Abstract
Traditionally, γ-aminobutyric acid (GABA) is best known for its role as a primary inhibitory neurotransmitter reducing neuronal excitability in the mammalian central nervous system (CNS), thereby producing calming effects. However, an emerging body of data now supports a function for GABA beyond neurotransmission as a potent factor regulating cancer cell growth and metastasis, as well as the anti-tumor immune response, by shaping the tumor microenvironment. Here, we review the current knowledge of GABA’s effects on the function of tumor cells, tumor-immune interactions, and the underlying molecular mechanisms. Since altered GABAergic signaling is now recognized as a feature of certain types of solid tumors, we also discuss the potential of repurposing existing GABAergic agents as a new class of anti-cancer therapy.
Keywords: GABAergic signaling, tumor growth, metastasis, anti-tumor immunity, cancer therapy
The role of GABA beyond its classic function as a neurotransmitter
It was initially believed that γ-aminobutyric acid (GABA) expression is restricted to the central nervous system (CNS) such that its release from GABAergic neurons (see Glossary) of the autonomic nervous system only regulates functions of involuntary cells and organs. In GABAergic neurons, GABA is synthesized from glutamate through the activity of glutamic acid decarboxylase (GAD) enzymes. Intracellularly, GABA can be catabolized to form succinic semialdehyde within the mitochondrial matrix by GABA transaminase (ABAT), allowing entry of the GABA carbon skeleton into the TCA cycle. Alternatively, GABA can be released into the postsynaptic space where it inhibits neuronal firing by engaging two classes of structurally and pharmacologically distinct cell surface receptors: GABA-A receptors, which are ligand-gated chloride channels, and heterodimeric GABA-B receptors. However, besides this role in neurotransmission, GABA is now considered to have a wide variety of additional functions. For example, it can serve as a trophic factor to affect cellular events including cell proliferation, migration, differentiation, synapse maturation, and cell death during nervous system development [1, 2]. In addition, GABA modulates pancreatic islet homeostasis, hormone secretion, and inflammation by controlling the function and proliferation of pancreatic β cells and immune cells [3, 4]. Notably, a growing body of studies have reported elevated GABA content in different neoplastic tissues as compared with normal tissues [5–10]. The reasonable range of GABA concentration for treatment the cultured cells in vitro is from 0.1 to 100 mM [11]. Moreover, various components of the GABAergic system have been shown to regulate tumor growth and metastasis, strongly suggesting a relationship between GABA and oncogenesis. With recent evidence also implicating GABA in immune evasion within the tumor microenvironment (TME) [5, 12], an improved understanding of GABA’s impacts on cancer progression could prove beneficial in identifying novel therapeutic interventions to improve the anti-tumor efficacy of immunotherapy.
This review will focus on the current state of knowledge regarding the role of GABA and GABAergic components in cancer cell growth, metastasis, and immune evasion, as well as advances in elucidating their underlying mechanisms. Given that GABA agents are already in widespread use for various nervous system disorders, we also discuss the pharmacological potential of repurposing such drugs as a new class of anti-cancer therapy to exert regulatory effects on immunosurveillance and tumorigenesis.
GABAergic signaling in tumor growth and progression
While GABA is known to be the main inhibitory neurotransmitter of the mammalian CNS, it also exerts regulatory effects on proliferation and migration in both neural and non-neuronal peripheral tissues, including brain and pancreatic islets, where GABA can accumulate in large quantities [2]. Moreover, increased GABA levels have also been observed in certain solid tumor types [5–10], where it has been found to promote cancer cell proliferation and migration by identified mechanisms. With growing evidence implicating the role of GABA in tumor progression and metastasis, a novel strategy to eliminate tumors with elevated GABA production may involve targeting components of the GABAergic system. In this section, we describe the function of the major GABAergic components including GABA metabolic enzymes and receptors, with a focus on their roles and therapeutic potentials in cancer cell proliferation and migration.
GABA metabolic enzymes in tumor growth and progression
Glutamic acid decarboxylase (GAD67)
GABA-positive tumors express GAD67, one of two GAD isoforms responsible for GABA synthesis. These GAD67-positive tumor cells often exhibit a greater proliferative and/or invasive potential; as a result, they have heightened carcinogenesis and tumor progression [5, 6, 10, 13, 14]. Consistent with these observations, inhibiting GAD67 expression or enzymatic activity has been shown to reduce cancer cell proliferation, invasiveness, and migratory ability [5, 14]. Thus, aberrantly upregulated GAD67 expression can play an important role in malignancy. Indeed, high GAD67 is associated with advanced tumor status and correlates with worse patient outcomes in multiple solid cancer types [5, 15–18], suggesting that GAD67 expression may serve as a biomarker for malignancy. It has been revealed that GAD67 rewires glutamine metabolism for GABA synthesis in cancer cells (Figure 1), confirming GAD67’s role in GABA accumulation in neoplastic tissues [5]. It should be noted that GAD65, which is normally expressed in the CNS and pancreas, has not yet been identified in tumors [5].
Figure 1. GABAergic components as potential targets to prevent cancer growth and metastasis.
