Abstract
Background
Despite the growing use of repetitive transcranial magnetic stimulation (rTMS) as a treatment for depression, there is a limited understanding of the mechanisms of action and how potential treatment-related brain changes help to characterize treatment response. To address this gap in understanding we investigated the effects of an approach combining rTMS with simultaneous psychotherapy on global functional connectivity.
Method
We compared task-related functional connectomes based on an idiographic goal priming task tied to emotional regulation acquired before and after simultaneous rTMS/psychotherapy treatment for patients with major depressive disorders and compared these changes to normative connectivity patterns from a set of healthy volunteers (HV) performing the same task.
Results
At baseline, compared to HVs, patients demonstrated hyperconnectivity of the DMN, cerebellum and limbic system, and hypoconnectivity of the fronto-parietal dorsal-attention network and visual cortex. Simultaneous rTMS/psychotherapy helped to normalize these differences, which were reduced after treatment. This finding suggests that the rTMS/therapy treatment regularizes connectivity patterns in both hyperactive and hypoactive brain networks.
Conclusions
These results help to link treatment to a comprehensive model of the neurocircuitry underlying depression and pave the way for future studies using network-guided principles to significantly improve rTMS efficacy for depression.
Keywords: rTMS, Depression, Functional connectivity, Self-system therapy
Introduction
Major depressive disorder (MDD) is a mental health disorder characterized by a variety of neuronal dysfunctions. Cognitive neuroscience approaches to the assessment and treatment of many mental disorders, including MDD, increasingly rely on a conceptual model grounded in the idea that neural processing abnormalities are not localized to specific brain regions such as the anterior cingulate cortex (ACC), medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC), ventromedial cortex (vmPFC), amygdala and hippocampus (for review, see Wise et al. 2014); but instead relates to deficits in integrated action between multiple distributed cortical and subcortical regions and abnormalities in complex, functionally integrated networks (Ge et al., 2017; Krug et al., 2022).
This transition in focus is especially evident in the growing use of repetitive transcranial magnetic stimulation (rTMS) for therapeutic purposes in association with neuroimaging data such as functional MRI (fMRI). rTMS is a non-invasive brain stimulation technique that offers the potential to directly modulate neural circuits hypothesized to underlie observed dysfunctions in mental disorders. Despite the increasing therapeutic use of rTMS, typical effect sizes of rTMS treatment have been modest (Berlim et al., 2014; Lefaucheur et al., 2014), and both methodological and conceptual challenges remain regarding the mechanisms of action underlying successful treatment (Daskalakis et al., 2008; Downar & Daskalakis, 2013; Noda et al., 2015). Recent TMS/fMRI work highlights that rTMS effects are not only found underneath the stimulation site, but instead spread out to a variety of brain networks. Consequently, rTMS protocols have been moving from localization approaches based on identifying a single node which shows strong activation on a univariate localizer, to connectivity-based targeting using resting state functional connectivity (Cash et al., 2021; Fox et al., 2014). These connectivity-based approaches highlight the growing appreciation that some of the strongest responses to TMS may be seen not only at the site of stimulation, where TMS may produce a strong mix of inhibitory and excitatory responses (Rafiei & Rahnev, 2022), but also within the regions connected to the site of stimulation (Luber et al., 2021; Momi et al., 2021, 2021). When combined with more recent accelerated sequences such as SAINT/SNT (Cole et al., 2022) such network-targeted approaches are providing hopeful pathways to increasing rTMS efficacy, though more work is needed to fully realize this goal.
However, these studies do not directly engage emotion regulation or other critical psychological processes (Aizenstein et al., 2009). Several behavioral tasks used in fMRI research elicit reliable, emotion-related activation patterns centered on the DLPFC. With respect to studying the etiology and treatment of MDD, these tasks are useful because they provide a reliable target for up- or down-regulation of relevant networks. Treatment-related alterations in cortical-limbic functional connectivity have been observed in MDD during a number of different emotional processing paradigms, including passive viewing of emotional stimuli (Beall et al., 2012; Schaefer et al., 2006), face processing (Chen et al., 2008; Fu et al., 2004), emotional interference tasks (Fales et al., 2009; Robertson et al., 2007). However, to our knowledge very few studies are using such fMRI task to guide rTMS targeting (Neacsiu et al., 2022). The focus of the present study was to retrospectively investigate the TMS-related response of whole-brain connectivity patterns to evaluate the network-level response to our combined neuromodulation and psychotherapy intervention. Towards this goal we evaluated connectivity within relevant canonical networks obtained from patients with MDD before and after individualized, fMRI-guided rTMS combined with SST, and compared their data with normative patterns of connectivity based on nonpsychiatric controls completing the same task.
