Abstract
Background
Obstructive sleep apnea (OSA) is associated with impaired sleep quality and autonomic dysfunction. Adenotonsillectomy significantly improves subjective and objective sleep quality in children with OSA. However, the postoperative changes in heart rate variability (HRV) indices (indicators of cardiac autonomic function) and their importance remain inconclusive in childhood OSA. This retrospective case series aimed to investigate the association of sleep HRV indices, total OSA-18 questionnaire score (a subjective indicator of sleep quality) and polysomnographic parameters (objective indicators of sleep quality), and effects of adenotonsillectomy on HRV indices, total OSA-18 questionnaire score and polysomnographic parameters in children with OSA.
Methods
Seventy-six children with OSA were included in baseline analysis, of whom 64 (84%) completed at least 3 months follow-up examinations after adenotonsillectomy and were included in outcome analysis. Associations between baseline variables, and relationships with treatment-related changes were examined.
Results
Multivariable linear regression models in the baseline analysis revealed independent relationships between tonsil size and obstructive apnea-hypopnea index (OAHI), adenoidal-nasopharyngeal ratio and very low frequency (VLF) power of HRV (an indicator of sympathetic activity), and normalized low frequency power (an indicator of sympathetic activity) and OAHI. The outcome analysis showed that adenotonsillectomy significantly improved standard deviation of all normal-to-normal intervals, and high frequency power, QoL (in terms of reduced total OSA-18 questionnaire score), OAHI and hypoxemia. Using a conceptual serial multiple mediation model, % change in OSA-18 questionnaire score and % change in VLF power serially mediated the relationships between change in tonsil size and % change in OAHI.
Conclusions
The improvement in OAHI after adenotonsillectomy was serially mediated by reductions in total OSA-18 questionnaire score and VLF power. These preliminary findings are novel and provide a direction for future research to investigate the effects of VLF power-guided interventions on childhood OSA.
Keywords: adenotonsillectomy, children, heart rate variability (HRV), mediation, obstructive sleep apnea, quality of life
1. Introduction
Over 4% of children worldwide suffer from obstructive sleep apnea (OSA) (1). OSA, characterized by snoring and abnormal breathing during sleep, is a chronic disorder with many comorbidities, including cardiovascular sequelae (2) and cognitive/behavioral problems (3). OSA considerably reduces sleep quality in children (4). Furthermore, childhood OSA has been associated with hypofunction in brain autonomic control regions (5), which can influence heart rate and heart rate variability (HRV) by the interposition of cortico-subcortical pathways to the sympathetic nervous system (SNS) and parasympathetic nervous system (PNS) (6).
Unlike clinical signs and symptoms, which are often direct presentations of a disease, HRV reflects more indirect underlying pathophysiological process, either causal, mediating, or reactive, which allows measurements of the HRV to serve as a biomarker in a wide range of health conditions (7). Time domain and frequency domain HRV analysis on electrocardiograms are useful for diagnosing different clinical and functional conditions (8). For example, 24-h HRV indices are significantly associated with sleep disturbance and depression symptoms of medical students (9). In children with OSA, sleep fragmentation, arousal, and hypoxemia may increase SNS activity (10). However, sleep stage-specific HRV measurements have shown significantly downregulated PNS activity in children with sleep-disordered breathing (11). Studies on HRV in children with OSA have reported inconsistent results (12–14), and thus further investigations on cardiac autonomic function in this population are warranted.
Hypertrophy of adenoids and tonsils is the most common cause of upper airway obstruction in children (15), and adenotonsillectomy is the first-line treatment for childhood OSA (12, 16). Adenotonsillectomy significantly reduces the severity of OSA in terms of apnea-hypopnea index (AHI) and sympathetic activity (17) and sustainably improved quality of life (18). However, approximately 70% of children have residual OSA (19), which still threatens children's health. Further, changes in OSA-related HRV indices are not related to changes in AHI and hypoxemia (14). Accordingly, the aims of this study were to evaluate the reproducibility of sleep HRV analysis, the associations of sleep HRV and sleep quality, and the changes in HRV indices after adenotonsillectomy in children with OSA, and understand how these changes relate to adenoid-tonsil size and improvements in polysomnographic parameters.
2. Materials and methods
2.1. Study participants
The Institutional Review Board of Chang Gung Medical Foundation approved this retrospective case series (No. 202200882B0). The requirement for written informed consent was waived because the current study was based on a secondary analysis of existing data. This study followed the World Medical Association's Declaration of Helsinki and the Strengthening the Reporting of Cohort Studies in Surgery guidelines (20).
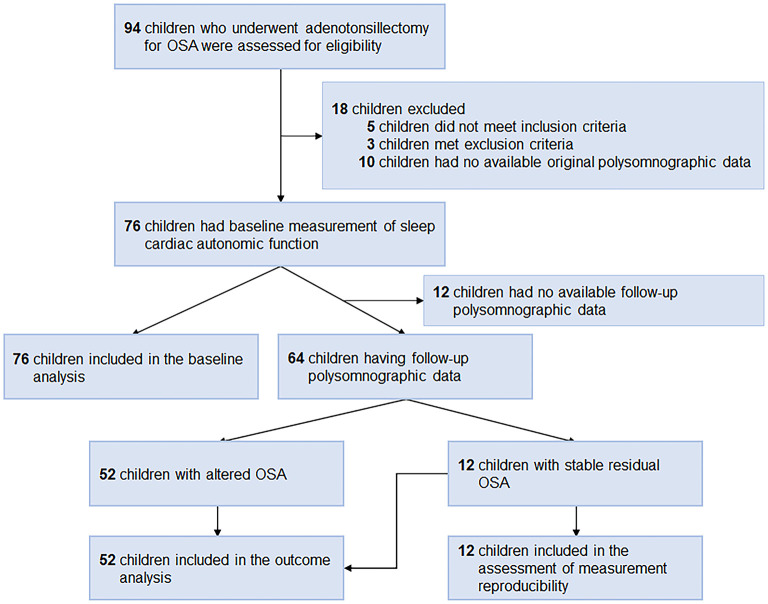
We included consecutive children who underwent adenotonsillectomy for OSA at Chang Gung Memorial Hospital, Linkou Main Branch (Taoyuan, Taiwan) between March 1, 2017 and September 30, 2021. The inclusion criteria were: (1) age 5–12 years, and (2) obstructive AHI (OAHI) ≥ 2.0 events/h or obstructive apnea index (OAI) ≥ 1.0 events/h (21, 22). The exclusion criteria were (1) patients with craniofacial, neuromuscular, or chronic inflammatory disorders (23, 24), or (2) patients without available polysomnographic data. All the children underwent extracapsular tonsillectomy with tonsillar pillar suturing and adenoidectomy that aimed to improve the upper airway obstruction by the principal investigator (L-AL) in a single stage under general anesthesia (25). Children with follow-up polysomnographic data were included in outcome analysis (Figure 1).
Figure 1.
Flow diagram. OSA, obstructive sleep apnea.
2.2. Clinical variables
Age, sex, body mass index (BMI), tonsil size, adenoidal-nasopharyngeal ratio (ANR) and evening blood pressure (BP) (2, 26), OSA-related quality of life, and polysomnographic parameters were recorded. All the clinical measurements were performed before and at least 3 months after adenotonsillectomy.
The tonsils were graded with a size scale from 1–4 (1: tonsils within the tonsillar; 2: tonsils visible outside the anterior pillars; 3: tonsils extending three-quarters of the way to the midline; 4: tonsils meeting at the midline) (27).
The ANR (distance from the point of maximal convexity of the adenoid shadow/the distance between the posterior border of the hard palate and the anteroinferior edge of the sphenobasioccipital synchondrosis) was measured on neck lateral view (28).
2.3. Sleep quality
2.3.1. Subjective measurement
All parents evaluated their children's OSA-related quality of life using the Chinese version of the OSA-18 questionnaire (29), which includes 18 items grouped into 5 domains: sleep disturbance (4 items), physical suffering (4 items), emotional distress (3 items), daytime problems (3 items), and caregiver concerns (4 items). Each item was scored using a 7-point ordinal scale. The total score was calculated as the sum of the 18 items (overall range, 18–126) and has been shown to have excellent test-retest reliability (30).
2.3.2. Objective measurement
All participants underwent full-night, in-laboratory polysomnography (Nicolet Biomedical Inc., Madison, WI, USA) (23). OAHI, OAI, arousal index, mean blood oxygen saturation (SaO2), minimal SaO2, sleep stages and total sleep time were scored and manually verified by the study investigators (L-PC and Y-SH) using a standard approach of the American Academy of Sleep Medicine (31). For example, the AHI was calculated by dividing the sum of all apneas (defined as a ≥ 90% reduction in airflow for a duration of ≥ 2 consecutive breaths) and hypopneas (defined as a ≥ 30% reduction in airflow in association with electroencephalographic arousal or a ≥ 3% reduction in SpO2 for a duration of ≥ 2 consecutive breaths) by the hours of total sleep time.
2.4. Sleep heart rate variability analysis
Electrocardiographic polysomnography signals were analyzed using HRV software (profusionSLEEPTM, version 4.5, build 502, Compumedics, Abbotsford, Australia). For artifact correction, automated annotations of electrocardiographic signals, such as loose leads, motion artifacts, and broken wires (32), were manually verified by trained technicians who had been certificated by the domestic board of the Taiwan Society of Sleep Medicine and shown substantial-to-almost perfect reliabilities in the scoring of respiratory events (intraclass correlation coefficients [ICCs] ranged from 0.66 to 0.98) (33). According to standard guidelines, time-domain indices, including standard deviation of all normal-to-normal (N-N) intervals (SDNN), number of pairs of adjacent N-N intervals differing by more than 50 ms in the entire recording divided by the total number of all N-N intervals (pNN50), and square root of the mean of the sum of the squares of differences between adjacent N-N intervals (RMSSD) were recorded. In addition, frequency-domain indices, including total power (0.0033–0.4 Hz), very low frequency (VLF) power (0.0033–0.04 Hz), low frequency (LF) power (0.04–0.15 Hz), normalized LF power (LF%), high frequency (HF) power (0.15–0.4 Hz), and LF/HF ratio were also recorded (Table 1) (34–39).
Table 1.
