Abstract
Erectile dysfunction is a common disease of the male reproductive system, which seriously affects the life quality of patients and their partners. At present, erectile dysfunction is considered as a social-psychological-physiological disease with complex etiology and various treatment methods. Oral PDE5I is the first-line treatment for erectile dysfunction with the advantages of high safety, good effect and non-invasiveness. But intracavernosal injection, hormonal replacement therapy, vacuum erection device, penile prosthesis implantation can also be alternative treatments for patients have organic erectile dysfunction or tolerance to PDE5I. With the rapid development of technologies, some new methods, such as low-intensity extracorporeal shock wave and stem cell injection therapy can even repair the organic damage of the corpora cavernosa. These are important directions for the treatment of male erectile dysfunction in the future. In this mini-review, we will introduce these therapies in detail.
Keywords: erectile dysfunction, phosphodiesterase 5 inhibitor, intracavernosal injection, hormonal replacement therapy, vacuum erection device, penile prosthesis implantation, low-intensity extracorporeal shock wave, stem cell injection therapy
Introduction
Erectile dysfunction (ED) is defined as the consistent inability to attain and maintain an erection sufficient to perform satisfactory sexual intercourse (1). ED is a common male problem at all ages that has a great impact on the quality of life of sufferers and their partners. More than 150 million men worldwide are reported to have ED in different extent (2). Due to racial and regional differences and different definitions of ED, there is a large gap in the existing epidemiological data of ED. In the United States, the incidence of ED is 25.9 cases per 1000 people, and it increases with age with more than 70% of men over 70 years old affected by ED. It is predicted that by 2025, 322 million men worldwide will have ED (3–5). Studies have shown that the occurrence of ED is associated with many comorbidities and risk factors, such as aging, smoking, obesity, decreased androgen levels, cardiovascular disease, depression, prostate surgery, and penile trauma (6–8).
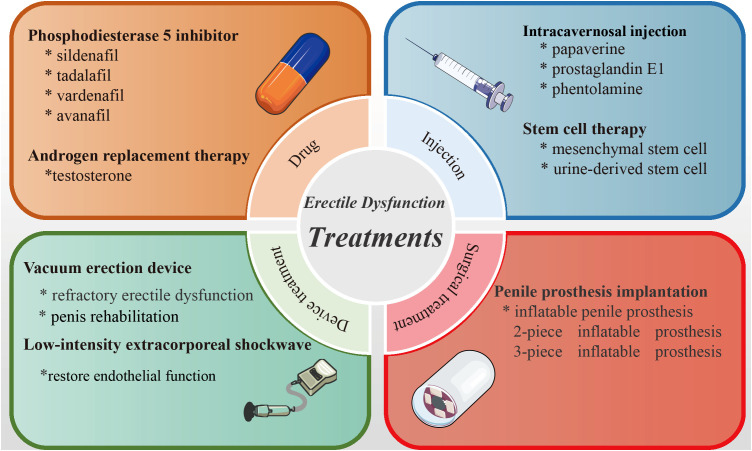
Normal penile erection is a neurovascular phenomenon controlled by psychological factors and coordinated by endocrine, vascular, and nervous system (9). The first step in management of ED is often making lifestyle changes, such as losing weight, reducing alcohol intake, and avoiding smoking. These psychosocial methods are effective when ED is mainly caused by emotional or psychological factors (10). Current therapies to treat ED mainly include oral phosphodiesterase 5 inhibitor (PDE5I), intracavernosal injection, hormonal replacement therapy, vacuum erection device, penile prosthesis, low-intensity extracorporeal shock wave (Li-ESW), and stem cell injection therapy (11) ( Figure 1 ). Under different conditions, any option can be the first line of treatment. To data, PDE5I is still the most popular treatment option due to its good efficacy, safety, and non-invasiveness. But, the growing number of patients with no or low response to PDE5I, and the potential of adverse reactions have prompted the development of safer and more effective treatments (12). In this paper, we will introduce various treatment methods for ED in detail based on current research progress.
Figure 1.
Summary diagram of the therapies of erectile dysfunction.
Drug therapy: Phosphodiesterase 5 inhibitors
Phosphodiesterase 5 (PDE5) is highly expressed in vascular smooth muscle and it is the most common PDE subtype in penile smooth muscle. Penile erection mainly depends on the activation of NO/cGMP signal pathway and the function of PDE5 is to block the decomposition of cyclic guanosine monophosphate (cGMP) (13, 14). the NO produced by non-adrenergic/non-cholinergic neurons and endothelial cells is released into the corpora cavernosa, resulting in an increase in the concentration of cGMP, thus promoting the relaxation of smooth muscle in the corpora cavernosa and the expansion of penile blood vessels, finally leading to vascular filling and penile erection (15). Therefore, PDE5I can enhance erectile response and treat erectile dysfunction by enhancing the downstream cGMP effect caused by NO.
