Abstract
5-aminolevulinic acid (ALA) is a clinically approved prodrug involved in intracellular Heme biosynthesis to produce the natural photosensitizer (PS) Protoporphyrin IX (PpIX). ALA based photodynamic therapy (PDT) has been used to treat various malignant and non-malignant diseases. However, natural ALA has disadvantages such as weak lipophilicity, low stability and poor bioavailability, greatly reducing its clinical performance. The emerging nanotechnology is expected to address these limitations and thus improve the therapeutic outcomes. Herein, we summarized important recent advances in the design of ALA-based prodrugs using nanotechnology to improve the efficacy of PDT. The potential limitations and future perspectives of ALA-based nanomedicines are also briefly presented and discussed.
Keywords: 5-aminolevulinic acid, biotransformation, photodynamic therapy, prodrug, nanotechnology
1 Introduction
Photodynamic therapy (PDT) is a clinically approved modality involving the combination of a photosensitizer (PS) and molecule oxygen to produce cytotoxic reactive oxygen species (ROSs), primarily singlet oxygen (1O2), thereby inducing cell death (Xiao et al., 2018; Li Q et al., 2020). The unique mechanism of action makes PDT safe, efficient, reproducible and minimally invasive. The inherent fluorescent features of PSs endow them with capabilities in fluorescence-guided surgery and diseases diagnosis (Ji et al., 2021; Xiang et al., 2021). Besides, with rich experience in anti-tumor PDT, a large number of researchers have applied PDT to fight against microorganisms, especially drug-resistant bacteria (Liu et al., 2016; Jia et al., 2019; Xiao et al., 2020; Xiao et al., 2021a).
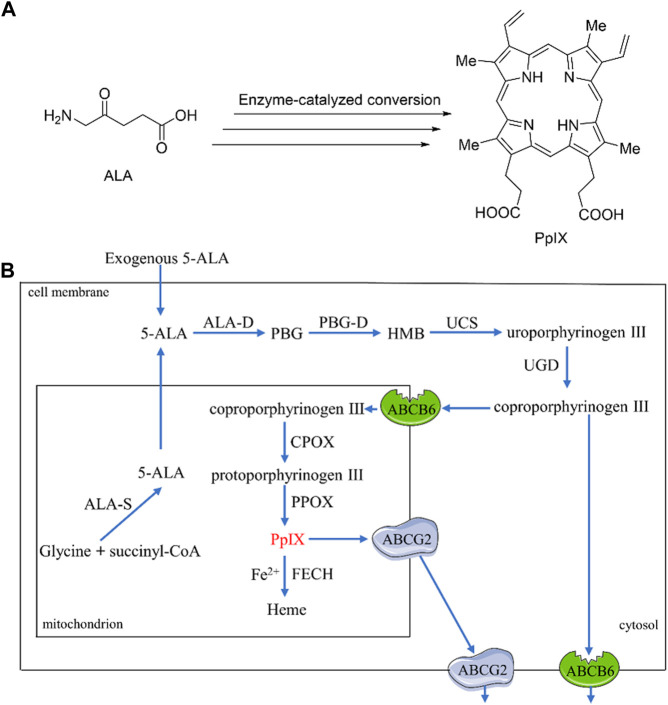
5-aminolevulinic acid (ALA) is a clinically approved prodrug of natural PS protoporphyrin IX (PpIX) (Figure 1A). Upon systematical or topical administration, ALA molecules penetrate into cells, where they participate in the biosynthesis of Heme and produce PpIX (Figure 1B). Briefly, the conversion involves a total of eight steps catalyzed by eight enzymes, of which four steps occur in the mitochondria and another four steps occur in the cytosol (Ponka, 1999). Firstly, ALA presented in the cytosol, including exogenous and endogenous molecules, are condensed to generate porphobilinogen (PBG) under catalysis by ALA dehydratase (ALA-D). Next, four units of PBG via mediation by porphobilogen deaminase (PBG-D) are connected to form tetrapyrrole intermediate hydroxymethylbilane (HMB), which is then cyclized to provide uroporphyrinogen III upon activation by uroporphyrinogen III cosynthase (UCS). The resulting uroporphyrinogen III is converted to coproporphyrinogen III through decarboxylation by uroporphyrinogen decarboxylase (UGD), and then migrates to mitochondria, where it is oxidized to protoporphyrinogen III by coproporphyrinogen III oxidase (CPOX). Finally, protoporphyrinogen III is oxidized by protoporphyrinogen III oxidase (PPOX) to produce PpIX. Further conversion of PpIX to the downstream substrate of Heme requires the participation of Fe2+ and a rate-limiting enzyme ferrochelatase (FECH). The endogenous ALA molecules are generated in mitochondria from succinyl-CoA and glycine mediated by ALA synthase (ALA-S). Collectively, the intracellular PpIX content under normal physiological condition is tightly regulated by the negative feedback of Heme itself. Nevertheless, external administration of ALA can make cells produce abundant PpIX, which cannot be rapidly converted into Heme by FECH, thereby accumulating within cells and making ALA one of the most successful prodrugs in cancer treatment (Vallejo et al., 2021).
FIGURE 1.
(A) Structures of ALA and PpIX; (B) A simplified mechanism of enzyme-catalyzed bio-transformation from ALA to PpIX.
ALA-based PDT has been widely used to treat a variety of neoplastic and non-neoplastic diseases, including bladder cancer, basal cell carcinoma, head and neck cancer, and skin disorders such as actinic keratosis, psoriasis, acne and Bowen’s disease (Allison and Sibata, 2010; Xiao et al., 2018). ALA-assisted fluorescence diagnosis has been used for visualization of bladder, lung and skin cancers (Peng et al., 1997). The clinically available ALA and its derivatives (e.g., methyl ester derivative, MAL; hexyl ester derivative, HAL) and their associated indications are summarized in Table 1.
TABLE 1.
Clinically used ALA and its derivatives in PDT.
| Compound | Trade name | Indications | Approved countries | Ref(s) |
|---|---|---|---|---|
| ALA | Levulan® or Ameluz® | Actinic keratosis, Human Papilloma Virus, head and neck cancer, etc. | United States, Canada, Europe, Japan, China, etc. | Jeffes (2002), Gupta et al. (2017) |
| Gliolan® | Detection of malignant glioma, etc. | Europe, Canada, Japan, etc. | Krammer and Plaetzer (2008) | |
| MAL | Metvix® | Actinic keratosis, Basal cell carcinoma, Bowen’s disease, etc. | United States, Canada, Europe, Australia, etc. | Gardlo and Ruzicka (2002) |
| HAL | Hexvic® or Cysview® | Detection of recurrent bladder cancer, etc. | United States, Europea, New Zealand, etc. | Compérat et al. (2011), Pietzak (2017) |
Increasing the drug concentration in the target tissue is beneficial to improve the therapeutic efficacy. The intracellular biosynthesis of PpIX is mediated by multiple enzymes and several important membrane transporters, including ATP-binding cassette sub-family B member 6 (ABCB6) and ATP-binding cassette sub-family G member 2 (ABCG2) (Yamamoto et al., 2020) (Figure 1B). ABCB6 present on the mitochondrial outer membrane are involved in transporting coproporphyrinogen III into mitochondria (Krishnamurthy et al., 2006), whilst those present in the cell membrane (Paterson et al., 2007) can transport coproporphyrinogen III out of the cells (Matsumoto et al., 2015). The net effect of ABCB6 on PpIX level is thus depending on the relative activity of ABCB6 in mitochondria and cell membrane. Besides, ABCG2, an efflux transporter present in mitochondria as well as cell membranes, is able to induce extracellular transport of PpIX synthesized in mitochondria (Kobuchi et al., 2012). Therefore, to improve the accumulation of PpIX in cells can generally be achieved by 1) enhancing the activities of CPOX by using upregulated molecules such as methotrexate (MTX) and vitamin D (Ortel et al., 2002; Sinha et al., 2006); 2) using Fe2+ chelators such as 1,2-diethyl-3-hydroxypyridin-4-one hydrochloride (CP94) or deferoxamine (DFO) to lower the conversion of PpIX to Heme (Richardson et al., 1994; Pye and Curnow, 2007); and 3) using ABCG2 inhibitors such as Fumitremorgin C (Robey et al., 2005), Ko143 (Palasuberniam et al., 2015), sunitinib (Mansi et al., 2022) or gefitinib (Palasuberniam et al., 2021) to reduce PpIX efflux.
However, the methods descried above are not cell-selective in accumulation of PpIX, and thus cannot distinguish tumor cells from normal cells. The inherent limitations of natural ALA such as weak lipophilicity, low stability and poor bioavailability, greatly reduce its clinical efficiency. The recently emerged nanotechnology provides new strategies to address these deficiencies, because nanocarriers-assisted drug delivery affords outstanding tumor selectivity, well-controlled drug release and good biocompatibility (Sun et al., 2020; Ji et al., 2022). Notably, nanomedicines are able to improve the therapeutic efficacy by combining multiple therapies into one platform (Ding et al., 2019). In this work, we summarized the important recent advances and strategies for designing ALA-based prodrugs using nanotechnology for enhanced PDT. The potential limitations and future perspectives of ALA-based nanomedicine are also briefly described and discussed.
2 ALA-based nanoprodrugs using nanotechnology
2.1 Stimuli-responsive ALA nanoprodrugs
Stimuli-responsive nanomedicines are agents that can recognize the microenvironment in living systems and release therapeutic molecules to the target tissues without affecting normal areas (Mura et al., 2013; Zhang et al., 2022). Such drug delivery systems are capable of controlling drug distribution, reducing potential side effects and enhancing the therapeutic efficacy. The responsive signals include endogenous stimuli such as low pH, increased glutathione (GSH) concentration and enhanced expression of certain enzymes, as well as exogenous stimuli such as light, temperature, ultrasound, and chemicals.
2.1.1 pH responsive systems
pH values in tumor tissues are usually 0.5–1.0 units lower than those in healthy tissues due to glucose consumption and lactic acid accumulation caused by rapid cell proliferation (Volk et al., 1993; Pang et al., 2016). The difference in pH is thus applied as an ideal trigger for selectively releasing therapeutics in tumor tissues and/or cells. To date, numerous of drug delivery systems with pH-sensitivity have been investigated, including nanoparticles (Gao et al., 2010), nanogels (Li et al., 2021), nanoclusters (Lin et al., 2021), liposomes (Barattin et al., 2018), and micelles (Liao et al., 2018).
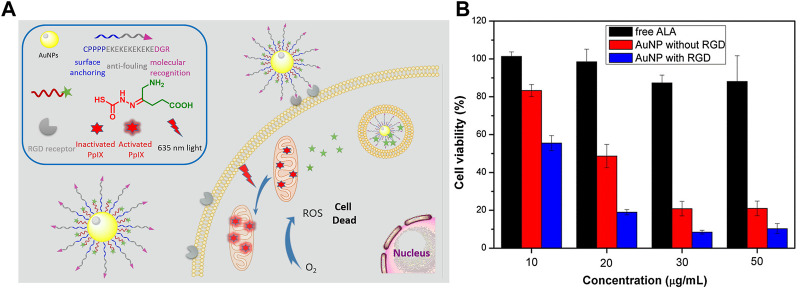
Gold nanoparticles (AuNPs) are a conventional type of inorganic nanoparticles used in drug delivery and biomedical imaging due to their outstanding drug loading and unique optical properties (Sperling et al., 2008; Cheng et al., 2013). The phase I clinical trials using AuNP to deliver tumor necrosis factor (TNF) have been successfully completed and no significant systematic toxicity using AuNPs was observed in animal model (Libutti et al., 2010). In 2017, Wu and coworkers reported pH-responsive zwitterionic stealth peptide-capped ALA prodrug nanoparticles for targeted PDT (Wu et al., 2017a). The nanoprodrugs were prepared by using thiolated stealth peptide sequence CPPPPEKEKEKEKEKEDGR and hydrazine-containing ALA to couple with AuNPs. The zwitterionic stealth peptide sequence EKEKEKEKEK endows nanoparticles with strong anti-fouling ability, and the RGD portion in peptide targets the RGD receptors overexpressed on the surface of cancer cells, thereby resulting in enhanced cellular uptake. The nanoparticles internalized by tumor cells release ALA in an acidic environment due to the presence of hydrazone bonds, thereby generating PpIX via a biosynthetic pathway (Figure 2A). The in vitro study against A549 tumor cells showed that the pH-responsive prodrug nanoparticles displayed better phototoxicity than free ALA after light irradiation (Figure 2B), suggesting that such pH-responsive ALA-loaded AuNPs have enhanced PDT effects.