Indices, units, descriptions, and meanings of heart rate variability.
| Variable | Unit | Description | Meaning |
|---|---|---|---|
| Time-domain indices | |||
| N-N | ms | Time interval between N-N heartbeats | |
| SDNN | ms | Standard deviation of all N-N intervals. | Total capacity of the regulation system (35) |
| pNN50 | % | Number of pairs of adjacent N-N intervals differing by more than 50 ms divided by the total number of all N-N intervals. | Increased parasympathetic activity (35) |
| RMSSD | ms | The square root of the mean of the sum of the squares of di?erences between adjacent N-N intervals. | Increased parasympathetic activity (36) |
| Frequency-domain indices | |||
| Total power | ms2 | The variance of N-N intervals over the approximately the temporal segment (approximately ≤ 0.4 Hz) | Total capacity of the regulation system (35) |
| VLF power | ms2 | Power in very low frequency range ( ≤ 0.04 Hz) | Sympathetic activity (36) |
| LF power | ms2 | Power in low frequency range (0.04–0.15 Hz) | Baroreceptor activity (37) |
| LF% | % | LF power / (Total Power–VLF power) × 100 | Sympathetic modulation (38) |
| HF power | ms2 | Power in high frequency range (0.15–0.4 Hz) | Parasympathetic modulation (39) |
| LF/HF ratio | Ratio LF [ms2]/HF [ms2] | Sympathovagal balance (34) | |
HF, high frequency; LF, low frequency; LF%, normalized LF power; N-N, normal-to-normal; pNN50, proportion of N-N50 divided by the total number of N-N intervals; RMSSD, square root of the mean of the sum of the squares of di?erences between adjacent N-N intervals; SDNN, standard deviation of all N-N intervals; VLF, very low frequency.
2.5. Reproducibility assessment
Reproducibility of the HRV measurements was assessed using ICCs (two-way random model; absolute agreement type) from data quantified from separate sleep HRV measurements performed at least 3 months apart in a sample of 12 children with stable residual OSA [defined as postoperative OAHI within (preoperative OAHI−5.6 events/h) to (preoperative OAHI + 6.8 events/h), compatible with the upper and lower limits of agreement of OAHI measured on the first and second night in children and adolescents] (40). This sample represented children who did not undergo adenotonsillectomy. ICCs evaluated reproducibility as “poor” (< 0.001), “slight” (0.001–0.020), “fair” (0.021–0.40), “moderate” (0.41–0.60), “substantial” (0.61–0.80), and almost perfect (0.81–1.00) (41).
2.6. Statistical analysis
Data were analyzed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 9.0 for Windows (Graph Pad Software Inc., San Diego, CA, USA). Changes in scores were calculated as postoperative minus preoperative values. Percentage change [(change in score/preoperative value) × 100] was calculated for variables of interest. Because all the children underwent extracapsular tonsillectomy, the change in tonsil size was equal to the negative value of tonsil size and used for further statistical analysis.
Using the Shapiro-Wilk test to examine normality, descriptive statistics were expressed as mean (standard deviation) for normally distributed continuous variables, median (interquartile range [IQR]) for skewed variables, and number (proportion) for categorical variables.
For continuous variables, the independent-samples t-test or Mann-Whitney U test was used to assess between-group changes; the paired-samples t-test or Wilcoxon signed-rank test was used to assess within-group changes as appropriate. Differences in categorical variables between two subgroups were analyzed using Fisher's exact test.
To facilitate comparisons with previous studies, linear regression models, or mediation and moderation analysis, non-normally distributed data of reference studies were transformed to normal after estimation from the sample size (n), median (m), and the first (q1) and third (q3) quartiles (42, 43). The sample standard deviation was estimated to be [(q3 – q1) / η] where η = η(n) = 2Φ−1[(0.75 × n – 0.125) / (n + 0.25)] (42). In addition, non-normally distributed continuous variables were transformed to normal using a two-step approach: fractional rank and inverse-normal transformation (44). For comparisons with reference values, the one-sample t-test was applied.
Relationships between variables of interest were assessed using Pearson and Point-Biserial correlation tests as appropriate. Multivariable linear regression models, including all variables, with manual selection based on a probability of F < 0.05 were used to identify independent variables. The variance inflation factor of each predictor was calculated to adjust for intervariable relationships within the model. The regression model was repeated after removing all variables with a variance inflation factor ≥ 5 to reduce multicollinearity (45).
Conditional process analysis was performed to evaluate the mediators and moderators between changes in tonsil size/ANR and % changes in polysomnographic parameters using the SPSS PROCESS macro (version 4.1) (46). Bias-corrected 95% confidence intervals (CIs) were estimated via bootstrapping (5,000 runs) to verify mediation, moderated mediation, or mediated moderation. A two-sided P < 0.05 was considered statistically significant.
3. Results
3.1. Participants' characteristics
Seventeen (22%) girls and 59 (78%) boys with OSA (median OAHI, 5.5 [IQR, 2.3–12.6] events/h) were included in the baseline analysis (Figure 1), of whom 64 (84%) were included in the outcome analysis and 12 (16%) were not included due to no available follow-up polysomnography. All baseline variables were comparable between these two subgroups (Table 2).
Table 2.
Demographics of the participants in the baseline analysis and those included and excluded from the outcome analysis.
| Variable | Participants included in the baseline analysis | Participants included in the outcome analysis | Participants excluded in the outcome analysis | P valuea |
|---|---|---|---|---|
| N | 76 | 64 | 12 | |
| Clinical variables | ||||
| Age at diagnosis (years) | 7 (6–9) | 6 (5–9) | 7 (6–10) | 0.417 |
| Male sex, n (%) | 59 [78] | 47 [73] | 12 (100) | 0.058 |
| BMI (kg/m2) | 17.4 (15.3–22.8) | 17.3 (15.0–21.9) | 21.1 (15.6–26.8) | 0.133 |
| Tonsil size | 3 (3–4) | 3 (3–4) | 3 (3–4) | 0.703 |
| ANR | 0.800 (0.697–0.872) | 0.800 (0.710–0.864) | 0.691 (0.585–0.848) | 0.093 |
| Systolic BP (mmHg) | 104.2 (18.0) | 103.5 (17.2) | 107.9 (22.1) | 0.440 |
| Diastolic BP (mmHg) | 65 (59–71) | 65 (59–71) | 66 (57–76) | 0.825 |
| Subjective sleep quality (assessed by the OSA-18 questionnaire) | ||||
| OSA-18 score | 81.3 (15.4) | 81.8 (15.7) | 78.4 (14.1) | 0.486 |
| Objective sleep quality (assessed by polysomnography) | ||||
| OAHI (events/h) | 5.5 (2.3–12.6) | 5.8 (2.4–13.1) | 6.3 (2.4–10.4) | 0.943 |
| OAI (events/h) | 0.4 (0.1–1.5) | 0.6 (0.2–1.7) | 0.5 (0.18–1.5) | 0.908 |
| Arousal index (events/h) | 9.8 (7.2–16.5) | 9.9 (7.3–16.9) | 9.0 (6.7–15.2) | 0.397 |
| Mean SaO2 (%) | 97 (97–98) | 97 (97–98) | 97 (96–98) | 0.480 |
| Minimal SaO2 (%) | 84 (90–92) | 90 (84–92) | 90 (83–92) | 0.797 |
| N1 sleep (%) | 10 (6–15) | 10 (6–16) | 9 (7–14) | 0.569 |
| N2 sleep (%) | 39.1 (8.6) | 39.2 (9.2) | 39.0 (4.8) | 0.911 |
| N3 sleep (%) | 28 (22–35) | 27 (22–35) | 29 (23–35) | 0.711 |
| REM sleep (%) | 19.3 (5.9) | 19.1 (5.8) | 20.2 (6.3) | 0.556 |
| TST (min) | 337 (321–352) | 337 (320–353) | 329 (321–349) | 0.437 |
| Sleep heart rate variability indices | ||||
| Heart rate (bpm) | 76 (70–82) | 76 (70–85) | 76 (70–82) | 0.770 |
| N-N interval (ms) | 791.8 (97.3) | 793.7 (95.6) | 781.6 (93.3) | 0.694 |
| SDNN (ms) | 96.6 (32.6) | 98.3 (34.1) | 87.4 (21.7) | 0.163 |
| pNN50 (%) | 36.9 (19.0) | 37.1 (19.2) | 35.8 (5.5) | 0.840 |
| RMSSD (ms) | 67 (50–105) | 63 (49–114) | 69 (53–80) | 0.680 |
| Total power (ms2) | 8688 (4614–14944) | 9454 (4499–15430) | 7560 (5233–9443) | 0.298 |
| VLF power (ms2) | 1509 (1095–2540) | 1509 (1095–2727) | 1479 (895–1833) | 0.340 |
| LF power (ms2) | 1236 (760–2150) | 1243 (734–2507) | 992 (804–1526) | 0.243 |
| LF% (%) | 37 (28–47) | 38 (29–48) | 33 (27–42) | 0.494 |
| HF power (ms2) | 2140 (1113–4228) | 1970 (1103–5155) | 2183 (1248–4412) | 0.669 |
| LF/HF ratio | 0.59 (0.40–0.90) | 0.62 (0.40–0.92) | 0.50 (0.40–0.70) | 0.459 |
Data are expressed as mean (standard deviation), median (interquartile range), or number (%).
aData were compared between participants included in the outcome analysis and those excluded using the independent-samples t-test, Mann-Whitney U test, or Fisher's exact test as appropriate.
ANR, adenoidal-nasopharyngeal ratio; BMI, body mass index; BP, blood pressure; bpm, beats per min; HF, high frequency; LF, low frequency; LF%, normalized LF power; N-N, normal-to-normal; OAHI, obstructive apnea-hypopnea index; OAI, obstructive apnea index; OSA, obstructive sleep apnea; pNN50, proportion of N-N50 divided by the total number of N-N intervals; REM, rapid eye movement; RMSSD, square root of the mean of the sum of the squares of di?erences between adjacent N-N intervals; SaO2, blood oxygen saturation; SDNN, standard deviation of all N-N intervals; TST, total sleep time; VLF, very low frequency.
