Abstract
Background
Policy makers, health staff and communities recognise that health services in lower‐ and middle‐income countries need to improve people's access to HIV treatment and retention to treatment programmes. One strategy is to move antiretroviral delivery from hospitals to more peripheral health facilities or even beyond health facilities. This could increase the number of people with access to care, improve health outcomes, and enhance retention in treatment programmes. On the other hand, providing care at less sophisticated levels in the health service or at community‐level may decrease quality of care and result in worse health outcomes. To address these uncertainties, we summarised the research studies examining the risks and benefits of decentralising antiretroviral therapy service delivery.
Objectives
To assess the effects of various models that decentralised HIV treatment and care to more basic levels in the health system for initiating and maintaining antiretroviral therapy.
Search methods
We conducted a comprehensive search to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress) from 1 January 1996 to 31 March 2013, and contacted relevant organisations and researchers. The search terms included 'decentralisation', 'down referral', 'delivery of health care', and 'health services accessibility'.
Selection criteria
Our inclusion criteria were controlled trials (randomised and non‐randomised), controlled‐before and after studies, and cohorts (prospective and retrospective) in which HIV‐infected people were either initiated on antiretroviral therapy or maintained on therapy in a decentralised setting in lower‐ and middle‐income countries. We define decentralisation as providing treatment at a more basic level in the health system to the comparator.
Data collection and analysis
Two authors applied the inclusion criteria and extracted data independently. We designed a framework to describe different decentralisation strategies, and then grouped studies against these strategies. Data were pooled using random‐effects meta‐analysis. Because loss to follow up in HIV programmes is known to include some deaths, we used attrition as our primary outcome, defined as death plus loss to follow‐up. We assessed evidence quality with GRADE methodology.
Main results
Sixteen studies met the inclusion criteria, all but one were from Africa, comprising two cluster randomised trials and 14 cohort studies. Antiretroviral therapy started at a hospital and maintained at a health centre (partial decentralisation) probably reduces attrition (RR 0.46, 95% CI 0.29 to 0.71, 4 studies, 39 090 patients, moderate quality evidence). There may be fewer patients lost to care with this model (RR 0.55, 95% CI 0.45 to 0.69, low quality evidence).
We are uncertain whether there is a difference in attrition for antiretroviral therapy started and maintained at a health centre (full decentralisation) compared to a hospital at 12 months (RR 0.70, 95% CI 0.47 to 1.02; four studies, 56 360 patients, very low quality evidence), but there are probably fewer patients lost to care with this model (RR 0.3, 95% CI 0.17 to 0.54, moderate quality evidence).
When antiretroviral maintenance therapy is delivered at home by trained volunteers, there is probably no difference in attrition at 12 months (RR 0.95, 95% CI 0.62 to 1.46, two trials, 1453 patients, moderate quality evidence).
Authors' conclusions
Decentralisation of HIV care aims to improve patient access and retention in care. Most data were from good quality cohort studies but confounding between site of treatment and outcomes cannot be excluded. Nevertheless, this review found that attrition appears to be lower in partial decentralisation models of treatment, where antiretrovirals were started at hospital and continued in the health centre; with antiretroviral drugs started and continued at health centres, no difference in attrition was detected, but there were fewer patients lost to care. For antiretroviral therapy provided at home by trained volunteers, no difference in outcomes were detected when compared to facility‐based care.
Keywords: Humans, Developing Countries, Anti‐HIV Agents, Anti‐HIV Agents/supply & distribution, Cohort Studies, Community Health Centers, Community Health Centers/statistics & numerical data, HIV Infections, HIV Infections/drug therapy, Health Services Accessibility, Health Services Accessibility/organization & administration, Health Services Accessibility/standards, Medication Adherence, Medication Adherence/statistics & numerical data, Patient Dropouts, Patient Dropouts/statistics & numerical data, Randomized Controlled Trials as Topic
Plain language summary
Providing antiretroviral therapy closer to patients homes to improve access to care in lower‐ and middle‐income countries
Background
Many people living with HIV who need antiretroviral therapy are unable to access or remain in care. This is often because of the time and cost required to travel to health centres. One approach to facilitating access and retention in care is to provide antiretroviral therapy close to people’s homes, ‘decentralising’ treatment from hospitals to health centres or even to the community. We wanted to assess whether decentralisation of antiretroviral therapy reduced the number of people lost to follow‐up. Because loss to follow‐up in HIV programmes is known to include some people who have died, our main outcome of interest was 'attrition', which is the number of people who have either died or been lost to follow‐up.
Study characteristics
We searched for studies up to March 2013. We found 16 studies, including two high quality randomised controlled trials and 14 studies collecting data from HIV care programmes. All but one study was conducted in Africa. The study participants included both adults and children who were followed‐up for up to two years.
We describe three types of care:
‐ Partial decentralisation: starting antiretroviral therapy at the hospital, then moving to a health centre to continue treatment
‐ Full decentralisation: starting and continuing treatment at a health centre
‐ Providing antiretroviral therapy in the community: antiretroviral therapy is started at a health centre or hospital and thereafter provided in the community
Key results
We found that if antiretroviral therapy was started at a hospital and continued in a health centre (partial decentralisation), there was probably less attrition and fewer patients were lost to care after one year (four studies, 39 090 patients).
Where antiretroviral therapy was started and continued at a health centre (full decentralisation), there was probably no difference in the number of deaths and patients lost to follow‐up (attrition), but overall, there were probably fewer patients lost to care after one year (four studies, 56 360 patients).
If antiretroviral therapy was provided in the community, by trained volunteers, there was probably no difference detected in death or losses to care when compared to care provided at a health centre after one year (two studies, 1 453 patients).
Overall, none of the models of decentralisation led to worse health outcomes. The research indicates that fewer patients are lost to care when they are continued on antiretroviral therapy at health centres rather than in hospitals. The research also did not detect a difference in the numbers of patients lost to care when they are treated in the community rather than in a health facility.
Summary of findings
Summary of findings for the main comparison. Antiretroviral therapy initiated in a hospital, maintained at a health centre for HIV infected patients.
| Antiretroviral therapy initiated in a hospital, maintained at a health centre for HIV infected patients | ||||||
| Patient or population: HIV infected patients Settings: Lower‐ and middle‐income countries Intervention: Antiretroviral therapy initiated in a hospital, maintained at a health centre | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antiretroviral therapy initiated in a hospital, maintained at a health centre | |||||
| Death or lost to care Follow‐up: 12 months | 218 per 1000 | 100 per 1000 (63 to 155) | RR 0.46 (0.29 to 0.71) | 39090 (4 studies) | ⊕⊕⊕⊝ moderate1,2,3 | |
| Lost to care Follow‐up: 12 months4 | 134 per 1000 | 74 per 1000 (60 to 93) | RR 0.55 (0.45 to 0.69) | 39090 (4 studies) | ⊕⊕⊝⊝ low2,5 | |
| Death Follow‐up: 12 months6 | 84 per 1000 | 28 per 1000 (11 to 73) | RR 0.34 (0.13 to 0.87) | 39090 (4 studies) | ⊕⊕⊝⊝ low2,7,8,9 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 No serious inconsistency. All four studies report a decrease in attrition at 12 months. 2 Not downgraded for indirectness. The studies included adults (two studies), children (1 study) or both (1 study); and were conducted in sub‐Saharan Africa (South Africa, Malawi). 3 Upgraded by 1 for large effect size. The effect estimate indicated a 54% decrease in attrition in the decentralised group. 4 Adjusted rates for Brennan 2011, Chan 2010 and Fatti 2010 are consistent with the crude proportions reported here. In Brennan 2011, the adjusted hazard ratio was 0.3 (95% CI 0.2 to 0.6)/ 100 person years indicating better outcomes at the health centre. Chan 2010 reported an adjusted odds ratio of 0.48 (95% CI 0.4 to 0.58) indicating better outcomes at the health centre. Fatti 2010 presented the results inversing the site of risk, the adjusted hazard ratio was 2.19 (1.94 to 2.24) indicating greater problems with patients failing to attend the hospital. 5 No serious inconsistency. Three of the four studies show benefit with varied effect sizes (39%. 51% and 66% reduction in patients lost to care), the smallest study reports no difference in clinic follow‐up at 12 months. 6 Adjusted rates for Brennan 2011, Chan 2010 and Fatti 2010 are consistent with the crude proportions reported here. In Brennan 2011, the adjusted hazard ratio was 0.2 (95% CI 0.04 to 0.8)/ 100 person years indicating better outcomes at the health centre. Chan 2010 reported an adjusted odds ratio of 0.19 (95% CI 0.15 to 0.25) indicating better outcomes at the health centre. Fatti 2010 presented the results inversing the site of risk, the adjusted hazard ratio was 1.6 (95% CI 1.3 to 1.99) indicating relatively increased risk of death in patients attending the hospital. 7 Not downgraded for methodological limitations. For one included study (Fatti 2010), the health centre group had balanced CD4 cell counts, but more severe illness ‐ 79% had WHO clinical stage III or IV disease compared with 58% in the hospital group. However, this would tend to favour the hospital group so we did not downgrade on baseline imbalance. 8 No serious inconsistency. All four studies show decrease in death at 12 months with varied effect sizes (10%, 74%, 77% and 81% reductions). 9 Not upgraded for large effect size, despite large effect size and narrow confidence interval, this review is not aiming to explore whether decentralisation decreases death, rather excluding that it increases death. The model of care down refers healthier patients for maintenance therapy, generally sicker patients remain at the hospital setting, this therefore favours decentralisation.
Summary of findings 2. Antiretroviral therapy started and maintained in a health centre for HIV infected patients.
| Antiretroviral therapy be started and maintained in health centre for HIV infected patients | ||||||
| Patient or population: HIV infected patients Settings: Lower‐ and middle‐income countries Intervention: Antiretroviral therapy be started and maintained in health centre | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antiretroviral therapy be started and maintained in health centre | |||||
| Death or lost to care Follow‐up: 12 months | 365 per 1000 | 256 per 1000 (172 to 373) | RR 0.7 (0.47 to 1.02) | 56360 (4 studies) | ⊕⊝⊝⊝ very low1,2,3,4 | |
| Lost to care Follow‐up: 12 months | 270 per 1000 | 81 per 1000 (46 to 146) | RR 0.3 (0.17 to 0.54) | 56360 (4 studies) | ⊕⊕⊕⊝ moderate3,5,6,7 | |
| Death Follow‐up: 12 months | 97 per 1000 | 106 per 1000 (61 to 185) | RR 1.1 (0.63 to 1.92) | 55099 (4 studies) | ⊕⊝⊝⊝ very low1,3,8,9 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded by 1 for methodological limitations. Bedelu 2008, McGuire 2013 and Massaquoi 2009 included sicker patients at the hospital setting, Assefa has unknown baseline risk as other baseline characteristics were not reported. This bias would tend to favour therapy provided at the health centre. 2 Not downgraded for inconsistency. Three studies report significantly reduced attrition with decentralisation (13%, 42% and 52%), while one study reported no difference. 3 Not downgraded for indirectness. The studies included adults (3 studies) or adults and children (1 study); and were conducted in sub‐Saharan Africa (South Africa, Malawi and Ethiopia). This model of care is probably applicable in better resourced settings where basic levels of healthcare are likely to be better resourced, favouring decentralisation. 4 Downgraded by 1 for imprecision. Although the sample sizes are large and event rates are high, the confidence interval is wide including both appreciable benefit and the null effect. 5 Not downgraded for risk of bias. Four retrospective cohorts provided data. Although there were differences in their baseline health status (Bedelu 2008, Massaquoi 2009 and McGuire 2012 included sicker patients at the hospital), this study limitation is not expected to impact on the attendance at the clinic. 6 Not downgraded for inconsistency. All four studies showed substantially better clinic attendance with decentralisation, however, the effect sizes varied, 24%, 63%, 80% and 89% reductions. 7 Upgraded by 1 for large effect size . The effect size indicates a 70% lower rate of failure to attend clinic follow‐up at the health centre compared to hospital. 8 Downgraded for inconsistency.There is qualitative heterogeneity, Bedelu 2008, Massaquoi 2009 and McGuire 2013 include sicker patients at the hospital, yet only McGuire showed increased death in that setting. Therefore the inconsistency is unexplained. 9 Downgraded by 1 for imprecision. Although the sample sizes are large and event rates are high, the confidence interval is wide including both appreciable benefit and harm.
Summary of findings 3. Decentralisation from the facility to the community for antiretroviral maintenance therapy for HIV‐infected patients on antiretroviral therapy.
| Decentralisation from the facility to the community for antiretroviral maintenance therapy for HIV‐infected patients | ||||||
| Patient or population: HIV‐infected patients Settings: Lower‐ and middle‐income countries Intervention: Decentralisation from the facility to the community for antiretroviral maintenance therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Decentralisation from the facility to the community for antiretroviral maintenance therapy | |||||
| Death or lost to care Follow‐up: 12 months | 106 per 1000 | 101 per 1000 (66 to 155) | RR 0.95 (0.62 to 1.46) | 709 (2 studies) | ⊕⊕⊕⊝ moderate1,2 | |
| Lost to care Follow‐up: 12 months3 | 26 per 1000 | 21 per 1000 (8 to 57) | RR 0.81 (0.3 to 2.21) | 709 (2 studies) | ⊕⊕⊕⊝ moderate1,2 | |
| Death Follow‐up: 12 months4 | Moderate | RR 1.03 (0.64 to 1.65) | 709 (2 studies) | ⊕⊕⊕⊝ moderate1,2 | ||
| 55 per 1000 | 57 per 1000 (35 to 91) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Not downgraded for indirectness. Note that the trials were conducted in Kenya and Uganda in adult populations. 2 Downgraded by 1 for imprecision. These two cluster trials have been pooled after adjusting for the design effect. The intra‐cluster co‐efficient was assumed, as it was not provided in the trial reports.The included studies have small sample sizes and wide confidence intervals which include appreciable harm and benefit. 3 The cluster randomised controlled trials Selke 2010 and Jaffar 2009 are included in this pooled analysis. Selke 2010 reports the adjusted incidence rate ratio for patients lost to care as IRR 1.15 (95% CI 0.24 to 3.03), P = 1.0 4 The cluster randomised controlled trials Selke 2010 and Jaffar 2009 are included in this pooled analysis. Jaffar 2009 reports the adjusted rate ratio for death, RR 0.95(95% CI 0.71 to 1.28); Selke 2010 did not provide adjusted rates for this outcome.
Background
Description of the condition
The spread and volume of HIV care and treatment services has increased markedly in lower‐ and middle‐income countries (LMIC). As of mid‐2011, around 8 million people were receiving antiretroviral therapy (ART) in LMIC. In spite of considerable progress in improving access to ART to date, global coverage for ART is still around 50% (UNAIDS 2011). There is high‐level political commitment to provide ART to 15 million people by 2015, but the current rate of enrolment of patients on ART may be insufficient to reach the global goal, therefore, adaptations to service delivery models are needed.
An effective service needs HIV testing and counselling services to be linked to HIV care and treatment; requires ART initiation as early as clinically indicated; and a service that retains patients. This will help decrease AIDS‐related morbidity and mortality, reduce costs and maximise efficiency gains, and avert new infections (Ford 2011). Yet there are a number of constraints at all of these steps. Recent systematic reviews have indicated that, for those who do initiate ART, retention in care is a major challenge, with around 25% of patients estimated to be lost to follow‐up within 24 months of initiating ART (Fox 2010). Barriers to access to care appear to be important drivers of poor retention, with transport costs, time spent travelling to health facilities, and time waiting for services at health facilities all cited as reasons for defaulting (Kagee 2011; Miller 2010; Ware 2009).
Description of the intervention
In order to increase access to care ‐ both to allow more people to be treated, and to improve retention among those in care ‐ a number of countries have introduced two important, linked adaptations to the traditional, "Western‐based" (i.e. hospital‐based and doctor‐led) model of care provision:
decentralisation of ART care delivery from hospitals to more peripheral health facilities (this review).
task shifting of treatment provision from highly trained specialists and medical practitioners to nurses and other non‐physician providers (Kredo 2012).
Decentralisation of care broadly means relocating services from centralised sites (i.e. hospitals) to peripheral health centres or lower levels of healthcare, generally geographically closer to the homes of patients. However, definitions of decentralised services vary considerably, and "community," "health post," "health centre" and "hospital services" all may vary in meaning between countries.
In this review, we define each "tier" in the health system according to their staffing configuration (Table 4). Thus, for community, care is provided by someone with only a few months training; for a health centre, this is led by a paramedic or nurse; for a hospital, it is led by a doctor or equivalent; and for an advanced hospital, there are specialist doctors present. In the table we also define community in three categories: family member, village volunteer, or a primary health care clinic with a nurse aide or community health worker. At community care level, systems may thus be established to deliver treatment at household level. This framework is to help describe different programmes. For HIV care, the emerging models are giving rise to a variety of terms, such as "full decentralisation", "partial decentralisation" (also sometimes referred to as down referral) and "full decentralisation with regular hospital support". To help classify models and allow cross study comparisons, we have developed a nomenclature (Table 5). This is not meant to be definitive and may need to be modified as the models of care develop, but provides a working framework for this review.
1. Health service nomenclature in lower‐ and middle‐income countries.
| Tier | Highest cadre | Terms often used | Facility and staff | Equipment facilities |
| Community | Individual with maximum of few months training; paid or unpaid | 1a. Family led care | Family member | |
| 1b. Village volunteer | Trained volunteer; health assistants | HIV tests, counselling, replenish drugs | ||
| 1c. Primary care clinic | Nurse aide or community health worker with a few months training | |||
| Health centre | clinical officer or nurse (2+ years training) | Health centres; district hospitals | Purpose built with at least one paramedic or nurse with some health assistants | HIV tests; antiretrovirals; opportunistic infections medicines; point of care laboratories |
| Health centre (enhanced) | Clinical officer or nurse (2 + years training) | Health centres, primary healthcare clinics, district hospitals | Purpose built with at least one paramedic or nurse with some health assistants, with input from a doctor (may be via mobile support service) | HIV tests; antiretrovirals; opportunistic infections medicines; point of care laboratories |
| Hospital | Doctor | Health centres; district hospitals | Purpose built with at least one medical doctor with nurses / paramedics and assistants | CD4 count Medicines Not viral load |
| Hospital (advanced) | Specialist doctor | District hospital; referral hospital | Purpose built with at least 2 specialist doctors with nurses / paramedics and assistants | Viral load and full investigations |
2. Models of HIV care.
| Our term | Initiation | Follow‐up |
| Standard hospital model | Hospital | Hospital |
| Partial decentralisation | Hospital | Health centre |
| Full decentralisation | Health centre | Health centre |
| Full decentralisation with regular hospital support | Health centre (weekly clinics with hospital staff) | Health centre (weekly clinics with hospital staff) |
| Community | Primary (tier 1c) Health centre |
Primary (tier 1c) (monitor six monthly by health centre) |
Task shifting is related to decentralisation, and is the process whereby specific tasks are transferred to different cadres of health workers who have had less training and have fewer qualifications (WHO 2008).Task shifting is being addressed by a separate Cochrane Review (Kredo 2012).
