Abstract
Aims
Quetiapine is widely used to treat psychiatric disorders such as major depression, generalized anxiety disorder, dysthymic disorder, and insomnia other than schizophrenia and bipolar disorder. This study investigated the diagnostic distribution of quetiapine use in patients in a psychiatric hospital, the doses of quetiapine prescribed, and the plasma concentrations (Cps) of quetiapine and active metabolites.
Methods
We enrolled 107 patients who had been prescribed quetiapine for at least 4 weeks. Diagnoses, demographics, and concomitant medications were recorded. Blood sampling was performed in the morning, approximately 12 h after the before‐bed dose of quetiapine.
Results
Diagnoses comprised schizophrenia (n = 25), bipolar disorder (n = 51), major depression (n = 15), dysthymic disorder (n = 9), and others (n = 7). The daily dose (DD) of quetiapine ranged from 25 to 800 (175.9 ± 184.4) mg, with the mean Cp being 105.6 ± 215.3 ng/ml, with a mean Cps/DD ratio of 0.58 ± 0.55 ng/ml/mg. There was a moderate positive linear correlation between the dose and Cps of quetiapine (r = 0.60), and the interpatient variation in Cps/DD ratio was up to 26‐fold.
Conclusion
Quetiapine is used in various doses to treat many psychiatric disorders other than psychosis, and it is usually prescribed as a secondary antipsychotic for symptoms such as insomnia or agitation. A wide interpatient variation of the Cps/DD ratio was noticed. Patients of East Asian descent may exhibit a 50% to 100% increase in the Cps/DD ratio for quetiapine compared with patients of Western descent.
Keywords: bipolar disorder, quetiapine, racial difference, schizophrenia, therapeutic drug monitoring
Quetiapine is used in various doses to treat many psychiatric disorders other than psychosis, and it is usually prescribed as a secondary antipsychotic for symptoms such as insomnia or agitation. Extended release quetiapine (200 and 50 mg) was more frequently prescribed to patients with schizophrenia or bipolar disorder compared with immediate release quetiapine (100 and 25 mg). We calculated the mean daily dose (DD) in each diagnosis and measured the plasma concentration (Cps) of quetiapine and the active metabolites. There was a moderate positive linear correlation between the dose and Cps of quetiapine (r = 0.60), and the interpatient variation in Cps/DD ratio was up to 26‐fold. Patients of East Asian descent may exhibit a 50% to 100% increase in the Cps/DD ratio for quetiapine compared with patients of Western descent.
1. INTRODUCTION
Quetiapine is a second‐generation antipsychotic used for the treatment of schizophrenia and bipolar disorder and as an adjunct treatment for major depressive disorder. Quetiapine has both immediate‐release (IR) and extended‐release (ER) forms. The recommended daily dose (DD) of IR quetiapine for schizophrenia is 150–750 mg, administered twice or thrice daily. The recommended DD of ER quetiapine is 400–800 mg. Quetiapine is also approved for the treatment of bipolar mania as either monotherapy or adjunct therapy and for the treatment of bipolar depression as monotherapy, with the recommended DDs being 400–800 and 300–600 mg, respectively. Additionally, quetiapine has proven efficacious for treating major depression 1 , 2 and generalized anxiety disorder. 3 , 4 Nevertheless, the most common off‐label use of quetiapine is for treating insomnia, 5 with the typical DD range being 12.5–150 mg/day.
Quetiapine is metabolized primarily through hepatic metabolism by cytochrome P450 (CYP) 3A4 6 and partially by the 2D6 system. 7 DeVane and Nemeroff 8 detailed the pharmacokinetics of quetiapine. In summary, food has minimal effects on quetiapine absorption, and smoking does not affect its pharmacokinetics. The elimination half‐life of quetiapine is approximately 7 h, and 11 metabolites formed through hepatic oxidation have been identified. The inactive sulfoxide metabolite and the parent acid metabolite accounted for 15.1% and 14.7%, respectively, and the two active metabolites 7‐hydroxyquetiapine (7‐OH‐quetiapine) and 7‐hydroxy‐N‐desalkylquetiapine (7‐OH‐N‐des quetiapine) constitute approximately 5% and 2% of the parent compound quetiapine, respectively. The suggested therapeutic Cps of quetiapine for schizophrenia range from 100 to 500 ng/ml, and N‐des quetiapine 100 to 250 ng/ml. 9
Both the pharmacokinetic and pharmacodynamic properties of a drug can affect the clinical response to an illness. In the treatment of schizophrenia, the blood levels of antipsychotics may affect the corresponding clinical responses, and these levels can be influenced by several individual factors, including medication adherence, body weight (BW) or body mass index, smoking habit, sex, individual genetic differences in the cytochrome P450 system, and the effect of drug–drug interaction. For example, the interpatient variation of Cps of clozapine was up to 12‐fold in a group of 62 patients who took the DD dose of 400 mg, 10 and up to 10‐fold in a group of 88 patients who took the DD of haloperidol 20 mg. 11
The aim of this study was to investigate the diagnostic distribution and prescribed dosages of quetiapine in patients at a psychiatric hospital and to monitor the Cps of quetiapine and its active metabolites 7‐OH‐quetiapine and 7‐OH‐N‐des quetiapine in these patients to explore the interpatient variations in Cps/DD.
