Dear Editor,
Nanotechnology-based therapeutic strategies have been proven effective in diseases including cancer, infection, inflammation, etc.1 However, the application of nanotechnology is greatly restricted in the treatment of central nervous system (CNS) disorders due to physiological CNS barriers. For example, the blood-brain barrier (BBB) can be the “Maginot line” for pharmacologically active molecules, blocking them out of the CNS. Besides, the neuroinflammation and non-directional differentiation of neural stem cells (NSCs) during neurological disorders could also affect neuronal activity and even produce fibrotic scars, thus hindering the CNS regeneration process.2
Synthetic nanocarriers, including liposomes, micelles, and nanoparticles, can be engineered through PEGylation and functional surface ligands to overcome the BBB and achieve CNS-targeted drug delivery.3,4 However, repeated administrations of synthetic nanoparticles may trigger unexpected immune responses and increase the blood clearance rate of nanocarriers. Inspired by nature, we focus on the naturally evolved biologic nanoparticles, such as extracellular vesicles (EVs), which are cell-secreted membrane-derived signaling nanoparticles, to address these issues. EVs contain biologically active molecules like lipids, DNA, RNAs, and proteins as natural intercellular signals, so they could serve as natural nanomedicine carriers.5 More importantly, EVs also demonstrate the ability to cross the BBB. Combined with the advantages of low immunogenicity and good biocompatibility, EVs are a promising class of biological nanocarriers that can be engineered to improve the treatment of neurological disorders.6
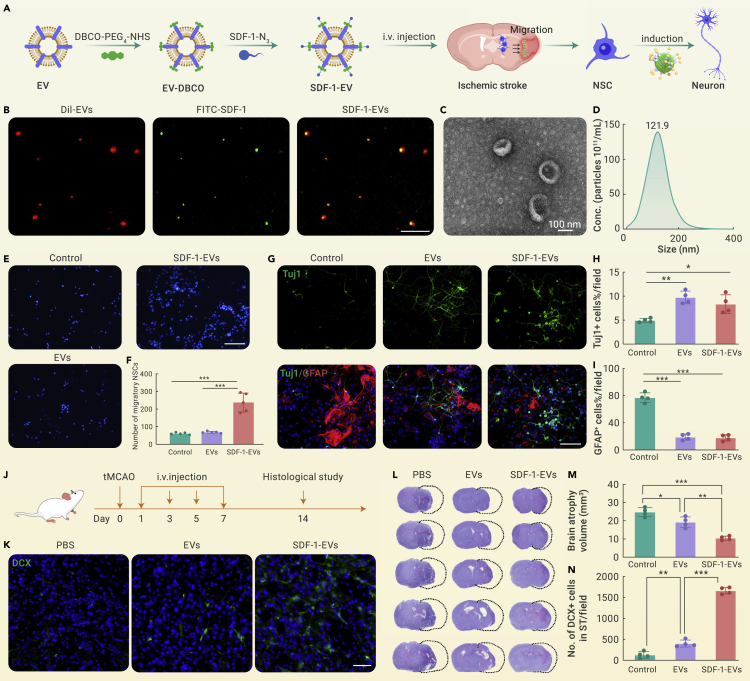
Taking ischemic stroke as an example, due to the limited number of NSCs in the injured area and the restricted neuronal differentiation efficiency, the neural regeneration in the cerebral ischemic region is inhibited.7 Thus, a biologically engineered nanomedicine that can transmit NSC recruitment signals and activate their neuronal differentiation ability was designed for ischemic stroke treatment. First, the M2 microglia-derived EVs were used as the core. Then stromal cell-derived factor 1 (SDF-1) was covalently grafted on the surface of EVs through copper-free click chemistry to emit NSC recruitment signals (SDF-1-EVs). When intravenously injected, SDF-1-EVs could cross the BBB and accumulate in the ischemic region (Figure 1A). The immunofluorescent assay (Figure 1B) showed that FITC-labeled SDF-1 could co-localize with Dil-red-labeled EVs, suggesting that SDF-1 was successfully modified on EVs. Besides, the morphology of SDF-1-EVs was in a cup shape with an approximate 120 nm diameter, identical to typical EVs (Figures 1C and 1D). Transwell experiments were carried out to verify the effect of SDF-1-EVs on the recruitment of NSCs. The results revealed that adding SDF-1-EVs in the lower chamber significantly induced NSCs in the upper chamber membrane to migrate to the opposite membrane compared with the control and unmodified