Abstract
BACKGROUND AND AIMS:
Destroying visceral sensory nerves impacts pancreatic islet function, glucose metabolism and diabetes onset, but how islet endocrine cells interact with sensory neurons has not been studied.
METHODS:
We characterized the anatomical pattern of pancreatic sensory innervation by combining viral tracing, immunohistochemistry, and reporter mouse models. To assess the functional interactions of beta cells with vagal sensory neurons, we recorded Ca2+ responses in individual nodose neurons in vivo while selectively stimulating beta cells with chemogenetic and pharmacologic approaches.
RESULTS:
We found that pancreatic islets are innervated by vagal sensory axons expressing Phox2b, substance P, calcitonin-gene related peptide, and the serotonin receptor 5HT3R. Centrally, vagal neurons projecting to the pancreas terminate in the commissural nucleus of the solitary tract. Nodose neurons responded in vivo to chemogenetic stimulation of beta cells and to pancreas infusion with serotonin, but were not sensitive to insulin. Responses to chemogenetic and pharmacological stimulation of beta cells were blocked by a 5HT3R antagonist and were enhanced by increasing serotonin levels in beta cells. We further confirmed directly in living pancreas slices that sensory terminals in the islet were sensitive to serotonin.
CONCLUSIONS:
Our study establishes that pancreatic beta cells communicate with vagal sensory neurons, likely using serotonin signaling as a transduction mechanism. Serotonin is co-released with insulin and may therefore convey information about the secretory state of beta cells via vagal afferent nerves.
Keywords: Visceral Sensory Innervation, Pancreatic Islet, Vagus Nerve, Serotonin
Short summary:
Our study established that insulin secreting beta cell of the pancreas communicate with sensory neurons of the vagus nerve, which in turns relay this information to the brain.
Graphical Abstract
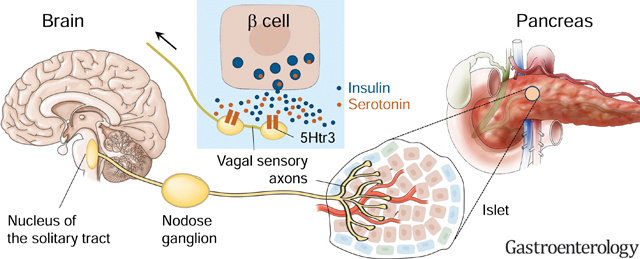
INTRODUCTION
Blood glycemia is a tightly regulated homeostatic parameter, controlled heavily by pancreatic islets of Langerhans, mini-organs whose endocrine cells secrete insulin and glucagon. At their discovery in the late 19th century, Paul Langerhans described that pancreatic islets are richly innervated.1 Claude Bernard had reported earlier that puncturing the floor of the fourth ventricle induces diabetes.2 Since then, it is generally assumed that the brain helps control glucose homeostasis, presumably via autonomic nerves that regulate pancreatic endocrine function. It is not until recently, however, that detailed neuroanatomical studies revealed how the efferent branches of the autonomic nervous system, the sympathetic and parasympathetic nerves, innervate distinct cell populations within the islet.3–5 By contrast, little is known about the sensory innervation of the islet. Because visceral sensory innervation is a crucial component of homeostatic regulatory circuits,6, 7 there is a need to understand how islets signal to sensory fibers.
A wide body of literature shows that chemical and genetic sensory denervation affects physiologic and pathophysiologic processes in the pancreas, including insulin secretion and diabetes onset.8–13 Little is known, however, about the basic patterns of pancreatic sensory innervation and its transduction mechanisms. Tracing studies showed that vagal afferent axons innervate the pancreas.14–18 In rodents, sensory fibers are localized preferentially to the periphery of the islet forming a dense superficial network.9, 19–21 Functional studies found that dispersed sensory afferent neurons of the vagal nodose ganglion respond to pancreatic and gastrointestinal stimuli, as well as to islet-derived stimuli.22, 23 These findings suggest that the vagus nerve could sense the islet microenvironment, yet this notion has not been explored. We still don’t know what activates the vagal islet-brain axis and how changes in the islet microenvironment affect vagus activity.
Serotonin is one of the strongest stimuli for vagal afferent neurons.24, 25 Within the pancreas, the insulin-producing beta cell is likely the sole source of serotonin. Beta cells secrete serotonin to communicate with neighboring cells.26–30 We therefore hypothesized that pancreatic islets use serotonin as a signaling molecule to communicate with the brain via vagal afferents. We used anatomical and physiological tools to characterize the sensory innervation patterns in the islet, to identify brain regions that receive pancreatic input, to examine neuronal response profiles in the nodose ganglion during pancreatic manipulation in vivo, and to demonstrate that vagal sensory neurons innervating the islet respond to serotonin released from beta cells.
MATERIAL AND METHODS
Mouse models
Experiments protocols were approved by the University of Miami Institutional Animal Care and Use Committee.
To characterize vagal sensory terminals, we crossed Phox2b-Cre mice (line3, Jax 016223) to floxed tdTomato reporter mice (Jax 007909). We used Htr3a-GFP mice [GENSAT, Tg(Htr3a-EGFP)DH30Gsat], which express GFP in cells expressing serotonin receptor 5HT3A. The pattern of transgene expression in both models was validated.31, 32 For in vivo Ca2+ imaging we used Snap25-GCaMP6s (Jax 025111) and Pirt-Cre mice crossed to floxed GCaMP6s mice (Jax 024106). For ex vivo Ca2+ imaging in peripheral sensory terminals, we used Pirt-GCaMP3 mice. While Pirt promoter is specific for sensory neurons,33 Snap25 is a pan-neuronal promoter.34 In these mouse models GCaMP was expressed in all neurons of vagal sensory ganglia. We used F1 heterozygous mice, from both sexes, 8–30 weeks old.
Nodose ganglion injection
Viral tracers were injected into the ganglion as previously described,6 delivering 2–4 pulses (69 nl each) of tracer. Animals were sacrificed 4 weeks post-surgery. For list of viral particles, see Supplementary Table 1.
Confocal imaging of the vagal sensory ganglion (nodose ganglion) in vivo
Mice were anesthetized with isoflurane (1.5–3%), fixed in a head holder (SG-4N, Narishige) in supine position, and tracheotomized to facilitate respiration. We accessed the left vagal ganglion by blunt dissection and imaged it on Leica TCS SP5 upright confocal microscope with resonant scanner, 10x/0.3NA dry objective (#11506505, Leica) in XYZT mode. The temporal resolution was 3 s, and spatial resolution of 512×512 pixels. GCaMP fluorescence was recorded at 488Ex/510–550Em. We checked ganglion blood perfusion by injecting intravenously DyLight 594 labeled lectin (DL-1067, Vector Labs, 594Ex/ 610–650Em) or 3kDa TRITC dextran (D3308, ThermoFisher, 568Ex/590–630Em).
Infusion of the pancreas through the common bile duct
We used a described surgical approach of pancreas infusion for tracer delivery to the pancreas and for pancreas stimulation during in vivo Ca2+ imaging.35 The pancreas was infused with tracers CTB (ThermoFisher, C22842), Fast Blue (Polysciences, 17740–1), AAVrg (Supplementary Table1) at a rate of 6 μl/min (total volume of 7.5 μl/g body weight). Tissues were collected one week after infusion of CTB and Fast Blue or four weeks after infusion of AAVrg. Tracers diffused throughout the pancreatic tissue but not beyond (Supplementary Figure 1).
For mechanical stimulation, we distended the pancreas with 300 μl of physiological buffer infused at 300 μl/min. For chemical stimulation without distension, 150 μl of stimulus was infused at 150 μl/min. Between stimuli, the pancreas was infused with buffer (5 μl/min, > 5 minutes) to wash out the stimulus and maintain ductal tonus. The volume infused per imaging session did not exceed 1 ml to avoid pancreatic tissue damage and leakage into the peritoneum.
Pancreas exteriorization and topical stimulation
Pancreas exteriorization and topical stimulation are illustrated in Supplementary Figure 2.
Chemogenetic stimulation of pancreatic beta cells
We injected Pirt-GCaMP6 mice intraperitoneally with an adeno-associated virus (AAV8-RIP-hM3Dq) encoding the excitatory DREADD receptor (hM3Dq) under a beta cell specific promoter. We used 414 basepairs of rat insulin-1 promoter followed by 691 basepairs of the rabbit beta-globin intron. This sequence achieves high levels of beta cell specific transgene expression.36, 37 We validated by immunohistochemistry that virally-delivered DREADD receptor was only expressed in pancreatic beta cells, and not in the surrounding exocrine tissue, duodenum, colon, liver, and spleen. Control animals were injected with an analogous virus encoding channel rhodopsin receptor instead of DREADD. Virally infected animals were used 4–7 weeks after injections.