2. METHODS
2.1. Patients
Patients aged between 20 and 65 years from Taipei City Hospital, who were prescribed quetiapine were included after obtaining written informed consent. To ensure steady‐state Cps, changing the dose of quetiapine within 2 weeks was excluded. The patients' diagnoses, demographics, and concomitant medications were recorded. This study was supported by the Taipei City Government (TCHIRB‐10402102).
2.2. Quetiapine assay
Blood samples were taken in the morning, approximately 12 h after the dosing of quetiapine. From each patient, venous blood samples (10 ml) were collected into K2EDTA‐containing tubes (BD, Plymouth, UK). The plasma quetiapine samples were analyzed through ultra‐performance liquid chromatography–tandem mass spectrometry (UPLC‐MS/MS; Waters, Milford, MA, USA). Chromatographic separation was performed on an Acquity UPLC BEH C18 column (1.7 μm; 2.1 × 50 mm; Waters) equipped with an Acquity UPLC BEH C18 VanGuard Pre‐column (1.7 μm; 2.1 × 5 mm; Waters). The mass spectrometer was a triple quadrupole Xevo TQ MS system (Waters). In the experiments, we utilized an electrospray ionization source in positive‐ionization mode. Chromatographic conditions were set as follows: a flow rate of 0.3 ml/min with a mobile phase composed of 10 mM ammonium formate buffer (pH 3.0; solution A) and MeOH (solution B). The gradient program started at an initial mixture of 84% solution A with 16% solution B. Solution B was increased to 47% at 6.0 min and then increased to 95% at 7.5 min and held for 0.5 min. MassLynx software (version 4.1; Waters) was used for data processing. The lower limit of quantification was 0.2 ng/ml. The interday and intraday precisions by relative standard deviation for quetiapine, 7‐OH‐quetiapine and 7‐OH‐N‐des quetiapine were 0.6%–5.0% and 1.1%–4.5%, 0.3%–5.4% and 1.0%–8.0%, and 0.7%–10.0% and 1.1%–4.4%, respectively.
2.3. Data analysis
spss software (version 20; IBM, Armonk, NY, USA) was used for all statistical data analyses in this study. Statistical significance was set at p < 0.05.
3. RESULTS
This study enrolled 107 patients (male patients: 51; mean age: 51.9 ± 13.0 years; mean body weight: 66.3 ± 13.3 kg). The sample comprised patients with schizophrenia (n = 25), bipolar disorder (n = 53), major depression (n = 15), dysthymic disorder (n = 9), and others (n = 7). Table 1 compares DD, Cps, and Cps/DD ratio in different diagnoses. There were 59 (55%) patients received the IR form, and 48 patients received the ER form of quetiapine.
TABLE 1.
Dose of quetiapine, plasma concentrations of quetiapine, and metabolites between different diagnoses
| Diagnosis | n (%) | Dose (mg) | Cps of QT (ng/ml) | Cps/DD (ng/ml/mg) | Cps of 7‐OH‐QT (ng/ml) | 7‐OH‐N‐des QT (ng/ml) |
|---|---|---|---|---|---|---|
| Schizophrenia | 25 (23) | 218.4 ± 195.7 | 178.9 ± 366.1 | 0.60 ± 0.52 | 6.1 ± 9.8 | 6.0 ± 6.3 |
| Bipolar disorder | 51 (48) | 228.4 ± 202.4 | 115.7 ± 160.6 | 0.55 ± 0.58 | 5.2 ± 6.0 | 5.6 ± 5.4 |
| Major depression | 15 (14) | 50.0 ± 46.3 | 30.5 ± 32.8 | 0.68 ± 0.42 | 0.8 ± 0.5 | 1.2 ± 0.5 |
| Dysthymic disorder | 9 (8) | 30.6 ± 11.0 | 15.7 ± 16.9 | 0.57 ± 0.69 | 0.6 ± 0.3 | 1.1 ± 0.4 |
| Others | 7 (7) | 128.6 ± 134.2 | 85.7 ± 142.3 | 0.53 ± 0.49 | 3.2 ± 3.6 | 3.0 ± 2.9 |
| Total | 107 (100) | 175.9 ± 184.4 | 105.6 ± 215.3 | 0.58 ± 0.55 | 4.1 ± 6.5 | 4.6 ± 5.1 |
Abbreviations: Cps, plasma concentration; DD, daily dose; N‐des, N‐desalkyl; QT, quetiapine.