FIGURE 2.
(A) Design and mechanism of action for peptide-modified AuNPs-loaded with pH-responsive ALA; (B) Cell viabilities treated with free ALA and AuNPs with or without RGD peptide under irradiation with 635 nm light. Adapted from ref. (Wu et al., 2017a). Copyright 2016 Elsevier Inc.
Upconverting nanoparticles (UCNPs), characterized by the upconversion of lower-energy photons into high-energy photons and thereby affording deep tissue penetration and low autofluorescence, have attracted considerable attention in biomedical applications including drug delivery (Chen et al., 2014; Pickel et al., 2018). In 2014, Punjabi et al developed a class of biocompatible UCNPs with largely amplified red emissions by dopping high percentage of Yb into the core and coating the outer shell with biocompatible CaF2. These UCNPs were next conjugated with pH-responsive ALA linkages to provide ALA-conjugated UCNPs (Punjabi et al., 2014). The developed UCNPs displayed 15-fold stronger in red-emission than that of the hexagonal phased counterparts, and demonstrated significant PDT effects for the treatment of deep-set tumors (>1.2 cm). Covalent binding of ALA to UCNPs via hydrazone bond is able to avoid possible leakage of ALA, thus increasing ALA bioavailability and enhancing singlet oxygen generation from PpIX produced by the internalized ALA.
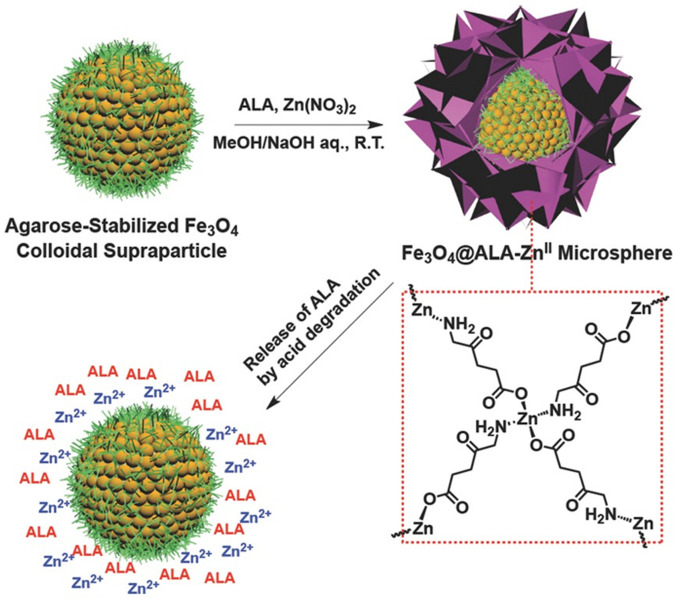
In 2015, Tan and coworkers reported core/shell-structured nanoparticles (Fe3O4@ALA-ZnII) with agarose-stabilized magnetite colloidal supraparticles (MCSPs) as cores and 5-ALA-ZnII coordination polymers as shells for PDT treatment of bladder cancer (Tan et al., 2015). The coordination polymers formed between Zn ions and the terminal NH2 and COOH of ALA was subsequently deposited on the surface of MNCPs to produce core/shell structure of Fe3O4@ALA-ZnII (Figure 3). At normal physiological pH, ALA molecules are stabilized on the shell of MCSPs whilst being released from the dynamic coordination bonds due to the acidic microenvironment of tumors. In addition to the cell inhibition by ALA-induced PDT, Zn ions also caused additional suppression due to their ability to induce cell apoptosis.
FIGURE 3.
Schematic illustration of the preparation of Fe3O4 @ALA-ZnII microsphere and pH-induced release of ALA. Adapted from ref. (Tan et al., 2015). Copyright 2015 WILEY-VCH Verlag GmbH & Co., KGaA, Weinheim.
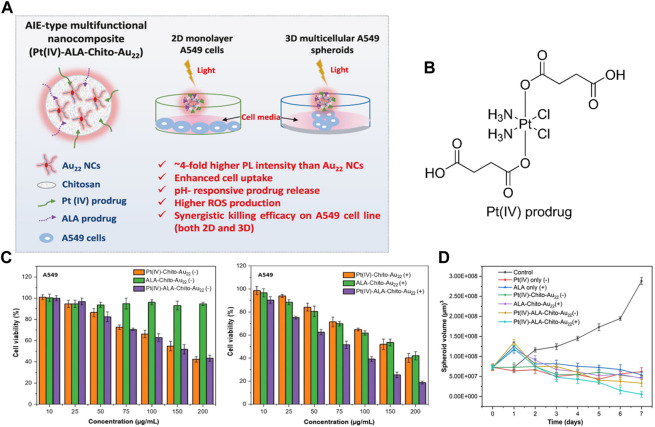
Chemo-photodynamic combined therapy is increasingly investigated in improved anticancer therapy as it can overcome tumor recurrence and multidrug resistance (Guo et al., 2018; Liu et al., 2021). In 2021, Srinivasulu et al. reported a multifunctional nanocomposite consist of Gold nanocluster (Au22), chitosan, chemotherapeutic platinum (Pt(IV)) prodrug and photodynamic ALA prodrug, denoted as Pt(IV)-ALA-Chito-Au22 (Srinivasulu et al., 2021). Pt(IV) and ALA prodrugs were coated on the amino-rich Au22-Chito nanocarriers via amide bonds and subsequently released in the acidic microenvironment of tumor cells (Figures 4A, B). This nanocluster-based prodrug system demonstrated good stability, enhanced cellular uptake and ROS generation. At acidic pH around 5.0, the cumulative drug release was more than 50% within 12 h. Especially, enhanced cell killing effects were observed with the dual nanoprodrug of Pt(IV)-ALA-Chito-Au22 than any single prodrug that conjugated with Chito-Au22 (Figures 4C, D). This study suggests that combining two different prodrugs into one single nanocarrier affords an alternative strategy for enhanced cancer treatment.
FIGURE 4.
(A) Design of dual nanoprodrug Pt(IV)-ALA-Chito-Au22; (B) Molecular structure of the Pt(IV) prodrug; (C) Cell viability of A549 cells after treatment with Pt(IV)-Chito-Au22, ALA-Chito-Au22 or Pt(IV)-ALA-Chito-Au22 without or with light exposure; (D) Quantification of 3D A549 spheroid volume change in different treatment groups over 7 days. Adapted from ref. (Srinivasulu et al., 2021). Copyright 2021 American Chemical Society.
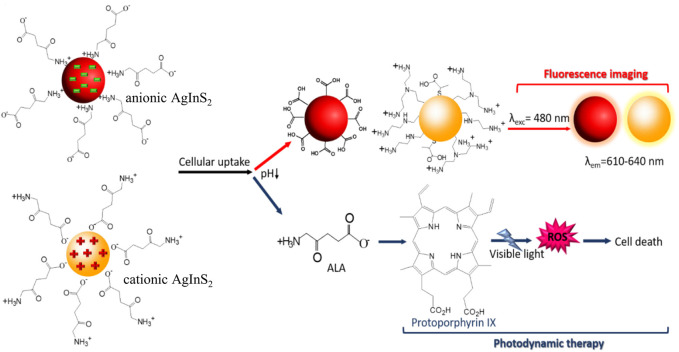
Quantum dots (QDs) are important semiconductor nanomaterials with fascinating optical properties and quantum confinement effects, making them potential candidates for nanoprobes and nanocarriers in biomedical applications, especially for the early detection, monitoring and treatment of certain localized diseases (Ruzycka-Ayoush et al., 2021). Recently, Hashemkhani and coworkers reported one-step aqueous synthesis of cationic and anionic silver-indium-sulfide quantum dots (AIS QDs), which were subsequently loaded with ALA through electrostatic interaction for enhanced PDT (Hashemkhani et al., 2022). The obtained AIS QDs demonstrated high quantum yields, long-term stability and excellent fluorescence imaging ability under light illumination, and thus can be used for efficient cell/tissue visualization. Importantly, these ALA-loaded AIS QDs showed sustained ALA release in the acid tumor microenvironment while protecting ALA at physiological pH, thereby achieving selective tumor destruction through intracellular bioconversion into PpIX (Figure 5). Although the delivery efficiency of cationic and anionic AIS-QDs was comparable, the anionic AIS-QDs appeared to be safer due to their relatively lower cytotoxicity.
FIGURE 5.
Schematic showing the design and responsive mechanism of ALA-loaded anionic and cationic AIS QDs. Adapted from ref. (Hashemkhani et al., 2022). Copyright 2022 American Chemical Society.
Besides, Tong and coworkers reported dual pH-responsive ALA pseudopolyrotaxane prodrug micelles for improved PDT (Tong et al., 2016). The pH-responsive ALA fragment was prepared by conjugating ALA to α-cyclodextrin (α-CD) via an acid-sensitive hydrazone linkage, and the acid-sensitive PEG fragment was prepared by connecting PEG to pH-responsive CPP R6H4 (RRRRRRHHHH), thereby affording the dual pH-responsive prodrug micelles through host guest interactions between α-CD and PEG fragments. The introduction of pH-sensitive CPPs can enhance the cellular uptake of micelles in an acid tumor microenvironment. Such dual-pH responsive ALA prodrug micelles thus afford improved selectivity and new opportunities for future ALA-based photodynamic antitumor therapy.
2.1.2 GSH-responsive systems
It is believed that the intracellular concentration of GSH in tumor cells is higher than that in the extracellular matrix and normal cells, and GSH-responsive prodrugs are usually designed to selectively deliver therapeutic molecules into the cytosol of tumor cells (Xiao et al., 2021b; Chen et al., 2021). Generally, GSH-mediated drug delivery can be achieved by directly introducing disulfide (S-S) bonds into the therapeutic molecules or indirectly preparing disulfide-containing carriers to encapsulate therapeutic molecules.