Subdiaphragmatic vagotomy
We performed subdiaphragmatic truncal vagotomy on anesthetized Phox2b-Cre-tdTomatofloxed mice. Stomach and esophagus were exteriorized from the peritoneal cavity, and the dorsal and subdiaphragmatic vagal trunk were separated from the esophagus and cut off. Vagotomy was validated by evaluating stomach enlargement.
Confocal imaging of the nodose ganglion explants in vitro
Left nodose ganglia were dissected from Pirt-Cre-GCaMP6 mice, placed in a perfusion imaging chamber (Warner Instruments) filled with physiological buffer (125 mM NaCl, 5.9 mM KCl, 2.56 mM CaCl2, 1 mM MgCl2, 25 mM HEPES, 0.1% BSA, pH 7.4), and imaged on a Leica TCS SP5 upright confocal microscope.
Preparation and imaging of living pancreatic slices
Acute pancreatic slices were prepared from Pirt-GCaMP3 transgenic mice as previously described.5, 38
Biosensor cells to detect serotonin secretion
Real time measurements of serotonin secretion were performed using fura-2 AM-loaded Chinese hamster ovary (CHO) cells expressing 5-HT2C receptors as previously described.30
Intraperitoneal glucose tolerance test
Intraperitoneal glucose-tolerance tests were performed after overnight fasting. Mice were injected with 200–300 μl glucose solution (2 g/kg body weight), blood glucose was monitored at predetermined time points, plasma insulin levels were measure by ELISA (Mercodia, 10–11-32–01).
Immunohistochemistry
Collected tissues (pancreas, duodenum, right and left vagal sensory ganglia, dorsal root ganglia, and brain) were processed for immunostaining as frozen sections on the slide as described.5 Sensory ganglia were sectioned at 15–30 μm, while pancreas, brain and duodenum were sectioned at 40–50 μm. See Supplementary Table 2 for full list of primary antibodies. Confocal images of immunostained sections were acquired on an inverted Leica TCS SP5 confocal microscope.
Quantification of sensory innervation
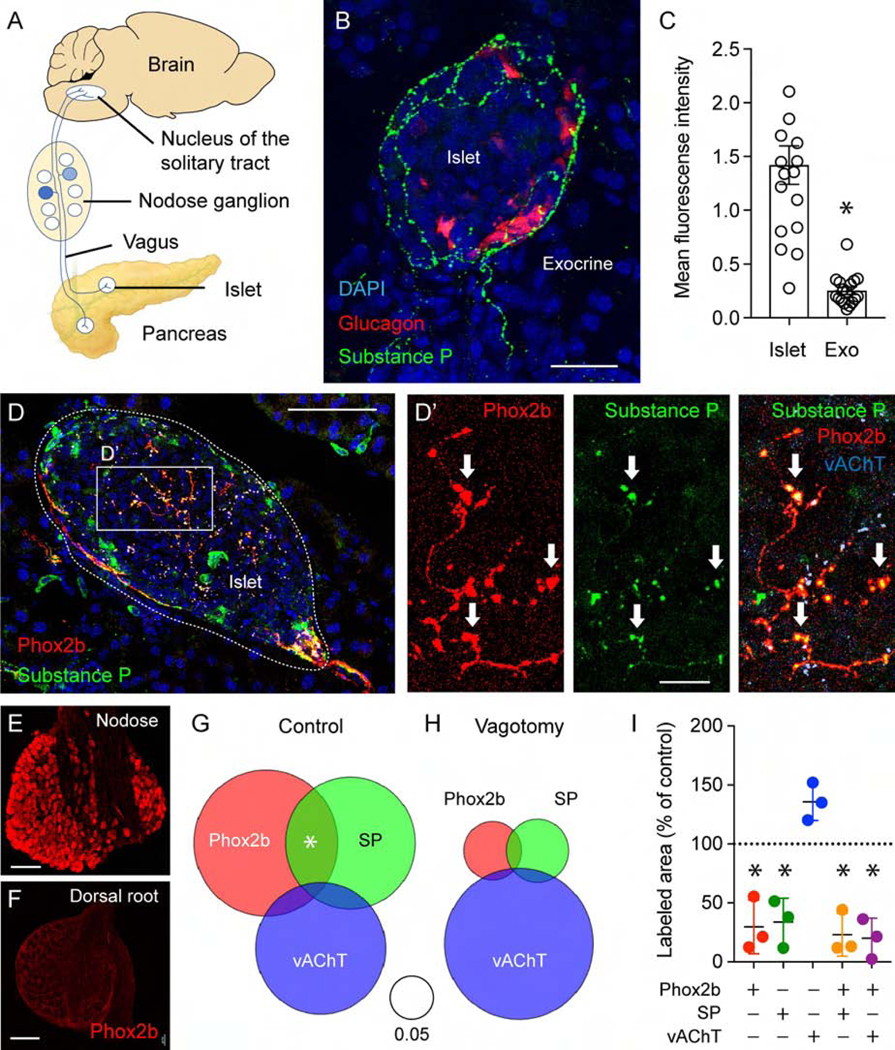
Immunohistochemistry images are presented as z-stack of confocal images, 8–10 images in a 40 μm section, z step ~2–4 μm. Regions of interest for pancreatic islets were selected based on DAPI staining, sensory innervation was quanified using ImageJ software (https://imagej.nih.gov/ij/). We quantified staining intensity as mean gray values of SP fluorescence (Figure 1C, Supplementary Figure 3) and staining areas for triple immunostaining for Phox2b, SP, and vAChT. Areas of each neuronal staining and overlap areas were normalized to islet area. Sums of normalized area values were plotted in Venn diagrams (Figure 1G–H).
Quantification of retrogradely traced neurons in the nodose ganglion
We used ImageJ software to estimate the number of neurons labeled with the retrograde tracer. Data were calculated as percentage of nodose ganglion neurons stained with general neuronal marker NeuN in confocal planes.
Quantification of cytosolic Ca2+ levels
We manually selected regions of interest (ROIs) around individual nodose ganglion neurons. ROI selection was biased towards bright and responding neurons. We measured changes in mean GCaMP fluorescence intensity using ImageJ. Changes in fluorescence intensity were expressed as percentage changes over baseline (dF/F). The baseline was defined as the mean of the intensity values during the non-stimulatory period. Data were displayed as heatmaps to include all recorded neurons. We further reported percentages of responding neurons based on inspection of individual Ca2+ traces by tree blinded independent observers.
To analyze response kinetics, we averaged traces of the 15 top responding neurons and normalized these averages to 100%. Using GraphPad Prism, we fitted a sigmoidal curve over each average trace and calculated slope coefficient and time to peak (Supplementary Table 3).
We are aware that our studies are biased by the use of a Ca2+ indicator to measure neuronal activity. The results may reflect the kinetics of the reporter, not those of electrical activity, and may not detect transduction mechanism that do not rely on changes in Ca2+ concentration (e.g. Na+).
Data analyses
Sample sizes are indicated in main text, figure legends, or heatmaps (numbers in parentheses). Significance was determined using an unpaired Student’s t-test (Figures 1C, 4D and 5D), a one sample t-test comparison to a hypothetical value of 100 (Figure 1I), or using a two-tailed Mann-Whitney test (Figure 7G). All experiments involved biological, not technical replicates. We considered statistical significance when P values were lower than 0.05.
RESULTS
The mouse pancreas is innervated by vagal afferent neurons
We investigated the pancreatic distribution of the sensory neuronal marker substance P across mouse strains using immunohistochemistry. The density of substance P immunoreactive fibers within the islet was significantly higher than in the surrounding exocrine tissue (Figure 1B–C). We did not observe gender or strain differences within the analyzed mouse strains (129, BALB6-C, C57Bl6; Supplementary Figure 3). However, males of the non-obese diabetic (NOD) mouse strain had a significantly lower density of sensory fibers within the islet (Supplementary Figure 3).
Figure 1.
Mouse pancreatic islets are innervated by vagal sensory fibers. (A) Cartoon of the vagal islet-brain axis. (B) Mouse pancreas section showing the innervation pattern of substance P(SP)-positive sensory axons (green) in an islet, alpha cells labeled for glucagon (red). Shown are z-stacks of confocal images. (C) Quantification of the mean fluorescence intensity of SP immunostaining in the islet and the surrounding exocrine tissue (n = 18 mice, 3–7 islets per animal; mean +/− SEM, unpaired Student’s t-test; for data showing innervation densities in mice of different genders and strains, see Supplementary Figure 1). (D-D’) Pancreatic section from a Phox2b-Cre-tdTomatofloxed transgenic mouse, immunostained for RFP (red), SP (green), and vAChT (light blue), Dapi (dark blue). Arrows point at colocalization of Phox2b with SP. (E-F) Sections of the nodose (E) and thoracic dorsal root ganglia (F) from a Phox2b-Cre-tdTomatofloxed transgenic mouse, immunostained for RFP (red). (G-I) Quantification of immunostaining of pancreatic innervation (as in D). Staining areas for each neuronal marker and their overlap were normalized to the corresponding islet area (control n = 4 mice, vagotomy n = 3 mice, 28 islets in each group). Venn diagrams depict sums of the normalized area values. (I) Means of neuronal staining areas for mice of the vagotomy group, shown as percentage of the average area of the control population (mean +/− SEM, one sample t-test comparison to a hypothetical value of 100). Scale bars, (B) 20 μm, (D) 50 μm, (D’) 10 μm, and (E, F) 100 μm.