How the intervention might work
Decentralisation aims to increase access to care and improve health outcomes, in particular retention in care. These benefits may result from a number of factors, including the improved patient care by nurses and counsellors due to lower workload (i.e. lower staff to patient ratios) compared to centralised sites; and reduced time and financial cost to patient due to greater proximity of services (Fatti 2010). On the other hand, there may be concern that providing care at less sophisticated levels of the health system may decrease quality of care and result in worse health outcomes (Decroo 2009). As a result of these uncertainties, countries and regions vary in the extent to which HIV/AIDS treatment is decentralised beyond hospitals, and there is a need for clarity around the risks and benefits of decentralising ART services in order to inform future operational guidance.
Objectives
To assess the effects of decentralised HIV care in relation to initiation and maintenance of antiretroviral therapy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, non‐randomised and controlled before‐and‐after studies.
Prospective and retrospective cohort studies with a comparison between standard and decentralised delivery. For cohort studies, comparators needed to be contemporaneous (delivered at the same time), in the same country, and geographically adjacent (i.e. within the same district, or in adjacent districts within a province).
Types of participants
HIV‐infected patients at the point of initiating treatment, and patients already on treatment requiring maintenance and follow‐up.
Types of interventions
Any form of decentralised care delivery model for the initiation of treatment, continuation of treatment, or both. Decentralisation is defined as the provision of treatment at a more basic level in the health system than the centralised site (Table 4), according to the definitions described above (Table 5).
Control
Care delivered at the centralised site (usually a hospital, or in the case of community interventions, any facility)
Types of outcome measures
Primary outcomes
Attrition, defined as a composite of loss to follow‐up or death.
Loss to follow‐up at set time points after the intervention has been introduced, as defined by the study authors.
Death, after being considered eligible for treatment, or during treatment.
Secondary outcomes
Time to starting antiretroviral treatment.
Patients diagnosed with tuberculosis after entry into HIV care.
Virologic response to ART (the proportion of participants that reach or maintain a pre‐defined level of viral load suppression, as defined by the study authors).
Immunologic response to ART (mean change in the concentration of CD4+ lymphocytes from baseline, as expressed in cells/mm3).
Occurrence of a new AIDS‐defining illness.
Patient satisfaction with care, as defined by the study authors. We will include qualitative data if available from the included studies.
Cost to the provider.
Cost to the patient and family.
Any negative impact on other programme and health care delivery reported by the authors.
Search methods for identification of studies
See the Cochrane HIV/AIDS Group search strategy.
Electronic searches
In collaboration with the trial search coordinator of the Cochrane HIV/AIDS Review Group, we developed a comprehensive search strategy to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress). We searched from 1 January 1996 (the advent of triple‐drug ART) to 11 March 2013. We searched the following electronic databases:
Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 1)
MEDLINE (Appendix 2)
EMBASE (Appendix 3)
LILACS
CINAHL
Web of Science
WHO Index Medicus
Key words included MeSH terms and free‐text terms relevant to decentralisation, down referral, delivery of health care, health services accessibility, and other relevant terms.
Searching other resources
Researchers and relevant organisations. We contacted individual researchers working in the field and staff of international organisations including the Joint United Nations Programme on HIV/AIDS (UNAIDS), and the World Health Organization (WHO), and Médecins Sans Frontières (MSF) to identify studies either completed or ongoing.
Reference lists. We checked the reference lists of all studies identified by the above methods and examined the bibliographies of any systematic reviews, meta‐analyses, or current guidelines identified during the search process.
Ongoing studies. We searched the WHO International Clinical Trials Registry Platform and clinicaltrials.gov search portals for information on unpublished and ongoing trials.
Data collection and analysis
The methodology for data collection and analysis was based on the guidance of Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2008). Abstracts of all trials identified by electronic or bibliographic scanning was examined by two authors working independently. Where necessary, the full text was obtained to determine the eligibility of studies for inclusion.
Selection of studies
We removed duplicate references using a reference management software. Following this, a Cochrane research specialist did a broad review of results, excluding those that were clearly irrelevant. TK and FBA independently selected potentially relevant studies by scanning the titles, abstracts, and descriptor terms of the remaining references and applied the inclusion criteria. We discarded irrelevant reports and obtained the full article or abstract for all potentially relevant or uncertain reports. TK and FBA independently applied the inclusion criteria using a standardised eligibility form. Studies were reviewed for relevance, based on study design, types of participants, exposures and outcomes measures. All authors contributed to a consensus decision for any uncertainties or disagreements about inclusion.
Data extraction and management
After initial search and article screening, two authors independently double‐coded and entered information from each selected study onto standardised data extraction forms. Extracted information included:
Study details: citation, start and end dates, location, study design and details.
Participant details: study population eligibility (inclusion and exclusion) criteria, ages, population size, attrition rate, details of HIV care and disease progression and any clinical, immunologic or virologic staging, tuberculosis or laboratory information.
Intervention details: level of health service, cadre of health worker and other forms of patient support, including diagnosis of tuberculosis.
Outcome details: retention in care, mortality, tuberculosis case finding, AIDS‐related progression of disease, virological and immunological outcomes, patient satisfaction, cost of care.
The interventions were carefully and systematically described to ensure that all of the interventions and co‐interventions that were reported were captured.
Assessment of risk of bias in included studies
Two authors independently assessed the risk of bias within the included studies against criteria described below in accordance with methods recommended by the Cochrane Effective Practice and Organisation of Care (EPOC) Group and the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). The following judgments were used: low risk of bias, high risk of bias or unclear risk of bias (either due to lack of information or uncertainty over the potential for bias). We resolved disagreements by consensus.
Randomised controlled trials and cohort studies:
Standard criteria are suggested for all randomised controlled trials (RCTs), non‐randomised controlled trials (NRCTs) and controlled before‐after studies (CBA studies) from the EPOC group. Further information can be obtained from the Cochrane Handbook section on risk of bias (Higgins 2008a). We adapted these criteria, referring to the Newcastle‐Ottawa (Newcastle‐Ottawa Scale) and EPOC recommendations to best address the included studies and potential risk of bias presented by them as follows:
Adequate generation of the allocation sequence [trials]
Adequate allocation concealment [trials]
Baseline CD4 count measurements were similar [all studies]
Other baseline characteristics were similar [all studies]
The study was adequately protected against contamination [trials]
Data collection methods (i.e. retrospective of prospective) [cohorts]
The study was free from other risks of bias [ we have specified co‐interventions as possibly introducing bias] [all studies]
Patient selection bias [cohorts]
Assessment of overall quality of evidence We assessed the quality of evidence across particular models of care with Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology (Guyatt 2011), defining the quality of evidence for each outcome as “the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest” (Higgins 2008). The quality rating across studies has four levels: high, moderate, low or very low. Randomised controlled trials are initially categorised as providing high quality evidence, but the quality can be downgraded; similarly, other types of controlled trials and observational studies are categorised as providing low quality evidence but the quality can be upgraded if justified. Factors that decrease the quality of evidence include limitations in design, indirectness of evidence, unexplained heterogeneity or inconsistency of results, imprecision of results or high probability of publication bias. Factors that can increase the quality level of a body of evidence include studies with a large magnitude of effect, and studies in which all plausible confounding would lead to an underestimation of effect.
Measures of treatment effect
We used Review Manager software (Review Manager 2011) provided by the Cochrane Collaboration for statistical analysis and GRADEpro software (GRADEpro 2008) provided by the GRADE Working Group to produce GRADE Summary of Findings tables and GRADE Evidence Profiles. We summarised dichotomous outcomes for effect in terms of risk ratios (RRs) with their 95% confidence intervals. We calculated summary statistics using meta‐analytic methods, presented as forest plots, and presented findings in GRADE Summary of Findings tables and GRADE Evidence Profiles for all critical outcomes of interest.
Dealing with missing data
We contacted study authors to clarify data where needed, as in the case of the Bock 2008; Fatti 2010; Kipp 2010 and Morsheimer (unpublished) studies.
Assessment of heterogeneity
For the main outcomes death and loss to follow‐up, we conducted meta‐analyses. We examined heterogeneity by using the χ2 statistic with a significance level of 0.10, and the I2 statistic. We interpreted an I2 estimate of greater than 50% as indicating moderate to high levels of heterogeneity (Deeks 2008).
Data synthesis
We grouped data by the tiers of service and care configurations outlined in Table 4 and Table 5. The main comparisons for decentralisation included partial decentralisation, full decentralisation and referral to community maintenance care. When interventions and study populations were sufficiently similar across the different studies, we pooled the data across studies and estimated summary effect sizes using random‐effects models. We used the inverse variance method for analysis of cluster randomised designs.The inverse variance method assumes that the variance for each study is inversely proportional to its importance, therefore more weight is given to studies with less variance than studies with greater variance. We did not meta‐analyse data where different study designs were included, as the data were considered too heterogeneous for pooling.
We summarised the quality of evidence for the studies separately for each outcome, and for the different study designs, in the GRADE Summary of Findings tables and GRADE Evidence Profiles (Guyatt 2011).
Subgroup analysis and investigation of heterogeneity
We examined outcomes under the three models of care: partial decentralisation (initiated at hospital, continued at health centre), full decentralisation (initiated and treated at health centre) and community care for maintenance of ART (Table 5). We defined hospital and health centre strictly as established in the protocol (Table 4). Sub‐groups were formed by duration of follow‐up (i.e. 6 months, 12 months or 24 months).
Sensitivity analysis
A sensitivity analysis was conducted in which 40% of all patients lost to follow‐up were reclassified as being dead. This figure was chosen because, according to a systematic review of HIV infected patients in treatment programmes in low resource setting, 40% of patients lost to follow‐up were found upon tracing to have died (Brinkhof 2009).
Results
Description of studies
Results of the search
Searches were conducted in May 2012 and March 2013 and identified 3437 titles (see prisma flow diagram Figure 1). Twenty‐nine full‐text articles were closely examined by two authors (TK and FBA), including a trial, identified by checking references; an unpublished study provided by the co‐author, on hearing of this review in progress, however this is awaiting further data before inclusion; and a further study was suggested through contact with the technical team at the WHO.
1.
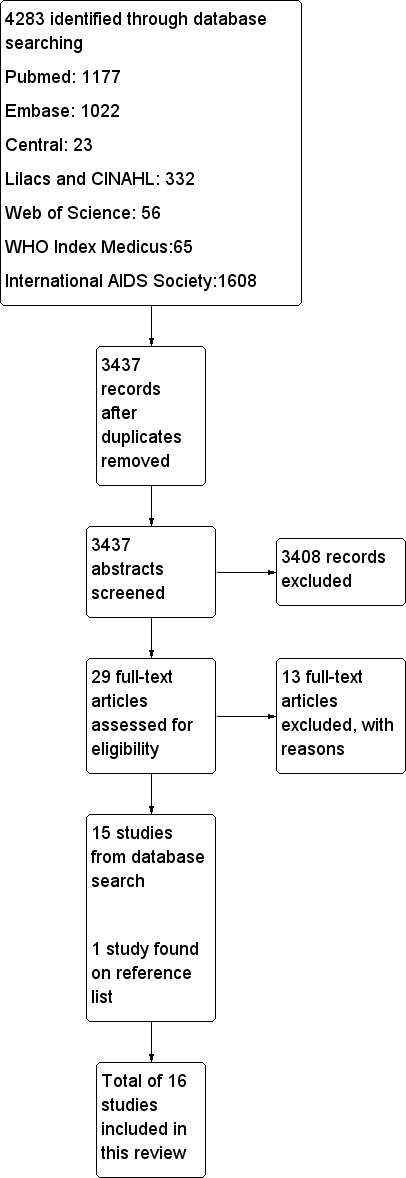
Study flow diagram.
We finally identified two cluster randomised controlled trials and 14 cohort studies that met our inclusion criteria for data extraction, coding and potential meta‐analysis. TK, FBA and NF independently extracted data for the included studies.
Included studies
Sixteen studies met the inclusion criteria, two cluster randomised controlled trials (Jaffar 2009; Selke 2010), two prospective cohorts (Humphreys 2010; Kipp 2010) and 12 retrospective cohort studies (Assefa 2012; Balcha 2010; Bedelu 2007; Bock 2008; Brennan 2011; Chan 2010; Fatti 2010; Fayorsey 2013; Hansudewechakul 2012; McGuire 2012; Massaquoi 2009; Odafe 2012).
Three studies took place in rural Malawi (Chan 2010; Massaquoi 2009;McGuire 2012), two in various settings including urban, peri‐urban and rural Ethiopia (Assefa 2012; Balcha 2010), two in rural Uganda (Jaffar 2009; Kipp 2010), one in rural and urban Kenya (Selke 2010), one in rural Swaziland (Humphreys 2010); four in various settings including urban, peri‐urban and rural settings in South Africa (Bedelu 2007; Bock 2008; Brennan 2011; Fatti 2010). One study examined data from five countries in Africa (Kenya, Lesotho, Mozambique, Rwanda and Tanzania (Fayorsey 2013) and one from Thailand (Hansudewechakul 2012)
All studies evaluated decentralisation of care from hospital level to more basic levels of care. In addition, eight studies included task shifting from doctors to non‐doctors (either nurses or clinical officers): Assefa 2012; Bedelu 2007; Brennan 2011; Humphreys 2010; Jaffar 2009; Kipp 2010; Massaquoi 2009; and Selke 2010.
Three studies examined treatment in children only (Bock 2008, Fayorsey 2013, Hansudewechakul 2012), two included adults and children (Chan 2010 and Massaquoi 2009) and the rest included adults only.
Finally, one additional study from Nigeria was included that compared treatment at tertiary and secondary hospital care (Odafe 2012). Whilst it met our inclusion criteria, the model evaluated was not considered directly relevant to the review question. Results from this study are reported separately.
Interventions
The three models of decentralisation of care that were pre‐specified in the protocol (Table 5) were further elaborated while reviewing the included studies as follows:
1. Partial decentralisation, in which ART is initiated in a hospital setting and patients are down referred for follow‐up at the health centre (also sometimes referred to as "down referral")
2. Full decentralisation, in which ART is initiated and maintained at a health centre rather than a hospital
In both of these models of care the health care provider at the health centre, or more basic level of care, was usually a nurse of clinical officer (health officer), except in the case of the paediatric studies in which doctors were generally providing care.
3. A community model, in which ART is initiated at the health centre or hospital, but maintenance occurs at the home, supported by community health workers
In this model, HIV care was delivered by community volunteers or field officers with specific training, to ensure they could monitor adherence, adverse effects and clinical symptoms at the home (e.g. computer aided devices or checklists).
The models of care in included studies are described in detail (Table 6) including relevant co‐interventions such as additional adherence support through peer educators, supervision on‐site or training for health workers.
3. Description of the models of care of included studies.
| Models of care | Provider details | Laboratory facilities | Community support | Training in ART initiation and maintenance | Supervision or mentoring | Referral | |
| Partial decentralisation | |||||||
| Bock 2008 | Health centres (enhanced) | Doctors | yes | not stated | not stated | specialists available | yes |
| Hospital (advanced) | Doctors | yes | not stated | yes | specialists available | not applicable | |
| Brennan 2011 | Health centres | Primary health care nurses | not stated | not stated | yes | yes ‐ telephonic | yes ‐ to hospital |
| Hospitals | Doctors | not stated | not stated | not applicable | not applicable | not applicable | |
| Chan 2010 | Health centres | Nurses and health surveillance assistants | no | Expert patients | yes | yes ‐ from hospital | not stated |
| Hospitals | Clinical officers, nurses and doctors | yes | Home‐based care volunteers | not applicable | not applicable | not stated | |
| Fatti 2010 | Health centres | Doctors | yes | Community‐based adherence counsellors | not stated | not stated | not stated |
| Hospitals | Doctors | yes | not stated | not stated | not stated | not stated | |
| Hansudewechakul 2012 | health centres | Nurses | yes | yes | yes | yes | not stated |
| Hospital | Doctors | yes | yes | yes | not applicable | not stated | |
| Humphreys 2010 | Health centres | Nurses | no | not stated | yes | yes ‐ monthly visit from nurse and counsellor | yes |
| Hospital | Doctors | yes | not stated | not applicable | not applicable | not applicable | |
| Full decentralisation | |||||||
| Assefa 2012 | Health centres | Health officers, nurse | not stated | Community health workers, adherence counselling, defaulter tracing, referral and linkage between facilities | not stated | not stated | yes ‐ to hospital |
| Hospitals | Doctors | not stated | none | not stated | not stated | not applicable | |
| Balcha 2010 | Health centres | Health officers, nurses, data clerk, pharmacy technicians | not stated | not stated | not stated | not stated | yes ‐ to hospital |
| Hospitals | Nurses, data clerks, pharmacists | not stated | not stated | not stated | not stated | not applicable | |
| Bedelu 2007 | Health centres | Nurses | no | Community health workers, adherence support, defaulter tracing | yes | yes ‐ mobile team | yes ‐ to hospital |
| Hospitals | Doctors | yes | no | not stated | not applicable | not applicable | |
| Fayorsey 2013 | health centres | doctors and nurses | 8/182 sites CD4 machines | variable by site | not stated | not stated | yes |
| Hospitals | doctors and nurses | 54/92 sites Cd4 machines | variable by site | not stated | not stated | not applicable | |
| Massaquoi 2009 | Health centres | Medical assistants and nurse | yes | yes | yes | yes | yes ‐ to hospital |
| Hospitals | Doctors | yes | not stated | yes | not applicable | not applicable | |
| McGuire 2012 | Health centres | Clinical officers, nurses and medical assistants | yes | yes | yes | not stated | yes |
| Hospitals | Clinical officers and nurses | yes | yes | not stated | not stated | not applicable | |
| Odafe 2012 | Hospitals | Medical doctors | yes | yes | not stated | not stated | not stated |
| Hospitals (advanced) | Medical specialists | yes | not stated | not stated | not applicable | not applicable | |
| Decentralisation from facility to community | |||||||
| Jaffar | Community | Field officers | no | not stated | yes | yes | yes |
| Health centres | Clinical staff | yes | not stated | yes | yes | not applicable | |
| Kipp | Community | Unpaid volunteers, >18 years old and literate | no | Treatment supporter to assist with adherence | yes | yes | yes |
| Health centres | Doctors | yes | no | not applicable | not stated | not applicable | |
| Selke | Community | Community care co‐ordinators | no | Computer aided devices | yes | yes | yes |
| Health centres | Clinical officers, doctor (1 day/ week) | yes | no | not applicable | not applicable | not applicable | |
Excluded studies
See Excluded studies
There were 13 excluded studies, nine of which were excluded on the basis of their study design (cross‐sectional surveys, qualitative or no contemporary arm for comparison), and two because they were conducted in high income countries.
