Abstract
Background
Paediatric community-acquired pneumonia (CAP) is a leading cause of paediatric morbidity. However, particularly for outpatients with paediatric CAP, data on aetiology and management are scarce.
Methods
The prospective pedCAPNETZ study multicentrically enrols children and adolescents with outpatient-treated or hospitalised paediatric CAP in Germany. Blood and respiratory specimens were collected systematically, and comprehensive analyses of pathogen spectra were conducted. Follow-up evaluations were performed until day 90 after enrolment.
Results
Between December 2014 and August 2020, we enrolled 486 children with paediatric CAP at eight study sites, 437 (89.9%) of whom had radiographic evidence of paediatric CAP. Median (interquartile range) age was 4.5 (1.6–6.6) years, and 345 (78.9%) children were hospitalised. The most prevalent symptoms at enrolment were cough (91.8%), fever (89.2%) and tachypnoea (62.0%). Outpatients were significantly older, displayed significantly lower C-reactive protein levels and were significantly more likely to be symptom-free at follow-up days 14 and 90. Pathogens were detected in 90.3% of all patients (one or more viral pathogens in 68.1%; one or more bacterial strains in 18.7%; combined bacterial/viral pathogens in 4.1%). Parainfluenza virus and Mycoplasma pneumoniae were significantly more frequent in outpatients. The proportion of patients with antibiotic therapy was comparably high in both groups (92.4% of outpatients versus 86.2% of hospitalised patients).
Conclusion
We present first data on paediatric CAP with comprehensive analyses in outpatients and hospitalised cases and demonstrate high detection rates of viral pathogens in both groups. Particularly in young paediatric CAP patients with outpatient care, antibiotic therapy needs to be critically debated.
Short abstract
This prospective multicentre study analysed outpatients and hospitalised children with CAP. Clinical phenotype, pathogen spectra and outcome were compared. A high rate of viral pathogens in young children questions the use of antibiotics in this group. https://bit.ly/3fyxw0O
Introduction
Paediatric community-acquired pneumonia (CAP) is one of the leading causes of childhood morbidity and mortality worldwide. Even in high-income countries, it causes up to 20% of paediatric hospital admissions [1]. In Germany, annual hospitalisation rates of children aged <5 years with paediatric CAP are estimated to be ∼90 per 10 000 [2]. However, a critical gap in the knowledge on aetiology and management of paediatric CAP exists. Most paediatric CAP studies thus far have focused on hospitalised paediatric CAP, and longitudinal data on nonsevere courses are scarce [3–5]. Detailed aetiological data in young age groups are particularly rare [4, 6, 7]. The diagnostic and predictive value of clinical paediatric CAP symptoms such as fever and tachypnoea, especially in infants, are under debate [8–11], and validated severity scores for paediatric CAP are pending. In recent years, implementation of anti-pneumococcal and anti-Haemophilus influenzae B vaccines and novel diagnostic nucleic acid amplification techniques led to a significant shift in pathogens identified in children with paediatric CAP [12–16]. However, even with updated diagnostic tools, clear differentiation between viral and bacterial paediatric CAP remains challenging [4, 5, 17–19]. Although viral infections are assumed to be responsible for a high proportion of paediatric CAP, particularly in infants, antibiotic therapy in paediatric CAP is still recommended in a large proportion of cases [11, 20–22]. To contribute to current knowledge on aetiology, phenotype and treatment regimens and improve paediatric CAP management, we initiated the prospective, multicentre pedCAPNETZ study [23]. Here, we present first data from this cohort with a particular focus on pathogen spectra in hospitalised and nonhospitalised paediatric CAP.
Methods
Study cohort
Setup and protocol of the pedCAPNETZ cohort is described in detail elsewhere [23]. The analysis presented herein includes data obtained between December 2014 and August 2020. In brief, patients were recruited in nine clinical centres in inpatient or outpatient settings as a convenience sample not following an established protocol to identify eligible patients. Inclusion criteria were age <18 years and diagnosis of paediatric CAP confirmed by lung ultrasound (LUS) or chest radiography. Proof of lung consolidation with air bronchograms by LUS was classified as pneumonia [24, 25]. If pleural effusion was present in LUS, extent and morphology were determined. Chest radiographs were reviewed by two independent, certified paediatric radiologists in accordance with World Health Organization guidelines [26, 27]. Exclusion criteria were hospitalisation for any reason or cytostatic therapy 28 days prior to inclusion, neutropenia (<1000 cells·μL−1), other relevant immunosuppressive treatment, congenital or acquired immunodeficiency, concomitant respiratory disease with impaired mucociliary clearance or tuberculosis. Respiratory insufficiency (demand for oxygen supplementation) was defined as peripheral oxygen saturation (SpO2) <92%.
Data collection
Demographic data and information on clinical symptoms, comorbidities, previous medical antibiotic treatment, inflammation parameters, diagnostic data, radiological findings and treatment were recorded in pseudonymised fashion in a central online database at study inclusion. Follow-up visits by phone interview or chart reviews were performed on days 14 and 90 after enrolment; follow-up on day 28 was performed when clinical symptoms persisted until day 14 after inclusion.
