Dear Editor,
We would like to focus the attention of the reader on the SARS-CoV-2 placentitis, an unappreciated, rare but serious complication of maternal SARS-CoV-2 Infection.
First, we report two cases of acute placental insufficiency and fetal distress emerging about 14 days after the diagnosis of maternal SARS-CoV-2 infection with paucisymptomatic course of disease presented to our attention during the dominance of the delta (B.1.617.2) variant of the virus. The discussion will be supported by the current knowledge on this item.
Case 1: a 34-year-old healthy pregnant woman (2G, 0P) at 23 weeks + 6 days of gestation, presented to our observation on the first day after her 14-day isolation due to a mild SARS-CoV-2 infection (B.1.617.2). Until that moment, the pregnancy had been uneventful. The fetal scan showed a FGR (Fetal Growth Restriction) with pathological maternal and fetal arterial flow (AREDF), but still positive pulsatility of the DV (Ductus Venosus). No other risks for placental insufficiency were known, the NIPT (non-Invasive Prenatal Test) showed a low risk for Trisomy 21, 18 and 13, CMV and Toxoplasmosis serology were uneventful.
The patient was normotensive, asymptomatic and without any indication for HELLP-Syndrome. The sFlt/PLGF quotient showed a high risk for preeclampsia. The parents were counseled about the very poor fetal outcome in case of delivery at that moment (23 weeks + 6 days), so that they preferred an expectant management, being aware of the high risk for IUD (Intrauterine fetal Death). We induced the fetal lung maturation (two doses of betamethasone 12 mg IM 24 h apart) and monitored the maternal arterial pressure and well-being without fetal monitoring. At 25 weeks of gestation, the earliest point at which delivery could be recommended, we started the fetal surveillance and found a deterioration of the DV velocimetry (AREDF) and a pathologic CTG tracing. The patient consented the urgent cesarean section despite the high risk of hypoxic-ischemic encephalopathy in the newborn. She delivered a 450 g boy, APGAR score 2/4/7, umbilical artery pH 6.99. The initial neonatal course was complicated by respiratory distress syndrome and other complications consistent with the prematurity, the extremely low birth weight, and the hypoxia-acidosis at delivery. The infant recovered slowly and could be discharged after 3 months in a relatively good clinical condition appropriate to his extreme prematurity.
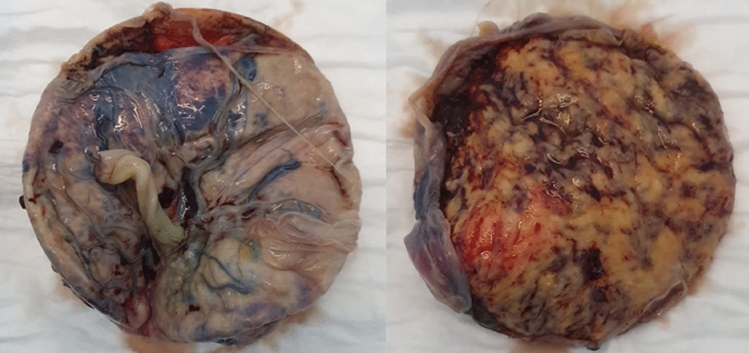
Macroscopically, the placenta was eutrophic, but diffusely affected by large irregular, solid, and whitish areas (Fig. 1). Microscopically, prominent villous trophoblastic necrosis (> 60%) and increased perivillous fibrin deposition could be observed. A positive smear RT-PCR (high viral load) was obtained from the fetal side of the placenta, although the neonatal nasopharyngeal swab (RT-PCR) was negative and the maternal one weakly positive (low viral load), suggesting a viral infection of the placenta (SARS-CoV-2 placentitis).
Fig. 1.
Fetal and maternal side of the Placenta (Case 1): eutrophic but diffusely affected by large irregular, solid, and whitish areas (widespread villous trophoblastic necrosis)
Case 2: a 31-year-old healthy pregnant woman (1G, 0P) at 35 weeks + 1 day of gestation was referred to our clinic because of a non-reassuring fetal heart rate tracing 14 days after her positive nasopharyngeal swab RT-PCR result for SARS-CoV-2, at just the first day after her isolation. She reported an asymptomatic course of the COVID-19 disease, but she noticed a reduction of the fetal movements in the last few days. Due to the B.1.617.2 (delta) variant dominance in this period, there was no sequencing of the SARS-CoV-2 genome. After a few minutes of FHR-registration (fetal heart rate) we suspected a fetal acidosis. A quick ultrasound scan showed a hypotrophic fetus with a pathological fetal Doppler flow and an oligohydramnios. Thus, we confirmed the suspicion of fetal acidosis and indicated an emergency cesarean section. We delivered a depressed, acidotic, hypotrophic girl (1800 g, APGAR score 1/5/7, umbilical artery pH 7.06). After an uncomplicated immediate care, she was transferred to the intermediate NICU for monitoring. She recovered quickly and could be discharged after a few days.