Risk of bias in included studies
Modified risk of bias criteria were used to evaluate the included studies. The criteria were developed to reflect the study designs included in this review ‐ prospective and retrospective cohort studies and randomised controlled trials (including cluster randomised controlled trials). See summary of risk of bias by study in Figure 2.
2.
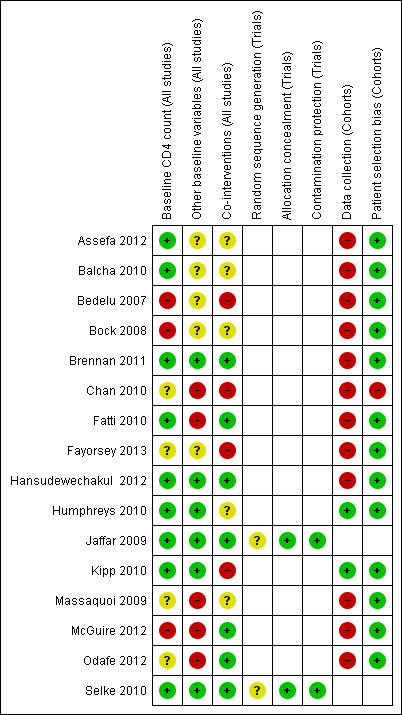
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Randomised controlled trials
The two randomised controlled trials assigned interventions by cluster (Jaffar 2009; Selke 2010). They were both well balanced for baseline CD4+ cell count, an important indicator of baseline morbidity, and were therefore rated as having a low risk of bias, they were also balanced for other baseline characteristics, including sex, age and WHO clinical stage, also rated as contributing a low risk of bias. Co‐interventions used to support participants (such as adherence support) were similar for both groups in the Jaffar 2009 and Selke 2010 trials and this was rated as having a low risk of bias. Sequence generation was not described in either included trial and therefore was rated as presenting an unclear risk of bias. Allocation concealment was well described and was unlikely to introduce bias, rated as a low risk of bias. Neither trial had any indication of contamination and it was unlikely that those in the control group were at risk of receiving the intervention. Overall, risk of bias was low, as judged by the modified risk of bias assessment tool.
Cohort studies
Prospective design:
Only two of the 14 cohort studies were prospective (Humphreys 2010, Kipp 2010). The other 12 studies were retrospective cohort studies, and therefore classified as high risk of bias for data collection.
Stated selection differential:
Chan 2010 stated that only stable patients were selected to receive ART at the health centre; this study was therefore rated as having a high risk of bias. No other selection bias was detected in any of the other cohorts.
Baseline comparability:
For partial decentralisation, four cohort studies had comparable CD4+ cell counts at baseline, with sicker children in the Bock 2008 study remaining at the hospital, and CD4+ cell count not reported in Chan 2010. For other baseline variables, two studies were not comparable, and one study did not report on other baseline characteristics (Bock 2008). In Chan 2010, patients at peripheral units were "healthier", which could lead to an erroneous conclusion that peripheral units were just as good as hospitals; and in Fatti 2010, where patients at peripheral units were sicker.
For full decentralisation, two cohort studies had comparable CD4+ cell counts, three did not report this, and in two the CD4+ cell counts indicated the patients were healthier at the peripheral units (Bedelu 2007; McGuire 2012). For other baseline factors, four did not report this, and three were not comparable as they reported that they included patients that were healthier at peripheral units (Massaquoi 2009; McGuire 2012; Odafe 2012).
For decentralisation from facility to community, all three studies were ranked as low risk of bias for CD4+ cell count and other baseline factors.
Effects of interventions
See: Table 1; Table 2; Table 3
Studies reported retention variably, sometimes including patients who were transferred out, but still in care, but this was not always clearly reported. We were more consistently able to extract data on whether patients were lost to care (defined within studies as failure to attend clinic follow‐up 3 months or 6 months after the expected appointment date), and this was therefore chosen as a more reliable measure than its inverse, retention. We also sought data on mortality; however, the majority of the included studies did not trace patients who were lost to care to determine if they were dead (and therefore classified as mortality) or alive and truly lost to follow‐up. This is a known problem with antiretroviral treatment programs with varying rates of mortality among patients who default from care. A recent systematic review showed a pooled mortality estimate of around 40% among HIV patients on antiretroviral programs who were successfully traced following default from care (Brinkhof 2009). We therefore report as our primary outcome the composite outcome of attrition (i.e. death or lost to care).
MAIN ANALYSIS BY THE THREE DECENTRALISATION MODELS
1. Partial decentralisation ‐ Initiated antiretroviral therapy at hospital and maintained in a health centre
See Table 1
There were no trials that examined this comparison. Data come from six observational cohorts reporting outcomes for three time points, including two cohorts of paediatric patients.
Attrition (death or lost to care)
Partial decentralisation reduced attrition at 12 months (RR 0.46, 95% CI 0.29 to 0.71, four cohort studies, 39 090 patients, moderate quality evidence, Figure 3). This benefit was consistent across all the four studies included in this analysis.
3.
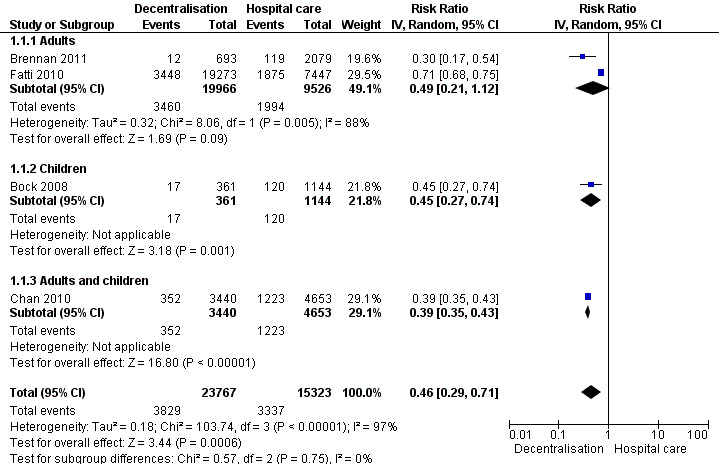
Forest plot of comparison: 1 Partial decentralisation ‐ initiation in hospital, maintenance at health centre, outcome: 1.1 Death or lost to care (12 months).
Lost to care
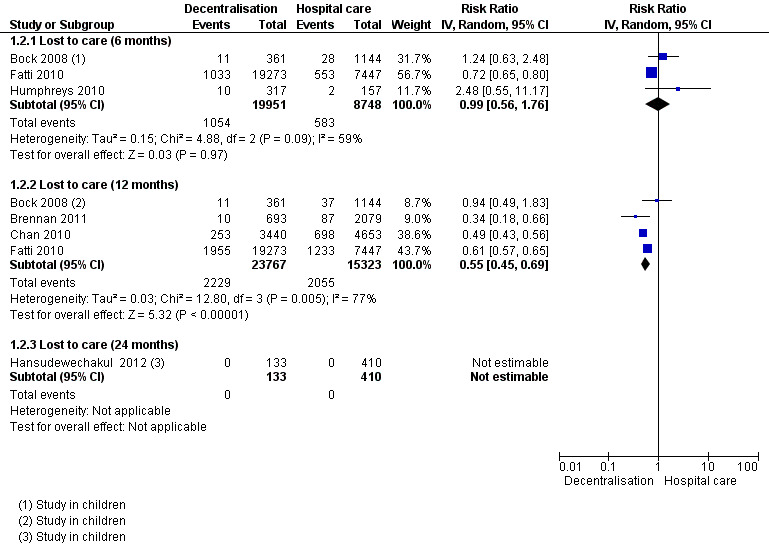
See Analysis 1.2 and Figure 4
1.2. Analysis.
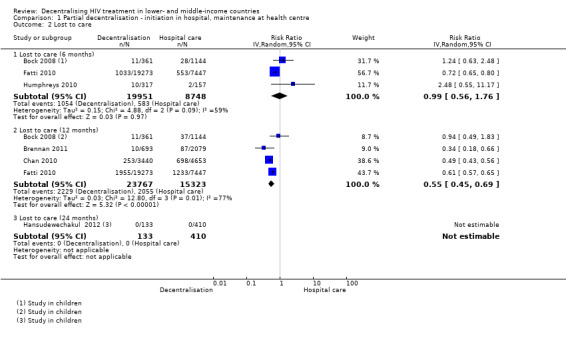
Comparison 1 Partial decentralisation ‐ initiation in hospital, maintenance at health centre, Outcome 2 Lost to care.
4.
Forest plot of comparison: 1 Partial decentralisation ‐ initiation in hospital, maintenance at health centre, outcome: 1.2 Lost to care.
Overall, partial decentralisation was found to lead to fewer numbers of patients lost to care at 12 months.
Two retrospective (Bock 2008; Fatti 2010) and one prospective cohort (Humphreys 2010) contributed to the 6 month data, including 28 699 patients in rural, peri‐urban and urban settings. Overall, from our unadjusted pooled analysis the relative risk of patients lost to care is RR 0.99 (95% CI 0.56 to 1.76; I2 = 59%).
Four retrospective cohorts contributed data on patients lost to care at 12 months (Bock 2008; Brennan 2011; Chan 2010; Fatti 2010) including 39 090 patients. Bock 2008 and Chan 2010 included children in their study. Overall, patients were 45% less likely to be lost to care at the decentralised site (RR 0.55, 95% CI 0.45 to 0.69; I2 = 77%). Thus there is low quality evidence that down referral may decrease the numbers of patients lost to care at 12 months.
Finally, one retrospective study in children reported data on children at 24 months (Hansudewechakul 2012), with no difference between models of care (no patients lost to care at any site).
Mortality
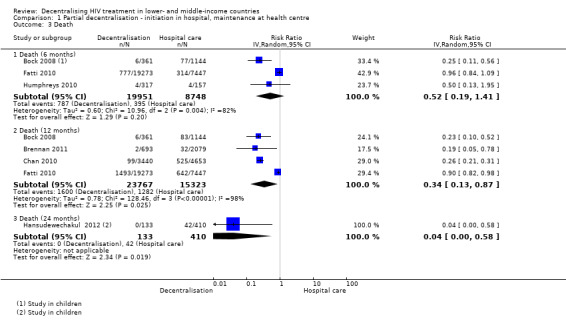
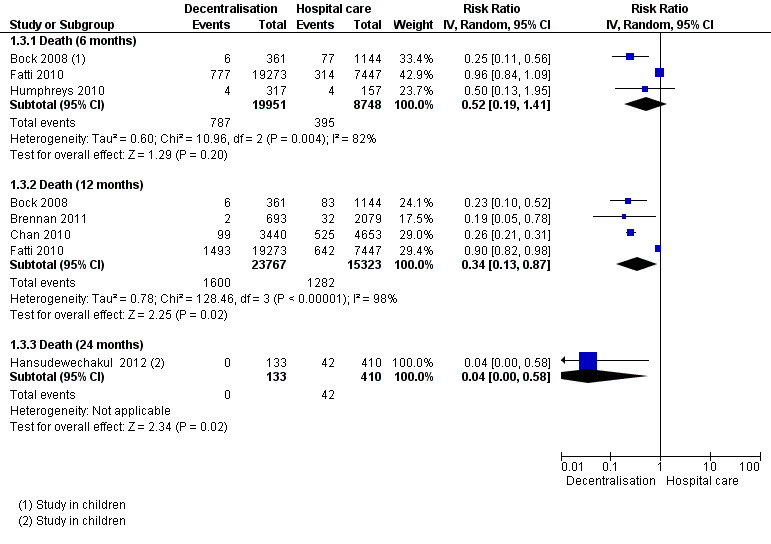
See Analysis 1.3 and Figure 5
1.3. Analysis.
Comparison 1 Partial decentralisation ‐ initiation in hospital, maintenance at health centre, Outcome 3 Death.
5.
Forest plot of comparison: 1 Partial decentralisation ‐ initiation in hospital, maintenance at health centre, outcome: 1.3 Death.
Overall, there is low quality evidence that partial decentralisation of care after initiation at a hospital may reduce death at 12 months.
The three cohorts reporting on patients lost to care at the clinic, also provide data for mortality at six months (Bock 2008; Fatti 2010; Humphreys 2010) The pooled risk of death at six months was RR 0.52 (95% CI 0.19 to 1.41; I2 = 82 %) .
The risk of bias inherent and reported in the cohort studies would be expected to favour the intervention in all studies, except in Fatti 2010. There is overall very low quality data for this outcome at six months, with wide confidence intervals.
The same four cohorts reporting losses to care at 12 months provide data for this outcome, with the same high potential risk of bias. The relative risk of mortality at 12 months was RR 0.34 (95% CI 0.13 to 0.87). There was substantial quantitative and qualitative heterogeneity, and an I2 of 98%, all of which was introduced by one study (Fatti 2010), which may be explained by the inclusion of patients with more advanced WHO clinical stages in the control arm of the study. As the direction of bias favours the intervention, the quality of the evidence was not downgraded for methodological limitations. The studies, although clinically heterogeneous, consistently favoured partial decentralisation, and were therefore judged to have no serious inconsistency. In a sensitivity analysis assuming 40% of patients lost to care had died, mortality at 12 months remained lower at decentralised sites (RR 0.41, 95% CI 0.22 to 0.76).
Thus, overall there is low quality evidence that partial decentralisation of care reduces death at 12 months. This should be seen in the context of substantial heterogeneity in the studies and high risk of bias. Overall, there was no excess of deaths seen in any of the studies with care decentralised to the health centres, after initiation at hospital level.
Finally, one study, done among children, reported lower mortality at the decentralised site at 24 months (RR 0.04, 95%CI 0.00 to 0.58) (Hansudewechakul 2012).
2. Full decentralisation ‐ Initiated and maintained antiretroviral therapy at a health centre
See Table 2
No trials examined this comparison. Data come from six observational cohorts reported outcomes at three time points, including one cohort of paediatric patients.
All studies included patients in rural settings. Antiretroviral therapy was initiated and maintained at the health centres and delivered by nurses or clinical officers, not doctors.
Attrition (Death or lost to care)
Overall, decentralisation to heath centres for initiation and maintenance of care appeared to reduce attrition at 12 months (RR 0.70, 95% CI 0.47 to 1.02, four cohort studies, 56 360 patients, very low quality evidence, Figure 6). This result was consistent across three of the studies included in the analysis while the fourth study showed no difference in attrition.
6.
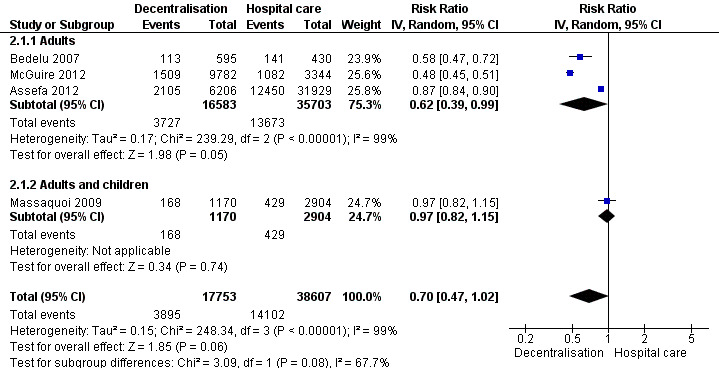
Forest plot of comparison: 2 Full decentralisation ‐ initiation and maintenance in health centre, outcome: 2.1 Death or lost to care (12 months).
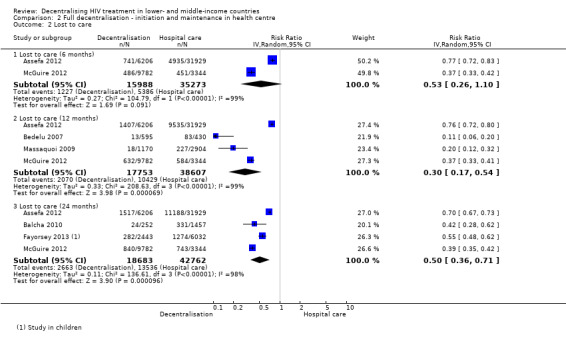
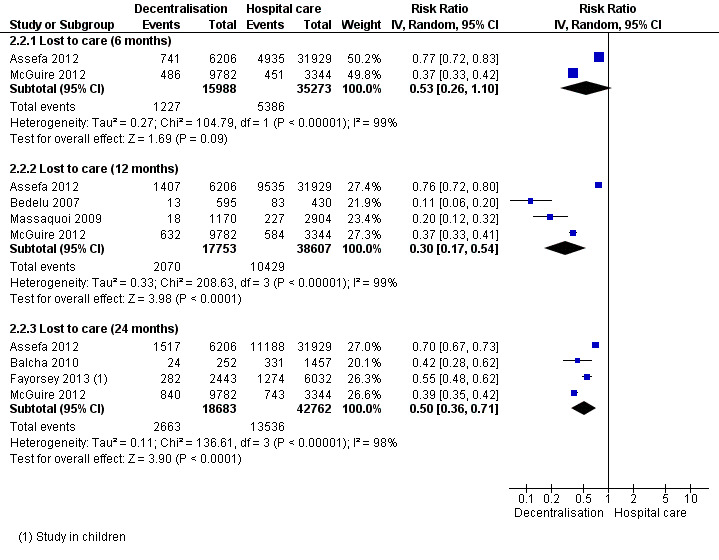
Lost to care
See Analysis 2.2 and Figure 7
2.2. Analysis.
Comparison 2 Full decentralisation ‐ initiation and maintenance in health centre, Outcome 2 Lost to care.
7.
Forest plot of comparison: 2 Full decentralisation ‐ initiation and maintenance in health centre, outcome: 2.2 Lost to care.
Overall there was moderate quality evidence that full decentralisation of care from hospitals to health centres for both initiation and maintenance of HIV care probably reduces the numbers of patients lost to care at 12 months.