To identify the vagal component of the sensory innervation of the islet, we crossed tdTomato reporter mice to mice of the Phox2b-Cre line, which expresses Cre-recombinase in vagal neuronal populations (nodose ganglia and dorsal motor nucleus of vagus) but not in the dorsal ganglion, sympathetic chain, parasympathetic ganglia, or enteric nervous system.32 Indeed, Phox2b was detected in the nodose ganglion but not in T9-T13 dorsal root ganglia (Figures 1E and 1F). Phox2b-positive axonal fibers were identified in over 50% of the inspected pancreatic islets. A subset of Phox2b-positive fibers (~20%) also stained for substance P (SP; Figure 1 and Supplementary Figure 4). Inside the islet, few Phox2b-positive fibers stained for the cholinergic marker vesicular acetylcholine transferase (vAChT) (Figure 1 and Supplementary Figure 4), which is in line with Phox2b not being expressed in postganglionic parasympathetic neurons (Figure 1D–H).32 The few cholinergic fibers that did co-stain for Phox2b were seen contacting neuroinsular ganglia (Supplementary Figure 5), suggesting that these were efferent projections from the dorsal motor nucleus of the vagus.39 Subdiaphragmatic vagotomy reduced the Phox2b-positive sensory innervation by 80 %, confirming its vagal origin (Figure 1G–I). SP-positive sensory fibers in the islet did not always colocalize with Phox2b, suggesting a possible contribution of spinal afferents to the sensory innervation of the islet. Our data indicate that a substantial part of the sensory innervation of the pancreatic islet is of vagal origin.
We performed anterograde tracing by injecting viral tracers into the nodose ganglion, as previously described.6 We identified anterogradely traced fibers inside pancreatic islets 4 weeks after AAV8-CMV-mCherry injection into the ganglia of 5HT3R-GFP mice (Supplementary Figure 6, also see below) thus confirming previous findings.17 Traced fibers were also seen in the brain and duodenum, matching the pattern previously reported (Supplementary Figure 6).7 AdiN-Syn-mCherry, encoding synaptophysin-mCherry fusion protein, was injected into Pirt-Cre mice. One week after viral injection, we visualized synaptophysin-resembling mCherry puncta in pancreatic islets that were also positive for SP, thus indicating presence of synaptic terminals of vagal afferents in pancreatic islets (Supplementary Figure 6).
Pancreatic afferents project to the commissural nucleus of the solitary tract
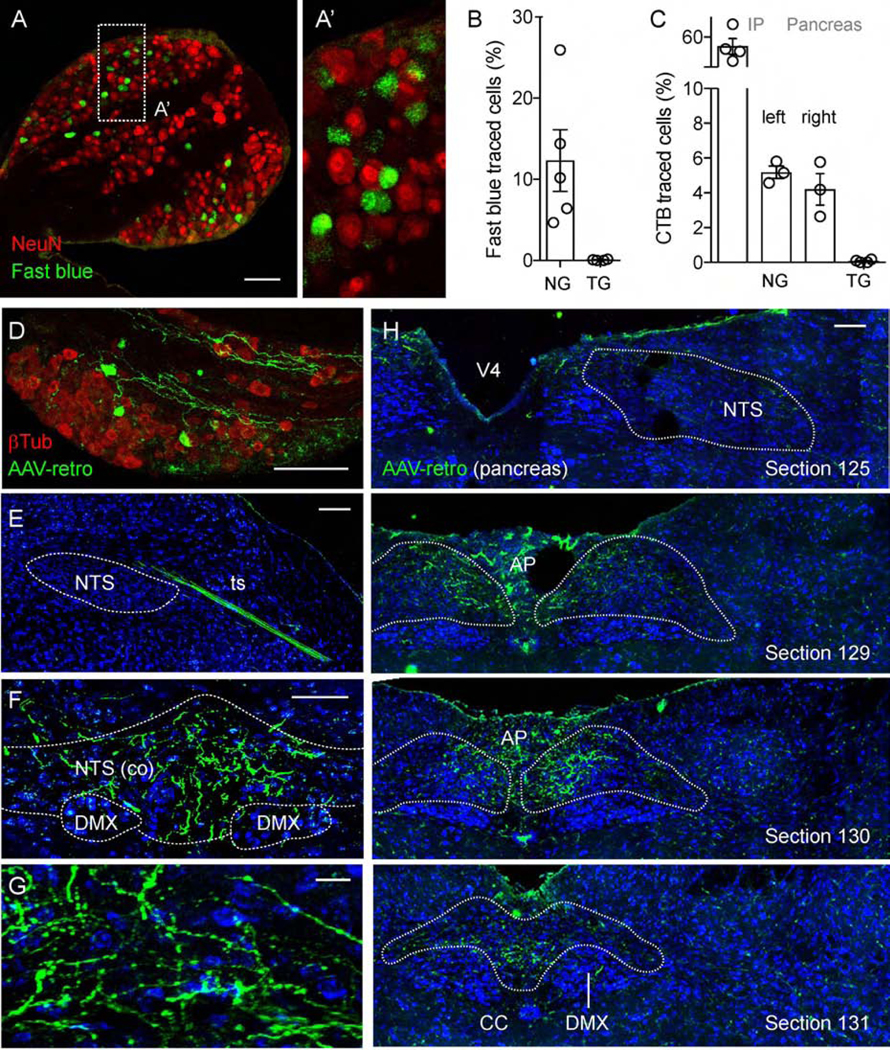
To identify vagal sensory neurons projecting to the pancreas and their terminal fields in the brain, we performed retrograde tracing by infusing the pancreas with tracers through the common bile duct, as previously described (Figure 2 and Supplementary Figure 1).35 We obtained similar results with fast blue (Figure 2A–B), cholera toxin subunit beta (CTB, Figure 2C), and retrograde AAV-hSyn-mCherry (Figure 2D) at the level of the nodose ganglion. CTB labeled 72 +/− 9 neurons in the right ganglion and 100 +/− 8 neurons in the left ganglion without a topographic organization. We estimated that 2–6% of nodose neurons were traced from the pancreas. By contrast, 50% of nodose ganglion neurons were traced when CTB was injected intraperitoneally (Figure 2C). Neurons in the trigeminal ganglion could not be traced from the pancreas (negative control, Figure 2C).
Figure 2.
Pancreatic vagal afferents projections to the nucleus of the solitary tract (NTS). (A-A’) Section of a mouse nodose ganglion showing retrogradely traced neurons (green), one week after intraductal pancreas infusion of the neuronal tracer fast blue. NeuN (red) visualizes all neurons. (B) Quantification of data as in A showing the percentage of neurons traced with Fast Blue in the left nodose ganglion (1034/8275 neurons) and trigeminal ganglion (0/5000 neurons, negative control); (n = 5 mice, 5–9 sections per ganglion). (C) Quantification of the percentage of neurons traced with cholera toxin B (CTB) in the left nodose ganglion after IP injection of the tracer (2252/4000 neurons, positive control); the left (348/6761 neurons) and right (178/4818 neurons) nodose ganglia and trigeminal ganglion (2/4000 neurons, negative control) after intraductal pancreas infusion of CTB (n = 3–4 mice, 5–9 sections per ganglion). (D-H) Mouse nodose ganglion (D) and brainstem sections (E-H) showing traced neurons and central terminals (green) 4 weeks after intraductal pancreas infusion of the neuronal tracer AAV-retro. β−Tubulin staining (red) visualizes all nodose neurons (D). Cytoarchitectural boundaries in E-H were identified with Nissl stain (blue, representative of 5 animals). A rostral brainstem section shows a traced neuronal bundle in the tractus solitarius (ts), not terminating in the rostral NTS (E). Sections at the level of the caudal commissural NTS contain terminal fields of neurons traced from the pancreas (F-G). Images of mouse brainstem sections from rostral to caudal are shown in H (section numbers correspond to those of the Allen reference atlas). Scale bars, (all but G) 100 μm and (G) 10 μm.
The retrograde AAV-hSyn-mCherry tracer efficiently traced the whole pathway from the pancreas to the brain (Figure 2E–H; Supplementary Figure 7). We found that the central projections of pancreas-innervating vagal sensory neurons mostly terminated in the commissural regions of the caudal nucleus of the solitary tract, but detailed analyses are still needed to determine the exact location of these terminals in the nucleus.
