigmodontis mouse model; as observed in other experimental filarial systems (6), the recovery rate drops a few hours after challenge inoculation (phase 1) and then remains stable (phase 2) for 2 months (24, 26, 29). In BALB/c mice vaccinated with irradiated larvae, the recovery rate follows the same kinetics; however, there is a stronger reduction during phase 1, amounting to 65 to 70% protection (24, 29). In both cases, the larvae that escape the inflammatory reaction in the subcutaneous tissue penetrate the lymphatic vessels (39) and migrate to the pleural cavity (6, 25, 26, 29). The late development in vaccinated mice (adult maturation and patent phase) was similar, except that the worm load and the cytokine production were lower (25).
While studying the mechanisms of the vaccination induced protection, we provided evidence that it may be due to high subcutaneous infiltration of eosinophils that degranulate within the first hours following the challenge inoculation (25, 29). We then estimated the filaricidial capacity of the eosinophils by treating vaccinated mice with anti-interleukin-5 (IL-5) to suppress the differentiation of eosinophils and their infiltration into the subcutaneous tissue (29); these mice were no longer protected. In nonpermissive filarial models (13, 23), eosinophils were also involved in protection. Another approach was to use primary-infected mice overexpressing IL-5 and thus eosinophilic. Filarial mortality in the pleural cavity was faster, occurring by the first half of phase 2 (28).
Since eosinophils can mediate a special type of antibody-dependent cell-mediated cytotoxicity directed against helminth parasites (7), we supposed that the antibodies produced before the challenge in vaccination and belatedly in primary infection would induce this eosinophil degranulation and subsequently the killing of filariae, either in the subcutaneous tissue or in the pleural cavity (25, 29). However, while antibody-dependent cell-mediated cytotoxicity against filariae can be easily demonstrated in vitro (8, 14), its role in host defense in vivo is not clearly established. Indeed, passive transfer of hyperimmune serum did not protect nude or BALB/c mice against a Brugia malayi larval inoculum (17, 37).
Working on mutant mice helps clarify progressively the mechanisms that control filarial development. Recently, factors like IL-4, IL-5, and gamma interferon (IFN-γ) have been shown to be protective (5, 28, 34, 38), and others, like NK cells, seem to promote the infection (3), probably through their cytokine production.
In this study, we used μMT mice, which lack mature B cells, to analyze the consequences of this mutation on the early subcutaneous events following vaccination and on the development of L. sigmodontis in primary infection. With this filaria, only the effect of the lack of B1 cells in primary infection had been studied (1), whereas B-cell deficiency has been studied only with the nonpermissive B. malayi mouse model in primary infection (4, 31, 33).
MATERIALS AND METHODS
Parasites and mice.
The maintenance of the filaria L. sigmodontis Chandler 1931 and recovery of infective larvae from the mite vector, Ornithonyssus bacoti, were carried out as previously described (12, 32). Homozygous mutant mice with a targeted disruption of the membrane exon of the immunoglobulin (Ig) μ chain gene (21) were used. These μMT mice backcrossed to BALB/c were a kind gift of Anne O'Garra, DNAX, Palo Alto, Calif. BALB/c wild-type mice were used as controls; they were obtained from Charles River, Cléon, France. Six-week-old female mice were used throughout the study.
Six groups of mice were constituted: vaccinated and challenged wild-type (wt) mice, vaccinated and challenged μMT mice, challenged-only (primary-infected) wt type mice challenged-only μMT mice, nonvaccinated nonchallenged μMT mice (naive), and nonvaccinated nonchallenged wild-type mice (naive). For each time point, six mice per group were used.
Parasitological techniques. (i) Vaccination and infection protocol.
As previously described (25), infected mites were irradiated at 450 Gy (cesium source) and dissected, and the larvae were recovered and checked for motility. A total of 25 irradiated larvae were inoculated subcutaneously into each vaccinated mouse, three times at weekly intervals. Challenged-only mice were inoculated with RPMI 1640 at the same time points.
At 14 days following the last inoculation, all infected mice were inoculated subcutaneously with 40 L3 larvae into the right lumbar area.
(ii) Time points of mouse necropsies and evaluation of filarial development.
As in to previous studies (24, 25, 27, 29), we limited our analysis to three time points for the vaccination: just before the challenge; 6 h after the challenge to study the subcutaneous tissue infiltrated cells; and 28 days after the challenge to measure the recovery rate. In primary infection, the study was carried up to the patent phase (day 60 postinoculation [p.i.]).
Filariae were recovered with pleural exudate cells (PLEC) by flushing the pleural cavity with 10 ml of phosphate-buffered saline (PBS)-1% fetal calf serum (FCS). The peritoneal cavity was also observed under a stereomicroscope in case some rare filariae might have been present. The location, motility, and aspect of the filariae were noted. Filariae were harvested, counted, and fixed in hot 70% ethanol for morphological analysis. The filarial development was evaluated by means of the following parameters: (i) percentage of mice with filarial worms (%F); (ii) recovery rate of filariae, expressed as 100 × number of worms recovered/number of larvae inoculated (F/L3); (iii) number of live worms partially surrounded by inflammatory cells (cF) (these worms were used to calculate the recovery rate); (iv) number of dead worms or pieces of worms in granulomas (K) (these were not included in the recovery rate); (v) size of worms; (vi) stage of worms; (vii) sex ratio of recovered worms, expressed as number of female worms/total worm burden; (viii) blood microfilaremia (Mf, expressed as the number of microfilariae/10 mm3) determined on day 60 p.i. on a 10-mm3-thick blood smear stained with Giemsa; (ix) and percentage of mice with blood microfilariae. Protection (%P) was expressed as F/L3 (primary infected − vaccinated) × 100/(F/L3 primary infected) (23, 24, 28).