EVs groups (Figures 1E and 1F). Additionally, in vitro NSC differentiation assays demonstrated (Figures 1G–1I) that SDF-1-EVs can effectively promote neuronal differentiation (Tuj1+ cells) of NSCs while inhibiting astrocytes (GFAP+) generation. Moreover, the induction effect was similar to the unmodified EVs group, indicating that the modification of SDF-1 did not affect their activity on NSC differentiation while conferring the migratory effect of EVs on NSCs. Next, a transient middle cerebral artery occlusion (tMCAO) mouse model was constructed to simulate clinical ischemic stroke. PBS, EVs, and SDF-1-EVs were intravenously injected respectively. 14 days post-stroke, brains were taken for histological analysis (Figure 1J). Compared with PBS and EVs groups, the significantly higher fluorescent signal of DCX-positive cells in the SDF-1-EVs group indicated that an increased number of immature neurons were migrating toward the ischemic area (Figures 1K and 1N), demonstrating that SDF-1-EVs could efficiently enhance NSC migration and its neuronal differentiation. Moreover, the brain atrophy volume obviously decreased under treatment with SDF-1-EVs (Figures 1L and 1M). The above results all revealed that the biologically engineered SDF-1-EVs exhibited an enhanced therapeutic effect on brain injury.
Figure 1.
Systemic administration of biologically engineered EVs for ischemic stroke
(A) Schematic diagram illustrating the construction of SDF-1-EVs via the copper-free click chemistry, which was intravenously injected into the tMCAO mice and traveled across the BBB to recruit NSCs and induce neuronal differentiation, eventually promoting neurogenesis.
(B) Fluorescent staining to verify the successful modification with FITC-labeled SDF-1 on the surface of Dil-red-labeled EVs; scale bar: 20 μm.
(C) Transmission electron microscope image and (D) nanoparticle tracking analysis (NTA) of SDF-1-EVs; scale bar: 100 nm.
(E) In vitro Transwell assay to assess the effect of NSC migration under different treatments. NSCs were seeded in the upper chamber. 48 h later, the NSCs on the upper membrane were removed, and those migrated to the lower membrane were stained with DAPI (blue). Scale bar: 200 μm.
(F) Quantification of the number of migratory NSCs using ImageJ software, n = 5.
(G) Immunofluorescent staining of in vitro NSC differentiation after treatment with blank medium, EVs, or SDF-1-EVs for 7 days. Neurons were stained with anti-Tuj1 antibody, and astrocytes were marked with anti-GFAP antibody. Scale bar: 100 μm.
(H and I) Quantitative analysis of the differentiated percentage of NSCs into neurons or astrocytes, n = 4.
(J) Scheme of in vivo animal experiment, where tMCAO mice were treated with PBS, EVs, and SDF-1-EVs, respectively. Medication was given every 2 days post-stroke at the dosage of 20 μg per mouse per time, and mice were sacrificed 14 days later for histological analysis.
(K and N) Representative fluorescent images and quantitative analysis of DCX-positive migrating immature neurons in the striatum; scale bar: 50 μm, n = 4.
