Abstract
Ticks are ectoparasites that impact the health and productivity in farm animals. They are also important vectors for pathogens transmitted to animals and humans. A cross-sectional study was conducted from November 2018 to April 2019 with the objectives of determining the prevalence, identifying genera and seasonal dynamics of adult ixodid ticks infesting cattle in and around Gondar, northwestern Ethiopia. Pearson Chi-square Test was used to evaluate the association of tick prevalence with explanatory variables. One way analysis of variance was used to compare mean tick density of cattle with the explanatory variables. The overall prevalence of cattle ixodid tick infestation was found to be 65.8% (95% CI = 60.0–71.2%). The effect of breed, sex, age and body condition score on tick prevalence was investigated. However, only the body condition score of study animals was found to have statistically significant effect on the prevalence of tick infestation. Monthly analysis of tick infestation showed statistically significant variation (χ2 = 36.17, p = 0.00) during the study period (November 2018 – April 2019). The highest monthly prevalence was recorded in March (85.0%), and the least in February (42.0%). A comparison of the tick prevalence across seasons showed statistically significant differences (χ = 34.10, p = 0.00), being highest (82.5%) during the short rainy season. During the study period, a total of 3796 adult ixodid ticks were collected from different body regions of the study animals. Three ixodid tick genera were identified, with the genus Rhipicephalus being the most prevalent (n = 2122 (55.9%) of the total adult ticks (3796). The overall mean tick density per host for all genera was 12.78 ticks, with a marked difference in tick density during the three study seasons. The highest MTD was recorded during the short rainy season (MTD = 18.58), (F = 19.71, p < 0.05). The present study has shown that ticks are highly prevalent in the study area. Therefore, an appropriate tick control program should be designed and implemented.
Keywords: Cattle, Gondar, Ixodid ticks, Prevalence, Risk factors, Seasons
1. Introduction
With 65 million cattle, 40 million sheep, 51 million goats, 8 million camels, and 49 million chickens in 2020, Ethiopia has the greatest livestock population in Africa (CSA, 2016). In the highland regions of Ethiopia, livestock especially cattle are an important source of animal protein, crop cultivation power, manure for crop production and household energy, economic security at the time of crop failure, and wealth creation (Feed The Future, 2021). Despite the importance of livestock to significant proportion of the population and the economy of the country, the sector has remained undeveloped and productivities are extremely low and its impact either at the household or national level has so far been limited compared to its size (The World Bank Livestock and Fisheries Sector Development Project (P159382: International Development Association Project Appraisal Document on a Proposed Credit to The Federal Democratic Republic of Ethiopia for a Livestock and Fisheries Sector Developme, 2017). Devastating diseases, poor management and recurrent and prolonged drought are the most important challenges affecting the viability of the sector in Ethiopia (Dinku, 2019).
Ectoparasites like ticks have substantial impact on the health, and productivity of animals. They inflict significant economic losses to the cattle industry and play a vital role in the transmission of some pathogens (Urge, 2020). Ixodidae or hard ticks are obligate blood-sucking parasites with worldwide distribution (Zhou et al., 2021). Epidemiologically they are important in the distribution and transmission of infectious and parasitic agents in humans, domestic and wild animals. They are vectors, reservoir and cyclic hosts for tick-borne pathogens (Olsthoorn et al., 2021). The geographical distribution of hard ticks is generally affected by different ecological and climatic conditions including temperature, precipitation, vegetation and relative humidity (Medlock et al., 2013).
Ticks are prevalent in the different agro-ecological areas of Ethiopia. Amblyomma, Haemaphysalis, Hyalomma, and Rhipicephalus are the main tick genera reported to be found in the country (Ayana et al., 2021). Apart from being disease vectors, bovine tick species have direct damaging effect on the skin and hide of infested animals and cause serious economic losses in the Ethiopian livestock industry (Wondimu and Bayu, 2021).
Relevant data on the distribution of ticks is essential for the development of effective tick and tickborne disease control strategies. Studying ticks on cattle under their natural conditions is also useful in understanding the host-parasite relationship (Lihou et al., 2020).
Hence, this study has been conducted with the objectives of determining the prevalence of adult ixodid ticks, identifying the major genera infesting cattle, assessing the associated risk factors, and analyzing the seasonal population dynamics of the adult ixodid tick genera identified from cattle in the study area.
