Abstract
Perimenopause is a time in a woman's life where fertility may vary depending upon her age and her reproductive stage and has been defined as the transition period prior to menopause that is characterized by irregular menses, hormonal changes, vasomotor symptoms, and declining fertility (Casper, 2020). Fertility tracking during this time in a woman's reproductive stage has not been widely studied. Employing the use of Luteinizing Hormone Urine Assay sticks, an electronic hormonal monitor device or mucus, we propose a set of guidelines to determine the potentially fertile times of a woman's cycle based on staging according to the Stages of Reproductive Aging Workshop (STRAW) criteria and illustrate their application with three case reports.
Keywords: Fertility awareness, Fertility awareness information technology, Fertility health, Menstrual cycle, Natural family planning, Women’s health, Women’s reproductive health, Women’s reproductive issues
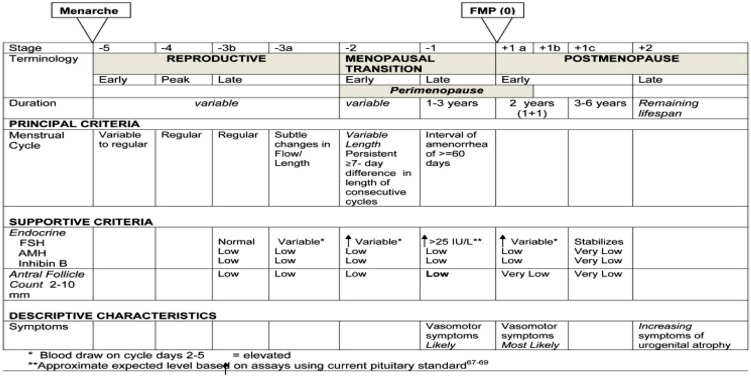
Perimenopause is a time in a woman’s life where fertility may vary depending upon her age and her reproductive stage. It has been defined as the transition period prior to menopause that is characterized by irregular menses, hormonal changes, vasomotor symptoms, and declining fertility (Casper, 2020, 1–2). The World Health Organization has described a woman’s fertility status based on her age: women aged 40–44 years old have a 10% fertility rate, women aged 45–49 years old have a 2% fertility rate, and women aged over or equal to 50 have a low fertility rate, but not equal to zero (Gray 1979, 97–115; Metcalf 1979, 39–48). Although this categorization is helpful in giving a woman of a certain age an estimate of her expected fertility, it is not as specific as characterizing a woman’s fertility based on her individual reproductive stage using her menstrual cycle characteristics. In 1994, the Study of Women’s Health Across the Nation (SWAN) began to investigate mid-life women’s various health issues, including their hormonal variations in later reproductive stages (Khoudary et al., 2019, 1213–1214), but did not look specifically at these women’s fertility or pregnancy risk. In 2001, the Stages of Reproductive Aging Workshop (STRAW) criteria were developed to characterize women’s menstrual cycle characteristics as they advanced toward menopause, and these guidelines were updated in 2011, STRAW + 10 (Harlow et al. 2012, 387–395) (see Figure 1). Instead of a woman’s age, this system takes into account the woman’s cycle length, cycle variability, and hormonal status to accurately describe her reproductive stage and likely fertility.
Figure 1.
Stages of Reproductive Aging Workshop + 10 Staging System for Reproductive Aging in Women).