The psychotherapy used in this study was a self-system therapy (SST, (Strauman et al., 2006). SST is an evidence-based brief structured therapy for depression that is similar to cognitive therapy (CT; Beck, 1997) but focuses primarily on self-evaluation as opposed to CT's primary focus on cognitive distortions. SST is hypothesized to work by altering maladaptive self-regulation, using techniques that include changing the availability and accessibility of personal goals, changing the importance and affective significance of such goals, and changing regulatory system engagement strength. The behavioral success of the combined approach in this sample has been addressed previously (Neacsiu et al., 2018); the current manuscript focuses on the changes on task-related functional connectivity. Based on a previously described model of self-regulation dysfunction in depression (Strauman, 2017), we hypothesized that the combination of rTMS and simultaneous SST would help to provide rapid, effective measures to normalize connectivity patterns in regions typically seen to be hypoactive in MDD (e.g., dorsal frontoparietal cortex) by boosting connectivity after treatment (Luber et al., 2017). Additionally, such a theory of neural normalization would also predict that any hyperactive regions should be attenuated by treatment. Such a result would help to link two disparate literatures by providing evidence for a mechanistic understanding of how individualized DLPFC stimulation results in efficacious treatments, while also demonstrating the conceptual and empirical value of a theoretical model guiding the selection and implementation of treatments (Strauman & Eddington, 2017).
Methods
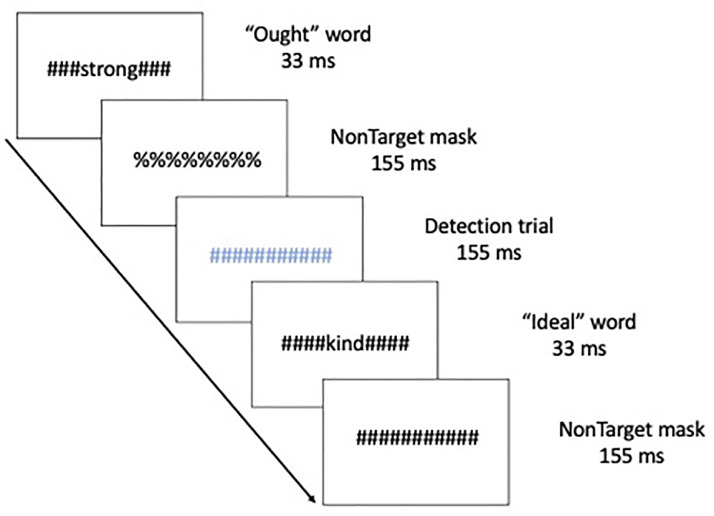
This manuscript is a re-analysis of multivariate connectivity information based on a previously univariate study (Neacsiu et al., 2018). Therefore, we summarize here only the main aspects of the original paper and turn our focus to new analysis of network information before any after our combined intervention. Five patients with major depressive disorders (40% female, Mage = 53.8, SDage = 4.32) received rTMS combined with psychotherapy. The control group consisted of seven healthy volunteers (HV), taken from a previously published study (Ritchey et al., 2011), with no personal or family history of affective disorder (64.3% female), ranging from 24 to 44 years old (Mage = 34.6, SDage = 6.9). Structural and functional MRI were collected on a 3.0 Tesla GE Signa EXCITE HD system at the Duke Brain Imaging and Analysis Center. The anatomical MRI was acquired using a 3D T1-weighted echo-planar sequence (TR = 12.2 ms; TE = 5.3 ms; FOV = 24 cm; image matrix = 2562; voxel size = 0.9375 x 0.9375 × 1.9 mm). fMRI data was recorded from subjects while they participated in a previously validated goal priming task (Pizzagalli et al., 2009). Stimuli were back-projected onto a screen located at the foot of the MRI bed using an LCD projector. Subjects viewed the screen via a mirror system located in the head coil. Task onset was electronically synchronized with the MRI acquisition computer. Task administration and collection of reaction times and accuracy data was computer controlled. Functional images sensitive to blood oxygenation-level dependent (BOLD) contrast were acquired using an inverse spiral pulse sequence (TR, 1.5 s; TE, 35 ms; FOV, 24 cm; image matrix, 642; 34 contiguous axial slices; voxel size 3.75 × 3.75 × 3.8 mm). Each of five runs consisted of the acquisition of a time series of 242 brain volumes. Four initial RF excitations were performed (and discarded) to achieve steady state equilibrium. A semi-automated high-order shimming program was used to ensure global field homogeneity. A rapid masked individualized goal priming task (Strauman et al., 2012) was used to quantify the promotion/prevention neural network activation in each participant such that sites of peak activation associated with a specific goal priming condition was used for subsequent rTMS targeting. Briefly, the event-related fMRI paradigm adapted from Diaz and McCarthy (2007) uses multiple rapid, masked exposures to words representing a participant's ideal (promotion) and ought (prevention) goals that the individual thinks they have successfully achieved or that they have not yet achieved as obtained from the Selves Questionnaire (Higgins et al., 1986). Thus, five sets of masked stimuli (2 in each category) were used embedded in strings of characters: promotion success, promotion failure, prevention success, prevention failure, and control words. The control words are goals generated by a different participant that are semantically unrelated to the goals generated by the target participant. Each stimulus presentation was 12 characters in length, with the target word or nonword in the center surrounded by pound or percent signs, which serve as the pattern mask. Participants saw a constantly changing visual display in which a critical stimulus occurred every 500 ms. Most trials were masked nonwords, and masked goals were presented once every 12–15 s (Fig. 1). Subjects were instructed to make a choice button press response when they detect a pound sign string presented in either blue or red font (to keep subjects engaged during the scanning). These target events occurred infrequently (mean interval = 25 s) and were not in close temporal proximity to the masked goal trials. For a more detailed description of these techniques please see Neacsiu et al. (2018).
Fig. 1.