Two large retrospective cohorts, one from Ethiopia (Assefa 2012) and another from Malawi (McGuire 2012) both reported a statistically significant reduction in patients lost to care at decentralised sites at six months, and the pooled relative risk, indicated a large reduction of 47% in the risk of being lost to care (RR 0.53, 95% CI 0.26 to 1.10, 51 261 patients).
There was also a reduction in the risk of patients being lost to care at decentralised sites at 12 months, with consistent reductions reported across four cohorts and a strong association reporting a reduction of 70% (RR 0.3, 95% CI 0.17 to 0.54, 56 360 patients) when pooling the data. A consistent benefit was also reported by four studies that reported lost to care at 24 months (RR 0.50, 95%CI 0.36 to 0.71, 61 445 patients).
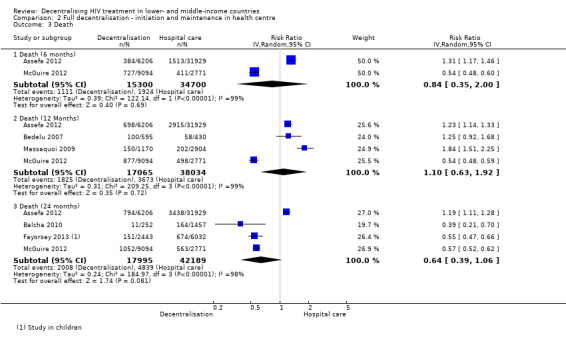
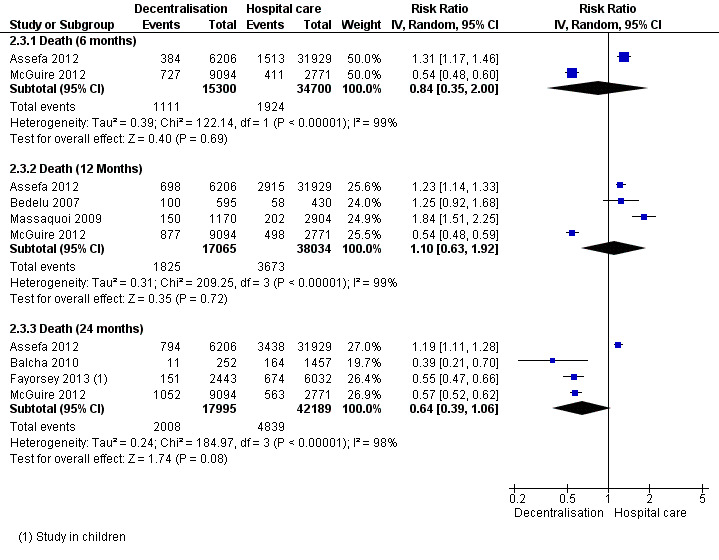
Mortality
See Analysis 2.3 and Figure 8.
2.3. Analysis.
Comparison 2 Full decentralisation ‐ initiation and maintenance in health centre, Outcome 3 Death.
8.
Forest plot of comparison: 2 Full decentralisation ‐ initiation and maintenance in health centre, outcome: 2.3 Death.
Overall, there is very low quality evidence that there was no difference in mortality for patients treated at the health centre or the hospital at 12 months.
The pooled risk of death in decentralised sites was (RR 0.84, 95%CI 0.35 to 2.00, two studies, 50 000 patients) at six months, (RR 1.10, 95%CI 0.63 to 1.92, four studies, 55 099 patients) at 12 months, and (RR 0.64, 95%CI 0.39 to 1.06, four studies, 60 184 patients) at 24 months. Although baseline CD4+ cell counts were similar between some groups, the studies were judged to have unclear bias related to co‐interventions and other baseline characteristics, as these were not well reported. In the sensitivity analysis, mortality at 12 months remained similar between sites (RR 0.86, 95% CI 0.58 to 1.29).
Other models of full decentralisation
Odafe 2012 examined attrition, death and loss to follow‐up of patients treated in secondary and tertiary providers in Nigeria. Whilst this loosely could be a study of "decentralisation" it was at a higher level of care and is not a current question for most health care systems in countries of Africa, where HIV treatment is currently standard at secondary level.
3. Decentralisation to the community ‐ maintained on antiretroviral therapy in the community
Two trials examined this comparison. Data come from these two trials and one observational cohort reporting outcomes at three time points.
All studies included patients in rural settings. Antiretroviral therapy was initiated at the health centres and maintained in the community with a trained community health worker.
Attrition (Death or lost to care):
Neither trial provided adjusted rates for analysis, therefore in order to adjust for the design effect, we required an intra‐cluster co‐efficient (ICC). We made a statistical assumption and used a liberal ICC of 0.05. Following this adjustment, the included studies had small sample sizes and wide confidence intervals, the evidence was thus downgraded for imprecision. Overall, there is moderate quality evidence that there is probably no difference in attrition rates at 12 months comparing community and facility‐based maintenance care (RR 0.95, 95% CI 0.62 to 1.46, two trials, 1453 participants, Figure 9).
9.
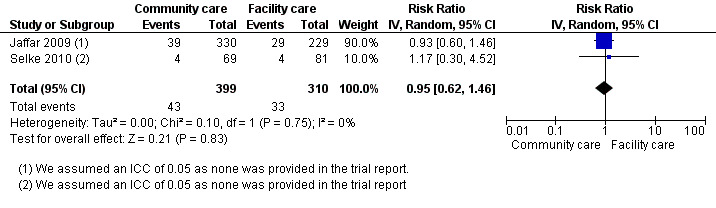
Forest plot of comparison: 3 Decentralisation ‐ from the facility to the community for antiretroviral maintenance therapy, outcome: 3.1 Death or lost to care (12 months).
Lost to care
See Analysis 3.2 and Figure 10
3.2. Analysis.
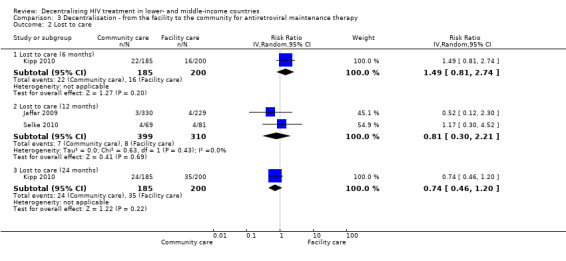
Comparison 3 Decentralisation ‐ from the facility to the community for antiretroviral maintenance therapy, Outcome 2 Lost to care.
10.
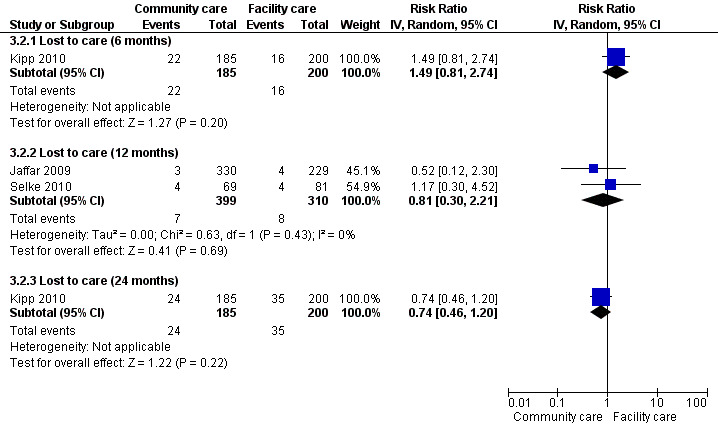
Forest plot of comparison: 3 Decentralisation ‐ to community from facility, outcome: 3.2 lost to care.
Two cluster randomised controlled trials (Jaffar 2009; Selke 2010) and a single cohort (Kipp 2010) provided the 6 and 12 month data for patients who were lost to follow‐up in the community. Overall, there was moderate quality evidence from the two cluster trials reporting that there was no difference at 12 months.
One prospective cohort reported on lost to care at 6 months (Kipp 2010). Overall the risk of bias in this included study was low. There was no difference in the rate of failure to attend follow‐up between the groups (RR 1.49, 95% CI 0.81 to 2.74, 385 participants).The same cohort also reported data at 24 months, and again the result was not significantly different between models (RR 0.74, 95%CI 0.46 to 1.20, 385 participants).
This lack of difference was also supported by data from the two randomised controlled trials at 12 months (RR 0.81, 95% CI 0.3 to 2.21, 1453 participants).The quality of the evidence was downgraded for imprecision due to the low event rates in both arms of both trials and wide confidence interval when combining these two trials.
Mortality
See Analysis 3.3 and Figure 11
3.3. Analysis.
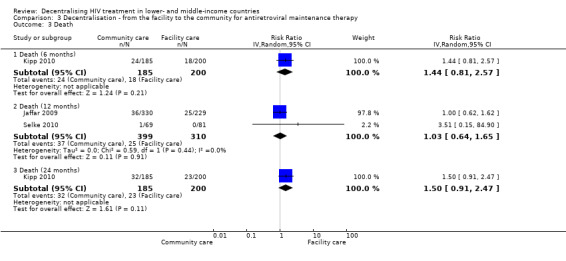
Comparison 3 Decentralisation ‐ from the facility to the community for antiretroviral maintenance therapy, Outcome 3 Death.
11.
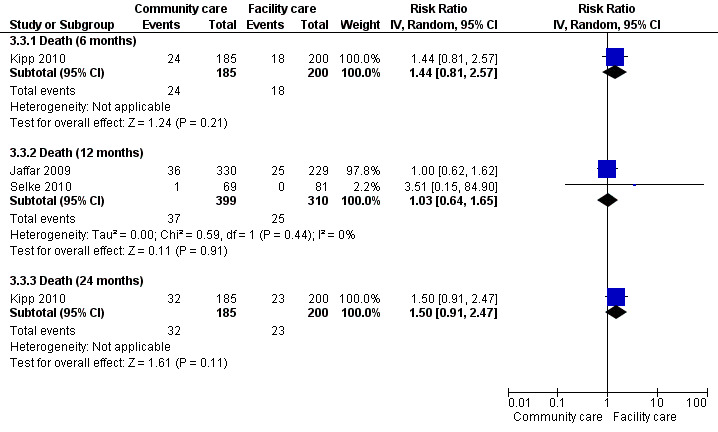
Forest plot of comparison: 3 Decentralisation ‐ to community from facility, outcome: 3.1 Death.
There was moderate quality evidence that rates of death at 12 months were similar whether maintenance care was delivered at the facility or in the community (RR 1.03, 95% CI 0.64 to 1.65, two trials, 1453 participants). Similarly, the risk of death was not significantly different in the cohort study at six months (RR 1.44, 95%CI 0.81 to 2.57, one study, 385 participants) and 24 months (RR 1.50, 95%CI 0.91 to 2.47, one study). All results of the sensitivity analysis were non‐significant (results not shown).
OTHER OUTCOMES
Studies reported on a variety of other outcomes that are reported below.
Immunological changes ‐ CD4+ cell count
Twelve cohorts report on change in CD4+ count, six provide median change at either six or 12 months (Assefa 2012; Balcha 2010; Brennan 2011; Jaffar 2009; Hansudewechakul 2012; Kipp 2010). As the studies reporting this outcome differed by model of care, time of reporting or threshold, this outcome is reported narratively. For all of these studies there is a consistent report of an increase in CD4+ cell count, with no statistical difference between decentralised or standard hospital treated groups.
Viral load suppression
Eight studies report the proportion of patients who are virologically suppressed (Bedelu 2007; Brennan 2011; Fatti 2010; Hansudewechakul 2012; Jaffar 2009; Kipp 2010; Selke 2010). The reported virological suppression rates were similar across the decentralised and control groups across these varied studies which could not be meta‐analysed.
Cost to providers and patients
One prospective cohort, Humphreys 2010, reports specifically on the cost of travel for patients (Analysis 1.4). The average cost for a decentralised patient was USD 0.74 compared to USD 1.5 for a patient seen at the hospital (P = 0.001).
1.4. Analysis.
Comparison 1 Partial decentralisation ‐ initiation in hospital, maintenance at health centre, Outcome 4 Cost of travel.
| Cost of travel | |||
|---|---|---|---|
| Study | Down referred patient | Hospital care patient | P‐value |
| cost of travel | |||
| Humphreys 2010 | Average cost for follow up care USD 0.74 | Average cost for follow up care USD 1.5 | P = 0.001 |
Two studies provided data on overall cost to patient (Analysis 3.4). Both reports come from community‐based treatment compared to standard hospital‐based treatment (Jaffar 2009; Kipp 2010). Both studies indicate substantial increase in cost to patient when they are required to travel to the hospital, which is usually further from their homes. Kipp 2010 reports a doubling of cost to patients when accounting for transport only. Jaffar 2009, the cluster trial, reports a three times increase in costs, including transport, lost work time, child‐care costs and food.
3.4. Analysis.
Comparison 3 Decentralisation ‐ from the facility to the community for antiretroviral maintenance therapy, Outcome 4 Cost to patient.
| Cost to patient | ||
|---|---|---|
| Study | Home based care | Hospital based care |
| Jaffar 2009 | total cost per year for transport, lunch, childcare costs, lost work time: $18/year (after first year) | total cost per year for transport, lunch, childcare costs, lost work time: $54/ year (after the first year) |
| Kipp 2010 | Transport cost $0.74/ visit for home based care | Transport cost $1.5/ visit for facility based care |
Costs to the health service are also reported. Jaffar 2009 reported costs to health service for community versus hospital‐based groups. These included staff, transport, drugs, laboratory, training, supervision, capital and utilities costs and was a mean of US$ 793 / year for each patient in the home‐based group compared to US$ 838 / year / patient in the hospital‐based group.
Initiation of tuberculosis treatment, time to initiation of ART, new AIDS defining illness, any negative impact on the health delivery
No study reported data on initiation of tuberculosis treatment, or time to initiation of antiretroviral treatment. Selke 2010 reported on new WHO clinical stage 3 or 4 diseases, indicating no difference between groups in these reported clinical events. No study reported information indicating a negative impact on healthcare delivery.
Patient satisfaction with care
Assefa 2012 and Humphreys 2010 included a qualitative component to their studies which reports on patient satisfaction with the model of care by group.
Assefa 2012 evaluated patient satisfaction with care by conducting two hour long focused group discussions (57 patients in 7 groups). This study looked predominantly at the issue of task shifting and its acceptability amongst patients and healthcare providers. Patients reported that nurse and health officer (clinical officer) services were 'generally well accepted, and reduced waiting time', they also reported that they were 'more comfortable with nurses than with physicians because nurses were friendlier and more supportive'. Patients emphasised that nurses and health officers spent more time with them discussing their medical problems and took enough time examining them. Patients identified three additional benefits of being involved in ART delivery: their life experience helped them to provide appropriate counselling; it helped combat stigma and discrimination in society; and it provided them with an opportunity for employment. In the same study, focused‐group discussions were held with programme managers and healthcare providers who agreed the model including task shifting provided a timely solution for Ethiopias needs. They also agreed that nurses and health officers can provide high quality care given adequate training and supervision.
Humphreys 2010 was a prospective cohort that used the model of down referral and included the assessment of patient satisfaction as a primary outcome. Those attending the intervention clinic were asked about their level of satisfaction, and 25 of the 31 respondents said that they were very satisfied with the care received. Reasons provided included the reduced cost of transport, being nearer to home, shorter queue, being treated better by staff, receiving better care and that they would not be talked about. The two respondents who were not satisfied with the care complained about the lack of doctor, saying they did not have money to get to the main clinic, and that there was a delay because staff from the hospital arrived late at the health centre.
Discussion
Summary of main results
Three models of care were assessed in the included studies of this review.
In the first model, antiretroviral therapy is initiated at a hospital, and maintained at a health centre (partial decentralisation). When pooling the data, we found moderate quality evidence, favouring lower rates of patient attrition, and low quality evidence of lower rates of patients lost to care and mortality at 12 months (four observational studies). Although the results of the meta‐analysis report lower risk of death and better follow up rates at the health centres, there is substantial uncertainty about these results.
In the second model antiretroviral therapy is initiated and maintained at the health centre (full decentralisation). Our meta‐analysis found moderate quality evidence of lower rates of patients lost to care at the health centre compared to the hospital at 12 months (four observational cohorts). The observational cohort data was upgraded due to the large association reporting a 70% (95% CI 0.17 to 0.54) relative risk reduction in patients lost to care if they attended the health centre, rather than the hospital, for HIV services. There is however the possibility that additional evidence will substantially alter this result. There is very low quality evidence that attrition or death differed between arms whether care was delivered at the hospital or health centre. The quality of the data was downgraded for methodological limitations, as patients tended to be sicker at the hospital, favouring the intervention. These results were also consistent for paediatric populations.
For the third model, community volunteers with basic training delivered antiretroviral healthcare to participants at their homes (two cluster randomised controlled trials). We found moderate quality evidence reporting no difference for death or losses to care at 12 months of follow‐up. The risk of bias in the trials was overall low. Overall we have moderate confidence that there is no difference in these outcomes when community care is introduced, but there is the possibility that further trials may change the results.
With respect to other relevant outcomes, the cohorts reporting CD4+ cell counts showed increases in immunological status, but no difference between models of care was found. Similar results were found for changes in viral load, a marker of the effectiveness of antiretroviral therapy, with studies reporting comparable virological suppression regardless of the model of care employed.
Costs, reported by three studies are considerably reduced in decentralised care for both the patient and provider making decentralisation an attractive option for patients, and possibly assisting in uptake of care closer to home. This is also reflected in the high level of acceptability to patients reported by the two studies in which this was assessed.
Overall completeness and applicability of evidence
There is a recognised need to address HIV health service delivery backlogs and ensure expanded access to HIV care. For this reason, several governments and implementing partners have implemented a decentralised approach to care. The majority of the data included in this review were from retrospective cohorts describing programmes that have rolled out across sub‐Saharan Africa using facility‐based models utilising lower levels of health care, and often accompanied by task shifting to non‐doctors for ART provision.
Retrospective data have several inherent biases. A key concern for the interpretation of these studies is that individuals could choose whether to be down‐referred or not, or their healthcare provider could allocate them according to both objective and subjective assessments, but the methods for this decision was not always clearly reported. In addition, the quality of data collection in these studies is variable, and generally based on a secondary analysis of routinely collected programme data. Another concern is that the models of care, including healthcare provider, training, supervision and mentoring provided and the necessary organisational planning that is required, were likely to differ across the studies. Even within the cohorts, there is a possibility that decentralisation did not occur in a systematic way. These are pragmatic issues that a study design that includes randomisation and concealed allocation of the intervention could address to provide higher quality evidence. The community models of care were evaluated using the cluster trial design which provides moderate quality evidence that community ART delivery can result in acceptable outcomes.
