Abstract
Background/Objectives:
Routine intraoperative cholangiography (IOC) for laparoscopic cholecystectomy (LC) remains controversial. The primary outcomes of this meta-analysis were detection rates of choledocholithiasis, bile duct injuries (BDI), and missed stones in LCs.
Methods:
A systematic literature search was conducted for the time period January 1, 1990 to July 31, 2022. Some studies reported LCs with conversion to open therefore subgroup analysis in BDI rates was performed for studies which included LCs with and without conversion to open. Studies including primary open cholecystectomies were excluded. I2 statistics were used for heterogeneity analysis.
Results:
Fourteen studies involving 440659 patients were included. In studies comparing routine and selective IOC policies in LC, 61.1% of patients underwent routine IOC; 38.9% underwent selective IOC. In studies comparing IOC to no IOC in LC, 17.3% of patients had IOC; 82.7% did not. Between the selective and routine IOC groups there was no difference in choledocholithiasis detection rate (odds ratio [OR] = 1.33, p = 0.20, 95% confidence interval [CI] = 0.86 – 2.04), no difference in the rate of missed stones (OR = 1.59, p = 0.58; 95% CI = 0.31 – 8.29), and no difference in BDI rates in selective compared to routine IOC (OR = 0.92, p = 0.92; 95% CI = 0.20 – 4.22). There was no difference in the BDI detection rates in LC with and without IOC (OR = 1.12, p = 0.77; 95% CI = 0.52 – 2.38).
Conclusion:
This is the largest meta-analysis on this topic to date. There was no statistically significant difference in choledocholithiasis detection, missed stones, or BDI rates in the analyzed groups.
Keywords: Bile duct injury, Choledocholithiasis, Intraoperative cholangiography, Laparoscopic cholecystectomy, Routine and selective
INTRODUCTION
Laparoscopic cholecystectomy (LC) is the gold standard for management for patients with symptomatic gallstones.1–4 The addition of an intraoperative cholangiography (IOC), either routine or selective, is used to detect choledocholithiasis, which is found in approximately 8% – 15%.5 Furthermore, IOC has also been justified to delineate biliary tract anatomy and the early detection of bile duct injury (BDI).
The proponents of routine IOC argue that all patients should be screened for CBD stones and BDI, as early detection can drastically improve patient morbidity and mortality.6,7 Proponents of selective IOC use it only in patients at high risk of choledocholithiasis, based on a variety of criteria such as a history of pancreatitis, jaundice, abnormal liver function tests, or biliary dilatation on imaging.8,9 The precise criteria for the use of selective IOC continues to be controversial and based on institution and surgeon preference.
The arguments against the routine use include the increased intraoperative time required, high false positive rates requiring unnecessary additional interventions like endoscopic retrograde cholangiopancreatography, a failure rate of 3% – 17%, and increased costs with resource utilization.1,7,10 Furthermore, there are the risks relating to ionising radiation exposure to patients and operating theater personnel.11
The aim of this study was to evaluate efficacy of IOC in LC by systematically evaluating and synthesizing the evidence from the literature. This study investigated both the comparison of routine IOC to selective IOC in patients undergoing LC as well as analyzing the LC outcomes in patients without the use of IOC. The primary outcomes of interest were choledocholithiasis detection, missed stones, and the rate of BDI.
METHODS
Search Strategy
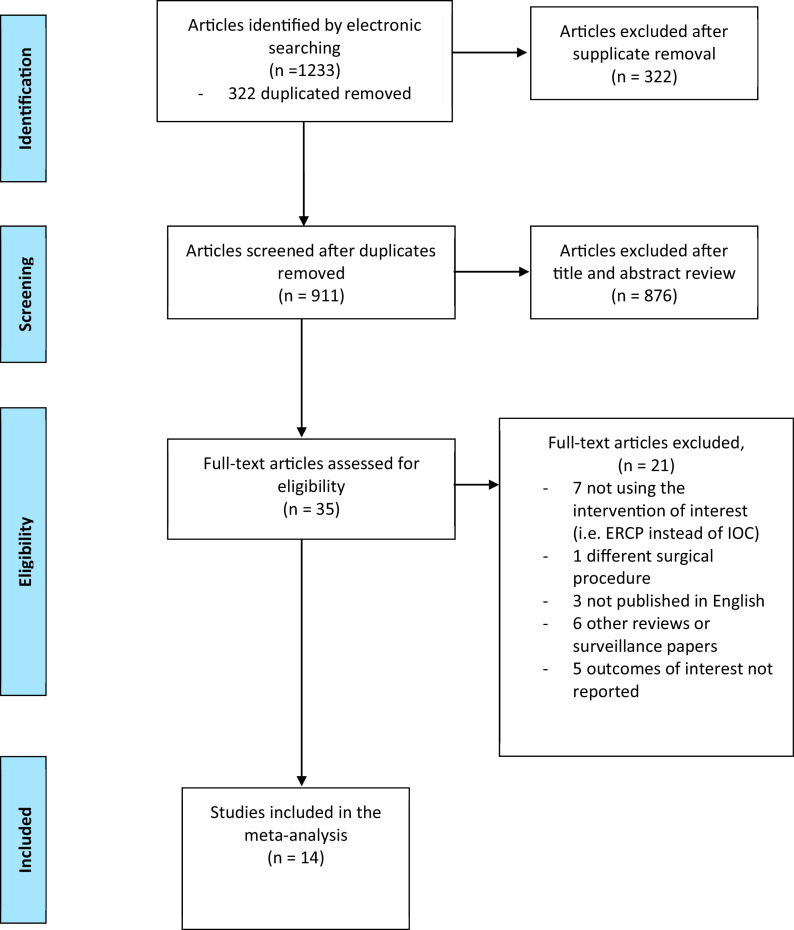
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)12 criteria were adapted (Figure 1). A comprehensive electronic literature search of Ovid MEDLINE, PubMed, EMBASE, and Cochrane Library was conducted for the dates January 1, 2000 – July 31, 2022. A grey literature search was conducted using the GoogleScholar search engine. A manual search of relevant journals and bibliographies of potential articles was performed for additional references. The search was completed independently by authors CH and SA.
