Abstract
Synucleinopathies are characterized by the deposition of alpha-synuclein (α-syn) aggregates in brain tissue. Pathological α-syn aggregates propagate in a prion-like manner and display prion-like biochemical properties. Using RT-QuIC, we measured α-syn seeding activity from brains of Dementia with Lewy body (DLB) patients post autoclave. Here, we show that autoclaving at 121 °C removes one to two log10 of α-syn seeding activity but the remaining 50% seeding dose (SD50) is more than 107/mg tissue. DLB brain samples autoclaved at 132 °C still revealed an SD50 of approximately 106/mg tissue. Our data suggest that DLB α-syn seeds are incompletely inactivated by standard autoclave, thus highlighting the need for evaluating laboratory procedures that fully inactivate them.
Keywords: Alpha-synuclein, Seeds, Inactivation, Autoclave, RT-QuIC, Prion
Highlights
-
•
Alpha-synuclein seeding activity in DLB brains can be quantitated using RT-QuIC.
-
•
Autoclave fails to completely inactivate alpha-synuclein seeding activity in DLB.
-
•
DLB brain samples autoclaved at 121 °C still contain more than 7 log10 SD50/mg tissue.
1. Introduction
Synucleinopathies including Parkinson’s disease (PD) and dementia with Lewy body (DLB) are characterized by the deposition of pathologically misfolded alpha-synuclein (α-syn) associated with neuronal or glial cells in brain tissue [1]. Pathological α-syn aggregates are known to propagate in a prion-like manner by acting as proteopathic seeds [2]. Prions are proteinaceous infectious agents that are composed of a pathological isoform of native prion protein, termed PrPSc [3]. Pathological α-syn aggregates and prions share biochemical properties such as detergent-insolubility and resistance to limited proteolysis [4]. Prions are notorious for their resistance to routine protocols that are used to effectively inactivate most other pathogens [5]. Misfolded α-syn aggregates are likewise resistant to inactivation methods such as formalin fixation and autoclave treatment [6,7].
Seeding activity for both α-syn and PrPSc aggregates can be detected by real-time quaking-induced conversion (RT-QuIC), which is an in vitro cyclic amplification technique initially developed to detect PrPSc with high sensitivity and specificity [8]. RT-QuIC has also been adapted for the detection of pathological α-syn aggregates from various synucleinopathies [9]. The RT-QuIC assay can be used for the quantitation of seeding activity in proteopathic seeds by determining those dilutions yielding positive responses in 50% of the replicates (SD50) [8]. Relative amounts of seeding activity can be expressed as the SD50 concentration. In this study, we used α-syn RT-QuIC to quantitate α-syn seeding activity from DLB patient brain tissue after selected autoclave protocols. We additionally examined PrPSc seeding activity from the brain tissue of CWD-affected elk. Our results show that autoclaving at 121 °C for 20 min removes one to two log10 of α-syn seeding activity with the remaining 50% seeding dose (SD50) more than 107/mg tissue, and that the removed seeding activity following this condition of autoclave are quantitatively similar between the two proteopathic seeds.
2. Materials and methods
Human brain tissue was obtained from the Edinburgh Brain and Tissue Bank (EBTB) in Edinburgh, Scotland, UK. Brain samples derived from four patients with DLB (Brodmann area [BA] 9 and BA39) and from one non-demented control (frontal cortex [FC]) were used. Ethical approvals for the acquisition and use of human brain samples were obtained from the Institutional Review Board of Korea Brain Research Institute (KBRI). Elk brain tissue was obtained in a cervid farm in which CWD was identified in the Republic of Korea. Whole brains derived from three CWD-positive elk were used in this study. Positive CWD diagnosis for these animals was made by the Animal and Plant Quarantine Agency (APQA) in the Republic of Korea. Brain tissue was homogenized in PBS, pH 7.4, supplemented with complete EDTA-free protease and phosphatase inhibitors (Roche). Homogenization for CWD brains was achieved by manual grinding of a whole brain with mortar and pestle. DLB brain tissue was homogenized by using the Precellys 24 tissue homogenizer (Bertin instrument). The 10% (w/v) brain homogenates were clarified by centrifugation at 2000 g and then kept at −80 °C until needed. Brain homogenates were autoclave-treated either at 121 °C or 132 °C for 20 min and then stored at −80 °C until needed. Autoclaving of DLB homogenates was conducted using a laboratory autoclave ES-315 (TOMY) in KBRI, and autoclaving CWD homogenates was performed using HA-305 M (Hirayama) in APQA. Prior to autoclaving, the cap of tubes containing samples were slightly loosened to allow steam penetration.
