Abstract
Interactions between the brain and the stomach shape both cognitive and digestive functions. Recent human studies report spontaneous synchronization between brain activity and gastric slow waves in the resting state. However, this finding has not been replicated in any animal models. The neural pathways underlying this apparent stomach-brain synchrony is also unclear. Here, we performed functional magnetic resonance imaging while simultaneously recording body-surface gastric slow waves from anesthetized rats in the fasted vs. postprandial conditions and performed a bilateral cervical vagotomy to assess the role of the vagus nerve. The coherence between brain fMRI signals and gastric slow waves was found in a distributed “gastric network ”, including subcortical and cortical regions in the sensory, motor, and limbic systems. The stomach-brain coherence was largely reduced by the bilateral vagotomy and was different between the fasted and fed states. These findings suggest that the vagus nerve mediates the spontaneous coherence between brain activity and gastric slow waves, which is likely a signature of real-time stomach-brain interactions. However, its functional significance remains to be established.
1. Introduction
The stomach and the brain interact with one another. The brain regulates food ingestion and digestion (Konturek et al., 2004; Holtmann and Tally, 2014; Clemmensen et al., 2017). The stomach affects intuition, emotion, and cognition (Mayer, 2011; Klarer et al., 2014, 2018). Such interactions are partly mediated by bidirectional neural signaling along the so-called stomach-brain neuroaxis (Powley et al., 2019; Liu et al., 2018; Kaelberer et al., 2018; Levinthal and Strick, 2020; Browning and Travagli, 2014; Holtmann and Talley, 2014), which includes neural circuits and pathways in both peripheral and central nervous systems (Powley et al., 2019; Mayer et al., 2019; Rebollo et al., 2021).
Prior studies typically characterize the stomach-brain neuroaxis by applying stimulation to the stomach or the brain while recording the resulting brain or gastric responses, respectively. For example, gastric distention can activate the subcortical and cortical networks involved in processing gastric sensation (Ladabaum et al., 2001; Mayer et al., 2019; Wang et al., 2008; Vandenbergh et al. 2005; Stephan et al., 2003). Gastric electrical stimulation can activate brainstem nuclei and cortical regions and reveal their functional selectivity (Cao et al., 2019; 2021). Likewise, brain stimulation can also modulate gastric motor function (Lyubashina, 2004; Hurley-Gius and Neafsey, 1986). Although these studies offer valuable insights, the stimulation settings used are often artificial, narrowly focused, prone to physiological confounds, and atypical of natural interactions between the stomach and the brain.
Recent studies demonstrate spontaneous interactions between brain activity and gastric electrical activity (Rebollo et al., 2018; Rebollo et al., 2021; Choe et al., 2021; Richter et al., 2017; Todd et al., 2021). In their seminal work, Rebollo et al. reports that resting state brain activity observed with functional magnetic resonance imaging (fMRI) is phase locked with gastric slow waves observed with electrogastrography (EGG) (Rebollo et al., 2018). This finding has been replicated by their follow-up study (Rebollo et al., 2021) and an independent group (Choe et al., 2021). In human brains, the stomach-brain synchrony is most pronounced in sensorimotor areas, default-mode network, and cerebellum (Rebollo et al., 2018; Choe et al., 2021) and likely extends further to other networks or systems. In addition, the phase of gastric slow waves is coupled with the amplitude of alpha oscillations in magnetoencephalography (MEG) (Richter et al., 2017) and electroencephalogram (EEG) (Todd et al., 2021), suggesting that the brain activity coupled with gastric activity is likely of neural origin.
It is also unclear how brain and gastric rhythms are synchronized in real-time despite a long distance between the two organs. The vagus is central to how the brain modulates gastric motor function, such as gastric tone (Azpiroz and Malagelada, 1987), accommodation (Takahashi and Owyang, 1997), motility, and emptying (Lu et al., 2018, 2020), primarily through the dorsal vagal complex in the brainstem that mediates the vagovagal reflexes (Powley et al., 2019; Travagli and Anselmi, 2016; Harper et al., 1959; Shapiro and Miselis, 1985; Roger et al., 1995; 1996). The dorsal vagal complex interacts with the hypothalamus, thalamus, amygdala, insular cortex, medial prefrontal cortex, and sensorimotor cortex (Browning and Travagli, 2014; Rinaman and Schwartz, 2004; Han et al., 2018; Levinthal and Strick, 2020; Mayer et al., 2019). Moreover, a subtype of vagal afferent receptors, namely intramuscular arrays (Berthoud and Powley 1992; Brookes et al., 2013), may directly innervate interstitial cells of Cajal (ICCs) (Powley et al., 2019). The ICCs initiate the slow wave and pace the stomach (Cajal 1893; Sanders et al., 1996). Taken together, the vagal pathways and their extension in the brain are plausible substrates that bring widespread brain activity into partial synchronization with gastric slow waves.
Here, we hypothesize that the vagal nerves mediate the stomach-brain synchrony in the resting state. We acquired EGG and fMRI simultaneously in rats and evaluated their coherence at the frequency of the gastric slow wave in an attempt to replicate the findings in prior human studies (Rebollo et al., 2018; Choe et al., 2021). Importantly, the use of the animal model allowed us to apply bilateral vagotomy and assess its effects on the apparent stomach-brain synchrony. In addition, we also tested the effect of fasted vs. fed (postprandial) states.
2. Methods and materials
2.1. Animals
A total of 18 Sprague-Dawley rats (male, 250–350 g, Envigo RMS, Indianapolis, IN) were used in this study. All animals were housed in a controlled environment with a temperature at 21 ± 1 °C and light on from 6 AM to 6 PM. After diet training and overnight fasting, the animals were divided into three groups for simultaneous acquisition of fMRI and EGG under three different conditions: fed with intact vagal nerves (n = 8) or vagotomy (n = 5), or fasting with the intact vagus (n = 5). All animal procedures followed a protocol approved by the Institutional Animal Care and Use Committee and the Laboratory Animal Program at Purdue University.
2.2. Animal preparation
Through diet training, every animal was preconditioned to be able to voluntarily consume a test meal before the experiment. The test meal was a fixed quantity (5 g) of dietgel (DietGel Recovery, ClearH2O, ME, USA). The test meal had 5.6 kcal, containing 0.03 g protein, 1.16 g carbohydrates, 1 g sugars, 0.055 g dietary fibers, 0.095 g fat, and 0.01 g saturated fat. As described elsewhere (Lu et al., 2017), the diet training took about five days. In the first two days, 56 g dietgel was placed in the animal cage together with regular chow. In the next three days, the animal was first fasted for 18 hrs from 6 PM to 12 PM on the next day and fed only with dietgel (56 g) at 12 PM. The animal was allowed to eat the dietgel from 12 PM to 6 PM; until then it was fasted again for the next day.
After diet training, all animals were fasted overnight. Food was removed from the cage at 6 PM to prepare the animal for the experiment starting at about 12 PM the next day. One group of animals (fasted, n = 5) remained fasted during the experiment. Other animals (n = 13) were provided a 5 g test meal (dietgel) and were able to quickly consume the meal at the beginning of the experiment. Among those that consumed the test meal, one group of animals had additional vagotomy (n = 5) whereas the rest had an intact vagus (n = 8). The animals were anesthetized for the vagotomy (if applicable), electrode placement, and fMRI-EGG acquisition in that order.
2.3. Anesthesia
Every animal was initially anesthetized using an induction chamber with 5% isoflurane mixed in oxygen delivered at 1 L/min for up to 5 min. Then the animal was transferred to a surgical bench, where 1.5–2.5% isoflurane mixed in oxygen was administered at 1 L/min through a nose cone during surgical vagotomy (for 5 animals after the test meal) and placement of EGG electrodes (for all 18 animals). Then the animal was transferred to a rat holder placed in the MRI scanner and was set up to receive isoflurane through a nose cone. During the set-up process, isoflurane was kept between 1.5–2.5% to maintain the appropriate depth of anesthesia. After the animal was set up for MRI, a bolus of the dex-domitor (Zoetis, Parsippany-Troy Hills, New Jersey, USA, 12.5 μg/kg, 0.05 mg/ml) was administered at 1 L/min through subcutaneous injection. About 15 min later, continuous subcutaneous infusion of the dex-domitor started at 12.5 μg/(kg × hour) and 0.05 mg/ml, while isoflurane was reduced to 0.1–0.5%. Following that, the infusion rate of the dex-domitor was increased by 12.5 μg / (kg × hour) for every additional hour to maintain a stable physiological condition over the course of fMRI-EGG acquisition. That is, the heart rate at 250–350 beats per minute, the respiratory rate at 20–60 times per minute, SpO2 above 96%, and the rectal temperature at 36.5–37.5 C°. These physiological parameters were monitored using two systems: Pulse Oximeter and Rat & Mouse Heart Rate Monitor Module (mouseSTAT, Kent scientific, Torrington, CT, USA), and the monitoring and gating system for small animals (model1030, SA instruments, Stony Brook, NY, USA). A heating pad was also controlled to keep the animal warm inside the MRI scanner.