The DDs of quetiapine ranged from 25 to 800 mg, with a mean value of 175.9 ± 184.4 mg. The mean Cp of quetiapine was 105.6 ± 215.3 ng/ml, and the Cps of 7‐OH‐quetiapine and 7‐OH‐N‐des quetiapine were 4.1 ± 6.5 (3.9%) and 4.6 ± 5.1 ng/ml (4.4%), respectively. Figure 1 illustrates the plot of quetiapine dose and Cps of quetiapine, 7‐OH‐quetiapine, and 7‐OH‐N‐des quetiapine. The Pearson correlation coefficients (r) between the DD and Cps of quetiapine, 7‐OH‐and 7‐OH‐N‐des quetiapine were 0.60, 0.76, and 0.86, respectively. In addition, the interindividual pharmacokinetic variability of quetiapine—derived as the fold difference between the 5th and 95th percentiles of the Cps/DD ratio—was up to 26‐fold. For 7‐OH‐quetiapine and 7‐OH‐N‐des quetiapine, the variations were up to 10 folds and 25 folds, respectively. Male patients received a slightly higher DD of quetiapine than female patients did (200.0 ± 209.2 vs. 154.0 ± 157.2 mg, p = 0.199), but no difference in the Cps/DD ratio (0.63 ± 0.62 vs. 0.53 ± 0.46 ng/ml/mg, p = 0.356) was observed between sex. The mean Cps/DD ratio derived for quetiapine was 0.58 ± 0.55 ng/ml/mg.
FIGURE 1.
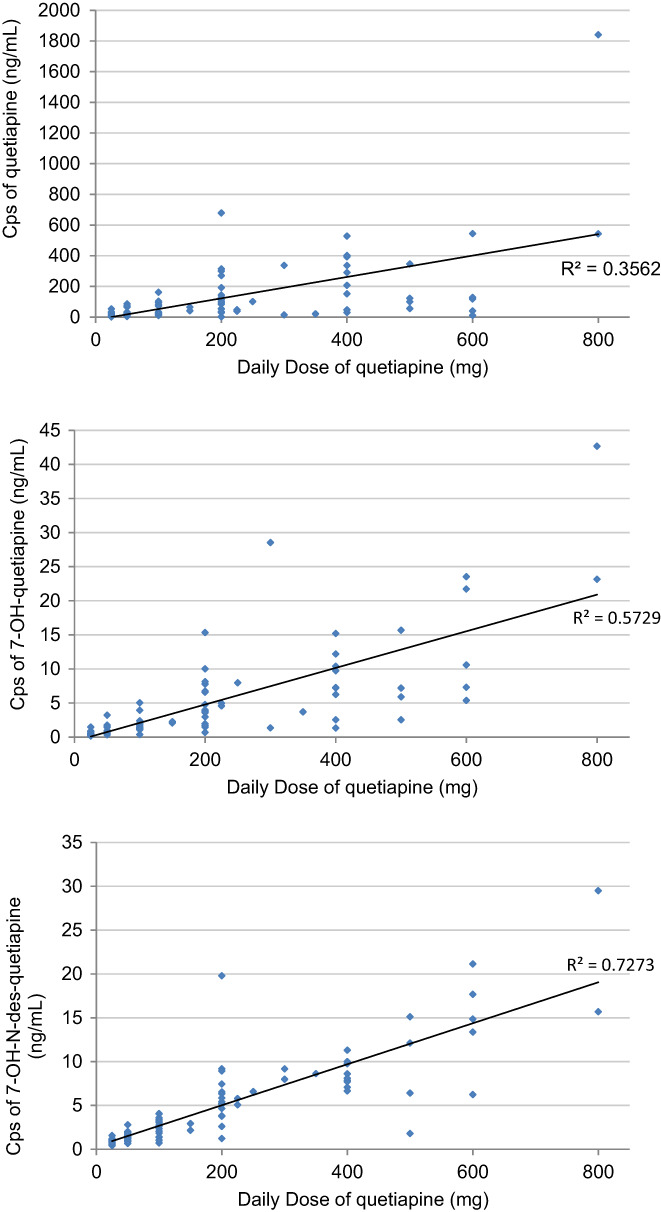
Plot of quetiapine dose and plasma concentrations (cps) of quetiapine, 7‐OH‐quetiapine, and 7‐OH‐N‐des quetiapine
Quetiapine was prescribed concomitantly with carbamazepine, a strong CYP 3A4 inducer, 12 in five patients; the mean DD was 285.0 ± 231.5 mg, and the Cp and Cps/DD ratio were 14.7 ± 10.3 ng/ml and 0.07 ± 0.05 ng/ml/mg, respectively. Moreover, quetiapine was prescribed concomitantly with lamotrigine in five patients; the mean DD was 60.0 ± 22.5 mg, and the Cp and Cps/DD ratio were 15.4 ± 9.2 ng/ml and 0.29 ± 0.20 ng/ml/mg, respectively. Besides, 30 patients were prescribed concomitantly with valproic acid, with mean DD, Cps, and Cps/DD ratio as 266 ± 225.5 mg, 234.2 ± 354.3 ng/ml, and 0.79 ± 0.72 ng/ml/mg.