Schematic of the experimental task, displaying a typical sequence of priming trials. The sequence for an individual trial consisted of alternating pound signs and percent signs, in between which a word or non-word was inserted. Ought, Ideal, and yoked-control priming stimuli were inserted throughout the run. Incidental to those stimuli visible colored symbol stimuli (detection trials) were displayed to which participants were instructed to respond with a button press as quickly as possible.
All treatments were conducted in the Noninvasive Neuromodulation Neuroscience Laboratory in the Duke Department of Psychiatry and Behavioral Sciences. Resting motor threshold (rMT) was assessed on the first day of each week for four weeks, before applying the FDA-approved 10 Hz rTMS protocol over the brain region within the left DLPFC showing the strongest activation in the Ideal > Ought contrast was defined as the individual localizer (individual results from this contrast can be found in Neacsiu et al. 2018). The center of mass of the resulting cluster of active voxels was used as the target site for rTMS and uploaded on a neuronavigation software (Brainsight, Rogue Research, Canada). During all sessions except the first introductory session (i.e., 1 + 20), an approximately 40-minute SST therapy session was begun simultaneously with the 37.5 min TMS application.
Data analysis
Functional connection matrices representing task-related connection strengths were estimated for both groups using a correlational psychophysical interaction (cPPI) analysis used previously by our group (Davis et al., 2017) and others (Fornito et al., 2012) to estimate task-related connectivity. Briefly, the model relies on the calculation of a PPI regressor for each of the 471 regions defined by the HOA atlas (Davis et al., 2019), based on the product of that region's time course and a task regressor of interest, in order to generate a term reflecting the psychophysical interaction between the seed region's activity and the specified experimental manipulation. In the current study the convolved Ideal and Ought task regressors from the univariate model were used as the psychological regressor, which were originally coded as either (a) events representing the display of promotion stimuli, (b) events representing the display of prevention stimuli, (c) events comprising display of control words. Notice that in this analysis we collapsed across success and failure in order to provide reliable estimates of connectivity; all regressors are mean-adjusted in FSL. We then computed the partial correlation , removing the variance , which includes both the psychological regressor and the time courses for regions I and , as well as constituent noise regressors including 6 motion parameters and noise regressors coding for the concurrent signal in white matter and CSF during each run. This cPPI analysis resulted in two separate output matrices, comprising connectivity delineated by prevention or promotion goals. Task-related connectivity was estimated from the resulting output matrices; negative connections were included in these analyses, as they may inform important, explicit interpretations about how networks may be segregated (Braun et al., 2012). However, given that the SST therapy focuses on the promotion goals, the analyses will focus only on functional connectivity associated with these trials.
Results
As reported in the original manuscript, all five patients demonstrated dramatic improvements in their score on the Hamilton Depression Rating Scale during simultaneous rTMS/psychotherapy treatment (Pre-treatment averages: 19.8 ± 5.8; post-treatment averages: 3.4 ± 1.34). For the current analysis, our primary goals were to investigate differences between patients and HV at baseline to determine patients’ connectivity differences to a normative response, and then examine the effects of combined rTMS and psychotherapy on functional connectivity before and after treatment. The results below focused only on whole brain connectivity changes rather than changes in specific brain areas – that were presented in Neacsiu et al. (2018) – to better align with the current shift in focus from univariate activation to multivariate connectivity in the TMS field.
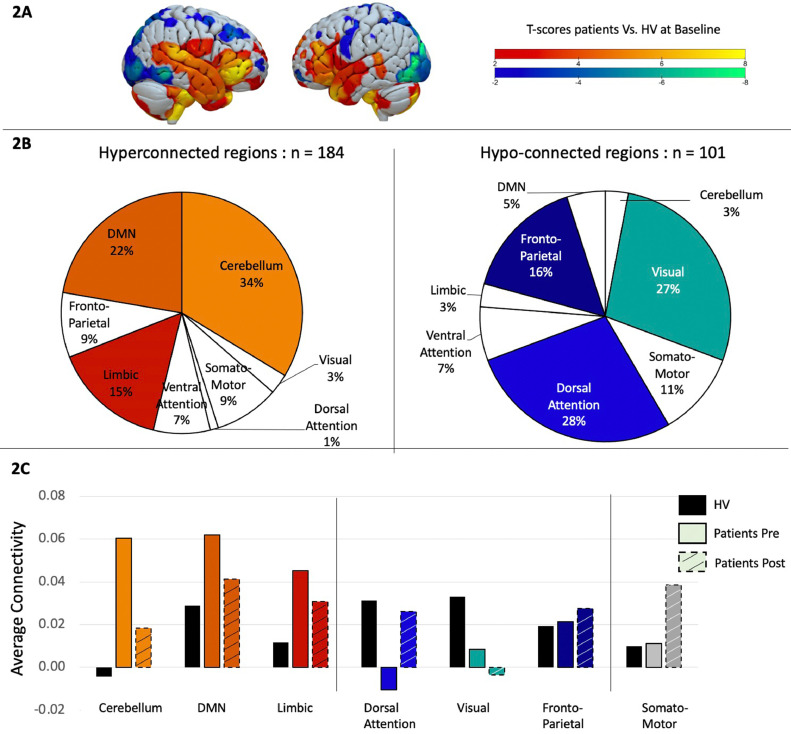
Patients vs. Healthy volunteers at baseline. We first conducted a two-sample t-test to examine differences in functional connectivity matrices between patients and healthy volunteers. Results were filtered to only display t-values larger than −2 or bigger than 2 as significant differences between groups, even though no correction for multiple comparison was done. Out of the 471 regions, 184 showed hyperconnectivity in patients compared to healthy volunteers and 101 were significantly hypo-connected (Fig. 2A). To better understand these results, the 7-network Yeo atlas was used to define to which network those brain regions belonged to, and demonstrated that the regions hyperconnected in patients were mainly located in the cerebellum (34%), the default mode network (22%) and the limbic system (15%), while the regions showing more hypoconnectivity compared to the control group were located in the dorsal-attention network (28%), visual (27%), and fronto-parietal network (16%) (Fig. 2B).