Fertility tracking methods have been successful in finding the fertile window of women who have regular ovulatory menstrual cycles. Most studies that have been done in fertility tracking employ women who are fertile, not at the end of their reproductive stages where fertility and pregnancy is less likely. There have been just a few retrospective studies looking at pregnancy risk in women in their later stages of reproduction. A study reviewing Israeli pregnant women’s birth data found that only 209 out of 104,659 women delivered a baby after the age of 45, a rate of 0.2% (Laufer et al. 2004, 1329). Another small study looking at fertility monitoring and advanced age included 36 users of the Sympto-thermal form of natural family planning (NFP); it was found that of the women between the ages of 45 and 53, 33% were potentially fertile and 61% of the 177 total menstrual cycles were potentially fertile (Flynn et al. 1991, 1987–1989). In 2014, Marquette researchers looked at pregnancy rates of 160 women 40–55 years of age who were using the Marquette Method of NFP for pregnancy avoidance. They found that the correct use survival pregnancy rate was 1.5 per 100 users over 12 months of use. The typical user survival pregnancy rate was 6 out of 100 users in 12 months of use. However, there were only 15 women over the age of 45 in the cohort studied and no pregnancies occurred in women over the age of 44 (Fehring and Mu 2014, 354–355).
To date, there have been no studies looking specifically at the pregnancy rates of perimenopausal women depending upon their reproductive stage defined by the STRAW criteria. However, in research published by K. O’Connor et al. (2009, 1178–1187), hormonal variations and the likelihood of an ovulatory event based on a woman’s reproductive staging were studied. Their results showed that although most menstrual cycles in late perimenopause (defined as amenorrhea intervals of greater than 60 days, STRAW-1) were anovulatory, 25% of those cycles longer than 60 days were ovulatory. Ovulation was determined by an algorithm and confirmed with 4 days of sustained rise in pregnanediol glucuronide (PdG), the urinary metabolite found during the postovulatory luteal phase of the menstrual cycle. The study also found that the average day of ovulation (mean Cycle Day 27) was later in the late perimenopause stage (STRAW-1) compared to women who were pre-perimenopausal. Follicle stimulating hormone (FSH) and luteinizing hormone (LH) were higher in perimenopause stages, secondary to negative feedback mechanisms to the pituitary gland, in response to dwindling follicular development. Estrone-3-glucuronide (E1G) levels, indicative of ovarian follicular development, were lower in anovulatory compared to ovulatory cycles. PdG was also decreased in late perimenopause. They concluded that women in the perimenopause late stages of reproduction have hormonal differences compared to pre-perimenopausal women but are still at risk of conception due to some ovulatory cycles (O’Connor et al. 2009, 1178–1187). Even though 25% of women had ovulatory cycles late in the perimenopause transition, these ovulatory cycles may have lower probability of pregnancy due to aged eggs and unsupportive PdG levels with aging luteal dysfunction. To determine the actual pregnancy risk of these women, research is needed to delineate if these late perimenopausal women’s ovulatory events could result in a viable pregnancy if conception occurred.
Perimenopausal women who use fertility tracking to determine their fertile period usually have completed their family size and desire to use it to help with pregnancy avoidance. When looking at the STRAW criteria for reproductive staging and characteristics of the menstrual cycles as one gets older, one can deduce important fertility tracking guidance based on these variables. We propose a set of guidelines to define the fertile periods based on specific STRAW stages (Figure 2) and illustrate their application with three case studies.
Figure 2.
Pregnancy Avoidance Perimenopause Protocols for STRAW Stages-3a, -2, and -1.
Prior to perimenopause, the late reproductive stage (STRAW-3a) menstrual cycles usually have subtle changes in flow or length that vary by less than 7 days. These cycles are often reduced in length with earlier ovulation compared to the woman’s usual cycle length. In order to use fertility tracking successfully for pregnancy avoidance during this stage, an appropriate protocol may be to only have postovulatory intercourse, after second Peak + 3 days. The second Peak on the hormonal monitor device or the last Peak mucus day plus three full days is the end of the fertile window according to most natural family planning methods; it gives the most conservative estimate for the end of the fertile window after ovulation has occurred and the released ovum is no longer viable. This should not cause undue hardship of early abstinence for the couple as these cycles are shorter anyway (Figures 3–5).
Figure 4.
47-year-old client using LH sticks and Premom T/C ratios, mucus, and temperature: STRAW-1.