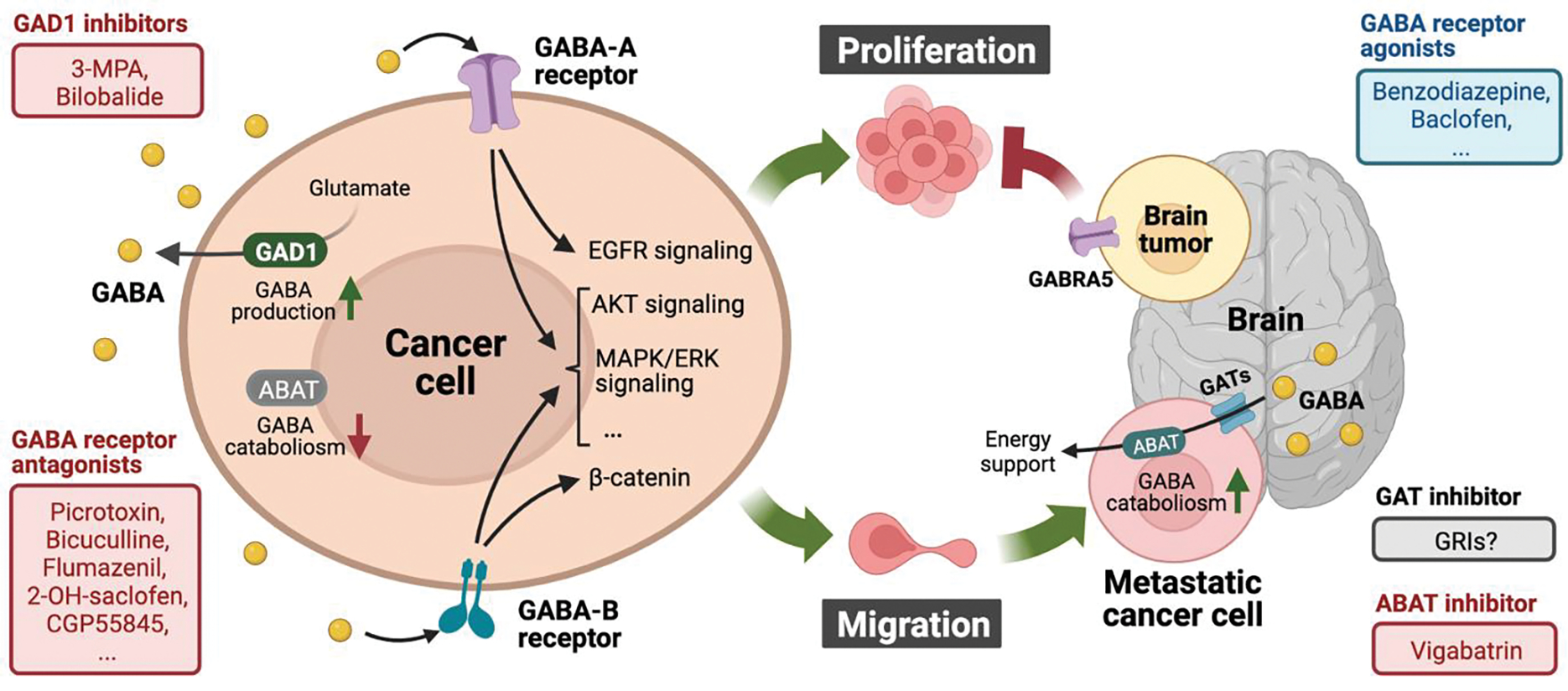
The cartoon depicts GABA, GABA metabolic enzymes, and GABA receptor-mediated signaling pathway functions in regulating tumor cell proliferation and migration. In non-brain cancer cells (left), highly expressed GAD1-mediated GABA synthesis and ABAT absence-impaired GABA degradation contribute to GABA accumulation within the TME. Activated GABA receptors facilitate cancer cell proliferation and/or migration by stimulating identified oncogenic signaling pathways. Therefore, targeting GABAergic signaling by inhibiting GAD1 or GABA receptors could exert anti-tumor effects in certain cancers. Whereas ABAT is commonly downregulated in peripheral primary tumors (left), metastasized brain tumors upregulate ABAT expression to catabolize neuronal GABA for energy production (right). Hence, pharmacological blockade of GABA utilization by targeting ABAT or GABA transporters might provide clinical benefits for brain cancer and metastasis (right, bottom). Moreover, GABA-A receptor activation appears to be tumor suppressive in brain tumors, such that GABA receptor agonism may have potential applications for cancer therapy (right, top). Figure created with BioRender.
Mechanistic investigations of GAD67 regulation in cancer cells identified a close relationship with β-catenin, the pivotal transcription factor in the Wingless/int1 (WNT) signaling pathway. Specifically, GAD67-dependent GABA production mediates β-catenin stabilization and localization to nuclei to facilitate the transcription of molecules involved in proliferation [5] and migration [14]. GAD67 has also been identified as a β-catenin target gene in cancer cells [19, 20], suggesting a positive feedback loop between GAD67 expression and β-catenin activation. Another possible mechanism underlying abnormal GAD67 expression in cancer cells is DNA methylation-mediated gene regulation: reducing GAD67 promoter methylation by inhibiting DNA methyltransferase causes transcriptional GAD67 activation in brain metastatic tumor cells [21]. A similar GAD67 regulatory mechanism was reported in GABAergic interneurons [22]. However, in contrast to the notion that DNA methylation always suppresses mammalian gene expression, two independent groups reported that hypermethylated GAD67 promoter region leads to transcriptional activation of GAD67 in certain cancer types [16–18]. This unusual effect of DNA methylation on GAD67 induction could be due to unknown cancer-specific transcriptional modulations. While increased levels of GAD67 in various solid tumor types have now been clearly demonstrated, further work is needed to identify the key regulator(s) involved in GAD67’s aberrant upregulation for targeting GABAergic signaling in cancer cells.
GABA-transaminase (ABAT)
ABAT catabolizes GABA to produce succinic semialdehyde, which can be used to generate NADPH for anabolic reducing equivalents as well as succinate for ATP production via its entry into the TCA cycle. Several studies have reported that expression of ABAT is downregulated in breast, liver, and kidney cancer cells [23–25]. Given GAD67’s high expression in certain cancers, impaired GABA degradation due to an absence of ABAT could accelerate GABA accumulation within tumors (Figure 1). Supporting the role of GABA in promoting tumor progression, decreasing intratumoral GABA levels by ectopically expressing ABAT is known to attenuate cancer cell proliferation and migration [23, 25]. Furthermore, loss of ABAT correlates with poor overall survival in patient with primary cancers [23–25], suggesting that low ABAT could be predictive of poor prognosis.