3.2. Sleep heart rate variability
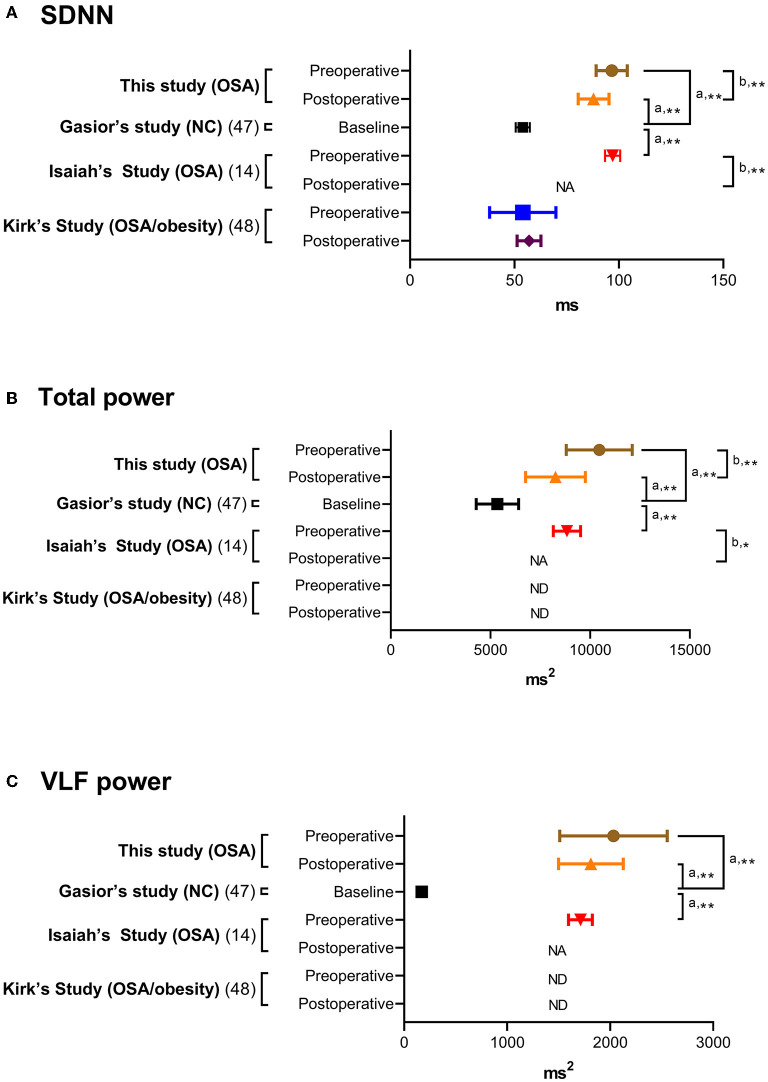
Distributions of HRV indices in baseline analysis are summarized in Table 2. For comparing with previous studies, the HRV indices in this study (full-night), normal controls (full-night) (47), children with OSA (full-night) (14), children with moderate-to-severe OSA (N3 sleep) (13, 17), and children with OSA/obesity (full-night) (48) are summarized in Table 3. Comparing with three representative full-night HRV studies (14, 47, 48), SDNN, total power and VLF power in the children with OSA were significantly higher than normal values (Figure 2).
Table 3.
Sleep heart rate variability indices in normal controls and in children and adolescents with OSA.
| Variable | Our study | Gasior's study (47) | Isaiah's study (14) | Muzumdar's study (17) | Nisbet's study (13) | Kirk's Study (48) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Publication year | 2020 | 2020 | 2011 | 2013 | 2020 | ||||||||
| Nation | Taiwan | Poland | USA | USA | Australia | Canada | |||||||
| Participants | OSA | NC | OSA | M-S OSA | Mild OSA | M-S OSA | OSA/obesity | ||||||
| Case number | 76 | 312 | 404 | 18 | 39 | 29 | 12 | ||||||
| Age (years) | 7.2 (2.1) | 10.1 (2.5) | 6.0 (4.5) | 4.9 (2.4) | 4.3 (0.1) | 4.2 (0.2) | 12.8 (5.1) | ||||||
| Male sex, n (%) | 59 (78) | 159 (51) | 195 (48) | 13 (72) | 26 (67) | 18 (62) | 10 (83) | ||||||
| OAHI (events/h) | 10.2 (12.6) | NA | 4.6 (4.6)* | 31.9 (24.8)* | 3.1 (0.9) | 15.5 (12.3) | 13.8 (14.5) | ||||||
| Sleep stage | FN | FN | FN | N1, N2 | N3 | REM | N1, N2 | N3 | REM | N1, N2 | N3 | REM | FN |
| Heart rate (bpm) | 77 (9) | NA | NA | 100 (17)a | 100 (15)a | 107 (16)a | NA | NA | 82 (7) | ||||
| N-N interval (ms) | 792 (97) | NA | NA | 630 (120)b | 620 (100)b | 580 (110)a | NA | NA | 732 (62) | ||||
| SDNN (ms) | 97 (33) | 54 (30) | 97 (37) | NA | NA | NA | 54 (25) | ||||||
| pNN50 (%) | 37 (19) | 34 (52) | 36 (24) | NA | NA | NA | NA | ||||||
| RMSSD (ms) | 76 (38) | 71 (91) | 81 (45) | 61 (55)b | 43 (29)b | 76 (57)b | NA | NA | NA | ||||
| Total power (ms2) | 10,460 (7,238) | 5,348 (9,534) | 8,838 (6,990) | NA | 7,632 (5,908) | 6,021 (6,145) | 5,287 (4,103) | 9,040 (5,905)a | 6,600 (6,144) | 5,827 (4,789)a | NA | ||
| VLF power (ms2) | 2,032 (2,284) | 171 (284) | 1,712 (1,186)a | NA | NA | NA | NA | ||||||
| LF power (ms2) | 1,727 (1,457) | 2,023 (3,789) | 1,382 (1,186) | 360 (342) | 184 (148)b | 307 (293) | 1,298 (1,024) | 758 (781) | 909 (606) | 1,959 (1,023)a | 848 (780) | 1,075 (603)a | NA |
| LF% (%) | 39 (15) | NA | NA | NA | NA | NA | 41 (17) | ||||||
| HF power (ms2) | 3,149 (3,029) | 3,766 (6,993) | 2,742 (3,340) | 902 (1,202)b | 483 (497)b | 412 (449)b | 4,418 (3,610)a | 3,985 (3,859) | 2,416 (2,186)a | 7,382 (3,608)a | 4,126 (3,856) | 2,183 (2,186) | NA |
| LF/HF ratio | 0.80 (0.66) | 0.72 (0.82) | 0.53 (0.36) | 1.60 (2.7)a | 1.20 (1.60)a | 3.0 (5.4)a | 0.70 (0.62) | 0.40 (0.25) | 1.10 (0.62) | 0.70 (0.54) | 0.30 (0.27)b | 0.90 (0.54) | 1.40 (1.18) |
To facilitate comparisons with previous studies, data are summarized as mean (standard deviation) after estimation from the sample size, median, and interquartile range.
aHigher than controls, P < 0.05; bLower than controls, P < 0.05.
*Only AHI was available.
AHI, apnea-hypopnea index; bpm, beats per min; FN, full night; HF, high frequency; LF, low frequency; LF%, normalized LF power; M-S, moderate-to-severe; N3, stage 3 sleep; NC, normal controls; N-N, normal-to-normal; pNN50, proportion of N-N50 divided by the total number of N-N intervals; RMSSD, square root of the mean of the sum of the squares of differences between adjacent N-N intervals; SDNN, standard deviation of all N-N intervals; VLF, very low frequency.
Figure 2.
(A–C) Full-night heart rate variability indices in normal controls and children and adolescents with obstructive sleep apnea. Data are summarized as means and 95% confidence intervals. aThe one-sample t-test was applied for comparisons of related samples according to the reference values of normal control (47). bThe paired-samples t-test or Wilcoxon signed-rank test was used to compare related samples according to the original references (14, 48). *P < 0.05 and ≥0.01; **P < 0.01 and ≥0.001. NA, not available; ND, not detected; OSA, obstructive sleep apnea; SDNN, standard deviation of all normal-normal intervals; VLF, very low frequency.
3.3. Measurement reproducibility of sleep heart rate variability
To assess measurement reproducibility, we calculated ICCs using HRV indices measured at least 3 months apart in 12 patients with stable residual OSA after adenotonsillectomy (Table 4). Their variables of interest were comparable to the patients with altered OSA (Tables 5, 6). Most HRV measurements demonstrated moderate (N-N interval, SDNN, RMSSD, total power, LF%) or substantial (VLF power, LF power, LF/HF ratio) reproducibility. Further, the reproducibility of pNN50 and HF power were fair (41).
Table 4.
Reproducibility of sleep HRV measurements in twelve children with stable residual OSA after adenotonsillectomy.
| Variable | Preoperative | Postoperative | ICC | 95% CI | P-value |
|---|---|---|---|---|---|
| Time-domain indices | |||||
| N-N interval (ms) | 803 (685–870) | 829 (728–855) | 0.488 | −0.123–0.823 | 0.053 |
| SDNN (ms) | 83 (66–102) | 72 (59–111) | 0.553 | 0.011–0.846 | 0.026 |
| pNN50 (%) | 27 (21–47) | 27 (11–47) | 0.256 | −0.397–0.716 | 0.213 |
| RMSSD (ms) | 56 (44–83) | 55 (33–73) | 0.471 | −0.136–0.815 | 0.060 |
| Frequency-domain indices | |||||
| Total power (ms2) | 6,012 (3,350–13,102) | 4,604 (3,222–10,158) | 0.483 | −0.063–0.814 | 0.045 |
| VLF power (ms2) | 1,814 (1012–2797) | 1,901 (1,006–2,393) | 0.634 | 0.290–0.833 | 0.001 |
| LF power (ms2) | 935 (350–1688) | 645 (391–1,377) | 0.720 | 0.270–0.911 | 0.004 |
| LF% (%) | 40 (30–50) | 46 (31–59) | 0.557 | 0.034–0.846 | 0.023 |
| HF power (ms2) | 1,460 (677–2468) | 789 (358–2,547) | 0.335 | −0.318–0.756 | 0.146 |
| LF/HF ratio | 0.67 (0.43–1.01) | 1.047 (0.45–1.51) | 0.725 | 0.237–0.915 | 0.001 |
Bold font indicates statistically significant differences (P < 0.05).
CI, confidence interval; HF, high frequency; HRV, Heart rate variability; ICC, intraclass correlation coefficient; LF, low frequency; LF%, normalized LF power; N-N, normal-to-normal; OSA, obstructive sleep apnea; pNN50, proportion of N-N50 divided by the total number of N-N intervals; RMSSD, square root of the mean of the sum of the squares of di?erences between adjacent N-N intervals; SDNN, standard deviation of all N-N intervals; VLF, very low frequency.
Table 5.