2.4. Vagotomy
Bilateral cervical vagotomy was performed in a group of (n = 5) animals after they consumed the test meal. The surgery was performed when the animal was anesthetized with 1.5–2.5% isoflurane as described above. After a toe pinch test, the fur in the cervical area was shaved, followed by scrubbing the skin with 75% ethanol and betadine. Then, a 2–3 cm midline incision was made, starting from the jawline and moving caudally. Skin and soft tissue were separated for the (left or right) cervical vagus nerve to be exposed, isolated and cut. This procedure was performed first for the left side and then for the right side, resulting in bilateral cervical vagotomy. The incision was sutured closed to make the animal ready for attachment of EGG electrodes on the abdominal surface (as described in the next subsection).
2.5. Placement of body-surface electrodes
For all animals, an array of electrodes was attached to the abdominal surface for EGG recordings. The fur on the abdominal skin surface was carefully shaved. We also applied hair remover lotion and waited for 5 min and then removed the extra fur. The shaved skin was further cleaned with sterile saline to prepare for electrode attachment. The electrodes were a 5-by-6 array on a thin 45×60 mm2 perylene substrate (chronic EMG patch, Microprobes, Gaithersburg, MD, USA). Each electrode in the array was a 1 × 2 mm2 platinum contact, with the distance between adjacent electrodes of 7 mm. The array was attached to the skin surface and sutured at four corners and four edges. Additional skin tape was applied to further secure the electrode attachment. In the array, the rows were aligned from left to right and the columns were aligned from superior to inferior (Fig. 1A). The intersection of the second row and the third column was on top of the xiphoid. In addition, two adhesive carbon electrodes (Neotech, Valencia, CA, USA) were used as the reference and the ground for EGG, respectively. The reference was about 5 mm superior to the xiphoid on the central line. The ground was on the left hind limb.
Fig. 1. Experimental design and physiological variations across conditions.

A, B, & C illustrate the experimental design and timeline for three conditions (fed, vagotomy, and fasted). Examples of corresponding EGG time series, their time-frequency representations and power spectra are shown for each condition. D shows an example of the power spectrum of the EGG recording. Spectral peaks are annotated in terms of gastric, respiratory, and cardiac activities. E, F, G, H, & I compare the difference in the EGG dominant frequency, respiratory rate, heart rate, heart rate viability (standard deviation of the normal-to-normal interval, SDNN), and the standard deviation (s.t.d.) of the global fMRI signal across the fed, vagotomy, and fasted conditions (*, p < 0.05, *** p < 0.001, two-sample t-test).
2.6. EGG recording
The cutaneous EGG signals were recorded using an electrophysiology system (Tucker Davis Technologies, Alachua, FL, USA) while the animal was placed inside the MRI scanner during concurrent fMRI (see details in the next subsection). The electrodes were connected via Pt/Ir wires and an Omnetics 36 socket nano connector to a headstage (LP32CH-Z-32 Channel Chronic Headstage, Tucker Davis Technologies, Alachua, FL, USA) near the animal. The headstage was connected via a shielded copper cable to an amplifier (PZ5 NeuroDigitizer, Tucker Davis Technologies, Alachua, FL, USA) placed in the MRI room. The amplifier digitized the signals (sampling rate: 24 kHz; bandwidth: DC to 24 kHz; dynamic range: +/−500 mV; ADC resolution: 28 bits) and connected to a data acquisition system (RZ2 BioAmp processor, Tucker Davis Technologies, Alachua, FL, USA) outside the MRI room through an optical fiber. The data acquisition system also received and stored a TTL trigger from MRI to synchronize EGG and fMRI acquisition.
2.7. MRI and fMRI acquisition
FMRI was performed with the 7-tesla small-animal MRI system (BioSpec 70/30, Bruker, Billerica, MA, USA) with a volume transmit coil (86 mm inner diameter) and a quadrature surface receive coil. Each animal was placed on a rat holder in the prone position. The head was fixed with two ear bars and a bite bar. The brain’s structure was acquired using a 2-D rapid acquisition with relaxation enhancement sequence with the repetition time (TR) = 5804.6 ms, effective echo time (TE) = 32.5 ms, echo spacing = 10.83 ms, voxel size = 0.125×0.125×0.5 mm3, RARE factor = 8, 50 slices, and flip angle (FA) = 90°. FMRI was acquired using a 2-D single-shot gradient-echo (GE) echo-planar imaging (EPI) sequence with TR = 1 s, TE = 16.5 ms, the number of repetition = 1800, in-plane resolution = 0.5 × 0.5 mm2, slice thickness = 1 mm, 25 slices, FA = 55°, and duration = 30 min. Each animal in the fed state (with or without vagotomy) underwent two sessions of fMRI, whereas each animal in the fasted state underwent four sessions of fMRI. The total time of fMRI-EGG acquisition was up to 2 hr in every animal.
2.8. EGG preprocessing
The high sampling rate and broad bandwidth of the EGG recording system allowed us to complete ly separate MRI-induced artifacts and EGG signals in the frequency domain without any confounding aliasing. Specifically, the signal recorded at each channel was first demeaned and then detrended by regressing out up to 5th-order polynomial functions that spanned the 30-min duration of each session. A low-pass filter was applied to the detrended signal with the cut-off frequency at 0.45 Hz. The signal was resampled at 1 Hz such that EGG and fMRI shared the same sampling frequency. Although data was recorded from an array of electrodes, analysis in this paper focused on one electrode located at the intersection of the second row and the third column of the array (Fig. 1A). Choosing this electrode as the channel of interest was because it was above the antrum of the stomach with peristaltic contractions (Lu et al., 2017). Among all electrodes, the location of this electrode was also most consistent across animals, since it was intentionally positioned to be on top of the xiphoid in every animal.
We also recovered from EGG recordings the signals of respiratory and cardiac origins. Specifically, the signals recorded every 30 min were demeaned and detrended by regressing out the fifth-order polynomial fit. Cardiac and respiratory signals were extracted by applying 3–10 Hz and 0.25–2 Hz bandpass filtering, respectively, and then downsampled to 50 Hz. Cardiac and respiratory rates were calculated by first detecting peaks in the corresponding signal and then evaluating the inter-peak intervals, based on a robust method from our previous publication (Liu et al., 2012a). The heart rate or respiratory rate was based on the average inter-peak interval. The heart rate variability was calculated as the standard deviation of the inter-peak intervals or the standard deviation of the normal-to-normal interval (SDNN) (Shaffer and Ginsberg, 2017).
2.9. fMRI preprocessing
The fMRI data was pre-processed using FSL (Jenkinson, et al., 2012), AFNI (Cox, 1996), and in-house software developed in MATLAB. Specifically, we first performed motion correction by registering the fMRI volumes from individual time points to the first volume using 3dvolreg in AFNI. The first 10 volumes were then discarded to avoid a non-steady state of MR signal. The timing of every slice within each volume was corrected using slicetimer in FSL. To align fMRI data to the rat brain template (Valdes Hernandez et al., 2011), fMRI images were aligned to T2w anatomical images from the same animal using flirt in FSL; the T2w images were transformed to the template using fnirt in FSL; and applying the same transformation to the fMRI images coregistered individual animals’ fMRI data to the same template. The coregistered fMRI signals were further denoised by regressing out the parameters for head-motion correction and a 3rd-order polynomial fit of the slow drift. Lastly, data were spatially smoothed with a 3-D Gaussian kernel with a 0.5-mm full-width at half maximum (FWHM).
2.10. EGG-fMRI coherence
Coherence between EGG and fMRI signals was evaluated at the dominant frequency of gastric slow waves. The slow-wave frequency was identified separately for each animal every 256 s to account for its variation across animals and over time. The slow-wave frequency was determined as the frequency with the greatest power spectral density between 0.06 and 0.13 Hz. This frequency range was reasonable since gastric slow waves were expected to be around 5 c.p.m. (or 0.083 Hz) for rats (Tümer et al., 2008). We excluded data from the coherence analysis when the slow-wave frequency was outside the range of 0.06–0.13 Hz.
Coherence between EGG and fMRI signals was evaluated at the slow-wave frequency. It was calculated as the power of the cross-spectral density normalized by the auto-spectral density as expressed in the following equation.
where x is the EGG signal, y is the voxel-wise fMRI signal, f is the slow-wave frequency, Gxy(f) is the cross-spectral density, Gxx(f) and Gyy(f) are auto-spectral density, specifically evaluated at the frequency f. The resulting coherence, Cxy(f), reflects the power transfer between x and y. In the context of this study, the EGG-fMRI coherence measured the extent to which the gastric slow wave can predict or be predicted by brain activity at a voxel or region via linear systems. This measure was less sensitive to the time delay of the fMRI signal due to the hemodynamic impulse response function.