Alternatively, ABAT appears to play a tumor-promoting role in certain metastatic settings, particularly those involving the nervous system. Disseminated medulloblastoma (MB), a malignant pediatric brain tumor which commonly metastasizes to the leptomeninges, requires ABAT to form leptomeningeal metastases. Specifically, metastatic MB cells upregulate ABAT to maintain their viability in response to poor-nutrient conditions in the cerebrospinal fluid by exploiting GABA as an energy source, thereby facilitating metastasis [26]. This is in line with another study on breast cancer brain metastases (BCBMs), which reveals that, after traveling to the neural niche, breast cancer cells display a similar GABAergic phenotype as neuronal cells, with prominent induction of ABAT expression [27]. Given GABA’s abundance in nerve tissue, the enhanced GABA shunt provides a metabolic and proliferative advantage to metastatic cancer cells in the brain microenvironment. In sum, whereas ABAT is commonly downregulated in peripheral primary tumors such that GABA accumulates within the TME, metastasized brain tumors can utilize ABAT to exploit neuronal GABA as a readily available energy source (Figure 1).
Targeting GABA metabolic enzymes for cancer therapy
As discussed above, GAD67 and ABAT, the key enzymes in GABA metabolism, are involved in regulating cancer proliferation and migration via their enzymatic activities in GABA synthesis and degradation, respectively. While elevated GAD67 expression is invariably associated with enhanced cancer progression, ABAT is usually suppressed in tumors, except in the brain metastatic niche. Consistent with this, genetic GAD67 silencing [5, 13, 14, 18] and ABAT overexpression [23–25] have both been shown to inhibit tumor progression. Moreover, treatment with 3-mercaptopropionic acid (3-MPA), a GAD67 inhibitor and a convulsant, leads to diminished oral cancer cell migration [14] and reduced carcinoma cell proliferation [5]. As 3-MPA usage is currently limited to research settings, the FDA-approved drug bilobalide, also reported to block GABA synthesis via GAD inhibition [28], might be more applicable for future clinical studies (Figure 1). Indeed, bilobalide exerts anti-tumor effects in gastric cancer [29], where increased GABA levels and GAD67 activity have been observed around the tumor site [8].
While ABAT appears to be tumor suppressive in primary tumors, elevated ABAT expression has been observed in brain metastatic MB [26] and BCBMs [27], where it functions to satisfy energy metabolism and growth requirements. Treatment with vigabatrin, an FDA-approved anti-epileptic seizure drug targeting ABAT enzymatic activity, can abolish the proliferative effect of GABA on breast-to-brain metastases derived cells in vitro [27], indicating that these cells utilize the GABA metabolite as fuel even under cell culture conditions. While vigabatrin has also been shown to have promising therapeutic effects for treating brain metastases in xenograft mouse models [21], additional research is required to determine the safety and efficacy of inhibiting GABA catabolism in the brain metastasis setting. In addition, given that patients with primary and metastatic brain tumors frequently experience seizures due to GABA dysregulation [30], vigabatrin might provide an additional benefit of reducing tumor-induced seizures, which could be of interest for future clinical studies (Figure 1). A third class of GABA modulating drug, GABA reuptake inhibitors (GRIs), might also provide clinical benefits in certain cancer settings (Figure 1); however, to date only a few studies have investigated the expression and function of GABA transporters (GATs) in tumor cells [27], so this possibility requires additional research.
GABA receptors in tumor growth and progression
GABA-A receptors
GABA-A receptors are chloride channels composed of five protein subunits belonging to 19 different subunit isoforms, known as: α1–6, β1–3, γ1–3, δ, ε, θ, π, and ρ1–3 [31]. Chloride influx through GABA-A inhibits the firing of neuronal action potentials and underlies GABA’s sedative effects. A growing body of evidence indicates that GABA-A receptors are also expressed in several solid tumor types, where they appear to play divergent roles in regulating proliferation and migration depending on the tumor site [32, 33].
In nervous system tumors, GABA-A receptor activation is tumor suppressive in glioma, medulloblastoma, and neuroblastoma cells [34–37]. More specifically, two groups identified GABA-A’s α5 subunit (GABRA5) as a negative factor for brain cancer progression, such that pharmacologically enhancing GABRA5 activity results in decreased cell viability and induction of apoptosis [36, 37]. Based on their increased dependence on ABAT-dependent GABA catabolism for energy production (described above), it is possible that cancer cells residing in a neural microenvironment preferentially utilize GABA as metabolic fuel rather than as a neurotransmitter for receptor activation. Moreover, pharmacological activation of GABA-A receptors in neuroblastoma cells inhibits mitogenic signaling and induces apoptosis [35], suggesting that GABA-A receptor agonists in clinical use may have potential applications for brain cancer therapy (Figure 1).
Conversely, GABA-A receptors exhibit clear tumor promoting effects on cancers outside the nervous system. In hepatocellular carcinoma (HCC), GABRA3 expression is upregulated in cultured cells as well as in patient tissues, and silencing GABRA3 expression impairs the GABA-stimulated growth advantage of HCC cells [38]. Mechanistically, GABRA3 overexpression has been shown to promote breast cancer migration and metastasis by activating protein kinase B (AKT) signaling [39]. GABRA1 immunoreactivity is also elevated in prostate carcinomatous glands compared with normal prostate epithelium, and pharmacological inhibition of GABA-A receptors blocks GABA-induced EGFR signaling and cancer cell growth [40]. In pancreatic cancer, the GABA-A receptor π subunit (GABRP) is required for the growth-promoting effects of GABA treatment; this process seems to be triggered by GABRP-mediated induction of intracellular calcium and activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathway [13]. Moreover, GABRP is also upregulated in other cancer types. In particular, high GABRP expression correlates with poorer survival of patients with BLBC or ovarian cancer, and depletion of GABRP decreases MAPK/ERK-mediated migration [41, 42]. Similarity, GABRP also promotes oral squamous cell carcinoma proliferation by activating the MAPK pathway [43]. In sum, GABA-A receptor signal transduction, particularly through GABRP, supports the proliferative and aggressive behavior of advanced tumors, at least in part through activating oncogenic MAPK/ERK signaling (Figure 1).
To summarize the current knowledge, while GABA-A receptors appear to be tumor suppressive in brain cancers, GABA-A expression and activity associate with poorer outcomes in most solid tumors outside the nervous system, highlighting the location-dependent functions of GABA-A receptors on tumor growth and metastasis. Notably, since no evidence of the contribution of GABA-A receptor-controlled chloride flux in tumor progression has been reported to our knowledge, it is possible that the different protein subunits of GABA-A receptors may have specific targets to modulate their downstream signaling pathways.