PDE5Is are the first-line treatment for erectile dysfunction (16). At present, four PDE5I drugs have been approved by the FDA, namely sildenafil, tadalafil, vardenafil, and avanafil. These PDE5Is have different pharmacokinetic properties but have similar efficacy, safety, and tolerability (17). Among them, sildenafil is the first approved, safe, and effective oral drug for the treatment of ED. In a randomized, double-blind study, Goldstein et al. (18) included 532 male patients diagnosed with ED at least 6 months and randomly assigned 316 patients to the sildenafil group (25, 50 or 100ng) and 216 patients to the placebo control group. After 24 weeks, the erectile function of the oral sildenafil treatment group improved significantly, and men taking 100 mg of sildenafil showed a better therapeutic effect compared to the placebo control group. Sildenafil can also be used in combination with other drugs. In another randomized controlled study, 59 patients with organic ED were included in the study (19). One group received oral sildenafil 50 mg, the other group received oral sildenafil 50 mg and L-arginine 1g, after 8 weeks of treatment, patients in the combined treatment group showed better erectile function. These studies have proved the effectiveness of sildenafil in the treatment of ED and its potential in combination with other therapies. As a powerful and highly selective PDE5I, avanafil has been reported to have better effects and fewer adverse reactions. Kumar et al. (20) recently reported a randomized, controlled, double-blind clinical trial in which 220 patients with ED were randomly divided into two groups in a 1:1 ratio, they were given orally 100 mg of avanafil and 50 mg of sildenafil respectively. International index of erectile function (IIEF) score, sexual encounter profile (SEF), and adverse drug events were evaluated, and the results demonstrated that avanafil took effect quickly, and most people showed good erectile function after 15 minutes of medication.
In addition to acting on PDE5 of cavernous smooth muscle, PDE5I also inhibits PDE5 and its isozymes in blood vessels, viscera, skeletal muscle, platelets, and other tissues, causing reactions in multiple systems. During treatment, adverse reactions such as headache, blush, dyspepsia, and visual disturbance can occur (21). In addition, after taking tadalafil for 6 months, the weight of testis, sperm quantity and sperm activity of the aged male rats were significantly reduced (22), after 12 weeks of oral administration of sildenafil, tadalafil, and vardenafil in male rabbits, the number of sperm in sperm cells and testis were also decreased (23). In general, PDE5I is a safe, effective, and well-tolerated first-line treatment for ED, for most patients, 50 mg sildenafil is the preferred treatment, after drug tolerance, 10 mg tadalafil or 100 mg udenafil can be used instead (24). Not only that, but current studies also found new molecular mechanisms of ED beyond the ‘NO/cGMP’ pathway. For example, hyperglycemia and increased oxidative stress are main contributors to endothelial dysfunction in ED patients complicated with diabetes mellitus (25). The major regulatory unit of myosin light chain phosphatase MYPT1 regulated ED by G-protein couple receptor pathway, and the lotusine could recover the level of MYPT1 and improve the function of injured penile smooth muscles. These studies provide novel therapeutic targets for the treatment of ED in the future (26).
Intracavernosal injection
Intracavernosal injection (ICI) of vasoactive drugs such as papaverine and prostaglandin E1 to induce penis erection is a breakthrough in the treatment of ED and can also be used as a diagnostic method (27). ICI is an effective local drug therapy for ED, individualized treatment plans can be formulated according to the individual conditions and needs of the patients (28). The combination of different vasoactive drugs and different injection doses can significantly improve the treatment effect and reduce complications. The study showed that the patients injected with papaverine and prostaglandin E1 could achieve satisfactory erectile function, which was better than patients injected with prostaglandin E1 alone (29). In a retrospective study, the researcher included 105 middle-aged and elderly patients and found that after ICI treatment, the patient’s penis hardness increased, erectile function improved, and there were no obvious complications, this means that ICI therapy is safe and feasible (30). However, with the use of PDE5Is, the clinical application of ICI has gradually decreased, because it has a high dropout rate and is related to priapism, ecchymoses, hematoma, and penile fibrosis (31). At present, the combination of ICI and Doppler ultrasound is mainly used in the diagnosis of ED and the evaluation of penile hemodynamics (32).
Hormonal replacement therapy
Androgen plays an important role in promoting the normal growth of the penis and stimulating the secondary sexual characteristics of men. Androgen is mainly secreted by the testis, androgen deficiency will lead to a series of pathophysiological conditions, which will damage the sexual function and overall health of the body (33). Researchers found that serum total testosterone, especially free testosterone and bioavailable testosterone levels of men will gradually decrease with age. A study showed that 64% of men over 40 years of age will be diagnosed with moderate, severe and very serious ED and older men over 60 years of age are more likely to suffer from more serious ED (34). Similarly, Rabijewski et al. found that 53% of the elderly men over 65 years old had lower testosterone levels, and ED was more serious in these men. They also found that there was significant negative correlation between age and testosterone (r=-0.3328, p<0.05), IIEF score and testosterone (r=-0.3149, p<0.05), and age and IIEF score (r=-0.3463, p<0.05) (35).