2.11. Statistical analysis
The voxel-wise coherence was tested for significance by first converting it to a z score for each animal and then applying a one-sample t test to the voxel-wise z scores across animals. For each animal, the coherence measured at each voxel was compared against a null distribution, of which the samples were the coherence between the randomly phase-shuffled EGG signal and the original fMRI signal. Phase shuffling of a signal kept the magnitude but altered the phase of its spectrum. As shown in Fig. 2C, the phases of the recorded EGG signals were first shuffled in the frequency domain. Inverse Fourier transform was then used to create the phase-shuffled data. After the phase shuffling of EGG, their coherence with the fMRI signals was disrupted and reduced to a chance level subject to the same signal processing steps for evaluating either coherence or phase locking value (Fig. 2B). The phase-shuffling of EGG was performed 100 times to build the null distribution. The null distribution was separately built for each voxel, animal, and condition. The coherence between the original EGG and fMRI signals was compared to the null distribution and converted to a z score by subtracting the mean and dividing by the standard deviation of the null distribution. To test for the significance in the group level, a one-sample t test was applied to voxel-wise z scores obtained from each group of animals (one-sided p < 0.05 corrected with a cluster size greater than 10 voxels or 2.5 mm3).
Fig. 2. EGG-fMRI coherence analysis.

A shows an example EGG signal from the xiphoid and the concurrent fMRI signal from the right AID/AIV. The EGG and fMRI signals are coherent at a frequency around 5 c.p.m. B shows the corresponding signals after phase shuffling is applied to the recorded EGG. The fMRI and phase-shuffled EGG signals are less coherent with each other. C illustrates how phase shuffling is applied to a signal. The signal’s Fourier representation keeps the amplitude but shuffles the phase across frequency. D shows the average coherence between the recorded EGG and fMRI signals in the right AID/AIV (red) in contrast to the null coherence obtained with the phase-shuffled signals (black).
The coherence with EGG was summarized with respect to anatomical regions of interest (ROIs). We used 156 ROIs pre-defined according to the rat brain template and atlas. The ROIs included cortical regions (Valdes Hernandez et al., 2011), subcortical regions or nuclei (Papp et al., 2014), and hand-drawn masks for the nucleus tractus solitarius (NTS) and subnuclei in the thalamus (Paxinos and Watson, 2006). For each ROI, the voxel-wise coherence with EGG (in terms of the z score as described above) was first averaged within the ROI for each animal and then tested for significance across animals in the same group using a one-sample t test (with one-sided p < 0.05 and Bonferroni correction for multiple comparisons).
The contrast between different groups, e.g., fed vs. fasted or vagal innervation vs. de-innervation, was tested for significance using a non-parametric permutation test with p < 0.05. For a given voxel, we evaluated the difference in EGG-fMRI coherence between one condition (e.g., fed) and the other (e.g., fasted), whereas each condition under comparison included a set of samples (per session and animal). We randomly exchanged the samples between the two conditions and evaluated the difference between conditions. Repeating this permutation 1000 times resulted in a null distribution of the between-condition difference, against which the real difference was compared. The p value was calculated as the chance by which the samples in the null distribution were greater than the real effect observed.
3. Results
Prior studies suggested that the human brain had a gastric network, in which regional fMRI activity is phase-locked to the gastric slow wave - an intrinsic gastric rhythm at 0.05 Hz (Rebollo et al., 2018; Choe et al., 2021). Here, we asked whether rat brains had a similar gastric network, and if yes, whether and how this network relied on the vagal nerves that mediate stomach-brain interactions. To address these questions, we simultaneously acquired EGG and fMRI signals in 18 anesthetized rats and evaluated the EGG-fMRI coherence after they voluntarily consumed a 5 g test meal with either an intact vagus (“fed”, n = 8) or bilateral cervical vagotomy (“vagotomy”, n = 5), or when they remained fasted (“fasted”, n = 5). The stomach-brain coherence measured in this way was compared between different conditions (vagal innervation vs. de-innervation and fed vs. fasted) to address whether and how the gastric network relied on intact vagal nerves and varied across gastric states.
3.1. Rat brains also had a gastric network
The slow waves recorded from anesthetized rats after consuming a test meal were 5.36 ± 0.64 (average± standard deviation) c.p.m. (or 0.09 ± 0.01 Hz), in contrast to ~3 c.p.m. (or 0.05 Hz) in humans (Chen et al., 1999; Hamilton et al., 1986). We calculated the coherence between the EGG signal from a channel of interest above the antrum (Fig. 1A) and the fMRI signal from every voxel in the post-prandial (or fed) state. See Fig. 2A for example EGG and fMRI signals.
In animals with intact vagal nerves, the fMRI signals were coherent with EGG at the slow-wave frequency for 12% brain voxels (one-sided p < 0.025, one-sample t test), highlighting a network involving both subcortical and cortical regions (Fig. 3C). This brain network, herein referred to as the gastric network, covered the anterior cingulate cortex, insular cortex, medial prefrontal cortex, sensorimotor cortex, auditory and visual cortex, brainstem, and cerebellum (Fig. 3C). These regions were homologous to the human brain regions phase locked to gastric slow waves (Rebollo et al., 2018; Choe et al., 2021).
Fig. 3. fMRI activity coherent with gastric slow waves.

A & B illustrate concurrent fMRI and EGG in two groups of rats after consuming a test meal with either intact vagus nerves (A) or bilateral cervical vagotomy (B). C shows a map of voxels in which fMRI signals were significantly coherent with EGG (one-sided p < 0.025, one-way t-test, corrected for the number of clustered voxels). EGG-coherent cortical and subcortical regions are labeled according to existing rat brain atlases (Paxinos and Watson, 2006; Valdes Hernandez et al., 2011; Papp et al., 2014). D shows the EGG-coherent regions in animals with bilateral cervical vagotomy (one-sided p < 0.05, one-way t-test, corrected for the number of clustered voxels in individual clusters). In both C and D, the underlay shows the T2-weighted images in an existing rat brain MRI template (Valdes Hernandez et al., 2011).
The gastric network was asymmetric between the two hemispheres. As shown in Fig. 4A, the left hemisphere had more EGG-coherent regions than the right hemisphere. In the sensorimotor cortex, the EGG-fMRI coherence was spreaded across the cortical representations of multiple body parts, whereas the coherence was consistently weaker on the right hemisphere. In contrast, the right insular and auditory cortices were more coherent with EGG than their left counterparts (Fig. 4B). The EGG-fMRI coherence tended to be lateralized to the left hemisphere for subcortical regions or nuclei, especially for the cerebellum.
Fig. 4. Lateralization of the gastric network.
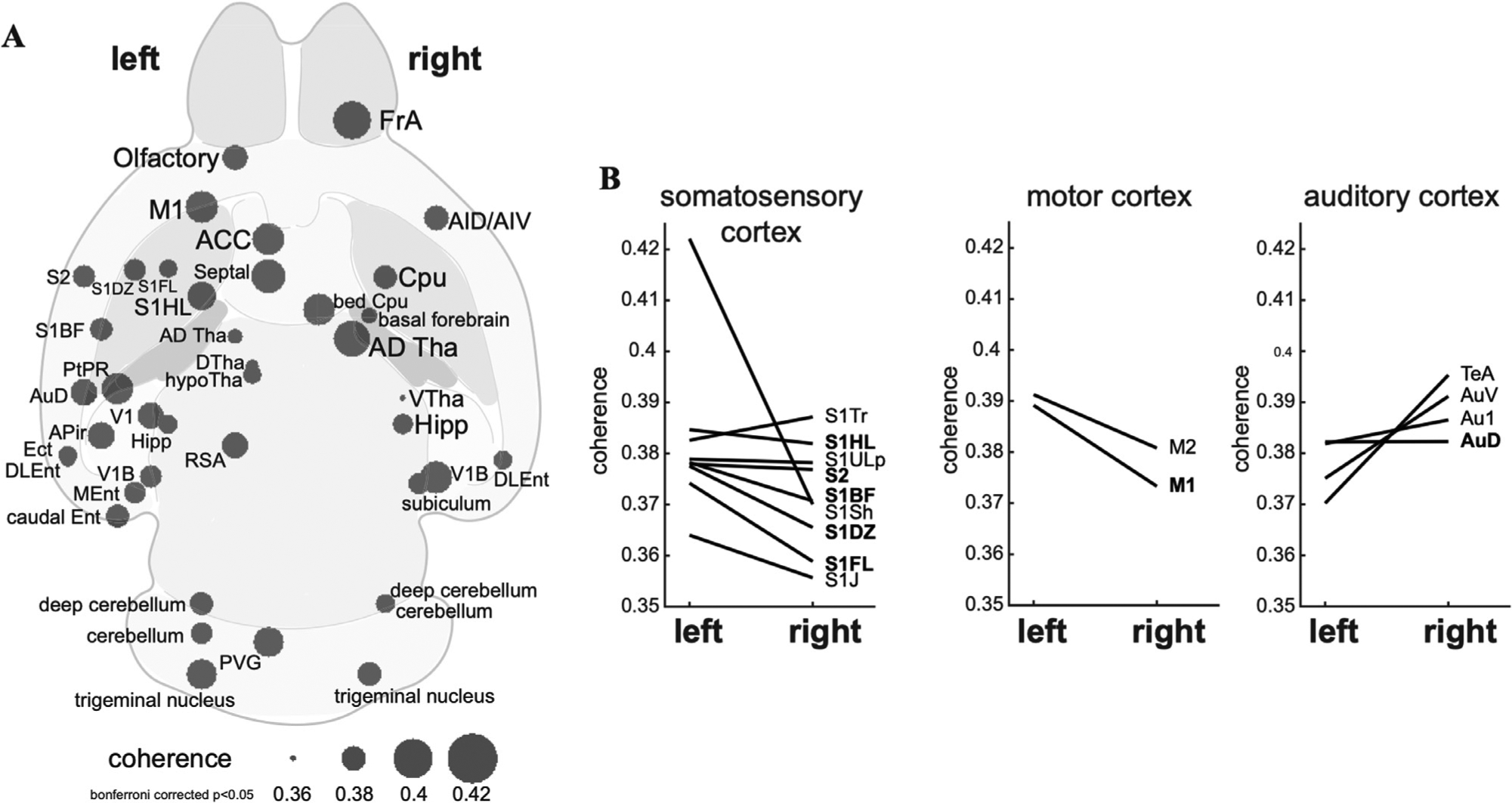
A plots the brain regions having significant phase-coupling between the stomach and the brain (one-sample t-test, bonferroni corrected p < 0.05). The statistical test was based on the averaged coherence in each region of interest. B shows the difference in phase-coupling with EGG between homologous ROIs in the left vs. right hemispheres. Results for the ROIs in the somatosensory cortex, motor cortex, and auditory cortex are shown in three separate subplots shown in the left, middle, and right, respectively.