(L and M) Representative cresyl violet staining and brain atrophy volume analysis in each group, n = 4. All the data are presented as mean ± SD, one-way ANOVA with Tukey’s multiple comparisons test, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
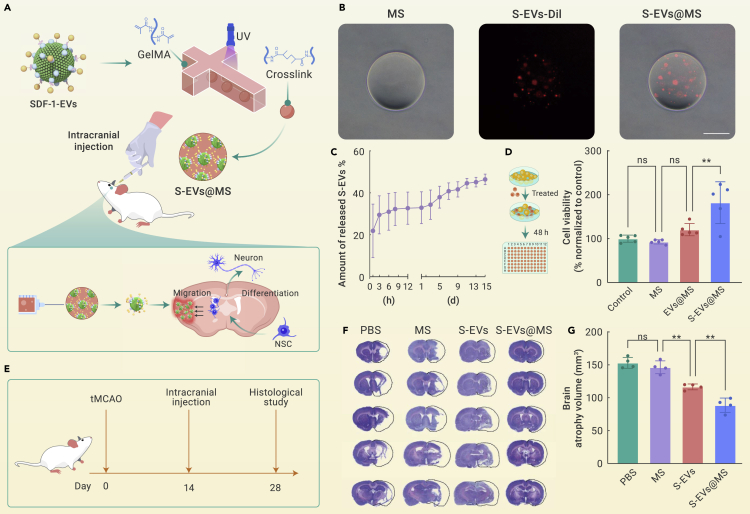
Though EVs can overcome BBB to treat neurological diseases, intravenous administration of EVs is still prone to be quickly cleared by the mononuclear phagocyte system, which is an obstacle to achieving therapeutic drug concentration. Therefore, it is expected to utilize the tissue engineering technique to construct a nanomedicine niche that could serve as the NSC signal transmitter in the specific area of the brain to recruit NSCs and direct their differentiation. Hydrogel microspheres (MSs) prepared using microfluidic techniques are excellent nanomedicine carriers due to their superior injectability, quality controllability, and sustained release.8 By loading SDF-1-EVs into the MSs, the SDF-1-EVs niche (S-EVs@MS) was constructed and stereotactically injected into the intracerebral cavity of the tMCAO model rat. This S-EVs@MS is expected to overcome obstacles of EVs blood clearance and achieve the long-term repair of stroke (Figure 2A). The MSs have a diameter around 200 μm, and they could effectively encapsulate Dil-red-labeled S-EVs (Figure 2B), and the loading efficiency was about 80%. The in vitro release results (Figure 2C) exhibited that S-EVs in S-EVs@MS were slowly released and reached about 46% after 15 days. Besides, S-EVs@MS was co-incubated with NSC in vitro to assess the cytotoxicity of S-EVs@MS. It was found that a MS itself had no toxic effect on cell proliferation and exhibited good biocompatibility (Figure 2D). Interestingly, S-EVs@MS was able to promote NSC proliferation, which may be attributed to the function of SDF-1. SDF-1 could promote NSC proliferation in a dose-dependent manner. Also, the growth factors like endothelial growth factor and basic fibroblast growth factor would further enhance the proliferative effect of SDF-1. In vivo studies were performed on the tMCAO model rat. The brain atrophy volume significantly decreased in the S-EVs@MS-treated group compared with the PBS and S-EVs groups, suggesting the importance of MS incorporation (Figures 2E and 2F).
Figure 2.
Hydrogel microsphere-functionalized SDF-1-EVs (S-EV@MS) for local treatment of ischemic stroke
(A) Preparation of SDF-1-EVs-loaded MSs using microfluidic technology and photo-crosslinking method, which were then intracranially injected into the tMCAO rats to locally emit signals for NSC recruitment and neuronal differentiation to promote neurogenesis.
(B) Microscopy images showed that Dil-red-labeled S-EVs were successfully loaded into microspheres. Scale bar: 100 μm.
(C) The release profile of S-EVs@MS at 37°C in PBS quantified by NTA, n = 3.
(D) Cytocompatibility of different formulations on NSCs at the dosage of 10 μg/mL EVs after incubation for 48 h, n = 5.
(E) Schematic illustration of in vivo animal experiment. tMCAO rats were intracranial injected with PBS, MS, S-EVs, and S-EVs@MS, respectively, 14 days post-surgery at the dosage of 50 μg EV per rat and sacrificed at day 28 for histological analysis.
(F) Representative cresyl violet staining section of brains in tMCAO rats 14 days post-injection of PBS, MS, S-EVs, or S-EVs@MS, respectively. S-EVs, or S-EVs@MS.