Histology and immunohistochemistry.
In the four infected groups, six mice each were sacrificed prior to the challenge inoculation (H0) and 6 h later (H6). Two skin and subcutaneous tissue samples from the inoculation area were removed post mortem from each mouse and fixed in 10% buffered formaldehyde, cut transversely with a razor blade into small pieces, and then paraffin embedded. For each mouse, two different 5-μm sections from two different sites distant from the puncture point were observed. Infiltrated cells in the connective tissue beneath the platysma muscle were analyzed at the highest magnification of the optical microscope, as described previously (25, 29).
(i) HES staining.
Sections were colored with hemalun-eosin-safran (HES) for counting the infiltrated eosinophils, neutrophils, mast cells, and lymphocytes. The characteristics of the nucleus and cytoplasm were used to identify eosinophils and neutrophils.
Eosinophils in general had annular or twisted, heterogeneously patched nuclei. However, they had different colorations of the cytoplasm; either it was stained with red-orange granules and the cell membrane was regular (Fig. 1A) or the cytoplasm was uncolored with extremely few granules and the cell membrane was irregular and locally disrupted (Fig. 1B). We called the first morphological type nondegranulated or intact eosinophils and the second type degranulated eosinophils. Neutrophils had a uniformly and densely stained lobed nucleus, and the cytoplasm was transparent (Fig. 1E). Eosinophils, neutrophils, mast cells, and lymphocytes were counted with a 100× objective in 10 consecutive fields (at this magnification, the diameter of each field is 200 μm) in two different places. In total, 20 fields were observed for each mouse. The mean number of cells per 10 fields was calculated for each group of mice at each time point.
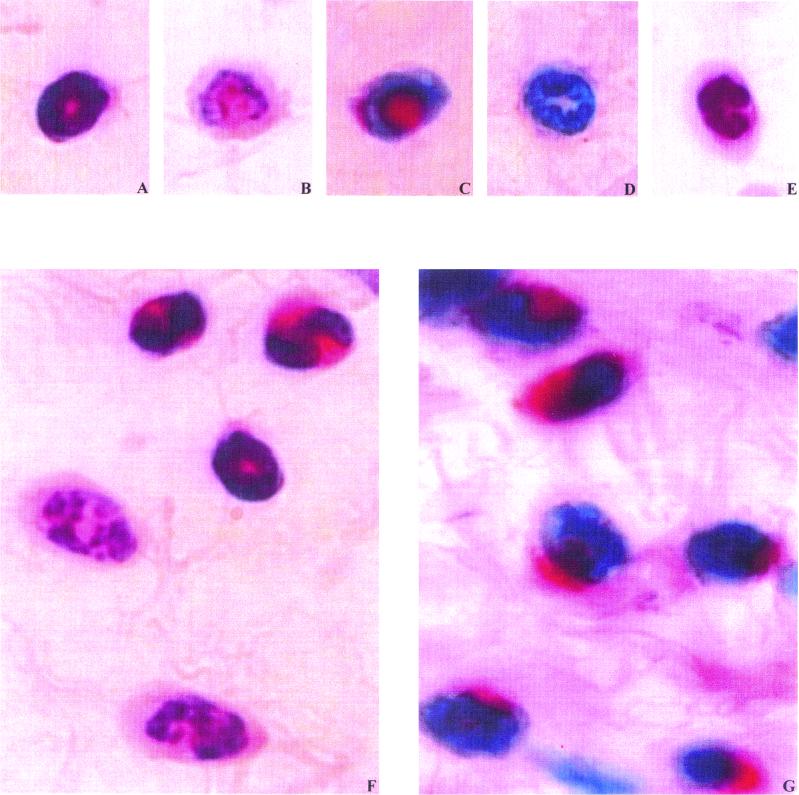
FIG. 1.
Polymorphonuclear infiltrated cells in the subcutaneous tissue of mice. (A and B) Intact (A) and degranulated (B) eosinophils, HES staining; (C and D) intact (A) and degranulated (B) eosinophils, MBP immunostaining; (E) neutrophil, HES staining; (F) three intact and two degranulated eosinophils in vaccinated wt mice 6 h p.i., HES staining; (G), intact eosinophils in vaccinated μMT mice 6 h p.i., MBP immunostaining.
(ii) Eosinophil MBP and neutrophil staining.