GABA-B receptors
In the mammalian CNS, GABA-B receptors are heterodimeric G-protein-coupled receptors composed of GABBR1 and GABBR2 subunits. Upon activation, GABA-B receptors associate specifically with Gi/o G-proteins to transduce GABA signaling and produce slow and prolonged inhibitory signals [44]. Outside the CNS, GABA-B receptors have also been shown to regulate cancer progression. Whereas some early reports suggested that GABA-B receptor activation can have tumor suppressive effects [45–47], evidence from more recent studies suggests that GABA-B receptors generally promote the proliferation and migration of numerous cancer types. Specifically, GABA-B receptors enhance the malignancy of human high-grade chondrosarcoma [48] and breast cancer cells [49] by promoting AKT signaling. Moreover, GABA-B receptors facilitate the invasion and migration of breast cancer cells by enhancing ERK1/2-mediated signaling and the subsequent expression of matrix metalloproteinase-2 (MMP-2) [50]. In addition, GABA activity has also been shown to promote both proliferation [40] and migration [51] in prostate cancer cells. While GABA-A receptors are highly expressed in prostate cancers where they stimulate proliferation [40, 52, 53], they do not appear to contribute prostate cancer cell migration [51]. Meanwhile, GABA-B receptor inhibition in prostate cancer can suppress GABA-induced MMP activation [51, 54] and gastrin peptide secretion [55], leading to decreased invasion and migration [52, 53]. Therefore, GABA-A and GABA-B receptors appear to selectively facilitate prostate cancer cell proliferation and migration, respectively (Figure 1).
While these studies indicate that GABA receptors play important roles in regulating the progression of multiple tumor types, the current data suggest that GABA receptor-mediated effects on proliferation and migration are likely dependent on both receptor class and physiological context. Of note, as measured in an oocyte expression system, GABA-A and GABA-B receptors appear to exhibit distinct pharmacodynamic responses to GABA ligand: to reach a half maximal response, GABA-B receptors (co-expression of GABBR1 and GABBR2) require a concentration of ~1 μM [56], whereas some subunits of GABA-A receptors (co-expression of βαγ and βα concatemeric receptor) need the ligand at ~34 μM [57]. Since the local concentration of GABA at the non-brain tumor cell membrane is lower than in CNS tissues [5, 12], tumoral GABA-B receptors might be preferentially activated as compared with specific GABA-A receptor subunits, resulting in their dominant biological impact on GABA-mediated tumor progression.
Targeting GABA receptors for cancer therapy
While many existing agonists and antagonists can selectively target GABA-A and GABA-B receptors [58–60], because expression of the different receptor subtypes on cancer cells can vary considerably, utilizing the most appropriate compound will be critical for optimizing anti-tumor efficacy. In medulloblastoma cells, GABRA5 activation with benzodiazepines, a class of psychoactive drugs, results in reduced cell viability via induction of apoptosis [36, 37]. This anti-tumor efficacy was also observed in a xenograft mouse model [61]. Moreover, two benzodiazepine derivatives (QH-II-066 and KRM-II-08) have also been found to reduce melanoma tumor growth and potentiate radiation therapy and anti-PD-L1 anti-tumor activity [62]. Nevertheless, because many benzodiazepines also have high affinity for the mitochondrial translocator protein (TSPO) and may work through their actions on TSPO [63], the utilization of benzodiazepines as GABA-A receptor agonists for anti-cancer therapy requires further investigation. Alternatively, GABA-A receptor antagonists have been found to exhibit anti-tumor efficacy in certain cancer types (Figure 1). For example, the GABA-A receptor antagonist picrotoxin blocks GABA-stimulated prostate cancer cell growth [40], and similar anti-proliferative effects have been achieved using other GABA-A inhibitors, including bicuculline and flumazenil [13, 52, 64].
Baclofen is a selective GABA-B receptor agonist which is clinically used to treat muscle spasms and chronic neuropathy. It has been reported that administration of baclofen causes reduced proliferation and/or migration of some human cancer types including liver and colon cancers [45, 47, 65]. However, baclofen can exert the opposite effect on cancer progression: studies in both breast and prostate cancers show that although baclofen treatment does not affect cell proliferation [49, 53], it can promote cellular migration and invasion [50, 51, 54, 55]. In agreement with this, several evidence showed that treatment with GABA-B receptor antagonists such as CGP55845, CGP54626 and CGP35348 diminishes GABA-B receptor-induced migration [50, 51, 54]. The apparently contradictory nature of GABA-B receptor activation using baclofen might be due to the different tumor types and models used in these studies.
It should be noted that many of the aforementioned results were obtained using cultured tumor cells grown in vitro. As described in more detail below, GABA is now understood to play an important role in suppressing anti-tumor immunity. For this reason, GABA-B receptor inhibition using 2-Hydroxy-saclofen synergizes with anti-PD-1 immunotherapy to eradicate lung and colon cancers in syngeneic mouse models [5]. In light of GABA’s immunosuppressive activity, future investigations seeking to leverage GABA receptor pharmacology as anti-cancer therapy should be conducted in vivo, using immunocompetent animals when possible, in order to maximize their therapeutic relevance to human disease.