2. Materials and methods
2.1. Description of study area
The study was conducted from November 2018 to April 2019 in and around Gondar. Gondar is found in Amhara regional state of Ethiopia, about 673 km north of Addis Ababa. It is located between 11°36ˈN 37° 23ˈ latitude and 11.600°N 37.38°E longitude with an altitude of 1800 m. a. s. l, annual rainfall of 1200–1600 mm and annual temperature of 27.5 °C. About 70% of the land is featured by plain plateaus covered by various bushes; low woods with mainly evergreen lands and some semi-humid and humid highland with major agricultural products like wheat, sorghum, maize and pulse crops (CSA, 2016).
2.2. Study animals
Cattle of different age group, sex, and breed in and around Gondar, North Gondar zone of Amhara region, were included in the study. The age of study animals was estimated according to a technique described by (DeLahunta and Habel, 1986), and animals were categorized into three age groups: young (≤ 2 years), adult (3–4 years), and old (≥ 5 years).The body condition score (BSC) of study animals was determined according to (Neary and Yager, 1996), and classified as poor, medium or good body condition score. The breed (local or cross) of study animals was also recorded.
2.3. Study design
A cross-sectional study was conducted from November 2018 to April 2019. The study period covered the three seasons of the year in the study area: Late Wet Season (November), Dry Season (December – February), and the Short Rainy Season (March–April).
2.4. Sample size determination and sampling technique
The total number of animals in the study was calculated by using the expected prevalence of tick infestation in Dembia district of North Gondar zone which is 81% by (Alemu et al., 2014) at a 95% confidence interval and desired absolute precision of 5% according to the formula given by (Thrusfield, 2005):
where: Pexp = expected prevalence (81%).
As per the above formula, the sample size was found to be 236 animals but to increase the accuracy of the study, a total of 295 animals were sampled.
To investigate the seasonal dynamics of the tick population, the study animals were divided among the number of months and seasons the study period covered. On average, about 50 animals were randomly selected in each month (about hundred animals in each season) and investigated for the prevalence of adult ixodid ticks and their seasonal dynamics.
2.5. Data collection
2.5.1. Adult tick collection and identification
After fully restraining the animal, all visible adult ticks were collected from half of the body. Ticks were removed by gentle horizontal pulling and holding the basic capitulum so as not to lose the tick mouthparts. The ticks were collected from the dewlap, belly, Brisket, ear, head, under tail, perineum, and udder/scrotum of the animal. Ticks from each animal and from each body site were collected in separate universal bottles containing 70% ethanol. The half body tick count was multiplied by two to obtain the whole-body tick count. Information of the date, site of collection, age, sex, breed and body condition score of the animal was recorded. The collected ticks were identified using stereomicroscope and forceps. Prior to examination, debris, hair and other foreign materials were removed from the ticks. They were then identified into different genera according to identification guidelines by (Estrada-peña et al., 2003).
2.6. Data analysis
The data collected were coded and imported into Statistical Package for Social Science (SPSS) version 20 for analysis. Descriptive statistics was used to estimate the tick prevalence while Pearson Chi-square Test was used to evaluate the association of tick prevalence with explanatory variables such as age, sex, breed and body condition score of study animals, and season of the year. One way analysis of variance (ANOVA) was used to compare mean tick density (MTD) of cattle with the explanatory variables. In all calculations, the confidence interval (CI) and the significance level were set at 95% and at 5% respectively.
3. Results
3.1. Prevalence of adult ixodid ticks on cattle in the study area
From the 295 study animals examined, 194 (65.8%) were found to be infested by varying number of ticks. The effects of the breed, sex, age and body condition of the study animals on prevalence were investigated. However, only the body condition score of the study animals was found to have statistically significant effect (P = 0.00) on the prevalence of ixodid ticks. The highest prevalence of adult ixodid ticks (85.2%, 95% CI = 72.9–93.3%) was found in cattle of poor body condition, compared to those of moderate (69.0%, 95% CI = 60.8–76.4%), and good body condition (50.0%, 95% CI = 39.6–60.4).
The study period was conveniently divided into three different seasons: Late Wet Season (November), Dry Season (December to February), and Short Rainy Season (March and April). The variation of tick prevalence in the different seasons was found to be statistically significant (χ = 34.10, P = 0.00), and the highest prevalence of ticks was recorded during the short rainy season (82.5%). (Table 1).
Table 1.