To detect major basic protein (MBP) and neutrophils, we used either rabbit antiserum to murine MBP (rbα-mMBP) (2, 14) or an anti-neutrophil monoclonal antibody NIMP-R14 (rat IgG2b) (12), (Fig. 1C to E). To block nonspecific binding of antibodies to FcɛRII or FcɛIII, sections were incubated for 1 h with anti-CD16/CD32 (5 μg/ml) (Pharmingen, Heidelberg, Germany) in 1% (wt/vol) bovine serum albumin and 0.1% (wt/vol) saponin in PBS. Sections were incubated either with rbα-mMBP at a 1:100 dilution in 1% bovine serum albumin in 0.05 M PBS or with NIMP-R14 (100 μl of a hybridoma culture supernatant containing 100 μg of protein per ml) at room temperature in a humidified chamber for 2 h. Thereafter, biotinylated goat anti-rabbit Ig (1:200) (Pharmingen) or biotinylated goat anti-rat Ig (10 μg/ml) (Pharmingen) was added for 30 min. The slides were further incubated with prediluted alkaline phosphatase-conjugated streptavidin (1:100) (Calbiochem, San Diego, Calif.). The color reaction was developed using Naphthol Fast Red (Sigma, Deisenhofen, Germany); slides were counterstained with Hemalun for 10 min and destained with tap water.
Immunological techniques. (i) Cytokine assays.
Concentrations of one type 1 cytokine, IFN-γ, and three type 2 cytokines, IL-4, IL-5, and IL-10 (which was shown to be deficient in mice lacking B1 B cells, 1), in serum were determined by specific two-site enzyme-linked immunosorbent assay (ELISA) using standard protocols on days 0, 2, 28 (same mice for these two time points), and 60 p.i. The monoclonal antibody pairs for the detection were purchased from Pharmingen (BVD4-1D11 and BVD6-24G2 for IL-4; TRFK5, and TRFK4 for IL-5; MP5-20F3 and MP5-32C11 for IL-10, and JES5-2A5 and SXC-1 for IFN-γ), as well as recombinant cytokines used as standards. AMDEX streptavidin-peroxidase conjugate (1:6,000) was added, and the reaction was revealed by addition of tetramethylbenzidine substrate-H2O2 (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) and stopped with a 1 M H2SO4 solution. The absorbance was read at 450 nm with an LP400 ELISA reader.
(ii) Ig assays.
Adult somatic extract antigen was obtained from mature female and male L. sigmodontis worms by the method previously described (25). L. sigmodontis-specific IgG1 and IgG2a in serum samples were quantified on days 28 and 60 p.i. by coating microtiter plates with 5 μg of adult somatic extract antigen per ml. After incubation with sera (1:400) and washing (using standard procedures), the plates were incubated with biotin-conjugated anti-mouse IgG1 and IgG2a at a dilution of 1:1,000 and 1:2,000 respectively. All ELISAs were developed as described above for cytokine ELISAs.
Other observations performed on mice. (i) Blood leukocyte counts.
Blood leukocyte counts were performed on days -21 and -15 before the challenge inoculation for vaccinated mice, on days 0, 2, and 28 p.i. for all groups, and on day 60 p.i. for both primary-infected groups. Smears of tail blood were stained with May-Grünwald-Giemsa, and the percentages of leukocytes were determined with 200 cells. Total leukocytes were enumerated with a Malassez cell, using Unopettes (Becton Dickinson). Leukocyte counts are expressed as a number per milliliter of blood.
(ii) Inflammatory cells in pleural cavity fluid on day 28 p.i.
PLEC were enumerated on a Malassez cell. After adjustment to 106 cells/ml in RPMI, 400 μl of the cell suspension was distributed in a flexiPERM (Unisyn) and centrifuged against the glass slide at 1,000 rpm (112 × g) for 15 min. The slides were stained with May-Grünwald-Giemsa and the inflammatory cells were differentially enumerated approximately on 400 cells. They are expressed in total numbers.
Statistical analysis.
The nonparametric Kruskall-Wallis H test and the Mann-Whitney U test were used to assess non-normally distributed parameters such as filarial recovery rates, cell numbers, and cytokine and Ig levels. Only significant differences (P < 0.05) are presented in the text unless otherwise specified.
RESULTS
Vaccine-induced protection is suppressed in μMT mice.
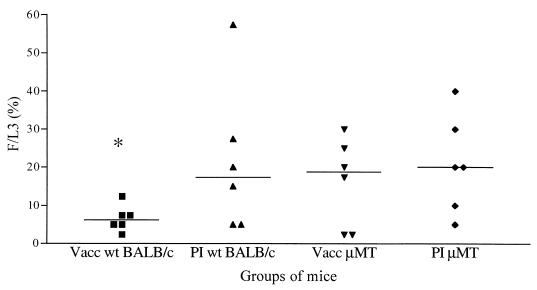
On day 28 p.i., μMT mice had the same recovery rate whether vaccinated or not (Fig. 2). In contrast, vaccinated wt mice had a lower recovery rate than the three other groups, and protection was within the range described previously (25), i.e., 65% (P = 0.03). Neither granulomas nor inflammatory cell-covered worms were found in all four groups of mice on day 28 p.i. The other parameters (length, stage, localization, and sex ratio) were also similar in the four groups.
FIG. 2.
L. sigmodontis recovery rate on day 28 p.i. in μMT and wt mice. vacc, vaccinated; PI, primary-infected. Individual values (n = 6) are presented with the median indicated (bar). ∗, significant difference between the vaccinated wt group and the others.
In primary-infected μMT mice, the development is dramatically impaired at on day 60 p.i. although the recovery rate is unchanged.
The recovery rate was similar in both primary-infected groups, μMT and wt, and its value did not vary between days 28 and 60 p.i. (Fig 2; Table 1). The length of the filariae was the same in both groups. However, all μMT mice were amicrofilaremic on day 60 p.i., none of the female filariae had uterine microfilariae, and one-quarter of the filariae were totally surrounded by inflammatory cells; these were still mobile but were damaged.