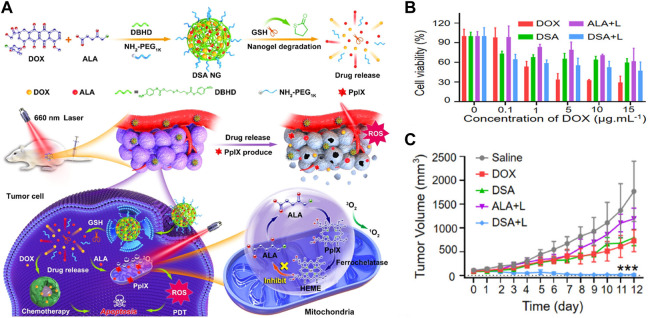
In 2020, Wang and coworkers reported GSH-responsive nanogels for chemo-photodynamic combined anticancer therapy (Wang et al., 2020a). Upon intermarriage with reduction-responsive cross-linker DBHD and hydrophilic chain NH2-PEG1k, ALA, and Doxorubicin (DOX) are cross-linked to form reduction-responsive nanogels (DSA NG), which has good stability, high drug loading and GSH-responsive properties (Figure 6A ). The internalized DSA NG release free ALA and DOX in response to high GSH concentration in tumor cells, thus inducing synergistic antitumor effects under red light irradiation (Figures 6B, C). The in vivo study in 4T1 tumor-bearing mice showed that DSA NG almost completely eradicated tumors (Figure 6C), suggesting GSH-sensitive nanogel might be a promising drug delivery platform to improve tumor therapy. Furthermore, this GSH-responsive prodrug strategy was also applied in chemo-immunotherapy, showing a robust antitumor immune response upon GSH activation (Ma et al., 2022).
FIGURE 6.
(A) Design and mechanism of GSH-responsive DSA NG; (B) Cell viability against 4T1 cells under different treatments; (C) Relative tumor volume over 12 days. Adapted from ref. (Wang et al., 2020b). Copyright 2020 American Chemical Society.
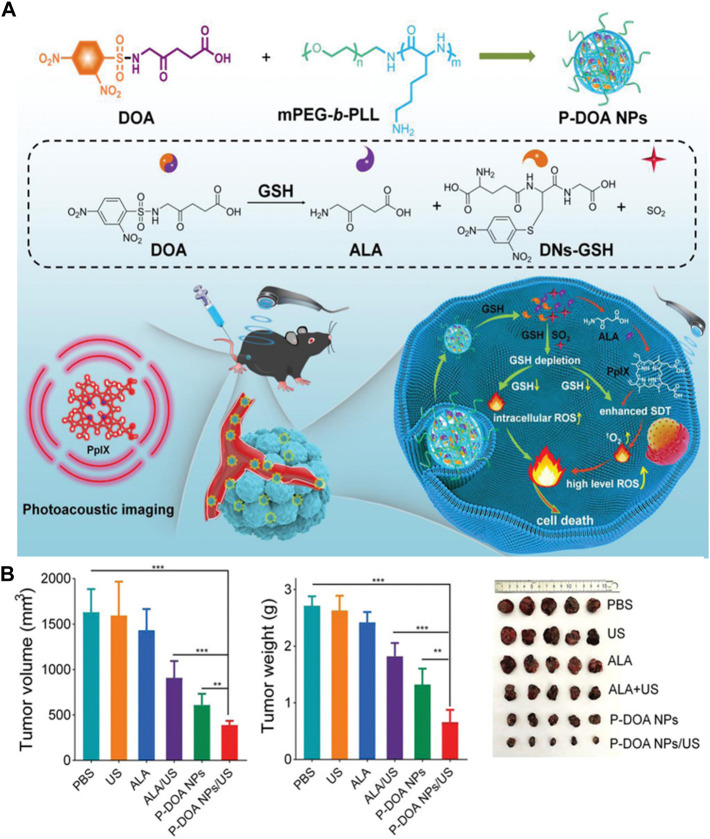
Sonodynamic therapy (SDT) is another relatively new cancer treatment that works by a similar mechanism of action as PDT (Gong and Dai, 2021; Zhang et al., 2021). However, high concentration of GSH in tumor cells would alleviate SDT-induced ROS damage, thus reducing the SDT effects (Kumar et al., 2022). To this end, Sun and coworkers reported a two-in-one GSH-responsive nanoprodrug for photoacoustic imaging-guided SDT for skin cancer (Sun et al., 2022). The two-in-one prodrug nanoparticles (P-DOA NPs) were co-assembled from methoxyl poly(ethylene glycol)-b-poly(l-lysine) (mPEG-b-PLL) and the GSH responsive DOA fragment was synthesized by 2,4-dinitrobenzenesulfonyl chloride (DNs-Cl) and ALA. The prodrug nanoparticles can simultaneously liberate ALA and SO2 under high concentration of GSH in tumor cells. The released ALA molecules were intracellularly converted into PpIX for photoacoustic imaging and SDT, whereas the released SO2 weakened the intracellular reductive microenvironment, thereby increasing intracellular ROS levels and resulting in enhanced SDT effects (Figure 7A). The in vivo study using a xenograft mouse model showed that the prodrug nanoparticles significantly suppressed tumor growth under ultrasound irradiation without obvious adverse effects (Figure 7B).
FIGURE 7.
(A) Design and mechanism of GSH-responsive P-DOA NPs; (B) Relative tumor volume, weight and photographs of resected tumors in B16F10 tumor-bearing mice after treatments on day 15. (Unite States: ultrasound). Adapted from ref. (Sun et al., 2022). Copyright 2022 WILEY-VCH Verlag GmbH & Co., KGaA, Weinheim.
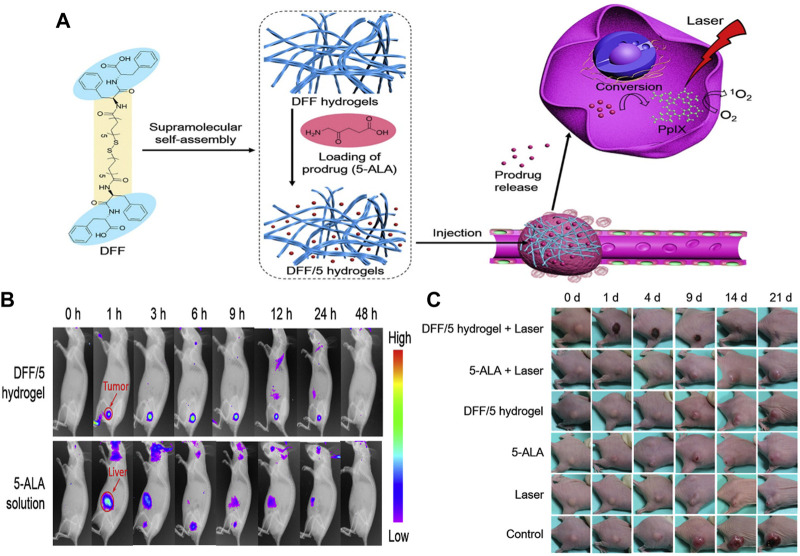
In addition, hydrogels, as a class of structurally controllable and easily degradable supramolecules, have received increasing attention in topical drug delivery (Li and Mooney, 2016). In 2020, Zou and coworkers reported an injectable and self-assembled bola-dipeptide (DFF) hydrogel for sustained and efficient ALA delivery (Zou et al., 2020). The structural unit of DFF is synthesized by coupling two hydrophilic peptides L-Phe-L-Phe (FF) via a disulfide-containing hydrophobic linker, which next encapsulate ALA molecules into the self-assembled hydrogel through electrostatic interactions (Figure 8A). The ALA-loaded DFF hydrogels exhibited robust mechanical properties and sustained ALA-release upon GSH activation. In particular, this ALA-loaded hydrogel can inhibit/reduce off-target leakage, thereby increasing local PpIX conversion, which in turn enhances tumor imaging and tumor ablation (Figures 8B, C). This study thus demonstrates that GSH-responsive hydrogels might be promising nanoplatforms for local and sustained drug delivery for cancer therapy.
FIGURE 8.
(A) Design and mechanism of GSH responsive DFF/ALA hydrogels; (B) Fluorescence images of mice at different time points after injection of DFF/ALA hydrogels and free ALA; (C) Images of tumor-bearing mice at different time points after treatments. Adapted from ref. (Zou et al., 2020). Copyright 2020 Elsevier B.V.
2.1.3 Enzyme-responsive systems
Enzymes are biological catalysts that selectively accelerate reactions and play important roles in physiological, pathological and metabolic processes. However, due to the heterogeneity between normal and pathological tissues, different distributions/expressions of certain enzymes are often observed in tissues/cells, making enzymes potential modules for designing stimuli-responsive drugs (Shahriari et al., 2019; Slor et al., 2021).
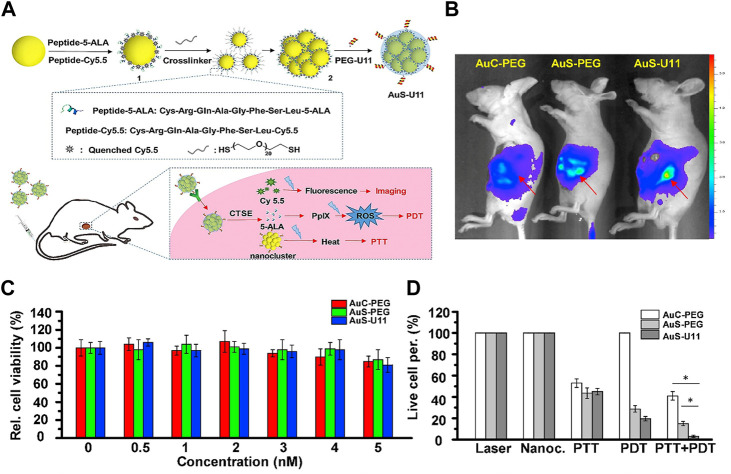
Cathepsin E (CTSE) is a tumor-associated intracellular enzyme, which is specifically overexpressed in a variety of cancers, including cervical, gastric, intestinal and pancreatic cancer (Dheer et al., 2019). In 2017, Li and coworkers reported a CTSE-triggered nanocluster prodrug (AuS-U11) for endomicroscopy-guided photodynamic and photothermal therapy of pancreatic ductal adenocarcinoma (Li Y et al., 2017). This nanocluster-based prodrug consists of gold nanoclusters as carriers, U11 peptide as a targeting ligand, a CTSE-sensitive PDT prodrug Cys-Arg-Gln-Ala-Gly-Phe-Ser-Leu-ALA (CRQAGFSL-ALA) and a CTSE-sensitive imaging agent Cys-Arg-Gln-Ala-Gly-Phe-Ser-Leu-Cy5.5 (CRQAGFSL-Cy5.5) (Figure 9A). The gold nanoclusters act not only as transporters but also as photothermal agents because the surface plasmon resonance peaks of gold nanoclusters shift to the near-infrared (NIR) region. Especially, this AuS-U11 prodrug demonstrated active targeting ability and enhanced cellular uptake due to the presence of U11 peptide. Stronger signal was observed in the same area under administration of AuS-U11 than that of AuS-PEG (without U11 peptide) and AuC-PEG (without both U11 peptide and ALA) (Figure 9B). Although the nanoclusters (AuS-U11, AuS-PEG and AuC-PEG) alone showed little cytotoxicity (Figure 9C), significant cell-killing effects were observed in AuS-U11 and AuS-PEG-treated group under PDT and PTT treatments. In particular, the AuS-U11-treated group demonstrated the highest cell-killing effects upon both PDT and PTT treatments, suggesting a powerful synergistic antitumor effect (Figure 9D).
FIGURE 9.
(A). Design and mechanism of CTSE-responsive AuS-U11 prodrug system; (B). In vivo images of mice treated with AuS-U11, AuS-PEG and AuC-PEG; (C). Dark toxicities of AuS-U11, AuS-PEG and AuC-PEG against pancreatic ductal adenocarcinoma cells; (D). Cell viabilities of AuS-U11, AuS-PEG and AuC-PEG upon PTT, PDT and combined PTT/PDT treatment under NIR irradiation. Adapted from ref. (Li H et al., 2017). Copyright 2017 Elsevier Ltd.