In vivo imaging of neuronal activity in the intact nodose ganglion in response to pancreas-specific stimulation
To characterize the sensory innervation of the pancreas physiologically, we adapted a technique for in vivo imaging of the nodose ganglion (Figure 3A–C, Movie S1).7 Specific sensory neuronal markers for the population of vagal neurons innervating the pancreas have not been identified yet. Thus, we recorded activity from all nodose neurons using mice expressing the Ca2+ indicator GCaMP6 driven by the general neuronal promoters Pirt or Snap25 (Figure 3C).33, 34 There was no difference in Ca2+ responses between the two strains. To stimulate the pancreas specifically and control for non-specific responses induced outside of the pancreas, we infused stimuli either through the common bile duct (intraductally) or applied them topically to the exteriorized pancreas (Figure 3A and Supplementary Figures 1 and 2). Intraductal administration of stimuli accesses the pancreas “inside out”, allowing the stimuli to fill the pancreatic ductal system and diffuse into the periductal space. Topical stimulus administration, by contrast, accesses the pancreas through “outside in” diffusion. Using these approaches, responses with a strong signal to noise ratio were recorded in nodose ganglion neurons (Figure 3D–E).
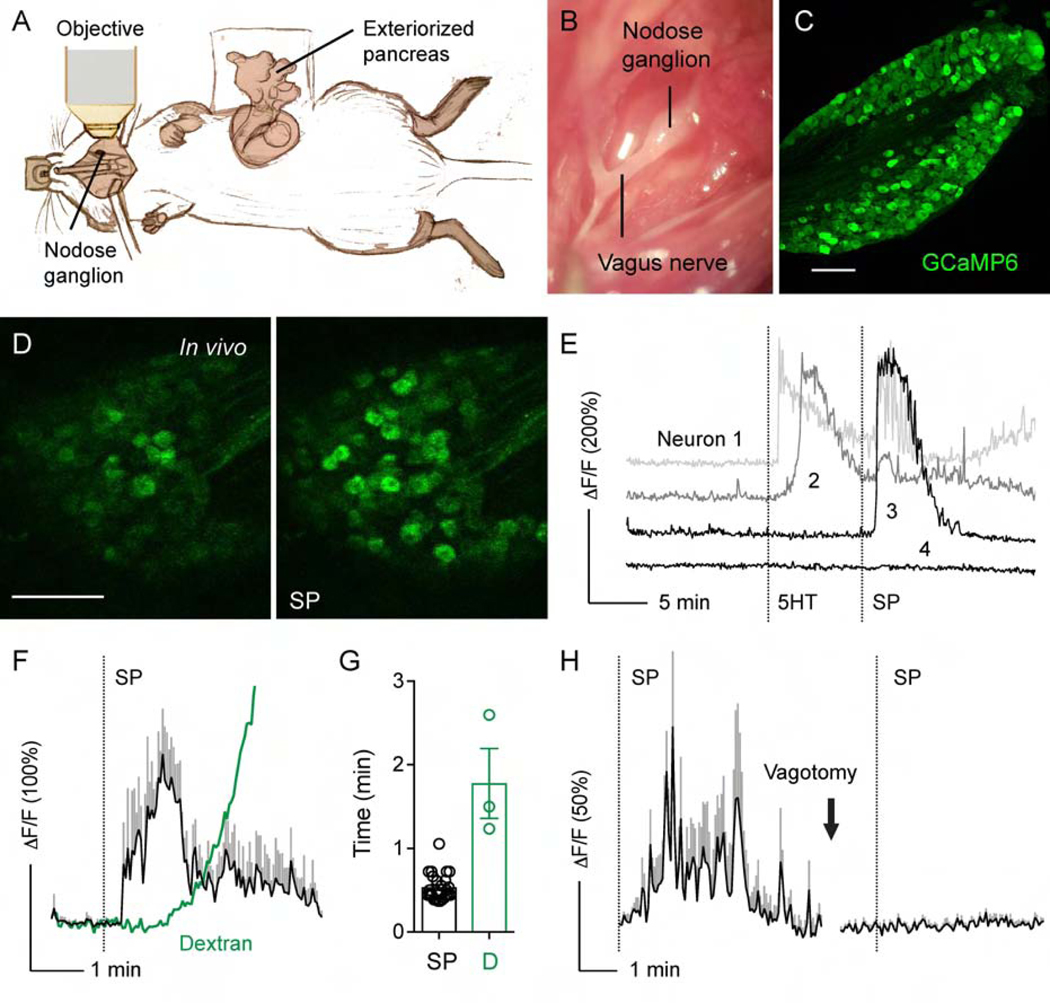
Figure 3.
In vivo imaging of the intact nodose ganglion in response to pancreas-specific stimulation. (A) Cartoon illustrating the experimental setup, where the intact nodose ganglion is exposed for Ca2+ imaging under a confocal microscope and the pancreas is exteriorized for topical stimulus application. See Supplementary Figures 1 and 2 for detailed visualization of pancreas stimulation approaches. (B) Microphotograph of the exposed nodose ganglion. (C) Section of the nodose ganglion from a Pirt-GCaMP6 mouse, which expresses the Ca2+ indicator GCaMP6 in all nodose ganglion neurons. (D) Sequential images of nodose ganglion neurons displaying Ca2+ responses (changes in GCaMP6 fluorescence, green) to topical stimulation of the pancreas with substance P (SP). (E) Representative traces of neurons recorded in D responding to 5HT (1 mM), SP (100 μM), or both. (F) Traces comparing the time course of neuronal Ca2+ responses to substance P (SP, 100 μM, black, average +/− SEM of 5 neurons) with that of the diffusion of fluorescent dextran (3 kDa) into the blood stream (green). A mixture of both substances was applied topically to the exteriorized pancreas and their fluorescence intensities measured in the nodose ganglion. (G) Quantification of data as in F showing the latencies of the Ca2+ response to SP and dextran diffusion into blood vessels of the nodose ganglion (D). Latency of fluorescence appearance was calculated as the first time point after topical application at which the mean fluorescence intensity was at least 3 SD above baseline fluorescence fluctuation (n = 3 animals). (H) Vagotomy abolished Ca2+ responses to substance P (SP). Trace shows an average of the same 10 neurons before and after vagotomy (representative of 3 animals). Scale bars, 100 μm.
Controls for tracer leakage are necessary when injecting the viscera with tracers or applying stimuli.40 For both the topical and ductal approach, we performed control experiments using injection or application of two dyes of varying sizes, namely crystal violet (408 Da) and fluorescent dextran (3 kDa) (Supplementary Figures 1 and 2). Control dyes were localized exclusively to the exteriorized pancreas region (topical approach), did not reach the duodenum, liver, stomach or spleen (ductal approach). Diffusion to the duodenum or liver was prevented by clamping the duct at different regions.
To control for systemic distribution of topically applied substances, we applied a fluorescent dextran (3 kDa) together with a chemical stimulus (substance P, 100 μM) to the exteriorized pancreas. We recorded neuronal Ca2+ responses and the appearance of the dextran fluorescence in the ganglion vasculature (Figure 3F–G). The latency of the neuronal response was smaller than that of the appearance of a dextran in the circulation (32 +/− 2 s versus 107 +/− 25 s; Figure 3G). Cervical vagotomy eliminated responses to topical administration of substance P (Figure 3H; Movie S2). These observations indicate that topical administration of chemical substances to the pancreas elicits specific responses in peripheral axonal terminals. As shown below, the combination of two different stimulation approaches (intraductal and topical) together with proper controls made it possible to identify and study sensory mechanisms of vagal sensory transmission from the pancreas.