TABLE 1.
L. sigmodontis infection on day 60 p.i. in primary-infected wt and μMT mice
| Group | F/L3 | % cF | Length (mm)
|
Sex ratioa | % Mf ut. | % Mfs | |
|---|---|---|---|---|---|---|---|
| Female worms | Male worms | ||||||
| wt | 17.8 ± 6.6 | 8.9 | 67.1 ± 1.9 | 22.7 ± 0.4 | 51.2 | 69 | 50 |
| μMT | 19.5 ± 4.2 | 26b | 59.8 ± 2.6 | 20.2 ± 0.4 | 51.1 | 0c | 0c |
Sex ratio is given as number of female worms/total number of worms.
% Mf ut., percentage of female worms with uterine microfilariae; % Mfs, percentage of mice with blood microfilariae.
Significantly different from wt.
Subcutaneous infiltrated eosinophils do not morphologically change in μMT-vaccinated mice after the challenge inoculation.
Just before challenge inoculation (H0), primary-infected groups, wt and μMT mice, had very few infiltrated cells in the subcutaneous tissue. In contrast, both vaccinated groups, wt and μMT, had a large number of infiltrated cells before the challenge inoculation. The majority of these cells were eosinophils. With the HES staining, the degranulated eosinophils were rarely identified in the wt mice or in the μMT mice (Table 2). With the MBP staining, the proportion of these degranulated eosinophils was around 30% higher in both vaccinated groups of mice. No neutrophils were identified in the four groups with HES staining or with NIMP R14 neutrophil-specific staining.
TABLE 2.
Number of eosinophils and neutrophils and percentage of degranulated eosinophils in the subcutaneous tissue of the inoculation area prior to the challenge (H0) and 6 h later (H6)a
| Groupb | Time | No. of neutrophils | No. of eosinophils | % of degranulated eosinophils |
|---|---|---|---|---|
| Vacc wt | H0 | 0 | 98 ± 11 | 0.3 ± 0.1 |
| H6 | 44 ± 6.6c | 101 ± 19 | 71.8 ± 5.4c | |
| Vacc μMT | H0 | 0 | 148 ± 32 | 0.1 ± 0.1 |
| H6 | 30 ± 3.4c | 163 ± 29 | 8.9 ± 2.5cd | |
| PI wt | H0 | 0 | 8.7 ± 2.4 | 0.4 ± 0.2 |
| H6 | 54 ± 9.2c | 7 ± 1.3 | 1.4 ± 0.8 | |
| PI μMT | H0 | 0 | 9.3 ± 3.4 | 0.1 ± 0.1 |
| H6 | 58 ± 9.2c | 14 ± 2.3 | 0.3 ± 02 |
Cells detected after HES staining. Values are means ± standard deviations.
Vacc, vaccinated mice; PI, primary-infected mice.
Significant difference between H0 and H6 within a group.
Significant difference between the two vaccinated groups at H6.
Six hours after the challenge, the neutrophil density was high and was similar in the four groups (HES and NIMP R14 staining); they were the only cells that were recruited in the primary-infected wt and μMT mice (Table 2). In both vaccinated groups, the eosinophil density remained unchanged compared to H0; nevertheless, the eosinophils in μMT mice were in a different state from those in wt mice. With HES staining, a high proportion of degranulated eosinophils was identified in the vaccinated wt mice whereas this proportion was low in the vaccinated μMT mice (71.8 and 8.9%, respectively; P = 0.01 [Table 2; Fig. 1F]). With MBP immunostaining, no extracellular MBP deposits were detected; however, the percentages of degranulated eosinophils (Fig. 1D) were 84 and 26% (P = 0.01), respectively, in vaccinated wt mice and vaccinated μMT mice (Fig. 1G); these percentages were not different between MBP and HES staining.
Mast cell and lymphocyte densities were extremely low, and no differences were observed among the four groups regardless of the time schedule. No larvae were detected in any samples.
The cellular immune response is decreased in vaccinated and primary-infected μMT mice.
No IL-4 was detected in the four groups on day 28 p.i.; on day 60 p.i., it was measurable in primary-infected wt mice and hardly present in primary-infected μMT mice (Table 3). The baseline level of IL-5 was lower in μMT mice; however, the kinetics were similar when the two groups of vaccinated mice were compared, as between the two groups of primary-infected mice (Table 3). IL-10 was detected only in wt mice: before challenge inoculation and 2 days p.i. in vaccinated wt mice and only on day 28 p.i. in primary-infected wt mice. IFN-γ levels recovered in μMT mice were lower in both vaccination and primary infection compared to those in wt mice.
TABLE 3.