An important limitation of the evidence base, particularly for observational studies, is the potential for misclassification of deaths among patients who are reported as lost to care. A meta‐analysis assessing this issue found that in studies that traced patients who were lost to care to determine their outcomes, 20% to 60% had in fact died (Brinkhof 2009). For this reason we include sensitivity analyses to account for possible deaths amongst those described as lost to care.
Finally, more information is needed on the package of care provided, including training and supervision, to support decentralisation of ART services, particularly to the community.
Quality of the evidence
In the GRADE system, well‐conducted randomised controlled trials (without additional limitations) provide high quality evidence, and observational studies without any special strengths (and without additional limitations) provide low‐quality evidence. The quality of evidence provided by a body of literature comprised exclusively of observational studies would thus generally be graded as low, except in circumstances where observational studies are upgraded.
This review largely comprises observational data, and we found that the quality of evidence reported for the facility based models of decentralisation of care was generally low or very low. Two exceptions to this were the outcome describing attrition in partial decentralisation and patients lost to care utilising the full decentralisation model where there was moderate quality evidence. This data was upgraded from 'low' due to the large effect size.
The model of care evaluating community follow‐up by trained field officers, including two high quality cluster randomised controlled trials provided moderate quality evidence that the care provided by clinicians or field officers was similar in terms of the rate of patients lost to care and death. The data was downgraded due to the low event rate across arms.
Potential biases in the review process
Biases in the review process were minimised by performing a comprehensive search of databases and conference proceedings, not limiting for language or time. In addition, we contacted expert researchers in the field and other experts associated with relevant organisations (e.g. WHO, MSF) for unpublished and ongoing studies. We did not explore publication bias by using funnel plots as there were too few studies to draw conclusions from this analysis.
Agreements and disagreements with other studies or reviews
To date this is the first systematic review evaluating decentralisation of ART care. Prior and ongoing reviews have evaluated task shifting, which is related to decentralisation in that lower‐level health services are generally staffed by lesser‐trained health workers. The findings of this review broadly agree with the task shifting reviews which have found similar outcomes comparing physician and non‐physician led care.
Authors' conclusions
Implications for practice.
The research to date provides no evidence that any model of decentralisation leads to a deterioration in health outcomes. Thus clinicians can be reassured that provision of HIV treatment at lower levels in the health system does not necessarily lead to a serious reduction in the quality of clinical care.
What is more, the findings of this review indicate that in some settings the loss to care is reduced, which is consistent with treatment being more accessible.
More broadly, studies show that decentralisation to lower levels in the health care system is feasible. The studies were of a reasonable size. Nevertheless, they were in the context of a range of support structures and investments to ensuring delivery, including training, supervision and additional devices such as computer‐aided or checklist‐based decision aids. Thus, policy makers and programme managers need to take into account adequate supervision and support when organising widespread delivery of HIV care through the more basic tiers in the health system. This would include referral systems to facilities being in place for those patients who experience complications. Importantly, several studies provide data indicating reduced costs for both patients and services when patients attend facilities closer to their homes.
Implications for research.
Additional high quality evidence from trials and prospective cohorts would provide valuable insight on the key outcomes including retention in care, survival, quality of care and costs of decentralising care from hospitals to other tiers of the health care system. Implementation science research, using pragmatic trial designs would be helpful in testing the types of packages of care that best suit various settings and provide high quality care at decentralised sites.
History
Protocol first published: Issue 7, 2012 Review first published: Issue 6, 2013
| Date | Event | Description |
|---|---|---|
| 8 June 2012 | Amended | New author, FBA added to team |
| 13 March 2009 | Amended | Converted to new review format. |
Acknowledgements
The Effective Health Care Research Consortium, including Paul Garner, are funded by UKaid from the UK Government for the benefit of developing countries. We would also like to acknowledge the assistance of the South African Cochrane Centre. Thanks for the support from the Cochrane HIV/AIDS Review Group, University of California, San Francisco.
Appendices
Appendix 1. Central search strategy
Database: CLIB Issue 4 of 12, April 2012 (1996 ‐ 2012)
Date: 3 May 2012
| ID | Search |
| #1 | MeSH descriptor HIV Infections explode all trees |
| #2 | MeSH descriptor HIV explode all trees |
| #3 | hiv OR hiv‐1* OR hiv‐2* OR hiv1 OR hiv2 OR HIV INFECT* OR HUMAN IMMUNODEFICIENCY VIRUS OR HUMAN IMMUNEDEFICIENCY VIRUS OR HUMAN IMMUNE‐DEFICIENCY VIRUS OR HUMAN IMMUNO‐DEFICIENCY VIRUS OR HUMAN IMMUN* DEFICIENCY VIRUS OR ACQUIRED IMMUNODEFICIENCY SYNDROME OR ACQUIRED IMMUNEDEFICIENCY SYNDROME OR ACQUIRED IMMUNO‐DEFICIENCY SYNDROME OR ACQUIRED IMMUNE‐DEFICIENCY SYNDROME OR ACQUIRED IMMUN* DEFICIENCY SYNDROME |
| #4 | MeSH descriptor Lymphoma, AIDS‐Related, this term only |
| #5 | MeSH descriptor Sexually Transmitted Diseases, Viral, this term only |
| #6 | (#1 OR #2 OR #3 OR #4 OR #5) |
| #7 | MeSH descriptor Antiretroviral Therapy, Highly Active, this term only |
| #8 | MeSH descriptor Anti‐HIV Agents explode all trees |
| #9 | MeSH descriptor Antiviral Agents, this term only |
| #10 | MeSH descriptor AIDS Vaccines, this term only |
| #11 | ANTI HIV OR ANTIRETROVIRAL* OR ANTI RETROVIRAL* OR AIDS VACCIN* |
| #12 | (#7 OR #8 OR #9 OR #10 OR #11) |
| #13 | (#6 AND #12) |
| #14 | MeSH descriptor Hospitals explode all trees |
| #15 | (healthcare NEAR/6 (facility OR facilities OR centre OR centres OR center OR centers)):ti,ab |
| #16 | (health NEAR/6 (facility OR facilities OR centre OR centres OR center OR centers)):ti,ab |
| #17 | hospital:ti,ab OR hospitals:ti,ab OR clinic:ti,ab OR clinics:ti,ab |
| #18 | home based:ti,ab OR facility based:ti,ab OR home care:ti,ab OR facility care:ti,ab |
| #19 | MeSH descriptor Health Facilities, this term only |
| #20 | (#14 OR #15 OR #16 OR #17 OR #18 OR #19) |
| #21 | MeSH descriptor Delivery of Health Care, this term only |
| #22 | MeSH descriptor Health Services Accessibility, this term only |
| #23 | MeSH descriptor Home Care Services, this term only |
| #24 | (healthcare NEAR/6 (accessibility OR access OR system OR systems OR delivery)):ti,ab |
| #25 | (health care NEAR/6 (accessibility OR access OR system OR systems OR delivery)):ti,ab |
| #26 | (health services NEAR/6 (accessibility OR access OR system OR systems OR delivery)):ti,ab |
| #27 | (#21 OR #22 OR #23 OR #24 OR #25 OR #26) |
| #28 | (#13 AND #20 AND #27) |
| #29 | (#13 AND #20 AND #27), from 1996 to 2012 |
Appendix 2. Medline search strategy
Database: PubMed (1996 ‐ 2012
Date: 2 May 2012
| Search | Query |
| #7 | Search #3 AND #4 AND #5 Limits: Publication Date from 1996/01/01 to 2012/05/02 |
| #6 | Search #3 AND #4 AND #5 |
| #5 | Search (health[tiab] OR healthcare[tiab] AND (facility[tiab] OR facilities[tiab] OR centre[tiab] OR centres[tiab] OR center[tiab] OR centers[tiab])) OR hospital[tiab] OR hospitals[tiab] OR clinic[tiab] OR clinics[tiab] OR health facilities[mh] OR home based[tiab] OR facility based[tiab] OR home care[tiab] OR facility care[tiab] |
| #4 | Search (healthcare[tiab] OR health care[tiab] OR health services[tiab] AND (access[tiab] OR accessibility[tiab] OR system[tiab] OR systems[tiab] OR delivery[tiab])) OR delivery of health care[mh:noexp] OR health services accessibility[mh:noexp] OR decentrali*[tiab] OR referr*[tiab] OR home care services[mh:noexp] |
| #3 | Search #1 AND #2 |
| #2 | Search Antiretroviral Therapy, Highly Active[MeSH] OR Anti‐Retroviral Agents[MeSH] OR Antiviral Agents[MeSH:NoExp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])) |
| #1 | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) |
Appendix 3. Embase search strategy
Database: EMBASE (1996 ‐ 2012)
Date: 3 May 2012
| No. | Query |
| #16 | #3 AND #8 AND #14 AND [humans]/lim AND [embase]/lim AND [1996‐2012]/py |
| #15 | #3 AND #8 AND #14 |
| #14 | #9 OR #10 OR #11 OR #12 OR #13 |
| #13 | 'home based':ab,ti OR 'facility based':ab,ti OR 'home care'/de OR 'home care' OR 'facility care':ab,ti |
| #12 | hospital:ab,ti OR hospitals:ab,ti OR clinic:ab,ti OR clinics:ab,ti |
| #11 | (health NEAR/6 (facility OR facilities OR centre OR centres OR center OR centers)):ab,ti |
| #10 | (healthcare NEAR/6 (facility OR facilities OR centre OR centres OR center OR centers)):ab,ti |
| #9 | 'health care facility'/exp OR 'health care facility' |
| #8 | #4 OR #5 OR #6 OR #7 |
| #7 | 'health care delivery'/de OR decentrali*:ab,ti OR referr*:ab,ti |
| #6 | ('health services' NEAR/6 (delivery OR accessibility OR system OR systems OR access)):ab,ti |
| #5 | ('health care' NEAR/6 (delivery OR accessibility OR system OR systems OR access)):ab,ti |
| #4 | (healthcare NEAR/6 (delivery OR accessibility OR system OR systems OR access)):ab,ti |
| #3 | #1 AND #2 |
| #2 | 'human immunodeficiency virus vaccine'/de OR 'human immunodeficiency virus vaccine' OR 'anti human immunedeficiency':ti OR 'anti human immunedeficiency':ab OR 'anti human immunodeficiency':ti OR 'anti human immunodeficiency':ab OR 'anti human immuno‐deficiency':ti OR 'anti human immuno‐deficiency':ab OR 'anti human immune‐deficiency':ti OR 'anti human immune‐deficiency':ab OR 'anti acquired immune‐deficiency':ti OR 'anti acquired immune‐deficiency':ab OR 'anti acquired immunedeficiency':ti OR 'anti acquired immunedeficiency':ab OR 'anti acquired immunodeficiency':ti OR 'anti acquired immunodeficiency':ab OR 'anti acquired immuno‐deficiency':ti OR 'anti acquired immuno‐deficiency':ab OR 'anti hiv':ti OR 'anti hiv':ab OR antiretrovir*:ti OR antiretrovir*:ab OR 'anti retroviral':ti OR 'anti retroviral':ab OR 'anti retrovirals':ti OR 'anti retrovirals':ab OR 'anti retrovirus':ti OR 'anti retrovirus':ab OR haart:ti OR haart:ab OR 'aids vaccine':ti OR 'aids vaccine':ab OR 'aids vaccines':ti OR 'aids vaccines':ab OR 'anti human immunodeficiency virus agent'/de OR 'anti human immunodeficiency virus agent' OR 'antiretrovirus agent'/de OR 'antiretrovirus agent' OR 'antivirus agent'/de OR 'antivirus agent' OR 'highly active antiretroviral therapy'/de OR 'highly active antiretroviral therapy' |
| #1 | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/de OR 'human immunodeficiency virus' OR 'b cell lymphoma'/de OR 'b cell lymphoma' OR hiv:ti OR hiv:ab OR 'hiv‐1':ti OR 'hiv‐1':ab OR 'hiv‐2':ti OR 'hiv‐2':ab OR 'human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab OR 'human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab OR 'human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab OR 'human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab OR 'acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab OR 'acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab OR 'acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab OR 'acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab |
Appendix 4. GRADE Evidence profile for partial decentralisation model
Question: Should antiretroviral therapy initiated in a hospital, maintained at a health centre be used in HIV infected patients? Settings: Lower‐ and middle‐income countries
| Quality assessment | No of patients | Effect | Quality | Importance | |||||||||
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Antiretroviral therapy initiated in a hospital, maintained at a health centre | Control | Relative (95% CI) | Absolute | |||
| Death or lost to care (follow‐up 12 months) | |||||||||||||
| 4 | observational studies | no serious risk of bias | no serious inconsistency1 | no serious indirectness2 | no serious imprecision | strong association3 | 3829/23767 (16.1%) | 3337/15323 (21.8%) | RR 0.46 (0.29 to 0.71) | 118 fewer per 1000 (from 63 fewer to 155 fewer) | ⊕⊕⊕Ο MODERATE | CRITICAL | |
| Lost to care (follow‐up 12 months4) | |||||||||||||
| 4 | observational studies | no serious risk of bias | no serious inconsistency5 | no serious indirectness2 | no serious imprecision | none | 2229/23767 (9.4%) | 2055/15323 (13.4%) | RR 0.55 (0.45 to 0.69) | 60 fewer per 1000 (from 42 fewer to 74 fewer) | ⊕⊕ΟΟ LOW | CRITICAL | |
| Death (follow‐up 12 months6) | |||||||||||||
| 4 | observational studies | no serious risk of bias7 | no serious inconsistency7,8 | no serious indirectness2 | no serious imprecision | none9 | 1600/23767 (6.7%) | 1282/15323 (8.4%) | RR 0.34 (0.13 to 0.87) | 55 fewer per 1000 (from 11 fewer to 73 fewer) | ⊕⊕ΟΟ LOW | CRITICAL | |
1 No serious inconsistency. All four studies report a decrease in attrition at 12 months. 2 Not downgraded for indirectness. The studies included adults (two studies), children (1 study) or both (1 study); and were conducted in sub‐Saharan Africa (South Africa, Malawi). 3 Upgraded by 1 for large effect size. The effect estimate indicated a 54% decrease in attrition in the decentralised group. 4 Adjusted rates for Brennan 2011, Chan 2010 and Fatti 2010 are consistent with the crude proportions reported here. In Brennan 2011, the adjusted hazard ratio was 0.3 (95% CI 0.2 to 0.6)/ 100 person years indicating better outcomes at the health centre. Chan 2010 reported an adjusted odds ratio of 0.48 (95% CI 0.4 to 0.58) indicating better outcomes at the health centre. Fatti 2010 presented the results inversing the site of risk, the adjusted hazard ratio was 2.19 (1.94 to 2.24) indicating greater problems with patients failing to attend the hospital. 5 No serious inconsistency. Three of the four studies show benefit with varied effect sizes (39%. 51% and 66% reduction in patients lost to care), the smallest study reports no difference in clinic follow‐up at 12 months. 6 Adjusted rates for Brennan 2011, Chan 2010 and Fatti 2010 are consistent with the crude proportions reported here. In Brennan 2011, the adjusted hazard ratio was 0.2 (95% CI 0.04 to 0.8)/ 100 person years indicating better outcomes at the health centre. Chan 2010 reported an adjusted odds ratio of 0.19 (95% CI 0.15 to 0.25) indicating better outcomes at the health centre. Fatti 2010 presented the results inversing the site of risk, the adjusted hazard ratio was 1.6 (95% CI 1.3 to 1.99) indicating relatively increased risk of death in patients attending the hospital. 7 Not downgraded for methodological limitations. For one included study (Fatti 2010), the health centre group had balanced CD4 cell counts, but more severe illness ‐ 79% had WHO clinical stage III or IV disease compared with 58% in the hospital group. However, this would tend to favour the hospital group so we did not downgrade on baseline imbalance. 8 No serious inconsistency. All four studies show decrease in death at 12 months with varied effect sizes (10%, 74%, 77% and 81% reductions). 9 Not upgraded for large effect size, despite large effect size and narrow confidence interval, this review is not aiming to explore whether decentralisation decreases death, rather excluding that it increases death. The model of care down refers healthier patients for maintenance therapy, generally sicker patients remain at the hospital setting, this therefore favours decentralisation.
Appendix 5. GRADE Evidence profile for full decentralisation model
Question: Should antiretroviral therapy be started and maintained in health centre be used in HIV infected patients? Settings: Lower‐ and middle‐income countries
| Quality assessment | No of patients | Effect | Quality | Importance | |||||||||
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Antiretroviral therapy be started and maintained in health centre | Control | Relative (95% CI) | Absolute | |||
| Death or lost to care (follow‐up 12 months) | |||||||||||||
| 4 | observational studies | serious1 | no serious inconsistency2 | no serious indirectness3 | serious4 | none | 3895/17753 (21.9%) | 14102/38607 (36.5%) | RR 0.7 (0.47 to 1.02) | 110 fewer per 1000 (from 194 fewer to 7 more) | ⊕ΟΟΟ VERY LOW | CRITICAL | |
| Lost to care (follow‐up 12 months) | |||||||||||||
| 4 | observational studies | no serious risk of bias5 | no serious inconsistency6 | no serious indirectness3 | no serious imprecision | strong association7 | 2070/17753 (11.7%) | 10429/38607 (27%) | RR 0.3 (0.17 to 0.54) | 189 fewer per 1000 (from 124 fewer to 224 fewer) | ⊕⊕⊕Ο MODERATE | CRITICAL | |
| Death (follow‐up 12 months) | |||||||||||||
| 4 | observational studies | serious1 | serious8 | no serious indirectness3 | serious9 | none | 1825/17065 (10.7%) | 3673/38034 (9.7%) | RR 1.1 (0.63 to 1.92) | 10 more per 1000 (from 36 fewer to 89 more) | ⊕ΟΟΟ VERY LOW | CRITICAL | |
1 Downgraded by 1 for methodological limitations. Bedelu 2008, McGuire 2013 and Massaquoi 2009 included sicker patients at the hospital setting, Assefa has unknown baseline risk as the CD4 counts and other baseline characteristics were not reported. This bias would tend to favour therapy provided at the health centre. 2 Not downgraded for inconsistency. Three studies report significantly reduced attrition with decentralisation (13%, 42% and 52%), while one study reported no difference. 3 Not downgraded for indirectness. The studies included adults (3 studies) or adults and children (1 study); and were conducted in sub‐Saharan Africa (South Africa, Malawi and Ethiopia). This model of care is probably applicable in better resourced settings where basic levels of healthcare are likely to be better resourced, favouring decentralisation. 4 Downgraded by 1 for imprecision. Although the sample sizes are large and event rates are high, the confidence interval is wide including both appreciable benefit and the null effect. 5 Not downgraded for risk of bias. Four retrospective cohorts provided data. Although there were differences in their baseline health status (Bedelu 2008, Massaquoi 2009 and McGuire 2012 included sicker patients at the hospital), this study limitation is not expected to impact on the attendance at the clinic. 6 Not downgraded for inconsistency. All four studies showed substantially better clinic attendance with decentralisation, however, the effect sizes varied, 24%, 63%, 80% and 89% reductions. 7 Upgraded by 1 for large effect size . The effect size indicates a 70% lower rate of failure to attend clinic follow‐up at the health center compared to hospital. 8 Downgraded for inconsistency.There is qualitative heterogeneity, Bedelu 2008, Massaquoi 2009 and McGuire 2013 include sicker patients at the hospital, yet only McGuire showed increased death in that setting. Therefore the inconsistency is unexplained. 9 Downgraded by 1 for imprecision. Although the sample sizes are large and event rates are high, the confidence interval is wide including both appreciable benefit and harm.