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram showing selection of article review.
The keywords used included the Medical Subheading (MeSH): laparoscopic cholecystectomy, cholecystectomy, cholangiography, per-operative cholangiography, routine, and selective. These terms were used in [MeSH] format for searching the Cochrane Library. For all other databases, the search was conducted by combining similar terms using the Boolean operator “OR” and terms were grouped using the Boolean operator “AND” to find appropriate results.
All citations found via electronic search were independently reviewed sequentially. Two authors (CH and SA) independently screened the abstract and title articles for potential inclusion. Shortlisted articles were then screened by full-text review for inclusion or exclusion based on selection criteria. Discrepancies in paper selection were resolved through group consensus and discussion with a third author (SG).
Inclusion and Exclusion Criteria
Randomized controlled trials (RCTs), controlled clinical trials (CCTs), retrospective and prospective cohort studies that compared IOC procedures in adults were included, namely: selective compared to routine IOC and use of IOC compared to no IOC for LC. We excluded: non-English studies, reviews (systematic and narrative), theses, editorials, case reports, case series containing < 30 patients, epidemiological studies, surveillance studies, economic evaluations, and conference proceedings. Studies including primary open cholecystectomies were excluded due to the risk of bias relating to the increased complexity or difficulties relating to these procedures.13,14 However studies investigating LCs that had some cases converted to open were included.
Quality Assessment for Included Studies
The studies were assessed independently by three authors (CH, SA, and RS) for quality using the Methodological Index for Non-Randomized Studies (MINORS).15 This assessment involved scoring all studies from 0 to 2 against 12 items. Tallied scores can range from 0 reflecting a low-quality study to 24 for a high-quality study. Any disparities in scores were resolved through discussion with a fourth author (SG) and group consensus.
Outcome Measures
The primary outcome of this study was to assess the difference in choledocholithiasis detection rates between routine and selective IOC groups. Successful IOC was used as the denominator in this analysis. Secondary outcomes were the incidence missed choledocholithiasis found on follow-up and the rate of BDI in routine compared to selective IOC policies and in LC with IOC compared to those without IOC, the denominator was all those allocated to each group.
Data Analysis
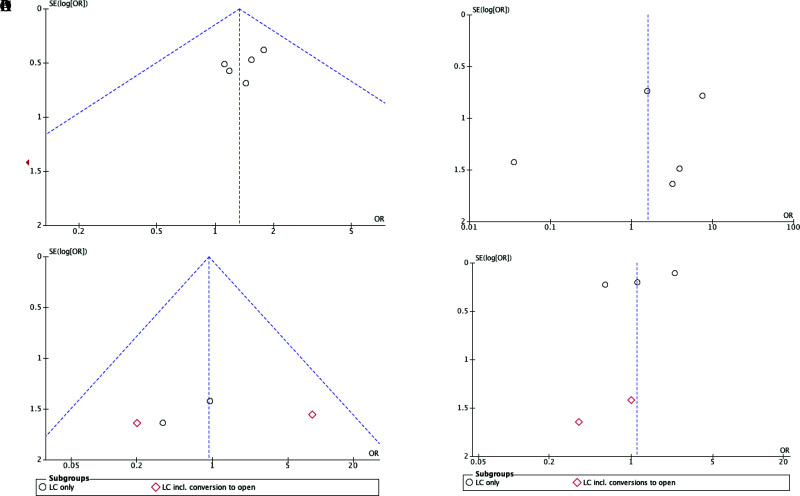
All statistical analysis was performed using Review Manager Software, Version 5.4.1 (The Cochrane Collaboration, Oxford, UK). Pooled analysis was performed comparing routine and selective IOC policies and IOC compared to no IOC policies to calculate the odds ratio (OR) and 95% confidence interval (CI). When analyzing BDI rates, subgroup analysis was performed with papers with LC only and papers reporting LC with some cases converted to open to acknowledge there may be bias relating to the increased complexity of these procedures. I2 statistics with P-value set to P < 0.10 for significance, were utilized to assess heterogeneity. I2 scores > 50% denoted significant heterogeneity. When the heterogeneity test was statistically significant, a random effects model was used; which assumes variation in treatment effects between studies, and estimates a more conservative overall treatment effect with wider confidence intervals. Where heterogeneity was not significant, a fixed effects model was used. Potential publication bias was evaluated using funnel plots; asymmetry within the plot implies that the results were subject to bias (Figure 2). Forest plots were created to demonstrate the pooled ORs and corresponding 95% CIs. P-values < 0.05 were considered statistically significant.
Figure 2.
Funnel plots for safety outcomes with ninety-five percent confidence limits: (A) common bile duct stone detection in routine versus selective intraoperative cholangiography (IOC) in laparoscopic cholecystectomy (B) missed stones on follow-up in routine and selective intraoperative cholangiography (C) bile duct injury rates in routine and selective intraoperative cholangiography (D) bile duct injury rates in intraoperative cholangiography versus no intraoperative cholangiography.
