Abstract
Background
It is known that bone marrow mesenchymal stem cells (BM-MSCs) could speed up the regeneration of diabetic corneal epithelium. To investigate the effect of exosomes derived from mouse BM-MSCs on corneal epithelium regeneration in diabetic mice.
Methods
Diabetic mouse models were established using streptozotocin (STZ), and their central corneal epithelium was scratched under a microscope. The diabetic mice were randomly divided into three groups: the control group was injected with subconjunctival phosphate buffer saline; the exosomes group was treated with a subconjunctival injection of exosomes derived from BM-MSCs; and the BM-MSCs group was treated with a subconjunctival injection of BM-MSCs. The corneal epithelium repair rates in the three groups were compared, and the distribution of the exosomes derived from BM-MSCs labeled with PKH-26 was observed by immunofluorescence. Hematoxylin-eosin staining of the corneal tissue was observed 72 h after the treatments in the three groups.
Results
The diabetic mice were successfully established by a blood glucose level >16.7 mmol/L after 8 weeks. The corneal epithelium healing rates in experimental groups 1 and 2 were significantly higher than those of the control group at 24, 48, and 72 h (P<0.05). However, there was no significant difference in the corneal epithelial healing rate between experimental groups 1 and 2 (P>0.05). The exosomes derived from BM-MSCs were found in the superficial corneal stroma in experimental groups 1 and 2, with the majority of the exosomes distributed in the limbal epithelium at the edge of the injury area. The proliferation of corneal epithelial cells in experimental groups 1 and 2 was more obvious than that in the control group.
Conclusions
The exosomes derived from BM-MSCs labeled with PKH-26 significantly promoted the repair of corneal epithelial injury in diabetic mice. These exosomes might be a substitute for BM-MSCs in the repair of diabetic keratopathy, suggesting a new idea for the repair of diabetic keratopathy with “cell-free” stem cell therapy, which will require a clinical study.
Keywords: Exosomes, bone marrow mesenchymal stem cells (BM-MSCs), corneal epithelial regeneration, diabetes mellitus, diabetic keratopathy
Highlight box.
Key findings
• The exosomes derived from BM-MSCs might be a substitute for BM-MSCs in the repair of diabetic keratopathy.
What is known and what is new?
• It was known that mice bone marrow mesenchymal stem cells (BM-MSCs) could speed up corneal epithelium regeneration in diabetic mice.
• It was found that the exosomes derived from mice BM-MSCs could also speed up corneal epithelium regeneration in diabetic mice.
What is the implication, and what should change now?
• These results suggested a novel idea for the repair of diabetic keratopathy with “cell-free” stem cell therapy, which will require a clinical study.
Introduction
With the increasing number of diabetic patients, diabetic keratopathy has become more common. It has been shown that 47–64% of patients with diabetes could have varying degrees of primary keratopathy. The main clinical manifestations are dry eye, decreased corneal sensitivity, difficult healing of the corneal epithelium after ocular surgery or trauma, neurotrophic corneal ulcers, etc. The main pathological mechanism of diabetic keratopathy could be stem cell dysfunction, abnormal function of the corneal epithelium, abnormal components in the basement membrane, deposition of the product of glycosylation, injury to the corneal nerve endings, abnormal corneal endothelium, oxidative stress, or inflammatory response (1-3).
Dry eye and delayed healing of the corneal epithelium are significant clinical problems for diabetic patients undergoing ocular surgery. Therefore, diabetic keratopathy is a major ocular complication in diabetic patients, which seriously affects their quality of vision and life. The progress of the treatment for corneal epithelium regeneration of diabetes includes stem cell therapy, autologous serum, artificial tear, growth factor eye drops, etc., but some patients have poor effects. The molecular mechanism of diabetic corneal injury and repair requires further study (4,5).
Exosomes are stably expressed, have a nano structure, do not form embolisms, have no tumorigenicity, and can freely cross biological barriers, which also are the characters of the exosomes derived from bone marrow mesenchymal stem cells (BM-MSCs) (6). Exosomes derived from stem cells have extensive application prospects in the repair of diabetic corneal trauma, the reduction of corneal scarring, and even as a new drug carrier and gene therapy vector for diabetic keratopathy and other diseases (5).