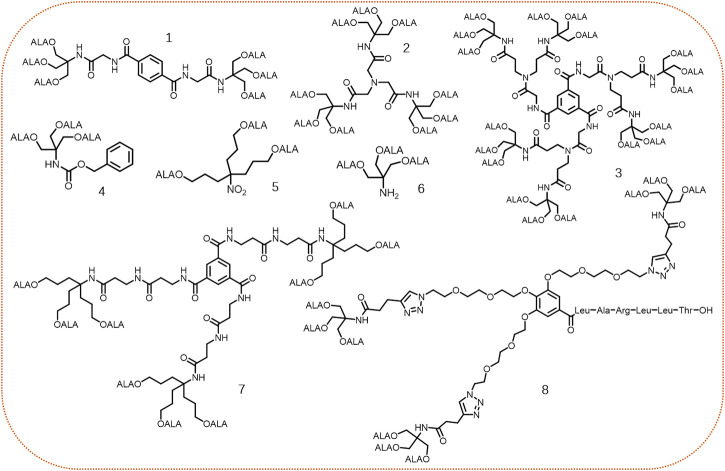
Besides, esterase, another common enzyme overexpressed in certain tumors, plays important roles in tumor growth, progression, invasion, and migration. (Wells and Grandis, 2003). For instance, the activity of esterase in colorectal tumor is around 2–4 folds than in normal tissues (Niu et al., 2012). Dendrimers, as a type of polymeric nanomaterials, possess hyper-branched 3D-structure, high drug loading, and easy control of size and lipophilicity, making them promising nanocarriers in drug delivery (Sherje et al., 2018). Typically, dendrimers constructed by ester linkages are hydrolyzed by intracellular hydrolases (e.g., lysosomal enzymes) to release free monomers. For example, the ALA-containing dendrimers with different core frameworks were synthesized and evaluated by Edwards’s group (Battah et al., 2001). Although these synthesized dendrimers could penetrate cell membrane and release free ALA intracellularly, there was no obvious correlation between the number of conjugated ALA residues in a dendrimer and the subsequent PpIX accumulation (Figure 10, compounds 1–3). Later, the same group synthesized another three ALA-based dendrimers (Figure 10, compounds 4–6), which all contained three ALA residues (Battah et al., 2006). The results showed that these three dendrimers produced PpIX more efficiently in cells compared with free ALA. Particularly, compound 5 bearing with a longer alkyl linker was the most effective dendrimer to generate PpIX, most likely due to its greater lipophilicity and less steric hindrance, suggesting lipophilicity and steric hindrance of ALA-based dendrimers might be the key factors affecting ALA release in cells.
FIGURE 10.
Representative structures of ALA-based dendrimers.
Recently, dendrimers 7 (Rodriguez et al., 2015) and 8 (Tewari et al., 2021) both containing 9 ALA residues via different linkers were reported for PDT (Figure 10, compounds 7–8). In contrast with the equimolar amount of free ALA-treated group, more PpIX production was observed in the group treated with ALA dendrimers (7 or 8). Notably, dendrimer 8 modified with a targeting peptide sequence demonstrated outstanding cell and tissue selectivity. However, most of the current ALA-based dendrimeric prodrugs mainly focus on the preparation and in vitro cell studies, further in vivo studies are highly needed to verify the effectiveness of dendrimer-based prodrug strategy.
2.1.4 Light-responsive systems
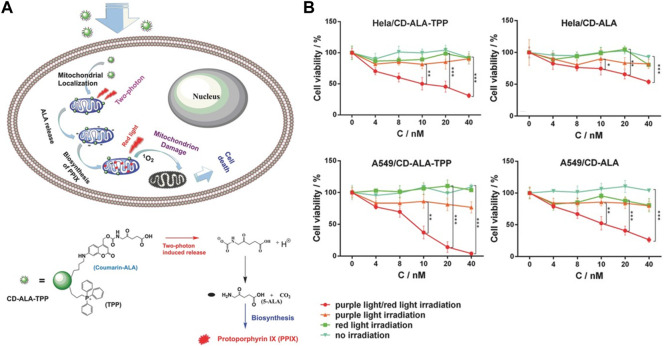
Light as an external stimulus with non-invasiveness, ease of use and high spatiotemporal control, has been used in light-responsive drug delivery systems for on-demand drug release (Linsley and Wu, 2017). In 2016, Wu and coworkers reported a mitochondria-targeted nanosystem capable of releasing ALA under two-photon irradiation to induce cell death (Wu et al., 2016). This nanosystem was constructed by covalently attaching a photo-triggerable coumarin-ALA conjugate and a mitochondria-targeting triphenylphosphonium (TPP) ligand onto carbon dots (Figure 11A). After internalization by tumor cells, the prodrugs preferentially accumulate in mitochondria due to the presence of TPP, and release ALA molecules under two-photo irradiation, which are next converted into PpIX through enzyme-catalyzed biotransformation. The in vitro study indicated that this nanosystem significantly suppressed tumor cell growth under light irradiation. In particular, the TPP-modified nanosystem demonstrated stronger cell-killing effects than the unmodified agent (Figure 11B), indicating mitochondria-targeting provides enhanced PDT effects.
FIGURE 11.
(A). Design and mechanism of light-responsive CD-ALA-TPP nanoprodrug; (B). Cell viabilities of the TPP targeted and non-targeted nanosystems against HeLa and A549 cells. Adapted from ref. (Wu et al., 2016). Copyright 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
2.1.5 Multi-stimuli-responsive systems
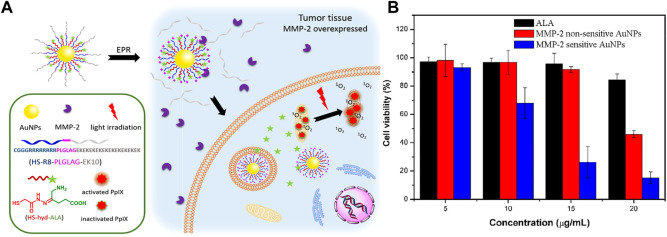
Simultaneous responses to more than one stimulus can greatly enhance the selectivity in drug delivery. In 2017, Ji’s group reported a pH-responsive AuNP for targeted delivery of ALA to kill A549 cancer cells (Wu et al., 2017b), and they later reported a pH and matrix metalloproteinase-2 (MMP-2) dual-responsive ALA prodrug nanoparticle for photodynamic killing of SCC-7 cancer cells (Wu et al., 2017a). This dual-responsive nanosystem was fabricated by connecting hydrazone-linked ALA and MMP-2-activatable cell-penetrating peptide (CPP) to AuNPs via Au-thiol interaction (Figure 12A). After being taken up by tumor cells, the shield CPPs attached on the nanosystem were removed by overexpressed MMP-2 in tumor cells, and the ALA molecules were liberated through cleavage the hydrazine bonds at an acidic environment in tumor cells. MMP-2-activatable CPP on the nanosystem can enhance cellular uptake, thereby increasing ALA accumulation and generating more PpIX in cells, which in turn results in enhanced PDT effects upon light irradiation (Figure 12B).
FIGURE 12.
(A). Design and mechanism of pH and MMP-2 dual-responsive AuNPs; (B). Cell viabilities treated with free ALA, MMP-2-sensitive and MMP-2-nonsensitive AuNPs under light irradiation. Adapted from ref. (Wu et al., 2017b). Copyright 2017 American Chemical Society.
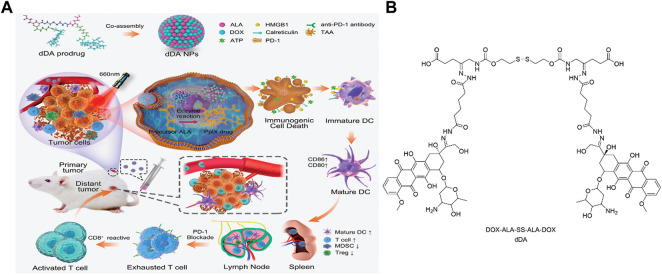
In 2020, Bai and coworkers reported pH/GSH dual-responsive prodrug nanoparticles for chemo-immunotherapy (Bai et al., 2020). In this work, the immunogenicity inducing drug DOX and photodynamic prodrug ALA were fabricated into one platform (DOX-ALA-SS-ALA-DOX, denoted as dDA) using pH and GSH responsive linker, respectively (Figures 13A, B), which were subsequently self-assembled into dDA nanoparticles via hydrophobic interaction. Under acidic (hydrazine bond hydrolysis) and high GSH concentration (disulfide bond cleavage) conditions in tumor cells, free Dox and ALA molecules are released to remodel the tumor microenvironment. This nanoprodrug system not only increases the production of PpIX in tumor cells, but also induces immunogenic cell death (ICD) and reduces the number of immunosuppressive immune cells, thereby improving the therapeutic efficacy.
FIGURE 13.
(A). Design and mechanism of pH/GSH dual responsive dDA nanoparticles; (B). Molecular structure of dDA. Adapted from ref. (Bai et al., 2020). Copyright 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
2.2 Non-responsive ALA nanoprodrugs
Non-responsive nanoprodrugs refer to prodrugs that are insensitive to external and endogenous stimuli. Generally, molecules encapsulated in nanocarriers are physically absorbed through intermolecular interactions such as hydrophobic interactions, H-bonds, van der Waals forces, electrostatic interactions and dipole-dipole interactions (Li X et al., 2020). After selective uptake by tumor cells through the EPR effect, the nanocarrier-encapsulated therapeutic drugs are released to the target site through natural leakage (Patra et al., 2018). Compared with responsive nanoprodrugs, non-responsive nanoprodrugs are relatively primitive, by which the transported cargos are physically encapsulated into nanocarriers including inorganic nanoparticles, organic nanoparticles, lipid-based nanocarriers and polymer-based nanocarriers. As a hydrophilic prodrug at physiological pH, ALA is encapsulated by nanocarriers mainly through electrostatic/ionic interaction.
Gold nanoparticles (AuNPs), a conventional type of inorganic nanoparticles used in drug delivery and biomedical imaging, possess outstanding drug loading and unique optical properties (Sperling et al., 2008; Cheng et al., 2013). A phase I clinical trial has successfully completed using AuNPs to deliver tumor necrosis factor (TNF), and it showed little systematic toxicity in animal model (Libutti et al., 2010). In 2013, Mohammadi and coworkers reported ALA coated AuNP for enhanced PDT (Mohammadi et al., 2013). In this study, ALA molecules were physically absorbed by AuNPs through electrostatic interactions between the positive-charged AuNPs and negative-charged ALA molecules. Compared with free ALA, AuNP-encapsulated ALA produced more PpIX in Mel-Rm cells, thus inducing a two-fold higher cell death. Similarly, Zhang and coworkers reported ALA-AuNPs-mediated PDT for killing human chronic myeloid leukemia K562 cells (Zhang et al., 2015). This study showed that AuNPs were able to increase the singlet oxygen generation (SOG) via a local field enhancement (LFE) effect induced by the localized surface plasmon resonance (LSPR) of AuNPs. However, LFE was dependent on the irradiation wavelength and the size of AuNPs, and the authors revealed that the cell-killing effect was attributed to the delivered ALA rather than the LSPR effect. In 2015, Oliveira et al. synthesized PEG-functionalized ALA-AuNPs for atherosclerosis (de Oliveira et al., 2015). PEGylation leads to an enhanced stability of the nanoparticles in aqueous solution and strong resistance to binding against various biomolecules. The PpIX fluorescence extracted from the rabbits’ blood and feces were significantly increased after ALA-AuNPs administration, indicating ALA was efficiently internalized by gold nanoparticles and rapidly transformed into PpIX. This study indicates that ALA-AuNPs might aid in the early diagnosis and treatment of atherosclerosis. Besides, Jiang and coworkers reported gold nanoshells-coated ALA liposome for photothermal-photodynamic antitumor therapy (Jiang et al., 2020). Apart from the photodynamic destruction induced by the delivered ALA, the photothermal effects from gold nanoshells also promoted antitumor effects.