Fig. 2.
A. Two-sample t-test comparing task-related functional connectivity between patients and healthy volunteers at baseline. Hot colors indicate hyper-connectivity in patients and cold colors indicate hypo-connectivity. B. Repartition of the brain regions showing hyperconnectivity (in hot colors on the left side) or hypo-connectivity (in cold colors on the right side) across brain networks, as defined by the Yeo atlas. C. Average connectivity across each brain networks between healthy volunteers in black, patients before treatment in solid color and patients in dashed colors.
Patients after vs. before rTMS. We then investigated how these specific brain regions changed after rTMS by performing a paired sample t-test between pre- and post- rTMS. Interestingly, 91% of the brain regions that were hyper-connected at baseline, showed a connectivity decrease after rTMS, while 83% of the regions that were hypo-connected at baseline showed an increase in connectivity after rTMS, suggesting a normalization of the connectivity pattern after rTMS. Even with this normalization, differences continued to be found between patients post rTMS and healthy volunteers. To get a better overview of these results we displayed the rTMS effect at a network level (Fig. 2C).
Discussion
The present study is a retrospective analysis of Neacsiu et al. (2018), in which we had proposed a novel multimodal treatment for MDD. While the univariate fMRI and behavioral results from this approach are already published, here the focus was the changes in functional connectomes with individualized fMRI-guided rTMS plus concurrent behavioral interventions, and the assessment of differences with a normative non-psychiatric population performing the same fMRI task: a goal-priming task used to observe the promotion/prevention neural network activation. While limited by the sample size, this study revealed an important insight: in comparison to controls, patients exhibited both a hypoconnectivity at baseline in the frontoparietal network and in the visual network; and a hyperconnectivity in cerebellar, limbic and DMN regions. After rTMS, this pattern appeared to normalize since the hyper-connected regions showed a decreased connectivity, while the hypo-connected ones showed an increase, both towards the normative pattern seen in our healthy volunteers.
To put this finding in its proper context, we offer three observations based on previously published work from other research teams. First, several fMRI studies have found that depressed individuals show a diminished engagement of PFC-amygdala circuitry when compared to control subjects, particularly in response to negative stimuli (Johnstone et al., 2007). In addition, it has been reported consistently that both antidepressant medication and brain stimulation treatments are associated with increases in this long-range connectivity (Aizenstein et al., 2009; Anand et al., 2005; Beall et al., 2012; Chen et al., 2008). Therefore, assessment of changes in limbic connectivity, broadly construed, is becoming a hallmark of treatment mechanism of action studies for MDD and related disorders. In the same way our results reproduce this pattern at the network level since we found hypoconnectivity of the executive networks (i.e.,” fronto-parietal, dorsal attention, and in the visual network) probably associated with the cognitive deficits associated with depression. The hyperconnectivity in the limbic and default mode networks could represent the difficulty with emotional regulation, with turning down excessive internally focused thoughts. An interesting result was the cerebellum hyperconnectivity. Indeed, while this structure has often been considered only for its involvement in motor behavior, studies suggest that the cerebellum due to its connectivity with other brain structures might act as a monitoring system integrating the information from the limbic system, and cortical structures and provide feedback to modulate behavior accordingly (Schutter et al., 2022), which could explain its hyperconnectivity observed at baseline.
Second, the valence of cognitive operations being probed using task-based fMRI is likely to have important consequences for the interaction of distant cortical regions. It is now well documented that a reliable feature of depression is the inability to effectively regulate negative mood, and the majority of fMRI task paradigms reflect this focus. However, it also has been documented that depression is associated with hypo-responsiveness of reward mechanisms in the presence of positive stimuli, such as those used in our goal priming task (Strauman et al., 2006). In fact, the task itself was designed to test a key component of regulatory focus theory, namely that activating different kinds of goals (e.g., promotion vs. prevention) induces different motivational states associated with distinct affective tendencies. The valence of the stimuli in our fMRI paradigm is thus in contrast with the majority of connectivity studies, which understandably have focused on emotion regulation following exposure negative stimuli. A growing number of studies have documented reward system dysfunction as a transdiagnostic mechanism in psychiatric disorders. MDD in particular is characterized by hypo responsivity of mesolimbic structures related to reward processing, supported by animal and human neuroimaging data (Pizzagalli et al., 2009; Smoski et al., 2009), and patients with depression demonstrate attenuated connectivity between the ventral striatum and other regions involved in reward processing (Satterthwaite et al., 2015). A greater focus on positive, goal-directed network identification – particularly using individually tailored stimuli – is likely to inform treatment development as well as identification of mechanisms for existing treatments (Strauman, 2017).