Table 2 presents a comparison of the numbers of, DDs prescribed for, Cps determined, and Cps/DD ratio between patients taking IR and ER form quetiapine. Higher doses were used in ER form, resulting in higher Cps of quetiapine. The Cps/DD in ER quetiapine was higher than that derived for IR quetiapine by approximately 44% after dosing for about 12 h (p < 0.05).
TABLE 2.
Mean ± SD of dose, cps, and cps/DD ratio of immediate release (IR) and extended release (ER) quetiapine
| Dosage form | n (%) | Dose (mg) | Cps (ng/ml) | Cps/DD (ng/ml/mg) |
|---|---|---|---|---|
| IR | 59 (55) | 78.4 ± 68.3 | 30.1 ± 31.2 | 0.48 ± 0.42 |
| ER | 48 (45) | 198.5 ± 295.6 | 198.5 ± 295.8 | 0.69 ± 0.65 |
4. DISCUSSION
Quetiapine is widely prescribed for many psychiatric disorders. 13 The recommended DD of quetiapine for schizophrenia treatment is 150–750 mg; however, in clinical practice, many patients receive polypharmacy (two or more types of antipsychotics). In our sample of 25 patients with schizophrenia, 19 (mean DD: 150.0 mg) received an additional antipsychotic, and only six were treated with monotherapy (mean DD: 433.3 mg). The suggested range of therapeutic Cps for quetiapine is 100 to 500 ng/ml 9 ; in our six patients who received monotherapy, the mean Cp was 456.3 ng/ml, which was within the suggested range.
Quetiapine is usually prescribed as a secondary antipsychotic for other symptoms such as insomnia or agitation. A comparison of the association of antipsychotic polypharmacy versus monotherapy with psychiatric rehospitalization conducted by Tiihonen et al. 14 revealed that quetiapine monotherapy was associated with the least successful rate, while low‐dose quetiapine combined with other antipsychotics was commonly prescribed with moderate effects.
Quetiapine was most commonly prescribed for bipolar disorder (recommended DD: 400–800 mg) in our sample (n = 51%, 49%). Most patients with bipolar disorder received quetiapine (mean DD: 229.2 mg) as an add‐on medication to mood stabilizers (valproic acid [n = 25], lithium [n = 10], carbamazepine [n = 5], and lamotrigine [n = 5]; total: 88%). A higher dose of quetiapine was prescribed for bipolar mania and mixed type (DD: 262.8 ± 208.0 mg, n = 41) than for bipolar depression (DD: 85.0 ± 47.4 mg, n = 10). None of the patients in the bipolar group received quetiapine as monotherapy.
A study by Castberg et al. 15 on the interactions of quetiapine with other drugs suggested that comedication with carbamazepine would significantly reduce Cps by 86%. In our sample, the mean Cps/DD ratio was 0.07 ± 0.05 ng/ml/mg in patients who were prescribed concomitantly carbamazepine. Carbamazepine is a strong CYP3A4 inducer, and coadministration of carbamazepine could decrease the Cps of quetiapine by increasing its clearance to 7.5‐fold. 16 A study by Andersson et al. 17 reported that the Cps/DD of quetiapine was around 58% lower in the lamotrigine group than in the control group, and suggested that induction of quetiapine glucuronidation by lamotrigine might be the reason for this decreasing effect. In our sample, the mean Cps/DD ratio was 0.29 ± 0.20 ng/ml/mg in patients who were prescribed concomitantly lamotrigine. Although valproic acid is a weak CYP 3A4 inhibitor, 18 Aichhorn et al. 19 reported that comedicated with valproic acid was associated with a 77% increase in quetiapine plasma levels. In our samples, the relative % of quetiapine Cps/DD ratio to the whole group (0.58 ng/ml/mg) of concomitant use of carbamazepine, lamotrigine and valproic acid were −87%, −45%, and +36%, respectively. Based on these findings, we agree with the aforementioned report and recommend that drug–drug interaction is an important clinical issue when prescribing quetiapine. Concomitant use of quetiapine with carbamazepine, and lamotrigine would decrease, and with valproic acid would increase the Cps of quetiapine.
The third group in our sample comprised major depression (n = 16). All patients in this group were treated with antidepressants and a low dose of quetiapine (mean DD: 50.0 mg). This small dose regimen was also administered to the fourth group of patients with dysthymia (n = 9, mean DD: 30.6 mg), indicating that the hypnotic–sedative effect was the major target in these two groups. Quetiapine is widely used off‐label as a treatment for insomnia. 5 , 20 A clinical guideline for the management of insomnia suggests that quetiapine should be used only in patients who have a comorbid psychiatric disorder as opposed to primary insomnia. 21 Insomnia is one of the common symptoms of major depression and dysthymia; therefore, the concomitant use of low‐dose quetiapine can ameliorate sleep disturbance, and the metabolite N‐des quetiapine can improve depressive mood. 22
Bakken et al. 23 ever monitored the Cps/DD ratio of quetiapine in 927 serum samples from 601 patients and reported a 15‐fold interpatient variation, while Hasselstrøm & Linnet 24 reported a 238‐fold variation in a group of 62 patients. The interpatient variation of the present study was 26‐fold. These large interpatient variations in the Cps of quetiapine might be due to the abovementioned factors, and also the drug–drug interaction might play a significant role since polypharmacy was popular in the study patients.