Selective stimulation of pancreatic beta cells induces a response in vagal sensory neurons
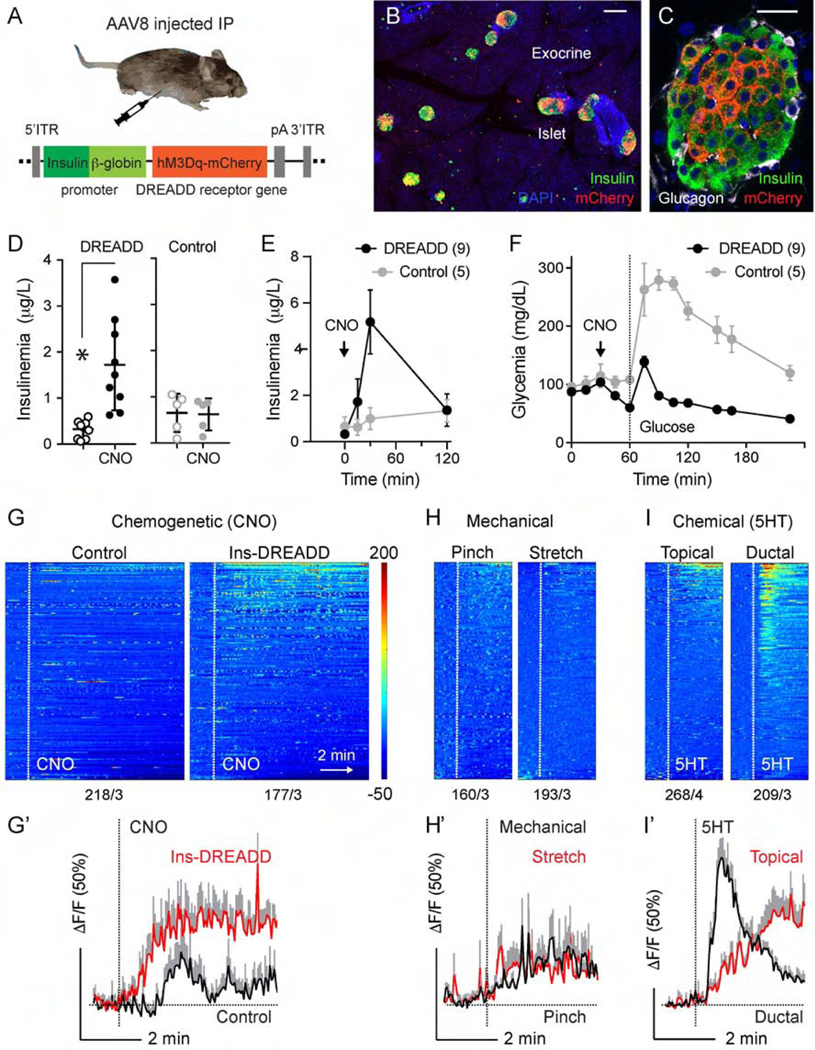
To selectively stimulate beta cells, we used a chemogenetic approach, where virally-delivered DREADD receptor was only expressed in the pancreatic beta cells (Figure 4B–C). Intraperitoneal injection of the DREADD agonist clozapine N-oxide (CNO; 5 mg/kg) increased insulin secretion under normoglycemic conditions and augmented insulin secretion upon glucose stimulation (Figure 4D–F). We recorded nodose ganglion responses to CNO-induced beta cell-specific stimulation in DREADD-expressing animals (> 20%, Figure 4G–I), responses to CNO were negligible in control mice (Figure 4G–G’) or in nodose ganglion explants in vitro (Supplementary Figure 8). Activity in CNO responsive neurons could be inhibited by the 5HT3R antagonist Y-25130 (1 mM, Supplementary Figures 9 and 12). Our results show that selective chemogenetic stimulation of pancreatic beta cells induces a robust response in nodose ganglion neurons.
Figure 4.
Chemogenetic stimulation of pancreatic beta cells activates vagal sensory neurons. (A) Cartoon of the chemogenetic AAV8 vector that was injected intraperitoneally into Pirt-GCaMP6 mice. (B-C) Pancreatic sections from AAV-8 infected mice, immunostained for RFP (DREADD-mCherry, red), insulin (green), Dapi (blue). (D) Changes in blood insulin levels in response to intraperitoneal injection of the DREADD agonist clozapine N-oxide (CNO, 5 mg/kg) in 12 h fasted DREADD-expressing (n = 9) and control (n = 5) mice (Student’s t-test). (E-F) Blood insulin levels and glucose excursion during an intraperitoneal glucose tolerance test preceded by CNO injection (arrow) in DREADD-expressing (n = 9) and control (n = 5) mice. (G-I) Heatmaps and average traces showing in vivo Ca2+ responses of nodose ganglion neurons to intraperitoneal administration of CNO in control and DREADD-expressing animals (G), to mechanical (pinch or stretch, H), and to chemical stimulation (5HT, I). Each row is a single cell, x-axis is time, and response magnitude dF/F (%) is shown in color scale, where fluorescence intensity increases from blue to red. Numbers of neurons/animals are indicated under each heatmap. G’-I’ are average traces (+/− SEM) of the top 15 responding neurons shown in G-I. Scale bars, (B) 100 μm and (C) 20 μm.
Because insulin and glucagon have been shown to activate nodose ganglion neurons,22, 23 we tested the effects of insulin and glucagon in vivo and in acutely dissected nodose ganglion explants in vitro (Supplementary Figure 8). A small percentage of nodose ganglion neurons showed small, delayed Ca2+ responses to hormonal stimulation in vitro, but responses could not be detected to ductal application of insulin in vivo. Our results suggest that pancreatic endocrine hormones may have a modulatory effect on sensory neurons of the nodose ganglion, but in vivo they are not likely to elicit direct and rapid neuronal activation.
Vagal afferent neurons innervating the pancreas are chemosensors
We assessed the responsiveness of vagal sensory neurons to mechanical and chemical stimulation of the pancreas (Figure 4, Supplementary Figure 8). Pancreata were stimulated mechanically either by a light pinch with forceps and gentle rinse of the exteriorized portion of the pancreas (mimicking the conditions of topical stimulus application) or by stretching the pancreas via intraductal injection of saline (twice the pressure at which chemical stimuli were applied intraductally). In contrast to what has been described for sensory neurons innervating the stomach, where ~70% of neurons respond to stretch,7 few neurons responded to mechanical distention of the pancreas (~16%, 31/193, n = 3 mice; Figure 4H–H’). The responses to mechanical stimuli were uncoordinated and were barely distinguishable from baseline neuronal activity. This lack of mechanical sensation is in line with the notion that the pancreas does not distend or contract physiologically.
We then explored the chemosensitive properties of pancreatic vagal afferents (Supplementary Figure 8). We selected stimuli known to activate receptors on sensory axons directly (substance P and serotonin), activate pancreas tissues (carbachol), or both (cerulein). Of 17 molecules tested, the aforementioned ones consistently elicited responses (Supplementary Table 4). We analyzed response kinetics by measuring time to peak and slope coefficients for each of the stimuli (Supplementary Table 3).
Mouse pancreatic islets contain and release serotonin
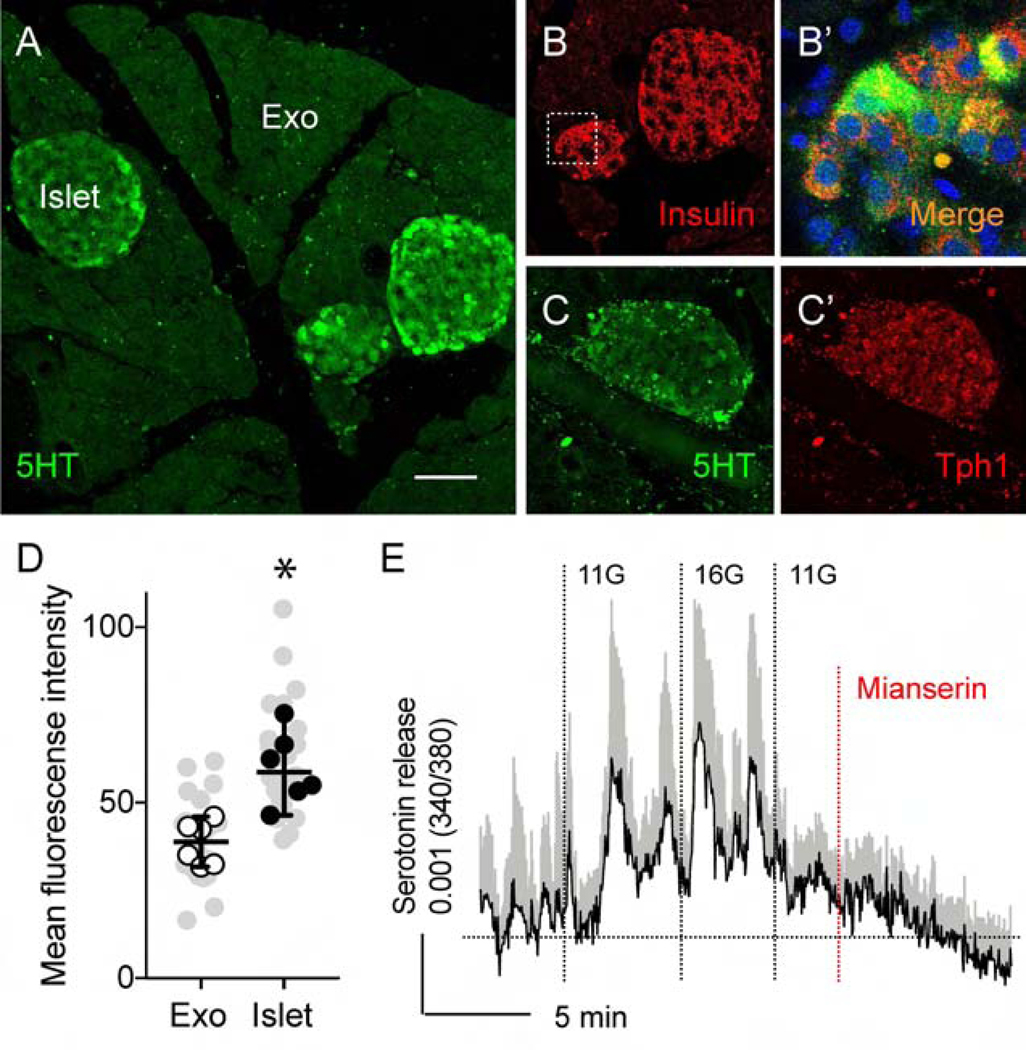
It is known that beta cells produce and secrete serotonin as a paracrine and autocrine signal.26, 27, 30 Basal beta-cell serotonin levels play a role in glucose stimulated insulin secretion, which is more pronounced in perinatal development, lacatation, pregnancy and under high fat diet-induced metabolic stress conditions.26, 27 We therefore investigated if under normal physiological conditions, serotonin is produced and released from mouse pancreatic islets (Figure 5). Serotonin immunostaining in pancreatic islets was present primarily in the beta cells (Figure 5B–B’) and was significantly higher than background levels in exocrine regions (Figure 5A and D, Supplementary Figure 10). We also detected the tryptophan hydroxylase-1 (Tph-1), the rate-limiting enzyme in serotonin synthesis, in islets (Figures 5C–C’).