These results suggest that rat brains also have a gastric network that manifests intrinsic coherence between EGG and fMRI signals, consistent with previous findings in human brains.
3.2. EGG-fMRI coherence relied on vagal nerves
We further attempted to identify the peripheral neural pathways underlying the apparent EGG-fMRI coherence. We chose to focus on the vagus nerve, which serves as the primary pathway for rapid and bi-directional neural interactions between the stomach and the brain (Travagli and Anselmi, 2016). Hence, we repeated the experiment in (n = 5) animals while applying bilateral cervical vagotomy before EGG-fMRI acquisition (Fig. 1B). The reduced heart rate viability and respiration rate confirmed the success of vagotomy (Fig. 1FH). With bilateral vagotomy, the slow-wave frequency was 4.95 ± 0.57 c.p.m., and the slow-wave amplitude was 10.34 ± 10.70, not differing much from 9.2 ± 8.4 with the intact vagus nerve. EGG-fMRI coherence significantly decreased but not completely diminished, while the coherence remained in the prelimbic and infralimbic cortex (Fig. 3D).
The contrast between the conditions without vs. with bilateral vagotomy was evaluated to characterize the effects of the vagus on EGG-fMRI coherence (Fig. 5). In the voxel level, bilateral vagotomy reduced the voxel-wise EGG-fMRI coherence in subcortical areas, such as the thalamus, amygdala, and inferior colliculus, as well as fewer cortical regions in the left dysgranular insular and sensorimotor cortex (Fig. 5A). Consistently on the ROI level, bilateral vagotomy significantly decreased the EGG-fMRI coherence (p < 0.05, permutation test, uncorrected) for most regions in the gastric network (Fig. 5B) whereas marginal but non-significant increases were found in fewer regions (Fig. 5C).
Fig. 5. Vagal contributions to postprandial stomach-brain phase-coupling.

A shows the voxel-level differences of coherence with a threshold of p < 0.05 (corrected for the number of clustered voxels) between the control group (intact vagal nerves) and vagotomy at the fed state. B plots the ROI-level differences in phase coupling (quantified as coherence) for regions where the coupling is stronger with the intact vagus than with vagotomy. The differences shown in this plot are statistically significant (permutation test, uncorrected p < 0.05). Similarly, C plots the differences for the regions where the coupling is weaker with the intact vagus than with vagotomy, although the differences are not statistically significant.
These results suggest that the vagus is the primary (not necessarily the only) peripheral nerve that mediates the coherence between gastric and brain activities observed with EGG and fMRI, respectively.
3.3. EGG-fMRI coherence depended on gastric states
For both rats and humans, the stomach shows continuous and peristaltic contractions in the post-prandial (fed) state and shows irregular and intermittent peristalsis (or migrating motor complex) in the fasted state (Deloose et al., 2012). We asked whether and how EGG-fMRI coherence depended on the fasted vs. fed state. To address this question, we repeated the same experiment in (n = 5) animals that remained fasted (after 18-hour fasting) during concurrent fMRI-EGG acquisition. In the fasted state, the slow-wave frequency was 5.02 ± 0.85 c.p.m.. It was comparable with but slightly lower than the slow-wave frequency of 5.36 ± 0.64 c.p.m. in the fed state (Fig. 1E).
We assessed the EGG-fMRI coherence for the fasted state in the same way as for the fed state. In the fasted state, EGG-fMRI coherence was observed at brain regions (Fig. 6B) that partially overlapped with those observed in the fed state (Fig. 3C). To better appreciate their relationships, Fig. 6C shows the intersection and distinction of EGG-coherent voxels observed in the fed vs. fasted states. In regions with an overlap between the two states, the coherence with EGG was generally higher in the fed state than in the fasted state (Fig. 6D). The ROI-level difference was significant in the primary somatosensory cortex, primary motor cortex, auditory cortex, olfactory nucleus, orbital cortex, septal nucleus, and thalamic nuclei (permutation test, p < 0.05, uncorrected).
Fig. 6. Stomach-brain phase coupling depends on the gastric state.

A illustrates the fed vs. fasted state. B shows the voxels in which fMRI signals are significantly coherent with EGG in the fasted state (one-sided p < 0.05, t-test, corrected for the number of voxels in individual clusters). C shows the overlapping voxels showing significant phase-coupling with EGG in both fasted and fed states with uncorrected p < 0.05. D plots the ROI-level differences in phase-coupling between the fed and fasted states, for the regions where such differences are statistically significant (permutation test, uncorrected p < 0.05).
These results suggest that EGG-fMRI coherence exists in both fasted and fed states but tends to cover complementary subdivisions of a similar set of cortical and subcortical regions.
4. Discussion
Here, we present evidence for the coherence between brain activity observed with fMRI and gastric activity observed with EGG in rats. Our findings add to prior observations in humans (Rebollo et al., 2018; Choe et al., 2021) and shed light on an intrinsic gastric network with its hallmark feature being the EGG-fMRI coherence at the gastric pace-making frequency. The substantial reduction in the EGG-fMRI coherence by the bilateral vagotomy supports a primary role of the vagus. The alteration in the gastric network from the fasted to fed state suggests its functional relevance to different gastric conditions.
4.1. Stomach-brain coherence
It is only until recently that the spontaneous interaction between brain activity and gastric activity was studied in humans by simultaneously acquiring EGG along with fMRI (Rebollo et al., 2018, 2022; Choe et al., 2021), MEG (Richter et al., 2017), or EEG (Todd et al., 2021). Gastric slow waves were found to be coupled with the amplitude of posterior alpha oscillations (Richter et al., 2017; Todd et al., 2021) or the phase of resting state fMRI activities in sensory and motor cortices (Rebollo et al., 2018; Choe et al., 2021). The findings obtained with EGG-fMRI are consistent with those with EGG and EEG/MEG, since the amplitude fluctuations of alpha oscillations are correlated with resting-state fMRI signals in sensory and motor cortices and thalamus (Liu et al., 2012a).
Our study confirms the spontaneous stomach-brain coupling reported in prior human studies. Note that gastric slow waves are of a higher frequency in anesthetized rats (~0.08 Hz) than awake humans (~0.05 Hz). Although resting state fMRI signals are sometimes filtered with a cutoff frequency at 0.08 Hz, a prior study shows that BOLD fMRI can follow visceral signals up to 0.8 Hz (Cao et al., 2019). Moreover, we used coherence to measure stomach-brain coupling, whereas prior studies used phase-locking value (Rebollo et al., 2018, 2022; Choe et al., 2021). Both coherence and phase-locking value measure how two signals are coupled at a specific frequency, i.e. the dominant frequency of the gastric slow wave. Coherence depends on both amplitude and phase relationships, whereas phase-locking value depends merely on the phase. We prefer coherence to phase-locking value, because both the amplitude and the phase reflect physiological information relevant to stomach-brain neural signaling. EGG arises from interstitial cells of Cajal (ICC) and smooth muscle cells (SMC). ICCs set the frequency and the phase of gastric slow waves and pace the activation of SMCs to drive muscle contractions as a peristaltic wave (Sanders, 1996). SMCs are under neural control by enteric motor neurons (Furness et al., 2020) that receive vagal efferent signals descending from the brain (Powley, 2021). As the electrical slow wave propagates in the stomach, the number of SMCs activated synchronously at the wavefront determines the strength of muscle contraction, as well as the amplitude of myoelectric activity observed with EGG. Vagal afferent nerves have intramuscular terminals in apposition with both SMC and ICC (Powley et al., 2019), providing a neural pathway to transmit, potentially, both the phasic change in ICC activity and the amplitude fluctuation in SMC activity.