In clinic, androgen replacement therapy can restore the serum testosterone level to normal, and improve the sexual desire of patients with hypogonadism. In addition, compared with the placebo group, after receiving androgen replacement therapy, the patients would get better mood and the depression was relieved (36). Another study showed that in older men with low testosterone levels older than 65 years, the frequency of sexual activity increased significantly and the sexual desire improved after one year of androgen replacement treatment (37). More importantly, androgen replacement therapy combined with PDE5I can effectively treat ED, and patients’ erectile function can even be maintained well after drugs withdrawal (38).
Vacuum erection device
Vacuum erection device (VED) is a mechanical device that can increase the blood flowing into the corpora cavernosa by creating a vacuum environment of up to 250 mmHg and there is a restraining ring at the root of the penis to maintain congestion, promote erection (39). VED is mainly used to treat patients with organic ED and it has high success rate and small side effects (40). In a recent study, 56 middle-aged and elderly patients with ED were treated with VED, 96% of the patients believed that the device could promote the ability of erection and 94% of the patients and their partners thought that they regained satisfactory sexual activities after VED treatment. Nevertheless, nearly 28.6% of the patients reported physical discomfort when using the device, usually due to the pain caused by using the restraining ring (41).
In addition to treating ED, VED can also treat penis atrophy after radical prostatectomy by enhancing oxygen saturation in the corpora cavernosa (42). Rats with penile atrophy and decreased erectile function were created by bilateral cavernous compression injury (BCNI). After 6 weeks of treatment with VED for BCNI rats, the penis diameter of the rats was increased, the degree of atrophy decreased, and the oxygen saturation in the corpora cavernosa increased. These results show that VED treatment has therapeutic effect by increasing the anti-hypoxia ability of corpora cavernosa (43). PDE5I tolerance is a common outcome of oral medication in ED patients and about 30% of ED patients have no obvious response to PDE5I treatment eventually (44). After using VED treatment for these patients, their erectile function improved and their sexual desire increased. VED is the second-line treatment for ED, but it should be considered as the first-line treatment for some men who have tolerance to PDE5I or need penis rehabilitation (45).
Penile prosthesis implantation
Penile prosthesis implantation (PPI) is currently the third-line treatment for ED. Because it can cause irreparable damage to the smooth muscle of the corpora cavernosa, it is usually considered when oral PDE5I drugs, intracavernosal injection, and VED therapy are ineffective (46). 3-piece inflatable prosthesis is the most common implant at present, and is also the most satisfactory (47). The 3-piece inflatable prosthesis can manually adjust the thickness, length, and hardness of the penis, and simulate the natural erection process. Therefore, it should be recommended for patients who choose PPI.
An early multi-center study reported that more than 90% of patients with ED and their partners can achieve normal sexual activity after receiving penis prosthesis implantation (48). Recent study has shown that penis prosthesis implantation is particularly suitable for ED patients secondary to Peyronie’s disease (49). Nevertheless, PPI is costly, traumatic, and prone to complications, such as prosthetic infection, pump migration, automatic inflation, secondary surgery, etc. This is the main reason why it cannot become a first-line treatment (50).
Low-intensity extracorporeal shock wave
Low-intensity extracorporeal shock wave (Li-ESW) is a physical shock wave that emits energy density lower than 0.1mj/mm2. As a non-invasive treatment technology, Li-ESW focuses on the target tissue area through the sound wave passing through the tissue structure (51). Studies have shown that one of the causes of ED is the decreased blood circulation in the corpora cavernosa, Li-ESW can stimulate the expression of eNOS, VEGF and other vascular growth factors in the corpora cavernosa, expand blood vessels, induce neovascularization, promote blood flow, and improve erectile function (52, 53).
Vardi et al. (54) treated 20 middle-aged patients with vascular ED with Li-ESW, the patients received 12 Li-ESW treatments within 6 weeks. The results showed that the erectile function of 75% of patients was significantly improved, and the IIEF score was obviously increased, the penile blood flow, erection duration and penile hardness of the patients were also increased. Li-ESW therapy has also been proved to be a safe and effective method for those patients with poor effect of PDE5I (55). Another study found that the use of Li-ESW can reverse the PDE5I tolerance, and more than 50% of the patients can achieve sufficient erectile stiffness (56).
Li-ESW is a promising treatment for refractory ED. It can restore the endothelial function of the penis and increase the blood flow of the corpora cavernosa. However, the therapeutic mechanism of Li-ESW is not yet completely clear, and more research and exploration are still needed.