Male were prescribed higher doses of quetiapine compared with female. However, we observed no significant differences in analyte Cps/DD between males and females. These results are similar to those reported in the literature by Fisher et al. 25 and Bakken et al., 23 in which both studies indicated that sex difference does not play a major role in the metabolism of quetiapine.
Quetiapine is metabolized primarily by the CYP 3A4 enzyme and partially by the CYP 2D6 enzyme. Both enzymes demonstrate different metabolic activities depending on racial characteristics, 26 , 27 , 28 and they exhibit relatively poor metabolic activities in patients of East Asian heritage. For example, for haloperidol, also metabolized primarily by the CYP 3A4 enzyme and partially by the CYP 2D6 enzyme, the Cps associated with the metabolic activities of these enzymes differed significantly between races, which is much higher in East Asian subjects. 29 , 30 A study conducted in Austria by Aichhorn et al. 19 reported the mean Cps/DD ratio derived for quetiapine (all IR) was 0.144 ± 0.016 in 36 males and 0.195 ± 0.017 in 58 females. We calculated the mean Cps/DD ratio as 0.18 ng/ml/mg. A study conducted in the United Kingdom by Fisher et al. 25 reported a mean Cp of quetiapine (IR or ER) as 195 ng/ml in 99 samples from 59 patients with a median DD 600 mg, yielding a Cps/DD ratio of approximately 0.33 ng/ml/mg. A study conducted in Norway by Bakken et al. 23 reported that the mean Cps/DD ratio derived for quetiapine (all IR) in 927 samples collected from 601 patients was 0.56 nmol/L/mg (0.21 ng/ml). Another study from the United Kingdom by Handley et al. 31 used therapeutic drug monitoring (TDM) data for 946 quetiapine samples (IR or ER) collected from 487 patients. The study revealed that the DD of quetiapine was 401–600 mg in 124 patients and 601–800 mg in 126 patients, with the corresponding mean Cps being 215 and 296 ng/ml, respectively. Based on these dose ranges, we calculated the mean Cps/DD ratio in these two groups was approximately 0.42 ng/ml/mg. A study in Denmark by Hasselstrøm & Linnet 24 also used TDM data for 62 patients and revealed a median Cps/DD ratio of 0.41 nmol/L/mg (0.16 ng/ml/mg) for quetiapine (all IR). Ostad Haji et al. 32 reported the mean Cps/DD ratio of quetiapine (43 IR) as 0.34 ± 0.24 ng/ml/mg in 105 depressive patients by the mean DD of 222 ± 125 mg.
A study in China by Li et al. 33 used TDM data for 21 patients who were prescribed a fixed dose of 200 mg quetiapine (all IR) twice daily. The mean steady‐state Cp of quetiapine was 147 ± 142 ng/ml, and the mean Cps/DD ratio was 0.37 ng/ml/mg. Another study in China by Li et al. 34 monitored the Cps of quetiapine (all ER) in patients who were prescribed DDs of 300 mg (n = 13), 600 mg (n = 13), and 800 mg (n = 14), revealing the Cps as 212, 320, and 551 ng/ml, respectively. The mean Cps/DD ratio of quetiapine for the whole sample was 0.64 ng/ml/mg. In the present study, the mean Cps/DD ratio of quetiapine (59 IR and 48 ER) was 0.58 ng/ml/mg. After comparing the results of these studies, we determined that patients of Chinese heritage may have approximately 50% to 100% higher Cps/DD ratio (0.37–0.64 ng/ml/mg) of quetiapine than Caucasian patients (0. 16–0.42 ng/ml/mg). Table 3 summarizes the published data of quetiapine DD, Cps, and Cps/DD from different countries.
TABLE 3.
Comparison of published data of quetiapine daily dose, plasma concentration and plasma concentration/daily dose
| Authors/country | Patient number (IR/ER) | Mean/median DD (mg) | Mean cps (ng/ml) | Mean cps/DD (ng/ml/mg) |
|---|---|---|---|---|
| Aichhorn et al. 19 /Austria | 94 (IR) | 381 (mean) | 68 (mean) | 0.18 (mean) |
| Hasselstrøm and Linnet 24 /Denmark | 62 (IR) | 400 (median) | 66 (median) | 0.16 (mean) |
| Bakken et al. 23 /Norway | 601 (IR) | 500 (median) | 104 (median) | 0.21(median) |
| Fisher et al. 25 /UK | 59 (IR or ER) | 600 (median) | 195 (mean) | 0.33 (mean) |
| Handley et al. 31 /UK | 487 (IR or ER) | 600 (median) | 242 (mean) | 0.42 (mean) |
| Ostad Haji et al. 32 /Germany | 105 (43/62) | 222 (mean) | 46 (mean) | 0.34 (mean) |
| Li et al. 33 /China | 21 (IR) | 400 (fixed dose) | 147 (mean) | 0.37 (mean) |
| Li et al. 34 /China | 40 (ER) | 572 (mean) | 365 (mean) | 0.64 (mean) |
| Present study/Taiwan | 107 (59/48) | 176 (mean) | 106 (mean) | 0.58 (mean) |
Abbreviations: DD, daily dose; ER, extended release; IR, Immediate release.