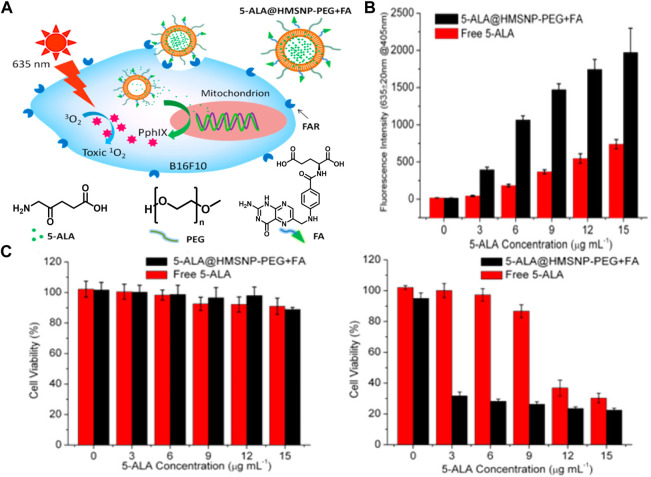
Hollow mesoporous silica nanoparticles (HMSNPs) are commonly used organic nanoparticles. The high surface area, good biocompability and high pore volume of HMSNP make them easy to chemically modify and load drugs (Li H et al., 2017). In 2015, Ma and coworkers designed and fabricated a versatile ALA-coated HMSNP for photodynamic treatment of skin cancer (Ma et al., 2015). In this study, folic acid was functionalized onto the nanoparticles, which could target folic acid receptor (FAR) that is overexpressed on the surface of most skin cancer cells and thus facilitate cellular uptake of HMSNP (Figure 14A). In addition, the nanoparticle was further functionalized with PEG to increase biocompatibility and prolong blood circulation. Such fabricated nanoparticles efficiently aid ALA to cross the lipophilic barrier of skin and selectively enter skin cancer cells. By contrast, more PpIX were produced in B16F10 skin cancer cells (Figure 14B) and induced higher PDT effects (Figure 14C) under light irradiation in ALA@HMSNP-PEG + FA-treated group than that of free ALA, suggesting HMSNP-based ALA delivery might be a promising approach in practical skin cancer treatment.
FIGURE 14.
(A) Design and mechanism of ALA@HMSNP-PEG + FA; (B) PpIX generation in B16F10 cancer cells after treatment with ALA@HMSNP-PEG + FA and free ALA; (C) Cell viabilities after treated with ALA@HMSNP-PEG + FA and free ALA without and with light irradiation. Adapted from ref. (Ma et al., 2015). Copyright 2015 American Chemical Society.
In 2020, Wang et al. constructed a biocompatible periodic mesoporous organosilica coated Prussian blue nanoparticles (PB@PMO) for loading ALA to treat glioma (Wang X et al., 2020). The encapsulated ALA could be delivered into malignant Glima cells and induce more PpIX production compared to free ALA. Besides, the PB core is able to catalyze H2O2 to generate oxygen in hypoxic tumor microenvironment, thereby attenuating tumor hypoxia and enhancing ALA-mediated PDT. The in vitro and in vivo results showed that PB@PMO-ALA-mediated PDT effectively suppressed tumor growth, thus providing a promising strategy for local glioma ablation.
Fullerenes (C60), as nanoscale carbon material with distinctive photochemical and electrochemical properties, have been used as drug delivery vehicles in nanomedicine for years (Markovic and Trajkovic, 2008; Torres et al., 2011). In 2014, Li and coworkers first evaluated the in vitro and in vivo PDT effects of fullerene-loaded ALA (Li et al., 2014). In this study, the prepared C60-ALA nanoparticles were stable in aqueous solution for over weeks, and cells treated with C60-ALA generated more PpIX, thereby inducing enhanced antitumor effect than free ALA in murine tumor model. In addition, Serda and coworkers reported water-soluble C60 nanomaterial modified with D-glucuronic acid for delivery of ALA (C60-GA-ALA) (Serda et al., 2022). Although this strategy led to an enhanced PpIX production in different tumor cells (e.g., MCF-7, HCT116 and A549 cells) upon treatment with C60-GA-ALA, the in vitro study using qRT-PCR analysis showed an increased concentration of ABCG2, which might promote PpIX efflux and reduce PDT efficacy. However, the authors verified that this side effect could be efficiently suppressed via co-incubation with ABCG2 inhibitor of Ko143.
Due to the unique physiochemical properties, lipids can form various nanomaterials, such as liposome, ethosome and niosome. The architecture of these lipid-based nanomaterials depends on the type of the components and/or the preparation method (Plaza-Oliver et al., 2021; Sguizzato et al., 2021). In 2008, Fang and coworkers compared ALA loaded liposome and ethosome for PDT (Fang et al., 2008). The results showed that the particle size of ethosomes was smaller than that of liposomes, and stronger PpIX fluorescence in the skin was observed for the ethosomes-treated group, suggesting the penetration ability of ethosomes was greater than that of liposomes. Afterwards, the same group used ALA-loaded ethosome to study the recovery of skin in a hyperproliferative murine model (Fang et al., 2009). The results showed that ethosomes penetrated to a depth of approximately 30–80 μm and produced about 3.64 times more PpIX than ALA alone. In addition, Bragagni and coworkers reported ALA loaded niosome for topical PDT treatment of skin malignancies (Bragagni et al., 2015). The penetration depth and drug retention were increased about 80% and 100% for the niosomal formulation compared to free ALA solution. Collectively, lipid-based nanoformulations possess good biocompatibility, high skin permeability and low cytotoxicity, providing potential benefits for ALA-based PDT treatment of skin diseases.
Besides, polymer-based nanomaterials are also frequently used for the delivery of ALA due to their high biological safety, good biodegradability and non-immunogenicity (Han et al., 2018). For instance, Chitosan-encapsulated ALA were used for PDT killing of colon cancer cells and melanoma cancer cells (Chung et al., 2013; Ferreira et al., 2013); poly(lactic-co-glycolic acid) (PLGA) encapsulated ALA were used for PDT killing of skin squamous cell carcinoma cells and 4T1 cells (Shi et al., 2013; de Andrade et al., 2017); and polylactic acid (PLA) -encapaulated ALA were used for PDT killing of HeLa cells (Di Martino et al., 2018). These polymer-based nanomaterials can significantly increase the accumulation of ALA molecules and their subsequent conversion to PpIX, thereby enhancing the PDT efficiency.
3 Conclusions and future perspectives
ALA, as a prodrug of PpIX, has been developed nearly 40 years since it was first used to treat skin-associated diseases such as actinic keratoses, basal cell carcinoma, Bowen’s disease, and basosquamous cell carcinoma (Kennedy et al., 1990; Kennedy et al., 1996). Although it is becoming increasingly mature and has been approved by FDA for the treatment of a variety of malignant diseases and for adjuvant surgical resection of high-grade gliomas, the weak lipophilicity, low stability and poor bioavailability of natural ALA reduce its clinical efficiency. Chemical modification of ALA to prepare the esterified derivatives (e.g., MAL, HAL) can improve its stability, tissue penetration and pharmacokinetic properties (Stepp and Waidelich, 2007; Lee and Baron ED, 2011). Besides, the recent emerged nanotechnology has opened another avenue to amplify the effects of ALA-based prodrug owing to the high drug loading, well-controlled drug release, excellent tissue targeting, and the ability to combine multiple treatments into one platform.
In this context, we summarized the important recent advances in the use of various ALA-based prodrug nanomedicines for PDT-related biomedical applications, predominately for cancer therapy (Table 2). Compared with non-responsive ALA nanoprodrugs, design of stimuli-responsive ALA nanoprodrugs may provide a more attractive approach for targeting delivery of ALA. A wide variety of endogenous and exogenous stimuli, as well as a large number of responsive materials capable of fabricating various architectures, provides great flexibility in the design of stimuli-responsive nanosystems. However, most endeavors on stimuli-responsive ALA nanosystems are focused on the in vitro studies, only a few have been tested in vivo preclinical study, and very few have entered the clinical stage. The difficulty in scaling-up preparation and the complexity in designing the architecture are likely to hamper the translation from bench to bedside. The potential toxicity of the fabricated nanosystem depends on the composition, physiochemical properties and dose. Moreover, lots of stimuli-responsive nanosystems have limited opportunities for translation due to refractory degradation and insufficient biocompatibility. In view of the tremendous work on ALA-based nanoprodrugs, more efforts should be paid on in vivo preclinical and clinical studies in future. Particularly, with rapid advances in material science, innovative structure design and multimodal smart responses, future development of easier, simpler, biocompatible and degradable nanosystems, will provide more opportunities to enter the clinical stage.
TABLE 2.
A summary of ALA-based prodrugs for improved PDT using nanotechnology.
| Stimulus | Nanocarrier | Applications | Ref(s) |
|---|---|---|---|
| pH | AuNPs | in vitro against A549 cells | Wu et al. (2017a) |
| UCNPs | in vitro and in vivo against HeLa cells | Punjabi et al. (2014) | |
| Core/shell-structured nanoparticles | in vitro against T24 cells | Tan et al. (2015) | |
| Gold nanoclusters | in vitro against A549 cells | Srinivasulu et al. (2021) | |
| Quantum dots | in vitro against HT29 and SW480 cells | Hashemkhani et al. (2022) | |
| Micelles | in vitro against HepG2 cells | Tong et al. (2016) | |
| GSH | Nanogels | in vitro and in vivo against 4T1 cells | Wang Y et al. (2020) |
| P-DOA NPs | in vitro and in vivo against B16F10 cells | Sun et al. (2022) | |
| DFF hydrogels | in vivo against MCF-7 cells | Zou et al. (2020) | |
| Enzyme | Gold nanoclusters | in vitro and in vivo against PANC-1 cells | Li Y et al. (2017) |
| Dendrimers | in vitro against PAM 212 cells | Battah et al. (2001) | |
| in vitro against PAM 212 cells | Battah et al. (2006) | ||
| in vitro against LM3 cells | Rodriguez et al. (2015) | ||
| in vitro against MDA-MB-231 cells | Tewari et al. (2021) | ||
| Light | Carbon dots | in vitro against HeLa and A549 cells | Wu et al. (2016) |
| pH/Enzyme | AuNPs | in vitro and in vivo against SCC-7 cells | Wu et al. (2017a) |
| pH/GSH | dDA nanoparticles | in vitro and in vivo against 4T1 cells | Bai et al. (2020) |
| Non-stimuli | AuNPs | in vitro against Mel-Rm cells | Mohammadi et al. (2013) |
| in vitro against K562 cells | Zhang et al. (2015) | ||
| atherosclerosis | de Oliveira et al. (2015) | ||
| HMSNPs | in vitro against B16F10 cells | Ma et al. (2015) | |
| PB@PMO nanoparticles | in vitro and in vivo against U87MG cells | Wang Y et al. (2020) | |
| Liposomes | in vitro and in vivo against SKOV3 cells | Jiang et al. (2020) | |
| Ethosomes | in vivo for enhancing ALA accumulation/penetration | Fang et al. (2008) | |
| in vivo for enhancing ALA accumulation/penetration | Fang et al. (2009) | ||
| Niosomes | in vivo against skin malignancies | Bragagni et al. (2015) | |
| Fullerenes | in vitro and in vivo against B16F10 cells | Li et al. (2014) | |
| in vitro against MCF-7 cells | Serda et al. (2022) | ||
| Polymer-based nanomaterials | in vitro against CT26 cells | Chung et al. (2013) | |
| in vitro against B16F10 cells | Ferreira et al. (2013) | ||
| in vitro against A431 cells | Shi et al. (2013) | ||
| in vitro against 4T1 cells | de Andrade et al. (2017) | ||
| in vitro against HeLa cells | Di Martino et al. (2018) |
Funding Statement
This work was supported by the National Natural Science Foundation of China (21877130 and U1801681), Natural Science Foundation of Guangdong Province (2021A1515010357), and Shenzhen Science and Technology Program (JCYJ20210324124411032).