Third, our findings highlight the value of using task-based functional activity for studying psychotherapy outcome and process (Carrig et al., 2009). Specifically, we used a task-based approach to functional connectivity, relying on a novel PPI-based method which sought to characterize whole-brain multivariate connectivity patterns during neural processing associated with well-characterized psychological states. To date, the majority of whole-brain network approaches to MDD have focused on the use of resting state data, collected while subjects passively view a blank screen. While it is certainly the case that resting-state paradigms avoid task-related confounds, such as performance, ceiling and floor effects, effort, and task strategies (Fischer et al., 2016), the ultimate justification for the use of any physiological measure is its relation to the psychological constructs of interest (Cacioppo et al., 2003). Affective disorders that have been linked to alterations of what have come to be known as ‘canonical networks’, including changes to the default mode network, affective network, the salience network, and the cognitive control network (Delaveau et al., 2011; Dutta et al., 2014). However, the interpretability of these network changes is limited by our understanding of their operations within and across individuals and situations – a challenge for which psychological theory can be a valuable asset. By grounding our predictions, as well as the fMRI task itself, in a well-validated behavioral model of goal pursuit, we were able to link specific neural patterns associated with emotional regulation with both behavioral and fMRI findings in normal and clinically diagnosed samples (Eddington et al., 2007; Eddington et al., 2009). Of course, we acknowledge that the broad range of emotional regulation tasks reviewed above may be a barrier to the adoption of a reliable and feasible functional neuroimaging-based treatment protocol. Just as obtaining an MRI or EEG is the standard of care for diagnosing dementia or epilepsy, fMRI-based information has the potential to aid in more precise diagnosis and treatment in depression (Carrig et al., 2009). While the complexities associated with administering a task-based paradigm are considerable, we feel that such an approach is necessary to reliably target individualized networks within a clinical setting.
Finally, while the present study was able to delineate some neural correlates of treatment response to rTMS + simultaneous SST, there are several limitations that should be acknowledged. This study's principal limitation was the open-label design and a lack of power, that prevented us investigating the effects of other covariates that could have a drastic impact on our results such as participants’ age or sex at birth. Relatedly, the current design was not fully balanced: while we were fortunate to be able to compare the connectivity patterns in MDD patients in the combined rTMS + SST condition with a healthy volunteer group performing the same task, clearly alternative intervention conditions should be tested to differentiate the relative effects of TMS and therapy, for example specifically employing TMS only and SST + Sham TMS conditions in both MDD and healthy controls. Our group is currently conducting a double-blind study that directly compares the effects of active rTMS to the effects of sham stimulation with a much larger sample size (n = 40, NCT03289923). This ongoing study will inform us regarding the superiority of this approach compared to a sham treatment and will allow assessment of the effects of other variables such as age, sex at birth, or education level. Lastly, our outcome measures were relatively sparse; the Hamilton-17 scores could be complimented in the future by a more extensive testing battery of mood questionnaires such as the MADRS, or even other questionnaires that capture important features of depressive symptoms such as quality of life and functional capacity to capture other meaningful treatment effects (Rabin et al., 2022). This would allow identification of different clusters of depressive symptoms (Siddiqi et al., 2020) and test how these clusters correlate with rTMS effect and connectome changes. Lastly, our use of the goal priming task was based on evidence that this task activates a reliable network of cortical and limbic regions, and predicts chronic regulatory focus (Eddington et al., 2007). In our data, task-related connectivity was in fact increased in MDD, relative to controls (Fig. 2). Nonetheless, alternative tasks may have emphasized different networks, and resulted in different connectivity outcomes.
In addition, while this study relied on the classical 10 Hz rTMS protocol applied for 37 min, which more easily accommodates performing the therapy during the stimulation, more recent accelerated iTBS protocols last only a few minutes and may even be repeated several times a day for only one week to boost therapeutic efficacy (Cole et al., 2020, 2022). While the timing of adjuvant psychotherapy with neuromodulation is necessarily different in such approaches, we believe that these newer protocols do not conflict with our approach. In fact, joining more potent forms of patterned stimulation and session scheduling with individualized targeting and concurrent therapy may prove to generate even stronger remediation of psychiatric illness. Indeed, several studies are already combining TBS as a treatment for addiction with exposure therapy performed right before the treatment (McCalley et al., 2022) and have shown promising effects. Also, in terms of targeting TMS for MDD, a variety of rTMS studies for depression have suggested the use of resting state functional connectivity as a targeting approach while we are using task-related connectivity. While further studies will be needed to work out the relative merits of these two methods, we believe that resting state imaging suffers from high inter-individual variability and that using a task involving emotion regulation is more reliable for targeting and engaging functional networks involved in MDD.
Larger, blinded, randomized sham-controlled trials will be required to test the possibility that this approach of fully engaging brain networks and controlling their functional state (here via simultaneous psychotherapy) represents a more efficacious approach to treating depression than the present FDA-approved method of using TMS alone, using scalp-based targeting. This issue is particularly important when considering the broader therapeutic focus of neurally-based treatment regimens. While an overarching goal of brain stimulation and psychotherapy approaches is to normalize behaviors, the current theory-based study helps to address what mechanistic changes might underly such a successful clinical response—namely, a shift in both hyper- and hypoactive regions towards a more normative connection pattern.