Higher Cps in Oriental patients have also been reported for clozapine, a substrate of CYP 1A2 and 2C19, 10 , 35 aripiprazole, a substrate of CYP2D6 and 3A4, 36 and lurasidone, a substrate of CYP 3A4. 37 These differences have been observed among different racial populations. For example, Shimada et al. 38 compared the CYP enzymes in the liver microsomes including CYP 1A2, 2A6, 2B6, 2C, 2D6, 2E1, and 3A between Japanese and Caucasians and found the total CYP content was higher in Caucasian than in Japanese populations. McGraw and Waller 39 have reviewed the CYP450 variations in different ethnic populations including CYP1A2, 2C8, 2C9, 2C19, 2D6, 3A4, and 3A5 single nucleotide polymorphisms (SNPs), and suggested that racial/ethnic differences in metabolic phenotype can be explained by differences in SNP distribution.
We compared the Cps and Cps/DD ratio of IR and ER quetiapine (Table 2). Our results indicate that ER quetiapine (200 and 50 mg) was more frequently prescribed to patients with schizophrenia or bipolar disorder compared with IR quetiapine (100 and 25 mg). We also observed that the Cps/DD ratio derived for ER quetiapine was higher than that derived for IR quetiapine by approximately 44% after dosing for about 12 h (p < 0.05). The study by Fisher et al. 25 also reported this finding. A study by Figueroa et al. 40 reported that the least squares mean of the ratio of the area under the plasma concentration‐time curve over a‐24 h dosing interval for ER and IR quetiapine was 1.04 (0.92–1.19), suggesting similar bioequivalence between the IR and ER forms.
The limitations of the present study are the small sample size, the convenience sampling method, and concerns about medication adherence. Because quetiapine is widely used in clinical practice for off‐label purposes, most patients in our sample were not within the recommended dose range indicated for the treatment of schizophrenia and bipolar disorder. 31 Nevertheless, our results reflect the real‐world prescription of quetiapine. Another limitation was that we did not measure the active metabolite of N‐desalkylquetiapine, and did not perform a well‐controlled or head‐to‐head comparison of racial differences in the Cps/DD ratio for quetiapine; therefore, our findings warrant further verification.
In conclusion, although quetiapine is categorized as an antipsychotic, it is widely used to treat many psychiatric disorders other than psychosis at various doses, and a wide interpatient variation of the Cps/DD ratio was noticed. Based on our comparison of Asian and Western studies, we believe that individuals of East Asian descent may exhibit a 50% to 100% increase in the Cps/DD ratio for quetiapine compared with patients of Western descent.
AUTHOR CONTRIBUTIONS
CYH and SKL designed this project. CYH, YFL, CRC, and SKL recruited participants and collected blood samples, and findings were interpreted by all the authors. SKL prepared the first draft, which was revised by all authors. All authors approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was approved by the Human Subject Committee of Taipei City Hospital (TCHIRB‐10402102).
INFORMED CONSENT
Every participant gave written informed consent after receiving the study explanation.
ACKNOWLEDGMENT
This study was supported by the Taipei City Government. The authors thank Mr. Yan‐Lung Chiou for assistance with data management. This manuscript was edited by Wallace Academic Editing.
Huang C‐Y, Lin Y‐F, Chen C‐R, Lin S‐K. Post‐therapy plasma concentrations of quetiapine in Taiwanese patients. Neuropsychopharmacol Rep. 2023;43:50–56. 10.1002/npr2.12303
DATA AVAILABILITY STATEMENT
Under the restriction of the Human Subject Committee of Taipei City Hospital, the data supporting this study's findings are available on request from the corresponding author on a reasonable basis, via an application for approval from the Human Subject Committee.