Appendix 6. GRADE evidence profile community model of care
Question: Should decentralisation from the facility to the community for antiretroviral maintenance therapy be used in HIV‐infected patients on antiretroviral therapy? Settings: Lower‐ and middle‐income countries
| Quality assessment | No of patients | Effect | Quality | Importance | |||||||||
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Decentralisation from the facility to the community for antiretroviral maintenance therapy | Control | Relative (95% CI) | Absolute | |||
| Death or lost to care (follow‐up 12 months) | |||||||||||||
| 2 | randomised trials | no serious risk of bias | no serious inconsistency | no serious indirectness1 | serious2 | none | 43/399 (10.8%) | 33/310 (10.6%) | RR 0.95 (0.62 to 1.46) | 5 fewer per 1000 (from 40 fewer to 49 more) | ⊕⊕⊕Ο MODERATE | CRITICAL | |
| Lost to care (follow‐up 12 months3) | |||||||||||||
| 2 | randomised trials | no serious risk of bias | no serious inconsistency | no serious indirectness1 | serious2 | none | 7/399 (1.8%) | 8/310 (2.6%) | RR 0.81 (0.3 to 2.21) | 5 fewer per 1000 (from 18 fewer to 31 more) | ⊕⊕⊕Ο MODERATE | CRITICAL | |
| Death (follow‐up 12 months4) | |||||||||||||
| 2 | randomised trials | no serious risk of bias | no serious inconsistency | no serious indirectness1 | serious2 | none | 37/399 (9.3%) | 5.5% | RR 1.03 (0.64 to 1.65) | 2 more per 1000 (from 20 fewer to 36 more) | ⊕⊕⊕Ο MODERATE | CRITICAL | |
1 Not downgraded for indirectness. Note that the trials were conducted in Kenya and Uganda in adult populations. 2 Downgraded by 1 for imprecision. These two cluster trials have been pooled after adjusting for the design effect. The intra‐cluster co‐efficient was assumed, as it was not provided in the trial reports.The included studies have small sample sizes and wide confidence intervals which include appreciable harm and benefit. 3 The cluster randomised controlled trials Selke 2010 and Jaffar 2009 are included in this pooled analysis. Selke 2010 reports the adjusted incidence rate ratio for patients lost to care as IRR 1.15 (95% CI 0.24 to 3.03), P = 1.0 4 The cluster randomised controlled trials Selke 2010 and Jaffar 2009 are included in this pooled analysis. Jaffar 2009 reports the adjusted rate ratio for death, RR 0.95(95% CI 0.71 to 1.28); Selke 2010 did not provide adjusted rates for this outcome.
Data and analyses
Comparison 1. Partial decentralisation ‐ initiation in hospital, maintenance at health centre.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or lost to care (12 months) | 4 | 39090 | Risk Ratio (IV, Random, 95% CI) | 0.46 [0.29, 0.71] |
| 1.1 Adults | 2 | 29492 | Risk Ratio (IV, Random, 95% CI) | 0.49 [0.21, 1.12] |
| 1.2 Children | 1 | 1505 | Risk Ratio (IV, Random, 95% CI) | 0.45 [0.27, 0.74] |
| 1.3 Adults and children | 1 | 8093 | Risk Ratio (IV, Random, 95% CI) | 0.39 [0.35, 0.43] |
| 2 Lost to care | 6 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Lost to care (6 months) | 3 | 28699 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.56, 1.76] |
| 2.2 Lost to care (12 months) | 4 | 39090 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.45, 0.69] |
| 2.3 Lost to care (24 months) | 1 | 543 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Death | 6 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Death (6 months) | 3 | 28699 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.19, 1.41] |
| 3.2 Death (12 months) | 4 | 39090 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.13, 0.87] |
| 3.3 Death (24 months) | 1 | 543 | Risk Ratio (IV, Random, 95% CI) | 0.04 [0.00, 0.58] |
| 4 Cost of travel | Other data | No numeric data | ||
| 4.1 cost of travel | Other data | No numeric data |
1.1. Analysis.
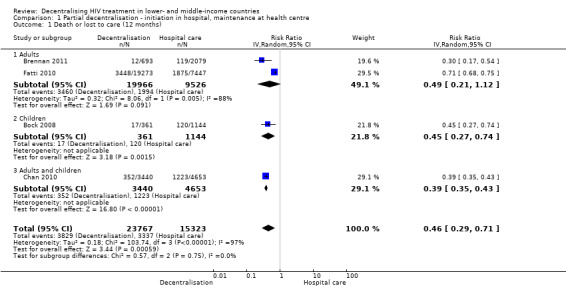
Comparison 1 Partial decentralisation ‐ initiation in hospital, maintenance at health centre, Outcome 1 Death or lost to care (12 months).
Comparison 2. Full decentralisation ‐ initiation and maintenance in health centre.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or lost to care (12 months) | 4 | 56360 | Risk Ratio (IV, Random, 95% CI) | 0.70 [0.47, 1.02] |
| 1.1 Adults | 3 | 52286 | Risk Ratio (IV, Random, 95% CI) | 0.62 [0.39, 0.99] |
| 1.2 Adults and children | 1 | 4074 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.82, 1.15] |
| 2 Lost to care | 6 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Lost to care (6 months) | 2 | 51261 | Risk Ratio (IV, Random, 95% CI) | 0.53 [0.26, 1.10] |
| 2.2 Lost to care (12 months) | 4 | 56360 | Risk Ratio (IV, Random, 95% CI) | 0.30 [0.17, 0.54] |
| 2.3 Lost to care (24 months) | 4 | 61445 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.36, 0.71] |
| 3 Death | 6 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Death (6 months) | 2 | 50000 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.35, 2.00] |
| 3.2 Death (12 Months) | 4 | 55099 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.63, 1.92] |
| 3.3 Death (24 months) | 4 | 60184 | Risk Ratio (IV, Random, 95% CI) | 0.64 [0.39, 1.06] |
2.1. Analysis.
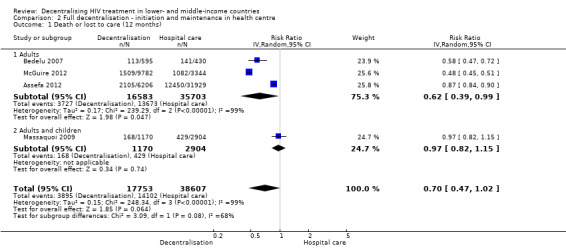
Comparison 2 Full decentralisation ‐ initiation and maintenance in health centre, Outcome 1 Death or lost to care (12 months).
Comparison 3. Decentralisation ‐ from the facility to the community for antiretroviral maintenance therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or lost to care (12 months) | 2 | 709 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.62, 1.46] |
| 2 Lost to care | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Lost to care (6 months) | 1 | 385 | Risk Ratio (IV, Random, 95% CI) | 1.49 [0.81, 2.74] |
| 2.2 Lost to care (12 months) | 2 | 709 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.30, 2.21] |
| 2.3 Lost to care (24 months) | 1 | 385 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.46, 1.20] |
| 3 Death | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Death (6 months) | 1 | 385 | Risk Ratio (IV, Random, 95% CI) | 1.44 [0.81, 2.57] |
| 3.2 Death (12 months) | 2 | 709 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.64, 1.65] |
| 3.3 Death (24 months) | 1 | 385 | Risk Ratio (IV, Random, 95% CI) | 1.50 [0.91, 2.47] |
| 4 Cost to patient | Other data | No numeric data |
3.1. Analysis.
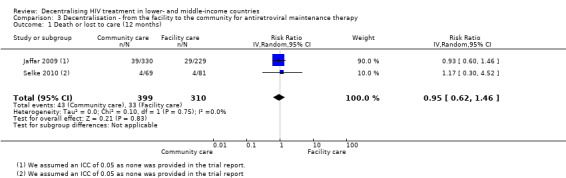
Comparison 3 Decentralisation ‐ from the facility to the community for antiretroviral maintenance therapy, Outcome 1 Death or lost to care (12 months).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Assefa 2012.
| Methods | Design: Retrospective cohort Duration of study: Recruitment Sept 2006‐2008, censored March 2009 (minimum 6 months, maximum 24 months follow‐up) |
|
| Participants | Country: Ethiopia Setting: Nationwide, 30 hospitals, 25 health centres Inclusion and exclusion criteria: None described Comparable CD4 count or clinical stage at baseline: Similar CD4 count |
|
| Interventions | Intervention: Patients initiated and maintained at health centres by nurses and health officers. Severe manifestations, treatment failures were referred to hospital Control: Initiated and followed up at hospital with physicians. Co‐interventions: Community health workers performed counselling, referrals and linkage between facilities and defaulter tracing, not clear if this was provided at all sites |
|
| Outcomes | Mortality, loss to follow‐up, retention, and median CD4+ cell count, Assessed at 6, 12 and 24 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Low risk | Similar median CD4 counts at baseline in both groups |
| Other baseline variables (All studies) | Unclear risk | Not described |
| Co‐interventions (All studies) | Unclear risk | Community health workers delivered adherence and referral services from health centres to hospitals, unclear whether this was for both groups for the health centre group only |
| Data collection (Cohorts) | High risk | Retrospective cohort |
| Patient selection bias (Cohorts) | Low risk | Randomly selected folders in all included sites in both groups |
Balcha 2010.
| Methods | Design: Retrospective cohort Duration: February 2007 ‐ February 2009, 6 months post censor follow‐up |
|
| Participants | Country: Ethiopia Setting: Rural and urban, in one region, 3 health centres, 2 hospitals Inclusion criteria: Adults eligible for antiretroviral treatment (CD4 <200cell/mm3 or WHO clinical stage 3 or 4) and on treatment for < 6 months Exclusion: HIV infected, but not on antiretroviral therapy Comparable CD4 count or clinical stage at baseline: Similar CD4 count |
|
| Interventions | Intervention: Initiation on antiretrovirals in health centre, maintenance in health centre; provided by nurses and health officers Control: Initiated and maintained in hospital, provided by doctors Co‐interventions: None described |
|
| Outcomes | Currently alive and on treatment, loss to follow‐up, transferred out, mortality Assessed at 24 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Low risk | Similar median CD4 in both groups |
| Other baseline variables (All studies) | Unclear risk | Only data on sex reported by arm |
| Co‐interventions (All studies) | Unclear risk | No co‐intervention information described |
| Data collection (Cohorts) | High risk | Retrospective cohort |
| Patient selection bias (Cohorts) | Low risk | No selection bias identified |
Bedelu 2007.
| Methods | Design: Retrospective cohort Duration: January 2004 ‐ June 2005, completed 12 months follow‐up by July 2006 |
|
| Participants | Country: South Africa Setting: Rural, 12 health centres, 1 hospital Inclusion criteria: Adults, eligible for ART CD4+ cell count < 200cells/mm3, WHO clinical stage 4 Exclusion criteria: None described Comparable CD4+ cell count or clinical stage at baseline: CD4+ cell counts differed at baseline (sicker at hospital) |
|
| Interventions | Intervention: ART initiated and maintained at health centre by nurses, physician support with mobile team, adherence counsellors and patient support groups available Control: ART initiated and maintained at hospital by doctors |
|
| Outcomes | Mortality, loss to follow‐up, CD4 count, viral load Assessed at 12 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | High risk | CD4+ cell counts differed between groups at baseline, lower CD4+ cell counts remained at the hospital |
| Other baseline variables (All studies) | Unclear risk | Only reported on sex of included participants |
| Co‐interventions (All studies) | High risk | Model differed by group: The health centre group received additional adherence support and visits from a mobile support team of experienced clinicians |
| Data collection (Cohorts) | High risk | Retrospective cohort |
| Patient selection bias (Cohorts) | Low risk | No selection bias identified |
Bock 2008.
| Methods | Design: Retrospective cohort Duration: April 2004 ‐ April 2006, no follow‐up post censor described |
|
| Participants | Country: South Africa Setting: Urban, peri‐urban, 20 health centres (enhanced), 16 hospitals, 3 advanced hospitals Inclusion criteria: Children <15 years, eligible for ART (modified WHO stage 2, 3 disease, or low CD4% by age group ‐ <20% if <18 months old, or <15% if >18 months old), recurrent hospitalisation >4 weeks, and identifiable caregiver Exclusion criteria: Previous exposure to ART for >1 month (treatment experienced), transferred in or out of antiretroviral treatment site Comparable CD4+ cell count or clinical stage at baseline: Sicker children with lower CD4 % at hospital and advanced hospital |
|
| Interventions | Intervention (Primary health care clinics): ART initiated at advanced hospital, maintained at enhanced health centres, by doctors Intervention (Level 1 district hospitals): ART initiated at advanced hospital, maintained at hospital, by doctors Control (level 2 and 3 facilities): ART initiated and maintained at advanced hospital, by doctors and specialists |
|
| Outcomes | Death, loss to follow‐up, virological suppression, CD4 % changes, change to second line treatment Assessed at 6, 12 and 18 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | High risk | Sicker children with lower CD4 % at hospital and advanced disease remained in hospital |
| Other baseline variables (All studies) | Unclear risk | Not described |
| Co‐interventions (All studies) | Unclear risk | Not described |
| Data collection (Cohorts) | High risk | Retrospective cohort |
| Patient selection bias (Cohorts) | Low risk | Although stable patients could be transferred to the PHC group while sicker children requiring specialist care were transferred to advanced hospital, this group of patients were excluded from the analysis. |
Brennan 2011.
| Methods | Design: Retrospective matched cohort analysis Duration: April 2004 ‐ January 2009 |
|
| Participants | Country: South Africa Setting: Peri‐urban, urban, 1 hospital, 1 clinic Inclusion criteria: Stable on antiretroviral treatment for at least 11 months, no opportunistic infections, CD4+ cell count > 200cells/mm3, stable weight and virologically suppressed <400 copies/mL. Considered good candidates by doctors and agree to down‐referral Exclusion criteria: Refused down referral Comparable CD4 count or clinical stage at baseline: Control matched on sex, age, months on therapy, treatment regimen, BMI, HB and CD4+ cell count (propensity scoring) |
|
| Interventions | Intervention: ART initiated at advanced hospital by doctors, maintained at health centre by nurses, seen every 2 months for medicine pick up. "Up referred" if default (>7 days), toxicity, detectable viral load Control: ART initiated and maintained by doctor at advanced hospital, seen 6 monthly, pick up medicines every 2 months Co‐interventions: Adherence counselling provided at both facilities |
|
| Outcomes | Death, loss to follow‐up, mean CD4+ cell count, viral load rebound Assessed at 12 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Low risk | Matched by propensity scores on all baseline characteristics |
| Other baseline variables (All studies) | Low risk | Matched by propensity scores on all baseline characteristics |
| Co‐interventions (All studies) | Low risk | Both groups received adherence counselling |
| Data collection (Cohorts) | High risk | Retrospective cohort |
| Patient selection bias (Cohorts) | Low risk | All participants were equally eligible for down referral, and were matched using propensity scores on baseline characteristics |
Chan 2010.
| Methods | Design: Retrospective cohort Duration: October 2004 ‐ 31 December 2008, censored 31 December 2008, maximum follow‐up 50 months |
|
| Participants | Country: Malawi Setting: Rural, Zomba district, 16 health centres and 1 hospital Inclusion criteria: Adults and older children eligible for antiretroviral therapy (CD4+ cell count <250 cells/mm3, WHO clinical stage 3 or 4), on treatment for >3 months and stable, no evidence of opportunistic infections or drug intolerance, provider confidence in patient adherence, live closer to health centre than hospital Exclusion criteria: None described Comparable CD4+ cell count or clinical stage at baseline: Earlier stage disease at intervention site, more men, children and advanced disease at control site |
|
| Interventions | Intervention: ART initiated at advanced hospital, maintained at health centre, seen by nurses and clinical officers, home‐based peer support and health surveillance assistants for defaulter tracing, expert patients, nutrition counsellors, volunteers from the community Control: ART initiated and maintained at hospital, by clinical officers, adherence counsellor and specialist support Co‐intervention: Paediatric and adult specialist support at both sites |
|
| Outcomes | Death, loss to follow‐up (not seen at facility for >3m) Unknown time of outcome reporting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Unclear risk | Not reported |
| Other baseline variables (All studies) | High risk | Healthier at peripheral site: Earlier WHO stage, more women and adults, differing baseline characteristics. |
| Co‐interventions (All studies) | High risk | Paediatric and adult specialist infectious diseases support at both sites, via mobile visits for health centres. In addition, the intervention group had many antiretroviral therapy counsellors health surveillance assistants, peer home based care providers and community volunteers to support adherence. |
| Data collection (Cohorts) | High risk | Retrospective cohort |
| Patient selection bias (Cohorts) | High risk | Only stable patients, on treatment for > 3 months with no opportunistic infections or signs of adverse effects of the medication were decentralised to intervention arm. |
Fatti 2010.