GABA in the anti-tumor immune response
Multiple neurotransmitters, including GABA, have been shown to modulate the mammalian immune system. Indeed, the presence of GABAergic signaling machinery has now been identified in multiple immune cell types including T cells, natural killer (NK) cells, B cells, macrophages, and dendritic cells, where it impacts critical immune cell functions including migration, cytokine secretion, activation, and cytotoxic responses [66]. Although GABAergic signaling has been reported to have anti-inflammatory and immunosuppressive effects by directly suppressing the functions of certain peripheral immune cells, such as macrophages and lymphocytes [67, 68], its role in immune evasion within the TME is not fully understood. Recently, several studies demonstrated that GABAergic signaling shapes the intratumoral immune landscape to facilitate cancer progression. Similar to previous findings [13, 42, 43], one study showed that expression of GABRP, a GABA-A receptor subunit, is upregulated during malignant progression and predicts a poor prognosis in pancreatic cancer [69]. Moreover, GABRP is positively correlated with the intratumoral density of macrophages, a cell type thought to promote cancer progression by suppressing immunocompetent cells in the TME [70]. Mechanistically, intracellular calcium level is modulated by GABRP in a GABA-independent manner to activate cancer-intrinsic nuclear factor κB (NF-κB) signaling in pancreatic cancer cells, ultimately facilitating intratumoral macrophage infiltration via cytokine induction (Figure 2). Another study [5] further details GABA’s functional role and molecular mechanism in suppressing anti-tumor immunity. Rather than providing metabolic fuel or building blocks, cancer cell-derived GABA activates GABA-B receptors to stabilize β-catenin, thereby suppressing CCL4 and CCL5 expression to prevent the recruitment of dendritic cells (Figure 2), which are critical for the generation of a T-cell-inflamed TME [71]. Through this process, autocrine GABA signaling generates an immunologically “cold” TME that is favorable for tumor progression. In addition to GABAergic cancer cells, B cells have also been shown to produce GABA as a metabolic product which exerts direct immunoregulatory effects on other immune cells [12]. Specifically, B cell-derived GABA limits anti-tumor responses by suppressing the cytotoxic activity of tumor-infiltrating CD8+ T and facilitating the differentiation and survival of macrophages with anti-inflammatory properties (Figure 2). In addition to the indirect roles of GABA-mediated cytokine production, evidence also indicates that GABA itself might act as a chemoattractant to modulate the function of the TME’s immune composition [72].
Figure 2. GABA signaling shapes the intratumoral immune landscape to facilitate cancer progression.

GABRP, which is increased in pancreatic ductal adenocarcinoma, activates cancer-intrinsic nuclear factor κB (NF-κB) signaling and ultimately increases the infiltration of intratumoral tumor-associated macrophages (TAM) by inducing CXCL5 and CCL20 expression in a GABA-independent manner. In lung and colon cancers, cancer cells with aberrant GAD1 expression rewire glutamine metabolism for the synthesis of GABA. Consequently, cancer cell-derived GABA activates the GABA-B receptor to facilitate β-catenin-mediated CCL4/5 inhibition, leading to the suppression of dendritic cell (DC) recruitment and T cell infiltration. In addition to cancer cells, activated B cell with GAD1 expression can also synthesize and secret GABA to promote TAM survival and inhibit CD8+ T cell killer function. As a result, both cancer cell- and B cell-derived GABA dampen antitumor immune responses within the TME. Figure created with BioRender.
Together, the existing evidence suggests that GABAergic signaling provides tumors with at least two functions critical for immune evasion: first, by shaping immune cell infiltration through cancer-intrinsic mechanisms [5, 69]; second, by directly affecting the functional properties of infiltrated immune cells [12]. In addition, multiple reports also indicate that GABAergic signaling can regulate the functions of certain peripheral immune cells, including macrophages [12] and lymphocytes [67, 68]. While these findings support the repurposing of existing GABA pathway inhibitors for the treatment of solid tumors, much remains to be learned regarding the detailed molecular mechanisms of how GABA and the GABAergic signaling modulate immune cell functions within the TME. This notwithstanding, because GABA conditions the host toward an immune tolerant TME permissive for tumor growth, targeting GABA production or GABAergic signaling has been shown to enhance anti-tumor immunity and tumor clearance [5, 12, 69].
The clinical efficacy of tumor immunotherapy has been exemplified by the unprecedented success of immune checkpoint intervention. However, molecular mechanisms that dictate the efficacy of checkpoint targeting need to be identified to develop novel therapeutic strategies synergizing with current treatments. Despite the context-dependent roles of autocrine GABA signaling in regulating cancer cell proliferation and migration, the current consensus indicates that GABA functions to suppress immune responses to protect tumors from attack [5, 12, 69]. Thus, GABA-mediated immunosuppression appears to be at least one cause of tumor “coldness”, such that antagonizing GABA signaling may enhance the anti-tumor function of multiple immune cell populations. In this regard, targeting GABA signaling provides a plausible synergistic strategy to improve the clinical efficacy of various immunotherapies including checkpoint blockade. Indeed, in tumor-bearing immunocompetent mice, administration of the GABA-B receptor antagonist 2-OH-saclofen in combination with anti-PD-1 antibody leads to significant reductions in tumor burden and improved overall survival compared with either monotherapy alone [5]. This result is supported by previous reports demonstrating that GABA-B receptor activation has anti-inflammatory effects [73, 74]. Given that immune cell expression of GABAergic components and their contribution to inflammatory responses is being increasingly recognized [66], distinct receptor sub-types or other GABAergic components may also be targeted pharmacologically to modulate anti-tumor immune responses. Although no clinical application of GABAergic agents in oncotherapy for humans has yet been reported, the current evidence suggests that full and partial GABAergic agents with immunomodulatory functions in various disease models are clinically safe and may be efficacious against multiple solid tumor types [74–76].
Concluding remarks
This review presents the existing knowledge regarding how GABAergic signaling contributes to cancer development and remodeling of the tumor immune microenvironment. However, many questions remain (see Outstanding questions), one being how GABAergic components are induced by cancer cells as well as the specific downstream target(s) of GABAergic signaling during cancer progression and metastasis. Improved understanding in these areas will be critical for the development of new agents to modulate GABAergic signaling as well as the repurposing of existing drugs. For example, GABA activates GABA-B receptor to stabilize β-catenin protein, thereby providing a Wnt-independent pathway for tumors to sustain β-catenin activity. This new signaling axis can be pharmacologically targeted using GABAergic pathway antagonists. While there has been growing interest in pharmacological strategies to target GABAergic signaling for anti-cancer therapy, further understanding of the interplay between GABAergic signaling and TME remodeling will accelerate research efforts in this field. Most previous studies have been focused on the roles of GABA or GABAergic components in contributing to autonomous tumor growth. However, an increasing body of evidence suggests that GABAergic signaling also functions to shape the immune landscape of the TME. Since expression of GABAergic components has been observed in several immune cell types, including T lymphocytes, myeloid lineage cells, and NK cells, GABAergic signaling may mediate microenvironmental crosstalk between these intra-tumoral cells. To elucidate the roles of GABAergic components and downstream signaling pathways involved in immune regulation, it will be important to dissect the underlying molecular mechanisms of how GABA functions in key immune cell processes, including cytokine secretion, proliferation, cytotoxicity, migration, and chemotaxis. Given that multiple anti-GABAergic agents, including clinically used drugs, exhibit anti-tumor effects in mouse models, clinical trials and rational patient selection could validate the therapeutic potential of these emerging antineoplastic agents.