RESULTS
The PRISMA diagram exhibiting the different phases of the study selection is shown in Figure 1.12 A total of 1,232 articles were retrieved from primary electronic searches. After removal of duplicates, 910 citations were screened using title and abstract. Forty-one articles were selected for final evaluation. Full texts of these articles were retrieved and three reviewers performed the final selection. Of these, 14 articles (four RCTs, four prospective studies, and six retrospective longitudinal studies) fulfilled the inclusion criteria.
A total of 440,659 pooled patients were included. In studies comparing routine versus selective IOC cohorts there were 3,605 (61.1%) patients and 2,294 (38.9%) patients, respectively. In studies that compared outcomes of IOC in LC, 17.3% of patients had IOC during LC (n = 75,211) and 82.7% patients had LC only (n = 359,549), with most of these patients coming from large retrospective studies.
Three of the seven papers comparing routine to selective IOC reported LCs that were converted to open. The rates of conversion were in the single figures2,16,17 other than in Kohn's18 paper which had a selective IOC conversion to open rate of 18%, compared to 0% among the routine IOC group. Of the seven papers that compared IOC to no IOC in LC, three papers reported rates of conversion to open ranging from 0% to 3%.4,19,20
The characteristics of included studies are summarised in Tables 1 and 2.
Table 1.
Summary of Included Studies – Laparoscopic Cholecystectomy with and without Intraoperative Cholangiography (IOC)
| Author, Year Country, Type of study | Study Design | Study period | Use of IOC | % BDI With or Without IOC | IOC vs. No IOC | Number of patients | LC converted to open | Patient demographics | Outcome: BDI |
|---|---|---|---|---|---|---|---|---|---|
| Altieri, 2017, USA21 | Retrospective analysis | 2000 – 2014 | 13.30% | With IOC: 0.25% | IOC | 43688 | Nil | NR | 108/43688 |
| Without IOC: 0.10% | No IOC | 327860 | Nil | NR | 343/327860 | ||||
| Ding, 2015, China4 | Randomized trial | 2012 – 2014 | 49.87% | With IOC: 0.54% | IOC | 182 | 4/182 | Mean age = 58.22 ± 8.41 years | 1/182 |
| Without IOC:0.54% | No IOC | 182 | 3/182 | Mean age = 57.43 ± 7.15 years | 1/182 | ||||
| Flum, 2001, USA40 | Retrospective study | 1991 – 1998 | 63.71% | With IOC : 0.20% | IOC | 19514 | Nil | NR | 39/19514 |
| Without IOC: 0.33% | No IOC | 11116 | Nil | NR | 37/11116 | ||||
| Giger, 2011, Switzerland41 | Retrospective analysis of prospectively collected database | 1995 – 2005 | 36.60% | With IOC: 0.34% | IOC | 11642 | Nil | Mean age 54.4 ± 15.9 years. M:F 10:22 | 40/11642 |
| Without IOC: 0.3% | No IOC | 20196 | Nil | 61/20196 | |||||
| Khan, 2011, UK19 | Randomized trial | 2003 – 2007 | 47.89% | With IOC: 0% | IOC | 91 | 0/91 | Mean age = 59 years, M:F ratio = 15:76 | 0/91 |
| Without IOC: 1% | No IOC | 99 | 1/99 | Mean age = 53 years, M:F ratio = 1:3 | 1/99 | ||||
| Soper, 1992, USA42 | Randomized trial | 1991 – 1992 | 48.70% | With IOC: 0% | IOC | 56 | nil | Mean age = 48 ± 2 years, M:F ratio = 1:3 | 0/56 |
| Without IOC: 0% | No IOC | 59 | nil | Mean age = 51 ± 2 years, M:F ratio = 27:73 | 0/59 | ||||
| Verma, 2016, Australia20 | Prospective study | 2013 – 2014 | 50.7% | With IOC: 0% | IOC | 38 | 2/75 (not defined which group) | M:F ratio = 3:7 | 0/38 |
| Without IOC: 0% | No IOC | 37 | 0/37 |
Abbreviations: LC, Laparoscopic Cholecystectomy; IOC, Intraoperative Cholangiography; BDI, bile duct injury; CBD, common bile duct; M: F ratio, male to female ratio; NR, not reported; RCT, Randomized controlled trial; UK, United Kingdom; USA, United States of America.
Results are reported as mean (standard deviation) or as a fraction.
Table 2.