Stem cell injection therapy
Stem cells can differentiate into different types of cells under the stimulation of complicated external environments and cytokines. They can be divided into totipotent stem cells, pluripotent stem cells, multipotent stem cells, and unipotent stem cells (57). Studies have shown that stem cells can also promote angiogenesis, tissue healing, and anti-apoptosis through paracrine action, which is also the theoretical basis of the therapy for ED (58). In the current scheme of stem cell therapy, it mainly includes mesenchymal stem cell (MSC), adipose tissue-derived stem cell (ADSC), urine-derived stem cell (UDSC), and muscle-derived stem cell (MDSC) (58, 59). In 2004, Bochinski et al. first discovered that injection of neural embryonic stem cells into the corpus cavernosa of male rats with neurogenic impotence could improve the erectile function (60). In the BCNI rat model, injection of autologous ADSC cells into the corpora cavernosa of rats can effectively prevent erectile dysfunction caused by cavernous nerve injury, enhance the ratio of smooth muscle over collagen content, and promote the neuronal nitric oxide synthase-positive nerve regeneration (61, 62). In rat model of erectile dysfunction, intracavernous injection of ADSCs can ameliorate ultrastructural damage and systemic oxidative stress states caused by chronic tobacco exposure or hyperlipidemia (63, 64). In addition, intracavernous injection of ADSCs-derived microtissues improves erectile function in STZ-induced diabetic rats via expressing vascular endothelial growth factor (VEGF), nerve growth factor (NGF), and tumor necrosis factor-stimulated gene-6 (TSG-6) (65). Furthermore, the exosomes secreted by stem cells have also been proved to have an effect on improving erectile dysfunction in rats. The study found that the injection of ADSC-derived exosomes into the cavernous body of rats can promote the growth of endothelial cells and smooth muscle cells, inhibit cell apoptosis, alleviate tissue hypoxia, and promote the recovery of erectile function in rats (66, 67).
Although many animal experiments have confirmed the effectiveness of stem cell therapy in treating ED, there are only a few clinical trials on stem cell therapy. In an open-label phase 1 clinical trial, 17 male patients with ED after radical prostatectomy were insensitive to PDE5I and ICI. The researchers injected autologous adipose-derived regenerative cells (ADRCs) into the corpora cavernosa of patients, the results showed that 73% of the patients recovered their erectile function within 3 months after treatment (68). According to animal experiments and a small number of clinical studies on stem cell therapy for ED, stem cell therapy is safe and reliable. Stem cell therapy has a broad prospect in the treatment of ED, but more clinical trials are still needed before clinical application.
Conclusions and outlook
ED is a common disease in men and seriously affects the life quality of patients and their partners. Currently, oral PDE5I is the first-line treatment of ED which has the advantages of high safety and good effect. For patients with low response to PDE5I, other treatments include intracavernosal injection, hormonal treatment, vacuum erection device, and penile prosthesis implantation can also be alternative methods. In recent years, some new therapies like low-intensity extracorporeal shock wave and stem cell injection therapy proved to have exciting effect and can even reverse the organic damage of the corpora cavernosa. In fact, there are other advanced therapies, such as gene therapy (69), 3D-printed hydrogel scaffolds (70), and gene edited stem cells (71), they have all been shown to improve erectile function in animal experiments.
Despite these promising therapies are important directions for treatment of ED in the future, but only impressive animal studies proving the benefit. Large-scale, randomized, placebo-controlled studies are desperately needed for these novel therapeutics. In summary, ED is a complex disease associated with multiple risk factors, and effective therapy should be taken according to the etiology and individual conditions. The safety and efficacy of the promising therapies still need to be evaluated through a number of clinical trials with ethical support and fully informed consent.
Author contributions
This mini review is contributed by all authors. XH and JX conceived and designed the study. CW and BW wrote the manuscript. CW, BW, PX, JX, and XH reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding Statement