Prevalence of adult Ixodid ticks in relation to the assessed risk factors.
| Factor | Category | Number examined | Number positive | Prevalence % (95% CI)a | χ2 statisticb | P-valuec |
|---|---|---|---|---|---|---|
| Breed | Local | 253 | 169 | 66.8% (60.6–72.6) | 0.85 | 0.36 |
| Cross | 42 | 25 | 59.5% (43.3–74.4) | |||
| Age group | Young | 34 | 19 | 55.9% (37.9–72.8) | 0.52 | 0.08 |
| Adult | 133 | 82 | 61.7% (52.8–69.9) | |||
| Old-aged | 128 | 93 | 72.1% (64.6–80.2) | |||
| Sex | Male | 98 | 68 | 69.4% (59.3–78.3) | 0.86 | 0.36 |
| Female | 197 | 126 | 64.0% (56.8–70.7) | |||
| Body condition | Poor | 54 | 46 | 85.2% (72.9–93.3) | 20.30 | 0.00 |
| Moderate | 145 | 100 | 69.0% (60.8–76.4) | |||
| Good | 96 | 48 | 50.0% (39.6–60.4) | |||
| Month | Nov-2018 | 75 | 50 | 66.7% (54.8–77.1) | 36.17 | 0.00 |
| Dec-2018 | 25 | 12 | 48.0% (27.8–68.7) | |||
| Jan-2019 | 25 | 12 | 48.0% (27.8–68.7) | |||
| Feb-2019 | 50 | 21 | 42.0% (28.2–56.8) | |||
| Mar-2019 | 100 | 85 | 85.0% (76.5–91.4) | |||
| Apr-2019 | 20 | 14 | 70.0% (45.7–88.1) | |||
| Season | Late Wet Season | 75 | 50 | 66.7% (54.8–77.1) | 34.10 | 0.00 |
| Dry Season | 100 | 45 | 45.0% (35.0–55.3) | |||
| Short Rainy Season | 120 | 99 | 82.5% (74.5–88.8) | |||
| Overall | 295 | 194 | 65.8% (60.0–71.2) |
Prevalence was calculated as number of positive animals divided by number of examined multiplied by 100, and exact binomial 95% CI of the prevalence was determined.
Pearson's Chi-Square (χ2) generated from independent univariable analysis of each factor.
A P-value ≤0.05 indicates observed differences in prevalence (column proportions) among the different levels of nominal or ordinal factors that are statistically significant at the 0.05 level.
3.2. Distribution of adult ixodid tick genera on cattle in the study area
A total of 3796 adult ixodid ticks were collected from different body regions of the 194 tick infested animals found in this study. Three ixodid tick genera (Ambylomma, Hyalomma and Rhipicephalus) were identified with Rhipicephalus being the most commonly identified genus (55.9%, n = 2122) (Table 2).
Table 2.
Distribution and density of adult ixodid ticks by genera.
| Genus | No. of Adult Ticks | Mean Tick Density (95% CI) | Percentage (%) |
|---|---|---|---|
| Rhipicephalus | 2122 | 7.19 | 55.9% |
| Ambylomma | 1388 | 4.71 | 36.6% |
| Hyalomma | 286 | 0.97 | 7.5% |
| Total | 3796 | 12.87 | 100.0% |
Analysis of variance (ANOVA) was performed to compare mean tick density of cattle with the different explanatory variables (breed, sex, age and body condition) of the study animals. The result shows that mean tick density per host was higher in local breed animals (mean = 13.72) than in Cross breeds (mean = 7.71) (F = 6.40, p = 0.012); in old-aged group (mean = 16.70) than in adults (mean = 10.53) and in young age group (7.59) (F = 9.07, p = 0.00); and in poor body condition animals (20.81) than in moderate (12.92) and in good (8.31) body condition groups. The result showed that age, breed and body condition of the study animals had statistically significant effect on the mean tick density(P < 0.005). However, sex of the study animals was found to have no statistically significant effect on tick density (p > 0.05) (Table 3).
Table 3.
Density of adult ixodid ticks and the associated risk factors.