Author contributions
LL, SZ, and ST wrote the first draft; MX and WW assisted in searching literatures; CY and LG revised the manuscript; QX outlined and revised the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Allison R. R., Sibata C. H. (2010). Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagnosis Photodyn. Ther. 7 (2), 61–75. 10.1016/j.pdpdt.2010.02.001 [DOI] [PubMed] [Google Scholar]
- Bai S., Yang L. L., Wang Y., Zhang T., Fu L., Yang S., et al. (2020). Prodrug-based versatile nanomedicine for enhancing cancer immunotherapy by increasing immunogenic cell death. Small 16 (19), e2000214. 10.1002/smll.202000214 [DOI] [PubMed] [Google Scholar]
- Barattin M., Mattarei A., Balasso A., Paradisi C., Cantù L., Del F. E., et al. (2018). pH-controlled liposomes for enhanced cell penetration in tumor environment. ACS Appl. Mat. Interfaces 10 (21), 17646–17661. 10.1021/acsami.8b03469 [DOI] [PubMed] [Google Scholar]
- Battah S. H., Chee C. E., Nakanishi H., Gerscher S., Macrobert A. J., Edwards C. (2001). Synthesis and biological studies of 5-aminolevulinic acid-containing dendrimers for photodynamic therapy. Bioconjugate Chem. 12 (6), 980–988. 10.1021/bc010027n [DOI] [PubMed] [Google Scholar]
- Battah S., O'Neill S., Edwards C., Balaratnam S., Dobbin P., Macrobert A. J. (2006). Enhanced porphyrin accumulation using dendritic derivatives of 5-aminolaevulinic acid for photodynamic therapy: An in vitro study. Int. J. Biochem. Cell Biol. 38 (8), 1382–1392. 10.1016/j.biocel.2006.02.001 [DOI] [PubMed] [Google Scholar]
- Bragagni M., Scozzafava A., Mastrolorenzo A., Supuran C. T., Mura P. (2015). Development and ex vivo evaluation of 5-aminolevulinic acid-loaded niosomal formulations for topical photodynamic therapy. Int. J. Pharm. 494 (1), 258–263. 10.1016/j.ijpharm.2015.08.036 [DOI] [PubMed] [Google Scholar]
- Chen G., Qiu H., Prasad P. N., Chen X. (2014). Upconversion nanoparticles: Design, nanochemistry, and applications in theranostics. Chem. Rev. 114 (10), 5161–5214. 10.1021/cr400425h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Liu D., Liu F., Wu Y., Peng X., Song F. (2021). Recent advances of redox-responsive nanoplatforms for tumor theranostics. J. Control. Release. 332, 269–284. 10.1016/j.jconrel.2021.02.030 [DOI] [PubMed] [Google Scholar]
- Cheng J., Gu Y. J., Cheng S. H., Wong W. T. (2013). Surface functionalized gold nanoparticles for drug delivery. J. Biomed. Nanotechnol. 9 (8), 1362–1369. 10.1166/jbn.2013.1536 [DOI] [PubMed] [Google Scholar]
- Chung C. W., Chung K. D., Jeong Y. I., Kang D. H. (2013). 5-aminolevulinic acid-incorporated nanoparticles of methoxy poly(ethylene glycol)-chitosan copolymer for photodynamic therapy. Int. J. Nanomed. 8, 809–819. 10.2147/IJN.S39615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compérat E., Conort P., Rouprêt M., Camparo P., Mazerolles C. (2011). [Pathologic diagnosis and management of flat lesions of urothelium detected with aminolevulinic acid (Hexvix®)]. Prog. Urol. 21 (3), 157–165. 10.1016/j.purol.2010.11.003 [DOI] [PubMed] [Google Scholar]
- de Andrade L. R., Primo F. L., Da S. J., Tedesco A. C., Lacava Z. (2017). In vitro assessment of anti-tumorigenic mechanisms and efficacy of NanoALA, a nanoformulation of aminolevulic acid designed for photodynamic therapy of cancer. Photodiagnosis Photodyn. Ther. 20, 62–70. 10.1016/j.pdpdt.2017.08.011 [DOI] [PubMed] [Google Scholar]
- de Oliveira G. K., Da S. M., Sicchieri L. B., de Oliveira S. F., de Matos R. A., Courrol L. C. (2015). Aminolevulinic acid with gold nanoparticles: A novel theranostic agent for atherosclerosis. Analyst 140 (6), 1974–1980. 10.1039/c4an02166e [DOI] [PubMed] [Google Scholar]
- Dheer D., Nicolas J., Shankar R. (2019). Cathepsin-sensitive nanoscale drug delivery systems for cancer therapy and other diseases. Adv. Drug Deliv. Rev. 151-152, 130–151. 10.1016/j.addr.2019.01.010 [DOI] [PubMed] [Google Scholar]
- Di Martino A., Trusova M. E., Postnikov P. S., Sedlarik V. (2018). Branched poly (lactic acid) microparticles for enhancing the 5-aminolevulinic acid phototoxicity. J. Photochem. Photobiol. B-Biol. 181, 80–88. 10.1016/j.jphotobiol.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Ding J. X., Chen J. J., Gao L. Q., Jiang Z. Y., Zhang Y., Li M. Q., et al. (2019). Engineered nanomedicines with enhanced tumor penetration. Nano Today 29, 100800. 10.1016/j.nantod.2019.100800 [DOI] [Google Scholar]
- Fang Y. P., Huang Y. B., Wu P. C., Tsai Y. H. (2009). Topical delivery of 5-aminolevulinic acid-encapsulated ethosomes in a hyperproliferative skin animal model using the CLSM technique to evaluate the penetration behavior. Eur. J. Pharm. Biopharm. 73 (3), 391–398. 10.1016/j.ejpb.2009.07.011 [DOI] [PubMed] [Google Scholar]
- Fang Y. P., Tsai Y. H., Wu P. C., Huang Y. B. (2008). Comparison of 5-aminolevulinic acid-encapsulated liposome versus ethosome for skin delivery for photodynamic therapy. Int. J. Pharm. 356 (1-2), 144–152. 10.1016/j.ijpharm.2008.01.020 [DOI] [PubMed] [Google Scholar]
- Ferreira D. M., Saga Y. Y., Aluicio-Sarduy E., Tedesco A. C. (2013). Chitosan nanoparticles for melanoma cancer treatment by Photodynamic Therapy and electrochemotherapy using aminolevulinic acid derivatives. Curr. Med. Chem. 20 (14), 1904–1911. 10.2174/0929867311320140007 [DOI] [PubMed] [Google Scholar]
- Gao W., Chan J. M., Farokhzad O. C. (2010). pH-Responsive nanoparticles for drug delivery. Mol. Pharm. 7 (6), 1913–1920. 10.1021/mp100253e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardlo K., Ruzicka T. (2002). Metvix (PhotoCure). Curr. Opin. Investig. Drugs 3 (11), 1672–1678. [PubMed] [Google Scholar]
- Gong Z., Dai Z. (2021). Design and challenges of sonodynamic therapy system for cancer theranostics: From equipment to sensitizers. Adv. Sci. 8 (10), 2002178. 10.1002/advs.202002178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D., Xu S., Huang Y., Jiang H., Yasen W., Wang N., et al. (2018). Platinum(IV) complex-based two-in-one polyprodrug for a combinatorial chemo-photodynamic therapy. Biomaterials 177, 67–77. 10.1016/j.biomaterials.2018.05.052 [DOI] [PubMed] [Google Scholar]
- Gupta A. K., Versteeg S. G., Abramovits W. (2017). AMELUZ(™) (BF-200 ALA). Skinmed 15 (2), 133–135. [PubMed] [Google Scholar]
- Han J., Zhao D., Li D., Wang X., Jin Z., Zhao K. (2018). Polymer-based nanomaterials and applications for vaccines and drugs. Polymers 10 (1), 3110010031. 10.3390/polym10010031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemkhani M., Loizidou M., Macrobert A. J., Yagci A. H. (2022). One-step aqueous synthesis of anionic and cationic AgInS(2) quantum dots and their utility in improving the efficacy of ALA-based photodynamic therapy. Inorg. Chem. 61 (6), 2846–2863. 10.1021/acs.inorgchem.1c03298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffes E. W. (2002). Levulan: The first approved topical photosensitizer for the treatment of actinic keratosis. J. Dermatol. Treat. 13, S19–S23. 10.1080/095466302317414663 [DOI] [PubMed] [Google Scholar]
- Ji B., Wei M., Yang B. (2022). Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics 12 (1), 434–458. 10.7150/thno.67300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji F., Jiang M., Yu Q., Hao X., Zhang Y., Zhu J., et al. (2021). Ionic conductive organohydrogel with ultrastretchability, self-healable and freezing-tolerant properties for wearable strain sensor. Front. Chem. 9, 758844. 10.3389/fchem.2021.758844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Q., Song Q., Li P., Huang W. (2019). Rejuvenated photodynamic therapy for bacterial infections. Adv. Healthc. Mat. 8 (14), e1900608. 10.1002/adhm.201900608 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Liu Y., Fang S., Ji M. (2020). Gold nanoshells coated 5-aminolevulinic liposomes for photothermal-photodynamic antitumor therapy. J. Nanosci. Nanotechnol. 20 (1), 1–14. 10.1166/jnn.2020.16886 [DOI] [PubMed] [Google Scholar]
- Kennedy J. C., Marcus S. L., Pottier R. H. (1996). Photodynamic therapy (PDT) and photodiagnosis (PD) using endogenous photosensitization induced by 5-aminolevulinic acid (ALA): Mechanisms and clinical results. J. Clin. Laser Med. Surg. 14 (5), 289–304. 10.1089/clm.1996.14.289 [DOI] [PubMed] [Google Scholar]
- Kennedy J. C., Pottier R. H., Pross D. C. (1990). Photodynamic therapy with endogenous protoporphyrin IX: Basic principles and present clinical experience. J. Photochem. Photobiol. B-Biol. 6 (1-2), 143–148. 10.1016/1011-1344(90)85083-9 [DOI] [PubMed] [Google Scholar]
- Kobuchi H., Moriya K., Ogino T., Fujita H., Inoue K., Shuin T., et al. (2012). Mitochondrial localization of ABC transporter ABCG2 and its function in 5-aminolevulinic acid-mediated protoporphyrin IX accumulation. PLoS One 7 (11), e50082. 10.1371/journal.pone.0050082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer B., Plaetzer K. (2008). ALA and its clinical impact, from bench to bedside. Photochem. Photobiol. Sci. 7 (3), 283–289. 10.1039/b712847a [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P. C., Du G., Fukuda Y., Sun D., Sampath J., Mercer K. E., et al. (2006). Identification of a mammalian mitochondrial porphyrin transporter. Nature 443 (7111), 586–589. 10.1038/nature05125 [DOI] [PubMed] [Google Scholar]
- Kumar Y. A., Kumar N., Talim K. A., Kushwaha R., Banerjee S. (2022). Sonodynamic therapy with metal complexes: A new promise in cancer therapy. ChemMedChem 17 (2), e202100615. 10.1002/cmdc.202100615 [DOI] [PubMed] [Google Scholar]