Figure 5.
Mouse pancreatic islets contain and release serotonin. (A) Section of mouse pancreas showing that serotonin immunostaining (green) is limited to the islets and not detected in the exocrine tissue. (B) Section of mouse pancreas shown in A, immunostained for insulin (red). (B’) Enlarged view of the pancreatic islet shown in A and B, reflecting overlap in serotonin and insulin immunostaining (yellow). (C and C’) Section of mouse pancreas showing islet immunostained for serotonin (green, C) and Tph1 (red, C’). (D) Quantification of serotonin immunostaining as shown in (A and Supplementary Figure 10). Immunostaining levels in endocrine regions (islet) were compared to those of surrounding exocrine regions (Exo) (n = 6 mice, 4–7 regions per animal; mean +/− SEM, paired t-test). Grey symbols represent values from all examined regions. (E) Stimulating mouse islets with increases in glucose concentration (3 mM to 11 mM to 16 mM) elicits serotonin secretion, as measured by biosensor cells (average of 9 cells +/− SEM). Responses are inhibited by the serotonin 5HT2C receptor antagonist mianserin (10 μM). Scale bar, 50 μm.
We used a biosensor cell approach to detect serotonin release from mouse islets.30 We placed mouse islets on top of serotonin biosensor cells (CHO cells) expressing the serotonin receptor 2C (5-HT2C) loaded with the Ca2+ indicator fura-2. Stimulating islets with high glucose concentrations or with KCl, elicited Ca2+ responses in biosensor cells (Figure 5E, Supplementary Figure 11). These responses were blocked by the 5HT2 antagonist mianserin, confirming that the biosensor cells had detected serotonin. These data indicate that mouse pancreatic islets contain and release serotonin in a glucose-dependent manner.
Serotonin activates axonal terminals in the pancreatic islet
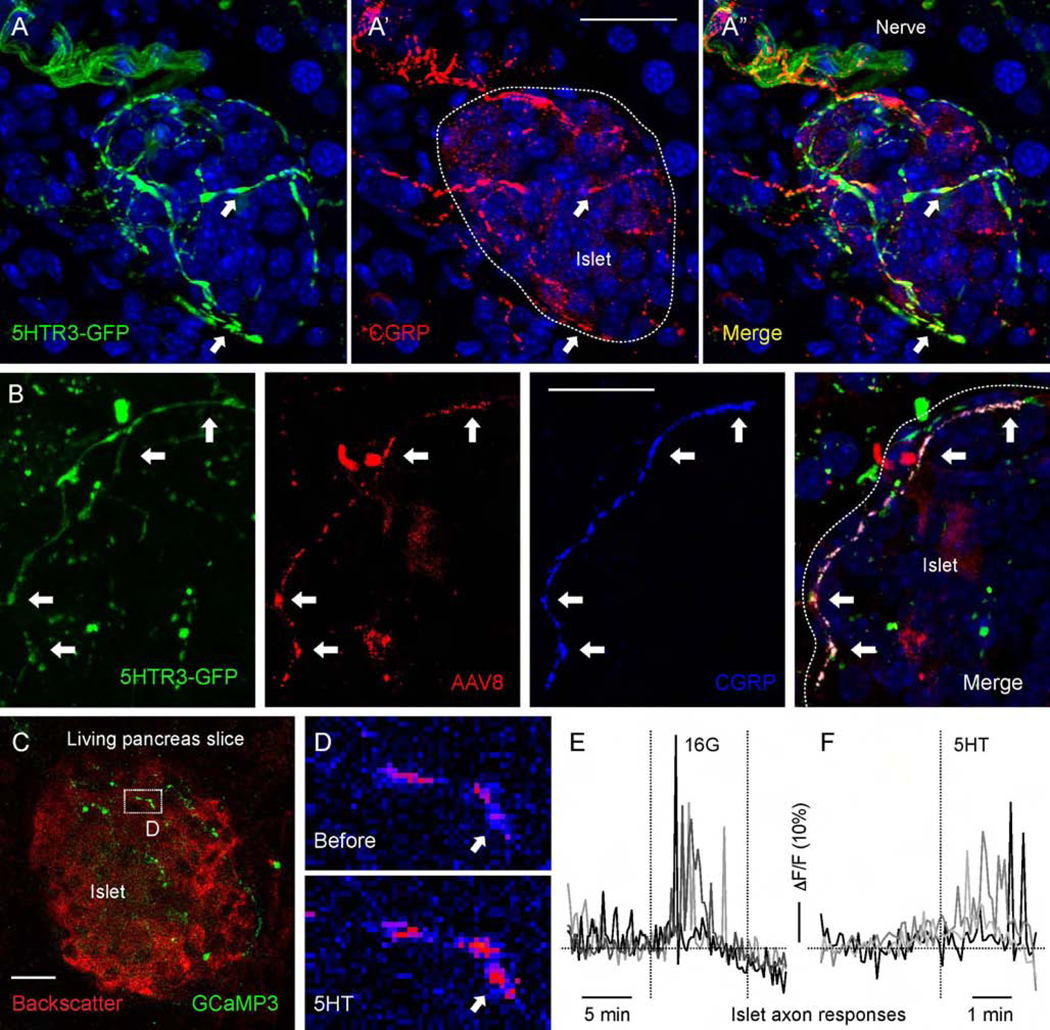
The ionotropic 5HT3 receptor is a prominent serotonin receptor in vagal afferent neurons.24, 25 To assess that neurons expressing this receptor project to the pancreatic islet we used 5HT3R-GFP mice.31 We found a high density of GFP-labeled fibers around and inside most examined islets (Figure 6A). These fibers also expressed the sensory axon marker CGRP and could be traced from the nodose ganglion (Figure 6A–B). To directly determine whether serotonin elicited responses in sensory fibers we recorded activity of intra-islet sensory fibers in pancreas slices from Pirt-GCaMP3 mice (Figure 6C–F, Movie S3).5, 38 We found that sensory fibers responded to stimulation of islet endocrine cells with high glucose concentration as well as to stimulation with serotonin (50 μM; Figure 6C–F).
Figure 6.
Serotonin activates axonal terminals in the mouse pancreatic islet. (A-A”) Pancreatic section from a 5HT3r-GFP reporter mouse immunostained for GFP (green) and CGRP (red). (B) Pancreatic section from a 5HT3r-GFP mouse with fibers anterogradely traced by injecting AAV8-mCherry into the nodose ganglion, immunostained for GFP (green), RFP (red), and CGRP (blue). (C) z-stack of confocal images of a living pancreatic slice from a Pirt-GCaMP3 mouse. Sensory fibers (green) can be seen in an islet visualized by backscatter (red). (D) Sequential images of sensory fiber shown in B displaying a Ca2+response to serotonin (5HT, 50 μM) perfused over the slice. Arrow points to a region showing an increase in GCaMP3 fluorescence (from blue to red in pseudocolor scale). (E and F) Representative traces of mean fluorescence intensity changes in axonal terminals inside the pancreatic islet demonstrating that axonal terminals respond to an increase in glucose concentration (from 3 mM to 16 mM) and to 5HT (50 μM) stimulation. Scale bars, 20 μm.
These findings show that vagal sensory neurons innervating the islet express 5HT3R. Although we could not establish if responses were elicited in vagal or spinal sensory neurons, vagal sensory axons innervating the islet are equipped to sense the serotonin generated within the endocrine pancreas.
