Abstract
Background
Elongation factor 1 A (EF1A), an essential regulator for protein synthesis, has been reported to participate in abiotic stress responses and environmental adaption in plants. However, the role of EF1A in abiotic stress response was barely studied in Medicago truncatula. Here, we identified elongation factor (EF) genes of M. truncatula and studied the salt stress response function of MtEF1A1 (MTR_6g021805).
Results
A total of 34 EF genes were identified in the M. truncatula genome. Protein domains and motifs of EFs were highly conserved in plants. MtEF1A1 has the highest expression levels in root nodules and roots, followed by the leaves and stems. Transgenic Arabidopsis thaliana overexpressing MtEF1A1 was more resistant to salt stress treatment, with higher germination rate, longer roots, and more lateral roots than wild type plant. In addition, lower levels of H2O2 and malondialdehyde (MDA) were also detected in transgenic Arabidopsis. Similarly, MtEF1A1 overexpressing M. truncatula was more resistant to salt stress and had lower levels of reactive oxygen species (ROS) in leaves. Furthermore, the expression levels of abiotic stress-responsive genes (MtRD22A and MtCOR15A) and calcium-binding genes (MtCaM and MtCBL4) were upregulated in MtEF1A1 overexpressing lines of M. truncatula.
Conclusion
These results suggested that MtEF1A1 play a positive role in salt stress regulation. MtEF1A1 may realize its function by binding to calmodulin (CaM) or by participating in Ca2+-dependent signaling pathway. This study revealed that MtEF1A1 is an important regulator for salt stress response in M. truncatula, and provided potential strategy for salt-tolerant plant breeding.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-023-04139-5.
Keywords: Medicago truncatula, Elongation factor, Transgenic, Salt stress, Calmodulin
Background
Elongation factors (EFs) are common cellular proteins that are responsible for the extension of peptide chains during protein synthesis. There are three prokaryotic extension factors: EF thermo unstable (EF-Tu), EF thermo stable (EF-Ts), and elongation factor G (EF-G). Eukaryotic EFs can be divided into the eEF1, eEF2, and eEF3 families. The eEF1 complex causes conformational changes in ribosomes and catalyzed aa-tRNA, resulting in the hydrolysis of GTP and the release of eEF1A·GDP. eEF1 contains four subunits: eEF1A, eEF1Bα, eEF1Bβ, and eEF1Bγ [1]. eEF1A is equivalent to prokaryotic EF-Tu, accounting for 1–2% of total cellular protein content [2]. The guanine nucleotide exchange factor (GEF) eEF1B promotes the exchange of GDP and GTP, allowing eEF1A to participate in another round of peptide chain extension [3]. eEF1Bα is also involved in plant phosphorylation and is related to serine [4]. In Arabidopsis, the silencing of eEF1Bβ leads plant dwarfing and reduced total lignin and crystalline cellulose, respectively [5]. eEF1Bγ has been demonstrated to participate in oxidative stress response and may be related to its glutathione transferase (GST) properties [6]. eEF2 is a monomer that catalyzes the movement of ribosomes bound to mRNA. Through the phosphorylation and inactivation of eukaryotic extension factor-2 kinase (eEF2K), extension of peptide chains is hindered and protein synthesis was inhibited [7]. In eukaryotes, eEF3 can assist eEF1A delivering aa-tRNA and removing deacyl-tRNA from the E-site, ensuring peptide chain extension [8]. Together with two paralogous genes, YEF3 (YLR249W) and HEF3 (YNL014W), eEF3 also plays an important role in detoxification of reactive oxygen species (ROS) [8].
Arabidopsis thaliana contains a family of four EF1A genes (AtEF1A1–4) that are divided into two subfamilies based on sequence similarity and physical location within the genome [9]. The four EF1A genes in soybean (Glycine max) can also be divided into two subfamilies, type A and type B; Al and A2 are highly expressed in green organs (leaves and stems), whereas there is little difference in expression levels of the four genes in the roots [10]. Eukaryotic cells contain two isoforms of EF1A, EF1A1 and EF1A2, that have structural differences [11]. Some studies have shown that the structure and sequences of plant EF1A genes are relatively conserved across species. It has been previously reported that AtEF1A2 (AT1G07930) and AtEF1A4 (AT5G60390) have stable structures and high expression levels in Arabidopsis [12, 13]. Analysis with NormFinder, which compares potential internal reference genes, showed that EF1A was expressed stably under pyrene and heavy metal treatments in mangrove (Aegiceras corniculatum) [14]. Furthermore, EF1A was stably expressed under salt, osmotic, and metal stress conditions in cucumber (Cucumis sativus) [15] and under drought stress in Chinese cabbage (Brassica rapa) [15, 16].