Summary of Included Studies – Routine and Selective Intraoperative Cholangiography (IOC) Use in Laparoscopic Cholecystectomy
| Author, Year of publication, country | Study Design | Study period | Policy of IOC | Incidence of BDI with and without IOC | IOC policy | Number of patients | Conversions to open | Patient demographics | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BDI | CBD stone detection incidence | Missed stones | |||||||||
| Amott, 2005, Australia43 | Prospective Randomized study | 1995 – 2002 | Routine and Selective | Routine: 0.68% | Routine | 148 | nil | NR | 1/148 | 12/94 | 3/152 |
| Selective 0.65% | Selective | 155 | nil | NR | 1/155 | 5/34 | 5/163 | ||||
| Carlson, 1993, USA44 | Prospective study | Not stated | Routine and Selective | Routine: 0.61% | Routine | 164 | nil | NR | 1/164 | NR | 0/164 |
| Selective: 0% | Selective | 155 | nil | NR | 0/155 | NR | 1/155 | ||||
| Guerra-Filho, 2007, Brazil17 | Prospective study | 1992 – 1994 | Routine and selective | Not recorded | Routine | 127 | nil | Mean age = 48.8 (13 – 83) years, M:F ratio = 1:3 approximately | NR | 11/102 | NR |
| Selective | 127 | nil | Mean age = 47.9 (12 – 91) years, M:F ratio = 1:3 approximately | NR | 7/59 | NR | |||||
| Kohn, 2004, USA18 | Retrospective study | 1999 – 2000 | Routine and Selective | Not recorded | Routine | 38 | 0/38 | Mean age = 49 years, M:F ratio = 12:27 | NR | 5/38 | 0/38 |
| Selective | 112 | 20/112 | Mean age = 47 years, M:F ratio = 43:69 | NR | 5/28 | 5/112 | |||||
| Ladosci, 1997, England16 | Randomized trial | 1991 – 1993 | Routine and Selective | Routine: 0.36%Selective: 0% | Routine | 276 | 19/276 | Mean age = 48.1 ± 16.4 years, M:F ratio = 24.6:73.6 | 1/276 | 13/187 | 0/276 |
| Selective | 458 | 15/458 | Mean age = 49 ± 16.3 years, M:F ratio = 1:3 approximately | 0/458 | 8/78 | 0/458 | |||||
| Nickkholgh, 2006, Iran2 | Retrospective study | 1992 – 2001 | Routine and Selective | Routine: 0%Selective: 0.25% | Routine | 1330 | 61 total, not described which group | NR | 0/1330 | 37/1330 | 2/1330 |
| Selective | 800 | NR | 2/800 | 9/159 | 9/800 | ||||||
| Snow, 2001, USA45 | Retrospective study | 1989 – 1998 | Routine and Selective | 0% both | Routine | 1522 | nil | NR | 0/1522 | 136/1517 | 42/1522 |
| Selective | 139 | nil | NR | 0/487 | 0/138 | 0/487 | |||||
Abbreviations: IOC, Intraoperative Cholangiography; BDI, bile duct injury; CBD, common bile duct.
Methodological Quality of the Included Studies
There was heterogeneity among the included trials in terms of methodological quality and assessment methods. Overall, the quality of the studies scored using the MINORS criteria was good to medium (Table 3). The overall mean for the included trials methodological quality scores was 17.9 ± 1.7 and ranged from 15 – 21 out of 24. Half of the non-RCTs had flaws in their methodological design with a high risk of bias related to their group allocation procedure, outcome assessors, and outcome analysis. Moreover, 10 studies were limited to a single facility. In those reporting on stones on follow-up, follow-up time points varied with three having greater than 12 months and three article not specifically stating follow-up timing, but rather referred to ‘stones on follow-up’.
Table 3.
Methodological Index for Non-Randomized Studies Assessment of Bias
| MINORS items | Altieri 201721 | Amott, 200542 | Carlson, 199344 | Ding, 20154 | Flum 200140 | Giger 201141 | Guerra-Filho 200717 | Khan, 201119 | Kohn, 200418 | Ladocsi, 199716 | Nickkholgh, 20052 | Snow 200045 | Soper, 199242 | Verma, 201620 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. A clearly stated aim | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 2 |
| 2. Inclusion of consecutive patients | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 3. Prospective collection of data | 1 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 4. Endpoints appropriate to aim | 1 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| 5. Unbiased assessment of the study endpoint | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 6. Appropriate follow-up period | 1 | 2 | 2 | 2 | 0 | 1 | 0 | 2 | 2 | 2 | 2 | 1 | 1 | 2 |
| 7. Loss to follow up less than 5% | 0 | 1 | 2 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 |
| 8. Prospective calculation of the study size | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| 9. An adequate control group | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 10. Contemporary groups | 2 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 0 | 1 | 2 | 1 |
| 11. Baseline equivalence of groups | 2 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 2 | 2 | 1 | 0 | 1 | 0 |
| 12. Adequate statistical analyses | 2 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| TOTAL SCORE | 19 | 17 | 18 | 20 | 15 | 18 | 16 | 19 | 20 | 21 | 17 | 16 | 17 | 17 |
MINORS, Methodological Index for Non‐Randomized Studies.
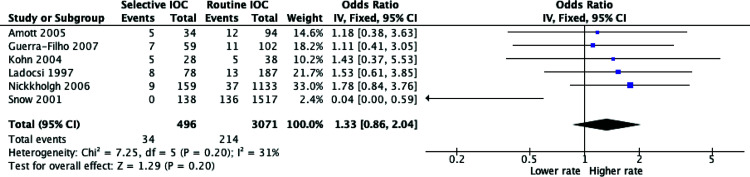
Common Bile Duct Stone Detection Incidence
Rates of common bile duct (CBD) stone detection rates were evaluated in six of the included studies. As the heterogeneity was low to moderate, (p = 0.20, I2 = 31%), a fixed effects model was used for statistical analysis. As demonstrated in Figure 3, there was no statistically significant difference in choledocholithiasis detection rates in successful IOC between the routine and selective groups, though there was a trend towards higher CBD stone detection rate in the selective group (OR = 1.33, P = 0.20, 95% CI = 0.86 – 2.04). Additionally, the false positive rate of IOC differed among the studies ranging from 0% to 5.5%.2,16
Figure 3.