Vagal sensory neurons respond to serotonin secreted from activated beta cells
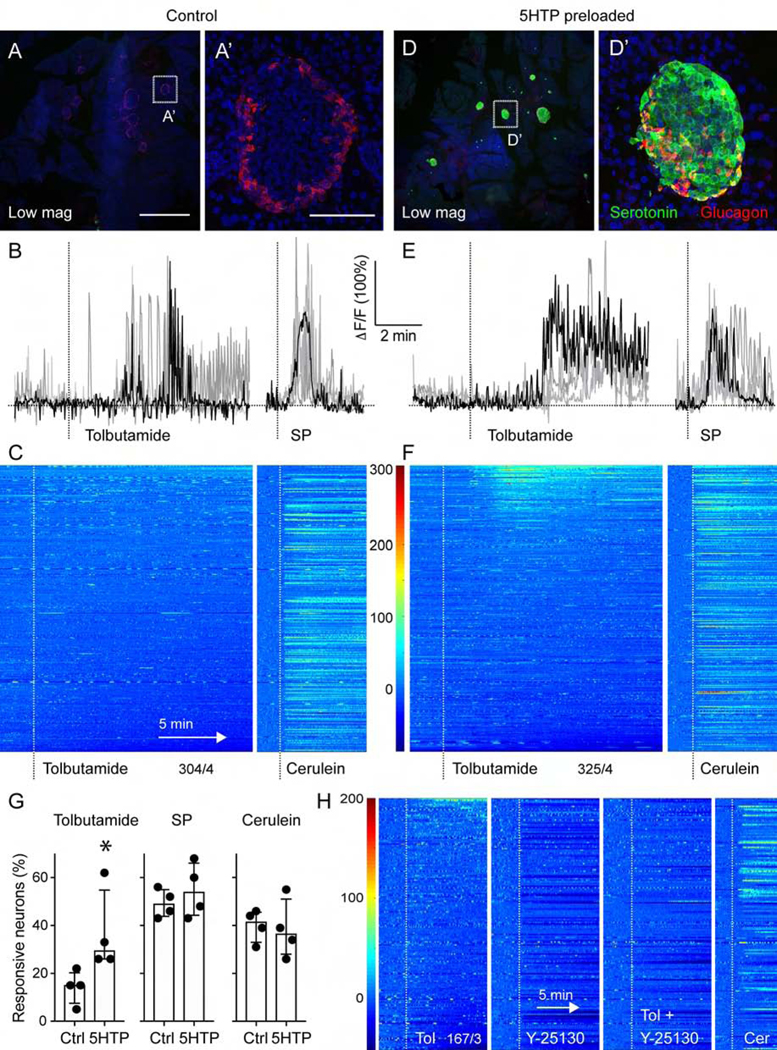
We next assessed if vagal sensory neurons respond to serotonin derived from stimulated beta cells. Using in vivo Ca2+ imaging of the nodose ganglion, we recorded neuronal activity in response to topical application of tolbutamide to the pancreas (5 mM; Figure 7). Tolbutamide is a sulfonylurea that closes KATP channels, thus stimulating beta cells. Applying tolbutamide elicited small responses in a subpopulation of vagal sensory neurons (17%, n = 4 mice; Figures 7B, C, G, and H). Responses were delayed compared to those to substance P, suggesting that tolbutamide was acting indirectly via activation of beta cells. Topical application of the 5HT3R antagonist Y-25130 inhibited responses to tolbutamide, but not to cerulein (Figure 7H).
Figure 7.
Vagal sensory neurons responses to beta cell stimulation are amplified by 5HTP-preloading. (A, D) z-stacks of confocal images of pancreatic sections from untreated mice (A) and mice preloaded with 5HTP (30 mg/kg; D). Serotonin immunostaining (green) is increased in islets (glucagon in red) from treated mice. A’ and D’ are higher magnifications of A and D. Note that endogenous levels of serotonin in control tissue are not visible because imaging settings were adjusted for the increased serotonin levels in 5HTP-preloaded tissue. (B, E) Representative traces of Ca2+ responses of nodose ganglion neurons to topical stimulation of the pancreas with tolbutamide (5 mM) and substance P (100 μM) in control (B) and 5HTP-preloaded (E) animals. (C, F) Heatmaps showing responses of nodose ganglion neurons to pancreatic stimulation with tolbutamide and cerulein in control mice (C, 304 neurons, n = 4 mice) and mice preloaded with 5HTP (F, 325 neurons, n = 4 mice). Each row is a single cell, x-axis is time, dF/F is color. (G) Quantification of the percentage of neurons responding to pancreatic stimulation in control and 5HTP preloaded animals (n = 4 for each group, Mann Whitney test, median +/− 95% CI). (H) Average trace of 16 nodose ganglion neurons that showed an increase in activity in response to tolbutamide stimulation of the pancreas. The Htr3 receptor antagonist Y-25130 (1 mM) inhibited responses to tolbutamide and lowered overall neuronal baseline activity level. Responses to cerulein were not affected. Scale bars, (A, D) 1 mm and (A’, D’) 50 μm.
To amplify the effects of beta cell stimulation, we injected mice with the serotonin precursor 5-hydroxytryptophan (5HTP, 30 mg/kg, IP).41 This treatment strongly and selectively increased serotonin levels in islets (Figure 7D–7D’). In 5HTP-preloaded mice, the size and incidence of the responses to tolbutamide increased (>30% of vagal sensory neurons; Figures 7E–G; Movies S4 and S5), while magnitude and incidence of the responses to substance P and cerulein remained unaffected. It is very likely that 5HTP-preloading also increases serotonin production in other tissues innervated by vagal sensory neurons (e.g. duodenum). It is unknown if serotonin secretion in those tissues can be stimulated by tolbutamide, but tolbutamide has been shown to preferentially activate pancreatic beta cells.42 Furthermore, we applied tolbutamide and the 5HT3R antagonist topically to ensure their effects remained mostly local. The caveats notwithstanding, our results can thus be interpreted to mean that beta cells use serotonin to communicate with vagal sensory neurons.
DISCUSSION
Our study establishes that pancreatic beta cells communicate with vagal afferent neurons. Using anatomical and physiological approaches we show that (a) pancreatic islets are densely innervated by sensory neurons, many of vagal origin, (b) selective chemogenetic activation of beta cells elicits responses in vagal sensory neurons of the nodose ganglion, and (c) serotonin signaling is likely one of the transduction mechanisms. While sensitive to beta cell stimulation, vagal afferents turned out to be insensitive to insulin. This contrasts with previous reports23 but is in line with insulin receptor signaling not being suited for quick activation of neurons. Beta cell communication, however, does not rely solely on insulin. Of the many other peptides and neurotransmitters beta cells secrete, serotonin deserves attention.
Based on our findings we propose that serotonin is co-released with insulin to activate vagal afferent nerves. This notion is supported by our findings showing that (a) vagal sensory axons innervating the islet express serotonin receptors, (b) beta cells produce and release serotonin from insulin granules, (c) antagonizing serotonin receptors inhibits vagal sensory neuron responses to selective beta cell stimulation, and (d) increasing islet serotonin levels in beta cells amplifies neuronal responses to beta cell stimulation.
In our model, intra-islet serotonin changes the activity rate of sensory neurons in ways that reflect the secretory status of beta cells. In normal physiology, serotonin contributes to paracrine control of hormone secretion, promoting the state of the islet which favors glucose clearance from the blood (via stimulation of insulin and inhibition of glucagon release).28, 30 Local islet serotonin is also known to promote islet adaptations to maintain appropriate glucose clearance during pregnancy and high fat diet challenge.26, 27, 29 Our results indicate that regular levels of islet serotonin are physiologically relevant and sufficient to stimulate vagal afferent neurons. Activating sensory axons thus seems to be an important element in the functional repertoire of islet serotonin. Vagal activation by islet serotonin therefore informs the brain about how much insulin is secreted in normal physiology as well as about the functional adaptations of the islet to physiological challenges. Evidence for serotonin production and secretion from human beta cells, however, is controversial. We reported that human beta cells synthesize and release serotonin30, but a single cell profiling data base shows that the enzymes required for making serotonin are expressed in very few islet cells (TPH1) or absent (TPH2)43. It thus remains to be determined if the mechanism described in this study translates to human beings.
Unlike intestinal enterochromaffin cells, whose massive release can increase serotonin levels systemically, beta cells secrete serotonin most likely for local consumption only. Given that the islet is the predominant source of serotonin in the pancreas, sensory axons need to be close to the islet if they are to detect serotonin. Indeed, we found that islets have the highest density of sensory axons in the pancreas. These axons are peripheral terminals of vagal sensory neurons expressing Htr3 receptors and respond to serotonin and activation of beta cells. Thus, by reaching into and around the islet, serotonin-sensitive axons put themselves in an ideal position to be exposed to the spatially restricted intra-islet serotonin.
Under certain physiological conditions that involve changes in beta cell mass (e.g. pregnancy), serotonin synthesis increases tremendously, and serotonin serves as a paracrine signal that promotes beta cell proliferation.27 Neural transmission initiated by islet serotonin thus has the potential to inform the brain about the beta cell’s secretory state with high temporal resolution as well as the ability to communicate chronic fluctuations in islet function. How sensory input from the islet influences homeostatic responses in the central nervous system remains to be determined.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT
It has been proposed that information exchange between the nervous system and the endocrine pancreas is important for glucose homeostasis but there is yet no evidence about how this works.
NEW FINDINGS
We established that beta cells are anatomically and functionally connected to vagal sensory neurons and likely use the neurotransmitter serotonin to activate their axon terminals in the islet.
LIMITATIONS
We still don’t know how the brain processes information relayed by the sensory vagus and how it impacts glucose homeostasis.