We also used PLV to measure stomach-brain synchrony (see the results in the fed state in Supplementary Fig. 1). Significant PLV was more spatially confined, highlighting a subset of the regions with significant coherence. Compared to the results from prior human studies, the rat gastric network observed in this study covers similar regions, including the somatosensory cortex, motor cortex, visual cortex, auditory cortex, cerebellum, and insula cortex. This finding is also consistent with a previous study demonstrating that gastric electrical stimulation can induce both neural and fMRI responses in sensory and motor cortices (Cao et al., 2019). Together, results from this and prior studies suggest that EGG-fMRI coupling at the gastric slow-wave frequency is an intrinsic phenomenon possibly shared across different species.
Compared to the human gastric network, the EGG-fMRI coherence observed in rats covers more subcortical regions, including the nucleus tractus solitarius and nuclei in the basal ganglia, e.g., the globus pallidus, substantia nigra. These regions reside along the pathways between the autonomic nervous system and the limbic system (Albin et al., 1989; Browning and Travagli, 2014; Parent and Hazrati, 1995) and are likely involved in both autonomic control and emotion regulation (Andresen and Kunze, 1994; Pazo and Belforte, 2002; Pierce and Péron, 2020). In particular, the nucleus tractus solitarius in the brainstem receives vagal afferents that innervate the stomach and is well suited to relay gastric information to the brain (Browning and Travagli, 2014; Powley et al., 2019).
4.2. Supporting anatomical and functional evidence
The EGG-coherent regions spread across multiple resting state networks in rats (Liang et al., 2011, 2012; Liu et al., 2020). Although the gastric network is not straightforward to interpret as a whole, most of its constituent regions reported herein have been shown to be structurally connected or functionally associated with the stomach. Neural tracing studies have shown that the insular cortex, medial prefrontal cortex, and cerebellum receive gastric inputs via ascending vagal pathways (Browning and Travagli, 2014). The rostral insula is the major cortical source of parasympathetic control of the stomach, whereas sensorimotor cortical areas are the major source of sympathetic control of the stomach (Levinthal and Strick, 2020).
The involvement of visual and auditory cortex as well as hippocampus in the gastric network is supported by indirect functional evidence from the literature. During slow-wave sleep, the firing rate of neurons in the visual cortex was found to be dependent on the phase of duodenal myoelectrical activity (Pigarev et al., 2013) or be responsive to both direct and transcutaneous stimulation of the gut (Pigarev et al., 1994, 2006). Lateral geniculate nuclei receive direct projections from parabrachial nuclei and further relay gastric signals to the visual cortex (Erişir et al., 1997; Uhlrich et al., 1988). Similarly, gastric-branch vagus nerve stimulation or gastric electrical stimulation can modulate neural activity in the auditory cortex (Shetake et al., 2012; Engineer et al., 2015; Cao et al., 2019). Neurons in the hippocampus can be activated by gastric distension or gastric electrical stimulation (Xu et al., 2008, 2009; Wang et al., 2006a, 2006b), playing a role in sensing satiety and regulating appetite (Davidson et al., 2007, 2009; Kanoski and Grill, 2017).
Our results suggest that the gastric network involves the somatosensory cortex. Visceral signals converge to the somatotopic area of body representation (Krames and Foreman, 2007; Willis et al., 2004; Sikandar, S. and Dickenson, A.H., 2012). Gastric distention can activate both the primary and secondary somatosensory cortices (Geeraerts et al., 2011; Ly et al., 2017; Van Oudenhove et al., 2010; Vandenbergh et al., 2005, 2007) likely via an ascending spinothalamic pathway (Dum et al., 2009). The primary somatosensory cortex also innervates the stomach through a descending sympathetic spinal pathway (Levinthal and Strick, 2020). It is thus possible that the spinal and spinothalamic pathways may mediate the coherence between gastric slow waves and somatosensory cortical activity. Our results support this possibility. Cutting the vagal nerves diminished the EGG-fMRI coherence at most, but not all, brain regions. While our study focuses on the vagus nerve, future studies are desirable to fully characterize the role of the thoracic splanchnic nerve.
4.3. Role of the vagus in the stomach-brain synchrony
Our results suggest that the vagus mediates the apparent stomach-brain synchrony. The neural mechanisms underlying the stomach-brain interaction involve both peripheral and central neural circuits. The peripheral component involves the vagus nerve and the thoracic splanchnic nerve. In general, the former is central to regulating gastric motor events (Travagli et al., 2006; Travagli and Anselmi, 2016), and the latter is more involved in visceral pain (Ness and Gebhart, 1990). Bilateral cervical vagotomy abolishes both vagal afferent and efferent pathways that support vagovagal reflexes. The fact that the vagotomy largely diminished the EGG-fMRI coherence suggests that neural signaling along the vagus nerve is central to maintaining the coupling between the brain and the stomach. The effects of vagotomy likely extend beyond the dorsal vagal complex, where vagal afferents end and efferents start, through connections between the dorsal vagal complex and other brain regions (Tsurugizawa et al., 2009). Also supporting the role of the vagus is the prior finding that vagus nerve stimulation could activate a similar set of brain regions as the regions highlighted in this study (Cao et al., 2017).
It is likely that the stomach ascends its intrinsic rhythm to the brain and thus results in the stomach-brain coherence at the gastric frequency. Gastric slow waves observed with EGG reflect an intrinsic rhythm that can be generated solely by the stomach itself. This intrinsic rhythm is thought to originate from and propagate through ICC (Cajal 1893; Sanders et al., 1996), but likely involves other mechanisms as well (Yin and Chen, 2008; Sarna, 1985). ICCs within the circular and longitudinal muscle layers of the stomach (ICC-IM) are innervated by vagal afferent nerves that branch into intramuscular arrays (Powley and Robert, 2011; Powley et al., 2019), which serve as the receptors to transmit sensory information to the brainstem (Cao et al., 2021). The vagovagal circuitry in the brainstem may pass the gastric rhythm as the bottom-up input to other regions in the gastric network. Taken together, there is a plausible bottom-up pathway to allow intrinsic gastric rhythms to be passed to the brain and account for the apparent brain-stomach synchrony.
It is possible but less likely that brain activity controls the phase of gastric rhythm in order for the brain to be the cause of stomach-brain synchrony. Given the vagotomy, the rat stomach continued to generate a rhythmic gastric slow wave, despite a slightly lower frequency. The stomach maintains peristaltic contractions even after the bilateral vagotomy.Although the stomach receives inputs from sensorimotor cortical regions (Levinthal and Strick, 2020), there is no direct evidence suggesting that top-down neural inputs control the phase of the gastric slow wave. The vagal efferent nerves innervate gastric enteric neurons (Powley et al., 2019), which further innervate gastric SMCs (Furness et al., 2020). It is not clear whether and how the brain controls the ICCs and gastric pace-making activity initiated by the ICCs. However, this does not imply that the brain does not influence EGG. As a gross measure of gastric electrical activity, EGG can result from any synchronized current sources from the stomach, including SMCs and ICCs. The brain may modulate the excitability of SMCs and their contributions to the amplitude of EGG, without directly influencing the frequency or phase presumably determined by ICCs. In line with this speculation, a previous study showed that the amplitude of EGG at its dominant frequency varies across different waking-to-sleep stages (Orr et al., 1997). We speculate that the brain senses gastric pace-making activity relayed through vagal afferent nerves, whereas the source of the synchronized rhythms is intrinsic to the gut, as opposed to the brain. This does not imply that the vagus only transmits gastric rhythms. The vagus may also transmit information about gastric volume, intragastric pressure, nutrients (Ladabaum et al., 2001; Ly et al., 2017; Spetter et al., 2014; Wang et al., 2008), resulting in changes to regional activity or network interactions in the brain (Tsurugizawa et al., 2019).
4.4. Gastric mechanical versus electrical activity
It should be noted that body-surface EGG may reflect both gastric pace-making activity and gastric contractions. The gastric pace-making activity is the electrical activity generated by ICCs; it remains largely stationary and continuous across all gastric states. In contrast, gastric contractions vary over time, being strong and continuous after a meal and becoming silent or intermittent in the fasted state (Sarna, 1985; Deloose et al., 2012; Abell and Malagelada, 1988; Brandstaeter et al., 2019; Kim and Malagelada, 1986). Our results obtained with the fasted state suggest that intrinsic pace-making activity explains, at least partially, the stomach-brain synchrony.