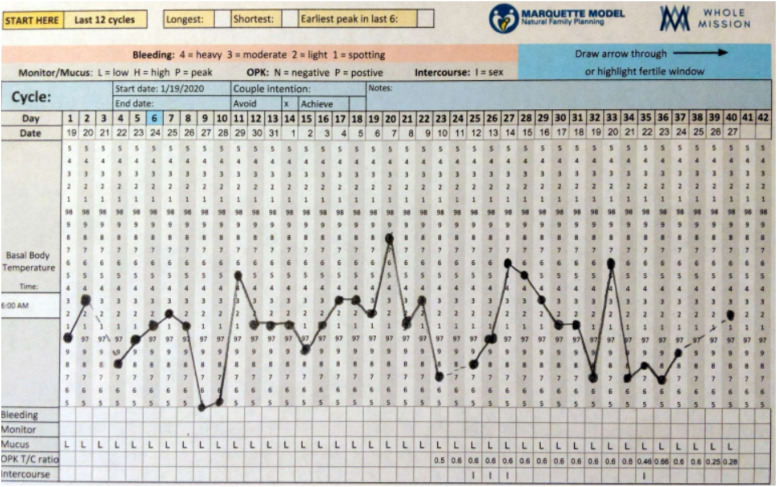
Figure 3.
46 yo perimenopausal woman using LH sticks and Premom T/C ratios: STRAW-1.
Figure 5.
52 year old using LH sticks, Premom T/C ratios, and mucus to chart fertility: STRAW-2.
In the early perimenopausal transition (STRAW-2), cycles can vary persistently by 7 days, with some being shorter and some being longer, and have higher probability of anovulation. FSH can increase and anti-Mullerian hormone (AMH) can decrease at this stage, indicating decreased but not nonexistent ovarian reserve. A plausible fertility tracking protocol may be to begin fertility monitoring with the Clearblue (CB) electronic hormonal monitor, LH urinary assay sticks, or with mucus observations of the menstrual cycle beginning on CD 6 and have relations only if two or more of these indicators read LOW (reflecting a lack of estrogenic activity and/or no impending ovulation evidenced by either LOW mucus or LOW T/C ratios). The couple should abstain on days with HIGH reading from any two or more of the three fertility indicators: mucus, CB monitor, or LH sticks. This may circumvent unnecessary abstinence and hardship on the couple in those longer cycles where there may be many LOW days prior to a late ovulation or no ovulation at all. Anovulation can be confirmed with consecutive negative PdG levels. According to one study, once a woman reaches cycle variability of 40 days or more during perimenopause, she is likely infertile (Metcalf 1979, 39–48).
In late perimenopause (STRAW-1), menstrual cycles are longer than 60 days apart and most will be anovulatory; however, some women may still desire to track their fertility because motivation for pregnancy avoidance is high. As stated earlier, it is known from previous research that a small percentage of these cycles may be ovulatory, up to 25% of them (O,Connor, 2009, 1183). Using the aforementioned protocol may be plausible in this time period as well. Additional studies of FSH and AMH can help a woman know her fertility status; early follicular phase FSH > 25 IU/L and AMH <0.4 ng/mL indicate very low fertility and low ovarian reserve (Barad, Weghofer and Gleicher 2009; Harlow et al. 2012, 392). LH can be elevated at this time due to negative feedback mechanisms on the pituitary gland, but if a cycle is anovulatory, LH may not reach Peak level on an electronic hormonal monitor or LH urine assay sticks and HIGH status may not be indicative of any impending ovulatory event. A woman’s fertility at this point would be quite low and probably unlikely to result in a pregnancy. The case studies that follow illustrate application of these guidelines.
Case 1
History: 46-year-old woman with family history of women undergoing menopause <50 years of age. Client has multiple sclerosis. Her last menstrual cycle was September 2020 and she contacted a Marquette instructor December 2020 because she and her husband had not had relations since menses cessation 3 months prior as she was unable to decipher her fertility status. She had previously used Creighton method of NFP but found her cervical mucus unreliable. Her husband is 58 years old and they have three children and the last has trisomy 21. They were highly motivated to avoid pregnancy.