EF1A genes are induced by environmental stress in many plants. EF1A expression was found to be induced by heat and cold stress in barley (Hordeum vulgare) and maize (Zea mays) [17–19]. In wheat (Triticum aestivum), EF1A expression increases in response to heat; this may be due to the involvement of EF1A in protein folding as a molecular chaperone, protecting heat-resistant proteins from heat damage or enhancing heat resistance by promoting protein synthesis or transcriptional activation [20, 21]. Subsequent studies have found that two or three basic peptides or subtypes of EF1A can increase EF1A abundance under heat stress [22]. Yeast hybrid and northern blot assays showed that AcEF1A of Bruguiera sexangula enhances tolerance to salt and osmotic stress in Escherichia coli [23]. A cDNA clone of AtEF1A2 was isolated from a salt-sensitive calcineurin (CaN) yeast deletion mutant library. This gene has companion protein activity promoting refolding of unfolded proteins in cell or in vitro which enhances salt tolerance in yeast, and overexpression in plants confers salt tolerance [24]. In recent years, researchers have studied the regulatory network of miRNAs responsive to salt stress in barley; they found that EF1A plays a role in salt tolerance as a molecular companion and is the target gene of miR169i and the novel miRNA PC-mir124 [25].
Plants respond and adapt to salt stresses by osmotic stress and ion stress, which can reduce the cell volume and the cell division rate, slow the growth of leaves and roots at different stages, and cause inhibition of plant photosynthesis [26, 27]. Salt stress accumulation can also promote the accumulation of reactive oxygen species (ROS) and damage the cell membrane structure [28]. Compared with the control group, the overexpression of soybean GmEF4 significantly delayed leaf wilting, prolonged root system, increased biomass and decreased ROS accumulation under drought and salt treatment [29]. Meanwhile, EF1A has rarely been reported to be involved in Medicago truncatula salt stress signaling. In our previous study, we conducted an iTRAQ proteomic analysis in M. truncatula ecotype R108 and M. sativa cv. Zhongmu3. From those data, an elongation factor protein (MTR_6g021805) was found to be induced by salt stress (1.51-fold and 1.20-fold change in M. truncatula and M. sativa, respectively) [30, 31]. Here, we cloned MtEF1A1 from M. truncatula and explored the molecular mechanisms through which it participates in salt stress responses. We found that MtEF1A1 overexpression enhanced salt tolerance in Arabidopsis and M. truncatula, and a model of MtEF1A1 regulation under salt stress was proposed, likely through calcium signaling pathways. These results position MtEF1A1 as a promising candidate gene for breeding salt tolerance in forage plants.
Results
Structural analysis of MtEF1A1
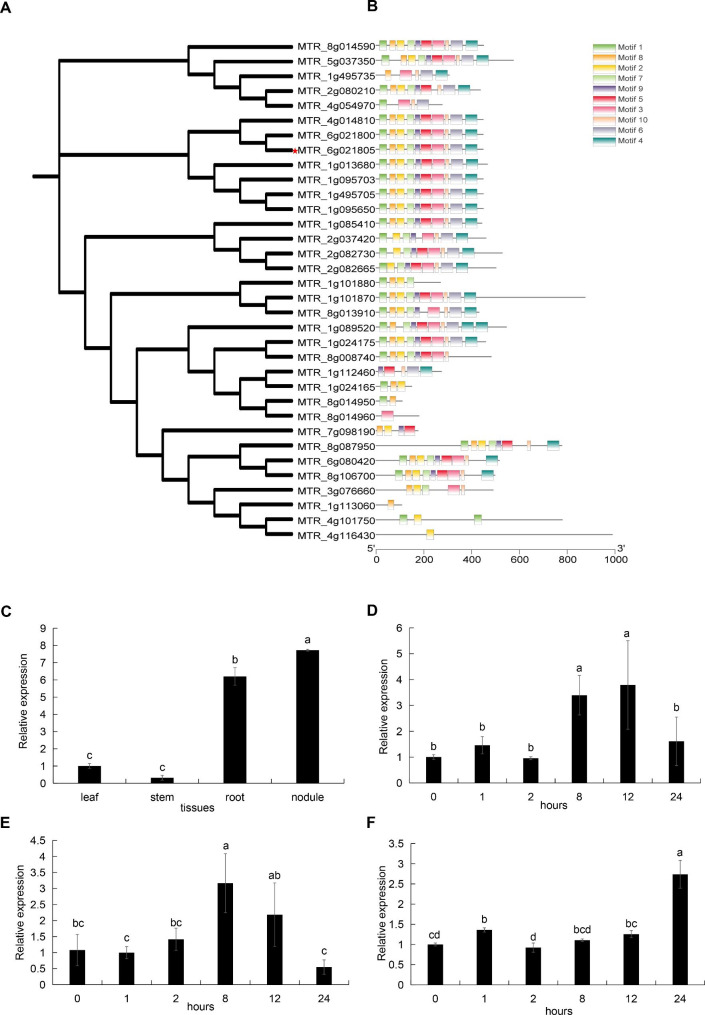