Pathogen detection and determination of biomarkers
An extended description can be found elsewhere [23]; in brief, study inclusion and biomaterial collection were performed within 24 h after radiological or sonographic proof of pneumonia. Bacterial cultures were performed in all available blood, pleural fluid, deep throat swab, sputum (with or without previous hypertonic saline inhalation) and bronchoalveolar lavage fluid (BALF) specimens upon study inclusion. Diagnostically valuable sputum was obtained in children aged >6 years. PCR assays were performed in nasopharyngeal swabs (NPS) or nasopharyngeal aspirates (NPA) to detect Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella, Bordetella pertussis and a panel of respiratory viruses (adenovirus, coronavirus 229E/HKU1/NL63/OC43 (not severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)), human metapneumovirus (HMPV), human rhinovirus, influenza A and B (flu), parainfluenza virus (PIV) types 1–4, bocavirus, enterovirus, parechovirus and respiratory syncytial virus (RSV)) at study inclusion. Available blood samples were tested for differential blood cell counting, C-reactive protein (CrP), creatinine, sodium, urea, blood gas and C. pneumoniae and M. pneumoniae immunoglobulins (Ig) in routine clinical testing. At the time of study inclusion, the results of NPS- or NPA-PCR, bacterial cultures and sputum as well as serology results of C. pneumoniae and M. pneumoniae were not available for the attending physicians according to daily clinical practice and analysis duration. Results of differential blood cell counting, CrP value, creatinine, sodium, urea and blood gas analysis were available for the treating physicians at the time of study inclusion.
The gained biomaterials were either directly measured at the participating centres (differential blood cell count, CrP value, creatinine, sodium, urea, blood gas analysis, blood culture, sputum, bronchoalveolar lavage, pleural fluid, serology for M. pneumoniae and C. pneumoniae) or immediately stored at −80°C and send to the study centre (NPA, NPS).
Statistical analysis
All statistical analyses were performed employing Statistical Software Package for Social Sciences (SPSS 24; IBM, Armonk, NY, USA), Excel or GraphPad Prism V9 (San Diego, CA, USA). Descriptive data were calculated as mean±sd or interquartile range (IQR). Categorical data were reported as frequencies and percentages. Differences between groups were analysed by t- or Mann–Whitney U-testing and Chi-quadrat or Fisher's exact testing for nominally distributed data. p-values <0.05 were considered statistically significant.
Ethical approval
The pedCAPNETZ study was approved by ethical review boards of all participating centres (e.g. lead study centre approval number MHH#2356–2014). All patients and their legal guardians provided written informed consent.
Results
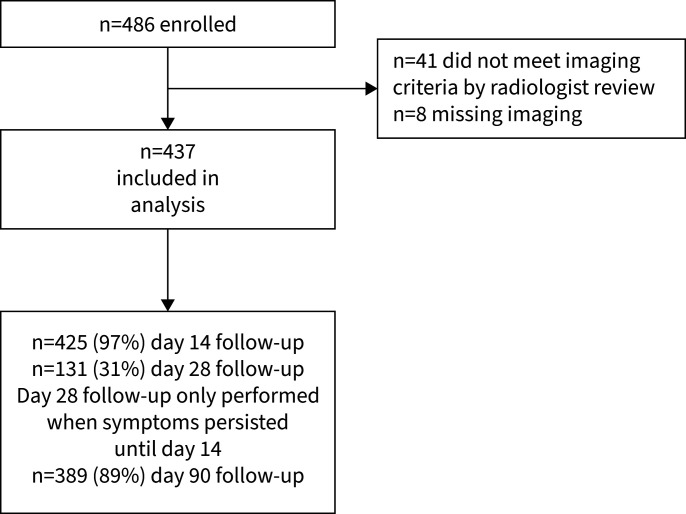
486 children were screened for study participation. 41 (8.4%) of these did not meet radiographic or sonographic inclusion criteria, and in eight (1.6%) cases thoracic imaging was missing. Accordingly, 437 patients were included in the analysis (figure 1). For demographic data see table 1. 92 (21.1%) were treated as outpatients, whereas 345 (78.9%) patients required hospitalisation (table 1). The mean (IQR) duration from symptom onset until study inclusion was 7 (3–9) days; 7.5 days in outpatients and 6.8 days in hospitalised cases (p=0.36).
FIGURE 1.
Study flowchart.
TABLE 1.
Cohort characteristics
| Total cohort | 437 |
| Age years | 4.5 (1.6–6.6) |
| Female | 207 (47) |
| Hospitalisation rate | 336 (79) |
| Intensive care unit admission | 35 (8) |
| Mechanical ventilation | 12 (2) |
| Death | 0 |
| CrP <10 mg·L−1 | 115 (27) |
| CrP >100 mg·L−1 | 93 (22) |
| Cough | 426 (98) |
| Fever (>38.0°C) | 380 (89) |
| Tachypnoea | 271 (62) |
| Auscultation abnormalities | 369 (91) |
| Wheeze, prolonged expiration | 157 (37) |
| Thoracic retractions | 188 (56) |
| Oxygen demand (SpO2 <92%) | 106 (25) |
| Reduced liquid intake | 179 (42) |
Data are presented as n, mean (interquartile range) or n (%). CrP: C-reactive protein; SpO2: peripheral oxygen saturation.
Among the 426 cases with paediatric CAP confirmation by chest radiography, alveolar infiltrates were the most prevalent pathological finding, occurring in 399 (93.6%) patients. Detailed radiographic differences of children treated as inpatients and outpatients are given in table 2. In 11 cases, CAP was diagnosed by LUS showing lung consolidation with air bronchograms.
TABLE 2.