Funding
This research was supported by MH052281, MH067447 and DA031579 granted to the corresponding author. The authors would like to thank Lori Kwapil for her various roles in this project. LB, BL, SHL were supported by the NIMH Intramural Research Program (ZIAMH002955).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Network-level dynamics underlying a simultaneous rTMS and psychotherapy treatment for major depressive disorder: An exploratory network analysis.
Dinámicas a nivel de red subyacentes a un tratamiento combinado de EMTr y psicoterapia para el trastorno depresivi mayor: un análisis exploratorio de la red.
References
- Aizenstein H.J., Butters M.A., Wu M., Mazurkewicz L.M., Stenger V.A., Gianaros P.J., et al. Altered functioning of the executive control circuit in late-life depression: Episodic and persistent phenomena. The American Journal of Geriatric Psychiatry : Official Journal of the American Association for Geriatric Psychiatry. 2009;17(1):30–42. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A., Li Y., Wang Y., Wu J., Gao S., Bukhari L., et al. Activity and connectivity of brain mood regulating circuit in depression: A functional magnetic resonance study. Biological Psychiatry. 2005;57(10):1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Beall E.B., Malone D.A., Dale R.M., Muzina D.J., Koenig K.A., Bhattacharrya P.K., et al. Effects of electroconvulsive therapy on brain functional activation and connectivity in depression. The Journal of ECT. 2012;28(4):234–241. doi: 10.1097/YCT.0b013e31825ebcc7. [DOI] [PubMed] [Google Scholar]
- Beck A.T. The past and future of cognitive therapy. The Journal of Psychotherapy Practice and Research. 1997;6(4):276–284. https://www.ncbi.nlm.nih.gov/pubmed/9292441 [PMC free article] [PubMed] [Google Scholar]
- Berlim M.T., van den Eynde F., Tovar-Perdomo S., Daskalakis Z.J. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: A systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychological Medicine. 2014;44(2):225–239. doi: 10.1017/S0033291713000512. [DOI] [PubMed] [Google Scholar]
- Braun U., Plichta M.M., Esslinger C., Sauer C., Haddad L., Grimm O., et al. Test-retest reliability of resting-state connectivity network characteristics using fMRI and graph theoretical measures. NeuroImage. 2012;59(2):1404–1412. doi: 10.1016/j.neuroimage.2011.08.044. [DOI] [PubMed] [Google Scholar]
- Cacioppo J.T., Berntson G.G., Lorig T.S., Norris C.J., Rickett E., Nusbaum H. Just because you're imaging the brain doesn't mean you can stop using your head: A primer and set of first principles. Journal of Personality and Social Psychology. 2003;85(4):650–661. doi: 10.1037/0022-3514.85.4.650. [DOI] [PubMed] [Google Scholar]
- Carrig M.M., Kolden G.G., Strauman T.J. Using functional magnetic resonance imaging in psychotherapy research: A brief introduction to concepts, methods, and task selection. Psychotherapy Research : Journal of the Society for Psychotherapy Research. 2009;19(4–5):409–417. doi: 10.1080/10503300902735864. [DOI] [PubMed] [Google Scholar]
- Cash R.F.H., Cocchi L., Lv J., Wu Y., Fitzgerald P.B., Zalesky A. Personalized connectivity-guided DLPFC-TMS for depression: Advancing computational feasibility, precision and reproducibility. Human Brain Mapping. 2021 doi: 10.1002/hbm.25330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.H., Suckling J., Ooi C., Fu C.H., Williams S.C., Walsh N.D., et al. Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2008;33(8):1909–1918. doi: 10.1038/sj.npp.1301593. [DOI] [PubMed] [Google Scholar]
- Cole E.J., Phillips A.L., Bentzley B.S., Stimpson K.H., Nejad R., Barmak F., et al. Stanford Neuromodulation Therapy (SNT): A Double-Blind Randomized Controlled Trial. The American Journal of Psychiatry. 2022;179(2):132–141. doi: 10.1176/appi.ajp.2021.20101429. [DOI] [PubMed] [Google Scholar]
- Cole E.J., Stimpson K.H., Bentzley B.S., Gulser M., Cherian K., Tischler C.…Williams N.R. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. American Journal of Psychiatry. 2020;177(8):716–726. doi: 10.1176/appi.ajp.2019.19070720. [DOI] [PubMed] [Google Scholar]
- Daskalakis Z.J., Levinson A.J., Fitzgerald P.B. Repetitive transcranial magnetic stimulation for major depressive disorder: A review. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie. 2008;53(9):555–566. doi: 10.1177/070674370805300902. [DOI] [PubMed] [Google Scholar]
- Davis S.W., Luber B., Murphy D.L.K., Lisanby S.H., Cabeza R. Frequency-specific neuromodulation of local and distant connectivity in aging and episodic memory function. Human Brain Mapping. 2017 doi: 10.1002/hbm.23803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S.W., Szymanski A., Boms H., Fink T., Cabeza R. Cooperative contributions of structural and functional connectivity to successful memory in aging. Network Neuroscience. 2019;3(1):173–194. doi: 10.1162/netn_a_00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaveau P., Jabourian M., Lemogne C., Guionnet S., Bergouignan L., Fossati P. Brain effects of antidepressants in major depression: A meta-analysis of emotional processing studies. Journal of Affective Disorders. 2011;130(1–2):66–74. doi: 10.1016/j.jad.2010.09.032. [DOI] [PubMed] [Google Scholar]
- Diaz M.T., McCarthy G. Unconscious word processing engages a distributed network of brain regions. Journal of Cognitive Neuroscience. 2007;19(11):1768–1775. doi: 10.1162/jocn.2007.19.11.1768. [DOI] [PubMed] [Google Scholar]
