Abstract
Background:
Despite of long-lasting efforts, in more than 50% of cases, the etiology of recurrent spontaneous abortion (RSA) remains unknown. Leukemia inhibitory factor (LIF) has an essential role in the reproductive process, such as modulating inflammatory responses. This study aimed to evaluate the relationship between the LIF gene expression as well as serum levels of inflammatory cytokines and occurrence of RSA in infertile women with a history of RSA.
Materials and Methods:
In this case-control study, the relative gene expression levels of LIF, concentrations of tumor necrosis factor-alpha (TNF-α), and interleukin (IL)-17 were measured in peripheral blood and serum of women with a history of RSA (N=40) compared with non-pregnant and fertile women as the control group (N=40) using quantitative real-time polymerase chain reaction and the enzyme-linked immunosorbent assay, respectively.
Results:
The mean age of patients and controls was 30.1 ± 4.28 and 30.03 ± 4.23, respectively. Patients had a history of at least 2 and at most 6 abortions. The mRNA levels of LIF were significantly lower in the women with RSA in comparison with the healthy participant (P=0.003). Regarding cytokine levels, no significant difference was seen between the two groups (P≥0.05). There was no correlation - between the LIF mRNA levels and TNF-α and IL-17 serum concentrations. The U-Mann-Whitney test and the Pearson correlation coefficient were applied to comparison variables between groups as well as a correlation between LIF mRNA and cytokine levels in serum.
Conclusion:
Despite a significant reduction in the LIF gene mRNA level in patients with RSA, it was not associated with increases in inflammatory cytokines. Dysfunction in the production of LIF protein may be involved in the onset of RSA disorder.
Keywords: Interleukine-17, Leukemia Inhibitory Factor, Recurrent Abortion, Tumor Necrosis Factor-Alpha
Introduction
The failure of two or more clinically known pregnancies before 20-24 weeks of gestation is called recurrent spontaneous abortion (RSA). The average prevalence of RSA is 1 to 4% of women of childbearing age. The main risk factors defined in RSA are parental genetics aberrations, uterine abnormalities, thrombophilia, infectious, endocrine, immunological, and unexplained causes (1-3).
Changes in the mother’s immune system are needed for implantation, the continuation of pregnancy, and delivery. During a normal pregnancy, the mother’s immune system responses have deviated to T helper (Th) 2 cells (4). The inability to shift responses from the T helper type 1 (Th1) cells to the Th2 cells during pregnancy is associated with the predominance of the Th1 cell response, that leads to complications such as preeclampsia, implantation failure, RSA, and preterm delivery (5).
Many women with RSA suffer from immune cell disorders, including an increase in the Th1/Th2 cell ratio, an increase in the number or cytotoxicity of natural killer (NK) cells, and a change in the Treg/Th17 ratio (6).
One of the pro-inflammatory cytokines generated by immune cells, such as Th1 cells, is tumor necrosis factor alpha (TNF-α). It is involved in the pathogenesis of some autoimmune and inflammatory diseases. The TNF-α is also thought to be a risk factor for RSA (7, 8).
LIF is a pleiotropic glycoprotein associated with cytokines in the interleukin (IL)-6 family (9). This cytokine plays an important role in the implantation, maintenance of pregnancy, and continuation of fetal growth (10). Based on a recent primary report, there were low levels of LIF protein in the blood and endometrial tissue of women with recurrent pregnancy loss (11).
The current study aimed to assess TNF-α and IL-17 serum levels, as well as LIF gene expression, in the peripheral blood of Iranian women with RSA. The findings on the relationships between these variables can provide a better understanding of the RSA etiology.
Materials and Methods
Sample collection and study population
This case-control study was performed on the peripheral blood mononuclear cells (PBMC) and serum samples of women who were referred to the Obstetrics and Gynecology clinic, Shahid Sadoughi Hospital, Yazd, Iran. The study was approved by the Ethical Committee of the Science and Arts University, Mashhad, Iran (IR.ACECR. JDM.REC.1400.022). Informed consent was obtained from all the participants.
The control group included 40 non-pregnant women who had no history of abortion, and underlying diseases, or at least one successful pregnancy. Women with a history of two or more RSAs were recruited as the case group. The sample size in each group was considered 40 (α=0.05, β=0.2).