Concentrations of cytokines in serum in μMT and wt mice
| Groupa | Time (days) p.i. | Concnb (pg/ml) of:
|
|||
|---|---|---|---|---|---|
| IL-4 | IL-5 | IL-10 | IFN-γ | ||
| Vacc wt | 0 | 0 | 253 (56–426)c | 61 (0–213)c | 419 (271–513)c |
| 2 | 0 | 283 (207–334)c | 275 (204–445)c | 332 (285–447)c | |
| 28 | 0 | 198 (158–406) | 0 | 1,175 (885–1908)c | |
| Vacc μMT | 0 | 0 | 150 (127–299) | 0 | 32 (0–64) |
| 2 | 0 | 123 (84–207) | 0 | 132 (112–168) | |
| 28 | 0 | 165 (99–284) | 0 | 0 (0–818) | |
| Naive wt | 0 | 0 | 17.3 (14–24)d | 0 | 0 |
| PI wt | 2 | 0 | 29 (28–35) | 0 | 144 (77–277)d |
| 28 | 0 | 128 (62–180)d | 85 (59–114)d | 465 (65–1023)d | |
| 60 | 3.3 (2.1–3.8)d | 84 (44–138)d | 0 | 3,046 (1057–5091)d | |
| Naive μMT | 0 | 0 | 1.3 (0–18) | 0 | 0 |
| PI μMT | 2 | 0 | 26 (22–42) | 0 | 105 (70–165) |
| 28 | 0 | 88 (78–139) | 0 | 0 (0–1560) | |
| 60 | 0.2 (0.02–0.41) | 19 (15–29) | 0 | 147 (52–249) | |
Vacc, vaccinated; PI, primary infected; naive, noninfected.
Results are expressed as medians, and ranges are given in parentheses.
Significant difference between vaccinated μMT mice and vaccinated wt mice.
Significant difference between PI μMT mice and PI wt mice.
As expected, specific antibodies were absent in both vaccinated and primary-infected groups of μMT mice. In contrast, IgG1 and IgG2a production was elevated in vaccinated wt mice by 28 days p.i. However, they were not detected on day 28 p.i. and were highly elevated on day 60 p.i. in primary-infected wt mice (data not shown).
Blood leukocytes.
Blood leukocyte counts were lower in μMT mice than in wt mice, as seen on day 28 p.i. (6.8 × 106/ml in vaccinated μMT mice versus 12.9 × 106/ml in vaccinated wt mice, and 7.2 × 106/ml in primary-infected μMT mice versus 10 × 106/ml in primary-infected wt mice [P < 0.05]) and day 60 p.i. (6.9 × 106/ml in primary-infected μMT mice versus 10.4 × 106 in primary-infected wt mice [P < 0.05]). The number of lymphocytes was half that in wt vaccinated or primary-infected mice. Blood leukocyte counts rose significantly in wt mice after challenge infection, whereas they did not rise in μMT mice because the lymphocyte numbers did not increase. Eosinophil numbers were similar in both vaccinated groups and in both primary-infected groups (0.6 × 106 to 0.8 × 106/ml).
Inflammatory pleural cells.
PLEC were less numerous in μMT mice than in wt mice, whether naive, vaccinated, or primary-infected, on day 28 p.i. (P < 0.05), but eosinophil recruitment was similar in wt and μMT mice. Neutrophils appeared to be more numerous in primary-infected μMT mice than in primary-infected wt mice (Table 4).
TABLE 4.
Inflammatory cells in pleural cavity fluid on day 28 p.i.
| Groupa | No. of cells (106)b
|
|||
|---|---|---|---|---|
| PLEC | Eosinophils | Neutrophils | Lymphocytes plus macrophages | |
| Naive wt | 2.7 ± 0.5 | 0.03 ± 0.003 | 0 | 2.7 ± 0.04 |
| Naive μMT | 0.7 ± 0.1c | 0.12 ± 0.01 | 0.002 ± 0.002 | 0.6 ± 0.01c |
| PI wt | 24.6 ± 3.8 | 6.5 ± 1 | 0.07 ± 0.02 | 17.4 ± 1.2 |
| PI μMT | 15.4 ± 2.4c | 9.8 ± 0.6 | 0.3 ± 0.1c | 5.3 ± 0.6c |
| Vacc wt | 30 ± 4.9 | 10.4 ± 1.4 | 0.8 ± 0.5 | 18.8 ± 1.9 |
| Vacc μMT | 19.7 ± 4.8c | 12.1 ± 0.5 | 0.5 ± 0.1 | 7.1 ± 0.8c |
Vacc, vaccinated; PI, primary infected; naive, noninfected.
Values are means ± standard deviation represents.
Significant difference between the μMT group and its control wt group.
DISCUSSION
The effects of deficiency of B cells and antibodies are first discussed in the vaccination protocol (25, 29). We focused on the early events because our previous data have shown that protection is established at the subcutaneous site within 2 days following the challenge inoculation (25, 29). We observed that no protection was induced by vaccination in μMT mice (Fig. 2) whereas in wt mice, as supported by parasitological and histological studies, the expected 65% protection was demonstrated 1 month p.i.
The quantity and nature of the infiltrated cells in the subcutaneous tissue were equivalent between wt and μMT vaccinated mice and consisted of large amounts of eosinophils before and after the challenge and arrival of neutrophils within a few hours following the challenge inoculation. The lack of B cells and/or antibodies therefore has no influence on the recruitment of eosinophils in the subcutaneous tissue of vaccinated mice (Table 2), as seen in the nonparasitic airway hyperresponsiveness models (22). In contrast, the state of the eosinophils after the challenge inoculation differed. A delicate question arose about the diagnosis of their degranulation, especially since there is a controversy about the capacity of murine eosinophils to degranulate, i.e., in asthma models (11, 15, 36; G. J. Gleich, personal communication); the activation and life span of eosinophils in tissues appear extremely complex and diverse depending on the tissue involved.