- Lee Y., Baron E. D. (2011). Photodynamic therapy: Current evidence and applications in dermatology. Semin. Cutan. Med. Surg. 30 (4), 199–209. 10.1016/j.sder.2011.08.001 [DOI] [PubMed] [Google Scholar]
- Li H., Wang P., Deng Y., Zeng M., Tang Y., Zhu W. H., et al. (2017). Combination of active targeting, enzyme-triggered release and fluorescent dye into gold nanoclusters for endomicroscopy-guided photothermal/photodynamic therapy to pancreatic ductal adenocarcinoma. Biomaterials 139, 30–38. 10.1016/j.biomaterials.2017.05.030 [DOI] [PubMed] [Google Scholar]
- Li J., Mooney D. J. (2016). Designing hydrogels for controlled drug delivery. Nat. Rev. Mat. 1 (12), 16071. 10.1038/natrevmats.2016.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Li X., Zhao C. (2020). Strategies to obtain encapsulation and controlled release of small hydrophilic molecules. Front. Bioeng. Biotechnol. 8, 437. 10.3389/fbioe.2020.00437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Lovell J. F., Yoon J., Chen X. (2020). Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 17 (11), 657–674. 10.1038/s41571-020-0410-2 [DOI] [PubMed] [Google Scholar]
- Li Y., Li N., Pan W., Yu Z., Yang L., Tang B. (2017). Hollow mesoporous silica nanoparticles with tunable structures for controlled drug delivery. ACS Appl. Mat. Interfaces. 9 (3), 2123–2129. 10.1021/acsami.6b13876 [DOI] [PubMed] [Google Scholar]
- Li Z., Huang J., Wu J. (2021). pH-Sensitive nanogels for drug delivery in cancer therapy. Biomater. Sci. 9 (3), 574–589. 10.1039/d0bm01729a [DOI] [PubMed] [Google Scholar]
- Li Z., Pan L. L., Zhang F. L., Zhu X. L., Liu Y., Zhang Z. Z. (2014). 5-Aminolevulinic acid-loaded fullerene nanoparticles for in vitro and in vivo photodynamic therapy. Photochem. Photobiol. 90 (5), 1144–1149. 10.1111/php.12299 [DOI] [PubMed] [Google Scholar]
- Liao Z. S., Huang S. Y., Huang J. J., Chen J. K., Lee A. W., Lai J. Y., et al. (2018). Self-assembled pH-responsive polymeric micelles for highly efficient, noncytotoxic delivery of doxorubicin chemotherapy to inhibit macrophage activation: In vitro investigation. Biomacromolecules 19 (7), 2772–2781. 10.1021/acs.biomac.8b00380 [DOI] [PubMed] [Google Scholar]
- Libutti S. K., Paciotti G. F., Byrnes A. A., Alexander H. J., Gannon W. E., Walker M., et al. (2010). Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin. Cancer Res. 16 (24), 6139–6149. 10.1158/1078-0432.CCR-10-0978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z. K., Goswami N., Xue T. T., Chai O., Xu H. J., Liu Y. X., et al. (2021). Engineering metal nanoclusters for targeted therapeutics: From targeting strategies to therapeutic applications. Adv. Funct. Mat. 31 (51), 2105662. 10.1002/adfm.202105662 [DOI] [Google Scholar]
- Linsley C. S., Wu B. M. (2017). Recent advances in light-responsive on-demand drug-delivery systems. Ther. Deliv. 8 (2), 89–107. 10.4155/tde-2016-0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Zhou Y., Wang L., Han L., Lei J., Ishaq H. M., et al. (2016). Photodynamic inactivation of Klebsiella pneumoniae biofilms and planktonic cells by 5-aminolevulinic acid and 5-aminolevulinic acid methyl ester. Lasers Med. Sci. 31 (3), 557–565. 10.1007/s10103-016-1891-1 [DOI] [PubMed] [Google Scholar]
- Liu P., Xie X., Liu M., Hu S., Ding J., Zhou W. (2021). A smart MnO(2)-doped graphene oxide nanosheet for enhanced chemo-photodynamic combinatorial therapy via simultaneous oxygenation and glutathione depletion. Acta Pharm. Sin. B 11 (3), 823–834. 10.1016/j.apsb.2020.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Qu Q., Zhao Y. (2015). Targeted delivery of 5-aminolevulinic acid by multifunctional hollow mesoporous silica nanoparticles for photodynamic skin cancer therapy. ACS Appl. Mat. Interfaces 7 (20), 10671–10676. 10.1021/acsami.5b03087 [DOI] [PubMed] [Google Scholar]
- Ma X., Yang S., Zhang T., Wang S., Yang Q., Xiao Y., et al. (2022). Bioresponsive immune-booster-based prodrug nanogel for cancer immunotherapy. Acta Pharm. Sin. B 12 (1), 451–466. 10.1016/j.apsb.2021.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansi M., Howley R., Chandratre S., Chen B. (2022). Inhibition of ABCG2 transporter by lapatinib enhances 5-aminolevulinic acid-mediated protoporphyrin IX fluorescence and photodynamic therapy response in human glioma cell lines. Biochem. Pharmacol. 200, 115031. 10.1016/j.bcp.2022.115031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic Z., Trajkovic V. (2008). Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials 29 (26), 3561–3573. 10.1016/j.biomaterials.2008.05.005 [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Hagiya Y., Endo Y., Nakajima M., Ishizuka M., Tanaka T., et al. (2015). Effects of plasma membrane ABCB6 on 5-aminolevulinic acid (ALA)-induced porphyrin accumulation in vitro: Tumor cell response to hypoxia. Photodiagnosis Photodyn. Ther. 12 (1), 45–51. 10.1016/j.pdpdt.2014.12.008 [DOI] [PubMed] [Google Scholar]
- Mohammadi Z., Sazgarnia A., Rajabi O., Soudmand S., Esmaily H., Sadeghi H. R. (2013). An in vitro study on the photosensitivity of 5-aminolevulinic acid conjugated gold nanoparticles. Photodiagnosis Photodyn. Ther. 10 (4), 382–388. 10.1016/j.pdpdt.2013.03.010 [DOI] [PubMed] [Google Scholar]
- Mura S., Nicolas J., Couvreur P. (2013). Stimuli-responsive nanocarriers for drug delivery. Nat. Mat. 12 (11), 991–1003. 10.1038/nmat3776 [DOI] [PubMed] [Google Scholar]
- Niu R., Jing H., Chen Z., Xu J., Dai J., Yan Z. (2012). Differentiating malignant colorectal tumor patients from benign colorectal tumor patients by assaying morning urinary arylsulfatase activity. Asia-Pac. J. Clin. Oncol. 8 (4), 362–367. 10.1111/j.1743-7563.2012.01545.x [DOI] [PubMed] [Google Scholar]
- Ortel B., Sharlin D., O'Donnell D., Sinha A. K., Maytin E. V., Hasan T. (2002). Differentiation enhances aminolevulinic acid-dependent photodynamic treatment of LNCaP prostate cancer cells. Br. J. Cancer. 87 (11), 1321–1327. 10.1038/sj.bjc.6600575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palasuberniam P., Kraus D., Mansi M., Howley R., Braun A., Myers K., et al. (2021). Small molecule kinase inhibitors enhance aminolevulinic acid-mediated protoporphyrin IX fluorescence and PDT response in triple negative breast cancer cell lines. J. Biomed. Opt. 26 (9), 098002. 10.1117/1.JBO.26.9.098002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palasuberniam P., Yang X., Kraus D., Jones P., Myers K. A., Chen B. (2015). ABCG2 transporter inhibitor restores the sensitivity of triple negative breast cancer cells to aminolevulinic acid-mediated photodynamic therapy. Sci. Rep. 5, 13298. 10.1038/srep13298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X., Jiang Y., Xiao Q., Leung A. W., Hua H., Xu C. (2016). pH-responsive polymer-drug conjugates: Design and progress. J. Control. Release. 222, 116–129. 10.1016/j.jconrel.2015.12.024 [DOI] [PubMed] [Google Scholar]
- Paterson J. K., Shukla S., Black C. M., Tachiwada T., Garfield S., Wincovitch S., et al. (2007). Human ABCB6 localizes to both the outer mitochondrial membrane and the plasma membrane. Biochemistry 46 (33), 9443–9452. 10.1021/bi700015m [DOI] [PubMed] [Google Scholar]
- Patra J. K., Das G., Fraceto L. F., Campos E., Rodriguez-Torres M., Acosta-Torres L. S., et al. (2018). Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 16 (1), 71. 10.1186/s12951-018-0392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q., Berg K., Moan J., Kongshaug M., Nesland J. M. (1997). 5-Aminolevulinic acid-based photodynamic therapy: Principles and experimental research. Photochem. Photobiol. 65 (2), 235–251. 10.1111/j.1751-1097.1997.tb08549.x [DOI] [PubMed] [Google Scholar]
- Pickel A. D., Teitelboim A., Chan E. M., Borys N. J., Schuck P. J., Dames C. (2018). Apparent self-heating of individual upconverting nanoparticle thermometers. Nat. Commun. 9 (1), 4907. 10.1038/s41467-018-07361-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietzak E. J. (2017). The impact of blue light cystoscopy on the diagnosis and treatment of bladder cancer. Curr. Urol. Rep. 18 (5), 39. 10.1007/s11934-017-0685-8 [DOI] [PubMed] [Google Scholar]
- Plaza-Oliver M., Santander-Ortega M. J., Lozano M. V. (2021). Current approaches in lipid-based nanocarriers for oral drug delivery. Drug Deliv. Transl. Res. 11 (2), 471–497. 10.1007/s13346-021-00908-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponka P. (1999). Cell biology of heme. Am. J. Med. Sci. 318 (4), 241–256. 10.1097/00000441-199910000-00004 [DOI] [PubMed] [Google Scholar]
- Punjabi A., Wu X., Tokatli-Apollon A., El-Rifai M., Lee H., Zhang Y., et al. (2014). Amplifying the red-emission of upconverting nanoparticles for biocompatible clinically used prodrug-induced photodynamic therapy. ACS Nano 8 (10), 10621–10630. 10.1021/nn505051d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pye A., Curnow A. (2007). Direct comparison of delta-aminolevulinic acid and methyl-aminolevulinate-derived protoporphyrin IX accumulations potentiated by desferrioxamine or the novel hydroxypyridinone iron chelator CP94 in cultured human cells. Photochem. Photobiol. 83 (3), 766–773. 10.1562/2006-05-30-RA-906 [DOI] [PubMed] [Google Scholar]
- Richardson D., Ponka P., Baker E. (1994). The effect of the iron(III) chelator, desferrioxamine, on iron and transferrin uptake by the human malignant melanoma cell. Cancer Res. 54 (3), 685–689. [PubMed] [Google Scholar]
- Robey R. W., Steadman K., Polgar O., Bates S. E. (2005). ABCG2-mediated transport of photosensitizers: Potential impact on photodynamic therapy. Cancer Biol. Ther. 4 (2), 195–202. 10.4161/cbt.4.2.1440 [DOI] [PubMed] [Google Scholar]