The concentration of α-syn in the cleared 10% brain homogenates was measured using SensoLyteTM Anti-alpha-Synuclein Quantitative ELISA Kit (AS-55550-H, Anaspec). The homogenates were first diluted appropriately with sample dilution buffer supplied in the Kit to measure α-syn concentrations within the linear range of the standard curve, and then analyzed as described in the manufacturer’s instructions.
For the Western blot analysis of α-syn, the cleared 10% brain homogenates were mixed with NuPAGE LDS sample buffer (Invitrogen) to a final concentration of 1X and incubated for 10 min in a heating block set at 100 °C. Proteins were separated in 12% Bis-Tris gels (Invitrogen) and then blotted onto polyvinylidene difluoride (PVDF) membranes (Millipore). After blocking with 0.2% I-Block (Applied Biosystems) in TBST (20 mM Tris-HCl[pH 7.4], 150 mM NaCl, and 0.1% Tween 20), the membranes were probed with anti-alpha-synuclein antibody MJFR1 (abcam) at a dilution of 1:10,000 or with anti-beta-actin antibody AC-15 (abcam) at a dilution of 1:5000. The membranes were then incubated with horseradish peroxidase (HRP)-labeled anti-rabbit IgG antibody (KPL 074–1516; for MJFR1) or anti-mouse IgG antibody (KPL 074–1806; for AC-15), both at a dilution of 1:10,000. The blots were developed either with Supersignal West Pico PLUS Chemiluminescent Substrate or TMB-Blotting Substrate Solution (Thermo Scientific).
RT-QuIC for the detection of α-syn seeding activity was conducted as described previously [9,10] with minor modifications. Briefly, 10% brain homogenates (10-1 dilution) were sonicated with 5 cycles of 30 s sonication and 30 s rest in Microsonix 4000 (Sonix) set at 80% potency, as described previously [11]. Sonication was conducted in Cup Horns in an indirect manner (i.e. without the use of probe). Subsequently, sonicated samples were subjected to serial tenfold dilutions in the homogenization buffer as indicated. Recombinant wild-type full-length human α-syn (rPeptide #S-1001-1) was used as substrate. Black 96-well plates with clear bottom (Thermo #265301) were preloaded with 4 glass beads (1.0–1.25 mm in diameter). 100 μL reaction mixtures containing 40 mM phosphate, pH 8.2, 10 μM ThT, 170 mM NaCl, 0.00125% SDS, 0.1 mg/mL recombinant human α-syn and 2 μL seeds at specified dilutions were loaded in quadruplicate, and then incubated at 37 °C in a FLUOstar Omega plate reader (BMG Labtech) with cycles of 1-min shake (400 rpm, double orbital) and 1-min rest for 50 h. ThT fluorescence in relative fluorescence units (rfu) was recorded at the starting point of the assay and then at 1-h intervals from bottom of wells (gain of 1,900, 440 nm excitation and 480 nm emission). Samples were deemed positive when two or more of four replicates exceeded the threshold fluorescence values, as determined by averaging fluorescence values of the first five measurements for all samples plus ten standard deviations. Protein aggregation rates (PAR) were obtained by taking the inverse of hours required to reach the threshold fluorescence (lag phase). SD50 values were calculated using the Spearman-Kärber method [8,10]. RT-QuIC for the detection of prion seeding activity was performed as described previously [12] with minor modifications. Briefly, 10% brain homogenates were serially diluted from 10-2 to 10-9. A volume of 98 μL reaction mixtures (10 mM phosphate, pH 7.4, 1 mM EDTA, 10 μM ThT, 300 mM NaCl and 0.1 mg/ml recombinant Syrian hamster prion protein [residues 23–231, Impact Bio #201-01]) was loaded in quadruplicate, with 2 μL of diluted brain homogenates finally added to individual wells. The plate was incubated in the FLUOstar Omega plate reader at 42 °C for 71 h with cycles of 1-min shake (700 rpm, double orbital) and 1-min rest. Samples were deemed positive when at least three of four replicates exceeded 20,000 rfu.