In our filarial model, both HES and MBP staining showed a significant difference in the number of degranulated eosinophils between vaccinated wt and μMT mice 6 h p.i. HES staining showed that in vaccinated μMT mice intact eosinophils were prevalent (Table 2) and rich in red-orange granules (Fig. 1A); in contrast, eosinophils that were poor in granules or even had none (Fig. 1B) were prevalent in vaccinated wt mice (Table 2). With the MBP immunostaining, two states of eosinophils could also be distinguished: one with a red intracellular coloration (Fig. 1C), representing 74% of all eosinophils in vaccinated μMT mice in contrast to 16% in vaccinated wt mice, and the other with no intracellular coloration (Fig. 1D). We interpreted this result as a much weaker degranulation in the μMT group of mice, although, even in wt mice, we did not observe any tissue deposits of MBP around the eosinophils. However, the possibility exists that our assays were not sufficiently sensitive to detect them, taking into account the fact that eosinophils are dispersed in the connective tissue.
Close to the larvae, it is possible that the adherence of the eosinophils to the filariae and maybe even their degranulation were promoted in the subcutaneous tissue of vaccinated mice in which mobility of filariae was reduced (S. Babayan, C. Martin, J. Dufaux, G. Guiffant, J. C. Gantier, and O. Bain., submitted for publication). On histological sections, eosinophils were studied distant from the antigenic sources, the infective larvae, but the medium inoculated with them contains excretory-secretory antigens. At the time of challenge inoculation, the antibodies were present only in the vaccinated wt mice (reference 25 and unpublished data) in which there was a high density of degranulated eosinophils 6 h later. The murine eosinophils do not express cell surface receptors that bind IgE (10, 20), but IgG receptors are present and sufficient to activate the degranulation; indeed, it has been shown in vitro in a Candida albicans infection that human eosinophil degranulation can be induced by IgG only (19). The B-cells deficiency induces complex events such as a decrease in type 1 and type 2 responses (Table 3) and especially the lack of antibodies, as well as particularly low eosinophil degranulation ability. This strongly suggests, along with our previous results (24, 25, 29), that protection against filarial infection in vaccinated mice can be associated with (i) a high level of eosinophil recruitment in the subcutaneous tissue and (ii) an antibody-dependent degranulation of these eosinophils within a few hours following the challenge inoculation.
Considering the primary infection, we show that the mature B-cell deficiency on BALB/c background does not induce a change of the recovery rate during the first 2 months of observation. However, 2 months p.i., the μMT mutation has a negative effect on the development of the filariae: female maturation was abnormal, since none of the filariae was able to produce microfilariae. Furthermore, one of four worms was covered with inflammatory cells from head to tail. The B-cell deficiency has different parasitological consequences from those of the lack of the B1-cell subset in BALB/c Xid mice, in which the L. sigmodontis recovery rate and the microfilaremia are higher (1); in the nonpermissive B. pahangi CBA/N Xid model, the recovery rate is also improved. Therefore, B1 and B2 cells may have opposite effects on filarial infection. However, this mutation induces important modifications that can modify the filarial survival: the composition of the pleural inflammatory cells shows that the number of lymphocytes plus macrophages, but not that of the eosinophils, is markedly diminished (Table 4). Neutrophils are present in these mice but are rare in wt mice on day 28 p.i. As shown in an IFN-γ-deficient mouse study (34), increased numbers of neutrophils are associated with impaired filarial development.
Furthermore, B cells seem to regulate the immune response to filariae, since wt mice harbored higher cytokine levels in serum than did μMT mice, in which both type 1 and type 2 cytokine levels were decreased (Table 3). This can be linked to the antigen-presenting role of the B cells, as well as to their capacity to produce cytokines. Two different subsets of efficient B cells have indeed been identified, producing either type 1 or type 2 cytokines, depending on the immunological environment (16). These facts could explain the global decrease of cytokine production. The negative effect of the lack of B cells on the late maturation of L. sigmodontis seems to indicate a positive effect of the B2 cells on that part of the development.
ACKNOWLEDGMENTS
We are very grateful to J. J. Lee (Mayo Clinic Scottsdale, Ariz.) for providing the MBP antibodies.
This work was supported by a CE grant (ICA4-1999-10007) and the German Research Foundation (DFG HO2009/1-3).