Staging: Her menstrual cycle lengths were > 60 days putting her into STRAW-1 reproductive staging. Her FSH in August 2020 was 14 IU/L (unknown if this was taken appropriately in the first 3–5 days of the menstrual cycle which signifies a more accurate level). Her AMH level was 0.04 ng/mL in 2018, 2 years prior, indicating very low ovarian reserve.
Protocol: The couple was counseled that her fertility status was low according to WHO criteria; women aged 45–49 have a fertility rate of 2%. She decided to use more economical LH urine assay strips to chart her fertility along with the Premom electronic app which reads the LH level and assigns a numerical value of urine test to control color ratio (T/C ratio) to quantify LOW, HIGH, and PEAK levels of LH activity. T/C ratios of <0.5 indicate low levels of LH activity, >0.5 indicates some level of LH activity that is approaching the LH peak surge, and a ratio of 1 indicates the strongest LH surge or peak activity (Karl 2019). She used the protocol appropriate for late perimenopause stage (STRAW-1). She was able to have relations on any LOW day on the LH stick (<0.5) and abstain on any HIGH (>0.5) days and until Peak + 3. When the LH sticks were giving consistently HIGH values and/or Peak level, she used the PdG urine sticks to try to confirm ovulation. She did not have any positive confirmatory urine PdG tests, indicating her levels as anovulatory.
Observations: As women advance in STRAW stages of reproduction, they may have continual LH surges. This is a compensatory feedback mechanism from the pituitary gland to try stimulate ovulation, albeit unsuccessfully due to depleted ovarian reserve. One of the limitations of this fertility tracking is that there can be variation of T/C numerical ratios even when captured 1 minute apart on the electronic app. One may have a T/C ratio of 0.5 at 6:00 a.m. and then 0.85 at 6:01 a.m., using the same LH sample urine stick. The Premom electronic application seems to do a better job at looking at trends in LH levels leading up to the LH Peak and then declining after it. The client is now 6 months without a menstrual cycle and close to menopause. It is highly probable that this woman who exhibits anovulation could resume relations on any day without incurring the risk of pregnancy.
Case 2
History: 47 year old married for 25 years. Her husband is 48. They have used the Sympto-thermal method of NFP for 20 years. They have no children due to their own personal choice (not because of infertility). They are avoiding pregnancy. With advancement in age, she had basal body temperature fluctuations that made it hard to decipher her fertility status, thus looked for another method.
Staging: She had no menses for 8 months when contacting me in February 2020, consistent with STRAW-1 reproductive stage of cycles > 60 days in length. In March of 2020, she had an FSH level of 150 IU/L and an estradiol level of 11.5 pg/mL. Normal range estradiol levels for premenopausal women are 30–800 pg/mL, and in postmenopausal women, the levels vary from 0 to 20 pg/mL (Stanczyk and Clarke 2014, 56–58). She did not obtain an AMH level.
Protocol: She still had a desire to chart despite her probability of low fertility, so she opted for the more economical LH sticks to see if she could capture a Peak LH surge or ovulation. She also added the Premom app to give T/C ratio numerical values to the LH levels. She continued to chart her basal body temperature and mucus.
Observations: In this late STRAW-1 reproductive stage with 8 months of amenorrhea, one can see that her LH levels are consistently HIGH at 0.6 T/C ratio with no mucus production or sensation (due to low estradiol levels). Her basal body temperature was erratic without an established pattern. They were instructed that her fertility status was very low, indicated by low estradiol and very elevated FSH (150 IU/L). They felt reassured of her low fertility status and resumed relations even on HIGH LH days. No Peak on LH sticks was captured, indicating anovulation. They feel confident in pregnancy avoidance with the knowledge of her hormonal data and usage of the Premom electronic application.