However, gastric contractions may also influence the stomach-brain synchrony, since the pattern of EGG-fMRI coherence was different between the fasted and fed states. It is not straightforward to fully disentangle how gastric pace-making activity and contractions cause the stomach-brain synchrony within the scope of this study, because they are often coupled and their coupling is influenced by the extrinsic neural control. Addressing this question would ideally require simultaneous electromechanical recordings by placing both mechanical and electrical sensors on the stomach surface during concurrent fMRI. Another way is to attempt to separate electrical slow waves attributed to ICCs and myoelectrical activity attributed to SMCs (Kim and Malagelada, 1986; Sanders and Publicover, 1994; Stern et al., 2007).
4.5. Functional significance of the stomach-brain coherence
The functional significance of the stomach-brain coherence is unclear and awaits to be established. Speculatively, this phenomenon is an indication of how the brain monitors the gut in real-time and regulates food intake and digestion (Holtmann and Talley, 2014). It might also indicate how the gut exerts interoceptive influences on brain and behaviors (Critchley and Harrison, 2013; Azzalini et al., 2019). To establish its functional significance, it is necessary to relate the stomach-brain coherence to behaviors, such as eating behavior, perceptual performance, and cognitive performance. It would also be valuable to evaluate the stomach-brain coherence beyond normal conditions or healthy population and explore its relationship to various symptoms in diseased conditions.
4.6. Limitations
The nerves passing through the cervical vagus branch out to innervate lungs, heart, esophagus, stomach, etc. The right vagus nerve innervates the lung and modulates the respiratory rate and depth. The left vagus innervates the heart and modulates the cardiac rate and its variability. In this study, bilateral vagotomy was applied to the cervical level, affecting cardiac and respiratory patterns. It reduced the respiratory rate and heart rate variability, but not the heart rate itself. Such respiratory and cardiac effects may confound BOLD activity through non-neuronal vascular mechanisms (Birn et al., 2006; Chang et al., 2013), potentially causing non-specific effects on the whole brain as opposed to the gastric network. However, the global signal obtained by averaging the standardized voxel time series across the whole brain did not show any significant difference across three conditions of interest (fed, vagotomy, and fasted). Thus it is unlikely that non-specific systemic effects could explain the reduced EGG-fMRI coherence given vagotomy or the difference between the fed and fasted conditions. However, we cannot fully eliminate this potential confound. In future studies, it is highly desirable to apply subdiaphragmatic vagotomy to only the gastric branch of the vagus.
Another limitation pertains to comparing the vagal intact group and the vagotomy group. In this study, animals in the vagotomy group received acute thoracic surgery, while animals in the vagal-intact group did not receive any surgery. Although the vagotomy surgery was brief and did not directly perturb the stomach, the difference attributable to the surgical procedure could potentially be a confounding factor. It would be ideal to use another sham control group in which animals receive sham thoracic surgery without vagotomy.
5. Conclusion
In healthy and anesthetized rats, spontaneous brain activity in the “gastric network” is coherent with the intrinsic slow wave from the stomach, consistent with recent findings from humans (Rebollo et al., 2018; Choe et al., 2021). This network involves a number of subcortical and cortical regions that span across the sensory, motor, and limbic systems. Importantly, the vagus nerve is the primary pathway that mediates the stomach-brain coherence.
Supplementary Material
Acknowledgement
The authors thank Drs. John Furness, Terry Powley, Leo Cheng, Ulrich Scheven for their valuable discussions. This study is supported by the National Institutes of Health (OD023847, OD030538, AT011665), the University of Michigan, and Purdue University.
Nomenclature
Abbreviations
- AcbC
accumbens nucleus, core
- ACC
anterior cingulate cortex
- ADTha
anterior dorsal thatlmus
- AID
agranular insular cortex, dorsal region
- AIP
agranular insular cortex, posterior part
- AIV
agranular insular cortex, ventral region
- Amy
amygdala
- Au1
primary auditory cortex
- AuD
secondary auditory cortex, dorsal area
- AuV
secondary auditory cortex, ventral area
- CA3
field CA3 of hippocampus
- caudal Ent
caudal entorhinal field
- Cpu
caudate putamen (striatum)
- DI
dysgranular insular cortex
- DIEnt
dorsal-intermediate entorhinal field
- DLEnt
dorsal-lateral entorhinal field
- DLO
dorsolateral orbital cortex
- DSC
deep superior colliculus
- DTha
dorsal thalamus
- Ect
ectorhinal cortex
- Ent
entorhinal cortex
- FrA
frontal association cortex
- FrL
prelimbic cortex
- GI
granular insular cortex
- GP
globus pallidus
- Hipp
hippocampus
- hypoTha
hypothalamus
- IC
inferior colliculus
- IPN
interpeduncular nucleus
- LGP
lateral globus pallidus
- LGTha
dorsal lateral geniculate nucleus
- LPTha
lateral posterior thalamus
- LSV
lateral septal nucleus, ventral part
- M1
primary motor cortex
- septal
septal nucleus
- M2
secondary motor cortex
- MGP
medial globus pallidus
- NTS
nucleus tractus solitarius
- Olfactory
olfactory bulb
- PnO
pontine reticular nucleus, oral part
- PRh
perirhinal cortex
- PrL
prelimbic cortex
- PtA
parietal association cortex
- PTha
posterior thalamus
- PtPD
dorsal posterior parietal cortex
- PtPR
rostral posterior parietal cortex
- RSC
retrosplenial cortex
- RSGa
retrosplenial granular a cortex
- S1
primary somatosensory cortex
- S1BF
primary somatosensory cortex, barrel field
- S1DZ
primary somatosensory cortex, dysgranular region
- S1FL
primary somatosensory cortex, forelimb region
- S1HL
primary somatosensory cortex, hindlimb region
- S1J
primary somatosensory cortex, jaw region
- S1Sh
primary somatosensory cortex, shoulder region
- S1Tr
primary somatosensory cortex, trunk region
- S1ULp
primary somatosensory cortex, upper lip region
- S2
secondary somatosensory cortex
- SC
superior colliculus
- septal
septal nucleus
- SN
substantia nigra
- TeA
temporal association cortex
- Tha
thalamus
- TS
triangular septal nucleus
- V1B
primary visual cortex, binocular area
- V1M
primary visual cortex, monocular area
- V2
secondary visual cortex
- V2L
secondary visual cortex
- VATha
ventral anterior thalamic nucleus
- VTha
ventral thalamus
Footnotes
Credit authorship contribution statement
Jiayue Cao: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft. Xiaokai Wang: Data curation, Formal analysis, Writing – original draft. Jiande Chen: Funding acquisition. Nanyin Zhang: Funding acquisition. Zhongming Liu: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Funding acquisition, Writing – original draft.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.neuroimage.2022.119628.
Data and code availability statement
The data and code used in this paper will be shared to interested readers upon request.