Outstanding questions.
What are the molecular mechanisms responsible for GABAergic components’ aberrant expression in tumor cells?
What are the specific downstream targets of GABAergic components during cancer progression and metastasis?
Do GABA transporters play a role in regulating cancer progression as well as immune invasion in the TME?
How does GABAergic signaling mediate microenvironmental crosstalk with anti-tumor immune responses? And what are the underlying mechanisms on GABAergic signalling-controlled functions on peripheral immune cells?
Highlights.
Aberrant GABA signaling plays critical roles in solid tumor growth and progression.
GABA accumulates in certain types of solid tumors, where it can drive cancer cell proliferation and metastasis.
Brain tumors can catabolize GABA for energy production.
GABAergic signaling in the tumor microenvironment contributes to immune evasion.
Targeting GABAergic components using existing medications may provide new avenues for therapeutic intervention against cancer.
Acknowledgements
We thank the funding support from the National Cancer Institute (R33-CA-225325 to Q.-J.L. and R01- CA233205 to Q.-J.L. and X.-F.W.). We would like to apologize to colleagues whose work was not discussed in this review because of space limits.
Glossary
- 2-Hydroxy-saclofen
a potent competitive antagonist of GABA-B receptor-mediated actions in the central and peripheral nervous system.
- Baclofen
a GABA-B receptor agonist which is approved to treat muscle spasticity.
- Benzodiazepines
a class of medicines that increase the activity of GABA-A receptors. They are widely used to treat generalized anxiety disorder, insomnia, seizures, social phobia, and panic disorder.
- Bicuculline
a GABA-A receptor antagonist that can cause convulsions. It is experimentally utilized in the study of epilepsy.
- Chemoattractant
a substance that creates chemical concentration gradients for the movement of an organism or entity.
- Epileptic seizure
a neurological disorder due to abnormally excessive or synchronous neuronal activity in the brain, causing periods of unusual behavior, sensations and sometimes loss of awareness.
- Flumazenil
a GABA-A receptor antagonist which is used in the treatment of benzodiazepine overdose.
- GABAergic neuron
a type of neuron that can produce and secret GABA.
- GABA shunt
a metabolic process with the dual purpose of GABA biosynthesis and catabolism.
- G-protein-coupled receptors
the largest family of human cell surface receptors that converts extracellular signals into intracellular responses, including responses to hormones and neurotransmitters.
- Immune evasion
is a process by which pathogenic organisms or tumors are able to bypass or evade a host’s immune response.
- Trophic factor
a small protein that supports the growth and viability of cells.
Footnotes
Declaration of Interests
Qi-Jing Li is a scientific co-founder and shareholder of TCRCure Biopharma. The remaining authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Represa A and Ben-Ari Y (2005) Trophic actions of GABA on neuronal development. Trends Neurosci 28 (6), 278–283. [DOI] [PubMed] [Google Scholar]
- 2.Young SZ and Bordey A (2009) GABA’s control of stem and cancer cell proliferation in adult neural and peripheral niches. Physiology (Bethesda) 24, 171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soltani N et al. (2011) GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc Natl Acad Sci U S A 108 (28), 11692–11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang QH et al. (2019) GABAergic regulation of pancreatic islet cells: Physiology and antidiabetic effects. Journal of Cellular Physiology 234 (9), 14432–14444. [DOI] [PubMed] [Google Scholar]
- 5.Huang et al. (2022) Cancer-cell-derived GABA promotes beta-catenin-mediated tumour growth and immunosuppression. Nat Cell Biol 24 (2), 230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleinrok Z et al. (1998) GABA content and GAD activity in colon tumors taken from patients with colon cancer or from xenografted human colon cancer cells growing as s.c. tumors in athymic nu/nu mice. J Physiol Pharmacol 49 (2), 303–310. [PubMed] [Google Scholar]
- 7.Maemura K et al. (2003) Gamma-amino-butyric acid immunoreactivity in intramucosal colonic tumors. J Gastroenterol Hepatol 18 (9), 1089–1094. [DOI] [PubMed] [Google Scholar]
- 8.Matuszek M et al. (2001) GABA content and GAD activity in gastric cancer. Med Sci Monit 7 (3), 377–381. [PubMed] [Google Scholar]
- 9.Mazurkiewicz M et al. (1999) GABA level and GAD activity in human and mouse normal and neoplastic mammary gland. J Exp Clin Cancer Res 18 (2), 247–253. [PubMed] [Google Scholar]
- 10.Opolski A et al. (2000) The role of GABA-ergic system in human mammary gland pathology and in growth of transplantable murine mammary cancer. J Exp Clin Cancer Res 19 (3), 383–390. [PubMed] [Google Scholar]
- 11.Zhou X et al. (2018) High-level production and purification in a functional state of an extrasynaptic gamma-aminobutyric acid type A receptor containing alpha4beta3delta subunits. PLoS One 13 (1), e0191583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang B et al. (2021) B cell-derived GABA elicits IL-10(+) macrophages to limit antitumour immunity. Nature 599 (7885), 471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takehara A et al. (2007) Gamma-aminobutyric acid (GABA) stimulates pancreatic cancer growth through overexpressing GABAA receptor pi subunit. Cancer Res 67 (20), 9704–9712. [DOI] [PubMed] [Google Scholar]
- 14.Kimura R et al. (2013) Glutamate acid decarboxylase 1 promotes metastasis of human oral cancer by beta-catenin translocation and MMP7 activation. BMC Cancer 13, 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YY et al. (2016) Glutamate Decarboxylase 1 Overexpression as a Poor Prognostic Factor in Patients with Nasopharyngeal Carcinoma. Journal of Cancer 7 (12), 1716–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soejima S et al. (2021) GAD1 expression and its methylation as indicators of malignant behavior in thymic epithelial tumors. Oncology Letters 21 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuboi M et al. (2019) Prognostic significance of GAD1 overexpression in patients with resected lung adenocarcinoma. Cancer Medicine 8 (9), 4189–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan H et al. (2016) DNA methylation reactivates GAD1 expression in cancer by preventing CTCF-mediated polycomb repressive complex 2 recruitment. Oncogene 35 (30), 3995–4008. [DOI] [PubMed] [Google Scholar]