The yield of exosomes derived from BM-MSCs is very high, and such exomes could effectively perform the immunomodulatory role of stem cells and avoid the risk of embolisms, in addition to circumventing the ethical challenges of stem cell therapy (7).
Therefore, the present study explores the novel idea of repairing diabetic keratopathy with “cell-free” stem cell therapy using exosomes derived from BM-MSCs by studying the repair of corneal epithelial injury in diabetic mice. The distribution of exomes labeled with PKH-26 in the corneal tissue and the characteristics of corneal epithelial regeneration were observed using immunofluorescence. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6644/rc).
Methods
Main reagents
Streptozotocin (STZ) was placed in an Eppendorf (EP) tube and the EP pipe orifice was wrapped with sealing film and tin foil to protect it from light exposure. STZ was dissolved in citric acid phosphate-buffered saline (PBS) before injection and used within 15 min.
Study methods
A total of 40 C57 mice (including 20 males and 20 females aged 6–8 weeks and weighing approximately 30–40 g) were randomly assigned to a normal control group (n=10) or a STZ diabetes group (n=30). A protocol was prepared before the study without registration. Experiments were performed under a project license (No. 2020-117-01) granted by Animal Ethics Committee of Guangzhou Red Cross Hospital of Jinan University, in compliance with institutional guidelines for the care and use of animals.
Diabetic mice were injected intraperitoneally with l% STZ for five consecutive days after fasting for 8 h. All mice were given free exercise and drinking water (dietary glucose water). The diabetic animal model was successfully established, as represented by a blood glucose level >16.7 mmol/L for blood that was collected from the caudal vein after 8 weeks. To establish the mouse corneal epithelial injury model, 0.3–0.5 mL of anesthetic was injected intraperitoneally based on the weight of the mouse. Each anaesthetized mouse was then placed on the operating table. Procaine hydrochloride eye drops were used for surface anesthesia and the corneal epithelium was marked with a 3 mm corneal ring drill, which produced a 3 mm scratch on the central corneal epithelium, with the corneal apex as the central point. The mouse corneal epithelial nerve injury model was also successfully established using this method.
Normal C57 mice were used as the blank normal group (n=10). The diabetic mice were randomly divided into three groups: the control group was treated with a subconjunctival PBS injection (n=10); the exosomes group was treated with a subconjunctival injection of 20 µL 25 mg/mL exosomes derived from BM-MSCs according to the preliminary experiment (n=10); and the BM-MSCs group was treated with a subconjunctival injection of 20 µL BM-MSCs (n=10).
Histopathology and immunohistochemistry
Corneal tissue from the three groups was randomly collected for immunohistological analysis at 24 and 48 h after surgery. Immunohistochemical staining was performed according to the manufacturer’s instructions and included the following steps: the tissue was fixed with neutral formic acid for 24 h, dried in an automatic dehydrator, and then embedded in wax and sectioned. The slice thickness was 1.5 mm. The slices were baked for 1 h; dewaxed for 15 min each in xylene I, II, and III; washed with distilled water once after eosin staining for 5 minutes; and subsequently passed through an alcohol gradient dehydration series: 75% ethanol → 95% ethanol → absolute ethanol for 5 seconds each. The slices were dried, sealed with neutral resin, and observed and photographed under a microscope.
Evaluation index
(I) The healing rate of the corneal epithelium in each group was observed using corneal fluorescein staining and a slit lamp. (II) The exosomes derived from BM-MSCs labeled with PKH-26 were traced in vivo, and the corneal distribution was observed by immunofluorescence. (III) The repair of mouse corneal injury was observed using hematoxylin-eosin (HE) staining and histopathology after corneal epithelial scratching at 24, 48, and 72 h.
Statistical analysis
The SPSS 22.0 statistical package (Chicago, IL, USA) was used for the statistical analysis. All data were expressed as the mean ± standard deviation (mean ± SD). An independent sample t-test was used among the groups, and the counting data were expressed as a percentage (P<0.05). All tests were two-tailed and a probability value of 0.05 was considered statistically significant.
Results
Comparison of the healing rates of diabetic corneal epithelia in the three groups
At 24, 48, and 72 h after surgery, the healing rate of diabetic corneal epithelia in the control group was slower than that in the normal mouse group (P<0.05). The healing rates of corneal epithelia in experimental groups 1 and 2 were significantly higher than that in the control group (P<0.05). However, there was no notable difference in the healing rate of corneal epithelia between experimental groups 1 and 2 (P>0.05) (Figure 1).