| Factor | Category | Number examined | Number of Adult Ticks | Mean Tick Density (95% CI)a | ANOVA† |
|
|---|---|---|---|---|---|---|
| F statisticb | P-value | |||||
| Breed | Local | 253 | 3472 | 13.72 (11.87–15.58) | 6.40 | 0.012 |
| Cross | 42 | 324 | 7.71 (5.02–10.41) | |||
| Age | Young | 34 | 258 | 7.59 (3.94–11.24) | 9.07 | 0.000 |
| Adult | 133 | 1400 | 10.53 (8.42–12.63) | |||
| Old-aged | 128 | 2138 | 16.70 (13.85–19.56) | |||
| Sex | Male | 98 | 1248 | 12.73 (10.09–15.38) | 0.013. | 0.911 |
| Female | 197 | 2548 | 12.93 (10.83–15.04) | |||
| Body condition | Poor | 54 | 1123 | 20.81 (16.14–25.49) | 14.22. | 0.00 |
| Moderate | 145 | 1873 | 12.92 (10.79–15.05) | |||
| Good | 96 | 797 | 8.31 (5.71–10.91) | |||
| Month | Nov-18 | 75 | 836 | 11.15 (8.10–14.20) | 8.59. | 0.00 |
| Dec-18 | 25 | 256 | 10.24(4.10–16.381) | |||
| Jan-19 | 25 | 192 | 7.68(3.16–12.20) | |||
| Feb-19 | 50 | 282 | 5.64(3.20–8.08) | |||
| Mar-19 | 100 | 1790 | 17.90(15.06–20.74) | |||
| Apr-19 | 20 | 440 | 22.00(12.44–31.56) | |||
| Season | Late wet season | 75 | 836 | 11.15(8.10–14.20 | 19.713 | 0.00 |
| Dry season | 100 | 730 | 7.30(5.10–9.50) | |||
| Short rainy season | 120 | 2229 | 18.58(15.7921.38) | |||
| Overall | 295 | 3796 | 12.87 (11.22–14.52) | |||
Mean tick density for a particular group was calculated as the total number of adult ticks counted divided by total number of animals in that group, and was given as mean (± 95% CI).
Analysis of variance (ANOVA) for comparison of mean tick density between different levels of a nominal or an ordinal factor.
3.3. Seasonal dynamics of adult ixodid tick genera infesting cattle in the study area
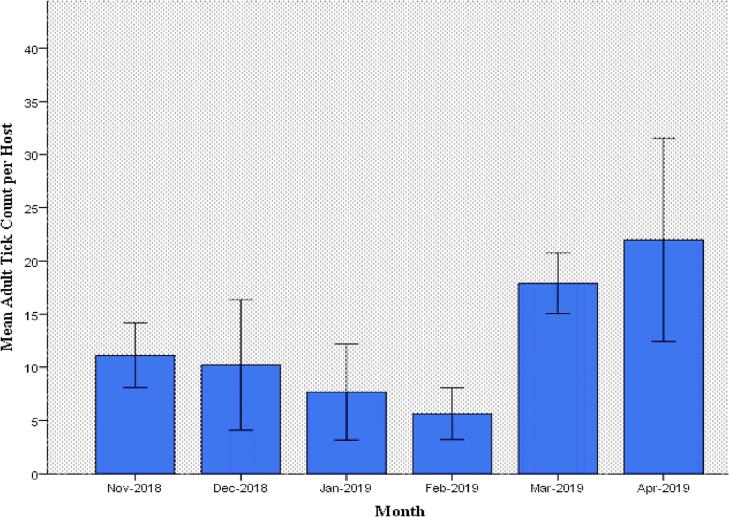
The study revealed that mean tick density per host was significantly (p < 0.05) different across months (Fig. 1). Analysis of mean tick density by season gave a similar statistically significant difference (p < 0.05) but more visible variation than among months. Tick density in short rainy season (18.58) was higher than during the preceding two seasons (Table 3).
Fig. 1.
Monthly mean adult ixodid tick count on cattle in the study area from November 2018 to April 2019.
4. Discussion
From the total 295 study animals examined, 194 (65.8%) were found to be infested by varying number of ticks. This result is in agreement with the findings of 61.0% by (Wasihun and Doda, 2013) in Humbo, Southern Nations, Nationalities and People's Region (SNNPR) of Ethiopia. A similar prevalence of 60.07% was also reported from Uttar Pradesh, India by (Patel et al., 2013). However, in contrast to these, (Kebede and Fetene, 2012) reported a higher prevalence of 89.4% from Western Amhara Region. A different prevalence was also reported by (Ayana et al., 2021) in the in pastoral areas of Yabello district, Borana zone, Oromia, Ethiopia. The difference in the prevalence of tick infestation in the different areas are due to changes in socio-economic and environmental factors, climatic conditions like temperature and humidity, availability of tick control programs and method of study and sample selection (Domşa et al., 2016; Paul et al., 2016).