3. Results and discussion
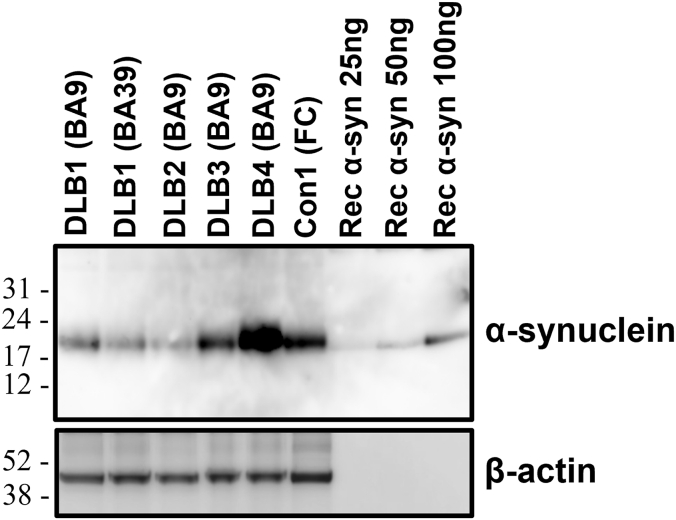
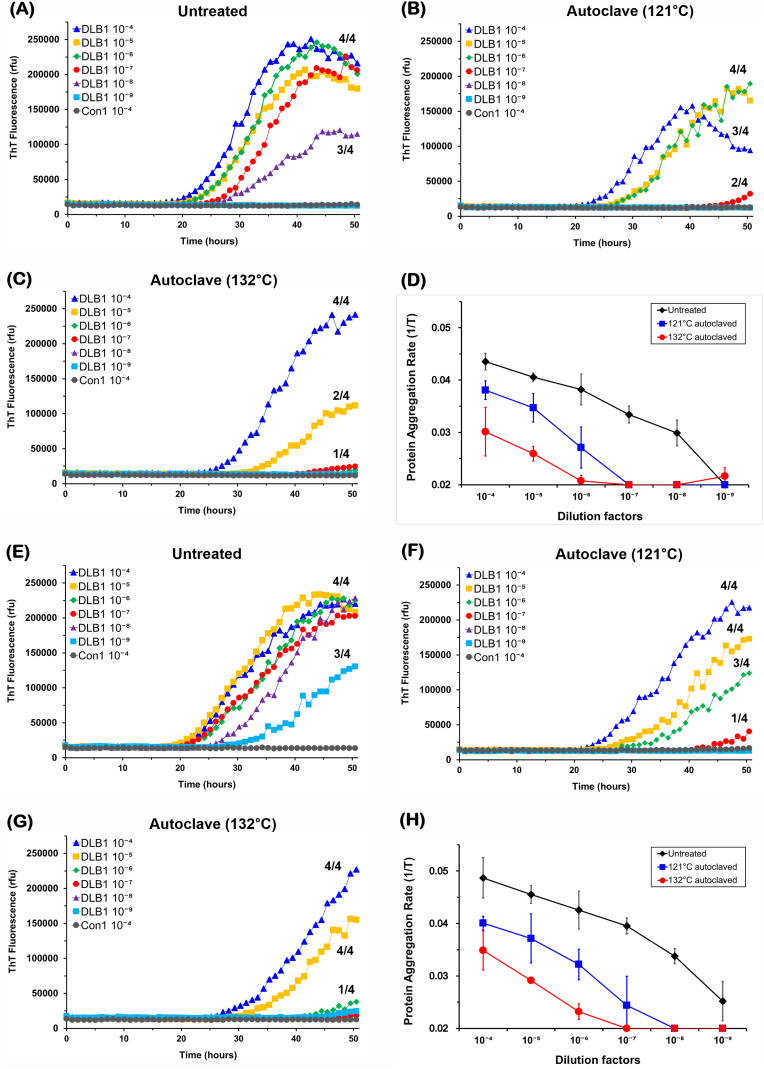
We first measured α-syn concentrations by ELISA in the brain homogenates used for seeding. Concentrations of α-syn were measured in the range of 0.9 μg/ml to 2.1 μg/ml in the 10% homogenates, showing some variation between samples (Table 1). Similarly, immunoblot band intensities of α-syn migrating approximately at 18–19 KDa also displayed minor variation between samples (Fig. 1). We then examined α-syn seeding activity in non-autoclaved DLB brain homogenates with serial dilutions up to 10–11. Flat responses were seen in control (Con1) reactions and positive responses rising above baseline were detected in reactions with 10–4 to 10-8 DLB1 BA9 tissue dilutions (Fig. 2A) or 10-4 to 10-9 DLB1 BA39 tissue dilutions (Fig. 2E). The SD50 values reached 8.78 log10 SD50/mg tissue (BA9) or 9.7 log10 SD50/mg tissue (BA39), respectively. We then analyzed α-syn seeding activity in the BA9 tissue of three more DLB cases. Positive RT-QuIC responses were detectable in reactions seeded with up to 10-7 to 10-8 dilutions, with relative concentrations of α-syn seeding activity in the range of 8–9 log10 SD50/mg tissue (Table 1).
Table 1.
Inactivation effects of autoclave treatment against α-syn or prion seeding activity.