Figure 1.
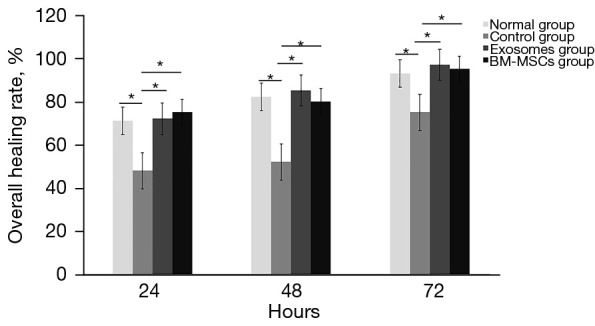
Comparison of the healing rates of diabetic corneal epithelia in the three groups. * indicated the healing rate of diabetic corneal epithelia in the control group was slower than that in the normal mouse group (P<0.05). The healing rates of corneal epithelia in experimental groups 1 and 2 were significantly higher than that in the control group (P<0.05) at 24, 48, and 72 h after surgery. BM-MSC, bone marrow mesenchymal stem cell.
Tracing the exosomes derived from BM-MSCs using PKH-26 in vivo
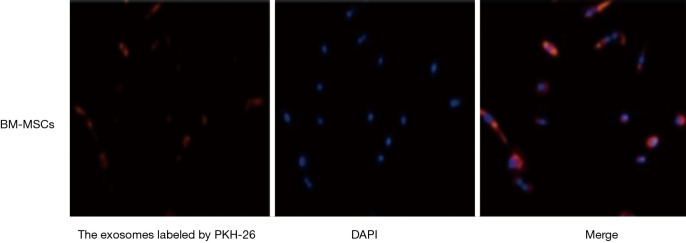
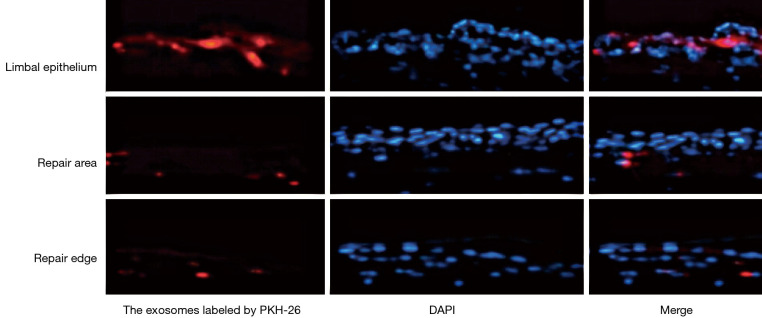
The exosomes derived from BM-MSCs were successfully marked by PKH-26 (Figure 2). The labeled PKH-26 was inoculated into the right subconjunctiva of diabetic mice. The corneal tissue was harvested to facilitate quick-frozen sections at 72 h. The exosomes derived from BM-MSCs labeled with red fluorescence were distributed in the limbal epithelium, repair area, and repair edge (Figure 3). Exomes were found in the superficial corneal stroma of mice in experimental groups 1 and 2, and there was more division and proliferation of corneal epithelial cells in these two groups than in the control group.
Figure 2.
The exosomes derived from BM-MSCs can be labeled with PKH-26. Magnification times: 100×. BM-MSC, bone marrow mesenchymal stem cell; DAPI, 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride staining.
Figure 3.
The exosomes derived from BM-MSCs labeled with red fluorescence were distributed in the limbal epithelium, repair area, and repair edge. Magnification times: 100×. BM-MSC, bone marrow mesenchymal stem cell; DAPI, 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride staining.
HE histopathology results
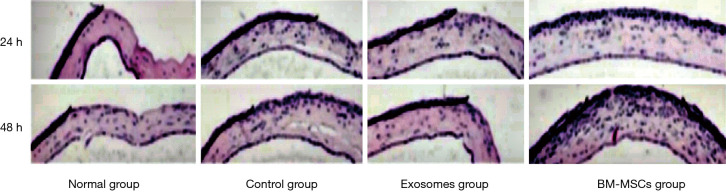
The repair of the corneal injury in diabetic mice was observed using HE staining at 24 and 48 h post-operation. The number of infiltrating inflammatory cells began to increase at 24 h after corneal epithelial scratching in the control group, and this number was further increased at 48 h. Compared to that in the control group, the infiltration of inflammatory cells in experimental groups 1 and 2 was significantly decreased (Figure 4).