Case: 3
History: 52-year-old client married to 58-year-old husband for 23 years. They have four children, ranging from 14 to 22 and one miscarriage at 44 years of age. They used the Sympto-thermal method for the first 12 years of marriage and then Marquette Method NFP for the past 11 years. They were not using any method when conception occurred at 44 years of age. They are avoiding pregnancy.
Staging: Approximately at the age of 51, she began having cycle variation with some cycles being 25 days long and others being 45 days long. This puts her into a STRAW-2 reproductive stage (cycle variation >7 days). Her FSH level in March 2020 was 55 IU/L and AMH was <0.11 ng/mL, indicating compensatory pituitary feedback and low ovarian reserve.
Protocol: They followed the protocol of abstaining on all HIGH days captured by mucus or T/C ratio on the Premom app until after second Peak + 3.
Observations: This client is 52 years old. According to the WHO, she has less than 2% fertility rate, although not zero. She is older in age than the two previous case studies; however, she is in an earlier STRAW-2 perimenopausal stage than they are. This is an important consideration as age alone is not as specific in determining fertility status as are cycle characteristics. Her T/C ratios on Premom show very low values of 0.17 and 0.26 five days prior to Peak LH, indicating that those values alone may not give enough forewarning for impending ovulation. Although her FSH is >25 IU/L (it is 55 IU/L) and her AMH is low at <0.11 ng/mL, indicating low ovarian reserve, she is still able to pick up Peak LH levels according to the Premom T/C ratio with two cycles and is able to confirm ovulation with a positive PdG post-Peak with at least two of her cycles. She did not perform the PdG samples at any standardized time during the length of her cycle, but during random post-Peak days to confirm ovulation. This indicates that despite her age and her hormone levels, she indeed is probably still ovulating at least on some cycles.
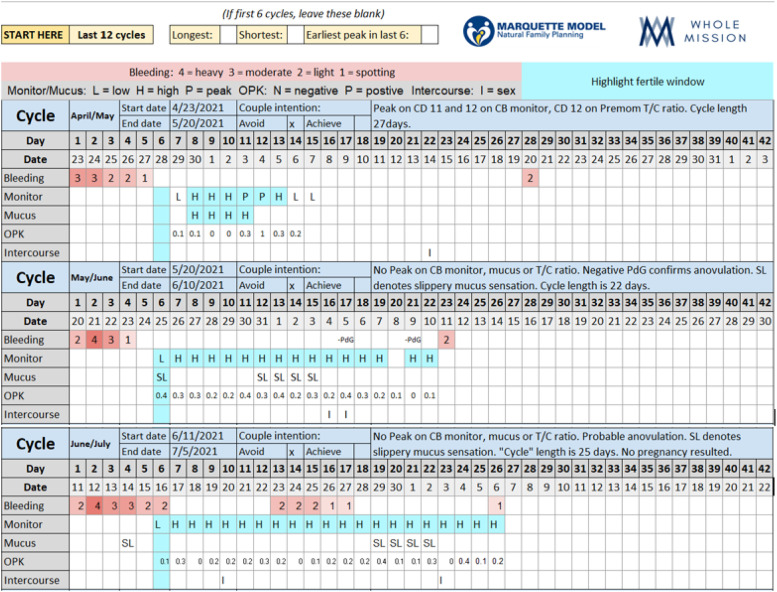
During her cycles from May through July, she used the CB monitor, T/C LH ratio, and mucus observations (Figure 6). She was able to capture CB monitor Peak on the April/May cycle. On the May through July charting, she had no discernable LH surge from the CB monitor and her T/C ratio of LH depicted LOW values and no upward trend that would have indicated a possible LH surge. She noted slippery mucus that did not stretch as “SL,” LOW mucus. She also had negative PdG levels on the May/June cycle, indicating probable anovulation. The couple did not have relations on any day that had more than one HIGH indicator of fertility. No pregnancy resulted from relations during only one HIGH indicator of fertility.