- 19.Li CM et al. (2004) CTNNB1 mutations and overexpression of Wnt/beta-catenin target genes in WT1-mutant Wilms’ tumors. Am J Pathol 165 (6), 1943–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz DR et al. (2003) Novel candidate targets of beta-catenin/T-cell factor signaling identified by gene expression profiling of ovarian endometrioid adenocarcinomas. Cancer Res 63 (11), 2913–2922. [PubMed] [Google Scholar]
- 21.Schnepp PM et al. (2017) GAD1 Upregulation Programs Aggressive Features of Cancer Cell Metabolism in the Brain Metastatic Microenvironment. Cancer Res 77 (11), 2844–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veldic M et al. (2004) DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc Natl Acad Sci U S A 101 (1), 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X et al. (2019) Loss of ABAT-Mediated GABAergic System Promotes Basal-Like Breast Cancer Progression by Activating Ca(2+)-NFAT1 Axis. Theranostics 9 (1), 34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han H et al. (2021) ABAT targeted by miR-183–5p regulates cell functions in liver cancer. International Journal of Biochemistry & Cell Biology 141. [DOI] [PubMed] [Google Scholar]
- 25.Lu J et al. (2020) ABAT and ALDH6A1, regulated by transcription factor HNF4A, suppress tumorigenic capability in clear cell renal cell carcinoma. J Transl Med 18 (1), 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martirosian V et al. (2021) Medulloblastoma uses GABA transaminase to survive in the cerebrospinal fluid microenvironment and promote leptomeningeal dissemination. Cell Rep 36 (4), 109475. [DOI] [PubMed] [Google Scholar]
- 27.Neman J et al. (2014) Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proceedings of the National Academy of Sciences of the United States of America 111 (3), 984–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki K et al. (1999) Effects of bilobalide on gamma-aminobutyric acid levels and glutamic acid decarboxylase in mouse brain. Eur J Pharmacol 367 (2–3), 165–173. [DOI] [PubMed] [Google Scholar]
- 29.Liu J et al. (2021) Sesquiterpenoid bilobalide inhibits gastric carcinoma cell growth and induces apoptosis both in vitro and in vivo models. J Biochem Mol Toxicol 35 (5), e22723. [DOI] [PubMed] [Google Scholar]
- 30.MacKenzie G et al. (2016) Compromised GABAergic inhibition contributes to tumor-associated epilepsy. Epilepsy Research 126, 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen RW and Sieghart W (2009) GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology 56 (1), 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhattacharya D et al. (2021) Therapeutically leveraging GABAA receptors in cancer. Exp Biol Med (Maywood) 246 (19), 2128–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao R et al. (2022) Ligand-Gated Ion Channels as Targets for Treatment and Management of Cancers. Front Physiol 13, 839437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blanchart A et al. (2017) Endogenous GABAA receptor activity suppresses glioma growth. Oncogene 36 (6), 777–786. [DOI] [PubMed] [Google Scholar]
- 35.Hackett CS et al. (2014) Expression quantitative trait loci and receptor pharmacology implicate Arg1 and the GABA-A receptor as therapeutic targets in neuroblastoma. Cell Rep 9 (3), 1034–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kallay L et al. (2019) Modulating native GABAA receptors in medulloblastoma with positive allosteric benzodiazepine-derivatives induces cell death. J Neurooncol 142 (3), 411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sengupta S et al. (2014) alpha5-GABAA receptors negatively regulate MYC-amplified medulloblastoma growth. Acta Neuropathol 127 (4), 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y et al. (2008) Gamma-aminobutyric acid promotes human hepatocellular carcinoma growth through overexpressed gamma-aminobutyric acid A receptor alpha 3 subunit. World Journal of Gastroenterology 14 (47), 7175–7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gumireddy K et al. (2016) The mRNA-edited form of GABRA3 suppresses GABRA3-mediated Akt activation and breast cancer metastasis. Nat Commun 7, 10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu W et al. (2014) Linking gamma-aminobutyric acid A receptor to epidermal growth factor receptor pathways activation in human prostate cancer. Mol Cell Endocrinol 383 (1–2), 69–79. [DOI] [PubMed] [Google Scholar]
- 41.Sizemore GM et al. (2014) GABA(A) Receptor Pi (GABRP) Stimulates Basal-like Breast Cancer Cell Migration through Activation of Extracellular-regulated Kinase 1/2 (ERK1/2). Journal of Biological Chemistry 289 (35), 24102–24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sung HY et al. (2017) Aberrant epigenetic regulation of GABRP associates with aggressive phenotype of ovarian cancer. Experimental and Molecular Medicine 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma J et al. (2016) Proliferative effects of gamma-amino butyric acid on oral squamous cell carcinoma cells are associated with mitogen-activated protein kinase signaling pathways. Int J Mol Med 38 (1), 305–311. [DOI] [PubMed] [Google Scholar]
- 44.Terunuma M (2018) Diversity of structure and function of GABAB receptors: a complexity of GABAB-mediated signaling. Proc Jpn Acad Ser B Phys Biol Sci 94 (10), 390–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joseph J et al. (2002) The neurotransmitter gamma-aminobutyric acid is an inhibitory regulator for the migration of SW 480 colon carcinoma cells. Cancer Res 62 (22), 6467–6469. [PubMed] [Google Scholar]
- 46.Tatsuta M et al. (1990) Inhibition by gamma-amino-n-butyric acid and baclofen of gastric carcinogenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine in Wistar rats. Cancer Res 50 (16), 4931–4934. [PubMed] [Google Scholar]