Cohort characteristics for hospitalised and nonhospitalised children
| Inpatients | Outpatients | p-value | |
| Proportion of total study group | 345 (79) | 92 (21) | |
| Age years | 4.3 (1.5–5.7) | 5.5 (2.6–7.4) | <0.001 |
| CrP mg·L−1 | 30.5 (4.9–52) | 74.9 (10–107) | <0.001 |
| Radiography images | 340 (99) | 86 (93) | |
| Alveolar infiltrates | 322 (95) | 77 (90) | 0.08 |
| Consolidations | 213 (63) | 26 (30) | <0.001 |
| Atelectasis | 65 (19) | 8 (9) | 0.01 |
| Hyperinflation | 84 (25) | 7 (8) | <0.001 |
| Interstitial infiltrates | 68 (20) | 7 (8) | 0.01 |
| Pleural effusion | 98 (29) | 20 (23) | 0.35 |
| Symptoms | |||
| Cough | 313 (91) | 88 (96) | 0.14 |
| Fever (>38.0°C) | 306 (93) | 84 (97) | 0.572 |
| Tachypnoea | 221 (64) | 50 (54) | 0.092 |
| Thoracic retractions | 199 (66)# | 19 (21) | <0.0001 |
| Pulmonary obstruction | 106 (31) | 12 (13) | <0.001 |
| Abdominal pain | 82 (27)¶ | 15 (17) | 0.052 |
| Reduced liquid intake | 167 (50)+ | 12 (13) | <0.001 |
| Clinical course | |||
| Healthy day 14 follow-up | 177 (58)¶ | 74 (83)§ | <0.001 |
| Healthy day 90 follow-up | 270 (89)ƒ | 84 (99)## | 0.002 |
| Re-diagnosis paediatric CAP day 90 follow-up | 20 (6.6)ƒ | 7 (8.3)## | 0.63 |
Data are presented as n (%) or mean (interquartile range), unless otherwise stated. CrP: C-reactive protein; CAP: community-acquired pneumonia. Data were available in #: n=303, ¶: n=307, +: n=335, §: n=89, ƒ: n=304, ##: n=85.
Overall, the most prevalent symptom at study inclusion was cough, occurring in 91.8% of patients (95.6% of outpatients, 91.0% of hospitalised cases; p=0.14), followed by fever (89.2% of all patients; 91.3% of outpatients versus 88.7% of hospitalised cases; p=0.57), tachypnoea (62.0% of all patients; 54.3% of outpatients versus 64.1% of hospitalised cases; p=0.09) and chest retractions (55.5% of all patients and 21.3% of outpatients versus 65.7% of hospitalised cases; p<0.0001). In addition, fluid intake reduction occurred more often in hospitalised cases than their outpatient counterparts (49.8% versus 1.03%, p<0.001). Auscultation abnormalities occurred in 91.1% of patients (91.3% of outpatients, 90.7% of hospitalised cases; p=1.0), with wheezing and/or extended expiration in 27.0% of all patients and 13.0% of outpatients versus 30.7% of hospitalised cases (p<0.001) (table 2).
For 425 (97%) children, data on antibiotic management were available. In 31.4% of these cases (33.4% of outpatients versus 22.8% of hospitalised cases; p=0.06), antibiotic treatment for paediatric CAP had already been initiated prior to study inclusion. Upon study inclusion, antibiotic treatment was changed or initiated in 87.4% (92.4% of outpatients versus 86.1% of hospitalised cases; p=0.11). Aminopenicillins were the most common initial antibiotic treatment (71.2% in all patients; 74.4% in inpatients, 60.0% in outpatients; p=0.01), followed by macrolides, which were prescribed in 19.6% of all cases. Macrolides were prescribed significantly more frequently in outpatients (31.8%) than in hospitalised patients (14.8%, p=0.001).
At day 14 after enrolment, 251 (65.4%) cases were reported to be healthy. Outpatients were more frequently symptom-free (83.1%) than hospitalised cases (57.7%, p<0.001) (table 2). In line with this, 90 days after inclusion, 91.0% of all patients were symptom-free, with a significantly higher rate in outpatients (98.8%) than in those with hospital treatment (88.8%, p=0.002) (table 2). At study inclusion, normal CrP values (<10 mg·L−1) were observed in 27% of cases. Mean (IQR) CrP value of all patients was 65.4 (8.9–84.5) mg·L−1, with significantly higher levels in hospitalised cases than outpatients (mean (IQR) 30.5 (4.9–52) mg·L−1 versus 74.9 (10–107) mg·L−1, p<0.001).