Clinical variables, subjective quality and objective sleep quality of the study sample by altered OSA status in the outcome analysis.
| Variable | All participants | Stable residual OSA | Altered OSA | P Valuea |
|---|---|---|---|---|
| n | 64 | 12 | 52 | |
| Clinical variables | ||||
| Age at diagnosis (years) | 6 (5–9) | 8 (6–10) | 6 (5–8) | 0.132 |
| Male sex, n (%) | 47 (73) | 10 (83) | 37 (79) | 0.490 |
| BMI (kg/m 2 ) | ||||
| Preoperative | 17.3 (15.0–21.9) | 17.4 (15.3–23.0) | 16.6 (14.7–21.9) | 0.711 |
| Postoperative | 17.4 (15.1–22.8) | 18.8 (16.4–24.5) | 17.2 (15.0–22.2) | 0.225 |
| Change | 4.0 (−0.4–1.4) | 1.5 (−0.7–3.0) | 0.4 (−0.4–1.1) | 0.148 |
| % Change | 3 (−2–8) | 7 (−4–16) | 2 (−2–7) | 0.135 |
| Systolic BP (mmHg) | ||||
| Preoperative | 103.5 (17.2) | 105 (100–113) | 103 (94–113) | 0.558 |
| Postoperative | 105.3 (15.3) | 106 (96–120) | 102 (94–115) | 0.642 |
| Change | 1.8 (14.9) | −1 (−9–10) | 3 (−10–11) | 0.783 |
| % Change | 3.2 (15.7) | −1 (−7–10) | 3 (−9–11) | 0.680 |
| Diastolic BP (mmHg) | ||||
| Preoperative | 65 (59–71) | 61 (58–72) | 65 (59–71) | 0.444 |
| Postoperative | 64 (58–72) | 64 (56–72) | 64 (59–75) | 0.530 |
| Change | −1 (−7–7) | 2 (−4–14) | −2 (−8–6) | 0.136 |
| % Change | −2 (−11–12) | 4 (−6–25) | −3 (−12–9) | 0.120 |
| Objective sleep quality (assessed by the OSA-18 questionnaire) | ||||
| OSA-18 score | ||||
| Preoperative | 81.8 (15.7) | 89 (77–94) | 82 (70–92) | 0.404 |
| Postoperative | 52.0 (13.2) | 54 (49–67) | 50 (40–60) | 0.127 |
| Change | −26.4 (22.7) | −20 (−30–−6) | −32 (−43–−14) | 0.072 |
| % Change | −31 (24) | −27 (−34–−3) | −39 (−48–−22) | 0.037 |
| Subjective sleep quality (assessed by polysomnography) | ||||
| OAHI (events/h) | ||||
| Preoperative | 5.8 (2.4–13.1) | 5.0 (3.1–6.7) | 7.7 (2.3–17.1) | 0.318 |
| Postoperative | 1.4 (0.6–2.5) | 3.0 (2.4–6.4) | 1.2 (0.5–1.8) | < 0.001 |
| Change | −3.7 (−11.2–−1.2) | −1.8 (−3.4–0.9) | −5.9 (−12.6–−1.6) | 0.006 |
| % Change | −75 (−92–−45) | −32 (−46–−20) | −87 (−94–−64) | < 0.001 |
| OAI (events/h) | ||||
| Preoperative | 0.6 (0.2–1.7) | 0.4 (0.1–1.5) | 0.4 (0–1.5) | 0.931 |
| Postoperative | 0 (0–0.3) | 0.5 (0–0.8) | 0 (0–0.2) | 0.008 |
| Change | −0.3 (−1.0–0) | −0.2 (−0.9–0.3) | −0.3 (−1.1–0) | 0.393 |
| % Change | −79 (−100–0) | −55 (−98–95) | −85 (−100–0) | 0.185 |
| Arousal index (events/h) | ||||
| Preoperative | 9.9 (7.3–16.9) | 10.4 (8.0–15.5) | 9.6 (7.2–17.7) | 0.959 |
| Postoperative | 7.1 (6.0–9.2) | 7.6 (6.0–9.0) | 6.8 (6.0–9.8) | 0.624 |
| Change | −2.9 (−8.9–−3.3) | −2.2 (−7.3–−0.6) | −3.1 (−10.7–−0.3) | 0.371 |
| % Change | −33 (−53–−5) | −23 (−49–−6) | −34 (−53–−5) | 0.667 |
| Mean SaO2 (%) | ||||
| Preoperative | 97 (97–98) | 98 (97–98) | 97 (97–98) | 0.508 |
| Postoperative | 98 (97–98) | 98 (97–98) | 98 (97–98) | 0.927 |
| Change | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.731 |
| % Change | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.589 |
| Minimal SaO2 (%) | ||||
| Preoperative | 90 (84–92) | 90 (88–92) | 90 (84–92) | 0.904 |
| Postoperative | 92 (89–94) | 91 (89–93) | 92 (89–93) | 0.316 |
| Change | 2 (−1–5) | 1 (−2–6) | 2 (0–5) | 0.329 |
| % Change | 2 (−1–6) | 1 (−2–6) | 2 (0–6) | 0.331 |
| N1 sleep (%) | ||||
| Preoperative | 10 (6–16) | 11 (6–16) | 10 (7–16) | 0.918 |
| Postoperative | 9 (7–12) | 7 (6–10) | 9 (7–13) | 0.212 |
| Change | −2 (−9–2) | −4 (−8–1) | −2 (−9–3) | 0.594 |
| % Change | −24 (−51–41) | −30 (−55–23) | −20 (−50–52) | 0.439 |
| N2 sleep (%) | ||||
| Preoperative | 39.2 (9.2) | 46 (40–54) | 39 (31–44) | 0.012 |
| Postoperative | 41.6 (8.8) | 47 (36–52) | 41 (35–46) | 0.232 |
| Change | 2.4 (10.5) | 1 (−11–9) | 4 (−2–10) | 0.225 |
| % Change | 11.3 (32.3) | 2 (−21–25) | 10 (−8–32) | 0.203 |
| N3 sleep (%) | ||||
| Preoperative | 27 (22–35) | 25 (21–28) | 28 (22–37) | 0.081 |
| Postoperative | 25 (21–31) | 27 (20–30) | 25 (21–32) | 0.810 |
| Change | −1 (−8–5) | 2 (−6–8) | −4 (−9–4) | 0.180 |
| % Change | 3 (−30–24) | 7 (−18–32) | −12 (−31–18) | 0.235 |
| REM sleep (%) | ||||
| Preoperative | 19.1 (5.8) | 19 (15–22) | 21 (16–23) | 0.636 |
| Postoperative | 22.4 (6.3) | 22 (21–26) | 22 (18–27) | 0.925 |
| Change | 3.3 (7.4) | 6 (−1–9) | 3 (−3–8) | 0.564 |
| % Change | 28.4 (53.0) | 27 (−7–53) | 14 (−14–56) | 0.667 |
| TST (min) | ||||
| Preoperative | 337 (320–353) | 338 (309–354) | 337 (320–353) | > 0.999 |
| Postoperative | 336 (321–350) | 335 (278–352) | 337 (321–350) | 0.763 |
| Change | −6 (−32–21) | −8 (−50–15) | −4 (−32–23) | 0.536 |
| % Change | −2 (−9–6) | −3 (−13–5) | −1 (−9–8) | 0.547 |
Data are expressed as median (interquartile range) or number (%). Bold font indicates statistically significant differences (P < 0.05).
BMI, body mass index; BP, blood pressure; OAHI, obstructive apnea-hypopnea index; OAI, obstructive apnea index; OSA, obstructive sleep apnea; REM, rapid eye movement; SaO2, blood oxygen saturation; TST, total sleep time.
Table 6.
HRV indices of the study sample by altered OSA status in the outcome analysis.
| Variable | All participants | Stable residual OSA | Altered OSA | P valuea |
|---|---|---|---|---|
| n | 64 | 12 | 52 | |
| Time-domain indices | ||||
| N-N interval (ms) | ||||
| Preoperative | 793.7 (95.6) | 803 (685–870) | 786 (735–849) | 0.945 |
| Postoperative | 827.0 (100.5) | 829 (728–855) | 828 (763–915) | 0.390 |
| Change | 33.3 (108.8) | −25 (−112–107) | 36 (−16–96) | 0.216 |
| % Change | 5.1 (13.9) | −3 (−13–15) | 5 (−2–12) | 0.279 |
| SDNN (ms) | ||||
| Preoperative | 98.3 (34.1) | 83 (66–102) | 102 (75–125) | 0.194 |
| Postoperative | 87.9 (29.6) | 72 (59–111) | 85 (69–110) | 0.282 |
| Change | −10.4 (30.1) | −5 (−29–9) | −13 (−29–13) | 0.712 |
| % Change | −4.8 (33.8) | −5 (−28–14) | −12 (−29–10) | 0.606 |
| pNN50 (%) | ||||
| Preoperative | 37.1 (19.2) | 27 (21–47) | 38 (25–54 | 0.340 |
| Postoperative | 36.6 (21.5) | 27 (11–47) | 40 (20–53) | 0.242 |
| Change | −0.5 (19.0) | 1 (−24–15) | −1 (−12–14) | 0.612 |
| % Change | 28.7 (135.5) | 5 (−55–86) | −2 (−30–39) | 0.843 |
| RMSSD (ms) | ||||
| Preoperative | 63 (49–114) | 56 (44–83) | 70 (49–116) | 0.371 |
| Postoperative | 61 (38–85) | 55 (33–73) | 64 (41–94) | 0.249 |
| Change | −9 (−30–10) | −2 (−36–13) | −10 (−28–8) | 0.891 |
| % Change | −9 (−32–22) | −1 (−40–38) | −10 (−32–17) | 0.945 |
| Frequency-domain indices | ||||
| Total power (ms 2 ) | ||||
| Preoperative | 9,454 (4,499–15,430) | 6,012 (3,350–13,102) | 9,505 (4,831–16,300) | 0.169 |
| Postoperative | 6,437 (4,058–10,980) | 4,605 (3,222–10,158) | 6,739 (4,503–12,675) | 0.180 |
| Change | −2261 (−5568–1741) | −608 (−7189–1401) | −2,606 (−5,547–1,741) | 0.904 |
| % Change | −18 (−50–17) | −11 (−57–32) | −23 (−50–17) | 0.655 |
| VLF power (ms 2 ) | ||||
| Preoperative | 1,509 (1,095–2,727) | 1,345 (771–2,718) | 1,535 (1,157–2,727) | 0.399 |
| Postoperative | 1,485 (1,032–2,102) | 1,456 (852–3,691) | 1,485 (1,063–2,102) | 0.823 |
| Change | 92 (−734–540) | 193 (−387–990) | 42 (−779–497) | 0.318 |
| % Change | 3 (−41–38) | 19 (−30–56) | 0 (−41–36) | 0.460 |
| LF power (ms 2 ) | ||||
| Preoperative | 1,243 (734–2,507) | 935 (350–1,688) | 1,286 (791–2,657) | 0.144 |
| Postoperative | 942 (474–1,406) | 645 (391–1,377) | 1,022 (671–1,535) | 0.279 |
| Change | −155 (−924–231) | 40 (−818–206) | −285 (−925–248) | 0.439 |
| % Change | −9 (−63–29) | 9 (−56–96) | −20 (−63–19) | 0.249 |
| LF% (%) | ||||
| Preoperative | 38 (29–48) | 40 (30–50) | 38 (28–48) | 0.570 |
| Postoperative | 40 (32–50) | 46 (31–59) | 39 (32–49) | 0.327 |
| Change | 2 (−8–13) | 4 (−3–13) | 2 (−10–13) | 0.570 |
| % Change | 5 (−19–42) | 10 (−13–33) | 3 (−22–43) | 0.823 |
| HF power (ms 2 ) | ||||
| Preoperative | 1,970 (1,103–5,155) | 1,460 (677–2,468) | 2,234 (1,113–5,353) | 0.122 |
| Postoperative | 1,364 (636–3,360) | 789 (358–2547) | 1,624 (812–3,420) | 0.164 |
| Change | −531 (−1,870–301) | −284 (−1601–282) | −663 (−2229–301) | 0.536 |
| % Change | −26 (−66–21) | −15 (−70–120) | −28 (−66–15) | 0.559 |
| LF/HF ratio | ||||
| Preoperative | 0.62 (0.40–0.92) | 0.67 (0.43–1.01) | 0.61 (0.40–0.92) | 0.570 |
| Postoperative | 0.70 (0.48–1.19) | 1.05 (0.45–1.51) | 0.66 (0.48–1.05) | 0.294 |
| Change | 0.08 (−0.08–0.40) | 0.16 (−0.07–0.49) | 0.07 (−0.13–0.36) | 0.310 |
| % Change | 13 (−12–70) | 20 (−10–69) | 13 (−14–72) | 0.606 |
Data are expressed as median (interquartile range) or number (%).