- Downar J., Daskalakis Z.J. New targets for rTMS in depression: A review of convergent evidence. Brain Stimulation. 2013;6(3):231–240. doi: 10.1016/j.brs.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Dutta A., McKie S., Deakin J.F. Resting state networks in major depressive disorder. Psychiatry Research. 2014;224(3):139–151. doi: 10.1016/j.pscychresns.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Eddington K.M., Dolcos F., Cabeza R., KR R.K., Strauman T.J. Neural correlates of promotion and prevention goal activation: An fMRI study using an idiographic approach. Journal of Cognitive Neuroscience. 2007;19(7):1152–1162. doi: 10.1162/jocn.2007.19.7.1152. [DOI] [PubMed] [Google Scholar]
- Eddington K.M., Dolcos F., McLean A.N., Krishnan K.R., Cabeza R., Strauman T.J. Neural correlates of idiographic goal priming in depression: Goal-specific dysfunctions in the orbitofrontal cortex. Social Cognitive and Affective Neuroscience. 2009;4(3):238–246. doi: 10.1093/scan/nsp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales C.L., Barch D.M., Rundle M.M., Mintun M.A., Mathews J., Snyder A.Z., et al. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. Journal of Affective Disorders. 2009;112(1–3):206–211. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A.S., Keller C.J., Etkin A. The clinical applicability of functional connectivity in depression: Pathways toward more targeted intervention. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2016;1(3):262–270. doi: 10.1016/j.bpsc.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Fornito A., Harrison B.J., Zalesky A., Simons J.S. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(31):12788–12793. doi: 10.1073/pnas.1204185109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Buckner R.L., Liu H., Chakravarty M.M., Lozano A.M., Pascual-Leone A. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(41):E4367–E4375. doi: 10.1073/pnas.1405003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C.H., Williams S.C., Cleare A.J., Brammer M.J., Walsh N.D., Kim J., et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: A prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61(9):877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Ge R., Blumberger D.M., Downar J., Daskalakis Z.J., Dipinto A.A., Tham J.C.W., et al. Abnormal functional connectivity within resting-state networks is related to rTMS-based therapy effects of treatment resistant depression: A pilot study. Journal of Affective Disorders. 2017;218:75–81. doi: 10.1016/j.jad.2017.04.060. [DOI] [PubMed] [Google Scholar]
- Higgins E.T., Bond R.N., Klein R., Strauman T. Self-discrepancies and emotional vulnerability: How magnitude, accessibility, and type of discrepancy influence affect. Journal of Personality and Social Psychology. 1986;51(1):5–15. doi: 10.1037/0022-3514.51.1.5. [DOI] [PubMed] [Google Scholar]
- Johnstone T., van Reekum C.M., Urry H.L., Kalin N.H., Davidson R.J. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of Neuroscience. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug S., Muller T., Kayali O., Leichter E., Peschel S.K.V., Jahn N., et al. Altered functional connectivity in common resting-state networks in patients with major depressive disorder: A resting-state functional connectivity study. Journal of Psychiatric Research. 2022;155:33–41. doi: 10.1016/j.jpsychires.2022.07.040. [DOI] [PubMed] [Google Scholar]
- Lefaucheur J.P., Andre-Obadia N., Antal A., Ayache S.S., Baeken C., Benninger D.H., et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology. 2014;125(11):2150–2206. doi: 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- Luber B., Davis S.W., Deng Z.D., Murphy D., Martella A., Peterchev A.V., et al. Using diffusion tensor imaging to effectively target TMS to deep brain structures. NeuroImage. 2021;249 doi: 10.1016/j.neuroimage.2021.118863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luber B.M., Davis S., Bernhardt E., Neacsiu A., Kwapil L., Lisanby S.H., et al. Using neuroimaging to individualize TMS treatment for depression: Toward a new paradigm for imaging-guided intervention. NeuroImage. 2017;148:1–7. doi: 10.1016/j.neuroimage.2016.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCalley D.M., Kaur N., Wolf J.P., Conteras I.E., Book S.W., Smtih J.P., et al. Medial prefrontal cortex theta burst stimulation improves treatment outcomes in alcohol use disorder: A double-blind, sham-controlled neuroimaging study. Biological Psychiatry Global Open Science. 2022 doi: 10.1016/j.bpsgos.2022.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momi D., Ozdemir R.A., Tadayon E., Boucher P., Di Domenico A., Fasolo M., et al. Perturbation of resting-state network nodes preferentially propagates to structurally rather than functionally connected regions. Scientific Reports. 2021;11(1):12458. doi: 10.1038/s41598-021-90663-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momi D., Ozdemir R.A., Tadayon E., Boucher P., Shafi M.M., Pascual-Leone A., et al. Network-level macroscale structural connectivity predicts propagation of transcranial magnetic stimulation. NeuroImage. 