| Disease (protein) | Case ID in this study | EBTB’s reference ID | Sex | Age at death (in years) | Brain region | α-syn concentration (μg/ml) | log10 SD50/mg brain tissue (Mean ± SD) 1) |
log10 SD50 decrease following steam sterilization (Mean ± SD) | |
|---|---|---|---|---|---|---|---|---|---|
| Untreated | 121 °C | ||||||||
| DLB (α-syn) |
DLB1 | SD26/13 | M | 65 | BA39 2,3) | 1.1 | 9.70 ± 0.35 | 7.28 ± 0.12 | 2.42 ± 0.31 |
| BA9 4) | 1.1 | 8.78 ± 0.24 | 7.12 ± 0.42 | 1.67 ± 0.42 | |||||
| DLB2 | SD19/14 | M | 60 | BA9 | 1.6 | 8.82 ± 0.13 | 7.57 ± 0.38 | 1.25 ± 0.25 | |
| DLB3 | SD50/12 | M | 61 | BA9 | 2.1 | 8.95 ± 0.35 | 7.70 ± 0.20 | 1.25 ± 0.54 | |
| DLB4 | SD24/16 | F | 86 | BA9 | 0.9 | 8.07 ± 0.13 | 7.20 ± 0.00 | 0.88 ± 0.13 | |
| CWD (prion) | C22-1 | – | M | 3 | whole Brain | – | 6.82 ± 0.13 | 5.45 ± 0.25 | 1.38 ± 0.18 |
| C22-2 | – | F | 5 | whole Brain | – | 6.32 ± 0.13 | 5.07 ± 0.38 | 1.25 ± 0.35 | |
| C22-3 | – | M | 5 | whole Brain | – | 6.57 ± 0.13 | 5.20 ± 0.25 | 1.38 ± 0.18 | |
1) Data shown represent the average values ± SD of two to three independent experiments.
2) In one of three experiments where a true end-point was not reached, we assumed that the next 10-fold dilution would have given 0 positive reactions in order to calculate the SD50 values.