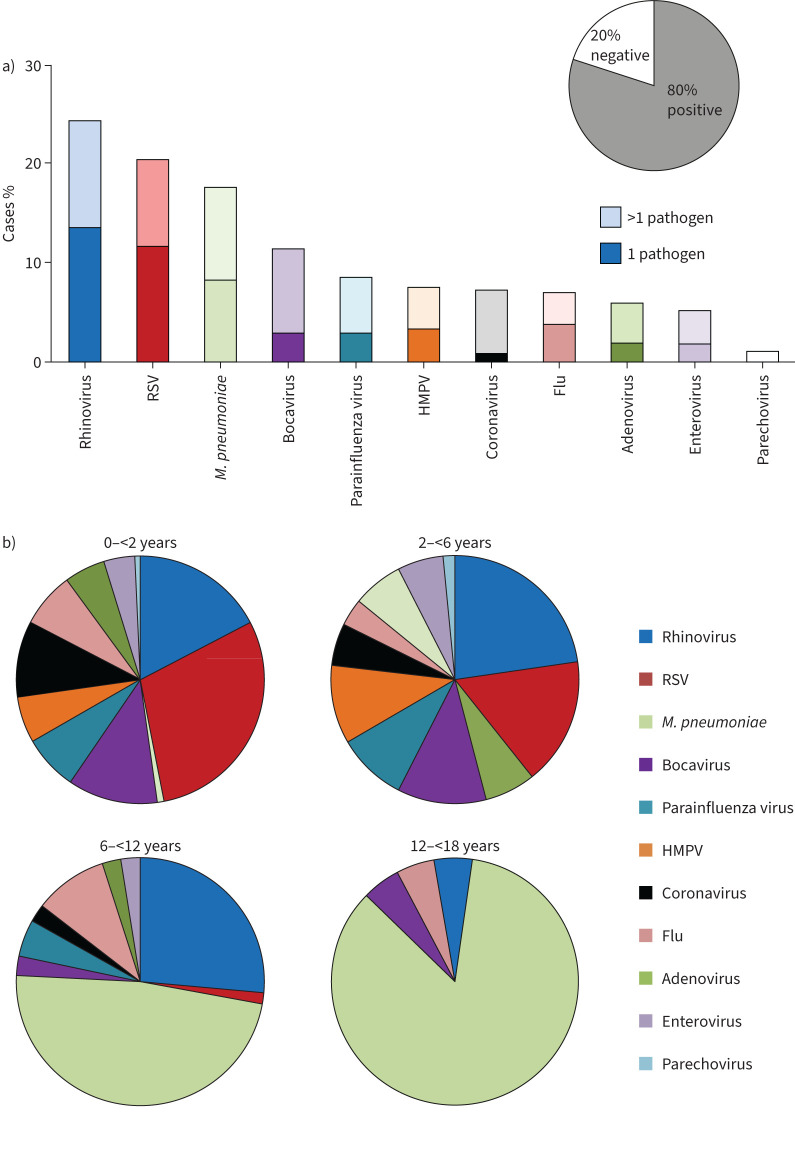
When we analysed pathogen occurrence within our cohort, at least one pathogen was detected in 90% of all cases. PCR testing of NPA or NPS was performed in 91.7% of cases, yielding positive results in 80.3% (figure 2a). The most commonly detected viral pathogens in PCR analyses were rhinovirus (24.2%), RSV (20.3%), bocavirus (11.3%), PIV (8.5%), HMPV (7.5%), coronavirus (not SARS-CoV-2; 7.2%), flu (6.9%), adenovirus (5.9%), enterovirus (5.1%) and parechovirus (1.0%) (figure 2a). In children aged <6 years (preschoolers), detection of viruses was significantly more common than in older children (82.1% versus 34.2%, p<0.001). RSV was the most common virus among preschoolers with paediatric CAP, with a significantly higher incidence in cases aged <6 years than in older patients (28.4% versus 0.9%, p<0.0001) (figure 2b). A similar pattern was observed for HMPV, which was detected in 10.5% of preschoolers, but in none of the older children in our cohort (p<0.001), for bocavirus (14.9% versus 2.6%, p<0.001), and for rhinovirus (25.8% versus 20.2%, p=0.3) (figure 2b). There was no statistical difference in the prevalence of flu between preschoolers and older children (6.5% and 7.9%, p=0.66) (figure 2b).
FIGURE 2.
Pathogen detection within the cohort. a) Results of pathogen detection by PCR analysis of nasopharyngeal specimens of the entire cohort. b) Age-dependent subgroup analysis of results. Bars display cumulative percentage with the darker/lower part of each bar representing singular pathogen and the lighter/upper part of each bar representing co-occurrence in mixed pathogen detection. RSV: respiratory syncytial virus; M. pneumoniae: Mycoplasma pneumoniae; HMPV: human metapneumovirus.
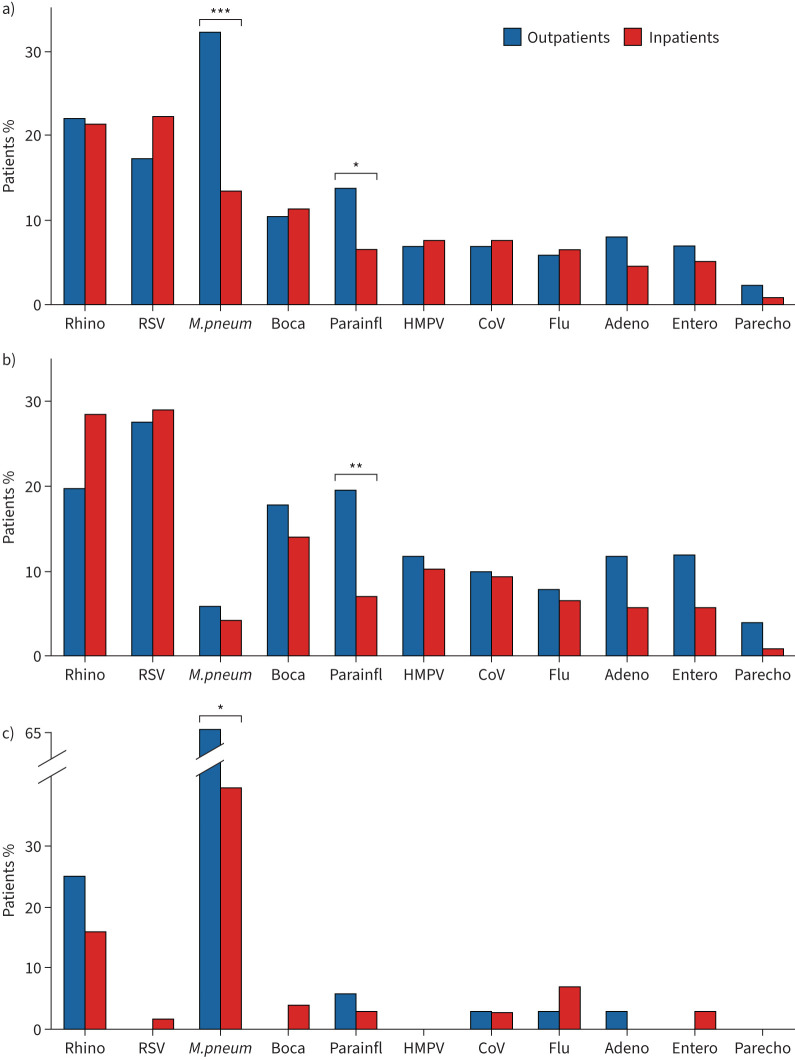
Virus pathogen detection was performed in 97.8% of outpatients and 86.7% of inpatients. Again, when we compared detection rates of viral pathogens via NPA- or NPS-PCR in outpatients versus hospitalised children, a higher rate of virus detection was observed for both patient groups in patients aged <6 years (86.0% for outpatients and 82.2% for inpatients, p=0.6768, and 28.6% for outpatients and 26.5% for inpatients aged >6 years, p=0.31) (figure 3b,c). For the entire cohort, M. pneumoniae and PIV occurred significantly more frequently in outpatients (31.1% versus 13.0% in hospitalised cases, p<0.01, and 13.3% versus 6.4% in hospitalised cases, p<0.05) (figure 3a). RSV was the leading viral pathogen in both hospitalised and non-hospitalised preschoolers (27.4% for outpatients and 29% for inpatients versus 0% in outpatients and 1% inpatients aged >6 years; p=0.0003 and p<0.0001, respectively), whereas rhinovirus was the most prevalent virus in inpatients and outpatients aged >6 years (15.8% and 25.0% versus 20.0% and 28.5% in outpatients and inpatients aged <6 years; p=0.29 and p=0.3, respectively) (figure 3b). Most virus detection rates were not significantly different between patients with or without hospital treatment, but PIV was detected significantly more frequent in non-hospitalised preschoolers (20.0% versus 7.0% of hospitalised pre-schoolers; p=0.04) (figure 3b).
FIGURE 3.