This study was supported by the National Natural Science Foundation of China (81901545) and the Natural Science Foundation of Anhui Provincial (1908085QH315).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. European Association of urology guidelines on sexual and reproductive health-2021 update: Male sexual dysfunction. Eur Urol (2021) 80(3):333–57. doi: 10.1016/j.eururo.2021.06.007 [DOI] [PubMed] [Google Scholar]
- 2. McCabe MP, Sharlip ID, Lewis R, Atalla E, Balon R, Fisher AD, et al. Incidence and prevalence of sexual dysfunction in women and men: A consensus statement from the fourth international consultation on sexual medicine 2015. J Sex Med (2016) 13(2):144–52. doi: 10.1016/j.jsxm.2015.12.034 [DOI] [PubMed] [Google Scholar]
- 3. Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int (1999) 84(1):50–6. doi: 10.1046/j.1464-410x.1999.00142.x [DOI] [PubMed] [Google Scholar]
- 4. Yafi FA, Jenkins L, Albersen M, Corona G, Isidori AM, Goldfarb S, et al. Erectile dysfunction. Nat Rev Dis Primers (2016) 2:16003. doi: 10.1038/nrdp.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med (2007) 120(2):151–7. doi: 10.1016/j.amjmed.2006.06.010 [DOI] [PubMed] [Google Scholar]
- 6. Zeleke M, Hailu D, Daka D. Erectile dysfunction and associated factors among diabetic patients at, hawassa, southern, Ethiopia. BMC Endocr Disord (2021) 21(1):139. doi: 10.1186/s12902-021-00807-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bauer SR, Breyer BN, Stampfer MJ, Rimm EB, Giovannucci EL, Kenfield SA. Association of diet with erectile dysfunction among men in the health professionals follow-up study. JAMA Netw Open (2020) 3(11):e2021701. doi: 10.1001/jamanetworkopen.2020.21701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Q, Zhang Y, Wang J, Li S, Cheng Y, Guo J, et al. Erectile dysfunction and depression: A systematic review and meta-analysis. J Sex Med (2018) 15(8):1073–82. doi: 10.1016/j.jsxm.2018.05.016 [DOI] [PubMed] [Google Scholar]
- 9. MacDonald SM, Burnett AL. Physiology of erection and pathophysiology of erectile dysfunction. Urol Clin North Am (2021) 48(4):513–25. doi: 10.1016/j.ucl.2021.06.009 [DOI] [PubMed] [Google Scholar]
- 10. Najari BB, Kashanian JA. Erectile dysfunction. Jama (2016) 316(17):1838. doi: 10.1001/jama.2016.12284 [DOI] [PubMed] [Google Scholar]
- 11. Raheem OA, Natale C, Dick B, Reddy AG, Yousif A, Khera M, et al. Novel treatments of erectile dysfunction: Review of the current literature. Sex Med Rev (2021) 9(1):123–32. doi: 10.1016/j.sxmr.2020.03.005 [DOI] [PubMed] [Google Scholar]
- 12. Cayetano-Alcaraz AA, Tharakan T, Chen R, Sofikitis N, Minhas S. The management of erectile dysfunction in men with diabetes mellitus unresponsive to phosphodiesterase type 5 inhibitors. Andrology (2022) 11(2):257–69. doi: 10.1111/andr.13257 [DOI] [PubMed] [Google Scholar]
- 13. Amin KM, El-Badry OM, Abdel Rahman DE, Abdellattif MH, Abourehab MAS, El-Maghrabey MH, et al. Scaffold repurposing reveals new nanomolar phosphodiesterase type 5 (PDE5) inhibitors based on pyridopyrazinone scaffold: Investigation of In vitro and in silico properties. Pharmaceutics (2022) 14(9):1954. doi: 10.3390/pharmaceutics14091954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hamed MA, Akhigbe TM, Akhigbe RE, Aremu AO, Oyedokun PA, Gbadamosi JA, et al. Glutamine restores testicular glutathione-dependent antioxidant defense and upregulates NO/cGMP signaling in sleep deprivation-induced reproductive dysfunction in rats. BioMed Pharmacother (2022) 148:112765. doi: 10.1016/j.biopha.2022.112765 [DOI] [PubMed] [Google Scholar]
- 15. Bobin P, Belacel-Ouari M, Bedioune I, Zhang L, Leroy J, Leblais V, et al. Cyclic nucleotide phosphodiesterases in heart and vessels: A therapeutic perspective. Arch Cardiovasc Dis (2016) 109(6-7):431–43. doi: 10.1016/j.acvd.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 16. Al Demour S, Jafar H, Adwan S, AlSharif A, Alhawari H, Alrabadi A, et al. Safety and potential therapeutic effect of two intracavernous autologous bone marrow derived mesenchymal stem cells injections in diabetic patients with erectile dysfunction: An open label phase I clinical trial. Urol Int (2018) 101(3):358–65. doi: 10.1159/000492120 [DOI] [PubMed] [Google Scholar]
- 17. Hatzimouratidis K, Salonia A, Adaikan G, Buvat J, Carrier S, El-Meliegy A, et al. Pharmacotherapy for erectile dysfunction: Recommendations from the fourth international consultation for sexual medicine (ICSM 2015). J Sex Med (2016) 13(4):465–88. doi: 10.1016/j.jsxm.2016.01.016 [DOI] [PubMed] [Google Scholar]
- 18. Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction. sildenafil study group. N Engl J Med (1998) 338(20):1397–404. doi: 10.1056/NEJM199805143382001 [DOI] [PubMed] [Google Scholar]