REFERENCES
- 1.Al Qaoud K M, Taubert A, Zahner H, Fleischer B, Hoerauf A. The Xid defect imparts susceptibility to experimental murine filariasis—association with a lack of antibody and IL-10 production by B cells in response to phosphorylcholine. Int Immunol. 1998;10:17–25. doi: 10.1093/intimm/10.1.17. [DOI] [PubMed] [Google Scholar]
- 2.Al Qaoud K M, Taubert A, Zahner H, Fleischer B, Hoerauf A. Interleukin-5 is required for neutrophil-mediated worm elimination in murine filariasis. Int Immunol. 2000;12:899–908. doi: 10.1093/intimm/12.6.899. [DOI] [PubMed] [Google Scholar]
- 3.Babu S, Porte P, Klei T R, Shultz L D, Rajan T V. Host NK cells are required for the growth of the human filarial parasite Brugia malayi in mice. J Immunol. 1998;161:1428–1432. [PubMed] [Google Scholar]
- 4.Babu S, Shultz L D, Klei T R, Rajan T V. Immunity in experimental murine filariasis: roles of T and B cells revisited. Infect Immun. 1999;67:3166–3167. doi: 10.1128/iai.67.6.3166-3167.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babu S, Ganley L M, Klei T R, Shultz L D, Rajan T V. Role of gamma interferon and interleukin-4 in host defense against the human filarial parasite Brugia malayi. Infect Immun. 2000;68:3034–3035. doi: 10.1128/iai.68.5.3034-3035.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bain O, Wanji S, Vuong P N, Maréchal P, Le Goff L, Petit G. Larval biology of six filariae of the sub-family Onchocercinae in a vertebrate host. Parasite. 1994;1:241–254. doi: 10.1051/parasite/1994013241. [DOI] [PubMed] [Google Scholar]
- 7.Butterworth A E. Cell-mediated damage to helminths. Adv Parasitol. 1984;23:143–235. doi: 10.1016/s0065-308x(08)60287-0. [DOI] [PubMed] [Google Scholar]
- 8.Chandrashekar R, Rao U R, Subrahmanyam D. Antibody-mediated cytotoxic effects in vitro and in vivo of rat cells on infective larvae of Brugia malayi. Int J Parasitol. 1990;20:725–730. doi: 10.1016/0020-7519(90)90005-8. [DOI] [PubMed] [Google Scholar]
- 9.Chippaux J P, Boussinesq M, Fobi G, Lafleur C, Auduge A, Banos M T, Ngosso A, Prod'hon J. Effect of repeated ivermectin treatments on ocular onchocerciasis: evaluation after six to eight doses. Ophthalmic Epidemiol. 1999;6:229–234. doi: 10.1076/opep.6.4.229.4185. [DOI] [PubMed] [Google Scholar]
- 10.De Andrès B, Rakasz E, Hagen M, McCormik M L, Mueller A L, Elliot D, Metwali A, Sandor M, Britigan B E, Weinstock J V, Lynch R G. Lack of Fcɛ-receptors on murine eosinophils: implication for the functional significance of elevated IgE and eosinophils in parasitic infections. Blood. 1997;89:3826–3836. [PubMed] [Google Scholar]
- 11.Denzler K L, Farmer S C, Crosby J R, Borchers M, Cieslewicz G, Larson K A, Cormier-Regard S, Lee N A, Lee J J. Eosinophil major basic protein-1 does not contribute to allergen-induced airways pathologies in mouse models of asthma. J Immunol. 2000;165:5509–5517. doi: 10.4049/jimmunol.165.10.5509. [DOI] [PubMed] [Google Scholar]
- 12.Diagne M, Petit G, Liot P, Cabaret J, Bain O. The filaria Litomosoides galizai in mites: microfilarial distribution in the host and regulation of the transmission. Ann Parasitol Hum Comp. 1990;65:193–199. doi: 10.1051/parasite/1990654193. [DOI] [PubMed] [Google Scholar]
- 13.Folkard S G, Hogarth P J, Taylor M J, Bianco A E. Eosinophils are the major effector cells of immunity to microfilariae in a mouse model of onchocerciasis. Parasitology. 1996;112:323–329. doi: 10.1017/s0031182000065847. [DOI] [PubMed] [Google Scholar]
- 14.Greene B M, Taylor H R, Aikawa M. Cellular killing of microfilariae of Onchocerca volvulus: eosinophil- and neutrophil-mediated immune serum-dependent destruction. J Immunol. 1981;127:1611–1618. [PubMed] [Google Scholar]
- 15.Hall L R, Mehlotra R K, Higgins A W, Haxhiu M A, Pearlman E. An essential role for interleukin-5 and eosinophils in helminth-induced airway hyperresponsiveness. Infect Immun. 1998;66:4425–4430. doi: 10.1128/iai.66.9.4425-4430.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris D P, Haynes L, Sayles P C, Duso D K, Eaton S M, Lepak N M, Johnson L L, Swain S L, Lund F E. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nature Immunol. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi Y, Nogami S, Nakamura M, Shirasaka A, Noda K. Passive transfer of protective immunity against Brugia malayi in BALB/c mice. Jpn J Exp Med. 1984;54:183–187. [PubMed] [Google Scholar]
- 18.Hoffmann W H, Petit G, Schulz-Key H, Taylor D, Bain O, Le Goff L. Litomosoides sigmodontis in mice: reappraisal of an old model for filarial research. Parasitol Today. 2000;16:387–388. doi: 10.1016/s0169-4758(00)01738-5. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda Y, Mita H, Kudo M, Hasegawa M, Akiyama K. Degranulation of eosinophils by IgG antibody to Candida antigen. Arerugi. 1999;48:546–553. [PubMed] [Google Scholar]