Two groups were matched in terms of age. A gynecologist examined all of the women in the case group. The presence of male factor etiologies (determined via sperm analysis and andrological examinations), anatomical and endocrine problems, and the abnormal chromosomal karyotype, positive antiphospholipid antibodies (APA), presence of anticardiolipin antibodies (ACA), thrombophilia, and TORCH syndrome were considered as exclusion criteria for the case group. Peripheral blood was taken from all the participants to evaluate the LIF gene expression and IL-17 and TNF-α concentrations.
Enzyme-linked immunosorbent assay (ELISA)
Using ELISA Kits, serum concentrations of IL-17 and TNF-α (Karmania Pars Gen Co., Kerman, Iran) of participants were detected according to the manufacturer’s instructions. The minimum detectable concentrations for IL-17 and TNF-α was 1.2 ng/ml.
RNA extraction and cDNA synthesis
Total RNA was extracted from the PBMC samples using an RNX-Plus kit (Sinaclon Bioscience, Cat. No.: RN7713C, IRAN) according to the manufacturer’s instructions. The purity and concentrations of each extracted RNA were measured by using a spectrophotometer (2000C, Thermo Scientific, Waltham, MA, USA) in the optical density of 260/280 and 260 nm, respectively. The RNA values between 1.8 and 2.0 was applied for normalization before cDNA synthesis (12). All cDNA was synthesized according to the protocol guidance of RevertAid First Strand cDNA Synthesis Kit (Cat. No: A101161, Parstous biotechnology, Iran). The cDNA product was kept at -20°C until it was used.
Relative gene expression
A quantitative real-time polymerase chain reaction (qRT-PCR) technique was used to assess the mRNA levels of LIF in all samples. To evaluate the LIF expression levels, the primer sequences were summarized in Table 1. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as a reference gene. Briefly, PCR reaction was done according to standard protocol (2). The 2-ΔΔCt method was applied to determine the relative gene expression level.
Statistical analysis
Different software, GraphPad Prism 8 (San Diego, CA) and SPSS 20 (Chicago, IL, USA), were used for the statistical analysis in this study. The Shapiro-Wilk and Kolmogorov-Smirnov tests were used to ensure that the data distribution was normal. Then, the U-Mann-Whitney test was applied to compare variables between cases and controls. Moreover, the Pearson correlation coefficient was used to investigate any possible correlation between the z RNA expression level and serum level of TNF-α and IL-17 cytokines in each group. The data were presented as mean ± standard deviation, and P<0.05 was considered a significant value.
Table 1.
Primer sequences used for the real-time polymerase chain reaction
|
| |||
|---|---|---|---|
| Gene | Primer sequence (5´-3´) | Accession No. | PCR product (bp) |
|
| |||
| LIF | F: CCAACGTGACGGACTTCCC | NM_001257135.2 | 82 |
| R: TACACGACTATGCGGTACAGC | |||
| GAPDH | F: AAATCAAGTGGGGCGATGCTG | NM_001357943.2 | 118 |
| R: GCAGAGATGATGACCCTTTTG | |||
|
| |||
PCR; Polymerase chain reaction, LIF; Leukemia inhibitory factor, and GAPDH; Glyceraldehyde-3-phosphate dehydrogenase.