Macroscopically, the placenta was mildly hypotrophic (10° percentile) and inhomogeneously affected by large irregular, solid and whitish areas. Microscopically, prominent villous trophoblastic necrosis (40–45%) and increased intervillous inflammatory infiltrate with increased amount of macrophages and CD8 positive T cells (villitis and intervillositis) could be observed (Figs. 2, 3). A positive smear RT-PCR (high viral load) was obtained from the fetal side of the placenta even though the neonatal and maternal nasopharyngeal swab (RT-PCR) were negative, as well as in case 1, suggesting a viral infection of the placenta (SARS-CoV-2 placentitis).
Fig. 2.
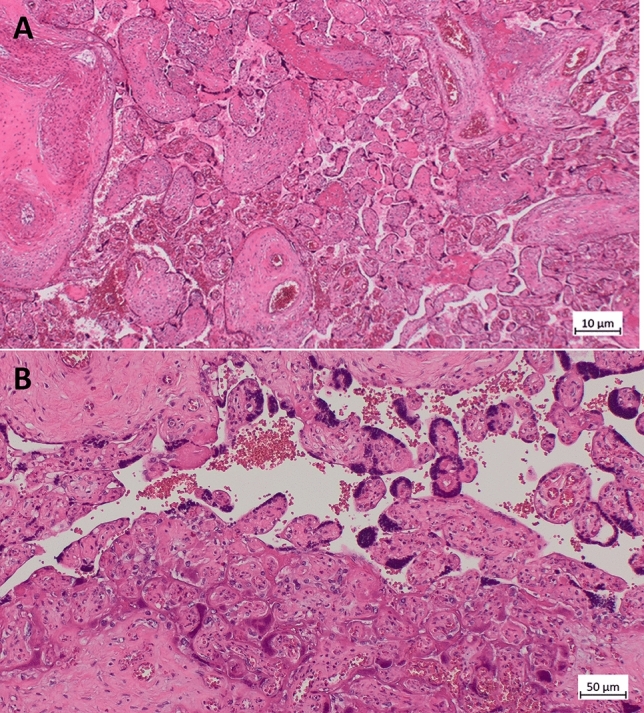
Histology (microscopic) of the placenta from Case 2: A H&E section of a placenta demonstrating necrotic syncytiotrophoblast collapse and fibrin. (200×). B collapse of the intervillous space and trophoblast necrosis (lower part), on the same image vital functioning villi (upper part) (200×)
Fig. 3.
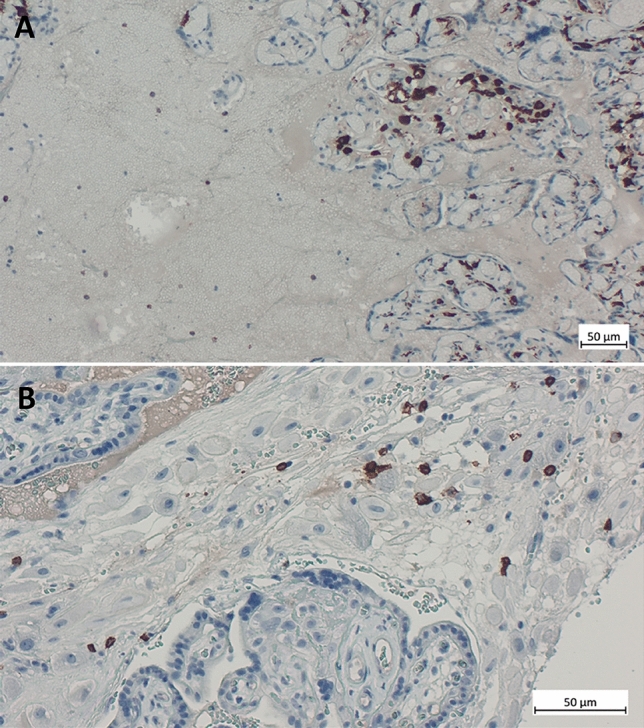
A Immunohistochemistry for CD163 on the placenta showing increased macrophages demonstrating the histiocytic intervillositis (200×). B Immunohistochemistry for CD8 demonstrating increased activity of cytotoxic T cells during infection against SARS-CoV-2 (400×)
Discussion
At the beginning of the outbreak of COVID-19 pandemic the impact of the SARS-CoV-2 infection in pregnancy raised great concern about the possible risks for the mothers and their unborn children.