- 20.Kaneko M, Swanson M C, Gleich G J, Kita H. Allergen-specific IgG1 and IgG3 through Fc gamma RII. J Clin Investig. 1995;95:2813–2821. doi: 10.1172/JCI117986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 22.Korsgren M, Erjefalt J S, Korsgren O, Sundler F, Persson C G A. Allergic eosinophil-rich inflammation develops in lungs and airways of B-cell-deficient mice. J Exp Med. 1997;185:885–892. doi: 10.1084/jem.185.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lange A M, Yutanawiboonchai W, Scott P, Abraham D. IL-4 and IL-5 dependent protective immunity to Onchocerca volvulus infective larvae in BALB/cBYJ mice. J Immunol. 1994;153:205–211. [PubMed] [Google Scholar]
- 24.Le Goff L, Maréchal P, Petit G, Taylor D W, Hoffmann W, Bain O. Early reduction of the challenge recovery rate following immunization with irradiated infective larvae in a filaria mouse system. Trop Med Int Health. 1997;2:1170–1174. doi: 10.1046/j.1365-3156.1997.d01-218.x. [DOI] [PubMed] [Google Scholar]
- 25.Le Goff L, Martin C, Oswald I P, Vuong P N, Petit G, Ungeheuer M N, Bain O. Parasitology and immunology of mice vaccinated with irradiated Litomosoides sigmodontis larvae. Parasitology. 2000;120:271–280. doi: 10.1017/s0031182099005533. [DOI] [PubMed] [Google Scholar]
- 26.Maréchal P, Le Goff L, Petit G, Diagne M, Taylor D W, Bain O. The fate of the filaria Litomosoides sigmodontis in susceptible and naturally resistant mice. Parasite. 1996;3:25–31. doi: 10.1051/parasite/1996031025. [DOI] [PubMed] [Google Scholar]
- 27.Maréchal P, Le Goff L, Hoffmann W, Rapp J, Oswald I P, Ombrouck C, Taylor D W, Bain O, Petit G. Immune response to the filaria Litomosoides sigmodontis in susceptible and resistant mice. Parasite Immunol. 1997;19:273–279. doi: 10.1046/j.1365-3024.1997.d01-209.x. [DOI] [PubMed] [Google Scholar]
- 28.Martin C, Le Goff L, Ungeheuer M N, Vuong P N, Bain O. Drastic reduction of a filarial infection in eosinophilic interleukin-5 transgenic mice. Infect Immun. 2000;68:3651–3656. doi: 10.1128/iai.68.6.3651-3656.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin C, Al Qaoud K M, Ungeheuer M N, Paehle K, Vuong P N, Bain O, Fleischer B, Hoerauf A. IL-5 is essential for vaccine induced protection and for resolution of primary infection in murine filariasis. Med Microbiol Immunol. 2000;189:67–74. doi: 10.1007/pl00008258. [DOI] [PubMed] [Google Scholar]
- 30.Ottesen E A, Duke B O L, Karan M, Behbehani K. Strategies and tools for the control-elimination of lymphatic filariasis. Bull W H O. 1997;75:491–503. [PMC free article] [PubMed] [Google Scholar]
- 31.Paciorkowski N, Porte P, Shultz L D, Rajan T V. B1 B lymphocytes play a critical role in host protection against lymphatic filarial parasites. J Exp Med. 2000;191:731–736. doi: 10.1084/jem.191.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petit G, Diagne M, Maréchal P, Owen D, Taylor D W, Bain O. Maturation of the filaria Litomosoides sigmodontis in BALB/c mice: comparative susceptibility of nine other inbred strains. Ann Parasitol Hum Comp. 1992;67:144–150. doi: 10.1051/parasite/1992675144. [DOI] [PubMed] [Google Scholar]
- 33.Rajan T V, Shultz L D, Yates J, Greiner D L. B lymphocytes are not required for murine resistance to the human filarial parasite, Brugia malayi. J Parasitol. 1995;81:490–493. [PubMed] [Google Scholar]
- 34.Saeftel M, Volkmann L, Korten S, Brattig N, Al-Qaoud K, Fleischer B, Hoerauf A. Lack of interferon-y confers impaired neutrophil granulocyte function and imparts prolonged survival of adult filarial worms in murine filariasis. Microbes Infect. 2001;3:203–213. doi: 10.1016/s1286-4579(01)01372-7. [DOI] [PubMed] [Google Scholar]
- 35.Simonsen P E, Pedersen E M, Lemnge M M, Michael E. Lymphatic filariasis and research and control in Africa. Parasitol Today. 1997;13:413–415. [Google Scholar]
- 36.Stelts D, Egan R W, Falcone A, Garlisi C G, Gleich G J, Kreutner W, Kung T T, Nahrebne D K, Chapman R W, Minnicozzi M. Eosinophils retain their granule major basic protein in a murine model of allergic pulmonary inflammation. Am J Respir Cell Mol Biol. 1998;18:463–470. doi: 10.1165/ajrcmb.18.4.2957. [DOI] [PubMed] [Google Scholar]
- 37.Vickery A C, Vincent A L, Sodeman W A. Effect of immune reconstitution on resistance to Brugia pahangi in congenitally athymic nude mice. J Parasitol. 1983;69:478–485. [PubMed] [Google Scholar]
- 38.Volkmann L, Saeftel M, Bain O, Fisher K, Fleischer B, Hoerauf A. Interleukin-4 is essential for the control of microfilariae in murine infection with the filaria Litomosoides sigmodontis. Infect Immun. 2001;69:2950–2956. doi: 10.1128/IAI.69.5.2950-2956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wenk P. Der Invasionsweg der metazylischen Larven von Litomosoides carinii Chandler 1931 (Filariidae) Z Parasitenkd. 1967;28:240–248. doi: 10.1007/BF00260265. [DOI] [PubMed] [Google Scholar]