Meta-analysis of choledocholithiasis detection rates between routine versus selective intraoperative cholangiography (IOC) in laparoscopic cholecystectomy.
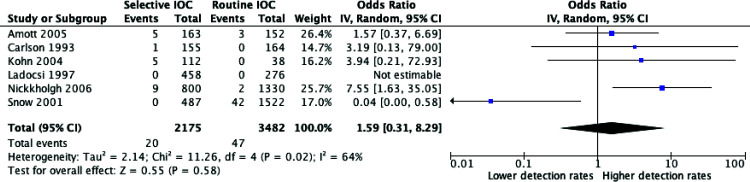
Missed Choledocholithiasis on Follow-up
Six studies reported rates of missed stones on follow-up. There was no difference between routine and selective IOC policies and missed stones (OR = 1.59, P = 0.58; 95% CI = 0.31 – 8.29). Due to heterogeneity (P = 0.02, I2 = 64%), a random effects model was used. The meta-analysis for missed stones on follow-up is illustrated in Figure 4.
Figure 4.
Meta-analysis of missed choledocholithiasis between routine and selective intraoperative cholangiography.
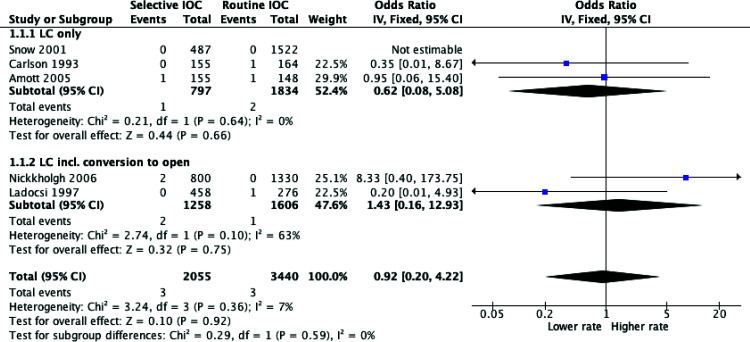
Bile Duct Injury
A total of five studies included data comparing BDI rates between routine and selective IOC use in LCs. Subgroup analyses were performed to account for potential bias between studies which included some LCs converted to open and those that were purely LCs. However, as demonstrated in Figure 5, there was no statistically significant result in each group or combined (OR = 0.92, P = 0.92; 95% CI = 0.20 – 4.22).
Figure 5.
Meta-analysis of bile duct injury rates between routine and selective intraoperative cholangiography.
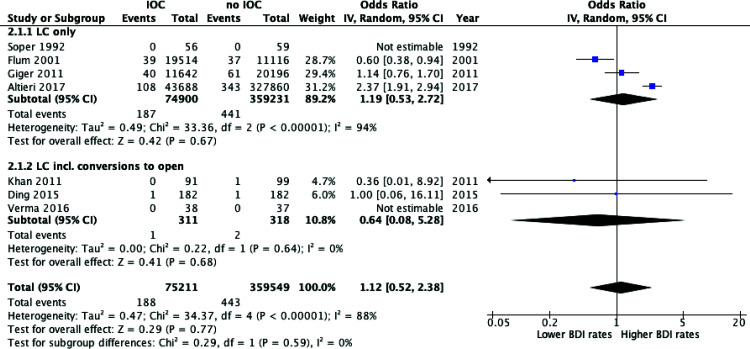
In comparing IOC to no IOC use in LC, the meta-analysis found no significant difference in the BDI detection rates (OR = 1.12, P = 0.77; 95% CI = 0.52 – 2.38). Similarly, there was no significant finding in each subgroup or combined for the rates of BDI. As there was significant heterogeneity (I2 = 88%, P < 0.00001) a random effects model was employed (Figure 6).
Figure 6.
Meta-analysis of bile duct injury rates between laparoscopic cholecystectomy with intraoperative cholangiography versus without intraoperative cholangiography.
DISCUSSION
This is the largest systematic review and meta-analysis to look at choledocholithiasis, missed stones, and BDI detection rates in laparoscopic cholecystectomies. There were no statistically significant findings in any outcome comparison group and there was significant heterogeneity within the studies. Essentially, larger, more robust studies are required to support IOC policy application one way or another.
Despite minimal evidence for the selective or routine application of IOC, there remain strong surgical cultures of routine and selective IOC worldwide. In 2014 – 2015, IOC was performed in 81% of LC cases in Australia1 whereas, in the United States of America the trend is towards selective IOC with only 10% to 12% of LCs in New York needed an IOC.21 Even within Australia, there are differences between IOC rates in the public and private healthcare systems; in the private sector, surgeons used IOC at the lower rate of 75%22 in LC compared to 81% in the public hospital system. Additionally, this report found that 60% of surgeons billed an IOC for some patients, this may be a reflection that 60% of surgeons in the private sector perform IOCs selectively in Australia.22 An additional factor is that studies report that surgeons with a high-volume caseload, operating in high volume hospitals are more likely to perform IOC.23–25
Indeed, the updated 2018 Tokyo Guidelines for surgical management of acute cholecystitis state that “there is no evidence for the value of intraoperative cholangiography”14 and due to insufficient evidence and mixed results,25–28 the guidelines suggest that IOC is optional.14 The findings of this meta-analysis are in line with these sentiments as there was no statistically significant difference in the rates of CBD stone detection, rates of missed stones, or BDI between the groups.