First published data has shown somewhat contradictory findings about the consequence of the infection for pregnancy outcome, but later reports indicated an unequivocally higher rate of preterm delivery, preeclampsia, stillbirth and maternal mortality in SARS-CoV-2 infected mothers after 20 weeks of gestation. On the other hand, the risk of vertical transmission is very low (0.3–3.2%), teratogenic effects have not been observed and the infected newborns mostly have a mild course of disease so that there is only little direct impact of the maternal infection on the unborn child [1–5].
In the last two years we observed several Covid-19 surges due to the development of the new SARS-CoV-2 variants and to the lack of herd immunity. The vaccination hesitancy before and during pregnancy has left a big part of childbearing women unprotected from the infection hence the number of infected pregnant women exploded, particularly during the dominance of the delta variant (in Germany between June 2021 and January 2022) exacerbating the evidence of rare complications.
The CDC reported a fourfold risk of abortion/stillbirth in infected mothers during the delta variant dominance, and the severely decreased placental function caused by SARS-CoV-2 placentitis seems to be a possible explanation [6].
SARS-CoV-2 placentitis is a rare but largely unvalued and serious complication of maternal infection, large series reported a prevalence of 1–2% in infected mothers, but higher prevalence was described during the delta variant surge [7–11].
Meanwhile pathognomonic histopathological patterns in cases of SARS-CoV-2-placentitis have been described (inflammatory intervillositis, increased perivillous fibrin, villous trophoblast necrosis) and pathophysiologic consequences for the fetus have been analyzed and well understood. An impaired trophoblast differentiation in addition to activation of antiviral and inflammatory CD8 T cells can be observed in the case of SARS COV-19 infection. This may lead to necrosis of the syncytiotrophoblast—as observed in the present case [12, 13]. The slightly increase of CD8 positive T cells exclude the chronic villitis of unknown etiology (VUE), where the increase would be high, and leads to an infectious origin. The Intrinsic genetic abnormality (interferon pathway mutations) and extrinsic factors (high virus load, focal placental hypoxia) seem to predispose to such uncontrolled placental cell apoptosis resulting in diffuse placental parenchymal destruction (50–90% involvement). The placental damage takes from several days up to two weeks after onset of the infection, therefore leading to an acute placental insufficiency and poor fetal outcome irrespective of fetal infection and of the severity of maternal disease [14–16].
Numerous guidelines on Covid-19 Infection in pregnancy have been published, but suggestions on fetal surveillance after maternal infection are vague, in part due to the rapidly evolving situation. Obstetric ultrasound and fetal surveillance in the first 2–3 weeks after maternal infection could be used to detect timely placental malperfusion before it would be incompatible with intrauterine survival, as reported in the above mentioned two cases.
Prerequisite for a SARS-CoV-2 placentitis is the presence of circulating viruses in the maternal bloodstream, the only possible way for the virus to reach trophoblast cells.
Viremia is rare, transient and with low viral load after SARS-CoV-2 infection (about 1% of symptomatic non pregnant individuals) and the few data on pregnant women show a similar trend, independently of the severity of maternal disease [16].
At the present time the Omicron variant surpasses other variants. Although different SARS-CoV-2 variants may have different pathogenicity, disease severity and pregnancy complications in unvaccinated women during the Omicron surge are similar to those observed during the pre-Delta period, while being the most severe during the time with Delta dominance [17].
To minimize the risks of SARS-CoV-2 infection and to prevent maternal viremia during pregnancy and the consequent placentitis, vaccination could play a significant role.
In conclusion, implementation of vaccination in childbearing women and a strict fetal surveillance in the first 2–3 weeks after infection of not immune pregnant women could be an effective preventive strategy to avoid fetal injury.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
LDC: conception and design of the study, acquisition of data and data analysis, manuscript writing and editing. IS: acquisition of data, manuscript writing and revision. MSD: manuscript writing, revision and editing. KB-G: acquisition of the data.
Funding
None.
Data availability
Clinical data and histology images supporting our paper are stored in the database of the perinatology tertiary center Olgahospital/Frauenklinik-Klinikum Stuttgart and are available on request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study used exclusively data from an existing database of the Hospital (perinatology tertiary center Olgahospital/Frauenklinik, Stuttgart). An institutional review board approval was not required.