3) BA39 = Brodmann area 39.
4) BA9 = Brodmann area 9.
Fig. 1.
Immunoblot analysis of α-syn in DLB brains. The brain homogenate from four DLB cases and one non-demented control case was subjected to immunoblot using the anti-alpha-synuclein antibody MJFR1, which recognizes residues 118–123 of α-syn. In each sample, 20 μg of total protein was loaded per lane. For quantitative estimation, recombinant α-syn protein at three different concentrations (25 ng, 50 ng, or 100 ng per lane) was included in the analysis. Molecular mass markers in kilodaltons were shown on the left.
Fig. 2.
RT-QuIC analysis of α-syn seeding activity in untreated or autoclaved DLB brain homogenates. DLB brain homogenates were prepared either from BA9 tissue (A, B, C, D) or BA39 tissue (E, F, G, H) of DLB1 patient. Frontal cortex homogenates from a non-demented patient (Con1) were used as a control. Brain homogenates remained untreated (A, E) or autoclaved for 20 min either at 121 °C (B, F) or 132 °C (C, G). Brain homogenates (2 μL) at indicated dilutions were used to seed α-syn RT-QuIC reactions in quadruplicate. Average ThT fluorescence values from quadruplicate wells were plotted against time (The negative replicates were taken for the average). (D, H) Protein aggregation rates (PAR) were obtained by taking the inverse of lag phase time (1/T). For reactions in which average ThT signals did not exceed the threshold values during the assay, lag phases were assigned as 50 h. Data shown represent the average values ± SD of three independent experiments.
Next, brain homogenates from DLB1 patient were autoclaved either at 121 °C or 132 °C for 20 min prior to RT-QuIC. At 121 °C, positive responses were identified in reactions seeded with up to 10-7 dilution of BA9 tissue but were not detectable with tissue dilutions of 10–8 to 10-9 (Fig. 2B). The remaining α-syn seeding activity was 107.12/mg tissue. At 132 °C, α-syn seeding activity was identified only in reactions seeded with 10–4 to 10-5 dilutions of BA9 tissue with the SD50 value of 105.95/mg tissue (Fig. 2C). When compared to non-autoclaved BA9 homogenate, SD50 values were reduced by 1.67 log10 following autoclave at 121 °C or by 2.83 log10 following 132 °C. Protein aggregation rates (PARs) were calculated as the inverse of the lag phase. Temperature-dependent reductions in PAR were observed at each dilution following autoclave treatment (Fig. 2D). Similar RT-QuIC kinetics were observed for the DLB1 BA39 homogenates (Fig. 2F, G and H). Following autoclave of the DLB1 BA39 homogenates, seeding activity titers decreased by 2.42 log10 (at 121 °C) or 3.13 log10 (at 132 °C) when compared to the corresponding non-autoclaved sample. The analysis of BA9 tissue from three additional DLB cases autoclaved at 121 °C showed similar results to DLB1, with the reduction of SD50 values in the range of 0.88–1.25 log10 (Table 1).
We then investigated prion seeding activity in CWD-positive elk brains before and after autoclave treatment. The SD50 values in non-autoclaved brain homogenates from CWD-positive elk were in the range of 6–7 log10 SD50/mg tissue (Table 1). Following autoclave treatment at 121 °C for 20 min, SD50 values were reduced by 1.25–1.38 log10 when compared to the corresponding non-autoclaved samples and the remaining prion seeding titers reached 5 to 5.5 log10 SD50/mg tissue.