- 47.Wang T et al. (2008) Baclofen, a GABAB receptor agonist, inhibits human hepatocellular carcinoma cell growth in vitro and in vivo. Life Sci 82 (9–10), 536–541. [DOI] [PubMed] [Google Scholar]
- 48.Kanbara K et al. (2018) GABAB receptor regulates proliferation in the high-grade chondrosarcoma cell line OUMS-27 via apoptotic pathways. BMC Cancer 18 (1), 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei B et al. (2021) GABAB1e promotes the malignancy of human cancer cells by targeting the tyrosine phosphatase PTPN12. iScience 24 (11), 103311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang D et al. (2014) GABAergic signaling facilitates breast cancer metastasis by promoting ERK1/2-dependent phosphorylation. Cancer Lett 348 (1–2), 100–108. [DOI] [PubMed] [Google Scholar]
- 51.Azuma H et al. (2003) gamma-Aminobutyric acid as a promoting factor of cancer metastasis; Induction of matrix metalloproteinase production is potentially its underlying mechanism. Cancer Research 63 (23), 8090–8096. [PubMed] [Google Scholar]
- 52.Ippolito JE et al. (2006) Linkage between cellular communications, energy utilization, and proliferation in metastatic neuroendocrine cancers. Proceedings of the National Academy of Sciences of the United States of America 103 (33), 12505–12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abdul M et al. (2008) Expression of gamma-aminobutyric acid receptor (subtype A) in prostate cancer. Acta Oncologica 47 (8), 1546–1550. [DOI] [PubMed] [Google Scholar]
- 54.Xia S et al. (2017) GABABR-Induced EGFR Transactivation Promotes Migration of Human Prostate Cancer Cells. Mol Pharmacol 92 (3), 265–277. [DOI] [PubMed] [Google Scholar]
- 55.Solorzano SR et al. (2018) GABA promotes gastrin-releasing peptide secretion in NE/NE-like cells: Contribution to prostate cancer progression. Sci Rep 8 (1), 10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones KA et al. (2000) Signal transduction by GABA(B) receptor heterodimers. Neuropsychopharmacology 23 (4 Suppl), S41–49. [DOI] [PubMed] [Google Scholar]
- 57.Akk G et al. (2018) GABA Type A Receptor Activation in the Allosteric Coagonist Model Framework: Relationship between EC50 and Basal Activity. Molecular Pharmacology 93 (2), 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Evenseth LSM et al. (2020) The GABAB Receptor-Structure, Ligand Binding and Drug Development. Molecules 25 (13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.George K and Sadiq NM (2020) GABA Inhibitors. In StatPearls. [PubMed] [Google Scholar]
- 60.Jewett BE and Sharma S (2022) Physiology, GABA. In StatPearls. [PubMed] [Google Scholar]
- 61.Jonas O et al. (2016) First In Vivo Testing of Compounds Targeting Group 3 Medulloblastomas Using an Implantable Microdevice as a New Paradigm for Drug Development. Journal of Biomedical Nanotechnology 12 (6), 1297–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pomeranz Krummel DA et al. (2021) Melanoma Cell Intrinsic GABAA Receptor Enhancement Potentiates Radiation and Immune Checkpoint Inhibitor Response by Promoting Direct and T Cell-Mediated Antitumor Activity. Int J Radiat Oncol Biol Phys 109 (4), 1040–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El Chemali L et al. (2022) The mitochondrial translocator protein (TSPO): a key multifunctional molecule in the nervous system. Biochem J 479 (13), 1455–1466. [DOI] [PubMed] [Google Scholar]
- 64.Xia D et al. (2018) Transition from androgenic to neurosteroidal action of 5alpha-androstane-3alpha, 17beta-diol through the type A gamma-aminobutyric acid receptor in prostate cancer progression. J Steroid Biochem Mol Biol 178, 89–98. [DOI] [PubMed] [Google Scholar]
- 65.Wang H et al. (2021) GABAB receptor inhibits tumor progression and epithelial-mesenchymal transition via the regulation of Hippo/YAP1 pathway in colorectal cancer. Int J Biol Sci 17 (8), 1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhandage AK and Barragan A (2021) GABAergic signaling by cells of the immune system: more the rule than the exception. Cellular and Molecular Life Sciences 78 (15), 5667–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhandage AK et al. (2018) GABA Regulates Release of Inflammatory Cytokines From Peripheral Blood Mononuclear Cells and CD4(+) T Cells and Is Immunosuppressive in Type 1 Diabetes. EBioMedicine 30, 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim JK et al. (2018) GABAergic signaling linked to autophagy enhances host protection against intracellular bacterial infections. Nat Commun 9 (1), 4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang SH et al. (2019) GABRP regulates chemokine signalling, macrophage recruitment and tumour progression in pancreatic cancer through tuning KCNN4-mediated Ca(2+) signalling in a GABA-independent manner. Gut 68 (11), 1994–2006. [DOI] [PubMed] [Google Scholar]
- 70.Qian BZ and Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141 (1), 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spranger S et al. (2017) Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell 31 (5), 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhandage AK et al. (2021) GABAergic signaling in human and murine NK cells upon challenge with Toxoplasma gondii. J Leukoc Biol 110 (4), 617–628. [DOI] [PubMed] [Google Scholar]
- 73.Liu F et al. (2019) GABAB receptor activation attenuates inflammatory orofacial pain by modulating interleukin-1beta in satellite glial cells: Role of NF-kappaB and MAPK signaling pathways. Brain Res Bull 149, 240–250. [DOI] [PubMed] [Google Scholar]
- 74.Duthey B et al. (2010) Anti-inflammatory effects of the GABA(B) receptor agonist baclofen in allergic contact dermatitis. Exp Dermatol 19 (7), 661–666. [DOI] [PubMed] [Google Scholar]
- 75.Tian J et al. (2021) GABAA-Receptor Agonists Limit Pneumonitis and Death in Murine Coronavirus-Infected Mice. Viruses 13 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tian J et al. (2021) GABAB-Receptor Agonist-Based Immunotherapy for Type 1 Diabetes in NOD Mice. Biomedicines 9 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]