Results
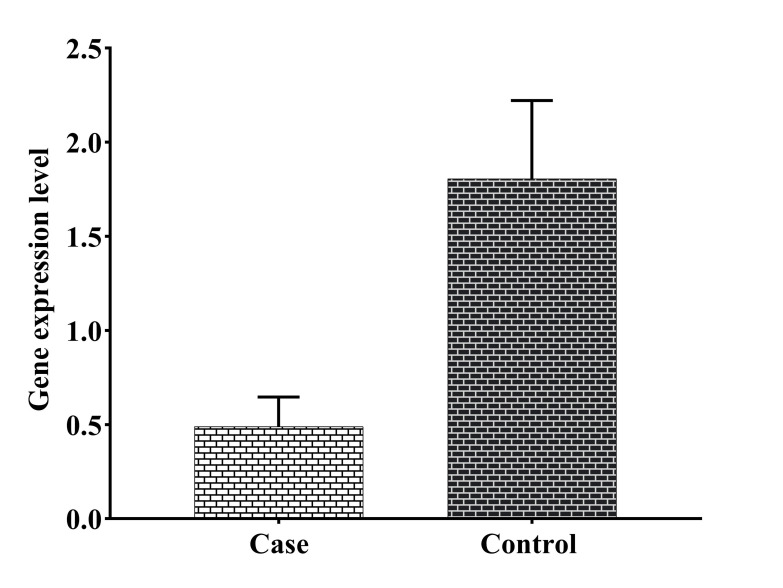
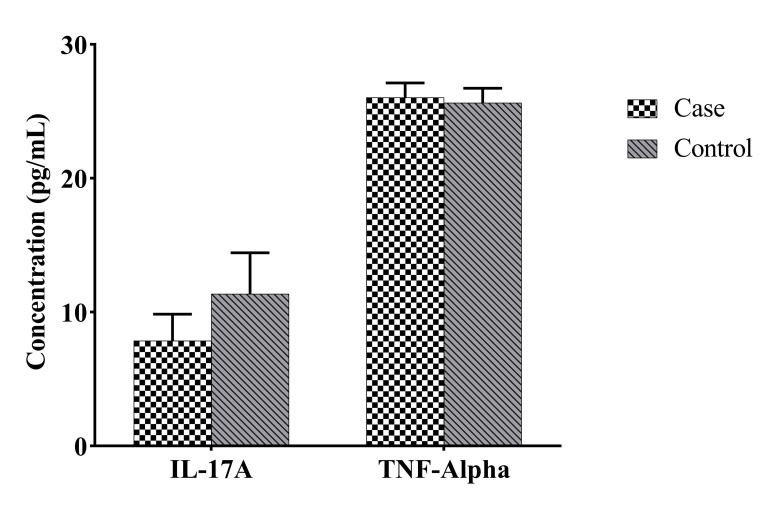
The mean age of the participants in the case and control groups was 30.1 ± 4.28 (22-39) and 30.03 ± 4.23 (20-38) years, respectively. The participants had at least two and at most six abortions. The number of successful pregnancies in the control group ranged from one to four. The LIF RNA expression level was significantly reduced in the case group in comparison with the control group (P=0.003, Fig .1). Despite the insignificant difference, serum level of IL-17 was lower in the case group than in the control group. Concerning the serum level of TNF-α, no statistically significant change was seen between both groups (Fig .2). Furthermore, no statistical relationship was found between LIF RNA expression level and serum TNF-α and IL-17 cytokine levels (Table 2).
Fig 1.
Comparison of leukemia inhibitory factor (LIF) RNA expression in blood samples of the study groups. Case: patient with recurrent spontaneous abortion, control: healthy fertile women. There was a significant increase in the Leukemia inhibitory factor (LIF) mRNA level in the case and control groups (P=0.003, mean ± SEM: 0.49 ± 0.16 and 1.81 ± 0.41, respectively). Non-parametric Mann-Whitney test was used for comparing cases and controls.
Fig 2.
Comparison of concentrations of IL-17A and TNF-α in serum samples of case and control groups. There was no significant change in the levels of TNF-α (P=0.41, mean ± SEM: 26.04 ± 1.08 and 25.64 ± 1.09, respectively) and IL-17A (P=0.14, mean ± SEM: 7.873 ± 1.974 and 11.37 ± 3.074, respectively) in serum samples of the case group in comparison with the controls. Non-parametric Mann-Whitney test was used for comparing cases and controls. Case; Patient with recurrent spontaneous abortion, Control; Healthy fertile women, TNF-α; Tumor necrosis factoralpha, and IL-17A; Interleukin-17A.
Table 2.
Correlation between serum level of TNF-α, IL-17A, and LIF RNA expression level in our groups
|
| ||
|---|---|---|
| Variable | Groups | LIF gene expression |
|
| ||
| TNF-α | Case | r=-0.02 |
| P=0.91 | ||
| CI=-0.35-0.31 | ||
| Control | r=-0.11 | |
| P=0.55 | ||
| CI=-0.43-0.241 | ||
| IL-17A | Case | r=0.09 |
| P=0.58 | ||
| CI=-0.25-0.41 | ||
| Control | r=0.68 | |
| P=0.9 | ||
| CI=0.45-0.83 | ||
|
| ||
r; Pearson correlation coefficient, P; P value, CI; Confidence interval, LIF; Leukemia inhibitory factor gene, TNF-α; Tumor necrosis factor-alpha, and IL-17A; Interleukin-17A. The Pearson correlation test was used.
Discussion
The RSA is considered a major problem for women of reproductive age. Though various causes have been reported for RSA, the cause of this disorder is still unknown for 50% of affected women (13, 14). A successful pregnancy requires a balance between the components of the immune system, and any deviation in this balance that can lead to RSA (15).