- 19. Goldstein I, Burnett AL, Rosen RC, Park PW, Stecher VJ. The serendipitous story of sildenafil: An unexpected oral therapy for erectile dysfunction. Sex Med Rev (2019) 7(1):115–28. doi: 10.1016/j.sxmr.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 20. Kumar M, Pathade AD, Gupta SV, Goyal S, Rath D, Thakre M, et al. Efficacy and safety of avanafil as compared with sildenafil in the treatment of erectile dysfunction: A randomized, double blind, multicenter clinical trial. Int J Urol (2022) 29(4):351–9. doi: 10.1111/iju.14785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corona G, Rastrelli G, Burri A, Jannini EA, Maggi M. The safety and efficacy of avanafil, a new 2(nd) generation PDE5i: comprehensive review and meta-analysis. Expert Opin Drug Saf (2016) 15(2):237–47. doi: 10.1517/14740338.2016.1130126 [DOI] [PubMed] [Google Scholar]
- 22. Khalaf MA, Abbas MF, El-Fakahany HM. Effects of chronic tadalafil use on the testes and sperm parameters of old albino rats. Andrologia (2012) 44(Suppl 1):370–5. doi: 10.1111/j.1439-0272.2011.01191.x [DOI] [PubMed] [Google Scholar]
- 23. Sheweita SA, Meftah AA, Sheweita MS, Balbaa ME. Erectile dysfunction drugs altered the activities of antioxidant enzymes, oxidative stress and the protein expressions of some cytochrome P450 isozymes involved in the steroidogenesis of steroid hormones. PloS One (2020) 15(11):e0241509. doi: 10.1371/journal.pone.0241509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen L, Staubli SE, Schneider MP, Kessels AG, Ivic S, Bachmann LM, et al. Phosphodiesterase 5 inhibitors for the treatment of erectile dysfunction: a trade-off network meta-analysis. Eur Urol (2015) 68(4):674–80. doi: 10.1016/j.eururo.2015.03.031 [DOI] [PubMed] [Google Scholar]
- 25. Castela Â, Costa C. Molecular mechanisms associated with diabetic endothelial-erectile dysfunction. Nat Rev Urol (2016) 13(5):266–74. doi: 10.1038/nrurol.2016.23 [DOI] [PubMed] [Google Scholar]
- 26. Zhao W, Sun J, Yao LY, Hang D, Li YQ, Chen CP, et al. MYPT1 reduction is a pathogenic factor of erectile dysfunction. Commun Biol (2022) 5(1):744. doi: 10.1038/s42003-022-03716-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim S, Cho MC, Cho SY, Chung H, Rajasekaran MR. Novel emerging therapies for erectile dysfunction. World J Mens Health (2021) 39(1):48–64. doi: 10.5534/wjmh.200007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bassiem MA, Ismail IY, Salem TA, El-Sakka AI. Effect of intracavernosal injection of prostaglandin E1 on duration and rigidity of erection in patients with vasculogenic erectile dysfunction: Is it dose dependent? Urology (2021) 148:173–8. doi: 10.1016/j.urology.2020.09.030 [DOI] [PubMed] [Google Scholar]
- 29. Richter S, Vardi Y, Ringel A, Shalev M, Nissenkorn I. Intracavernous injections: still the gold standard for treatment of erectile dysfunction in elderly men. Int J Impot Res (2001) 13(3):172–5. doi: 10.1038/sj.ijir.3900672 [DOI] [PubMed] [Google Scholar]
- 30. Bearelly P, Phillips EA, Pan S, O'Brien K, Asher K, Martinez D, et al. Long-term intracavernosal injection therapy: treatment efficacy and patient satisfaction. Int J Impot Res (2020) 32(3):345–51. doi: 10.1038/s41443-019-0186-z [DOI] [PubMed] [Google Scholar]
- 31. Belew D, Klaassen Z, Lewis RW. Intracavernosal injection for the diagnosis, evaluation, and treatment of erectile dysfunction: A review. Sex Med Rev (2015) 3(1):11–23. doi: 10.1002/smrj.35 [DOI] [PubMed] [Google Scholar]
- 32. Zhang J, Zhou W, Zhang Y, Zhang W, Zhang C. A novel method to quantify penile arterial blood supply using shear wave elastography during penile duplex ultrasound in men with erectile dysfunction. Med Sci Monit (2022) 28:e935232. doi: 10.12659/MSM.935232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schiffer L, Arlt W, Storbeck KH. Intracrine androgen biosynthesis, metabolism and action revisited. Mol Cell Endocrinol (2018) 465:4–26. doi: 10.1016/j.mce.2017.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Naidan N, Rivaad OE, Muukhai N, Janlav M. Testosterone deficiency with erectile dysfunction in mongolian men. World J Mens Health (2013) 31(2):170–5. doi: 10.5534/wjmh.2013.31.2.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rabijewski M, Papierska L, Kozakowski J, Zgliczyński W. The high prevalence of testosterone deficiency in population of polish men over 65 years with erectile dysfunctions. Aging Male (2012) 15(4):258–62. doi: 10.3109/13685538.2012.729233 [DOI] [PubMed] [Google Scholar]