- Rodriguez L., Vallecorsa P., Battah S., Di Venosa G., Calvo G., Mamone L., et al. (2015). Aminolevulinic acid dendrimers in photodynamic treatment of cancer and atheromatous disease. Photochem. Photobiol. Sci. 14 (9), 1617–1627. 10.1039/c5pp00126a [DOI] [PubMed] [Google Scholar]
- Ruzycka-Ayoush M., Kowalik P., Kowalczyk A., Bujak P., Nowicka A. M., Wojewodzka M., et al. (2021). Quantum dots as targeted doxorubicin drug delivery nanosystems in human lung cancer cells. Cancer Nanotechnol. 12 (1), 8. 10.1186/s12645-021-00077-9 [DOI] [Google Scholar]
- Serda M., Gawecki R., Dulski M., Sajewicz M., Talik E., Szubka M., et al. (2022). Synthesis and applications of [60]fullerene nanoconjugate with 5-aminolevulinic acid and its glycoconjugate as drug delivery vehicles. RSC Adv. 12 (11), 6377–6388. 10.1039/d1ra08499b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sguizzato M., Esposito E., Cortesi R. (2021). Lipid-based nanosystems as a tool to overcome skin barrier. Int. J. Mol. Sci. 22 (15), 8319. 10.3390/ijms22158319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahriari M., Zahiri M., Abnous K., Taghdisi S. M., Ramezani M., Alibolandi M. (2019). Enzyme responsive drug delivery systems in cancer treatment. J. Control. Release. 308, 172–189. 10.1016/j.jconrel.2019.07.004 [DOI] [PubMed] [Google Scholar]
- Sherje A. P., Jadhav M., Dravyakar B. R., Kadam D. (2018). Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 548 (1), 707–720. 10.1016/j.ijpharm.2018.07.030 [DOI] [PubMed] [Google Scholar]
- Shi L., Wang X., Zhao F., Luan H., Tu Q., Huang Z., et al. (2013). In vitro evaluation of 5-aminolevulinic acid (ALA) loaded PLGA nanoparticles. Int. J. Nanomed. 8, 2669–2676. 10.2147/IJN.S45821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A. K., Anand S., Ortel B. J., Chang Y., Mai Z., Hasan T., et al. (2006). Methotrexate used in combination with aminolaevulinic acid for photodynamic killing of prostate cancer cells. Br. J. Cancer. 95 (4), 485–495. 10.1038/sj.bjc.6603273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slor G., Olea A. R., Pujals S., Tigrine A., De La Rosa V. R., Hoogenboom R., et al. (2021). Judging enzyme-responsive micelles by their covers: Direct comparison of dendritic amphiphiles with different hydrophilic blocks. Biomacromolecules 22 (3), 1197–1210. 10.1021/acs.biomac.0c01708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R. A., Rivera G. P., Zhang F., Zanella M., Parak W. J. (2008). Biological applications of gold nanoparticles. Chem. Soc. Rev. 37 (9), 1896–1908. 10.1039/b712170a [DOI] [PubMed] [Google Scholar]
- Srinivasulu Y. G., Mozhi A., Goswami N., Yao Q., Xie J. (2021). Traceable nanocluster-prodrug conjugate for chemo-photodynamic combinatorial therapy of non-small cell lung cancer. ACS Appl. Bio Mat. 4 (4), 3232–3245. 10.1021/acsabm.0c01611 [DOI] [PubMed] [Google Scholar]
- Stepp H., Waidelich R. (2007). [Fluorescence diagnosis and photodynamic therapy in urology]. Aktuelle Urol. 38 (6), 455–464. 10.1055/s-2007-980149 [DOI] [PubMed] [Google Scholar]
- Sun S., Wang D., Yin R., Zhang P., Jiang R., Xiao C. (2022). A two-in-one nanoprodrug for photoacoustic imaging-guided enhanced sonodynamic therapy. Small 18 (26), e2202558. 10.1002/smll.202202558 [DOI] [PubMed] [Google Scholar]
- Sun Y., Zhao D., Wang G., Wang Y., Cao L., Sun J., et al. (2020). Recent progress of hypoxia-modulated multifunctional nanomedicines to enhance photodynamic therapy: Opportunities, challenges, and future development. Acta Pharm. Sin. B 10 (8), 1382–1396. 10.1016/j.apsb.2020.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J., Sun C., Xu K., Wang C., Guo J. (2015). Immobilization of ALA-Zn(II) coordination polymer pro-photosensitizers on magnetite colloidal supraparticles for target photodynamic therapy of bladder cancer. Small 11 (47), 6338–6346. 10.1002/smll.201502131 [DOI] [PubMed] [Google Scholar]
- Tewari K. M., Dondi R., Yaghini E., Pourzand C., Macrobert A. J., Eggleston I. M. (2021). Peptide-targeted dendrimeric prodrugs of 5-aminolevulinic acid: A novel approach towards enhanced accumulation of protoporphyrin IX for photodynamic therapy. Bioorg. Chem. 109, 104667. 10.1016/j.bioorg.2021.104667 [DOI] [PubMed] [Google Scholar]
- Tong H., Wang Y., Li H., Jin Q., Ji J. (2016). Dual pH-responsive 5-aminolevulinic acid pseudopolyrotaxane prodrug micelles for enhanced photodynamic therapy. Chem. Commun. 52 (20), 3966–3969. 10.1039/c6cc00450d [DOI] [PubMed] [Google Scholar]
- Torres V. M., Posa M., Srdjenovic B., Simplício A. L. (2011). Solubilization of fullerene C60 in micellar solutions of different solubilizers. Colloid Surf. b-biointerfaces. 82 (1), 46–53. 10.1016/j.colsurfb.2010.08.012 [DOI] [PubMed] [Google Scholar]
- Vallejo M., Moura N., Gomes A., Joaquinito A., Faustino M., Almeida A., et al. (2021). The role of porphyrinoid photosensitizers for skin wound healing. Int. J. Mol. Sci. 22 (8), 4121. 10.3390/ijms22084121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk T., Jähde E., Fortmeyer H. P., Glüsenkamp K. H., Rajewsky M. F. (1993). pH in human tumour xenografts: effect of intravenous administration of glucose. Br. J. Cancer. 68 (3), 492–500. 10.1038/bjc.1993.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Tian Y., Liao X., Tang Y., Ni Q., Sun J., et al. (2020). Enhancing selective photosensitizer accumulation and oxygen supply for high-efficacy photodynamic therapy toward glioma by 5-aminolevulinic acid loaded nanoplatform. J. Colloid Interface Sci. 565, 483–493. 10.1016/j.jcis.2020.01.020 [DOI] [PubMed] [Google Scholar]
- Wang Y., Zu M., Ma X., Jia D., Lu Y., Zhang T., et al. (2020). Glutathione-responsive multifunctional "trojan horse" nanogel as a nanotheranostic for combined chemotherapy and photodynamic anticancer therapy. ACS Appl. Mat. Interfaces. 12 (45), 50896–50908. 10.1021/acsami.0c15781 [DOI] [PubMed] [Google Scholar]
- Wells A., Grandis J. R. (2003). Phospholipase C-gamma1 in tumor progression. Clin. Exp. Metastasis. 20 (4), 285–290. 10.1023/a:1024088922957 [DOI] [PubMed] [Google Scholar]
- Wu H., Zeng F., Zhang H., Xu J., Qiu J., Wu S. (2016). A nanosystem capable of releasing a photosensitizer bioprecursor under two-photon irradiation for photodynamic therapy. Adv. Sci. 3 (2), 1500254. 10.1002/advs.201500254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Han H., Jin Q., Li Z., Li H., Ji J. (2017a). Design and proof of programmed 5-aminolevulinic acid prodrug nanocarriers for targeted photodynamic cancer therapy. ACS Appl. Mat. Interfaces. 9 (17), 14596–14605. 10.1021/acsami.6b15853 [DOI] [PubMed] [Google Scholar]
- Wu J., Lin Y., Li H., Jin Q., Ji J. (2017b). Zwitterionic stealth peptide-capped 5-aminolevulinic acid prodrug nanoparticles for targeted photodynamic therapy. J. Colloid Interface Sci. 485, 251–259. 10.1016/j.jcis.2016.09.012 [DOI] [PubMed] [Google Scholar]
- Xiang M., Zhou Q., Shi Z., Wang X., Li M., Jia Y., et al. (2021). A review of light sources and enhanced targeting for photodynamic therapy. Curr. Med. Chem. 28 (31), 6437–6457. 10.2174/0929867328666210121122106 [DOI] [PubMed] [Google Scholar]
- Xiao Q., Du W., Dong X., Du S., Ong S. Y., Tang G., et al. (2021a). Cell-penetrating mitochondrion-targeting ligands for the universal delivery of small molecules, proteins and nanomaterials. Chem.-Eur. J. 27 (47), 12207–12214. 10.1002/chem.202101989 [DOI] [PubMed] [Google Scholar]
- Xiao Q., Lin H., Wu J., Pang X., Zhou Q., Jiang Y., et al. (2020). Pyridine-embedded phenothiazinium dyes as lysosome-targeted photosensitizers for highly efficient photodynamic antitumor therapy. J. Med. Chem. 63 (9), 4896–4907. 10.1021/acs.jmedchem.0c00280 [DOI] [PubMed] [Google Scholar]
- Xiao Q., Mai B., Nie Y., Yuan C., Xiang M., Shi Z., et al. (2021b). In vitro and in vivo demonstration of ultraefficient and broad-spectrum antibacterial agents for photodynamic antibacterial chemotherapy. ACS Appl. Mat. Interfaces. 13 (10), 11588–11596. 10.1021/acsami.0c20837 [DOI] [PubMed] [Google Scholar]
- Xiao Q., Wu J., Pang X., Jiang Y., Wang P., Leung A. W., et al. (2018). Discovery and development of natural products and their derivatives as photosensitizers for photodynamic therapy. Curr. Med. Chem. 25 (7), 839–860. 10.2174/0929867324666170823143137 [DOI] [PubMed] [Google Scholar]
- Yamamoto J., Kitagawa T., Miyaoka R., Suzuki K., Takamatsu S., Saito T., et al. (2020). 5-Aminolevulinic acid: Pitfalls of fluorescence-guided resection for malignant gliomas and application for malignant glioma therapy. J. UOEH 42 (1), 27–34. 10.7888/juoeh.42.27 [DOI] [PubMed] [Google Scholar]
- Zhang J., Lin Y., Lin Z., Wei Q., Qian J., Ruan R., et al. (2022). Stimuli-responsive nanoparticles for controlled drug delivery in synergistic cancer immunotherapy. Adv. Sci. 9 (5), e2103444. 10.1002/advs.202103444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang X., Yang H., Yu L., Xu Y., Sharma A., et al. (2021). Advanced biotechnology-assisted precise sonodynamic therapy. Chem. Soc. Rev. 50 (20), 11227–11248. 10.1039/d1cs00403d [DOI] [PubMed] [Google Scholar]
- Zhang Z., Wang S., Xu H., Wang B., Yao C. (2015). Role of 5-aminolevulinic acid-conjugated gold nanoparticles for photodynamic therapy of cancer. J. Biomed. Opt. 20 (5), 51043. 10.1117/1.JBO.20.5.051043 [DOI] [PubMed] [Google Scholar]
- Zou Q., Chang R., Xing R., Yuan C., Yan X. (2020). Injectable self-assembled bola-dipeptide hydrogels for sustained photodynamic prodrug delivery and enhanced tumor therapy. J. Control. Release. 319, 344–351. 10.1016/j.jconrel.2020.01.002 [DOI] [PubMed] [Google Scholar]