References
- Abell TL, Malagelada JR, 1988. Electrogastrography - Current assessment and future perspectives. Digestive Diseases and Sciences 33 (8), 982–992. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB, 1989. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12 (10), 366–375. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Kunze DL, 1994. Nucleus tractus solitaries—gateway to neural circulatory control. Annu. Rev. Physiol 56 (1), 93–116. [DOI] [PubMed] [Google Scholar]
- Azpiroz F, Malagelada JR, 1987. Importance of vagal input in maintaining gastric tone in the dog. J. Physiol 384 (1), 511–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzalini D, Rebollo I, Tallon-Baudry C, 2019. Visceral signals shape brain dynamics and cognition. Trends Cogn. Neurosci 23 (6), 488–509. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Powley TL, 1992. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J. Comp. Neurol 319 (2), 261–276. [DOI] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA, 2006. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage 31 (4), 1536–1548. [DOI] [PubMed] [Google Scholar]
- Brandstaeter S, Fuchs SL, Aydin RC, Cyron CJ, 2019. Mechanics of the stomach: a review of an emerging field of biomechanics. GAMM-Mitteilungen 42 (3), e201900001. [Google Scholar]
- Brookes SJ, Spencer NJ, Costa M, Zagorodnyuk VP, 2013. Extrinsic primary afferent signalling in the gut. Nat. Rev. Gastroenterol. Hepatol 10 (5), 286–296. [DOI] [PubMed] [Google Scholar]
- Browning KN, Travagli RA, 2014. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr. Physiol 4 (4), 1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal, 1893. Sur les ganglions et plexus nerveux d’intestin. C R. Soc. Biol 5, 217–223 1893. [Google Scholar]
- Cao J, Lu K−H, Powley TL, Liu Z, 2017. Vagal nerve stimulation triggers widespread responses and alters large-scale functional connectivity in the rat brain. PLoS One 12 (12), e0189518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Lu KH, Oleson ST, Phillips RJ, Jaffey D, Hendren CL, Powley TL, Liu Z, 2019. Gastric stimulation drives fast BOLD responses of neural origin. Neuroimage 197, 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Wang X, Powley TL, Liu Z, 2021. Gastric neurons in the nucleus tractus solitarius are selective to the orientation of gastric electrical stimulation. J. Neural Eng 18 (5), 056066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Cunningham JP, Glover GH, 2013. Influence of heart rate on the BOLD signal: the cardiac response function. Neuroimage 44 (3), 857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JDZ, Zou X, Lin X, Ouyang S, Liang J, 1999. Detection of gastric slow wave propagation from the cutaneous electrogastrogram. Am. J. Physiol.-Gastrointest. Liver Physiol 277 (2), G424–G430. [DOI] [PubMed] [Google Scholar]
- Choe AS, Tang B, Smith KR, Honari H, Lindquist MA, Caffo BS, Pekar JJ, 2021. Phase-locking of resting-state brain networks with the gastric basal electrical rhythm. PLoS One 16 (1), e0244756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmensen C, Müller TD, Woods SC, Berthoud HR, Seeley RJ, Tschöp MH, 2017. Gut-brain cross-talk in metabolic control. Cell 168 (5), 758–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Harrison NA, 2013. Visceral influences on brain and behavior. Neuron 77 (4), 624–638. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC, 2009. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus 19 (3), 235–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Schier LA, Clegg DJ, Benoit SC, 2007. A potential role for the hippocampus in energy intake and body weight regulation. Curr. Opin. Pharmacol 7 (6), 613–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloose E, Janssen P, Depoortere I, Tack J, 2012. The migrating motor complex: control mechanisms and its role in health and disease. Nat. Rev. Gastroenterol. Hepatol 9 (5), 271–285. [DOI] [PubMed] [Google Scholar]
- Dum RP, Levinthal DJ, Strick PL, 2009. The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J. Neurosci 29 (45), 14223–14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CT, Engineer ND, Riley JR, Seale JD, Kilgard MP, 2015. Pairing speech sounds with vagus nerve stimulation drives stimulus-specific cortical plasticity. Brain Stimul. 8 (3), 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erişir A, Van Horn SC, Sherman SM, 1997. Relative numbers of cortical and brainstem inputs to the lateral geniculate nucleus. Proc. Natl. Acad. Sci 94 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB, Di Natale M, Hunne B, Oparija-Rogenmozere L, Ward SM, Sasse KC, Powley TL, Stebbing MJ, Jaffey D, Fothergill LJ, 2020. The identification of neuronal control pathways supplying effector tissues in the stomach. Cell Tissue Res. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeraerts B, Van Oudenhove L, Dupont P, Vanderghinste D, Bormans G, Van Laere K, Tack J, 2011. Different regional brain activity during physiological gastric distension compared to balloon distension: a H215O-PET study. Neurogastroenterol. Motility 23 (6), 533–e203. [DOI] [PubMed] [Google Scholar]
- Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, Ferreira TL, Quinn D, Liu ZW, Gao XB, Kaelberer MM, 2018. A neural circuit for gut-induced reward. Cell 175 (3), 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper AA, Kidd C, Scratcherd T, 1959. Vago-vagal reflex effects on gastric and pancreatic secretion and gastro-intestinal motility. J. Physiol 148 (2), 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmann G, Talley NJ, 2014. The stomach–brain axis. Best Practice Res. Clin. Gastroenterol 28 (6), 967–979. [DOI] [PubMed] [Google Scholar]
- Hurley-Gius KM, Neafsey EJ, 1986a. The medial frontal cortex and gastric motility: microstimulation results and their possible significance for the overall pattern of organization of rat frontal and parietal cortex. Brain Res 365 (2), 241–248. [DOI] [PubMed] [Google Scholar]
- Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, Bohórquez DV, 2018. A gut-brain neural circuit for nutrient sensory transduction. Science (6408) 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JW, Bellahsene BE, Reichelderfer M, Webster JG, Bass P, 1986. Human electrogastrograms. Dig. Dis. Sci 31 (1), 33–39. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Grill HJ, 2017. Hippocampus contributions to food intake control: mnemonic, neuroanatomical, and endocrine mechanisms. Biol. Psychiatry 81 (9), 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarer M, Arnold M, Günther L, Winter C, Langhans W, Meyer U, 2014. Gut vagal afferents differentially modulate innate anxiety and learned fear. J. Neurosci 34 (21), 7067–7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarer M, Krieger JP, Richetto J, Weber-Stadlbauer U, Günther L, Winter C, Arnold M, Langhans W, Meyer U, 2018. Abdominal vagal afferents modulate the brain transcriptome and behaviors relevant to schizophrenia. J. Neurosci 38 (7), 1634–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Malagelada JR, 1986. Electrical activity of the stomach: clinical implications. In: Mayo Clinic Proceedings, 61. Elsevier, pp. 205–210. [DOI] [PubMed] [Google Scholar]
- Krames ES, Foreman R, 2007. Spinal cord stimulation modulates visceral nociception and hyperalgesia via the spinothalamic tracts and the postsynaptic dorsal column pathways: a literature review and hypothesis. Neuromodulation: Technol.Neural Interface 10 (3), 224–237. [DOI] [PubMed] [Google Scholar]
- Ladabaum URI, Minoshima S, Hasler WL, Cross D, Chey WD, Owyang C, 2001. Gastric distention correlates with activation of multiple cortical and subcortical regions. Gastroenterology 120 (2), 369–376. [DOI] [PubMed] [Google Scholar]
- Levinthal DJ, Strick PL, 2020. Multiple areas of the cerebral cortex influence the stomach. Proc. Natl. Acad. Sci 117 (23), 13078–13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, King J, Zhang N, 2011. Uncovering intrinsic connectional architecture of functional networks in awake rat brain. J. Neurosci 31 (10), 3776–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, King J, Zhang N, 2012. Intrinsic organization of the anesthetized brain. J. Neurosci 32 (30), 10183–10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Perez PD, Ma Z, Ma Z, Dopfel D, Cramer S, Tu W, Zhang N, 2020. An open database of resting-state fMRI in awake rats. Neuroimage 220, 117094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, de Zwart JA, Yao B, van Gelderen P, Kuo LW, Duyn JH, 2012a. Finding thalamic BOLD correlates to posterior alpha EEG. Neuroimage 63 (3), 1060–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K−H, Cao J, Oleson ST, Ward MP, Phillips RL, Powley TL, Liu Z, 2018. Vagus nerve stimulation promotes gastric emptying by increasing pyloric opening measured with magnetic resonance imaging. Neurogastroenterol. Motil 30 (10), e13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K−H, Cao J, Phillips RJ, Powley TL, Liu Z, 2020. Acute effects of vagus nerve stimulation parameters on gastric motility assessed with magnetic resonance imaging. Neurogastroenterol. Motil 32 (7), e13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly HG, Dupont P, Van Laere K, Depoortere I, Tack J, Van Oudenhove L, 2017. Differential brain responses to gradual intragastric nutrient infusion and gastric balloon distension: a role for gut peptides? Neuroimage 144, 101–112. [DOI] [PubMed] [Google Scholar]
- Lyubashina OA, 2004. Possible mechanisms of involvement of the amygdaloid complex in the control of gastric motor function. Neurosci. Behav. Physiol 34 (4), 379–388. [DOI] [PubMed] [Google Scholar]
- Mayer EA, 2011. Gut feelings: the emerging biology of gut–brain communication. Nat. Rev. Neurosci 12 (8), 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Labus J, Aziz Q, Tracey I, Kilpatrick L, Elsenbruch S, Borsook D, 2019. Role of brain imaging in disorders of brain–gut interaction: a Rome Working Team Report. Gut 68 (9), 1701–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF, 1990. Visceral pain: a review of experimental studies. pain 41 (2), 167–234. doi: 10.1016/0304-3959(90)90021-5. [DOI] [PubMed] [Google Scholar]