| Methods | Design: Retrospective cohort Duration: December 2004 ‐ December 2007, followed up until March 2008 |
|
| Participants | Country: South Africa Setting: Four provinces, peri‐urban and rural, 47 health centres (enhanced), 9 hospitals and 3 advanced hospitals Inclusion criteria: Adults >16 years with CD4+ cell count <200cells/mm3 or WHO clinical stage 4, documented date of birth, gender and date of starting antiretroviral therapy Exclusion criteria: Missing demographic data, antiretroviral therapy experienced, starting antiretroviral therapy after 31 December 2007 Comparable CD4 cell count or clinical stage at baseline: CD4+ cell count clinically similar at baseline (median range 109‐113 cells/mm3), but more advanced disease (WHO clinical stage 3 or 4) at primary care facilities |
|
| Interventions | Intervention: ART initiated at hospital by doctor and maintained at health centre (enhanced) by doctors Control: ART initiated and maintained at hospital by doctors Co‐interventions: Adherence counselling by community support workers |
|
| Outcomes | Death. loss to follow‐up, virological suppression reported at 12, 24 and 36 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Low risk | Similar CD4+ cell counts between groups |
| Other baseline variables (All studies) | High risk | Health centres had patients with more advanced disease by WHO clinical stage (note bias in favour of control) |
| Co‐interventions (All studies) | Low risk | Adherence support provided by community‐based adherence counsellor, linking with losses and detecting deaths |
| Data collection (Cohorts) | High risk | Retrospective cohort |
| Patient selection bias (Cohorts) | Low risk | No evidence of patient selection bias |
Fayorsey 2013.
| Methods | Design: Retrospective cohort Duration:January 2008 to March 2010 |
|
| Participants | Country: Kenya. Lesotho, Mozambique, Rwanda and Tanzania Setting: 274 sites, all receiving funding from the Presidents Emergency Plan for AIDS Relief Inclusion criteria: Children < 15 years old Exclusion criteria: if initiated on therapy before study period or at another facility they were excluded from analysis Comparable CD4 cell count or clinical stage at baseline: no data on CD4 counts at baseline or other health related variables |
|
| Interventions | Intervention: ART initiated and maintained at health centres by doctor or nurse (43%) Control: ART initiated and maintained at hospital by doctors or nurse (42%) Co‐interventions:differed by country and site and included nutrition support, outreach services, support groups, PEER educator programme and adherence counselling. |
|
| Outcomes | Loss to follow‐up (not having made a clinic visit or pharmacy pick up in 90 days); mortality (documented death in clinic records) | |
| Notes | Primary health facilities (health centres in our model) included health centres and clinics, secondary health facilities (hospitals) included district, sub‐district and provincial hospitals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Unclear risk | Not stated |
| Other baseline variables (All studies) | Unclear risk | None provided |
| Co‐interventions (All studies) | High risk | Variable by country and setting, therefore not known |
| Data collection (Cohorts) | High risk | Retrospective cohort |
| Patient selection bias (Cohorts) | Low risk | No evidence of selection bias |
Hansudewechakul 2012.
| Methods | Design: Retrospective cohort Duration: February 2002 to April 2008 |
|
| Participants | Country: Thailand Setting: Community‐based paediatric HIV care and treatment network, training and supervision were provided Inclusion criteria: Children, stable on treatment prior to referral (absence of opportunistic infections and improved weight and CD4%) Exclusion criteria: ART experienced, follow up < 6 months, opportunistic infections, on protease inhibitor Comparable CD4 cell count or clinical stage at baseline: Median CD4% and viral load was similar, but more CDC stage C at health centre (25% at hospital and 40% at health centre) |
|
| Interventions | Intervention: ART initiated at hospital by doctors and maintained at health centres by nurses, under doctor attendance Control: ART initiated and maintained at hospital by doctors Co‐interventions: Team included nurses/ counsellors, people living with HIV, pharmacists and physicians, adherence monitoring conducted at both sites. This model included mentoring, emails, phone calls and discussion between health centres and hospital |
|
| Outcomes | Mortality, loss to follow‐up, weight for age score, adherence, CD4%, viral load change | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Low risk | Balanced CD4% at baseline |
| Other baseline variables (All studies) | Low risk | Baseline variables balanced. |
| Co‐interventions (All studies) | Low risk | There was mentoring and support for health centres health staff maintaining ART, however this is part of the model of care and not expected to bias the results. |
| Data collection (Cohorts) | High risk | Retrospective cohort |
| Patient selection bias (Cohorts) | Low risk | Stable patients were referred to the health centres, however, the analysis excluded patients who were ill with opportunistic infections, or who were on a protease inhibitor, and the baseline characteristics were similar |
Humphreys 2010.
| Methods | Design: Prospective cohort Duration: Started recruitment January 2007 ‐ June 2007, followed up until November 2007, minimum 6 months follow‐up |
|
| Participants | Country: Swaziland Setting: Rural setting, one district hospital, 30 nurse led health centres Inclusion criteria: Adults >14 years on antiretroviral therapy for at least 4 weeks, CD4+ cell count >100 cells/mm3 Exclusion criteria: refused to be down referred Comparable CD4+ cell count or clinical stage at baseline: CD4+ cell count and clinical stage similar at baseline |
|
| Interventions | Intervention: ART initiated at hospital by doctor and maintained at health centre by nurses Control: ART initiated and maintained at hospital by doctors Co‐interventions: Training for primary care centre nurses, monthly outreach support visit by at least one counsellor and nurse |
|
| Outcomes | Clinic attendance, patient experience, loss to follow‐up, change in CD4 count, weight, death | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Low risk | Similar mean CD4+ cell counts between groups |
| Other baseline variables (All studies) | Low risk | Age, sex, and weight were similar at baseline |
| Co‐interventions (All studies) | Unclear risk | No additional intervention described, other than mobile support team visits monthly which are part of the model of care |
| Data collection (Cohorts) | Low risk | Prospective Cohort |
| Patient selection bias (Cohorts) | Low risk | Assignment was based on catchment areas (intervention clinics / control clinics) |
Jaffar 2009.
| Methods | Design: Cluster randomised controlled trial, 22 clusters for each arm, median size 25 ‐ 36, inter‐cluster co‐efficient 0.2 Duration: February 2005 ‐ December 2006, follow‐up until 31 January 2009, median follow‐up 27 ‐ 28 months |
|
| Participants | Country: Uganda Setting: Urban, peri‐urban and rural, varying distance from the hospital Inclusion criteria: Adults >18 years old, CD4+ cell count <200cells/mm3, WHO clinical stage 3 or 4 Exclusion criteria: Living >100 km from facility Comparable CD4+ cell count or clinical stage at baseline: Similar, slightly lower CD4+ cell count for intervention arm |
|
| Interventions | Intervention: ART initiated at hospital by doctors, maintained in the community by field officers who delivered treatments every month on motorcycles, monitored adherence, drug toxicity and disease, they referred patients; had access to mobile phones for on‐site call to doctor. If patients was absent, followed up. Reviewed at hospital 6 monthly. Control: ART initiated and maintained at hospital. Monthly clinic visits to collect medicine, reviewed by medical officer 3 monthly, drop in clinic; if defaulted, followed up at home; household vouchers for counselling |
|
| Outcomes | Rate of virological failure, time to detectable viral load >500 copies/mL, time to detectable viral load >500 copies/mL at any visit from 12 months if it was <500 copies/mL at 6 months or increase in 1000 copies/mL between two consecutive tests in those who did not have viral load <500 copies/mL at 6 months, all cause mortality, admission, change to second line antiretrovirals, outpatient attendance, adherence in previous 28 days, cost incurred by health services and patients, patient diagnosed with TB at first admission, proportion of those with CD4+ cell count > 200cells/mm3 Timepoints of outcome assessment not clear |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Low risk | Similar at baseline |
| Other baseline variables (All studies) | Low risk | Comparable baseline characteristics |
| Co‐interventions (All studies) | Low risk | Both groups seen monthly, but provider and facility differed by group |
| Random sequence generation (Trials) | Unclear risk | Not described |
| Allocation concealment (Trials) | Low risk | Allocation cards labelled with stratum number and sealed in advance was drawn from a concealed box in the presence of all stakeholders |
| Contamination protection (Trials) | Low risk | No evidence of contamination |
Kipp 2010.
| Methods | Design: Prospective cohort Duration: 6 month results |
|
| Participants | Country: Rwimi, Uganda Setting: intervention in rural setting, control in urban setting Inclusion criteria: Adults >18 years, eligible for antiretroviral therapy, antiretroviral therapy naive, resident in the sub‐county Exclusion criteria: None described Comparable CD4 count or clinical stage at baseline: Similar CD4+ cell count at baseline |
|
| Interventions | Intervention: ART initiated at the health centre, maintained in community receiving care from volunteer community health workers who did weekly home visits ‐ delivering antiretrovirals monthly, monitoring and supporting adherence, monitoring adverse effects and clinical symptoms Control: ART initiated and maintained in hospital, by doctors Co‐intervention: an additional treatment support was required by those in the home‐based group to support adherence and disclosure |
|
| Outcomes | Mortality, viral load, increase in CD4+ cell count, cost to provider Assessed at 6 months and 24 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Low risk | Similar mean CD4+ cell count in both groups |
| Other baseline variables (All studies) | Low risk | Age, sex and educational status similar at baseline (although occupations different)., |
| Co‐interventions (All studies) | High risk | Treatment supporter was required by home‐based care group |
| Data collection (Cohorts) | Low risk | Prospective cohort |
| Patient selection bias (Cohorts) | Low risk | No selection bias identified |
Massaquoi 2009.
| Methods | Design: Retrospective cohort Duration: 1 June 2006 ‐ 31 June 2007, censored 31 June 2007 |
|
| Participants | Country: Thyolo District, Malawi Setting: Rural, 1 hospital, 9 health centres Inclusion criteria: HIV infected adults and children, eligible for antiretrovirals, CD4 count <250cells/mm3, WHO clinical stage 3 or 4 Exclusion criteria: None described Comparable CD4 count or clinical stage at baseline: More men and children and stage 4 disease at hospital, more patients with active tuberculosis at the health centre |
|
| Interventions | Intervention: ART initiated and maintained at health centre, by medical assistant, 1 nurse Control: ART initiated and maintained at hospital by clinical officer, medical assistants, nurses, counsellors Co‐interventions: Both have district mobile support teams |
|
| Outcomes | Attrition (dead, loss to follow‐up, stopped treatment), retention (alive and on treatment, and transferred out) Assessment of outcomes provided in person‐years of follow‐up |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Unclear risk | Not reported |
| Other baseline variables (All studies) | High risk | Sicker at peripheral site: More men and children, WHO clinical stage 4 disease, and fewer patients with active Tuberculosis at control site |
| Co‐interventions (All studies) | Unclear risk | The community‐based group received monthly visits from volunteer community support providers, and delivery of their medicines. They were also required to identify a treatment supporter at home, not clear if this applied to both groups |
| Data collection (Cohorts) | High risk | Retrospective cohort |
| Patient selection bias (Cohorts) | Low risk | No selection bias identified |
McGuire 2012.
| Methods | Design: Retrospective cohort Duration: August 2001 to December 2008, compared by year (e.g. 2001/2002; 2003/2004 etc) |
|
| Participants | Country: Malawi Setting: 10 peripheral health centres, 1 district hospital Inclusion criteria: HIV infected adults, eligible for antiretrovirals, CD4 count <250cells/mm3, WHO clinical stage 3 or 4 Exclusion criteria: None described Comparable CD4 count or clinical stage at baseline: CD4+ cell count similar at baseline median IQR 176 cells/mm3 [105 ‐229] at the health centre and 149 cells/mm3 [74 ‐ 219]; however more clinical stage 1 and 2 at the health centre which may favour the intervention, also more men at the hospital |
|
| Interventions | Intervention: ART initiated and maintained at health centre, by clinical officers. Medical assistants and nurses could prescribe after 2007 Control: ART initiated and maintained at hospital by clinical officer, medical assistants, nurses Co‐interventions: Lay community workers, peer counsellors for adherence support, group and individual counselling |
|
| Outcomes | Death, loss to follow‐up and attrition (death and loss to follow‐up) at 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | High risk | Healthier at peripheral site: CD4+ cell count higher. |
| Other baseline variables (All studies) | High risk | Healthier at peripheral site: (clinical stage 1 and 2). |
| Co‐interventions (All studies) | Low risk | default tracing by community health workers, seems to be same for both facilities |
| Data collection (Cohorts) | High risk | Retrospective cohort |
| Patient selection bias (Cohorts) | Low risk | No evidence of selection bias |
Odafe 2012.
| Methods | Design: Retrospective cohort Duration:Initiated therapy between January and December 2007, follow‐up data collected until 2010 |
|
| Participants | Country: Nigeria Setting: Secondary (medical officers, nurses, laboratory scientists, pharmacists and community heath officers) and tertiary hospitals (medical specialists) in Nigeria where majority of ART initiation occurs Inclusion criteria: HIV infected adults and children, eligible for antiretrovirals by Nigeria national recommendations, adapted from WHO recommendations Exclusion criteria: None described Comparable CD4 count or clinical stage at baseline: CD4+ cell count not reported, but more patients with clinical stage 3 and 4 at the hospital |
|
| Interventions | Intervention: ART initiated and maintained at health centre, by clinical officers. Medical assistants and nurses could prescribe after 2007 Control: ART initiated and maintained at hospital by clinical officer, medical assistants, nurses Co‐interventions: Adehrence counselling and pharmacy counselling received at every visit, at both tiers of health service |
|
| Outcomes | Primary outcome was attrition, which includes those stopping treatment,confirmed dead or lost to follow‐up at 12 and 24 months. Loss to follow‐up was defined as those absent from treatment for 90 days. | |
| Notes | As this comparison included tertiary vs secondary hospitals, this differed from other models in the analysis and is therefore described narratively, not included in analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Unclear risk | Not stated |
| Other baseline variables (All studies) | High risk | More patients with WHO clinical stage 3 disease at the secondary hospital, this is likely to increase bias in favour of control |
| Co‐interventions (All studies) | Low risk | Same approach to adherence support and counselling in both settings |
| Data collection (Cohorts) | High risk | Retrospecrive cohort |
| Patient selection bias (Cohorts) | Low risk | No selection bias identified |
Selke 2010.
| Methods | Design: Cluster randomised controlled trial. Unit of allocation: sub‐location, stratified by distance from road, inter cluster co‐efficient not found Duration: March 2006 ‐ April 2008, minimum 12 months follow‐up |
|
| Participants | Country: Kenya Setting: Rural, 24 sub‐locations Inclusion criteria: adults >18 years, clinically stable on antiretroviral therapy for 3 months with no adherence issues, disclosed to household members, live within area, informed consent given Exclusion criteria: Active WHO clinical stage 3 or 4 condition, pregnant, hospitalisation in previous 3 months, unable to understand informed consent process Comparable CD4 count or clinical stage at baseline:similar CD4+ cell count and WHO clinical stage |
|
| Interventions | Intervention: ART initiated at hospital by clinical officer, maintained in community by person living with HIV/AIDS ("community care coordinators" who had secondary education, were clinically stable, 100% adherent and "considered a good role model"; trained, given mobile computer decision aids, visited patients monthly at home and delivered medicines. Three monthly clinic visits seen by doctor or clinical officer Control: ART initiated at hospital by clinical officer or doctor (10% of visits), maintained at hospital. Visit clinic monthly, seen by nurse and doctor. Co‐interventions: community coordinators had computer decision aids to trigger referral for clinical or social concerns |
|
| Outcomes | Viral load, CD4+ cell count, number of clinic visits, Karnofsky score, stability of antiretroviral regimen, opportunistic infections, pregnancy, adherence to drugs, loss to follow‐up Assessed at 12 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Low risk | Similar at baseline |
| Other baseline variables (All studies) | Low risk | Similar baseline characteristics including age, sex and WHO clinical staging. |
| Co‐interventions (All studies) | Low risk | Other than the intervention (computerised decision aids and home‐based support) the groups were treated equally. |
| Random sequence generation (Trials) | Unclear risk | Not clearly described |
| Allocation concealment (Trials) | Low risk | Well described |
| Contamination protection (Trials) | Low risk | Unlikely that control group was exposed to intervention |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Babigumira 2009 | Decision analysis model using one patient as case study. |
| Bemelmans 2010 | Description of program outcomes. |
| Bolton‐Moore 2007 | No contemporaneous comparison group. |
| Boyer 2010 | Cross‐sectional survey. |
| Boyer 2012 | Cross‐sectional survey. |
| Chu 2010 | Randomised controlled trial conducted in high income country (United States of America). |
| Cohen 2009 | No contemporaneous comparison group. |
| Ingle 2010 | Cohort study with no clear comparison between standard level/model of care and a decentralised model |
| Lambdin 2013 | Intervention being evaluated was integration of care vs vertical care, both occurred at primary care setting. |
| Leon 2011 | Randomised controlled trial done in high income country (Spain). |
| Shumbusho 2009 | Cohort (task shifting) with no comparison group. |
| Stein 2007 | Qualitative data from health care workers only. |
| Tene 2013 | Cohort study with no clear comparison between standard level/model of care and a decentralised model |
Characteristics of studies awaiting assessment [ordered by study ID]
Assefa 2012b.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Presentation at International AIDS Conference, 2012. Awaiting additional information from authors. Querying whether this a sub‐study within Assefa 2012 or a different population. |
Labhart 2012.
| Methods | Data from International epidemiologic databases to evaluate AIDS in Southern Africa network (IeDEA‐SA). Programmatic data |
| Participants | 9 Hospitals and 40 health centres in 4 countries, 13 100 patients on ART in 2011 Inclusion criteria: >16 years old at start of ART, no previous ART exposure Baseline characteristics well balanced for sex and age, but not for CD4+ cell counts which were lower at hospital and WHO clinical stages which were more advanced at the hospitals Decentralisation model currently underway in Lesotho |
| Interventions | ART received at nurse run health centres |
| Outcomes | Loss to follow‐up defined as not returning to clinic >= 6 months, mortality. |
| Notes | Oral presentation at the International AIDS Conference, 2012. Additional data regarding loss to follow‐up needed. |
Miyano 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Presentation at International AIDS Conference, 2012. Seeking additional information about publication of this data to assess eligibility. |
Morsheimer (unpublished).
| Methods | Retrospective cohort |
| Participants | Children eligible for antiretroviral therapy in South Africa. |
| Interventions | Initiation and maintenance by paediatric medical officers in health centres (enhanced) compared to initiation at an advanced hospital by paediatric doctors and down referral to health centres |
| Outcomes | Mortality, retention, CD4 count |
| Notes | Awaiting feedback from author about outcomes of interest |
Van Dijk 2012.
| Methods | Cohort study in children Before and after results of decentralisation from hospital to 'outreach' site |
| Participants | Children in paediatric cohort in rural Zambia |
| Interventions | Decentralisation to outreach site, not clear when initiation of ART occurred |
| Outcomes | Cost, travel time, death, loss to follow‐up, viral load |
| Notes | Oral presentation at the International AIDS Conference, 2012. Concludes that outreach group less likely to achieve virological suppression, but travel costs and times lower. Need additional information from authors regarding study design and whether the arms of the study were evaluated contemporaneously. |
Characteristics of ongoing studies [ordered by study ID]
NCT 01414413.
| Trial name or title | Home assessment and initiation of ART: a cluster‐randomised controlled trial in Blantyre, Malawi |
| Methods | Randomised open‐label parallel arm trial |
| Participants | Adults >18 years of age, HIV positive and eligible for ART |
| Interventions | Intervention: Home assessment and initiation of ART Control: Clinic‐based ART assessment and initiation |
| Outcomes | Primary outcome: Antiretroviral initiation within first 6 months Secondary outcomes: Uptake of home‐based HIV testing, disclosure of HIV results, retention on ART, adherence to ART, mortality. |
| Starting date | January 2012 |
| Contact information | Peter MacPherson, p.macpherson@liverpool.ac.za |
| Notes |
Differences between protocol and review
Newcastle‐Ottawa tool for assessing quality of cohort studies was not used in the review as it did not adequately address the relevant items of quality included studies.