The biological involvement of the LIF gene in various features, including reproduction, bone remodeling, hypothalamus-pituitary-adrenal axis stress response, neuromuscular system, heart muscle regeneration, and cancer has been described previously (16). LIF has been shown to play an essential role in facilitating implantation, blastocyst development, and trophoblast invasion since the LIF gene mutation is associated with infertility and implantation failure (17). Ismail et al. (18) observed that a high RNA expression level of LIF also associates with a successful pregnancy. Considering that LIF and its receptor are required for early pregnancy and we also considered a control group for comparison, while the sampling time was a non-abortion time. In the present study, the RNA expression level of the LIF gene was significantly lower in the RSA affected while, Xu et al. (19) reported that the expression of endometrial LIF was not significantly different between RSA affected and control (healthy women). This difference may be due to the difference in samples or lower RNA expression level of LIF in the endometrium. A study by Piccinni et al. (20) on the decidual T cells of RSA patients showed that defective production of LIF and/or Th2 cytokines may be involved in the progression of abortion. Detecting protein levels of LIF as the complementary test could highlight the role of LIF protein in the occurrence of this disorder.
Previous research has also found a significant increase in embryo-toxic cytokines, such as TNF-α, in women with RSA in comparison with controls (women who had a normal early pregnancy but voluntarily decided to have an abortion) (18, 21). Li et al. (21) assessed the TNF-α level in peripheral blood and decidua. They observed that the level of TNF-α in both types of samples was significantly higher in the RSA group in comparison with the control group. They concluded that the concentration of this cytokine is much higher in the decidua tissue than the blood of RSA affected. TNF-α also causes abortion via placental thrombosis, and its production increases with RSA and the onset of labor (18). In our study, the serum levels of TNF-α showed a slight increase in the RSA group in comparison with the control group. The high inflammatory cytokine level in some studies suggests that TNF-α may play a remarkable role in the development of RSA (22-24). On the other hand, TNF-α inhibitors, increase the rate of live births, that may imply the potential role of TNF-α in the RSA pathogenesis (25). While some studies reported an increase in the serum IL-17 level in the RSA patients (26), there are studies that observed a decrease in in the serum IL-17 level in the same patients RSA affected (27, 28). Kaminski et al. (27) directed a study of the 16 healthy pregnant women and 13 women with RSA. They observed only an increase of the serum IL-17 level in the peripheral blood of healthy pregnant women. They also, observed no difference between groups regarding other cytokines. IL-17 is a proinflammatory cytokine that has a wide range of effects, including neovascularization and proangiogenic molecules generation. It has been shown to increase the production of the biologically active matrix metalloproteinase (MMP)-9, which is important for trophoblast invasion (29, 30). In addition, we found an increase in serum IL-17 levels in the control group in comparison with our case group, which is in line with findings of other studies (27, 28). The increase of this inflammatory cytokine in the physiological process of pregnancy may be due to its role in the neovascularization and trophoblast invasion (31, 32).
Conclusion
In the present study, it was shown that the expression of the LIF gene in the peripheral blood of women with RSA was significantly reduced compared to the control group, which was not associated with the serum concentration of inflammatory cytokines IL-17 and TNF-α. Thus, it seems that a defect in the production of LIF protein, as one of the measurable deficiencies in the blood of women with RSA, can contribute to unexplained recurrent abortions.
Acknowledgements
We thank all the patients and staff at Shahid Sadoughi Hospital, (Yazd, Iran) and the Clinic for their kind collaboration. The authors have declared that no financial support and conflict of interest exists.
Authors’ Contributions
S.M.M., F.Z.; Contributed to conception and design. F.F., F.Z., H.A., N.K.; Contributed to all experimental work, data and statistical analysis, and interpretation of data. S.M.M., F.Z.; Responsible for overall supervision. H.A., F.F.; Drafted the manuscript. S.M.M.; Revised the manuscript. All authors read and approved the final manuscript.