aData were compared using the Mann-Whitney U test or Fisher's exact test as appropriate.
HF, high frequency; HRV, Heart rate variability; LF, low frequency; LF%, normalized LF power; N-N, normal-to-normal; OSA, obstructive sleep apnea; pNN50, proportion of N-N50 divided by the total number of N-N intervals; RMSSD, square root of the mean of the sum of the squares of di?erences between adjacent N-N intervals; SDNN, standard deviation of all N-N intervals; VLF, very low frequency.
3.4. Associations between variables of interest at baseline
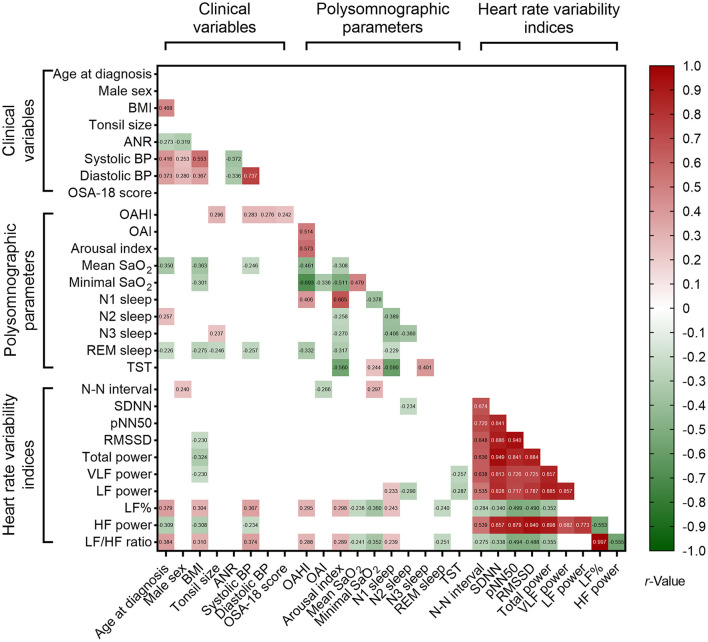
Nested data structure and significant correlations were found among the polysomnographic parameters, several clinical variables and HRV indices (Figure 3). However, total OSA-18 questionnaire score was not associated with variables of interest. Using multivariable linear regression models (Table 7), male sex, OAHI and N3 sleep were independently associated with tonsil size, and systolic BP, OAHI and VLF power were independently associated with ANR. Furthermore, tonsil size, diastolic BP and LF% were independently correlated with OAHI. Table 7 summarizes the independent associations of other polysomnographic parameters with the variables of interest.
Figure 3.
Associations of polysomnographic variables with clinical variables and sleep heart rate variability indices. Data are summarized as Pearson's or Point-Biserial rho, as appropriate. Blank spaces mean two-sided P ≥ 0.05.
Table 7.
Multivariable linear regression models of independent associations of tonsil/adenoid sizes and polysomnographic parameters with other variables in the baseline analysis.
| Baseline variables | Independent variables | β (95% CI) | VIF | P value | Adjusted R2 |
|---|---|---|---|---|---|
| Clinical variables | |||||
| Tonsil size | Male sex | −0.33 (-0.60-−0.06) | 1.00 | 0.019 | 0.216 |
| OAHI | 0.01 (0.001–0.02) | 1.01 | 0.027 | ||
| N3 sleep | 0.02 (0.01–0.04) | 1.01 | 0.005 | ||
| ANR | Systolic BP | −0.003 (-0.005-−0.002) | 1.14 | < 0.001 | |
| OAHI | 0.003 (0.001–0.005) | 1.16 | 0.005 | ||
| VLF power | −0.00001 (-0.00003-−0.000003) | 1.02 | 0.018 | ||
| Subjective sleep quality (polysomnographic parameters) | |||||
| OAHI | Tonsil size | 7.96 (3.03–12.89) | 1.02 | 0.002 | 0.243 |
| Diastolic BP | 0.29 (0.02–0.56) | 1.04 | 0.034 | ||
| LF% | 0.25 (0.07–0.44) | 1.06 | 0.008 | ||
| OAI | OSA-18 score | 0.07 (0.001–0.14) | 1.00 | 0.049 | 0.119 |
| N-N interval | −0.01 (-0.03-−0.003) | 1.00 | 0.015 | ||
| Arousal index | N-N interval | −0.05 (-0.08-−0.02) | 1.46 | 0.001 | 0.283 |
| LF power | 0.01 (0.003–0.01) | 2.72 | < 0.001 | ||
| HF power | −0.001 (-0.003-−0.0002) | 2.71 | 0.020 | ||
| Mean SaO2 | BMI | −0.10 (-0.15-−0.05) | 1.10 | < 0.001 | 0.228 |
| N-N interval | 0.004 (0.001–0.01) | 1.75 | 0.001 | ||
| VLF power | −0.0002 (-0.0004-−0.0001) | 1.84 | 0.007 | ||
| Minimal SaO2 | BMI | −0.50 (-0.74-−0.25) | 1.09 | < 0.001 | 0.319 |
| N-N interval | 0.04 (0.02–0.06) | 1.75 | < 0.001 | ||
| VLF power | −0.001 (-0.002-−0.001) | 1.83 | < 0.001 | ||
| N1 sleep | pNN50 | −0.22 (-0.38-−0.06) | 2.08 | 0.007 | 0.146 |
| LF power | 0.004 (0.002–0.01) | 2.08 | 0.001 | ||
| N2 sleep | LF power | −0.002 (-0.003-−0.0004) | 1.00 | 0.012 | 0.072 |
| N3 sleep | Tonsil size | 3.59 (0.04–7.14) | 1.00 | 0.048 | 0.123 |
| pNN50 | 0.15 (0.01–0.29) | 2.13 | 0.038 | ||
| VLF power | −0.001 (-0.003-−0.0001) | 2.12 | 0.030 | ||
| REM sleep | Age | −0.68 (-1.30-−0.05) | 1.00 | 0.036 | 0.116 |
| Tonsil size | −2.88 (-5.38-−0.39) | 1.00 | 0.027 | ||
| TST | LF power | −0.01 (-0.01-−0.002) | 1.00 | 0.013 | 0.070 |
ANR, adenoidal-nasopharyngeal ratio; BMI, body mass index; BP, blood pressure; CI, confidence interval; HF, high frequency; LF, low frequency; OAHI, obstructive apnea-hypopnea index; OAI, obstructive apnea index; OSA, obstructive sleep apnea; N-N, normal-to-normal; pNN50, proportion of N-N50 divided by the total number of N-N intervals; REM, rapid eye movement; SaO2, blood oxygen saturation; TST, total sleep time; VIF, variance inflation factor; VLF, very low frequency.
3.5. Changes in the variables of interest after adenotonsillectomy
The median follow-up period was 4 (IQR, 3–6) months. In outcome analysis, mean SaO2, minimal SaO2 and rapid eye movement sleep significantly increased, and OSA-18 score, OAHI, OAI, arousal index and N1 sleep significantly reduced after adenotonsillectomy (Table 5).
Regarding HRV indices, SDNN, total power and HF power significantly reduced after adenotonsillectomy (Table 6), and they were still significantly different from normal values (47) (Figure 2).
3.6. Associations of percentage changes in the variables of interest
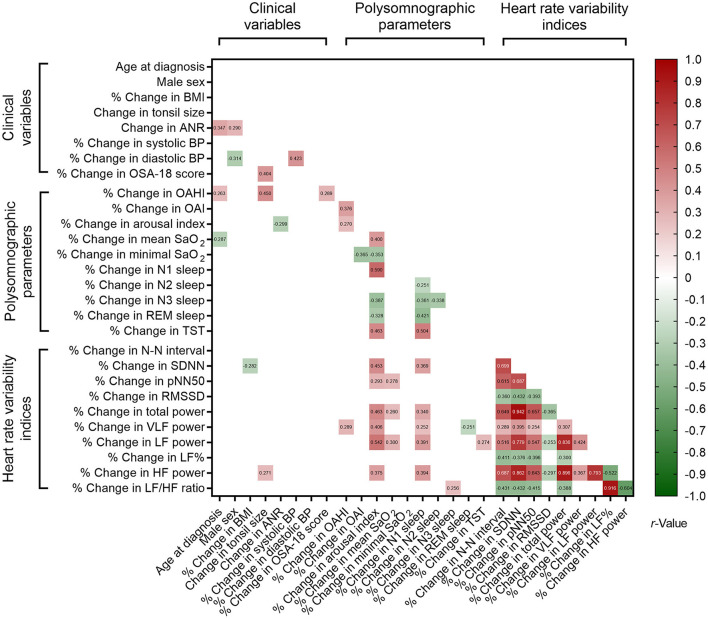
Correlations of % changes in polysomnographic parameters and % changes in clinical variables and HRV indices also revealed significant associations with nested data structure (Figure 4). Using multivariable linear regression models (Table 8), % changes in OSA-18 score, OAHI and HF power were independently associated with change in tonsil size. Age at diagnosis, male sex and % change in arousal index were independently associated with change in ANR, and change in tonsil size was independently correlated with % change in OAHI.
Figure 4.