Informed consent
We used only clinical data and tissue images, no identifiable personal data was gathered and published so that an informed consent is not necessary.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jamieson DJ, Rasmussen SA. An update on COVID-19 and pregnancy. Am J Obstet Gynecol. 2022;226(2):177–186. doi: 10.1016/j.ajog.2021.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villar J, Ariff S, Guiner R, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. Jama Pediatr. 2021;175(8):817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papegeorghiu A, Deruelle P, Gunier R, et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol. 2021;225(3):289.e1–289.e17. doi: 10.1016/j.ajog.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullins E, Hudak ML, Banerjee J, Getzlaff T, Townson J, Barnette K, Playle R, Perry A, Bourne T, Lees CC, PAN-COVID investigators and National Perinatal COVID-19 Registry Study Group Pregnancy and neonatal outcomes of COVID-19 coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet Gynecol. 2021;57:573–581. doi: 10.1002/uog.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Mascio D, Buca D, Berghella V, Khalil A, Rizzo G, Odibo A, Saccone G, Galindo A, Liberati M, D’Antonio F. Counseling in maternal-fetale medicine: SARS-CoV-2 infection in pregnancy. Ultarsound Obstet Gynecol. 2021;57:687–697. doi: 10.1002/uog.23628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Sisto CL, Wallace B, Simeone RM, et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization-Unites States, March 2020–September 2021. MMWR Morb Mortal Wkly Rep. 2021;70(47):1640–1645. doi: 10.15585/mmwr.mm7047e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redline R, Ravishankar S, et al. Diffuse and localized SARS-CoV-2 placentitis prevalence and pathogenesis of an uncommon complication of Covid-19 infection during pregnancy. Am J Surg Pathol. 2022;46(8):1036–1047. doi: 10.1097/PAS.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watkins JC, Torous VT, Roberts DJ. Defining severe acute respiratory Syndrome Coronavirus 2 (SARS-CoV-2) placentitis. Arch Pathol Lab Med. 2021;145(11):1341–1349. doi: 10.5858/arpa.2021-0246-SA. [DOI] [PubMed] [Google Scholar]
- 9.Lineham L, O’Donoghue K, Dineen S, White J, Higgins JR. SARS-CoV-2 placentitis: an uncommon complication of maternal COVID-19. Placenta. 2021;014:261–266. doi: 10.1016/j.placenta.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moltner S, de Vrijer B, Banner H. Placental infarction and intrauterine growth restriction following SARS-CoV-2 infection. Arch Gynecol Obst. 2021;304:1621–1622. doi: 10.1007/s00404-021-06176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shook L, Brigida S, Regan J, Flynn J, Mohammadi A, Etemad B, Siegel M, Clapp M, Li J, Roberts D, Edlow A. SARS-CoV-2 placentitis associated with B.1.6172.2 (Delta) variant and fetal distress or demise. J Infect Dis. 2022 doi: 10.1093/infdis/jiac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Du L, et al. Cellular and molecular atlas of the placenta from a COVID-19 pregnant woman infected at midgestation highlights the defective impacts on foetal health. Cell Prolif. 2022;55(4):e13204. doi: 10.1111/cpr.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornish EF, Mc Donnel T, Williams DJ. Chronic inflammatory placental disorders associated with recurrent adverse pregnancy outcome. Front Immunol. 2022;13:825075. doi: 10.3389/fimmu.2022.825075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrido Pontnou M, Navarro A, Camacho J, Crispi F, et al. Diffuse trophoblast damage is the hallmark of SARS-Cov-2-associated fetal demise. Mod Pathol. 2021;34(9):1704–1709. doi: 10.1038/s41379-021-00827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz D, et al. Placental tissue destruction and insufficiency from Covid-19 causes stillbirth and neonatal death from hypoxic-ischemic injury. Arch Patol Lab Med. 2022;146(6):660–676. doi: 10.5858/arpa.2022-0029-SA. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz D. Stillbirth after Covid-19 in unvaccinated mothers can result from SARS-Cov-2 placentitis, placental insufficiency, and hypoxic ischemic fetal demise, not direct fetal infection: potential role of maternal vaccination in pregnancy. Viruses. 2022;14(3):458. doi: 10.3390/v14030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birol Ilter P, Prasad S, et al. Maternal and perinatal outcomes of SARS-CoV-2 infection in unvaccinated pregnancies during delta and omicron waves. Ultrasound Obstet Gynecol. 2022;60:96–102. doi: 10.1002/uog.24916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Clinical data and histology images supporting our paper are stored in the database of the perinatology tertiary center Olgahospital/Frauenklinik-Klinikum Stuttgart and are available on request.