MtEF1A1 has a 447-bp coding sequence (CDS), which encodes a 49.24-kDa protein with a predicted isoelectric point (pI) of 8.02. EF1A has three conserved domains: GTP_EFTU (Pfam: PF00009), GTP_EFTU_D2 (Pfam: PF03144), and GTP_EFTU_D3 (Pfam: PF03143). There are 34 members of the MtEF gene family (Fig. 1A). Protein structure analysis showed that 14 of the 34 EF family genes contained 10 motifs, and the remaining 20 proteins had 1–9 motifs (Fig. 1B). Expression Logo of motifs in EF genes in M. truncatula were shown in Fig. S2. The amino acid sequence of MtEF1A1 was aligned with EF1A proteins from Arabidopsis, soybean, and rice (Oryza sativa). There was > 94% sequence similarity between MtEF1A1, GmEF1A (GLYMA_17G186600), OsEF1A (Os03g0177400), and AtEF1A1–4 (AT1G07920, AT1G07930, AT1G07940, and AT5G60390), indicating that EF1A was highly evolutionarily conserved. MtEF1A1 had four conserved regions (G-1 to G-4) predicted to be involved in GDP/GTP exchange and GTP hydrolysis based on sequence comparison to proteins in other species (Additional file 2: Fig. S1A). The average hydrophilic coefficient was − 0.645. MtEF1A1 was found to be a hydrophilic protein with no transmembrane structure or signal peptide (Additional file 2: Fig. S1B), and was predicted to be localized to the cytoplasm and nucleus. Tertiary protein structure of MtEF1A1 was predicted (Additional file 2: Fig. S1C). Random coils (37.14%) and alpha helixes (29.53%) were the dominant predicted secondary structures (Additional file 2: Fig. S1D).
Fig. 1.
Bioinformatic analyses of EF genes in M. truncatula and expression pattern of MtEF1A1. (A) Phylogenetic tree shows the relationships between the 34 EF gene family members in M. truncatula. MtEF1A (MTR_6g021805) is marked by a red pentagram. (B) Analysis of conserved motifs in EF family proteins. The conserved motifs 1–10 were indicated by different colors. (C) Expression levels of MtEF1A1 of different tissues in wild-type M. truncatula (R108) and expression levels were normalized to leaf. Different lowercase letters indicate significant differences (p < 0.05). Differential expression of MtEF1A1 in response to 200 mM NaCl for 0, 1, 2, 8, 12, 24 h in M. truncatula R108 of leaf (D), stem (E) and root (F). Each tissues expression level in salt-treated was normalized to that of plants at point 0 h. Values are means ± SD of three biological replicates. Different lowercase letters indicate significant differences (p < 0.05)
MtEF1A1 was salt stress-inducible
Quantitative real-time RT-PCR was used to detect detailed changes in MtEF1A1 expression in different tissues or response to salt treatment. By analyzing the expression levels in different tissues, the results showed that MtEF1A1 was expressed at higher levels in the root nodules than in the roots of M. truncatula (Fig. 1C). The results in response to salt treatment showed that MtEF1A1 was induced by salt stress in multiple tissues, and the expression level increased significantly after 8 h (Fig. 1D-F). In untreated plants, MtEF1A1 expression was highest in the roots, followed by the leaves and stems (Fig. 1C).