Associations of % changes in polysomnographic variables, % changes in clinical variables, and % changes in sleep heart rate variability indices. Blank spaces mean two-sided P ≥ 0.05.
Table 8.
Multivariable linear regression models of independent associations of changes in tonsil/adenoid size and % changes in polysomnographic parameters in the outcome analysis.
| Outcome variables | Independent variables | β (95% CI) | VIF | P Value | Adjusted R2 |
|---|---|---|---|---|---|
| Clinical variables | |||||
| Change in tonsil size | % Change in OSA-18 score | 0.01 (0.002–0.01) | 1.10 | 0.005 | 0.309 |
| % Change in OAHI | 0.002 (0.001–0.004) | 1.11 | 0.005 | ||
| HF power | 0.001 (0.0001–0.002) | 1.03 | 0.025 | ||
| Change in ANR | Age at diagnosis | 0.01 (0.001–0.03) | 1.06 | 0.030 | 0.203 |
| Male sex | 0.06 (0.004–0.12) | 1.04 | 0.037 | ||
| % Change in arousal index | −0.0002 (-0.0004-−0.0003) | 1.02 | 0.026 | ||
| Subjective sleep quality (polysomnographic parameters) | |||||
| % Change in OAHI | Change in tonsil size | 65.79 (32.37–99.20) | 1.00 | < 0.001 | 0.189 |
| % Change in OAI | None | ||||
| % Change in arousal index | Change in ANR | −317.27 (-593.74-−40.794) | 1.02 | 0.025 | 0.329 |
| % Change in LF power | 0.84 (0.49–1.18) | 1.02 | < 0.001 | ||
| % Change in mean SaO2 | Age at diagnosis | −0.17 (-0.31-−0.03) | 1.05 | 0.020 | 0.296 |
| % Change in N-N interval | −0.05 (-0.08-−0.03) | 1.74 | < 0.001 | ||
| % Change in pNN50 | 0.004 (0.002–0.01) | 1.78 | 0.002 | ||
| % Change in LF power | 0.004 (0.001–0.01) | 1.55 | 0.024 | ||
| % Change in minimal SaO2 | None | ||||
| % Change in N1 sleep | % Change in LF power | 0.55 (0.21–0.88) | 1.00 | 0.002 | 0.138 |
| % Change in N2 sleep | None | ||||
| % Change in N3 sleep | % Change in LF/HF ratio | 0.09 (0.002–0.18) | 1.00 | 0.045 | 0.050 |
| % Change in REM sleep | % Change in VLF power | −0.17 (-0.34-−0.004) | 1.00 | 0.045 | 0.048 |
| % Change in TST | % Change in LF power | 0.17 (0.02–0.32) | 1.00 | 0.031 | 0.060 |
BMI, body mass index; CI, confidence interval; HF, high frequency; LF, low frequency; OAHI, obstructive apnea-hypopnea index; OAI, obstructive apnea index; OSA, obstructive sleep apnea; N-N, normal-to-normal; pNN50, proportion of N-N50 divided by the total number of N-N intervals; REM, rapid eye movement; SaO2, blood oxygen saturation; TST, total sleep time; VIF, variance inflation factor; VLF, very low frequency.
3.7. Mediation and moderation analyses
Consistent relationships between “tonsil size and OAHI” and “change in tonsil size and % change in OAHI” were observed. Mediation and moderation analyses were performed from change in tonsil size to % change in OAHI, especially with regards to HRV indices, and only a significant conceptual serial multiple mediation model was identified: change in tonsil size (independent variable), % change in OSA-18 score (first mediator), % change in VLF power (second mediator), and % change in OAHI (dependent mediator) (Figure 5). The direct paths from change in tonsil size to % change in OAHI, change in tonsil size to % change in OSA-18 score, change in tonsil size to % change in VLF power, change in OSA-18 to % change in VLF power, and % change in VLF power to % change in OAHI were significant. In contrast, the direct paths from change in OSA-18 to % change in OAHI were not significant. The serial mediation model revealed a positive total effect (β = 65.78, standard error = 16.71, P < 0.001). The direct effect of change in tonsil size on % change in OAHI (β = 44.47, standard error = 18.90, P = 0.022) was significant. For the indirect effects, the first path from change in tonsil size to % change in OAHI through % change in OAS-18 score (effect = 12.44, 95% CI:−5.18–32.99) was not significant. The second path through % change in VLF power (effect = 13.74, 95% CI: 0.01–33.36), third path through % change in OAS-18 score and % change in VLF power (effect = −4.87, 95% CI:−13.69-−0.09), and indirect effect (effect = 21.32, 95% CI: 0.39–44.30) were significant.
Figure 5.
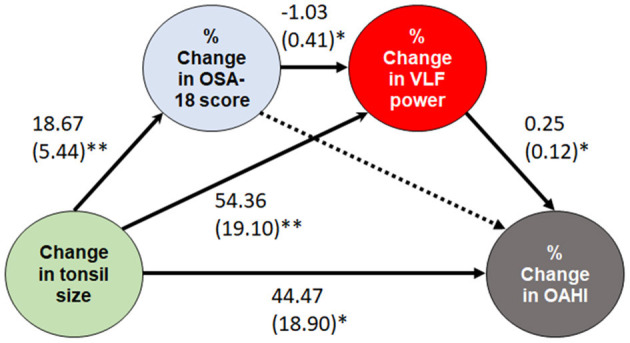
A serial multiple mediation model of the effect of change in tonsil size. Data are summarized as β and standard errors. *P < 0.05 and ≥ 0.01; **P < 0.01 and ≥ 0.001. Solid lines indicate significant paths, while a dotted line indicates a non-significant path. OAHI, obstructive apnea-hypopnea index; OSA, obstructive sleep apnea; VLF, very low frequency.
4. Discussion
This study is the first to report that OSA-related quality of life and VLF power were first and second mediators of the relationship between tonsil size and improvement in AHI using a conceptual serial multiple mediation model. Beyond providing important mechanistic insights, these results suggest that VLF power could be a new target for OSA therapy in children. For example, exercise training can decrease VLF power over time (49) and reduce AHI (50) in adults.
Our results confirmed the reproducibility of sleep HRV measurements at two time points. Most measures showed moderate or substantial reproducibility, except for pNN50 and HF power. The possible reason for this relatively lower reproducibility may be related to sleep stage and arousal index. To the best of our knowledge, no comprehensive reproducibility study has reported HRV measurements in children with OSA. Accordingly, the interpretations of sleep pNN50 and HF power should be made with caution in this population.
Using full-night HRV measurements, SDNN, total power, and VLF power in the children with OSA were significantly higher than normal values (Figure 2) (14, 47, 48). SDNN and total power represent total capacity of the regulation system, whilst VLF power represents sympathetic activity (36) (Table 1). Although SNS and PNS activities both contribute to SDNN and total power, long-term recordings have revealed that SNS activity is more related to these indices (51). The transition between normal and pathological respiration can enhance SNS activity rather than PNS activity in adults with OSA (52). Additionally, the results of this and previous studies (13, 14, 53) suggest that sympathetic activity increases during sleep in children with OSA; however, SDNN in the 12 obese children with OSA in this study was comparable to normal values (47) (Table 3). This discrepancy may be explained by the patients' weight status, since childhood obesity is significantly related to low SDNN (54). Furthermore, this study and Isaiah's study (14) found that % changes in SDNN and total power were not related to % change in OAHI. Therefore, these changes could not be simply due to improvements in OAHI after adenotonsillectomy.
The baseline values and % changes in tonsil size and ANR were not consistently associated with most HRV indices. Despite increased ANR being related to decreased VLF power in children with OSA, the causal relationship between adenoid hypertrophy and reduced sympathetic activity could not be supported by the post-operative changes. Nevertheless, our findings suggested a positive relationship between change in tonsil size and % change in HF power of HRV (parasympathetic modulation). We hypothesize that tonsillectomy may directly injure or cause scar formation, thereby reducing function of the lingual branch of the hypoglossal nerve, interrupting baroreceptor signaling at the carotid sinus, influencing vagus nerve function, eventually resulting in decreased parasympathetic modulation and increased sympathetic activity of cardiac autonomic function during sleep. This condition may further interfere with the relationships between % change in SDNN or total power and % change in OAHI.
Our results demonstrated significant relationships between the change in tonsil size and % change in OAHI as well as relationships between the change in tonsil size and % change in OSA-18 score as previous studies (29, 55). Tonsil size has been significantly associated with the change in OSA-18 score after tonsillectomy in children with sleep-disordered breathing (56). Although a change in AHI has been associated with a change in OSA-18 score (23), we found that this association was not independent in this study. In simple mediation and moderation models, % change in OSA-18 score neither mediated nor moderated the relationship between the change in tonsil size and % change in OAHI. However, in serial mediation analysis, the relationship between the change in tonsil size and % change in OAHI was mediated by % change in OSA-18 score and % change in VLF power in serial analysis, and also by % change in VLF power alone (Figure 5).
VLF rhythm is a cardiac intrinsic rhythm which is essential for health and happiness (36). Even though there is currently no agreement on the physiological mechanisms responsible for activity within the VLF band, low VLF power has been associated with adverse outcomes and all-cause mortality (57, 58). This band is generated by the stimulation of afferent sensory neurons in the heart (59). In animal models, stressful stimulation (60) and paradoxical sleep deprivation (61) have been shown to significantly reduce VLF power (60). In this study, the inverse relationship between % change in OSA-18 and % change in VLF power suggested that reduced OSA-specific stress and sleep disturbance may increase sleep VLF power.
However, VLF power is an independent predictor of AHI in humans (62). VLF power is significantly elevated during pathological respiration compared with normal respiration in OSA patients (52). Therefore, it is reasonable that reduced AHI may contribute to a decrease in VLF power after adenotonsillectomy. Our mediation model also highlighted the possibility that changes in VLF power may influence changes in AHI in children with OSA. Sympathetic abnormalities were shown to precede the development of mild OSA in a cohort of adults with no known diagnosis of OSA (63). Although further direct evidence is warranted, these studies suggest that a reduction in VLF power may help to alleviate the AHI in children with OSA.
Therefore, the mediation role of VLF power on the relationship between change in tonsil size and % change in AHI is of interest. Increasing exercise intensity can reduce awake VLF power (49), and morning exercise can increase sleep VLF power (64) in adults. Besides, exercise training (65) or aerobic exercise combined with resistance training can reduce AHI in adults. Therefore, VLF power is modifiable and may be a marker of therapeutic efficacy and a potential therapeutic target for OSA (66). However, in children with adenotonsillar hypertrophy, it may be unlikely that addressing the HRV independently will improve AHI unless there is a clear demonstration the neuromuscular tone is improved to the point that the tonsils do not medialize during sleep. Accordingly, future studies should focus on VLF power-lowering interventions and their effects on the severity of childhood OSA.