Despite the lack of convincing evidence on the matter, The Australian Medicare Benefits Schedule Review Taskforce Report for General Surgery from 2019 states that it is ‘best practice to perform an intraoperative cholangiogram at the time of cholecystectomy’ and that ‘performing a cholangiogram is incentivised’ at time of LC, with a higher fee attributed.29 An Australian study of The Medicare Benefits Scheme that analyzed trends of IOC, cholecystectomy, and BDI repair between January 1, 2001 and December 31, 2019 revealed a 31.8% increase in IOCs performed despite only a 7.0% increase in cholecystectomies.30 Additionally, there was a minimal change in the number of BDIs and thereby claimed that use of routine IOC to prevent or minimise BDI was unwarranted.30
A factor which may confound the results is that with routine cholangiography, the surgeon becomes more experienced and is more likely to perform it well and safely.31 Surgeons who do not perform IOC routinely may find the procedure difficult and consequently this may increase the potential for injury and misinterpretation.32 Furthermore, with the global trend of rising obesity, the technical challenge of IOC performance is amplified. Additionally, when there is a positive IOC finding in LC, there is a developing trend towards performing an LCBDE during LC rather than postoperative like endoscopic retrograde cholangiopancreatography. This is because additional anesthesia is not required and complications such as pancreatitis, cholangitis, and duodenal perforation are avoided.33 This is relevant because the skills associated with IOC provide an excellent foundation for LCBDE and therefore with routine application of IOC one may develop confidence in performing LCBDE.33
The arguments regarding increased operative time may be mitigated with the fact with routine performance of IOC the staff may be more adept with the set-up of the IOC equipment and shorter IOC procedure length compared to those who perform IOC occasionally. One large Swedish analysis found that for surgeons and teams performing IOC routinely, the procedure length is on average 12 min compared to 25 min for selective IOC.34 Additionally, while the cost of performing IOC during every LC may be higher in the immediate term, an economic analysis found that the cost to the healthcare system of missed BDI and stones with resultant readmission, further imaging, and return to operating theater in those without IOC during LC is far higher.35
The detection of CBD stones is one of the primary reasons for IOC in cholecystectomies.36 However, there was no difference between intraoperative choledocholithiasis detection rates between routine and selective IOC. Additionally, there was no difference in the rates of missed stones between the two groups, suggesting that there is no greater benefit in performing routine IOC in the hopes of detecting more potentially troublesome choledocholithiasis.
In total, 11 of the 14 included studies were RCTs and were of good quality (MINORS score >16). The remaining three articles consisted of one prospective and two retrospective studies and were of moderate to poor quality. Furthermore, each of the studies greatly varied in methodologies and only four were multicenter studies. To account for the heterogeneity of the data, random effects models were employed where appropriate. There are limitations in the methodology and the completeness of the retrieved literature in this review. Inclusion of the nonrandomized longitudinal studies may have introduced selection and recall bias. Only papers published in English were included which might have introduced a reference bias.
This is the largest meta-analysis investigating laparoscopic cholecystectomies and the use of IOC in detection of choledocholithiasis and BDI. Previous meta-analyses have included primary open-cholecystectomies,35,37 pediatric populations,13,35,37,38 misclassified routine and selective IOC when indeed they were papers comparing IOC to no IOC,35,36,38 had much smaller populations,39 and/or had narrower timeframes.37 Our results, therefore, provide the most comprehensive analysis to date to address the role of IOC during laparoscopic cholecystectomy with an all-inclusive timeframe and specific, well-defined inclusion criteria.
CONCLUSION
This study is the largest to investigate choledocholithiasis and BDI detection rates in IOC in LC. Despite surgical cultures and incentives to perform IOC routinely, this study supports that there is no strong evidence to suggest that intraoperative choledocholithiasis detection, missed stones, and BDI detection rates are lower with either routine or selective IOC. Due to significant heterogeneity in the analyses and, with large patient numbers from retrospective studies and a lack of large multicenter trials, no definitive conclusions about how IOC should be used can be drawn from this study. To add to the literature and to support routine IOC use one way or another, a large multicenter RCT with robust methodology is needed. Additionally, an outcome analysis should be performed based on clinical signs and biochemical markers in those who had routine and selective IOC in LC to inform a standard algorithm on who benefits most from an IOC during LC.
Footnotes
Acknowledgements: none.
Disclosure: none.
Conflict of interests: none.
Funding sources: none.
Informed consent: Dr. Catherine Hall declares that written informed consent was obtained from the patient/s for publication of this study/report and any accompanying images.
Contributor Information
Catherine Hall, Department of Surgery, The Canberra Hospital, Garran, Australia..
Slesha Amatya, Australian National University Medical School, The Australian National University, Canberra, Australia..
Ramesh Shanmugasundaram, Australian National University Medical School, The Australian National University, Canberra, Australia..
Ngee-Soon Lau, Department of Surgery, The Canberra Hospital, Garran, Australia..
Edwin Beenen, Department of Surgery, The Canberra Hospital, Garran, Australia..
Sivakumar Gananadha, Department of Surgery, The Canberra Hospital, Garran, Australia..
References:
- 1. Australian Institute of Health and Welfare. Laparoscopic cholecystectomy hospitalisations. The Second Australian Atlas of Healthcare Variation 2017. Available at: https://www.safetyandquality.gov.au/our-work/healthcare-variation/atlas-2017/atlas-2017-download-atlas. Accessed January 29, 2022.