Figure 6.
52 year old using LH sticks with Premom T/C ratios, mucus, and CB monitor to chart fertility: STRAW-2.
In conclusion, the perimenopausal state has varying hormonal levels and characteristics depending upon what STRAW stage a woman is in and is a helpful indicator of her reproductive status. An economical way for women to track their fertility during the perimenopausal state is the Premom LH urine assay sticks and T/C ratios and mucus. The accuracy of finding the fertile window with both LH urine assay sticks and mucus together has been demonstrated in previous research (Leiva, Bouchard and Abdullah 2017, 1–8); (Barron, Vanderkolk and Raviele 2018, 153–157); however, the population of women in these studies were in normal reproductive cycles and not perimenopausal. Also, LH urine assay stick T/C levels may not be accurate enough to determine the beginning of fertility in perimenopausal women but at best may be helpful to indicate anovulation if an upward trend toward an LH surge does not occur. Anovulation can also be confirmed with negative PdG levels. The numerical values that determine Low, High, and Peak T/C ratios are also under investigation and have not been clearly defined. To determine the start of fertility, using mucus signs in addition to the LH T/C ratio could be more predictive of impending ovulation. More research is needed to assess if other fertility trackers like quantitative hormonal devices or quantitative hormone sticks that measure actual levels of E3G or LH may be better indicators of beginning fertility and Peak LH during the perimenopausal stages. It would be helpful to accurately predict if and when it is best to tell women that their reproductive stage no longer warrants fertility tracking as the rate of fertility is so low that it is negligible. Finally, more research is needed to investigate if ovulatory cycles in the perimenopausal state translate to actual fertility and actual pregnancy rate.
Biographical Notes
Maria Meyers, MD, graduated from Louisiana State University Medical Center and completed her pediatric residency from Louisiana State UniversityHealthCenter in Shreveport in 1997. She is a pediatrician practicing at Jefferson County Department of Health for 23 years in Birmingham, Alabama; the health department has 3 strategic locations in underserved areas of Birmingham and her patients are mainly Medicaid recipients and those who live below the poverty level. She does chastity education for the youth on a daily basis in her clinic and has spoken at schools and youth groups as well. She teaches natural family planning (NFP) and became a certified Marquette Method of NFP in 2014. She teaches clients NFP within the diocese of Birmingham and at Whole Mission, an online MarquetteModel NFP service designed to teach the method remotely to couples. She is married to aviation artist husband, Wade Meyers, and they have 4 great children, ranging from 15 to 22 years of age.
Lauren Vitale, BSN, is a registered nurse and Marquette Method natural family planning instructor. She completed her Bachelor of Science in Nursing from Madonna University in 2009 and practiced as a pediatric nurse until 2015. In 2019, Lauren and her husband, Giovanni Vitale II, co-founded Whole Mission, an online natural family planning education service. Through Whole Mission, she works to connect couples with Marquette Method instructors and provide technical and practical support for natural family planning educators. Lauren continues to provide fertility education in the Marquette Method to couples and women around the world.