4.1. Strengths and limitations
Compared with previous studies (13, 14, 17, 48), the greatest strengths of this investigation were the inclusion of a sample of representative and well-characterized pediatric OSA patients. Our results provided a preliminary yet comprehensive documentation of the relationships of HRV indices with clinical variables and polysomnographic parameters before and after adenotonsillectomy, which showed some novel and interesting findings. However, limitations should be addressed. First, the HRV results may have been affected by certain psychophysiological changes (e.g., anthropometrics, lifestyle factors, acute or chronic diseases) other than adenotonsillectomy. However, the use of standardized, full-night, in-laboratory protocols with moderate-to-substantial reproducibility in most HRV indices among OSA patients with stable severity reduces this concern. Second, approximately half of our subjects received both adenotonsillectomy and medical treatment, and the heterogeneity of care may have had a confounding effect. Nevertheless, these interdisciplinary treatments are closer to real-world care for OSA, and a greater variability in AHI changes may contribute to better generalizability of this study. Third, 3 months may not be long enough to show cardiovascular changes, and studies with a longer follow-up period are warranted for this young population. Finally, in this study, there was no evidence of direct mediations of HRV on the relationship between adenotonsillectomy and AHI or AHI on the relationship between adenotonsillectomy and HRV indices (14), and this may be due to difficulties in measuring HRV across various sleep stages. Among school-age children, excessive body movements and parasomnia (67) make the researchers need 2-min epochs to analyze HRV and choose sleep periods free of respiratory events and movement artifacts (17). However, frequency-domain measurements, such as VLF power and LF/HF ratio, often require recording periods of at least 5 min (34). Therefore, measuring HRV across various stages in our study population is challenging. Nevertheless, averages may not be sensitive enough to detect sleep stage-specific mediating effects. In future studies, ultra-short-term HRV measurements during different sleep stages should be conducted to accurately assess the impact of nocturnal HRV changes on the management of OSA.
5. Conclusion
In conclusion, we confirmed that analysis of electrocardiographic polysomnography signals is a reliable method to measure HRV over 3 months in children with OSA. Adenotonsillectomy either reduced AHI or sympathetic activity during sleep. Improved OSA-specific quality of life and reduced sleep VLF power serially mediated the relationship between the change in tonsil size and % change in AHI. These findings suggest that HRV measurement may help monitor the sleep quality status and the disease burden of childhood OSA and many other venues. Our preliminary results also support applications of wireless HRV measurements with high-fidelity psychophysiology acquisition using edge computing in the patient's natural sleeping environment to overcome the highly obtrusive effects of visiting the sleep laboratory (68). This technology can potentially be a “platinum standard” of sleep studies instead of the traditional “gold standard” of in-laboratory polysomnography.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Chang Gung Medical Foundation, Taoyuan, Taiwan. Written informed consent for participation was not provided by the participants' legal guardians/next of kin because: the current study was based on a secondary analysis of existing data. We provided a copy of the approval by the Institutional Review Board of Chang Gung Medical Foundation (No. 202200882B0), which approved the waiver of the participants' consent.
Author contributions
L-AL, H-HC, TK, and CY conceptualized and designed the research project. L-AL, H-HC, H-SH, C-YW, TK, and CY interpreted the data. L-AL, H-HC, and H-SH collected the data and wrote the initial manuscript. C-YW, L-PC, H-YL, T-JF, Y-SH, G-SL, AY, TK, and CY contributed to writing and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors would like to thank Dr. I-An Jen (Department of Public Health, National Yang Ming Chiao Tung University, Taipei, Taiwan) for his kind helps in improving the statistical analysis of this stud and Ruo-Chi Wang and Chung-Fang Hsiao (Department of Otorhinolaryngology, Head and Neck Surgery, Chang Gung Memorial Hospital, Linkou Main Branch, Taoyuan, Taiwan) for their technical assistance.
Funding Statement
This study was supported by the National Science and Technology Council, Taiwan (Grant Number 109-2314-B-182-083-MY3 to L-AL) and the Chang Gung Medical Foundation, Taoyuan, Taiwan (Grant Number CMRPG3F1091-3 to L-AL).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. (2017) 34:70–81. 10.1016/j.smrv.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 2.Chuang HH, Hsu JF, Wang CY, Chuang LP, Chen MC, Chen NH, Huang YS, Li HY, Lee LA. Hypertension in children with obstructive sleep apnea syndrome-age, weight status, and disease severity. Int J Environ Res Public Health. (2021) 18:9602. 10.3390/ijerph18189602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang YS, Guilleminault C, Li HY, Yang CM, Wu YY, Chen NH. Attention-deficit/hyperactivity disorder with obstructive sleep apnea: a treatment outcome study. Sleep Med. (2007) 8:18–30. 10.1016/j.sleep.2006.05.016 [DOI] [PubMed] [Google Scholar]
- 4.Howarth TP, Gentin N, Reyes-Chicuellar N, Jonas C, Williamson B, Blecher G, et al. Sleep quality and obstructive sleep apnoea in Indigenous and non-Indigenous Australian children. Sleep Med. (2022) 98:68–78. 10.1016/j.sleep.2022.06.014 [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Tian L, Ma D, Wu P, Tang Y, Cui X, Xu Z. Autonomic nervous function and low-grade inflammation in children with sleep-disordered breathing. Pediatr Res. (2021) 91:1834–40. 10.1038/s41390-021-01691-4 [DOI] [PubMed] [Google Scholar]
- 6.Hayano J, Yuda E. Pitfalls of assessment of autonomic function by heart rate variability. J Physiol Anthropol. (2019) 38:3. 10.1186/s40101-019-0193-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drury RL, Porges S, Thayer J, Ginsberg JP. Editorial: Heart rate variability, health and well-being: a systems perspective. Front Public Health. (2019) 7:323. 10.3389/fpubh.2019.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siecinski S, Kostka PS, Tkacz EJ. Heart rate variability analysis on electrocardiograms, seismocardiograms and gyrocardiograms on healthy volunteers. Sensors (Basel). (2020) 20:4522. 10.3390/s20164522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo X, Su T, Xiao H, Xiao R, Xiao Z. Using 24-h heart rate variability to investigate the sleep quality and depression symptoms of medical students. Front Psychiatry. (2021) 12:781673. 10.3389/fpsyt.2021.781673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Driscoll DM, Horne RS, Davey MJ, Hope SA, Anderson V, Trinder J, et al. Increased sympathetic activity in children with obstructive sleep apnea: cardiovascular implications. Sleep Med. (2011) 12:483–8. 10.1016/j.sleep.2010.09.015 [DOI] [PubMed] [Google Scholar]
- 11.Lopes MC, Spruyt K, Azevedo-Soster L, Rosa A, Guilleminault C. Reduction in parasympathetic tone during sleep in children with habitual snoring. Front Neurosci. (2019) 12:997. 10.3389/fnins.2018.00997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li HY, Lee LA. Sleep-disordered breathing in children. Chang Gung Med J. (2009) 32:247–57. [PubMed] [Google Scholar]
- 13.Nisbet LC, Yiallourou SR, Nixon GM, Biggs SN, Davey MJ, Trinder J, et al. Nocturnal autonomic function in preschool children with sleep-disordered breathing. Sleep Med. (2013) 14:1310–6. 10.1016/j.sleep.2013.07.010 [DOI] [PubMed] [Google Scholar]
- 14.Isaiah A, Bertoni D, Pereira KD, Diaz-Abad M, Mitchell RB, Das G. Treatment-related changes in heart rate variability in children with sleep apnea. Otolaryngol Head Neck Surg. (2020) 162:737–45. 10.1177/0194599820907882 [DOI] [PubMed] [Google Scholar]
- 15.Chuang HH, Hsu JF, Chuang LP, Chen NH, Huang YS, Li HY, et al. Differences in anthropometric and clinical features among preschoolers, school-age children, and adolescents with obstructive sleep apnea-a hospital-based study in Taiwan. Int J Environ Res Public Health. (2020) 17:13. 10.3390/ijerph17134663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. (2012) 130:e714–55. 10.1542/peds.2012-1671 [DOI] [PubMed] [Google Scholar]
- 17.Muzumdar HV, Sin S, Nikova M, Gates G, Kim D, Arens R. Changes in heart rate variability after adenotonsillectomy in children with obstructive sleep apnea. Chest. (2011) 139:1050–9. 10.1378/chest.10-1555 [DOI] [PubMed] [Google Scholar]
- 18.Todd CA, Bareiss AK, McCoul ED, Rodriguez KH. Adenotonsillectomy for obstructive sleep apnea and quality of life: systematic review and meta-analysis. Otolaryngol Head Neck Surg. (2017) 157:767–73. 10.1177/0194599817717480 [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, Mitchell RB, Promchiarak J, Simakajornboon N, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. (2010) 182:676–83. 10.1164/rccm.200912-1930OC [DOI] [PubMed] [Google Scholar]
- 20.Mathew G, Agha R, STROCSS Group S. 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Ann Med Surg (Lond). (2021) 72:103026. 10.1016/j.amsu.2021.103026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcus CL, Moore RH, Rosen CL, Giordani B, Garetz SL, Taylor HG, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. (2013) 368:2366–76. 10.1056/NEJMoa1215881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuang HH, Huang CG, Chuang LP, Huang YS, Chen NH Li HY, et al. Relationships among and predictive values of obesity, inflammation markers, and disease severity in pediatric patients with obstructive sleep apnea before and after adenotonsillectomy. J Clin Med. (2020) 9:579. 10.3390/jcm9020579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang YS, Guilleminault C, Lee LA, Lin CH, Hwang FM. Treatment outcomes of adenotonsillectomy for children with obstructive sleep apnea: a prospective longitudinal study. Sleep. (2014) 37:71–6. 10.5665/sleep.3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chuang HH, Hsu JF, Chuang LP, Chiu CH, Huang YL, Li HY, et al. Different associations between tonsil microbiome, chronic tonsillitis, and intermittent hypoxemia among obstructive sleep apnea children of different weight status: a pilot case-control Study. J Pers Med. (2021) 11:486. 10.3390/jpm11060486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee LA Li HY, Lin YS, Fang TJ, Huang YS, Hsu JF, et al. Severity of childhood obstructive sleep apnea and hypertension improved after adenotonsillectomy. Otolaryngol Head Neck Surg. (2015) 152:553–60. 10.1177/0194599814561203 [DOI] [PubMed] [Google Scholar]