REFERENCES
- 1. Weisler R, Joyce M, McGill L, Lazarus A, Szamosi J, Eriksson H. Extended release quetiapine fumarate monotherapy for major depressive disorder: results of a double‐blind, randomized, placebo‐controlled study. CNS Spectr. 2009;14:299–313. [DOI] [PubMed] [Google Scholar]
- 2. Bortnick B, el‐Khalili N, Banov M, Adson D, Datto C, Raines S, et al. Efficacy and tolerability of extended release quetiapine fumarate (quetiapine XR) monotherapy in major depressive disorder: a placebo‐controlled, randomized study. J Affect Disord. 2011;128:83–94. [DOI] [PubMed] [Google Scholar]
- 3. Bandelow B, Chouinard G, Bobes J, Ahokas A, Eggens I, Liu S, et al. Extended‐release quetiapine fumarate (quetiapine XR): a once‐daily monotherapy effective in generalized anxiety disorder. Data from a randomized, double‐blind, placebo‐ and active‐controlled study. Int J Neuropsychopharmacol. 2010;13:305–20. [DOI] [PubMed] [Google Scholar]
- 4. Katzman MA, Brawman‐Mintzer O, Reyes EB, Olausson B, Liu S, Eriksson H. Extended release quetiapine fumarate (quetiapine XR) monotherapy as maintenance treatment for generalized anxiety disorder: a long‐term, randomized, placebo‐controlled trial. Int Clin Psychopharmacol. 2011;26:11–24. [DOI] [PubMed] [Google Scholar]
- 5. Anderson SL, Vande Griend JP. Quetiapine for insomnia: a review of the literature. Am J Health Syst Pharm. 2014;71:394–402. [DOI] [PubMed] [Google Scholar]
- 6. Nemeroff CB, Kinkead B, Goldstein J. Quetiapine: preclinical studies, pharmacokinetics, drug interactions, and dosing. J Clin Psychiatry. 2002;63(Suppl 13):5–11. [PubMed] [Google Scholar]
- 7. Le Daré B, Ferron PJ, Allard PM, Clément B, Morel I, Gicquel T. New insights into quetiapine metabolism using molecular networking. Sci Rep. 2020;10:19921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeVane CL, Nemeroff CB. Clinical pharmacokinetics of quetiapine: an atypical antipsychotic. Clin Pharmacokinet. 2001;40:509–22. [DOI] [PubMed] [Google Scholar]
- 9. Hiemke C, Bergemann N, Clement HW, Conca A, Deckert J, Domschke K, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51:e1. [DOI] [PubMed] [Google Scholar]
- 10. Chang WH, Lin SK, Lane HY, Hu WH, Jann MW, Lin HN. Clozapine dosages and plasma drug concentrations. J Formos Med Assoc. 1997;96:599–605. [PubMed] [Google Scholar]
- 11. Lane HY, Lin HN, Hwu HG, Jann M, Hu WH, Chang WH. Haloperidol plasma concentrations in Taiwanese psychiatric patients. J Formos Med Assoc. 1995;94:671–8. [PubMed] [Google Scholar]
- 12. Spina E, Pisani F, Perucca E. Clinically significant pharmacokinetic drug interactions with carbamazepine. An update. Clin Pharmacokinet. 1996;31:198–214. [DOI] [PubMed] [Google Scholar]
- 13. Zhornitsky S, Potvin S, Moteshafi H, Dubreucq S, Rompré PP, Stip E. Dose‐response and comparative efficacy and tolerability of quetiapine across psychiatric disorders: a systematic review of the placebo‐controlled monotherapy and add‐on trials. Int Clin Psychopharmacol. 2011;26:183–92. [DOI] [PubMed] [Google Scholar]
- 14. Tiihonen J, Taipale H, Mehtälä J, Vattulainen P, Correll CU, Tanskanen A. Association of Antipsychotic Polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiat. 2019;76:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Castberg I, Skogvoll E, Spigset O. Quetiapine and drug interactions: evidence from a routine therapeutic drug monitoring service. J Clin Psychiatry. 2007;68:1540–5. [DOI] [PubMed] [Google Scholar]
- 16. Grimm SW, Richtand NM, Winter HR, Stams KR, Reele SB. Effects of cytochrome P450 3A modulators ketoconazole and carbamazepine on quetiapine pharmacokinetics. Br J Clin Pharmacol. 2006;61:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andersson ML, Björkhem‐Bergman L, Lindh JD. Possible drug‐drug interaction between quetiapine and lamotrigine–evidence from a Swedish TDM database. Br J Clin Pharmacol. 2011;72:153–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wen X, Wang JS, Kivistö KT, Neuvonen PJ, Backman JT. In vitro evaluation of valproic acid as an inhibitor of human cytochrome P450 isoforms: preferential inhibition of cytochrome P450 2C9 (CYP2C9). Br J Clin Pharmacol. 2001;52:547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aichhorn W, Marksteiner J, Walch T, Zernig G, Saria A, Kemmler G. Influence of age, gender, body weight and valproate comedication on quetiapine plasma concentrations. Int Clin Psychopharmacol. 2006;21:81–5. [DOI] [PubMed] [Google Scholar]
- 20. Wiegand MH, Landry F, Brückner T, Pohl C, Veselý Z, Jahn T. Quetiapine in primary insomnia: a pilot study. Psychopharmacology. 2008;196:337–8. [DOI] [PubMed] [Google Scholar]