Pathogen detection in outpatient-treated versus hospitalised paediatric community-acquired pneumonia cases. a) Detection rates of pathogens in all outpatients versus hospital-treated. b,c) Detection rates of pathogens in patients aged b) <6 years and c) 6–18 years with outpatient and hospital treatment. Data are presented as cumulative percentages. Rhino: rhinovirus; RSV: respiratory syncytial virus; M. pneum: Mycoplasma pneumoniae; Boca: bocavirus; Parainfl: parainfluenza virus; HMPV: human metapneumovirus; CoV: coronavirus; flu: influenza; adeno: adenovirus; entero: enterovirus; parecho: parechovirus. *: p<0.05, **: p<0.01, ***: p<0.001.
In total, in 18.7% of all cases, bacterial pathogens were detected by airway specimen cultures or Ig-screening. NPA- or NPS-PCR for M. pneumoniae was positive in 17.5% of the total study cohort (13.0% of hospitalised cases and 31.1% of outpatients, p<0.001), with a significantly higher incidence in children aged ≥6 years than in preschoolers (47.4% versus 5.0%, p<0.001) (figure 3a) and the highest incidence in children aged ≥12 years (50.0%). In line with this, M. pneumoniae was also the leading pathogen in both inpatient and outpatients in the age group ≥6 years, where it was detected significantly more frequently in outpatients than in hospital-treated cases (63.9% versus 39.5%, p<0.05) (figure 3c). For further bacterial diagnostics, 116 blood cultures were performed (98.3% of them in hospitalised patients), which were positive for bacterial pathogens in 5.1% (all hospitalised cases). Here, Streptococcus pneumoniae was the most common strain (50% of positive cultures). With regard to lower respiratory tract analyses, 30 sputum specimens (n=9 in outpatients) and 13 BALF specimens were obtained. Overall, 54.5% of positive sputum cultures resulted from growth of Staphylococcus aureus, S. pneumoniae and H. influenzae (50.0% in outpatients and 57.1% hospitalised children). In 39% of patients with BALF sampling, bacterial pathogens were detected, with H. influenzae as the leading pathogen (40%). In those cases where pleural fluid was collected (n=24, all hospitalised patients), Streptococcus pyogenes and Staph. aureus were most commonly detected (22% each). In 4.5% of patients, both viral and bacterial pathogens were detected (4.6% in hospitalised children and 2.2% in outpatients).
Discussion
Our study is one of the first to address the full spectrum of radiographically confirmed paediatric CAP based on a cohort consisting of both non-hospitalised and hospitalised patients. The rates of clinical symptoms such as cough (91.8%), fever (89.2%) and chest retractions (55.5%) observed in our cohort were in accordance with those observed in a previous hallmark study on hospitalised paediatric CAP which found cough in 95.6%, fever in 91% and chest retractions in 45% of cases [5]. In accordance with previous studies [5, 28], paediatric CAP-related hospitalisation rates in our cohort were highest in infants and toddlers. As expected, hospitalised children were significantly younger and presented with significantly lower SpO2 and higher rates of cyanosis, pulmonary obstruction, chest retractions, impaired liquid intake and CrP levels than outpatients.