- 26.Ostchega Y, Hughes JP, Prineas RJ, Zhang G, Nwankwo T, Chiappa MM. Mid-arm circumference and recommended blood pressure cuffs for children and adolescents aged between 3 and 19 years: data from the National Health and Nutrition Examination Survey, 1999-2010. Blood Press Monit. (2014) 19:26–31. 10.1097/MBP.0000000000000008 [DOI] [PubMed] [Google Scholar]
- 27.Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin North Am. (1989) 36:1551–69. 10.1016/S0031-3955(16)36806-7 [DOI] [PubMed] [Google Scholar]
- 28.Fujioka M, Young LW, Girdany BR. Radiographic evaluation of adenoidal size in children: adenoidal-nasopharyngeal ratio. AJR Am J Roentgenol. (1979) 133:401–4. 10.2214/ajr.133.3.401 [DOI] [PubMed] [Google Scholar]
- 29.Huang Y-S, Hwang F-M, Lin C-H, Lee L-A, Huang P-Y, Chiu S-T. Clinical manifestations of pediatric obstructive sleep apnea syndrome: clinical utility of the chinese-version obstructive sleep apnea questionaire-18. Psychiatry Clin Neurosci. (2015) 69:752–62. 10.1111/pcn.12331 [DOI] [PubMed] [Google Scholar]
- 30.Franco RA, Rosenfeld RM, Rao M. Quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg. (2000) 123:9–16. 10.1067/mhn.2000.105254 [DOI] [PubMed] [Google Scholar]
- 31.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the (2007). AASM manual for the scoring of sleep and associated events deliberations of the sleep apnea definitions task force of the american academy of sleep medicine. J Clin Sleep Med. (2012) 8:597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Littmann L. Electrocardiographic artifact. J Electrocardiol. (2021) 64:23–9. 10.1016/j.jelectrocard.2020.11.006 [DOI] [PubMed] [Google Scholar]
- 33.Magalang UJ, Arnardottir ES, Chen NH, Cistulli PA, Gislason T, Lim D, et al. Agreement in the scoring of respiratory events among international sleep centers for home sleep testing. J Clin Sleep Med. (2016) 12:71–7. 10.5664/jcsm.5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. (2017) 5:258. 10.3389/fpubh.2017.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. (1998) 31:593–601. 10.1016/S0735-1097(97)00554-8 [DOI] [PubMed] [Google Scholar]
- 36.Shaffer F, McCraty R, Zerr CL, A. healthy heart is not a metronome: an integrative review of the heart's anatomy and heart rate variability. Front Psychol. (2014) 5:1040. 10.3389/fpsyg.2014.01040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein DS, Bentho O, Park MY, Sharabi Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol. (2011) 96:1255–61. 10.1113/expphysiol.2010.056259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuo TB, Lai CJ, Shaw FZ, Lai CW, Yang CC. Sleep-related sympathovagal imbalance in SHR. Am J Physiol Heart Circ Physiol. (2004) 286:H1170–6. 10.1152/ajpheart.00418.2003 [DOI] [PubMed] [Google Scholar]
- 39.Kuo TB, Chen CY, Hsu YC, Yang CC. EEG beta power and heart rate variability describe the association between cortical and autonomic arousals across sleep. Auton Neurosci. (2016) 194:32–7. 10.1016/j.autneu.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 40.Orntoft M, Andersen IG, Homoe P. Night-to-night variability in respiratory parameters in children and adolescents examined for obstructive sleep apnea. Int J Pediatr Otorhinolaryngol. (2020) 137:110206. 10.1016/j.ijporl.2020.110206 [DOI] [PubMed] [Google Scholar]
- 41.Wang SH, Keenan BT, Wiemken A, Zang Y, Staley B, Sarwer DB, et al. Effect of weight loss on upper airway anatomy and the apnea-hypopnea Index. The importance of tongue fat .Am J Respir Crit Care Med. (2020) 201:718–27. 10.1164/rccm.201903-0692OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi J, Luo D, Weng H, Zeng XT, Lin L, Chu H, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. (2020) 11:641–54. 10.1002/jrsm.1429 [DOI] [PubMed] [Google Scholar]
- 44.Templeton GF. A two-step approach for transforming continuous variables to normal: Implications and recommendations for IS research. Commun Assoc Inf Syst. (2011). 28:41–58. 10.17705/1CAIS.02804 [DOI] [Google Scholar]
- 45.Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography. (2013) 36:27–46. 10.1111/j.1600-0587.2012.07348.x [DOI] [Google Scholar]
- 46.Hayes AF. Introduction to mediation, moderation and conditional process analysis: a regression-based approach. Third ed New York: Guilford Press. (2022). [Google Scholar]
- 47.Gasior JS, Sacha J, Pawlowski M, Zielinski J, Jelen PJ, Tomik A, et al. Normative values for heart rate variability parameters in school-aged children: simple approach considering differences in average heart rate. Front Physiol. (2018) 9:1495. 10.3389/fphys.2018.01495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirk VG, Edgell H, Joshi H, Constantin E, Katz SL, MacLean JE. Cardiovascular changes in children with obstructive sleep apnea and obesity after treatment with noninvasive ventilation. J Clin Sleep Med. (2020) 16:2063–71. 10.5664/jcsm.8760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hunt KJ, Saengsuwan J. Changes in heart rate variability with respect to exercise intensity and time during treadmill running. Biomed Eng Online. (2018) 17:128. 10.1186/s12938-018-0561-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng J, Yuan Y, Zhao Y, Ren H. Effects of exercise on patients with obstructive sleep apnea: A systematic review and meta-analysis. Int J Environ Res Public Health. (2022) 19:10845. 10.3390/ijerph191710845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grant CC, van Rensburg DC, Strydom N, Viljoen M. Importance of tachogram length and period of recording during noninvasive investigation of the autonomic nervous system. Ann Noninvasive Electrocardiol. (2011) 16:131–9. 10.1111/j.1542-474X.2011.00422.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang J, Zhang X, Luo Y, Wang T, Sun L, Huang S. The impact of respiratory events on the autonomic nervous system during sleep. Int Heart J. (2018) 59:378–86. 10.1536/ihj.16-429 [DOI] [PubMed] [Google Scholar]
- 53.Baharav A, Kotagal S, Rubin BK, Pratt J, Akselrod S. Autonomic cardiovascular control in children with obstructive sleep apnea. Clin Auton Res. (1999) 9:345–51. 10.1007/BF02318382 [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Colon SM, Bixler EO Li X, Vgontzas AN, Liao D. Obesity is associated with impaired cardiac autonomic modulation in children. Int J Pediatr Obes. (2011) 6:128–34. 10.3109/17477166.2010.490265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee CH, Kang KT, Weng WC, Lee PL, Hsu WC. Quality of life after adenotonsillectomy for children with sleep-disordered breathing: a linear mixed model analysis. Int J Pediatr Otorhinolaryngol. (2014) 78:1374–80. 10.1016/j.ijporl.2014.05.038 [DOI] [PubMed] [Google Scholar]
- 56.Sohn H, Rosenfeld RM. Evaluation of sleep-disordered breathing in children. Otolaryngol Head Neck Surg. (2003) 128:344–52. 10.1067/mhn.2003.4 [DOI] [PubMed] [Google Scholar]
- 57.Schmidt H, Muller-Werdan U, Hoffmann T, Francis DP, Piepoli MF, Rauchhaus M, et al. Autonomic dysfunction predicts mortality in patients with multiple organ dysfunction syndrome of different age groups. Crit Care Med. (2005) 33:1994–2002. 10.1097/01.CCM.0000178181.91250.99 [DOI] [PubMed] [Google Scholar]
- 58.Endoh H, Kamimura N, Honda H, Nitta M. Early prognostication of neurological outcome by heart rate variability in adult patients with out-of-hospital sudden cardiac arrest. Crit Care. (2019) 23:323. 10.1186/s13054-019-2603-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Armour JA. Neurocardiology: Anatomical and Functional Principles. Boulder Creek, CA: Institute of HeartMath. (2003). [Google Scholar]
- 60.Zhukova EV. Teplyi DL. Effect of noradrenergic neurotoxin DSP-4 and maprotiline on heart rate spectral components in stressed and resting rats. Bull Exp Biol Med. (2017) 163:302–6. 10.1007/s10517-017-3790-2 [DOI] [PubMed] [Google Scholar]
- 61.Liu YP, Lin CC, Lin YC, Tsai SH, Tung CS. Effects of sinoaortic denervation on hemodynamic perturbations of prolonged paradoxical sleep deprivation and rapid cold stress in rats. J Integr Neurosci. (2022) 21:75. 10.31083/j.jin2103075 [DOI] [PubMed] [Google Scholar]
- 62.Gong X, Huang L, Liu X, Li C, Mao X, Liu W, et al. Correlation analysis between polysomnography diagnostic indices and heart rate variability parameters among patients with obstructive sleep apnea hypopnea syndrome. PLoS ONE. (2016) 11:e0156628. 10.1371/journal.pone.0156628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woodson BT, Brusky LT, Saurajen A, Jaradeh S. Association of autonomic dysfunction and mild obstructive sleep apnea. Otolaryngology–Head and Neck Surgery. (2016) 130:643–8. 10.1016/S0194-5998(03)01318-4 [DOI] [PubMed] [Google Scholar]
- 64.Yamanaka Y, Hashimoto S, Takasu NN, Tanahashi Y, Nishide SY, Honma S, et al. Morning and evening physical exercise differentially regulate the autonomic nervous system during nocturnal sleep in humans. Am J Physiol Regul. (2015) 309:R1112–21. 10.1152/ajpregu.00127.2015 [DOI] [PubMed] [Google Scholar]
- 65.Aiello KD, Caughey WG, Nelluri B, Sharma A, Mookadam F, Mookadam M. Effect of exercise training on sleep apnea: a systematic review and meta-analysis. Respir Med. (2016) 116:85–92. 10.1016/j.rmed.2016.05.015 [DOI] [PubMed] [Google Scholar]
- 66.Noda A, Hayano J, Ito N, Miyata S, Yasuma F, Yasuda Y. Very low frequency component of heart rate variability as a marker for therapeutic efficacy in patients with obstructive sleep apnea: preliminary study. J Res Med Sci. (2019) 24:84. 10.4103/jrms.JRMS_62_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kahn A, Dan B, Groswasser J, Franco P, Sottiaux M. Normal sleep architecture in infants and children. J Clin Neurophysiol. (1996) 13:184–97. 10.1097/00004691-199605000-00002 [DOI] [PubMed] [Google Scholar]
- 68.Drury RL. Geolocated wireless heart rate variability sentinel surveillance in immunological assessment, intervention and research concerning COVID-19 and other pandemic threats. Med Res Arch. (2022) 10:9. 10.18103/mra.v10i9.3021 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.