Abstract
Fecal incontinence (FI) has a significant long-term impact on patient quality of life for which there is a range of medical and surgical management alternatives. We report the preliminary outcome using the ForConti Contix Faecal Incontinence Management System (FIMS) in FI patients who had failed conservative therapy and who were recruited at 2 tertiary institutions between September 2018 and September 2020. Comparative assessments were made before and after 2 week periods of treatment using bowel diaries and subjective Wexner and Faecal Incontinence Quality of Life scores. Of 17 patients enrolled, 11 completed an 8-week assessment with a significant fall in the average percentage of FI days reported from 84% before treatment to 16.8% at the first posttreatment assessment and down to 13.2% by the second assessment period. This finding correlated with a similar reduction in the total weekly number of episodes of frank FI, minor soiling, and fecal urgency reported by patients along with concomitant improvements in the Wexner scores. For those using the device, there was less concern about accidental bowel leakage, high rates of satisfaction, and minimal problems with the device. Initial results are encouraging warranting further study.
Keywords: Fecal incontinence, Anal plug, Faecal Incontinence Management System, ForConti Contix, Accdental bowel leakage
INTRODUCTION
Fecal incontinence (FI), the involuntary expulsion of stool in someone over the age of 4 years, is both physically and psychologically disabling. FI directly impairs patients’ quality of life by creating a loss of confidence and self-esteem, which can bring about social isolation [1]. With the inclusion of diagnostic investigations and specific medical and surgical therapies, the full economic impact of FI is substantial, as it has a reported prevalence of 18% within the community and up to 47% among nursing home residents [2]. The true prevalence of FI and its overall social burden are substantially underestimated, with many barriers identified to patients seeking help, most notably a reluctance to report embarrassing symptoms and limited awareness by patients of effective therapies [3, 4].
The causes of FI are multifactorial; identified risk factors include female sex, sphincter injury associated with traumatic vaginal delivery, the aftermath of specific anorectal and perineal operations, and the effects of prolonged straining to pass stool [5, 6]. Traditionally, a stepwise approach has been employed for the management of FI, beginning with a range of conservative medical measures, including the use of antidiarrheal agents and dietary changes both aimed at reducing the number of stools and altering their consistency. Treatment typically progresses to pelvic floor rehabilitation techniques, the commonest of which is biofeedback. Lately, there has been a greater emphasis on sacral neuromodulation and posterior tibial nerve stimulation techniques with far less use of conventional surgical treatments such as sphincter reconstruction and muscle transposition. These operative approaches can still be used as a last resort, but they have considerable morbidity and poorer long-term functional success than neuromodulation [7].
As an alternative to surgery, there are particular containment devices and systems that work on the principle of blocking the inadvertent leakage of stool or rely on scheduled rectal emptying. These options operate in different ways and include a variety of anal plugs, transanal irrigation systems, rectal sealants, and pressure-regulated vaginal inserts. Although anal plugs have been shown to be effective, they can be difficult to tolerate largely because part of the plug lies in the most sensitive part of the anal canal below the dentate line. As this discomfort tends not to settle over time, many patients ultimately decide not to use an anal plug long-term despite its clinical effectiveness [8, 9]. The ForConti Contix Fecal Incontinence Management System (FIMS; Forconti Medical) was designed for self-insertion and retrieval, with the device placed in the insensitive area above the dentate line. Following approval by the Israel Ministry of Health, a pilot study was performed on 20 patients assessing the device’s safety and efficacy over short-term follow-up. This showed that the system was safe and comfortable, with a significant reduction in the incidence of fecal leakage (unpublished company-internal results). We present our initial preliminary patient experience with the Contix FIMS, describing the technical details and short-term outcomes.
TECHNIQUE
This single-arm, nonrandomized study format was approved by the local hospital ethics committees of the 2 participating tertiary referral institutions, the Laniado Medical Center and the Sheba Medical Center, Israel. Informed consent for device use was provided by all patients involved. Patients were included in the analysis who presented with FI (a minimum of 4 FI episodes over a 2-week baseline period) and in whom an adequate (≥ months) trial of conservative measures had failed. These measures typically included the use of antidiarrheal agents, lifestyle changes for aggravating foods and practices, and a range of physical therapies, including pelvic floor muscle retraining, biofeedback therapy, and selective neurostimulation. Patients were recruited between September 2018 and September 2020 to broadly evaluate the safety and effectiveness of the Contix FIMS. There are several points that should be noted regarding the device and its use.
1. The ForConti Contix FIMS device is slightly smaller in diameter than the average index finger and is designed for self-insertion and withdrawal.