Kathryn Elenchin, BSN, works as a nurse anesthetist in PA and NY states. She has been teaching the Marquette Method with her certificate of completion from 2016. She is a preceptor at FACTS (Fertility Appreciation Collaborative to Teach the Science), where she helps to educate medical students and healthcare professionals about fertility awareness. Kathryn is also a member of the chastity and NFP committee in the diocese of Erie, PA. She talks with engaged couples about the benefits of using NFP. She enjoys being a wife and mom of 6 in a rural community.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Maria G. Meyers https://orcid.org/0000-0002-3416-0032
References
- Barad D. H., Weghofer A., Gleicher N.. 2009. “How predictive of basic pregnancy potential are extremly low levels of anti-müllerian hormone (AMH)?” Fertility and Sterility 92, no. 3: S178–S179. 10.1016/j.fertnstert.2009.07.1361. [DOI] [Google Scholar]
- Barron M. L., Vanderkolk K., Raviele K.. 2018. “Finding the Fertile Phase: Low-Cost Luteinizing Hormone Sticks Versus Electronic Fertility Monitor.” American Journal of Maternal and Child Nursing 43, no. 3: 153–157. 10.1097/NMC.0000000000000422. [DOI] [PubMed] [Google Scholar]
- Casper R. F. 2020. Clinical manifestations and diagnosis of menopause. UpToDate. Literature review current through March 20, 2021. This topic last updated: March 20, 2020. Accessed April 28th, 2021 https://www.uptodate.com/contents/search. [Google Scholar]
- Fehring R. J., Mu Q.. 2014. “Cohort Efficacy Study of Natural Family Planning among Perimenopause Age Women.” Journal of Obstetric, Gynecologic & Neonatal Nursing 43: 351–358. 10.1111/1552-6909.12307. [DOI] [PubMed] [Google Scholar]
- Flynn A. M., James P., Collins W. P., Royston P.. 1991. “Symptothermal and hormonal markers of potential fertility in climacteric women.” American Journal of Obstetrics and Gynecology 165, 6: 1987–1989. 10.1016/S0002-9378(11)90560-2. [DOI] [PubMed] [Google Scholar]
- Gray R. H. 1979. “Biological and social interactions in the determination of late fertility.” Journal of Biosocial Science 11, Suppl. S6: 97–115. 10.1017/S0021932000024329. [DOI] [PubMed] [Google Scholar]
- Harlow S. D., Gass M., Hall J. E., Lobo R., Maki P., Rebar R. W., Sherman S., Sluss P. M., de Villiers T. J.. 2012. “Executive summary of the Stages of Reproductive Aging Workshop + 10.” Menopause 19, no. 4: 387–395. https://doi:10.1097/gme.0b013e31824d8f40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl Teri. 2019. “What is a T/C Ratio?”. https://premom.com/blogs/what-is-premom-and-why-premom/what-is-a-t-c-ratio?_pos=1&_sid=5c0240369&_ss=r.
- El Khoudary S. R., Greendale G., Crawford S. L., Avis N. E., Brooks M. M., Thurston R. C., Karvonen-Gutierrez C., Waetjen L. E., Matthews K.. 2019. “The menopause transition and women’s health at midlife: a progress report from the Study of Women’s Health Across the Nation (SWAN).” Menopause: The Journal of the North American Menopause Society 26, no. 10: 1213–1227. 10.1097/GME.0000000000001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiva R. A., Bouchard T. P., Abdullah S. H., Ecochard R.. 2017. “Urinary Luteinizing Hormone Tests: Which Concentration Threshold Best Predicts Ovulation?” Frontiers in Public Health 5: 320. 10.3389/fpubh.2017.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer N., Simon A., Samueloff A., Yaffe H., Milwidsky A., Gielchinsky Y.. 2004. “Successful spontaneous pregnancies in women older than 45 years.” Fertility and Sterility 81, no. 5: 1328–1332. 10.1016/j.fertnstert.2003.09.056. [DOI] [PubMed] [Google Scholar]
- Metcalf M. G. 1979. “Incidence of ovulatory cycles in women approaching the menopause.” Journal of Biosocial Science 11: 39–48. [DOI] [PubMed] [Google Scholar]
- O'Connor K. A., Ferrell R., Brindle E., Trumble B., Shofer J., Holman D. J., Weinstein M.. 2009. “Progesterone and ovulation across stages of the transition to menopause.” Menopause 16, no. 6: 1178–1187. 10.1097/gme.0b013e3181aa192d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczyk F. Z., Clarke N. J.. 2014. “Measurement of Estradiol-Challenges Ahead.” The Journal of Clinical Endocrinology & Metabolism 99, 1: 56–58. 10.1210/jc.2013-2905. [DOI] [PubMed] [Google Scholar]