- 36. Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, et al. Effects of testosterone treatment in older men. N Engl J Med (2016) 374(7):611–24. doi: 10.1056/NEJMoa1506119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cunningham GR, Stephens-Shields AJ, Rosen RC, Wang C, Bhasin S, Matsumoto AM, et al. Testosterone treatment and sexual function in older men with low testosterone levels. J Clin Endocrinol Metab (2016) 101(8):3096–104. doi: 10.1210/jc.2016-1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park MG, Yeo JK, Cho DY, Kim JW, Kim JW, Oh MM, et al. The efficacy of combination treatment with injectable testosterone undecanoate and daily tadalafil for erectile dysfunction with testosterone deficiency syndrome. J Sex Med (2015) 12(4):966–74. doi: 10.1111/jsm.12842 [DOI] [PubMed] [Google Scholar]
- 39. Sultana A, Grice P, Vukina J, Pearce I, Modgil V. Indications and characteristics of penile traction and vacuum erection devices. Nat Rev Urol (2022) 19(2):84–100. doi: 10.1038/s41585-021-00532-7 [DOI] [PubMed] [Google Scholar]
- 40. Lin H, Wang G, Wang R. Application of the vacuum erectile device in penile rehabilitation for erectile dysfunction after radical prostatectomy. Zhonghua Nan Ke Xue (2015) 21(3):195–9. [PubMed] [Google Scholar]
- 41. Beaudreau SA, Van Moorleghem K, Dodd SM, Liou-Johnson V, Suresh M, Gould CE. Satisfaction with a vacuum constriction device for erectile dysfunction among middle-aged and older veterans. Clin Gerontol (2021) 44(3):307–15. doi: 10.1080/07317115.2020.1823922 [DOI] [PubMed] [Google Scholar]
- 42. Lima TFN, Bitran J, Frech FS, Ramasamy R. Prevalence of post-prostatectomy erectile dysfunction and a review of the recommended therapeutic modalities. Int J Impot Res (2021) 33(4):401–9. doi: 10.1038/s41443-020-00374-8 [DOI] [PubMed] [Google Scholar]
- 43. Lin HC, Yang WL, Zhang JL, Dai YT, Wang R. Penile rehabilitation with a vacuum erectile device in an animal model is related to an antihypoxic mechanism: blood gas evidence. Asian J Androl (2013) 15(3):387–90. doi: 10.1038/aja.2013.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee M, Sharifi R. Non-invasive management options for erectile dysfunction when a phosphodiesterase type 5 inhibitor fails. Drugs Aging (2018) 35(3):175–87. doi: 10.1007/s40266-018-0528-4 [DOI] [PubMed] [Google Scholar]
- 45. Brison D, Seftel A, Sadeghi-Nejad H. The resurgence of the vacuum erection device (VED) for treatment of erectile dysfunction. J Sex Med (2013) 10(4):1124–35. doi: 10.1111/jsm.12046 [DOI] [PubMed] [Google Scholar]
- 46. Kohn TP, Rajanahally S, Hellstrom WJG, Hsieh TC, Raheem OA. Global trends in prevalence, treatments, and costs of penile prosthesis for erectile dysfunction in men. Eur Urol Focus (2022) 8(3):803–13. doi: 10.1016/j.euf.2021.05.003 [DOI] [PubMed] [Google Scholar]
- 47. Segal RL, Camper SB, Burnett AL. Modern utilization of penile prosthesis surgery: a national claim registry analysis. Int J Impot Res (2014) 26(5):167–71. doi: 10.1038/ijir.2014.11 [DOI] [PubMed] [Google Scholar]
- 48. Montorsi F, Rigatti P, Carmignani G, Corbu C, Campo B, Ordesi G, et al. AMS three-piece inflatable implants for erectile dysfunction: a long-term multi-institutional study in 200 consecutive patients. Eur Urol (2000) 37(1):50–5. doi: 10.1159/000020099 [DOI] [PubMed] [Google Scholar]
- 49. La Croce G, Schifano N, Pescatori E, Caraceni E, Colombo F, Bettocchi C, et al. Which patient may benefit the most from penile prosthesis implantation? Andrology (2022) 10(8):1567–74. doi: 10.1111/andr.13294 [DOI] [PubMed] [Google Scholar]
- 50. Lee DJ, Najari BB, Davison WL, Al Hussein Al Awamlh B, Zhao F, Paduch DA, et al. Trends in the utilization of penile prostheses in the treatment of erectile dysfunction in the united states. J Sex Med (2015) 12(7):1638–45. doi: 10.1111/jsm.12921 [DOI] [PubMed] [Google Scholar]
- 51. Dong L, Chang D, Zhang X, Li J, Yang F, Tan K, et al. Effect of low-intensity extracorporeal shock wave on the treatment of erectile dysfunction: A systematic review and meta-analysis. Am J Mens Health (2019) 13(2):1557988319846749. doi: 10.1177/1557988319846749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sokolakis I, Dimitriadis F, Teo P, Hatzichristodoulou G, Hatzichristou D, Giuliano F. The basic science behind low-intensity extracorporeal shockwave therapy for erectile dysfunction: A systematic scoping review of pre-clinical studies. J Sex Med (2019) 16(2):168–94. doi: 10.1016/j.jsxm.2018.12.016 [DOI] [PubMed] [Google Scholar]
- 53. Gruenwald I, Appel B, Vardi Y. Low-intensity extracorporeal shock wave therapy–a novel effective treatment for erectile dysfunction in severe ED patients who respond poorly to PDE5 inhibitor therapy. J Sex Med (2012) 9(1):259–64. doi: 10.1111/j.1743-6109.2011.02498.x [DOI] [PubMed] [Google Scholar]