IMPACT
Our work represents a first step in identifying the peripheral mechanisms and the afferent branch of neural circuits regulating glucose metabolism.
ACKNOWLEDGEMENTS
This work was supported by the Diabetes Research Institute Foundation and National Institutes of Health grants R56DK084321 (A.C.), R01DK084321 (A.C.), R01DK111538 (A.C.), R01DK113093 (A.C.), U01DK120456 (A. C.) R33ES025673 (A.C.) and R21ES025673 (A.C.), K01DK111757 (J.A.), R21DK114418 (R.R.-D.), F31DK112596 (M.M.), the Leona M. and Harry B. Helmsley Charitable Trust grants G-2018PG-T1D034 and G-1912-03552, and by the American Heart Association 19POST34450054 (J.W.). We thank Dr. Martin Myers for providing adenovirus and Dr. Steve Roper for providing transgenic mice.
Footnotes
DECLARATION OF INTERESTS: The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Langerhans Morrison. Contributions to the microscopic anatomy of the pancreas, 1937:39–39. [Google Scholar]
- 2.Bernard C. Magendie annonce à l’Académie des Sciences que Bernard a achevé une augmentation de glucose dans le sang par une blessure d’un certain point du cerveau. C R Hebd Acad Sci T 1849;28:393–4. [Google Scholar]
- 3.Taborsky GJ. Islets have a lot of nerve! Or do they? Cell metabolism 2011;14:5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Diaz R, Abdulreda MH, Formoso AL, et al. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell metabolism 2011;14:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almaça J, Weitz J, Rodriguez-Diaz R, et al. The pericyte of the pancreatic islet regulates capillary diameter and local blood flow. Cell metabolism 2018;27:630–644.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang RB, Strochlic DE, Williams EK, et al. Vagal sensory neuron subtypes that differentially control breathing. Cell 2015;161:622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams EKK, Chang RBB, Strochlic DEE, et al. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 2016;166:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bou Karam J, Cai W, Mohamed R, et al. TRPV1 neurons regulate β-cell function in a sex-dependent manner. Molecular Metabolism 2018;18:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gram DX, Ahrén B, Nagy I, et al. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. European Journal of Neuroscience 2007;25:213–223. [DOI] [PubMed] [Google Scholar]
- 10.Motter AL, Ahern GP. TRPV1-null mice are protected from diet-induced obesity. FEBS Letters 2008;582:2257–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Razavi R, Chan Y, Afifiyan FN, et al. TRPV1+ sensory neurons control β cell stress and islet inflammation in autoimmune diabetes. Cell 2006;127:1123–1135. [DOI] [PubMed] [Google Scholar]
- 12.Riera CE, Huising MO, Follett P, et al. TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell 2014;157:1023–1036. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson S, Sundler F, Ahrén B. Neonatal capsaicin-treatment in mice: effects on pancreatic peptidergic nerves and 2-deoxy-d-glucose-induced insulin and glucagon secretion. Journal of the Autonomic Nervous System 1992;39:51–59. [DOI] [PubMed] [Google Scholar]
- 14.Berthoud H-R, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Autonomic Neuroscience: Basic and Clinical 2000;85:1–17. [DOI] [PubMed] [Google Scholar]
- 15.Carobi C. Capsaicin-sensitive vagal afferent neurons innervating the rat pancreas. Neuroscience Letters 1987;77:5–9. [DOI] [PubMed] [Google Scholar]
- 16.Fasanella KE, Christianson JA, Chanthaphavong RS, et al. Distribution and neurochemical identification of pancreatic afferents in the mouse. Journal of Comparative Neurology 2008;509:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuhuber WL. Vagal afferent fibers almost exclusively innervate islets in the rat pancreas as demonstrated by anterograde tracing. Journal of the Autonomic Nervous System 1989;29:13–18. [DOI] [PubMed] [Google Scholar]
- 18.Sharkey KA, Williams RG. Extrinsic innervation of the rat pancreas: Demonstration of vagal sensory neurones in the rat by retrograde tracing. Neuroscience Letters 1983;42:131–135. [DOI] [PubMed] [Google Scholar]
- 19.Hannibal J, Fahrenkrug J. Pituitary adenylate cyclase-activating polypeptide in intrinsic and extrinsic nerves of the rat pancreas. Cell and Tissue Research 2000;299:59–70. [DOI] [PubMed] [Google Scholar]
- 20.Lindsay TH, Halvorson KG, Peters CM, et al. A quantitative analysis of the sensory and sympathetic innervation of the mouse pancreas. Neuroscience 2006;137:1417–1426. [DOI] [PubMed] [Google Scholar]
- 21.Pettersson M, Ahrén B, Böttcher G, et al. Calcitonin gene-related peptide: Occurrence in pancreatic islets in the mouse and the rat and inhibition of insulin secretion in the mouse. Endocrinology 1986;119:865–869. [DOI] [PubMed] [Google Scholar]
- 22.Ayush EA, Iwasaki Y, Iwamoto S, et al. Glucagon directly interacts with vagal afferent nodose ganglion neurons to induce Ca2+ signaling via glucagon receptors. Biochemical and Biophysical Research Communications 2015;456:727–732. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki Y, Shimomura K, Kohno D, et al. Insulin activates vagal afferent neurons including those innervating pancreas via insulin cascade and Ca2+ influx: Its dysfunction in IRS2-KO mice with hyperphagic obesity. PLoS ONE 2013;8:e67198–e67198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellono NW, Bayrer JR, Leitch DB, et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 2017;170:185–198.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Browning KN. Role of central vagal 5-HT3 receptors in gastrointestinal physiology and pathophysiology. Volume 9, 2015:413–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim K, Oh CM, Ohara-Imaizumi M, et al. Functional role of serotonin in insulin secretion in a diet-induced insulin-resistant state. Endocrinology 2015;156:444–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim H, Toyofuku Y, Lynn FC, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med 2010;16:804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohta Y, Kosaka Y, Kishimoto N, et al. Convergence of the insulin and serotonin programs in the pancreatic β-cell. Diabetes 2011;60:3208–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YG, Moon JH, Kim K, et al. β-cell serotonin production is associated with female sex, old age, and diabetes-free condition. Biochemical and Biophysical Research Communications 2017;493:1197–1203. [DOI] [PubMed] [Google Scholar]
- 30.Almaça J, Molina J, Menegaz D, et al. Human beta cells produce and release serotonin to inhibit glucagon secretion from alpha cells. Cell Reports 2016;17:3281–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inta D, Alfonso J, Engelhardt Jv, et al. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proceedings of the National Academy of Sciences 2008;105:20994–20999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott MM, Williams KW, Rossi J, et al. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J Clin Invest 2011;121:2413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YS, Chu Y, Han L, et al. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 2014;81:873–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madisen L, Garner AR, Shimaoka D, et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 2015;85:942–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao X, Guo P, Prasadan K, et al. Pancreatic cell tracing, lineage tagging and targeted genetic manipulations in multiple cell types using pancreatic ductal infusion of adeno-associated viral vectors and/or cell-tagging dyes. Nat Protoc 2014;9:2719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reissaus CA, Pineros AR, Twigg AN, et al. A Versatile, Portable Intravital Microscopy Platform for Studying Beta-cell Biology In Vivo. Sci Rep 2019;9:8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hay CW, Docherty K. Comparative analysis of insulin gene promoters: implications for diabetes research. Diabetes 2006;55:3201–13. [DOI] [PubMed] [Google Scholar]
- 38.Marciniak A, Cohrs CM, Tsata V, et al. Using pancreas tissue slices for in situ studies of islet of Langerhans and acinar cell biology. Nature Protocols 2014;9:2809–2822. [DOI] [PubMed] [Google Scholar]
- 39.Berthoud HR, Powley TL. Morphology and distribution of efferent vagal innervation of rat pancreas as revealed with anterograde transport of Dil. Brain Res 1991;553:336–41. [DOI] [PubMed] [Google Scholar]
- 40.Fox EA, Powley TL. Longitudinal Columnar Organization within the Dorsal Motor Nucleus Represents Separate Branches of the Abdominal Vagus. Volume 341, 1985:269–282. [DOI] [PubMed] [Google Scholar]
- 41.Zern RT, Bird JL, Feldman JM, et al. Effect of increased pancreatic islet norepinephrine, dopamine and serotonin concentration on insulin secretion in the Golden hamster. Diabetologia 1980;18:341–346. [DOI] [PubMed] [Google Scholar]
- 42.Gomis A, Valdeolmillos M. Regulation by tolbutamide and diazoxide of the electrical activity in mouse pancreatic β-cells recorded in vivo. British Journal of Pharmacology 1998;123:443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segerstolpe Å, Palasantza A, Eliasson P, et al. Single-Cell Transcriptome Profiling of Human Pancreatic Islets in Health and Type 2 Diabetes, Cell Metab. 2016. 24(4):593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.