In this study, we quantitated the decrease of seeding activity in autoclaved DLB α-syn aggregates or CWD prions using end-point RT-QuIC [8]. Our results show that autoclaving at 121 °C removes one to two log10 of α-syn seeding activity but the remaining seeding activity titer is more than 107/mg tissue. Consistent with our data, α-syn seeding activity from other synucleinopathies such as PD or multiple system atrophy (MSA) has also been reported as highly resistant to physiochemical inactivation. Tarutani and colleagues showed that MSA α-syn seeding activity measured using a cellular system was shown to be reduced by approximately 1 log10 SD50 following autoclaving at 134 °C for 20 min [6]. Another study performed by Pinder and colleagues reported that autoclaving PD brain homogenates at 134 °C removed 2.2 to 2.4 log10 of α-syn seeding titers irrespective of autoclave time [13]. Thus, our data suggest that α-syn seeding activity in DLB brains is more susceptible to inactivation by autoclave than that of PD or MSA brains. Notably, pathogenic α-syn aggregates derived from DLB, PD or MSA brains have been reported to differ in their physiochemical and biological properties [[14], [15], [16], [17]]. While further investigations are needed to more precisely quantify the levels of resistance of α-syn seeds from various synucleinopathies, remaining α-syn seeding activity after standard autoclave might be a public health problem given the structural, biochemical and mechanistic similarities between PrPSc and other proteopathic seeds [18,19]. There are growing concerns that neurodegenerative disorders such as Parkinson’s and Alzheimer’s disease (AD) might be transmissible between humans [20]. In fact, multiple cases of iatrogenic amyloid β transmission have been reported in recent years [21,22]. To the best of our knowledge, there is so far no evidence of α-syn transmission between humans. Nonetheless, incomplete inactivation of α-syn aggregates after standard autoclave procedures could be a concern in hospital environments. Therefore, more effective protocols for pathological α-syn aggregate inactivation are needed to fully remove any risk associated with human-to-human transmission. These inactivation methods could include treatments with 1–2 N sodium hydroxide, 20,000 ppm sodium hypochlorite, vaporized hydrogen peroxide, a mildly acidic formulation of hypochlorous acid or acidic SDS, and prolonged autoclaving at higher temperature [5,[23], [24], [25], [26], [27]], all of which were found to be highly effective in decontaminating prions.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by the research program of Korea Brain Research Institute funded by the Ministry of Science and ICT (20-BR-02-19) and by the Animal and Plant Quarantine Agency, Ministry for Agriculture, Food and Rural Affairs (B-1543085-22-24-01).
Contributor Information
Hyun-Joo Sohn, Email: shonhj@korea.kr.
Young Pyo Choi, Email: cyp0201@kbri.re.kr.
Data availability
Data will be made available on request.
References
- 1.Goedert M., Spillantini M.G., Del Tredici K., Braak H. 100 years of Lewy pathology. Nat. Rev. Neurol. 2013;9(1):13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- 2.Steiner J.A., Quansah E., Brundin P. The concept of alpha-synuclein as a prion-like protein: ten years after. Cell Tissue Res. 2018;373(1):161–173. doi: 10.1007/s00441-018-2814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prusiner S.B. Prions. Proc. Nat. Acad. Sci. USA. 1998;95(23):13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miake H., Mizusawa H., Iwatsubo T., Hasegawa M. Biochemical characterization of the core structure of alpha-synuclein filaments. J. Biol. Chem. 2002;277(21):19213–19219. doi: 10.1074/jbc.M110551200. [DOI] [PubMed] [Google Scholar]