In this study, sex, age and breed of study animals were found to have no statistically significant effect on the prevalence of ticks (P > 0.05) in the study area. However, the BSC was found to have statistically significant effect on the prevalence of tick infestation in the study area. The highest prevalence of adult ixodid ticks (85.2%, 95% CI = 72.9–93.3%) was found in cattle of poor body condition, compared to those of moderate (69.0%, 95% CI = 60.8–76.4%) and good body condition (50.0%, 95% CI = 39.6–60.4). This can be due to the fact that animals with poor body condition have lower resistance to tick infestation compared to animals with good and moderate body condition. Heavy tick infestation can also cause anemia and weight loss (Kemal et al., 2016). It also weakens the host immune system and the poor body condition can be indicative of the presence of a tickborne infection or co-infection of multiple tickborne diseases especially in animals infested by multiple tick genera or species (Chen et al., 2014).
In the present study, analysis of tick prevalence by season showed statistically significant difference among the three study seasons (χ = 34.10, P = 0.00). The study period covered three seasons: late wet season (November), dry season (December to February), and short rainy season (March and April). Even though ticks were prevalent throughout the study period, the highest prevalence of ticks (82.5%) was recorded during the short rainy season. The same findings were reported by (Urge, 2020). This higher prevalence of tick infestation during the rainy season indicated that rainfall (humidity) is a key climatic element that determines seasonal variation in tick infestation (Shoaib et al., 2022).
A total of 3796 adult ixodid ticks were collected from different body regions of the 194 tick infested animals found in this study. Three ixodid tick genera (Ambylomma, Hyalomma and Rhipicephalus) were identified with Rhipicephalus being the most commonly identified genus (55.9%, n = 2122). The same result was reported by (Yakhchali et al., 2011) in domestic ruminants in North and South of Iran. However, (Urge, 2020) found that Ambyloma to be the most prevalent genera in dairy calves reared by smallholder farmers in central areas of Ethiopia. The difference in the prevalence of the different tick genera infesting cattle is related to the biology and the feeding characteristics of the different tick genera, host resistance and the environmental and climatic conditions in the study area (Chepkwony et al., 2021).
5. Conclusion and recommendations
The present study has shown that ticks are highly prevalent in the study area. The body condition of the study animals and season of the year were found to have statistically significant association with the prevalence of ixodid ticks in cattle. Three ixodid tick genera were identified, with the genus Rhipicephalus being the most prevalent. The highest prevalence of ticks was recorded in the short rainy season. Ticks are known to cause significant economic and health problems in the infested animals, and affect the health and livelihoods of the cattle owners in the study area. Therefore, an appropriate control program should be designed and implemented in the study area especially during the period with low mean tick density as strategic intervention to prevent the high tick infestation in the period with the highest mean tick density (the short rainy season). Further researches on the economic and public health significance of ticks in the study area should also be done.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
References
- Alemu Getachew, Mersha Chanie D.M., BB. Prevalence of ixodid ticks on cattle in northwest Ethiopia. Acta Parasitol. Glob. 2014;5:139–145. [Google Scholar]
- Ayana M., Gelaye A., Fesseha H., Mathewos M. Study on the distribution of ixodid ticks of cattle in pastoral areas of Yabello district, Borana zone, Oromia, Ethiopia. Parasite Epidemiol. Control. 2021:12. doi: 10.1016/j.parepi.2021.e00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Liu Q., Liu J.Q., Xu B.L., Lv S., Xia S., et al. Tick-borne pathogens and associated co-infections in ticks collected from domestic animals in Central China. Parasit. Vectors. 2014;7:1–8. doi: 10.1186/1756-3305-7-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepkwony R., van Bommel S., van Langevelde F. Interactive effects of biological, human and environmental factors on tick loads in Boran cattle in tropical drylands. Parasit. Vectors. 2021;14:1–8. doi: 10.1186/s13071-021-04683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSA . vol. 09. Addis Ababa; Ethiopia: 2016. Crop and Livestock Product Utilization (Private Peasant Holdings, Meher Season) [Google Scholar]