Contributions of authors
TK and FBA conducted eligibility of the searches, and together with NF did data extraction, and quality assessment. PG resolved differences when needed. TK entered the data and conducted the analyses and wrote the first draft of the review. NF, FBA and PG provided regular feedback into the overall results and their interpretation. PG developed the framework for the nomenclature and models of health care delivery which were initially described in the protocol.
Sources of support
Internal sources
South African Cochrane Centre, South Africa.
External sources
-
World Health Organization, Department of HIV/AIDS, Switzerland.
Provided some funding support towards completing this review, as a subcontract through the University of California, San Francisco (UCSF)
-
UKaid from the UK Government for the benefit of developing countries (DFiD), UK.
Funding received through a grant from DFiD to the Effective Health Care Research Consortium
Declarations of interest
None declared.
New
References
References to studies included in this review
Assefa 2012 {published data only}
- Assefa Y, Kiflie A, Tekle B, Mariam DH, Laga M, Damme W. Effectiveness and acceptability of delivery of antiretroviral treatment in health centres by health officers and nurses in Ethiopia. Journal of health services research & policy 2012;17(1):24‐9. [PUBMED: 22096081] [DOI] [PubMed] [Google Scholar]
Balcha 2010 {published data only}
- Balcha TT, Jeppsson A. Outcomes of antiretroviral treatment: a comparison between hospitals and health centers in Ethiopia. Journal of the International Association of Physicians in AIDS Care (Chicago, Ill. : 2002) 2010;9(5):318‐24. [PUBMED: 20923956] [DOI] [PubMed] [Google Scholar]
Bedelu 2007 {published data only}
- Bedelu M, Ford N, Hilderbrand K, Reuter H. Implementing antiretroviral therapy in rural communities: the Lusikisiki model of decentralized HIV/AIDS care. The Journal of infectious diseases 2007;196 Suppl 3:S464‐8. [PUBMED: 18181695] [DOI] [PubMed] [Google Scholar]
Bock 2008 {published data only}
- Bock P, Boulle A, White C, Osler M, Eley B. Provision of antiretroviral therapy to children within the public sector of South Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene 2008;102(9):905‐11. [PUBMED: 18656217] [DOI] [PubMed] [Google Scholar]
Brennan 2011 {published data only}
- Brennan AT, Long L, Maskew M, Sanne I, Jaffray I, MacPhail P, et al. Outcomes of stable HIV‐positive patients down‐referred from a doctor‐managed antiretroviral therapy clinic to a nurse‐managed primary health clinic for monitoring and treatment. AIDS (London, England) 2011;25(16):2027‐36. [PUBMED: 21997488] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L, Brennan A, Fox MP, Ndibongo B, Jaffray I, Sanne I, et al. Treatment outcomes and cost‐effectiveness of shifting management of stable ART patients to nurses in South Africa: an observational cohort. PLoS medicine 2011;8(7):e1001055. [PUBMED: 21811402] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2010 {published data only}
- Chan AK, Mateyu G, Jahn A, Schouten E, Arora P, Mlotha W, et al. Outcome assessment of decentralization of antiretroviral therapy provision in a rural district of Malawi using an integrated primary care model. Tropical medicine & international health : TM & IH 2010;15 Suppl 1:90‐7. [PUBMED: 20586966] [DOI] [PubMed] [Google Scholar]
Fatti 2010 {published data only}
- Fatti G, Grimwood A, Bock P. Better antiretroviral therapy outcomes at primary healthcare facilities: an evaluation of three tiers of ART services in four South African provinces. PloS one 2010;5(9):e12888. [PUBMED: 20877631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fayorsey 2013 {published data only}
- Fayorsey, R. N, Saito, S, Carter, R. J, et al. Decentralization of pediatric HIV Care and Treatment in Five sub‐Saharan African Countries. Journal of Acquired Immune Deficiency Syndrome NLM. [DOI] [PMC free article] [PubMed]
Hansudewechakul 2012 {published data only}
- Hansudewechakul, R, Naiwatanakul, T, Katana, A, et al. Successful clinical outcomes following decentralization of tertiary paediatric HIV care to a community‐based paediatric antiretroviral treatment network, Chiangrai, Thailand, 2002 to 2008. Journal of International AIDS Society NLM. [DOI] [PMC free article] [PubMed]
Humphreys 2010 {published data only}
- Humphreys CP, Wright J, Walley J, Mamvura CT, Bailey KA, Ntshalintshali SN, et al. Nurse led, primary care based antiretroviral treatment versus hospital care: a controlled prospective study in Swaziland. BMC health services research 2010;10:229. [PUBMED: 20687955] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaffar 2009 {published data only}
- Amuron B, Coutinho A, Grosskurth H, Nabiryo C, Birungi J, Namara G, et al. A cluster‐randomised trial to compare home‐based with health facility‐based antiretroviral treatment in Uganda: study design and baseline findings. The open AIDS journal 2007;1:21‐7. [PUBMED: 18923692] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amuron B, Levin J, Birunghi J, Namara G, Coutinho A, Grosskurth H, et al. Mortality in an antiretroviral therapy programme in Jinja, south‐east Uganda: a prospective cohort study. AIDS research and therapy 2011;8:39. [PUBMED: 22018282] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffar S, Amuron B, Foster S, Birungi J, Levin J, Namara G, et al. Rates of virological failure in patients treated in a home‐based versus a facility‐based HIV‐care model in Jinja, southeast Uganda: a cluster‐randomised equivalence trial. Lancet 2009;374(9707):2080‐9. [PUBMED: 19939445] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kipp 2010 {published data only}
- Kipp W, Konde‐Lule J, Rubaale T, Okech‐Ojony J, Alibhai A, Saunders DL. Comparing antiretroviral treatment outcomes between a prospective community‐based and hospital‐based cohort of HIV patients in rural Uganda. BMC international health and human rights 2011;11 Suppl 2:S12. [PUBMED: 22166168] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipp W, Konde‐Lule J, Saunders LD, Alibhai A, Houston S, Rubaale T, et al. Results of a community‐based antiretroviral treatment program for HIV‐1 infection in Western Uganda. Current HIV research 2010;8(2):179‐85. [PUBMED: 20163349] [DOI] [PubMed] [Google Scholar]
- Kipp, W, Konde‐Lule, J, Saunders, L. D, et al. Antiretroviral treatment for HIV in rural Uganda: Two‐year treatment outcomes of a prospective health centre/community‐based and hospital‐based cohort. PLoSONE. [DOI] [PMC free article] [PubMed]
Massaquoi 2009 {published data only}
- Massaquoi M, Zachariah R, Manzi M, Pasulani O, Misindi D, Mwagomba B, et al. Patient retention and attrition on antiretroviral treatment at district level in rural Malawi. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009;103(6):594‐600. [PUBMED: 19298993] [DOI] [PubMed] [Google Scholar]
McGuire 2012 {published data only}
- McGuire, M, Pinoges, L, Kanapathipillai, R, et al. Treatment initiation, program attrition and patient treatment outcomes associated with scale‐up and decentralization of HIV care in rural Malawi. PLoSONE NLM. [DOI] [PMC free article] [PubMed]
Odafe 2012 {published data only}
- Odafe, S, Torpey, K, Khamofu, H, et al. The Pattern of Attrition from an Antiretroviral Treatment Program in Nigeria. PLoSONE. [DOI] [PMC free article] [PubMed]
Selke 2010 {published data only}
- Selke HM, Kimaiyo S, Sidle JE, Vedanthan R, Tierney WM, Shen C, et al. Task‐shifting of antiretroviral delivery from health care workers to persons living with HIV/AIDS: clinical outcomes of a community‐based program in Kenya. Journal of acquired immune deficiency syndromes (1999) 2010;55(4):483‐90. [PUBMED: 20683336] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Babigumira 2009 {published data only}
- Babigumira JB, Sethi AK, Smyth KA, Singer ME. Cost effectiveness of facility‐based care, home‐based care and mobile clinics for provision of antiretroviral therapy in Uganda. PharmacoEconomics 2009;27(11):963‐73. [PUBMED: 19888795] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bemelmans 2010 {published data only}
- Bemelmans M, Akker T, Ford N, Philips M, Zachariah R, Harries A, et al. Providing universal access to antiretroviral therapy in Thyolo, Malawi through task shifting and decentralization of HIV/AIDS care. Tropical medicine & international health : TM & IH 2010;15(12):1413‐20. [PUBMED: 20958897] [DOI] [PubMed] [Google Scholar]
Bolton‐Moore 2007 {published data only}
- Bolton‐Moore C, Mubiana‐Mbewe M, Cantrell RA, Chintu N, Stringer EM, Chi BH. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA : the journal of the American Medical Association 2007;298(16):1888‐99. [DOI] [PubMed] [Google Scholar]
Boyer 2010 {published data only}
- Boyer S, Eboko F, Camara M, Abe C, Nguini ME, Koulla‐Shiro S, et al. Scaling up access to antiretroviral treatment for HIV infection: the impact of decentralization of healthcare delivery in Cameroon. AIDS (London, England) 2010;24 Suppl 1:S5‐15. [PUBMED: 20023440] [DOI] [PubMed] [Google Scholar]
Boyer 2012 {published data only}
- Boyer S, Protopopescu C, Marcellin F, Carrieri MP, Koulla‐Shiro S, Moatti JP, et al. Performance of HIV care decentralization from the patient's perspective: health‐related quality of life and perceived quality of services in Cameroon. Health policy and planning 2012;27(4):301‐15. [PUBMED: 21690123] [DOI] [PubMed] [Google Scholar]
Chu 2010 {published data only}
- Chu C, Umanski G, Blank A, Grossberg R, Selwyn PA. HIV‐infected patients and treatment outcomes: an equivalence study of community‐located, primary care‐based HIV treatment vs. hospital‐based specialty care in the Bronx, New York. AIDS care 2010;22(12):1522‐9. [PUBMED: 20824549] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2009 {published data only}
- Cohen R, Lynch S, Bygrave H, Eggers E, Vlahakis N, Hilderbrand K, et al. Antiretroviral treatment outcomes from a nurse‐driven, community‐supported HIV/AIDS treatment programme in rural Lesotho: observational cohort assessment at two years. Journal of the International AIDS Society 2009;12:23. [PUBMED: 19814814] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ingle 2010 {published data only}
- Ingle SM, May M, Uebel K, Timmerman V, Kotze E, Bachmann M, et al. Differences in access and patient outcomes across antiretroviral treatment clinics in the Free State province: a prospective cohort study. South African Medical Journal 2010;100(10):675‐81. [PUBMED: 21080999] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lambdin 2013 {published data only}
- Lambdin, B. H, Micek, M. A, Sherr, K, et al. Integration of HIV Care and Treatment in Primary Health Care Centers and Patient Retention in Central Mozambique: A Retrospective Cohort Study. Journal of Acquired Immune Deficiency Syndrome NLM. [DOI] [PMC free article] [PubMed]
Leon 2011 {published data only}
- Leon A, Caceres C, Fernandez E, Chausa P, Martin M, Codina C, et al. A new multidisciplinary home care telemedicine system to monitor stable chronic human immunodeficiency virus‐infected patients: a randomized study. PLoS ONE 2011;6(1):e14515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shumbusho 2009 {published data only}
- Shumbusho F, Griensven J, Lowrance D, Turate I, Weaver MA, Price J, et al. Task shifting for scale‐up of HIV care: evaluation of nurse‐centered antiretroviral treatment at rural health centers in Rwanda. PLoS medicine 2009;6(10):e1000163. [PUBMED: 19823569] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 2007 {published data only}
- Stein J, Lewin S, Fairall L. Hope is the pillar of the universe: health‐care providers' experiences of delivering anti‐retroviral therapy in primary health‐care clinics in the Free State province of South Africa. Social science & medicine (1982) 2007;64(4):954‐64. [PUBMED: 17140716] [DOI] [PubMed] [Google Scholar]
Tene 2013 {published data only}
- Tene, G, Lahuerta, M, Teasdale, C, et al. High Retention among HIV‐Infected Children in Rwanda during Scale‐up and Decentralization of HIV Care and Treatment Programs, 2004‐2010. Pediatric Infectious Diseases Journal NLM. [DOI] [PMC free article] [PubMed]
References to studies awaiting assessment
Assefa 2012b {published data only}
- Assefa AY, Kiflie A, Tesfaye D, Rasschaert F, Lynen L, Damme W. Interventions to improve retention in antiretroviral treatment programmes in Ethiopia. XIX INTERNATIONAL AIDS CONFERENCE. Washington DC: International AIDS Society, July 2012. [WEPE727]
Labhart 2012 {unpublished data only}
- Labhardt N, Sello M, Mohlaba A, Keiser O, Pfeiffer K, Egger M, et al. Outcomes of antiretroviral treatment programs in rural Lesotho: health centres and hospitals compared.. XIX INTERNATIONAL AIDS CONFERENCE. Washington DC: International AIDS Society, JULY 2010. [WEA0205] [DOI] [PMC free article] [PubMed]
Miyano 2012 {published data only}
- Miyano S, Chipeta V, Sialubanje C, Utsushikawa M, Ishikawa N, Sikazwe I, et al. Decentralization of HIV care services in a rural district in Zambia: a comparative analysis of quality of care between the district hospital and rural health centre. XIX INTERNATIONAL AIDS CONFERENCE. Washington DC: International AIDS Society, JULY 2012. [WEPE722]
Morsheimer (unpublished) {unpublished data only}
- Morsheimer M, Dramowski A, Myer L, Cotton M, Rabie H. Successful pediatric HIV management within a South African decentralization model of ART delivery. (being submitted for publication) 2012.
Van Dijk 2012 {published data only}
- Van‐dijk JH, Moss WJ, Sinywimaanzi P, Hamangaba F, Sutcliffe CG. Scaling‐up access to antiretroviral treatment: a comparison of outcomes among HIV‐positive children receiving treatment at mobile and hospital‐based HIV clinics in rural Zambia. XIX INTERNATIONAL AIDS CONFERENCE. Washington DC: International AIDS Society, July 2012. [WEPDEO106]
References to ongoing studies
NCT 01414413 {published data only}
- Home Assessment and Initiation of Antiretroviral Therapy for HIV in Malawi (CONDA‐YAPA). International AIDS Society Conference.
Additional references
Brinkhof 2009
- Brinkhof M W, Pujades‐Rodriguez M, Egger M. Mortality of patients lost to follow‐up in antiretroviral treatment programmes in resource‐limited settings: systematic review and meta‐analysis. PLoS One 2009;4:e5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Decroo 2009
- Decroo T, Panunzi I, das Dores C, Maldonado F, Biot M, Ford N, et al. Lessons learned during down referral of antiretroviral treatment in Tete, Mozambique. Journal of the International AIDS Society 2009;12(1):6. [PUBMED: 19419543] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2008
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:276‐82. [Google Scholar]
Ford 2011
- Ford N, Calmy A, Mills EJ. The first decade of antiretroviral therapy in Africa. Globalization and Health 2011; Vol. 7:33. [PUBMED: 21958478] [DOI] [PMC free article] [PubMed]
Fox 2010
- Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub‐Saharan Africa, 2007‐2009: systematic review. Tropical Medicine & International Health 2010;15 Suppl 1:1‐15. [PUBMED: 20586956] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Schünemann H, Brozek J, Oxman A. GRADEpro. GRADE Working Group, 2008.
Guyatt 2011
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380‐2. [PUBMED: 21185693] [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from: http://www.cochrane‐handbook.org, (Accessed 20 February 2012). [Google Scholar]
Higgins 2008a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:187‐241. [Google Scholar]
Kagee 2011
- Kagee A, Remien RH, Berkman A, Hoffman S, Campos L, Swartz L. Structural barriers to ART adherence in Southern Africa: Challenges and potential ways forward. Global Public Health 2011;6(1):83‐97. [PUBMED: 20509066] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kredo 2012
- Kredo T, Bateganya M, Pienaar ED, Adeniyi FB. Task shifting from doctors to non‐doctors for initiation and maintenance of HIV/AIDS treatment. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2012, issue 9. [DOI: 10.1002/14651858.CD007331.pub2; CD007331] [DOI] [PMC free article] [PubMed]
Miller 2010
- Miller CM, Ketlhapile M, Rybasack‐Smith H, Rosen S. Why are antiretroviral treatment patients lost to follow‐up? A qualitative study from South Africa. Tropical Medicine & International Health 2010;15 Suppl 1:48‐54. [PUBMED: 20586960] [DOI] [PMC free article] [PubMed] [Google Scholar]
Newcastle‐Ottawa Scale
- Wells GA, Shea B, O'Connell D, Petersen J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of non‐randomised studies in meta‐analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed 20 February 2012).
Review Manager 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
UNAIDS 2011
- Global HIV/AIDS response: epidemic update and health sector progress towards Universal Access. Available from: http://www.who.int/hiv/pub/progress_report2011/hiv_full_report_2011.pdf (Accessed 22 February 2012). Geneva, Switzerland: WHO/UNAIDS. UNICEF.
Ware 2009
- Ware NC, Idoko J, Kaaya S, Biraro IA, Wyatt MA, Agbaji O, et al. Explaining adherence success in sub‐Saharan Africa: an ethnographic study. PLoS Medicine 2009;6(1):e11. [PUBMED: 19175285] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2008
- World Health Organization (WHO). Task shifting : rational redistribution of tasks among health workforce teams : global recommendations and guidelines (2008). Available from: http://www.who.int/healthsystems/task_shifting/en (Accessed 22 February 2012). WHO Press.