Figure 4.
HE staining of the cornea of diabetic mice in the different groups. It was showed that the number of infiltrating inflammatory cells began to increase at 24 h after corneal epithelial scratching in the control group, and this number was further increased at 48 h. Compared to that in the control group, the infiltration of inflammatory cells in experimental groups was significantly decreased. Magnification times: 100×. BM-MSC, bone marrow mesenchymal stem cell; HE, hematoxylin-eosin.
Discussion
Diabetes mellitus complications are detrimental to human health. Ocular complications mainly include diabetic keratopathy, retinopathy, and cataracts. It has been shown that 47–64% of diabetic patients have diabetic keratopathy, including corneal epithelial injury, decreased corneal perception, and neurotrophic corneal ulcers. Furthermore, corneal wound healing is delayed after ocular surgery and severe lesions can cause corneal opacity, infection, and even blindness (7-11).
It has been shown that lncRNA-H19 derived from the exosomes of BM-MSCs can significantly promote the healing of various chronic ulcers caused by diabetes mellitus. The exosomes derived from BM-MSCs can also reduce corneal fibrosis and inflammatory infiltration, thereby alleviating corneal scarring. Investigating the mechanism of exosome regulation of diabetic corneal injury and repair is crucial, as this could be very important for the maintenance of corneal transparency after corneal injury in diabetic patients (12-14). The use of exomes would provide a novel treatment for corneal blindness patients worldwide.
Special hydrogel implants, with a structure identical to that of the anterior corneal elastic layer, have been placed at the bottom of the corneal ulcer to act as a carrier. These implants could slowly release therapeutic exosomes to treat chronic corneal ulcers and reduce corneal fibrosis and inflammatory infiltration (15).
BM-MSCs are pluripotent stem cells with low immunogenicity, which play a role in immune regulation and stem cell homing and promote anti-inflammatory and tissue repair processes (9-13). It has been reported that the exosomes derived from BM-MSCs can promote the repair of corneal and conjunctival damage caused by chemical injuries and diabetic corneal epithelia; however, the underlying mechanism has not been elucidated (14-17). In this study, the exosomes derived from BM-MSCs were labeled with PKH-26. The exosomes labeled with red fluorescence were distributed in the limbal epithelium, repair area, and injury edge, and some exosomes were found in the corneal stroma, suggesting that these exosomes might support the proliferation and repair of corneal epithelial cells by nourishing the corneal stroma. However, the mechanism still needs to be investigated further.
The present study aimed to investigate the effects of exosomes derived from BM-MSCs on the repair of corneal epithelia in diabetic mice. The results showed that the healing rate of corneal epithelia in the control group was significantly lower at 24, 48, and 72 h after scratching, as compared to that of the diabetic corneal epithelia (P<0.05). Also, the healing rates of corneal epithelia in experimental groups 2 and 3 were significantly higher than that of the control group (P<0.05). However, there was no significant difference in the corneal epithelial healing rates between experimental groups 2 and 3 (P>0.05), suggesting that BM-MSCs and the exosomes both significantly promoted corneal epithelium injury repair in diabetic mice.
We also observed that the number of infiltrating inflammatory cells in experimental groups 1 and 2 decreased significantly compared to the control group, which suggests that the exosomes derived from BM-MSCs might promote corneal epithelium repair in diabetic mice through their anti-inflammatory effect.
Conclusions
The exosomes derived from BM-MSCs marked with PH-26 exerted an anti-inflammatory effect and promoted corneal epithelium repair in diabetic mice. The application of these exomes could avoid the risk of embolisms and circumvent the ethical debates surrounding the use of stem cells. However, further study is required to elucidate the mechanism involved.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was funded by the Western Medicine Project of Guangzhou Health Committee in 2021 (No. 20211A011022), and the Basic and Applied Basic Research Project of Guangzhou Science and Technology Plan jointly funded by the City and University (college) (No. 202201020008).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. 2020-117-01) granted by Animal Ethics Committee of Guangzhou Red Cross Hospital of Jinan University, in compliance with institutional guidelines for the care and use of animals.