- 21. Schutte‐Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- 22. Jensen NH, Rodriguiz RM, Caron MG, Wetsel WC, Rothman RB, Roth BL. N‐desalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5‐HT1A agonist, as a putative mediator of quetiapine's antidepressant activity. Neuropsychopharmacology. 2008;33:2303–12. [DOI] [PubMed] [Google Scholar]
- 23. Bakken GV, Rudberg I, Molden E, Refsum H, Hermann M. Pharmacokinetic variability of quetiapine and the active metabolite N‐desalkylquetiapine in psychiatric patients. Ther Drug Monit. 2011;33:222–6. [DOI] [PubMed] [Google Scholar]
- 24. Hasselstrøm J, Linnet K. Quetiapine serum concentrations in psychiatric patients: the influence of comedication. Ther Drug Monit. 2004;26:486–91. [DOI] [PubMed] [Google Scholar]
- 25. Fisher DS, Handley SA, Flanagan RJ, Taylor DM. Plasma concentrations of quetiapine, N‐desalkylquetiapine, o‐desalkylquetiapine, 7‐hydroxyquetiapine, and quetiapine sulfoxide in relation to quetiapine dose, formulation, and other factors. Ther Drug Monit. 2012;34:415–21. [DOI] [PubMed] [Google Scholar]
- 26. Hsieh KP, Lin YY, Cheng CL, Lai ML, Lin MS, Siest JP, et al. Novel mutations of CYP3A4 in Chinese. Drug Metab Dispos. 2001;29:268–73. [PubMed] [Google Scholar]
- 27. Yamaori S, Yamazaki H, Iwano S, Kiyotani K, Matsumura K, Saito T, et al. Ethnic differences between Japanese and Caucasians in the expression levels of mRNAs for CYP3A4, CYP3A5 and CYP3A7: lack of co‐regulation of the expression of CYP3A in Japanese livers. Xenobiotica. 2005;35:69–83. [DOI] [PubMed] [Google Scholar]
- 28. Liou YH, Lin CT, Wu YJ, Wu LS. The high prevalence of the poor and ultrarapid metabolite alleles of CYP2D6, CYP2C9, CYP2C19, CYP3A4, and CYP3A5 in Taiwanese population. J Hum Genet. 2006;51:857–63. [DOI] [PubMed] [Google Scholar]
- 29. Potkin SG, Shen Y, Pardes H, Phelps BH, Zhou D, Shu L, et al. Haloperidol concentrations elevated in Chinese patients. Psychiatry Res. 1984;12:167–72. [DOI] [PubMed] [Google Scholar]
- 30. Jann MW, Chang WH, Lam YW, Hwu HG, Lin HN, Chen H, et al. Comparison of haloperidol and reduced haloperidol plasma levels in four different ethnic populations. Prog Neuro‐Psychopharmacol Biol Psychiatry. 1992;16:193–202. [DOI] [PubMed] [Google Scholar]
- 31. Handley SA, Bowskill SV, Patel MX, Flanagan RJ. Plasma quetiapine in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 2000–2011. Ther Adv Psychopharmacol. 2013;3:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ostad Haji E, Wagner S, Fric M, Laux G, Pittermann P, Röschke J, et al. Quetiapine and norquetiapine serum concentrations and clinical effects in depressed patients under augmentation therapy with quetiapine. Ther Drug Monit. 2013;35:539–45. [DOI] [PubMed] [Google Scholar]
- 33. Li KY, Li X, Cheng ZN, Peng WX, Zhang BK, Li HD. Multiple dose pharmacokinetics of quetiapine and some of its metabolites in Chinese suffering from schizophrenia. Acta Pharmacol Sin. 2004;25:390–4. [PubMed] [Google Scholar]
- 34. Li Q, Su YA, Liu Y, Chen JX, Tan YL, Yang FD, et al. Pharmacokinetics and tolerability of extended‐release quetiapine fumarate in Han Chinese patients with schizophrenia. Clin Pharmacokinet. 2014;53:455–65. [DOI] [PubMed] [Google Scholar]
- 35. de Leon J, Rajkumar AP, Kaithi AR, Schoretsanitis G, Kane JM, Wang CY, et al. Do Asian patients require only half of the clozapine dose prescribed for Caucasians? A critical overview. Indian J Psychol Med. 2020;42:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin SK, Chen CK, Liu YL. Aripiprazole and dehydroaripiprazole plasma concentrations and clinical responses in patients with schizophrenia. J Clin Psychopharmacol. 2011;31:758–62. [DOI] [PubMed] [Google Scholar]
- 37. Huang CY, Lin SK. Therapeutic drug monitoring of lurasidone. Psychiatry Clin Neurosci. 2022;76:674–5. [DOI] [PubMed] [Google Scholar]
- 38. Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P‐450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–23. [PubMed] [Google Scholar]
- 39. McGraw J, Waller D. Cytochrome P450 variations in different ethnic populations. Expert Opin Drug Metab Toxicol. 2012;8:371–82. [DOI] [PubMed] [Google Scholar]
- 40. Figueroa C, Brecher M, Hamer‐Maansson JE, Winter H. Pharmacokinetic profiles of extended release quetiapine fumarate compared with quetiapine immediate release. Prog Neuro‐Psychopharmacol Biol Psychiatry. 2009;33:199–204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Under the restriction of the Human Subject Committee of Taipei City Hospital, the data supporting this study's findings are available on request from the corresponding author on a reasonable basis, via an application for approval from the Human Subject Committee.