The analyses of pathogen spectra within our cohort confirmed previous findings on the relevance of viral pathogens in paediatric CAP [4, 5]. In both inpatients and outpatients, viral pathogens were highly prevalent. Compared to previous studies, the incidence of viral pathogens observed in our analysis (68.1% of all patients; 82.1% of children aged <6 years) is within the range described in previous large paediatric CAP cohorts [4, 5], although a broad range of 18.7% to 91.0% detection rates of viral pathogens in paediatric CAP has been described [12]. In our examinations, rhinovirus and RSV were the most commonly detected viruses with around 25% detection rate each. This is in line with previous reports on the high prevalence of these pathogens in the airways of paediatric CAP patients [4, 5] and fits to previous reports on the relevance of RSV particularly in young children with paediatric CAP [5, 28]. Interestingly, we show that RSV belongs to the most frequent pathogens also in paediatric CAP outpatients, particularly in those aged <6 years. Our analyses also confirm previous reports on the high rate of M. pneumoniae positive paediatric CAP patients, particularly in older children and adolescents. In children aged ≥12 years, bacterial pathogens were more prevalent, with a 42.8% overall detection rate. The increasing relevance of bacterial pathogens, particularly of M. pneumoniae in older children and adolescents, is in accordance with previous publications on the high rate of M. pneumoniae paediatric CAP in this age group [5, 28]. Furthermore, our data illustrate that this pathogen is significantly more prevalent in mild paediatric CAP, as a striking 63.9% of children aged ≥6 years with outpatient treatment in our cohort displayed a positive NPA- or NPS-PCR for M. pneumoniae.
The particular strength of our cohort lies in the broad inclusion of paediatric CAP patients with and without hospitalisation. Only around one-fourth of CAP cases in Western Europe are hospitalised and the majority of paediatric CAP cases are treated as outpatients [5, 28]. Almost all recent studies in the field of paediatric CAP focused on hospitalised patients [4, 5, 16, 28–30], but this is insufficient to understand the full spectrum of paediatric CAP aetiology. Therefore, we also included outpatients in our study. As we did not focus on an epidemiological study and therefore did not perform systematic screening for paediatric CAP, outpatients are outnumbered. We systematically compare the occurrence of viral and bacterial infections in hospitalised and non-hospitalised paediatric CAP and show that in outpatients, viral pathogens are also highly prevalent, with pathogen spectra similar to hospitalised paediatric CAP cases. In fact, our age-specific comparative analyses of pathogen spectra in inpatients versus outpatients yielded few differences in the prevalence of specific pathogens. In spite of the high rate of viral infections, overall, 87.4% of children in our cohort received antibiotic treatment; aminopenicillins and macrolides were the most prevalent anti-infective agents administered to our patients. This is similar to observations of other groups, although antibiotics prescription behaviour of physicians varies strongly and is impacted by regional and economic differences [4, 5, 16, 28]. Particularly in the youngest group of our cohort (aged <2 years), with the highest detection rate of viral pathogens, a strikingly high rate of 83% antibiotic treatment occurred (data not shown). This discrepancy of high virus detection rates and frequent use of antibiotic therapy in paediatric CAP supports the notion of an assumed high rate of unjustified or ineffective antibiotic treatment in mild paediatric CAP and emphasises the need for updated management guidelines and advocate for stringent antibiotic stewardship in this disease [31].
Our study has certain limitations. Firstly, although our study design aims at a well-balanced patient recruitment with regard to hospitalised and non-hospitalised cases, only approximately one-fifth of patients were outpatient cases. As such, numbers in age- and pathogen-specific subgroups of this subgroup were low in some analyses. In addition, due to the high rate of patients recruited upon hospital admission, a high proportion of young children (75% aged <6 years) were enrolled, impeding statistical power in older age groups. Although our recruiting pattern somewhat reflects the previously described age distribution of paediatric CAP with high disease burden in infants [4, 5], it may have biased our analyses, particularly with regard to mild cases and older children. Although the pedCAPNETZ study group has partnered with paediatric outpatient practices to increase recruitment of patients with mild disease course, most cases were recruited in tertiary care centres. Moreover, recruitment did not follow a stringent screening routine, but were collected as a convenience sample. Both factors may lead to a patient selection with a more severe phenotype, and may also explain why pathogen detection is at the higher end of the range compared to previous studies [4, 5, 7, 12, 18].
Another limitation of our work may be the fact that pedCAPNETZ is a case-only study without recruitment of control probands. However, a plethora of case–control or healthy-only studies analysing airway pathogens in children exist and were taken into account in our work [4, 5, 7, 12, 18, 32–38].
Paediatric CAP still poses diagnostic, aetiological and therapeutic challenges. In future, improved viral diagnostic approaches will become increasingly available and may help to limit the overuse of antibiotics in children. Future analyses of the pedCAPNETZ study group will aim to correlate upper and lower airway tract pathogens, appropriateness and effectiveness of microbial treatment and pathogen-specific paediatric CAP outcomes.
Conclusion
The pedCAPNETZ study addresses the full spectrum of paediatric CAP. Here, we present first data of a cohort with hospitalised and non-hospitalised children. Despite significant clinical and CrP value differences, both groups show comparable viral pathogen distributions. Independently of hospitalisation status and pathogen detection, the majority of children in both groups received antibiotic treatment. Particularly in young patients with mild disease, viruses are the leading cause of paediatric CAP. This should be taken in account by clinicians when considering the use of antibiotics in the care of a child with pneumonia.