- 54. Vardi Y, Appel B, Jacob G, Massarwi O, Gruenwald I. Can low-intensity extracorporeal shockwave therapy improve erectile function? a 6-month follow-up pilot study in patients with organic erectile dysfunction. Eur Urol (2010) 58(2):243–8. doi: 10.1016/j.eururo.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 55. Grandez-Urbina JA, Rodríguez RP, Torres-Román JS, Saldaña-Gallo J, García-Perdomo HA. [Low-intensity extracorporeal shock wave treatment improves erectile function in non-responder PDEi5 patients: A systematic review]. Rev Int Androl (2021) 19(4):272–80. doi: 10.1016/j.androl.2020.04.004 [DOI] [PubMed] [Google Scholar]
- 56. Kitrey ND, Gruenwald I, Appel B, Shechter A, Massarwa O, Vardi Y. Penile low intensity shock wave treatment is able to shift PDE5i nonresponders to responders: A double-blind, sham controlled study. J Urol (2016) 195(5):1550–5. doi: 10.1016/j.juro.2015.12.049 [DOI] [PubMed] [Google Scholar]
- 57. Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev (2005) 19(10):1129–55. doi: 10.1101/gad.1303605 [DOI] [PubMed] [Google Scholar]
- 58. Matz EL, Terlecki R, Zhang Y, Jackson J, Atala A. Stem cell therapy for erectile dysfunction. Sex Med Rev (2019) 7(2):321–8. doi: 10.1016/j.sxmr.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 59. Kachgal S, Putnam AJ. Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis (2011) 14(1):47–59. doi: 10.1007/s10456-010-9194-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bochinski D, Lin GT, Nunes L, Carrion R, Rahman N, Lin CS, et al. The effect of neural embryonic stem cell therapy in a rat model of cavernosal nerve injury. BJU Int (2004) 94(6):904–9. doi: 10.1111/j.1464-410X.2003.05057.x [DOI] [PubMed] [Google Scholar]
- 61. Xu Y, Guan R, Lei H, Li H, Wang L, Gao Z, et al. Therapeutic potential of adipose-derived stem cells-based micro-tissues in a rat model of postprostatectomy erectile dysfunction. J Sex Med (2014) 11(10):2439–48. doi: 10.1111/jsm.12636 [DOI] [PubMed] [Google Scholar]
- 62. Chen Z, Han X, Ouyang X, Fang J, Huang X, Wei H. Transplantation of induced pluripotent stem cell-derived mesenchymal stem cells improved erectile dysfunction induced by cavernous nerve injury. Theranostics (2019) 9(22):6354–68. doi: 10.7150/thno.34008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Huang YC, Ning H, Shindel AW, Fandel TM, Lin G, Harraz AM, et al. The effect of intracavernous injection of adipose tissue-derived stem cells on hyperlipidemia-associated erectile dysfunction in a rat model. J Sex Med (2010) 7(4 Pt 1):1391–400. doi: 10.1111/j.1743-6109.2009.01697.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huang YC, Kuo YH, Huang YH, Chen CS, Ho DR, Shi CS. The effects of adipose-derived stem cells in a rat model of tobacco-associated erectile dysfunction. PloS One (2016) 11(6):e0156725. doi: 10.1371/journal.pone.0156725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhou F, Hui Y, Xin H, Xu YD, Lei HE, Yang BC, et al. Therapeutic effects of adipose-derived stem cells-based microtissues on erectile dysfunction in streptozotocin-induced diabetic rats. Asian J Androl (2017) 19(1):91–7. doi: 10.4103/1008-682x.182817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen F, Zhang H, Wang Z, Ding W, Zeng Q, Liu W, et al. Adipose-derived stem cell-derived exosomes ameliorate erectile dysfunction in a rat model of type 2 diabetes. J Sex Med (2017) 14(9):1084–94. doi: 10.1016/j.jsxm.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 67. Liang L, Zheng D, Lu C, Xi Q, Bao H, Li W, et al. Exosomes derived from miR-301a-3p-overexpressing adipose-derived mesenchymal stem cells reverse hypoxia-induced erectile dysfunction in rat models. Stem Cell Res Ther (2021) 12(1):87. doi: 10.1186/s13287-021-02161-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Haahr MK, Jensen CH, Toyserkani NM, Andersen DC, Damkier P, Sørensen JA, et al. Safety and potential effect of a single intracavernous injection of autologous adipose-derived regenerative cells in patients with erectile dysfunction following radical prostatectomy: An open-label phase I clinical trial. EBioMedicine (2016) 5:204–10. doi: 10.1016/j.ebiom.2016.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Matz EL, Terlecki RP. Stem cell and gene-based therapy for erectile dysfunction: Current status and future needs. Urol Clin North Am (2021) 48(4):611–9. doi: 10.1016/j.ucl.2021.06.014 [DOI] [PubMed] [Google Scholar]
- 70. An G, Guo F, Liu X, Wang Z, Zhu Y, Fan Y, et al. Functional reconstruction of injured corpus cavernosa using 3D-printed hydrogel scaffolds seeded with HIF-1α-expressing stem cells. Nat Commun (2020) 11(1):2687. doi: 10.1038/s41467-020-16192-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu Q, Cui Y, Lin H, Hu D, Qi T, Wang B, et al. MicroRNA-145 engineered bone marrow-derived mesenchymal stem cells alleviated erectile dysfunction in aged rats. Stem Cell Res Ther (2019) 10(1):398. doi: 10.1186/s13287-019-1509-1 [DOI] [PMC free article] [PubMed] [Google Scholar]