References
- 1.Dimitriadis E, Menkhorst E, Saito S, Kutteh WH, Brosens JJ. Recurrent pregnancy loss. Nat Rev Dis Primers. 2020;6(1):98–98. doi: 10.1038/s41572-020-00228-z. [DOI] [PubMed] [Google Scholar]
- 2.Tafti FD, Zare F, Miresmaeili SM, Fesahat F. Evaluating Vitamin D and foxp3 mRNA levels in women with recurrent spontaneous abortion. JBRA Assist Reprod. 2022;26(2):232–236. doi: 10.5935/1518-0557.20210062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fesahat F, Norouzi E, Seifati SM, Hamidian S, Hosseini A, Zare F. Impact of vitamin c on gene expression profile of inflammatory and anti-inflammatory cytokines in the male partners of couples with recurrent pregnancy loss. Int J Inflam. 2022;2022:1222533–1222533. doi: 10.1155/2022/1222533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi YK, Kwak-Kim J. Cytokine gene polymorphisms in recurrent spontaneous abortions: a comprehensive review. Am J Reprod Immunol. 2008;60(2):91–110. doi: 10.1111/j.1600-0897.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 5.Choudhury SR, Knapp LA. Human reproductive failure I: immunological factors. Hum Reprod Update. 2001;7(2):113–134. doi: 10.1093/humupd/7.2.113. [DOI] [PubMed] [Google Scholar]
- 6.Yang X, Meng T. Is there a role of intravenous immunoglobulin in immunologic recurrent pregnancy loss? J Immunol Res. 2020;2020:6672865–6672865. doi: 10.1155/2020/6672865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu H, You Q, Jiang Y, Mu F. Tumor necrosis factor inhibitors as therapeutic agents for recurrent spontaneous abortion (review) Mol Med Rep. 2021;24(6):847–847. doi: 10.3892/mmr.2021.12487. [DOI] [PubMed] [Google Scholar]
- 8.Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, et al. The role of tumor necrosis factor alpha (TNF-alpha) in Autoimmune disease and current TNF-alpha inhibitors in therapeutics. Int J Mol Sci. 2021;22(5):2719–2719. doi: 10.3390/ijms22052719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winship A, Correia J, Krishnan T, Menkhorst E, Cuman C, Zhang JG, et al. Blocking endogenous leukemia inhibitory factor during placental development in mice leads to abnormal placentation and pregnancy loss. Sci Rep. 2015;5(1):13237–13237. doi: 10.1038/srep13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosario GX, Stewart CL. multifaceted actions of leukaemia inhibitory factor in mediating uterine receptivity and embryo implantation. Am J Reprod Immunol. 2016;75(3):246–255. doi: 10.1111/aji.12474. [DOI] [PubMed] [Google Scholar]
- 11.Dhawan V, Malhotra N, Singh N, Dadhwal V, Dada R. Leukemia inhibitory factor in recurrent pregnancy loss: is there any role of lifestyle intervention? Fertil Steril. 2020;114(3):e195–e195. [Google Scholar]
- 12.Sadeghian-Nodoushan F, Aflatoonian R, Borzouie Z, Akyash F, Fesahat F, Soleimani M, et al. Pluripotency and differentiation of cells from human testicular sperm extraction: An investigation of cell stemness. Mol Reprod Dev. 2016;83(4):312–323. doi: 10.1002/mrd.22620. [DOI] [PubMed] [Google Scholar]
- 13.Fu YY, Ren CE, Qiao PY, Meng YH. Uterine natural killer cells and recurrent spontaneous abortion. Am J Reprod Immunol. 2021;86(2):e13433–e13433. doi: 10.1111/aji.13433. [DOI] [PubMed] [Google Scholar]
- 14.Yan Y, Fang L, Li Y, Yu Y, Li Y, Cheng JC, et al. Association of MMP2 and MMP9 gene polymorphisms with the recurrent spontaneous abortion: a meta-analysis. Gene. 2021;767:145173–145173. doi: 10.1016/j.gene.2020.145173. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Zheng L, Zhao D, Xu Y, Wang Y. The role of immune cells in recurrent spontaneous abortion. Reprod Sci. 2021;28(12):3303–3315. doi: 10.1007/s43032-021-00599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicola NA, Babon JJ. Leukemia inhibitory factor (LIF) Cytokine Growth Factor Rev. 2015;26(5):533–544. doi: 10.1016/j.cytogfr.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zare F, Amiri MM, Hadinedoushan H, Dehghan-Manshadi M, Mansouri F, Fesahat F, et al. Contraceptive and molecular function of a novel recombinant vaccine based human leukemia inhibitory factor on Balb/c mice: An experimental in vivo study. J Reprod Immunol. 2020;142:103195–103195. doi: 10.1016/j.jri.2020.103195. [DOI] [PubMed] [Google Scholar]