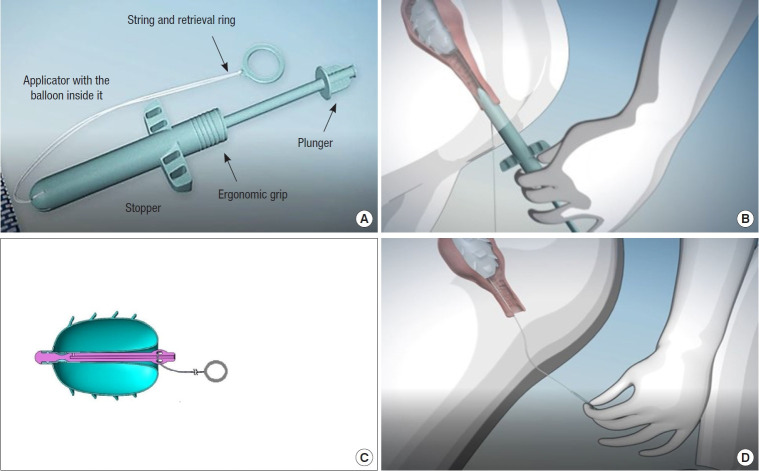
2. The balloon is inflated with air and is deployed above the dentate line for periods up to 12 hours. The structure of the device and its component parts are shown in Fig. 1.
Fig. 1.
The ForConti Contix Faecal Incontinence Management System (FIMS). (A) The component parts of the ForConti Contix FIMS. (B) The insertion process. (C) The jugs attached to the outer surface of the inflated balloon which resist displacement during peristalsis. (D) When the string is pulled for balloon retrieval, the balloon inverts exteriorizing the smooth inner surface.
3. The system comprises an applicator, which encases the balloon, and an inflation kit (a 60-mL syringe with a check valve and an extension cable), along with a string that has an external ring-pull permitting balloon withdrawal. The lubricated applicator is inserted into the rectum and advanced until the stopper reaches the anal margin.
4. By pushing the plunger on the end of the device as far as the barrel of the applicator, the balloon is expelled and exposed. The balloon is then inflated using a syringe filled with air, allowing secure placement above the dentate line. Soft jugs on the outer surface of the balloon assist in retaining it in place during peristalsis and in preventing accidental bowel leakage (ABL).
5. The ring-pull string remains outside the anal canal, so that when removal of the device is required, pulling on the string inverts the device into itself, exposing the smooth inner surface that readily slides out of the anal canal.
Patients were assessed daily using the Wexner and Faecal Incontinence Quality of Life (FIQL) domain questionnaires [10, 11], comparing the averages of the bowel diaries from the pretreatment (control) phase with 2 active 2-week treatment periods. The severity of FI was recorded as major or minor, along with recording fecal urgency. Pain on device insertion and removal was determined using a visual analogue scale (VAS). By definition for this study, the device was considered clinically effective if it showed a ≥ 50% reduction in weekly ABL episodes or of diary days with FI (when compared with baseline).
Results
Over the study period, 17 patients were enrolled, of whom 6 dropped out, leaving 11 patients examined with a complete 8-week follow-up (9 female patients; mean overall age, 62 years [range, 26–86 years]). The dropout cases were related to a reorganization in the team of investigators at one recruitment center. The mean percentage of FI days fell during the study period from 84% during the control phase to 16.8% after the first 2-week posttreatment assessment and down to 13.2% overall following the second assessment with the device. By the first assessment period, there was an overall reduction by 2.98 in the number of major FI events and by 3.83 after the final assessment period. Treatment response was defined as a reduction of ≥ 50% in FI episodes, and 2 additional responders became evident between the first and the final assessment periods (7 patients vs. 9 patients, respectively). There was a reduction in the number of days with FI (by 5.5 days on average) during the first posttreatment assessment, which improved by the final assessment (a 5.71-day reduction). Similar benefits were concomitantly evident in the reduction in the number of days with reported fecal urgency, although there was no additional improvement after a longer assessment period (7.88 vs. 7.55, respectively). In the patient cohort, the Wexner score fell from a pretreatment mean of 17 (range, 12–20) to a mean of 11 (range, 0–20). Patients used the device for an average of 5.4± 3.7 hours (range, 0.65–12.4 hours) over the first 2 weeks of treatment and for 5.5± 4.7 hours (range, 0.5–12.3 hours) during the second period of treatment.