(G) Quantitative analysis of brain atrophy volume, n = 4. Data are presented as mean ± SD, one-way ANOVA with Tukey’s multiple comparisons tests; ns, non-significance, ∗∗p < 0.01.
Overall, we constructed two different types of engineered EVs as signal transmitters for the recruitment and differentiation induction of NSCs and successfully achieved neural regeneration in the experimental rodent stroke model. We explored the possibility of biologically engineered EVs for neurological disorders treatment using both systemic and local delivery. Systemic therapy exhibits superior clinical compliance, but with the burdens of repeated administration and rapid blood clearance. Although the addition of sustained-release MSs overcomes these defects, the immunogenicity and biocompatibility of MS degradation products remain to be further investigated. Nevertheless, these kinds of engineered EVs therapies, together with related advanced biotechnologies or by itself, show great potential for clinical translation in the diagnosis, monitoring, treatment, and care of not only CNS diseases, but also cardiovascular disease, bone and joint regeneration, cancer, wound healing, and immune disorders.9,10
Acknowledgments
This research was supported by grants from the National Key R&D Program of China (2019YFA0112000), Zhejiang Provincial Natural Science Foundation of China (No. LQ21H300009), National Natural Science Foundation of China (81930051, 82003658, 82202785), and GuangCi Professorship Program of Ruijin Hospital Shanghai Jiao Tong University School of Medicine.
Declaration of interests
The authors declare no competing interests.
Published Online: February 16, 2023
Contributor Information
Gang Chen, Email: adcyy@aliyun.com.
Xingcai Zhang, Email: xingcai@mit.edu.
Yaohui Tang, Email: yaohuitang@sjtu.edu.cn.
Wenguo Cui, Email: wgcui80@hotmail.com.
References
- 1.Machtakova M., Thérien-Aubin H., Landfester K. Polymer nano-systems for the encapsulation and delivery of active biomacromolecular therapeutic agents. Chem. Soc. Rev. 2022;51:128–152. doi: 10.1039/d1cs00686j. [DOI] [PubMed] [Google Scholar]
- 2.Dorrier C.E., Aran D., Haenelt E.A., et al. CNS fibroblasts form a fibrotic scar in response to immune cell infiltration. Nat. Neurosci. 2021;24:234–244. doi: 10.1038/s41593-020-00770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terstappen G.C., Meyer A.H., Bell R.D., Zhang W. Strategies for delivering therapeutics across the blood-brain barrier. Nat. Rev. Drug Discov. 2021;20:362–383. doi: 10.1038/s41573-021-00139-y. [DOI] [PubMed] [Google Scholar]
- 4.Ruan H., Yao S., Wang S., et al. Stapled RAP12 peptide ligand of LRP1 for micelles-based multifunctional glioma-targeted drug delivery. Chem. Eng. J. 2021;403:126296. [Google Scholar]
- 5.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z.G., Buller B., Chopp M. Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 2019;15:193–203. doi: 10.1038/s41582-018-0126-4. [DOI] [PubMed] [Google Scholar]
- 7.Faiz M., Sachewsky N., Gascón S., et al. Adult neural stem cells from the subventricular zone give rise to reactive astrocytes in the cortex after stroke. Cell Stem Cell. 2015;17:624–634. doi: 10.1016/j.stem.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Z., Wang Z., Li G., et al. Injectable microfluidic hydrogel microspheres for cell and drug delivery. Adv. Funct. Mater. 2021;31:2103339. [Google Scholar]
- 9.Zheng D., Ruan H., Chen W., et al. Advances in extracellular vesicle functionalization strategies for tissue regeneration. Bioact. Mater. 2022 doi: 10.1016/j.bioactmat.2022.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng F., Li L., Zhang Z., et al. Biosynthetic neoantigen displayed on bacteria derived vesicles elicit systemic antitumour immunity. J. Extracell. Vesicles. 2022;11:12289. doi: 10.1002/jev2.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]