- DeLahunta A., Habel R.E. W. B. Saunders; Eastbourne: 1986. Veterinary Applied Anatomy. [Google Scholar]
- Dinku A. 2019. Assessment of Constraints and Opportunities in Small-scale Beef Cattle Fattening Business: Evidence from the West Hararghe Zone of Ethiopia. [DOI] [Google Scholar]
- Domşa C., Sándor A.D., Mihalca A.D. Climate change and species distribution: possible scenarios for thermophilic ticks in Romania. Geospat. Health. 2016;11:151–156. doi: 10.4081/gh.2016.421. [DOI] [PubMed] [Google Scholar]
- Estrada-peña A., Walker A., Bouattour A., Camicas J., Horak I., Latif A., et al. Bioscience Reports; Edinburgh Scotland,U.K: 2003. Ticks of Domestic Animals in Africa: A Guide to Identification of Species. [Google Scholar]
- Feed The Future . 2021. Ethiopia’s Livestock Systems: Overview and Areas of Inquiry. [Google Scholar]
- Kebede N., Fetene T. Population dynamics of cattle ectoparasites in Western Amhara National Regional State, Ethiopia. J. Vet. Med. Anim. Heal. 2012;4:22–26. doi: 10.5897/JVMAH11.006. [DOI] [Google Scholar]
- Kemal J., Tamerat N., Tuluka T. Infestation and identification of Ixodid tick in cattle: the case of Arbegona District, Southern Ethiopia. J. Vet. Med. 2016;2016:1–8. doi: 10.1155/2016/9618291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lihou K., Rose Vineer H., Wall R. Distribution and prevalence of ticks and tick-borne disease on sheep and cattle farms in Great Britain. Parasit. Vectors. 2020;13:1–10. doi: 10.1186/s13071-020-04287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlock J.M., Hansford K.M., Bormane A., Derdakova M., Estrada-peña A., George J., et al. Artikel Ixodes Ricinus Europa, 2013. Parasit. Vectors. 2013;6:1–11. doi: 10.1186/1756-3305-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary M., Yager A. 1996. Body Condition Scoring in Farm Animals. [Google Scholar]
- Olsthoorn F., Sprong H., Fonville M., Rocchi M., Medlock J., Gilbert L., et al. Occurrence of tick-borne pathogens in questing Ixodes ricinus ticks from wester Ross, Northwest Scotland. Parasit. Vectors. 2021;14:1–11. doi: 10.1186/s13071-021-04946-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel G., Shanker D., Jaiswal A.K., Sudan V., Verma S.K. Prevalence and seasonal variation in ixodid ticks on cattle of Mathura district, Uttar Pradesh. J. Parasit. Dis. 2013;37:173–176. doi: 10.1007/s12639-012-0154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R.E.L., Cote M., Le Naour E., Bonnet S.I. Environmental factors influencing tick densities over seven years in a French suburban forest. Parasit. Vectors. 2016;9:1–10. doi: 10.1186/s13071-016-1591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M., Rashid I., Akbar H., Sheikh A.A., Farooqi S.H., Khan M.A., et al. Prevalence of Ixodidae ticks and their association with different risk factors in Khyber Pakhtunkhwa, Pakistan. J. Anim. Plant Sci. 2022;32:413–420. doi: 10.36899/JAPS.2022.2.0438. [DOI] [Google Scholar]
- The World Bank Livestock and Fisheries Sector Development Project P159382: International Development Association Project Appraisal Document on a Proposed Credit to The Federal Democratic Republic of Ethiopia for a Livestock and Fisheries Sector Developme. 2017. p. 139. [Google Scholar]
- Thrusfield M. 2nd Editio. Blackwell Science Ltd, a Blackwell Publishing Company; Oxford, UK: 2005. Veterinary Epidemiology. [Google Scholar]
- Urge B. Vol. 26. 2020. Infestation of Ectoparasites in Dairy Calves Reared by Smallholder Farmers in Central Areas of Ethiopia. [DOI] [Google Scholar]
- Wasihun P., Doda D. Study on prevalence and identification of ticks in Humbo district, southern nations, nationalities, and People’s region (SNNPR), Ethiopia. J. Vet. Med. Anim. Heal. 2013;5:73–80. doi: 10.5897/JVMAH12.040. [DOI] [Google Scholar]
- Wondimu A., Bayu Y. Identification and prevalence of ixodid ticks of cattle in case of haramaya eastern hararghe, Ethiopia. Vet. Med. Int. 2021;2021 doi: 10.1155/2021/8836547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakhchali M., Rostami A., Esmailzadeh M. Diversity and seasonal distribution of ixodid ticks in the natural habitat of domestic ruminants in north and south of Iran. Rev. Med. Vet. (Toulouse) 2011;162:229–235. [Google Scholar]
- Zhou Q., Li J., Guo X., Liu J., Song Q., Gong X., et al. Records of three mammal tick species parasitizing an atypical host, the multi-ocellated racerunner lizard, in arid regions of Xinjiang, China. Parasit. Vectors. 2021;14:1–12. doi: 10.1186/s13071-021-04639-z. [DOI] [PMC free article] [PubMed] [Google Scholar]