- Orr WC, Crowell MD, Lin B, Harnish MJ, Chen JDZ, 1997. Sleep and gastric function in irritable bowel syndrome: derailing the gut-brain axis. Gut. 41, 390–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Hazrati LN, 1995. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidium in basal ganglia circuitry. Brain Res. Rev 20 (1), 128–154. [DOI] [PubMed] [Google Scholar]
- Pazo JH, Belforte JE, 2002. Basal ganglia and functions of the autonomic nervous system. Cell. Mol. Neurobiol 22 (5), 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JE, Péron J, 2020. The basal ganglia and the cerebellum in human emotion. Soc. Cogn. Affect. Neurosci 15 (5), 599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp EA, Leergaard TB, Calabrese E, Johnson GA, Bjaalie JG, 2014. Waxholm space atlas of the Sprague Dawley rat brain. Neuroimage 97, 374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2006. The Rat Brain in Stereotaxic coordinates: Hard Cover Edition. Elsevier. [Google Scholar]
- Pigarev I, Almirall H, Pigareva ML, Bautista V, Sánchez-Bahillo A, Barcia C, Her-rero MT, 2006. Visceral signals reach visual cortex during slow wave sleep. Study in monkeys. Acta Neurobiol. Exp. (Wars) 66 (1), 69. [DOI] [PubMed] [Google Scholar]
- Pigarev IN, 1994. Neurons of visual cortex respond to visceral stimulation during slow wave sleep. Neuroscience 62 (4), 1237–1243. [DOI] [PubMed] [Google Scholar]
- Pigarev IN, Bagaev VA, Levichkina EV, Fedorov GO, Busigina II, 2013. Cortical visual areas process intestinal information during slow wave sleep. Neurogastroentero. Motility 25 (3), 268–e169. [DOI] [PubMed] [Google Scholar]
- Powley TL, Jaffey DM, McAdams J, Baronowsky EA, Black D, Chesney L, Evans C, Phillips RJ, 2019. Vagal innervation of the stomach reassessed: brain−gut connectome uses smart terminals. Ann. N. Y. Acad. Sci 1454 (1), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powley TL, 2021. Brain-gut communication: vagovagal reflexes interconnect the two “brains. Am. J. Physiol. Gastrointest. Liver Physiol doi: 10.1152/ajpgi.00214.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powley Terry, Robert Robert, 2011. Vagal intramuscular array afferents form complexes with interstitial cells of Cajal in gastrointestinal smooth muscle: analogues of muscle spindle organs? Neuroscience 186, 188–200. doi: 10.1016/j.neuroscience.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollo I, Devauchelle AD, Béranger B, Tallon-Baudry C, 2018. Stomach-brain synchrony reveals a novel, delayed-connectivity resting-state network in humans. elife 7, e33321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollo I, Wolpert N, Tallon-Baudry C, 2021. Brain-stomach coupling: anatomy, functions, and future avenues of research. Curr. Opin. Biomed. Eng, 100270. [Google Scholar]
- Rebollo I, Tallon-Baudry C, 2022. The sensory and motor components of the cortical hierarchy are coupled to the rhythm of the stomach during rest. J. Neurosci 42 (11), 2205–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CG, Babo-Rebelo M, Schwartz D, Tallon-Baudry C, 2017. Phase-amplitude coupling at the organism level: the amplitude of spontaneous alpha rhythm fluctuations varies with the phase of the infra-slow gastric basal rhythm. Neuroimage 146, 951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Publicover NG, 1994. Excitation-contraction coupling in gastric muscles. Dig. Dis. Sci 39 (12), 69S–72S. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Schwartz G, 2004. Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. J. Neurosci 24 (11), 2782–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RC, McTIGUE DM, Hermann GE, 1995. Vagovagal reflex control of digestion: afferent modulation by neural and” endoneurocrine” factors. Am. J. Physiol. Gastrointest. Liver Physiol 268 (1), G1–G10. [DOI] [PubMed] [Google Scholar]
- Rogers RC, McTigue DM, Hermann GE, 1996. Vagal control of digestion: modulation by central neural and peripheral endocrine factors. Neurosci. Biobehav. Rev 20 (1), 57–66. [DOI] [PubMed] [Google Scholar]
- Sanders KM, 1996. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology 111, 492–515. [DOI] [PubMed] [Google Scholar]
- Sarna SK, 1985. Cyclic motor activity; migrating motor complex: 1985. Gastroenterology 89 (4), 894–913. [DOI] [PubMed] [Google Scholar]
- Shaffer F, Ginsberg JP, 2017. An overview of heart rate variability metrics and norms. Front. Public Health 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RE, Miselis RR, 1985. The central organization of the vagus nerve innervating the stomach of the rat. J. Comp. Neurol 238 (4), 473–488. [DOI] [PubMed] [Google Scholar]
- Shetake JA, Engineer ND, Vrana WA, Wolf JT, Kilgard MP, 2012. Pairing tone trains with vagus nerve stimulation induces temporal plasticity in auditory cortex. Exp. Neurol 233 (1), 342–349. [DOI] [PubMed] [Google Scholar]
- Sikandar S, Dickenson AH, 2012. Visceral pain–the ins and outs, the ups and downs. Curr. Opin. Support. Palliat. Care 6 (1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetter MS, de Graaf C, Mars M, Viergever MA, Smeets PA, 2014. The sum of its parts—effects of gastric distention, nutrient content and sensory stimulation on brain activation. PLoS ONE 9 (3), e90872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan E, Pardo JV, Faris PL, Hartman BK, Kim SW, Ivanov EH, Goodale RL, 2003. Functional neuroimaging of gastric distention. J. Gastrointest. Surg 7 (6), 740–749. [DOI] [PubMed] [Google Scholar]
- Stern R, Koch K, Levine M, Muth E, 2007. Gastrointestinal Response. In: Cacioppo J, Tassinary L, Berntson G (Eds.), Handbook of Psychophysiology. Cambridge University Press, Cambridge, pp. 211–230. [Google Scholar]
- Takahashi T, Owyang C, 1997. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J. Physiol 504 (2), 479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J, Cardellicchio P, Swami V, Cardini F, Aspell JE, 2021. Weaker implicit interoception is associated with more negative body image: evidence from gastric-alpha phase amplitude coupling and the heartbeat evoked potential. Cortex. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Anselmi L, 2016. Vagal neurocircuitry and its influence on gastric motility. Nat. Rev. Gastroenterol. Hepatol 13 (7), 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, Rogers RC, 2006. Brainstem circuits regulating gastric function. Annu. Rev. Physiol 68, 279–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tümer C, Oflazoğlu HD, Obay BD, Kelle M, Taşdemir E, 2008. Effect of ghrelin on gastric myoelectric activity and gastric emptying in rats. Regul. Pept 146 (1–3), 26–32. [DOI] [PubMed] [Google Scholar]
- Tsurugizawa T, Uematsu A, Nakamura E, Hasumura M, Hirota M, Kondoh T, Un-eyama H, Torii K, 2009. Mechanisms of neural response to gastrointestinal nutritive stimuli: the gut-brain axis. Gastroenterology 137 (1), 262–273. [DOI] [PubMed] [Google Scholar]
- Tsurugizawa T, Djemai B, Zalesky A, 2019. The impact of fasting on resting state brain networks in mice. Sci. Rep 9 (1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlrich DJ, Cucchiaro JB, Sherman SM, 1988. The projection of individual axons from the parabrachial region of the brain stem to the dorsal lateral geniculate nucleus in the cat. J. Neurosci 8 (12), 4565–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes Hernandez PA, Sumiyoshi A, Nonaka H, Haga R, Aubert Vasquez E, Ogawa T, Iturria-Medina Y, Riera JJ, Kawashima R, 2011. An in vivo MRI template set for morphometry, tissue segmentation, and fMRI localization in rats. Front. Neuroinform 5, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oudenhove L, Vandenberghe J, Dupont P, Geeraerts B, Vos R, Dirix S, Van Laere K, Bormans G, Vanderghinste D, Demyttenaere K, Fischler B, 2010. Regional brain activity in functional dyspepsia: a H215O-PET study on the role of gastric sensitivity and abuse history. Gastroenterology 139 (1), 36–47. [DOI] [PubMed] [Google Scholar]
- Vandenbergh J, DuPont P, Fischler B, Bormans G, Persoons P, Janssens J, Tack J, 2005. Regional brain activation during proximal stomach distention in humans: a positron emission tomography study. Gastroenterology 128 (3), 564–573. [DOI] [PubMed] [Google Scholar]
- Vandenberghe J, Dupont P, Van Oudenhove L, Bormans G, Demyttenaere K, Fischler B, Geeraerts B, Janssens J, Tack J, 2007. Regional cerebral blood flow during gastric balloon distention in functional dyspepsia. Gastroenterology 132 (5), 1684–1693. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Tomasi D, Backus W, Wang R, Telang F, Geliebter A, Korner J, Bauman A, Fowler JS, Thanos PK, Volkow ND, 2008. Gastric distention activates satiety circuitry in the human brain. Neuroimage 39 (4), 1824–1831. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Yang J, Volkow ND, Telang F, Ma Y, Zhu W, Wong CT, Tomasi D, Thanos PK, Fowler JS, 2006a. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proc. Natl. Acad. Sci 103 (42), 15641–15645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Yang J, Volkow ND, Telang F, Ma Y, Zhu W, Wong CT, Tomasi D, Thanos PK, Fowler JS, 2006b. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proc. Natl. Acad. Sci 103 (42), 15641–15645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD, Westlund KN, Carlton SM, 2004. The Rat Nervous System. Elsevier/Academic Press, San Diego, pp. 853–889. [Google Scholar]
- Xu L, Sun X, Lu J, Tang M, Chen JDZ, 2008. Effects of gastric electric stimulation on gastric distention responsive neurons and expressions of CCK in rodent hippocampus. obes. 16 (5), 951–957. [DOI] [PubMed] [Google Scholar]
- Xu L, Sun X, Tang M, Chen JDZ, 2009. Involvement of the hippocampus and neuronal nitric oxide synapse in the gastric electrical stimulation therapy for obesity. Obes. Surg 19 (4), 475–483. [DOI] [PubMed] [Google Scholar]
- Yin J, Chen JDZ, 2008. Roles of interstitial cells of Cajal in regulating gastrointestinal motility: in vitro versus in vivo studies. J. Cell. Mol. Med 12 (4), 1118–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and code used in this paper will be shared to interested readers upon request.