- 2. Nickkholgh A, Soltaniyekta S, Kalbasi H. Routine versus selective intraoperative cholangiography during laparoscopic cholecystectomy. Surg Endosc. 2006;20(6):868–874. [DOI] [PubMed] [Google Scholar]
- 3. Ausania F, Holmes LR, Ausania F, et al. Intraoperative cholangiography in the laparoscopic cholecystectomy era: why are we still debating? Surg Endosc. 2012;26(5):1193–1200. [DOI] [PubMed] [Google Scholar]
- 4. Ding GQ, Cai W, Qin MF. Is intraoperative cholangiography necessary during laparoscopic cholecystectomy for cholelithiasis? World J Gastroenterol. 2015;21(7):2147–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Institutes of Health Consensus Development Conference Statement on Gallstones and Laparoscopic Cholecystectomy. Am J Surg [Internet]. 1993;165(4):390–398. [DOI] [PubMed] [Google Scholar]
- 6. Boerma D, Rauws EA, Keulemans YC, et al. Impaired quality of life 5 years after bile duct injury during laparoscopic cholecystectomy: a prospective analysis. Ann Surg. 2001;234(6):750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Reuver PR, Sprangers MA, Rauws EA, et al. Impact of bile duct injury after laparoscopic cholecystectomy on quality of life: a longitudinal study after multidisciplinary treatment. Endoscopy [Internet]. 2008;40(8):637–643. [DOI] [PubMed] [Google Scholar]
- 8. Nies C, Bauknecht F, Groth C, et al. Intraoperative cholangiography as a routine method? A prospective, controlled, randomized study. Chirurg. 1997;68(9):892–897. [DOI] [PubMed] [Google Scholar]
- 9. Chan ACW, Chung SCS, Wyman A, et al. Selective use of preoperative endoscopic retrograde cholangiopancreatography in laparoscopic cholecystectomy. Gastrointest Endosc. 1996;43(3):212–215. [DOI] [PubMed] [Google Scholar]
- 10. Hainsworth PJ, Rhodes M, Gompertz RHK, et al. Imaging of the common bile duct in patients undergoing laparoscopic cholecystectomy. Gut [Internet]. 1994;35(7):991–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jamal KN, Smith H, Ratnasingham K, et al. Meta-analysis of the diagnostic accuracy of laparoscopic ultrasonography and intraoperative cholangiography in detection of common bile duct stones. Ann R Coll Surg Engl. 2016;98(4):244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. [DOI] [PubMed] [Google Scholar]
- 13. Törnqvist B, Waage A, Zheng Z, Ye W, Nilsson M. Severity of acute cholecystitis and risk of iatrogenic bile duct injury during cholecystectomy, a population-based case–control study. World J Surg. 2016;40(5):1060–1067. [DOI] [PubMed] [Google Scholar]
- 14. Wakabayashi G, Iwashita Y, Hibi T, et al. Tokyo Guidelines 2018: surgical management of acute cholecystitis: safe steps in laparoscopic cholecystectomy for acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci [ Sci]. 2018;25(1):73–86. [DOI] [PubMed] [Google Scholar]
- 15. Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. [DOI] [PubMed] [Google Scholar]
- 16. Ladocsi LT, Benitez LD, Filippone DR, Nance FC. Intraoperative cholangiography in laparoscopic cholecystectomy: a review of 734 consecutive cases. Am Surg. 1997;63(2):150–156. [PubMed] [Google Scholar]
- 17. Guerra-Filho V, Nunes TA, Araújo ID. Perioperative fluorocholangiography with routine indication versus selective indication in laparoscopic cholecystectomy. Arq Gastroenterol. 2007;44(3):271–275. [DOI] [PubMed] [Google Scholar]
- 18. Kohn A, Creech S, Shayani V. Indicated cholangiography in patients operated on by routine versus selective cholangiographers. Am Surg. 2004;70(3):203–206. [PubMed] [Google Scholar]
- 19. Khan OA, Balaji S, Branagan G, Bennett DH, Davies N. Randomized clinical trial of routine on-table cholangiography during laparoscopic cholecystectomy. Br J Surg. 2011;98(3):362–367. [DOI] [PubMed] [Google Scholar]
- 20. Verma S, Wichmann MW, Gunning T, Beukes E, Maddern G. Intraoperative cholangiogram during laparoscopic cholecystectomy: a clinical trial in rural setting. Aust J Rural Health [Health]. 2016;24(6):415–421. [DOI] [PubMed] [Google Scholar]
- 21. Altieri MS, Yang J, Obeid N, Zhu C, Talamini M, Pryor A. Increasing bile duct injury and decreasing utilization of intraoperative cholangiogram and common bile duct exploration over 14 years: an analysis of outcomes in New York State. Surg Endosc. 2018;32(2):667–674. [DOI] [PubMed] [Google Scholar]
- 22. Royal Australasian College of Surgeons and Medibank Private. Surgical Variance Report 2017 General Surgery. Available at: https://www.surgeons.org/Resources/reports-guidelines-publications/surgical-variance-reports#2017. Accessed May 10, 2022.