2021;229 doi: 10.1016/j.neuroimage.2020.117698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neacsiu A.D., Beynel L., Powers J.P., Szabo S.T., Appelbaum L.G., Lisanby S.H., et al. Enhancing cognitive restructuring with concurrent repetitive transcranial magnetic stimulation: A transdiagnostic randomized controlled trial. Psychotherapy and Psychosomatics. 2022;91(2):94–106. doi: 10.1159/000518957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neacsiu A.D., Luber B.M., Davis S.W., Bernhardt E., Strauman T.J., Lisanby S.H. On the concurrent use of self-system therapy and functional magnetic resonance imaging-guided transcranial magnetic stimulation as treatment for depression. The Journal of ECT. 2018;34(4):266–273. doi: 10.1097/YCT.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda Y., Silverstein W.K., Barr M.S., Vila-Rodriguez F., Downar J., Rajji T.K., et al. Neurobiological mechanisms of repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex in depression: A systematic review. Psychological Medicine. 2015;45(16):3411–3432. doi: 10.1017/S0033291715001609. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A., Holmes A.J., Dillon D.G., Goetz E.L., Birk J.L., Bogdan R., et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. The American Journal of Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin J.S., Nyman A.J., Davidson B., Zakzanis K.K., Giacobbe P., Hamani C., et al. Commonly used outcome measures in neurosurgical trials for major depressive disorder might not capture clinically meaningful treatment effects. Journal of Neurology, Neurosurgery, and Psychiatry. 2022;93(4):455–456. doi: 10.1136/jnnp-2021-327688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiei F., Rahnev D. TMS does not increase BOLD activity at the site of stimulation: A review of all concurrent TMS-fMRI studies. eNeuro. 2022;(4):9. doi: 10.1523/ENEURO.0163-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M., Dolcos F., Eddington K.M., Strauman T.J., Cabeza R. Neural correlates of emotional processing in depression: Changes with cognitive behavioral therapy and predictors of treatment response. Journal of Psychiatric Research. 2011;45(5):577–587. doi: 10.1016/j.jpsychires.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B., Wang L., Diaz M.T., Aiello M., Gersing K., Beyer J., et al. Effect of bupropion extended release on negative emotion processing in major depressive disorder: A pilot functional magnetic resonance imaging study. The Journal of Clinical Psychiatry. 2007;68(2):261–267. doi: 10.4088/jcp.v68n0212. https://www.ncbi.nlm.nih.gov/pubmed/17335325 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Kable J.W., Vandekar L., Katchmar N., Bassett D.S., Baldassano C.F., et al. Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2015;40(9):2258–2268. doi: 10.1038/npp.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer H.S., Putnam K.M., Benca R.M., Davidson R.J. Event-related functional magnetic resonance imaging measures of neural activity to positive social stimuli in pre- and post-treatment depression. Biological Psychiatry. 2006;60(9):974–986. doi: 10.1016/j.biopsych.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Schutter N., Holwerda T.J., Kuipers H., Van R.H.L., Stek M.L., Comijs H.C., et al. The association between loneliness and psychiatric symptomatology in older psychiatric outpatients. Journal of Geriatric Psychiatry and Neurology. 2022;35(6):778–788. doi: 10.1177/08919887211060022. [DOI] [PubMed] [Google Scholar]
- Siddiqi S.H., Taylor S.F., Cooke D., Pascual-Leone A., George M.S., Fox M.D. Distinct symptom-specific treatment targets for circuit-based neuromodulation. The American Journal of Psychiatry. 2020;177(5):435–446. doi: 10.1176/appi.ajp.2019.19090915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski M.J., Felder J., Bizzell J., Green S.R., Ernst M., Lynch T.R., et al. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. Journal of Affective Disorders. 2009;118(1–3):69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauman T.J. Self-regulation and psychopathology: Toward an integrative translational research paradigm. Annual Review of Clinical Psychology. 2017;13:497–523. doi: 10.1146/annurev-clinpsy-032816-045012. [DOI] [PubMed] [Google Scholar]
- Strauman T.J., Detloff A.M., Sestokas R., Smith D.V., Goetz E.L., Rivera C., et al. What shall I be, what must I be: Neural correlates of personal goal activation. Frontiers in Integrative Neuroscience. 2012;6:123. doi: 10.3389/fnint.2012.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauman T.J., Eddington K.M. Treatment of depression from a self-regulation perspective: Basic concepts and applied strategies in self-system therapy. Cognitive Therapy and Research. 2017;41(1):1–15. doi: 10.1007/s10608-016-9801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauman T.J., Vieth A.Z., Merrill K.A., Kolden G.G., Woods T.E., Klein M.H., et al. Self-system therapy as an intervention for self-regulatory dysfunction in depression: A randomized comparison with cognitive therapy. Journal of Consulting and Clinical Psychology. 2006;74(2):367–376. doi: 10.1037/0022-006X.74.2.367. [DOI] [PubMed] [Google Scholar]
- Wise T., Cleare A.J., Herane A., Young A.H., Arnone D. Diagnostic and therapeutic utility of neuroimaging in depression: An overview. Neuropsychiatric Disease and Treatment. 2014;10:1509–1522. doi: 10.2147/NDT.S50156. [DOI] [PMC free article] [PubMed] [Google Scholar]