- 18.Ismail AM, Agban MN, Hasanein AS, Rayan AA, Abbas AM. Role of Th-1 cell cytokines, leukemia inhibitory factor and hoxA genes in women with recurrent pregnancy loss. Middle East Fertil Soc J. 2017;22(4):300–304. [Google Scholar]
- 19.Xu B, Sun X, Li L, Wu L, Zhang A, Feng Y. Pinopodes, leukemia inhibitory factor, integrin-β3, and mucin-1 expression in the peri-implantation endometrium of women with unexplained recurrent pregnancy loss. Fertil Steril. 2012;98(2):389–395. doi: 10.1016/j.fertnstert.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 20.Piccinni MP, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med. 1998;4(9):1020–1024. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Wang L, Xing Z, Huang Y, Miao Z. Expression level of TNF-α in decidual tissue and peripheral blood of patients with recurrent spontaneous abortion. Cent Eur J Immunol. 2017;42(2):156–160. doi: 10.5114/ceji.2017.69357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill JA 3rd, Choi BC. Immunodystrophism: evidence for a novel alloimmune hypothesis for recurrent pregnancy loss involving Th1-type immunity to trophoblast. Semin Reprod Med. 2000;18(4):401–405. doi: 10.1055/s-2000-13730. [DOI] [PubMed] [Google Scholar]
- 23.Lim KJ, Odukoya OA, Ajjan RA, Li TC, Weetman AP, Cooke ID. The role of T-helper cytokines in human reproduction. Fertil Steril. 2000;73(1):136–142. doi: 10.1016/s0015-0282(99)00457-4. [DOI] [PubMed] [Google Scholar]
- 24.Yamada H, Kato EH, Kobashi G, Ebina Y, Shimada S, Morikawa M, et al. High NK cell activity in early pregnancy correlates with subsequent abortion with normal chromosomes in women with recurrent abortion. Am J Reprod Immunol. 2001;46(2):132–136. doi: 10.1111/j.8755-8920.2001.460203.x. [DOI] [PubMed] [Google Scholar]
- 25.Winger EE, Reed JL. Treatment with tumor necrosis factor inhibitors and intravenous immunoglobulin improves live birth rates in women with recurrent spontaneous abortion. Am J Reprod Immunol. 2008;60(1):8–16. doi: 10.1111/j.1600-0897.2008.00585.x. [DOI] [PubMed] [Google Scholar]
- 26.Cai J, Li M, Huang Q, Fu X, Wu H. Differences in cytokine expression and STAT3 activation between healthy controls and patients of unexplained recurrent spontaneous abortion (URSA) during early pregnancy. PLoS One. 2016;11(9):e0163252–e0163252. doi: 10.1371/journal.pone.0163252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaminski VL, Ellwanger JH, Matte MCC, Savaris RF, Vianna P, Chies JAB. IL-17 blood levels increase in healthy pregnancy but not in spontaneous abortion. Mol Biol Rep. 2018;45(5):1565–1568. doi: 10.1007/s11033-018-4268-7. [DOI] [PubMed] [Google Scholar]
- 28.Hosseini S, Shokri F, Ansari Pour S, Jeddi-Tehrani M, Nikoo S, Yousefi M, et al. A shift in the balance of T17 and Treg cells in menstrual blood of women with unexplained recurrent spontaneous abortion. J Reprod Immunol. 2016;116:13–22. doi: 10.1016/j.jri.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Pongcharoen S, Somran J, Sritippayawan S, Niumsup P, Chanchan P, Butkhamchot P, et al. Interleukin-17 expression in the human placenta. Placenta. 2007;28(1):59–63. doi: 10.1016/j.placenta.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Isaka K, Usuda S, Ito H, Sagawa Y, Nakamura H, Nishi H, et al. Expression and activity of matrix metalloproteinase 2 and 9 in human trophoblasts. Placenta. 2003;24(1):53–64. doi: 10.1053/plac.2002.0867. [DOI] [PubMed] [Google Scholar]
- 31.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101(7):2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 32.Wu HX, Jin LP, Xu B, Liang SS, Li DJ. Decidual stromal cells recruit Th17 cells into decidua to promote proliferation and invasion of human trophoblast cells by secreting IL-17. Cell Mol Immunol. 2014;11(3):253–262. doi: 10.1038/cmi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]