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6644/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6644/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6644/coif). The authors have no conflicts of interest to declare.
(English Language Editor: A. Kassem)
References
- 1.Yu FX, Lee PSY, Yang L, et al. The impact of sensory neuropathy and inflammation on epithelial wound healing in diabetic corneas. Prog Retin Eye Res 2022;89:101039. 10.1016/j.preteyeres.2021.101039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamed MA, Farag A, Zahran IS, et al. Pycnogenol a promising remedy for diabetic keratopathy in experimentally induced corneal alkali burns in diabetic rats. BMC Vet Res 2022;18:209. 10.1186/s12917-022-03307-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.So WZ, Qi Wong NS, Tan HC, et al. Diabetic corneal neuropathy as a surrogate marker for diabetic peripheral neuropathy. Neural Regen Res 2022;17:2172-8. 10.4103/1673-5374.327364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan L, Bai X, Zhou Q, et al. The advanced glycation end-products (AGEs)/ROS/NLRP3 inflammasome axis contributes to delayed diabetic corneal wound healing and nerve regeneration. Int J Biol Sci 2022;18:809-25. 10.7150/ijbs.63219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan L, Wu W, Wang Z, et al. Comparative study of the effects of recombinant human epidermal growth factor and basic fibroblast growth factor on corneal epithelial wound healing and neovascularization in vivo and in vitro. Ophthalmic Res 2013;49:150-60. 10.1159/000343775 [DOI] [PubMed] [Google Scholar]
- 6.Xiao F, Zuo B, Tao B, et al. Exosomes derived from cyclic mechanical stretch-exposed bone marrow mesenchymal stem cells inhibit RANKL-induced osteoclastogenesis through the NF-κB signaling pathway. Ann Transl Med 2021;9:798. 10.21037/atm-21-1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bian Y, Ma KK, Hall NE, et al. Neurotrophic Keratopathy in the United States: An Intelligent Research in Sight Registry Analysis. Ophthalmology 2022;129:1255-62. 10.1016/j.ophtha.2022.06.019 [DOI] [PubMed] [Google Scholar]
- 8.Ono M, Kosaka N, Tominaga N, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal 2014;7:ra63. 10.1126/scisignal.2005231 [DOI] [PubMed] [Google Scholar]
- 9.Tamama K, Fan VH, Griffith LG, et al. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells 2006;24:686-95. 10.1634/stemcells.2005-0176 [DOI] [PubMed] [Google Scholar]
- 10.Chang W, Wang J. Exosomes and Their Noncoding RNA Cargo Are Emerging as New Modulators for Diabetes Mellitus. Cells 2019;8:853. 10.3390/cells8080853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samaeekia R, Rabiee B, Putra I, et al. Effect of Human Corneal Mesenchymal Stromal Cell-derived Exosomes on Corneal Epithelial Wound Healing. Invest Ophthalmol Vis Sci 2018;59:5194-200. 10.1167/iovs.18-24803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu Z, Hao R, Du J, et al. A human cornea-on-a-chip for the study of epithelial wound healing by extracellular vesicles. iScience 2022;25:104200. 10.1016/j.isci.2022.104200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKay TB, Hutcheon AEK, Zieske JD, et al. Extracellular Vesicles Secreted by Corneal Epithelial Cells Promote Myofibroblast Differentiation. Cells 2020;9:1080. 10.3390/cells9051080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun B, Ding Y, Jin X, et al. Long non-coding RNA H19 promotes corneal neovascularization by targeting microRNA-29c. Biosci Rep 2019;39:BSR20182394. 10.1042/BSR20182394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo X, Li J, Yin L, et al. Role of microRNA 146a on the healing of cornea alkali burn treated with mesenchymal stem cells. Mol Med Rep 2018;18:3203-10. 10.3892/mmr.2018.9328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shojaati G, Khandaker I, Funderburgh ML, et al. Mesenchymal Stem Cells Reduce Corneal Fibrosis and Inflammation via Extracellular Vesicle-Mediated Delivery of miRNA. Stem Cells Transl Med 2019;8:1192-201. 10.1002/sctm.18-0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu W, Yu S, Feng S, et al. Effect of the TLR2/MyD88/NF-κB axis on corneal allograft rejection after penetrating keratoplasty. J Recept Signal Transduct Res 2016;36:45-52. 10.3109/10799893.2015.1016578 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as