- 5.Taylor D.M. Inactivation of prions by physical and chemical means. J. Hosp. Infect. 1999;43(Suppl):S69–S76. doi: 10.1016/s0195-6701(99)90067-1. [DOI] [PubMed] [Google Scholar]
- 6.Tarutani A., Arai T., Murayama S., Hisanaga S.I., Hasegawa M. Potent prion-like behaviors of pathogenic α-synuclein and evaluation of inactivation methods. Acta Neuropathol. Commun. 2018;6(1):29. doi: 10.1186/s40478-018-0532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woerman A.L., Kazmi S.A., Patel S., Freyman Y., Oehler A., Aoyagi A., Mordes D.A., Halliday G.M., Middleton L.T., Gentleman S.M., Olson S.H., Prusiner S.B. MSA prions exhibit remarkable stability and resistance to inactivation. Acta Neuropathol. 2018;135(1):49–63. doi: 10.1007/s00401-017-1762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilham J.M., Orru C.D., Bessen R.A., Atarashi R., Sano K., Race B., Meade-White K.D., Taubner L.M., Timmes A., Caughey B. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog. 2010;6(12) doi: 10.1371/journal.ppat.1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fairfoul G., McGuire L.I., Pal S., Ironside J.W., Neumann J., Christie S., Joachim C., Esiri M., Evetts S.G., Rolinski M., Baig F., Ruffmann C., Wade-Martins R., Hu M.T., Parkkinen L., Green A.J. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann. Clin. Transl. Neurol. 2016;3(10):812–818. doi: 10.1002/acn3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groveman B.R., Orru C.D., Hughson A.G., Raymond L.D., Zanusso G., Ghetti B., Campbell K.J., Safar J., Galasko D., Caughey B. Rapid and ultra-sensitive quantitation of disease-associated alpha-synuclein seeds in brain and cerebrospinal fluid by alphaSyn RT-QuIC. Acta Neuropathol. Commun. 2018;6(1):7. doi: 10.1186/s40478-018-0508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han J.Y., Jang H.S., Green A.J.E., Choi Y.P. RT-QuIC-based detection of alpha-synuclein seeding activity in brains of dementia with Lewy Body patients and of a transgenic mouse model of synucleinopathy. Prion. 2020;14(1):88–94. doi: 10.1080/19336896.2020.1724608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira N.C., Charco J.M., Plagenz J., Orru C.D., Denkers N.D., Metrick M.A., 2nd, Hughson A.G., Griffin K.A., Race B., Hoover E.A., Castilla J., Nichols T.A., Miller M.W., Caughey B. Detection of chronic wasting disease in mule and white-tailed deer by RT-QuIC analysis of outer ear. Sci. Rep. 2021;11(1):7702. doi: 10.1038/s41598-021-87295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinder P., Thomzig A., Schulz-Schaeffer W.J., Beekes M. Alpha-synuclein seeds of Parkinson's disease show high prion-exceeding resistance to steam sterilization. J. Hosp. Infect. 2021;108:25–32. doi: 10.1016/j.jhin.2020.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Ayers J.I., Lee J., Monteiro O., Woerman A.L., Lazar A.A., Condello C., Paras N.A., Prusiner S.B. Different α-synuclein prion strains cause dementia with Lewy bodies and multiple system atrophy. Proc. Natl. Acad. Sci. U.S.A. 2022;119(6) doi: 10.1073/pnas.2113489119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau A., So R.W.L., Lau H.H.C., Sang J.C., Ruiz-Riquelme A., Fleck S.C., Stuart E., Menon S., Visanji N.P., Meisl G., Faidi R., Marano M.M., Schmitt-Ulms C., Wang Z., Fraser P.E., Tandon A., Hyman B.T., Wille H., Ingelsson M., Klenerman D., Watts J.C. α-Synuclein strains target distinct brain regions and cell types. Nat. Neurosci. 2020;23(1):21–31. doi: 10.1038/s41593-019-0541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prusiner S.B., Woerman A.L., Mordes D.A., Watts J.C., Rampersaud R., Berry D.B., Patel S., Oehler A., Lowe J.K., Kravitz S.N., Geschwind D.H., Glidden D.V., Halliday G.M., Middleton L.T., Gentleman S.M., Grinberg L.T., Giles K. Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc. Natl. Acad. Sci. U.S.A. 2015;112(38):E5308–E5317. doi: 10.1073/pnas.1514475112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shahnawaz M., Mukherjee A., Pritzkow S., Mendez N., Rabadia P., Liu X., Hu B., Schmeichel A., Singer W., Wu G., Tsai A.L., Shirani H., Nilsson K.P.R., Low P.A., Soto C. Discriminating α-synuclein strains in Parkinson's disease and multiple system atrophy. Nature. 2020;578(7794):273–277. doi: 10.1038/s41586-020-1984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaunmuktane Z., Brandner S. Transmissible human proteopathies: an expanding field. Diagn. Histopathol. 2019;25(1):16–22. [Google Scholar]
- 19.Duyckaerts C., Clavaguera F., Potier M.C. The prion-like propagation hypothesis in Alzheimer's and Parkinson's disease. Curr. Opin. Neurol. 2019;32(2):266–271. doi: 10.1097/WCO.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 20.Asher D.M., Belay E., Bigio E., Brandner S., Brubaker S.A., Caughey B., Clark B., Damon I., Diamond M., Freund M., Hyman B.T., Jucker M., Keene C.D., Lieberman A.P., Mackiewicz M., Montine T.J., Morgello S., Phelps C., Safar J., Schneider J.A., Schonberger L.B., Sigurdson C., Silverberg N., Trojanowski J.Q., Frosch M.P. Risk of transmissibility from neurodegenerative disease-associated proteins: experimental knowns and unknowns. J. Neuropathol. Exp. Neurol. 2020;79(11):1141–1146. doi: 10.1093/jnen/nlaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamaguchi T., Ono K., Yamada M. Transmission of cerebral β-amyloidosis among individuals. Neurochem. Res. 2022;47(9):2469–2477. doi: 10.1007/s11064-022-03566-4. [DOI] [PubMed] [Google Scholar]
- 22.Lauwers E., Lalli G., Brandner S., Collinge J., Compernolle V., Duyckaerts C., Edgren G., Haïk S., Hardy J., Helmy A., Ivinson A.J., Jaunmuktane Z., Jucker M., Knight R., Lemmens R., Lin I.C., Love S., Mead S., Perry V.H., Pickett J., Poppy G., Radford S.E., Rousseau F., Routledge C., Schiavo G., Schymkowitz J., Selkoe D.J., Smith C., Thal D.R., Theys T., Tiberghien P., van den Burg P., Vandekerckhove P., Walton C., Zaaijer H.L., Zetterberg H., De Strooper B. Potential human transmission of amyloid β pathology: surveillance and risks. Lancet Neurol. 2020;19(10):872–878. doi: 10.1016/S1474-4422(20)30238-6. [DOI] [PubMed] [Google Scholar]
- 23.Taylor D.M. Inactivation of transmissible degenerative encephalopathy agents: a review. Vet. J. (London, England. 1997;159(1):10–17. doi: 10.1053/tvjl.1999.0406. 2000. [DOI] [PubMed] [Google Scholar]
- 24.Fichet G., Comoy E., Duval C., Antloga K., Dehen C., Charbonnier A., McDonnell G., Brown P., Lasmézas C.I., Deslys J.P. Novel methods for disinfection of prion-contaminated medical devices. Lancet. 2004;364(9433):521–526. doi: 10.1016/S0140-6736(04)16810-4. [DOI] [PubMed] [Google Scholar]
- 25.Peretz D., Supattapone S., Giles K., Vergara J., Freyman Y., Lessard P., Safar J.G., Glidden D.V., McCulloch C., Nguyen H.O., Scott M., Dearmond S.J., Prusiner S.B. Inactivation of prions by acidic sodium dodecyl sulfate. J. Virol. 2006;80(1):322–331. doi: 10.1128/JVI.80.1.322-331.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giles K., Glidden D.V., Beckwith R., Seoanes R., Peretz D., DeArmond S.J., Prusiner S.B. Resistance of bovine spongiform encephalopathy (BSE) prions to inactivation. PLoS Pathog. 2008;4(11) doi: 10.1371/journal.ppat.1000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughson A.G., Race B., Kraus A., Sangaré L.R., Robins L., Groveman B.R., Saijo E., Phillips K., Contreras L., Dhaliwal V., Manca M., Zanusso G., Terry D., Williams J.F., Caughey B. Inactivation of prions and amyloid seeds with hypochlorous acid. PLoS Pathog. 2016;12(9) doi: 10.1371/journal.ppat.1005914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.