Acknowledgements
The authors would like to thank all the patients and families for their contribution. They thank all persons involved in the recruitment and care of the patients, data storage, laboratory work and biobanking. Particularly, they thank Nadine Alfeis, Margarete Nawrocki, Nicole Rahmanian, Jana Bergmann, Anika Dreier, Malik Aydin and Maike Haas for technical support and assistance (Hannover Medical School, Hannover, Germany), and Rebecca Lopez-Winter (Cambridge, UK) for the linguistic revision as a native English speaker.
Provenance: Submitted article, peer reviewed.
Data availability: Data and results of biomaterials underlying this analysis will be available by formal request, which will be evaluated by the study board. Requests can be directed to the corresponding author by e-mail.
Conflict of interest: M. Wetzke received funding from the Young Academy Clinician/Scientist foundation Hannover Medical School, Germany and the Clinical Leave Clinician/Scientist program of the German Center for Infection Research (DZIF). K. Schütz and G. Hansen received funding from the Deutsche Forschungsgemeinschaft for the Cluster of Excellence EXC2155 “RESIST” project (ID 39087428). C. Happle received funding from the Young Academy Clinician/Scientist foundation and HiLF funding of Hannover Medical School, Germany, and the Excellence Cluster RESIST in infection research. M.V. Kopp, G. Hansen and T. Welte received funding from the German Center for Lung Research (DZL). The other authors received no additional funding. The authors have no conflicts of interests for this article to disclose.
Support statement: This project was supported by the German Center for Lung Research by funding for biosampling and infrastructure. The funder/sponsor did not participate in the work.
References
- 1.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015; 385: 430–440. doi: 10.1016/S0140-6736(14)61698-6 [DOI] [PubMed] [Google Scholar]
- 2.Gesundheitsberichterstattung des Bundes . Diagnosedaten der Krankenhäuser ab 2000 [Hospital Diagnostic Data Since 2000]. https://www.gbe-bund.de/gbe/!pkg_olap_tables.prc_set_orientation?p_uid=gast&p_aid=80676656&p_sprache=D&p_help=2&p_indnr=594&p_ansnr=30916473&p_version=4&D Date last accessed: 14 December 2022.
- 3.Weigl JA, Puppe W, Belke O. The descriptive epidemiology of severe lower respiratory tract infections in children in Kiel, Germany. Klin Padiatr 2005; 217: 259–267. doi: 10.1055/s-2004-820352 [DOI] [PubMed] [Google Scholar]
- 4.Pneumonia Etiology Research for Child Health (PERCH) Study Group. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet 2019; 394: 757–779. doi: 10.1016/S0140-6736(19)30721-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain S, Williams DJ, Arnold SR. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015; 372: 835–845. doi: 10.1056/NEJMoa1405870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madhi SA, De Wals P, Grijalva CG. The burden of childhood pneumonia in the developed world: a review of the literature. Pediatr Infect Dis J 2013; 32: e119–e127. doi: 10.1097/INF.0b013e3182784b26 [DOI] [PubMed] [Google Scholar]
- 7.Katz SE, Williams DJ. Pediatric community-acquired pneumonia in the United States: changing epidemiology, diagnostic and therapeutic challenges, and areas for future research. Infect Dis Clin North Am 2018; 32: 47–63. doi: 10.1016/j.idc.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardoso MR, Nascimento-Carvalho CM, Ferrero F. Adding fever to WHO criteria for diagnosing pneumonia enhances the ability to identify pneumonia cases among wheezing children. Arch Dis Child 2011; 96: 58–61. doi: 10.1136/adc.2010.189894 [DOI] [PubMed] [Google Scholar]
- 9.Shah SN, Bachur RG, Simel DL. Does this child have pneumonia? The rational clinical examination systematic review. JAMA 2017; 318: 462–471. doi: 10.1001/jama.2017.9039 [DOI] [PubMed] [Google Scholar]
- 10.Lynch T, Platt R, Gouin S. Can we predict which children with clinically suspected pneumonia will have the presence of focal infiltrates on chest radiographs? Pediatrics 2004; 113: e186–e189. doi: 10.1542/peds.113.3.e186 [DOI] [PubMed] [Google Scholar]
- 11.Rose MA, Barker M, Liese J. S2k-Leitlinie Management der ambulant erworbenen Pneumonie bei Kindern und Jugendlichen (pädiatrische ambulant erworbene Pneumonie, pCAP). [Guidelines for the management of community acquired pneumonia in children and adolescents (pediatric community acquired pneumonia, pCAP)]. Pneumologie 2020; 74: 515–544. doi: 10.1055/a-1139-5132 [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Cai F, Wu X. Incidence of viral infection detected by PCR and real-time PCR in childhood community-acquired pneumonia: a meta-analysis. Respirology 2015; 20: 405–412. doi: 10.1111/resp.12472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grijalva CG, Nuorti JP, Arbogast PG. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 2007; 369: 1179–1186. doi: 10.1016/S0140-6736(07)60564-9 [DOI] [PubMed] [Google Scholar]
- 14.Nelson JC, Jackson M, Yu O. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine 2008; 26: 4947–4954. doi: 10.1016/j.vaccine.2008.07.016 [DOI] [PubMed] [Google Scholar]
- 15.Griffin MR, Zhu Y, Moore MR. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013; 369: 155–163. doi: 10.1056/NEJMoa1209165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pírez MC, Algorta G, Chamorro F. Changes in hospitalizations for pneumonia after universal vaccination with pneumococcal conjugate vaccines 7/13 valent and Haemophilus influenzae type b conjugate vaccine in a pediatric referral hospital in Uruguay. Pediatr Infect Dis J 2014; 33: 753–759. doi: 10.1097/INF.0000000000000294 [DOI] [PubMed] [Google Scholar]