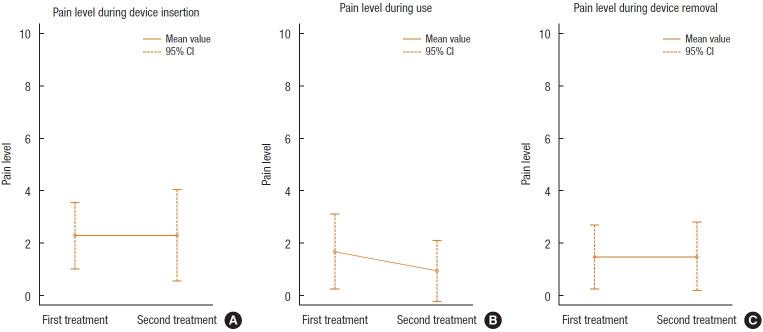
Fig. 2 shows the mean VAS pain reports of patients during device insertion, in general use, and upon device withdrawal. The pain was recorded as low in all cases and decreased even further over the duration of the assessment. Concerning the FIQL assessments, 8 of 11 patients reported fewer episodes of passive incontinence during treatment, with patients expressing less worry about the risk of ABL, less fear in leaving home, and an overall better feeling of wellbeing. Patients volunteered that they felt more in control of their bowel function and no longer scheduled their lives around the proximity of a toilet. As a result, some considered that they were more outgoing, more likely to visit friends, and even able to lead a more normal sex life. Adverse events were reported by 8 of 11 patients, with a total of 20 incidents, only 8 of which were device-related. Of these, 6 patients experienced some pain during insertion of the device and 2 noticed rectal bleeding for which no specific cause was identified on a rectoscopic examination performed the first day after the bleeding was reported by the patients. Normal recto-sigmoidoscopy done before the insertion of the device was an inclusion criterion. A second rectoscopy for nonbleeding cases was done a week after completion of the study. The problems attributed to the device spontaneously resolved in each case. Two patients withdrew from the study, 1 of whom cited pain on device insertion as a reason for withdrawal, while the other patient suffered an acute myocardial infarction during the pretreatment phase. Routine proctosigmoidoscopy during the study did not reveal any rectal or anal pathology that might have been considered to be device-related
Fig. 2.
Recorded pain level (visual analogue scale) with the ForConti Contix device. CI, confidence interval.
DISCUSSION
In conclusion, the ForConti Contix FIMS is safe, well tolerated, and efficient in its prevention of ABL. This is the first formal report of the initial clinical outcomes with this device used in a small number of patients presenting with FI who were unresponsive to conservative measures. Use of the device over a short period resulted in a reduction in the percentage of FI days and the number of discrete FI events, along with less minor soiling and fecal urgency. These effects were accompanied by improvements in the Wexner score and in quality of life and lifestyle parameters, which included personal confidence, a sense of bowel control, and less fear of leaving the house. Most patients experienced some initial discomfort with insertion of the device; however, this resolved once patients developed familiarity with repeated use. The FIMS represents a low-cost alternative to the treatment of FI and requires further study.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Conceptualization: MV. Data curation: CMR, YC. Formal analysis: MV, RYM, MBG. Investigation: RYM, CMR, DC. Methodology: YC, DC. Project administration: YC. Supervision: MBG. Writing–original draft: MV, RYM, CMR, YC. Writing–review & editing: DC, MBG. All authors have read and approved the final manuscript.
REFERENCES
- 1.Meyer I, Richter HE. Impact of fecal incontinence and its treatment on quality of life in women. Womens Health (Lond) 2015;11:225–38. doi: 10.2217/whe.14.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu X, Menees SB, Zochowski MK, Fenner DE. Economic cost of fecal incontinence. Dis Colon Rectum. 2012;55:586–98. doi: 10.1097/DCR.0b013e31823dfd6d. [DOI] [PubMed] [Google Scholar]
- 3.Brown HW, Rogers RG, Wise ME. Barriers to seeking care for accidental bowel leakage: a qualitative study. Int Urogynecol J. 2017;28:543–51. doi: 10.1007/s00192-016-3195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown HW, Guan W, Schmuhl NB, Smith PD, Whitehead WE, Rogers RG. If we don’t ask, they won’t tell: screening for urinary and fecal incontinence by primary care providers. J Am Board Fam Med. 2018;31:774–82. doi: 10.3122/jabfm.2018.05.180045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng KS, Sivakumaran Y, Nassar N, Gladman MA. Fecal incontinence: community prevalence and associated factors: a systematic review. Dis Colon Rectum. 2015;58:1194–209. doi: 10.1097/DCR.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 6.Hage-Fransen MA, Wiezer M, Otto A, Wieffer-Platvoet MS, Slotman MH, Nijhuis-van der Sanden MW, et al. Pregnancy- and obstetric-related risk factors for urinary incontinence, fecal incontinence, or pelvic organ prolapse later in life: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2021;100:373–82. doi: 10.1111/aogs.14027. [DOI] [PubMed] [Google Scholar]
- 7.Ivatury SJ, Wilson LR, Paquette IM. Surgical treatment alternatives to sacral neuromodulation for fecal incontinence: injectables, sphincter repair, and colostomy. Clin Colon Rectal Surg. 2021;34:40–8. doi: 10.1055/s-0040-1714285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norton C, Kamm MA. Anal plug for faecal incontinence. Colorectal Dis. 2001;3:323–7. doi: 10.1046/j.1463-1318.2001.00257.x. [DOI] [PubMed] [Google Scholar]
- 9.Deutekom M, Dobben AC. Plugs for containing faecal incontinence. Cochrane Database Syst Rev. 2015;2015:CD005086. doi: 10.1002/14651858.CD005086.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, et al. Fecal Incontinence Quality of Life Scale: quality of life instrument for patients with fecal incontinence. Dis Colon Rectum. 2000;43:9–17. doi: 10.1007/BF02237236. [DOI] [PubMed] [Google Scholar]
- 11.Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97. doi: 10.1007/BF02050307. [DOI] [PubMed] [Google Scholar]