- 23. Harrison EM, O'Neill S, Meurs TS, et al. Hospital volume and patient outcomes after cholecystectomy in Scotland: retrospective, national population based study. BMJ. 2012;344:e3330. [DOI] [PubMed] [Google Scholar]
- 24. Ingraham AM, Cohen ME, Ko CY, Hall BL. A current profile and assessment of North American cholecystectomy: results from the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;211(2):176–186. [DOI] [PubMed] [Google Scholar]
- 25. Ford JA, Soop M, Du J, Loveday BP, Rodgers M. Systematic review of intraoperative cholangiography in cholecystectomy. Br J Surg. 2012;99(2):160–167. [DOI] [PubMed] [Google Scholar]
- 26. Ludwig K, Bernhardt J, Steffen H, Lorenz D. Contribution of intraoperative cholangiography to incidence and outcome of common bile duct injuries during laparoscopic cholecystectomy. Surg Endosc. 2002;16(7):1098–1104. [DOI] [PubMed] [Google Scholar]
- 27. Slim K, Martin G. Does routine intra-operative cholangiography reduce the risk of biliary injury during laparoscopic cholecystectomy? An evidence-based approach. J Visc Surg. 2013;150(5):321–324. [DOI] [PubMed] [Google Scholar]
- 28. Sanjay P, Kulli C, Polignano FM, Tait IS. Optimal surgical technique, use of intra-operative cholangiography (IOC), and management of acute gallbladder disease: the results of a nation-wide survey in the UK and Ireland. Ann R Coll Surg Engl. 2010;92(4):302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Department of Health and Aged Care. The Australian Medicare Benefits Schedule Review Taskforce Report for General Surgery Items. Available at: https://www.health.gov.au/resources/publications/taskforce-final-report-general-surgery-items?language=en. Accessed on: May 15, 2022.
- 30. Mui J, Mayne DJ, Davis KJ, Cuenca J, Craig SJ. Increasing use of intraoperative cholangiogram in Australia: is it evidence‐based? ANZ J Surg. 2021;91(7–8):1534–1541. [DOI] [PubMed] [Google Scholar]
- 31. Sheffield KM, Riall TS, Han Y, Kuo YF, Townsend CM, Goodwin JS. Association between cholecystectomy with vs without intraoperative cholangiography and risk of common duct injury. JAMA [Internet]. 2013;310(8):812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Flum DR, Cheadle A, Prela C, Dellinger EP, Chan L. Bile duct injury during cholecystectomy and survival in Medicare beneficiaries. JAMA [Internet]. 2003;290(16):2168–2173. [DOI] [PubMed] [Google Scholar]
- 33. Czerwonko ME, Pekolj J, Uad P, et al. Laparoscopic transcystic common bile duct exploration in the emergency is as effective and safe as in elective setting. J Gastrointest Surg. 2019;23(9):1848–1855. [DOI] [PubMed] [Google Scholar]
- 34. Rystedt JM, Tingstedt B, Montgomery F, et al. Routine intraoperative cholangiography during cholecystectomy is a cost-effective approach when analysing the cost of iatrogenic bile duct injuries. HPB (Oxford). 2017;19(10):881–888. [DOI] [PubMed] [Google Scholar]
- 35. Rystedt JM, Wiss J, Adolfsson J, et al. Routine versus selective intraoperative cholangiography during cholecystectomy: systematic review, meta-analysis and health economic model analysis of iatrogenic bile duct injury. BJS open [Internet]. 2021;5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Photi ES, El-Hadi A, Brown S, et al. The routine use of cholangiography for laparoscopic cholecystectomy in the modern era. JSLS. 2017;21(3):e2017.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Donnellan E, Coulter J, Mathew C, et al. A meta-analysis of the use of intraoperative cholangiography; time to revisit our approach to cholecystectomy? Surg Open Sci. 2021;3:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Waage A, Nilsson M. Iatrogenic bile duct injury: a population-based study of 152,776 cholecystectomies in the Swedish Inpatient Registry. Arch Surg. 2006;141(12):1207–1213. Dec [DOI] [PubMed] [Google Scholar]
- 39. Kovács N, Németh D, Földi M, et al. Selective intraoperative cholangiography should be considered over routine intraoperative cholangiography during cholecystectomy: a systematic review and meta-analysis. Surg Endosc. 2022;36(10):7126–7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Flum DR, Koepsell T, Heagerty P, Sinanan M, Dellinger EP. Common bile duct injury during laparoscopic cholecystectomy and the use of intraoperative cholangiography: adverse outcome or preventable error? Arch Surg. 2001;136(11):1287–1292. [DOI] [PubMed] [Google Scholar]
- 41. Giger U, Ouaissi M, Schmitz SH, Krähenbühl S, Krähenbühl L. Bile duct injury and use of cholangiography during laparoscopic cholecystectomy. Br J Surg. 2011;98(3):391–396. [DOI] [PubMed] [Google Scholar]
- 42. Soper NJ, Dunnegan DL. Routine versus selective intra-operative cholangiography during laparoscopic cholecystectomy. World J Surg. 1992;16(6):1133–1140. [DOI] [PubMed] [Google Scholar]
- 43. Amott D, Webb A, Tulloh B. Prospective comparison of routine and selective operative cholangiography. ANZ J Surg. 2005;75(6):378–382. [DOI] [PubMed] [Google Scholar]
- 44. Carlson MA, Ludwig KA, Frantzides CT, et al. Routine or selective intraoperative cholangiography in laparoscopic cholecystectomy. J Laparoendosc Surg [Internet]. 1993;3(1):27–33. [DOI] [PubMed] [Google Scholar]
- 45. Snow LL, Weinstein LS, Hannon JK, et al. Evaluation of operative cholangiography in 2043 patients undergoing laparoscopic cholecystectomy: a case for the selective operative cholangiogram. Surg Endosc. 2001;15(1):14–20. [DOI] [PubMed] [Google Scholar]