- 17.Virkki R, Juven T, Rikalainen H. Differentiation of bacterial and viral pneumonia in children. Thorax 2002; 57: 438–441. doi: 10.1136/thorax.57.5.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juvén T, Mertsola J, Waris M. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J 2000; 19: 293–298. doi: 10.1097/00006454-200004000-00006 [DOI] [PubMed] [Google Scholar]
- 19.Cevey-Macherel M, Galetto-Lacour A, Gervaix A. Etiology of community-acquired pneumonia in hospitalized children based on WHO clinical guidelines. Eur J Pediatr 2009; 168: 1429–1436. doi: 10.1007/s00431-009-0943-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poole NM, Shapiro DJ, Kronman MP. Ambulatory antibiotic prescribing for children with pneumonia after publication of national guidelines: a cross-sectional retrospective study. Infect Dis Ther 2020; 9: 69–76. doi: 10.1007/s40121-019-00276-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Maat JS, Garcia Perez D, Driessen GJA. The influence of chest X-ray results on antibiotic prescription for childhood pneumonia in the emergency department. Eur J Pediatr 2021; 180: 2765–2772. doi: 10.1007/s00431-021-03996-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris M, Clark J, Coote N. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 2011; 66: Suppl. 2, ii1–ii23. doi: 10.1136/thoraxjnl-2011-200598 [DOI] [PubMed] [Google Scholar]
- 23.Wetzke M, Kopp MV, Seidenberg J. PedCAPNETZ – prospective observational study on community acquired pneumonia in children and adolescents. BMC Pulm Med 2019; 19: 238. doi: 10.1186/s12890-019-1013-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah VP, Tunik MG, Tsung JW. Prospective evaluation of point-of-care ultrasonography for the diagnosis of pneumonia in children and young adults. JAMA Pediatr 2013; 167: 119–125. doi: 10.1001/2013.jamapediatrics.107 [DOI] [PubMed] [Google Scholar]
- 25.Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics 2015; 135: 714–722. doi: 10.1542/peds.2014-2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voigt GM, Thiele D, Wetzke M. Interobserver agreement in interpretation of chest radiographs for pediatric community acquired pneumonia: findings of the pedCAPNETZ-cohort. Pediatr Pulmonol 2021; 56: 2676–2685. doi: 10.1002/ppul.25528 [DOI] [PubMed] [Google Scholar]
- 27.Cherian T, Mulholland EK, Carlin JB. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ 2005; 83: 353–359. [PMC free article] [PubMed] [Google Scholar]
- 28.Shan W, Shi T, Zhang X. Hospitalization rate and population-based incidence of hospitalization for community-acquired pneumonia among children in Suzhou, China. Pediatr Infect Dis J 2018; 37: 1242–1247. doi: 10.1097/INF.0000000000002016 [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Miao C, Chen Y. Age-specific risk factors of severe pneumonia among pediatric patients hospitalized with community-acquired pneumonia. Ital J Pediatr 2021; 47: 100. doi: 10.1186/s13052-021-01042-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feinstein Y, Greenberg D, Ben-Shimol S. Characterization of children younger than 5 years of age with severe community-acquired alveolar pneumonia (CAAP) requiring pediatric intensive care unit (PICU) admission. Pediatr Neonatol 2020; 61: 406–413. doi: 10.1016/j.pedneo.2020.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreitmeyr K, von Both U, Pecar A. Pediatric antibiotic stewardship: successful interventions to reduce broad-spectrum antibiotic use on general pediatric wards. Infection 2017; 45: 493–504. doi: 10.1007/s15010-017-1009-0 [DOI] [PubMed] [Google Scholar]
- 32.Self WH, Williams DJ, Zhu Y. Respiratory viral detection in children and adults: comparing asymptomatic controls and patients with community-acquired pneumonia. J Infect Dis 2016; 213: 584–591. doi: 10.1093/infdis/jiv323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin ET, Fairchok MP, Kuypers J. Frequent and prolonged shedding of bocavirus in young children attending daycare. J Infect Dis 2010; 201: 1625–1632. doi: 10.1086/652405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spuesens EB, Fraaij PL, Visser EG. Carriage of Mycoplasma pneumoniae in the upper respiratory tract of symptomatic and asymptomatic children: an observational study. PLoS Med 2013; 10: e1001444. doi: 10.1371/journal.pmed.1001444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf DG, Greenberg D, Shemer-Avni Y. Association of human metapneumovirus with radiologically diagnosed community-acquired alveolar pneumonia in young children. J Pediatr 2010; 156: 115–120. doi: 10.1016/j.jpeds.2009.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright PF, Deatly AM, Karron RA. Comparison of results of detection of rhinovirus by PCR and viral culture in human nasal wash specimens from subjects with and without clinical symptoms of respiratory illness. J Clin Microbiol 2007; 45: 2126–2129. doi: 10.1128/JCM.02553-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jartti T, Lee WM, Pappas T. Serial viral infections in infants with recurrent respiratory illnesses. Eur Respir J 2008; 32: 314–320. doi: 10.1183/09031936.00161907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honkinen M, Lahti E, Österback R. Viruses and bacteria in sputum samples of children with community-acquired pneumonia. Clin Microbiol Infect 2012; 18: 300–307. doi: 10.1111/j.1469-0691.2011.03603.x [DOI] [PMC free article] [PubMed] [Google Scholar]