MtEF1A1 overexpression improved salt tolerance in transgenic Arabidopsis
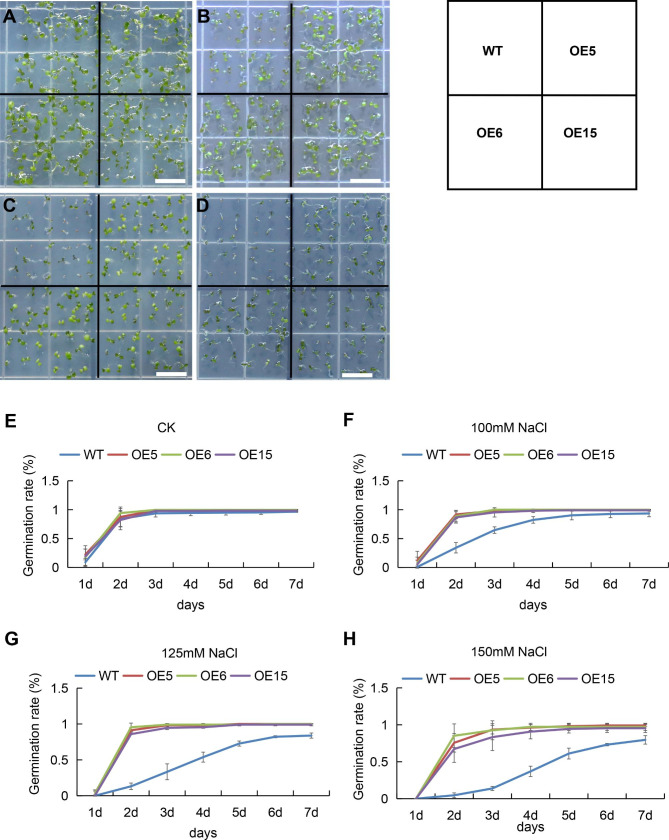
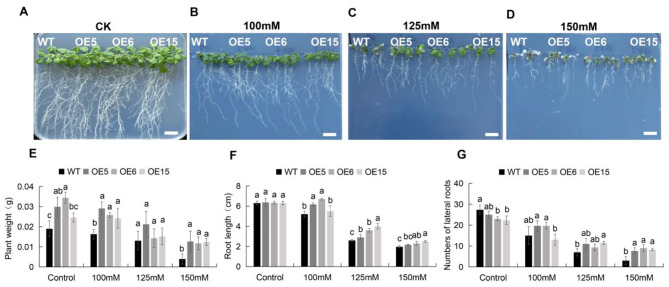
Fifteen Arabidopsis lines overexpressing MtEF1A1 were generated. Three of these overexpression (OE) lines (OE5, OE6, and OE15) were randomly selected and MtEF1A1 transcription was measured (Additional file 2: Fig. S3A). On media containing 0, 100, 125, or 150 mM NaCl, the MtEF1A1-OE lines exhibited significantly higher germination rates than the WT after 7 days. There were no significant differences in germination rates between WT and MtEF1A1-OE plants grown on agar plates without added salt (Fig. 2). WT, OE5, OE6, and OE15 plants were then grown on 1/2 Murashige and Skoog (MS) medium for 7 days, then transferred to vertical petri dishes containing 0, 100, 125, or 150 mM NaCl for 15 days to measure root growth. In salt-stressed plants, root growth inhibition was lower and leaf phenotypes were superior in the OE lines compared to the WT. Under the 150 mM NaCl treatment, the leaves of most MtEF1A1-OE plants remained green (Fig. 3D). In response to the range of salt stress conditions, the main root length was 6–54% higher in OE5, OE6, and OE15 than in WT seedlings. Without salt treatment, MtEF1A1-OE plants had fewer lateral roots than WT plants. However, as the salt concentration increased, OE plants had more lateral roots than the WT. The difference was significant at 150 mM NaCl, with the OE plants having 2.5–3 times as many lateral roots as WT plants. Plant weight was negatively correlated with the number of lateral roots without salt treatment (i.e., OE plants had heavier roots but fewer lateral roots than the WT). There was no significant difference in plant weight at 125 mM NaCl, but the plant weight was significantly higher for OE than WT plants at 100 mM NaCl and 150 mM NaCl (Fig. 3).
Fig. 2.
Germination of wild-type (WT) Arabidopsis plants and those overexpressing MtEF1A1. (A-D) Germination and growth of WT and MtEF1A1-overexpression (OE) Arabidopsis seedlings on medium containing NaCl at 0 mM (A), 100 mM (B), 125 mM (C), and 150 mM for 7 days (D). Scale bar = 1 cm. CK, control (0 mM NaCl). (E-H) Germination rates of WT and MtEF1A1-OE seeds sown on medium containing NaCl at 0 mM (E), 100 mM (F), 125 mM (G), and 150 mM (H). Values are means ± SD of three biological replicates
Fig. 3.
Salt tolerance analysis in seedlings grown on vertical plates for 15 days. (A-D) Images of wild-type (WT) and MtEF1A1-overexpression (OE) Arabidopsis seedling growth on vertical plates with media containing a range of salt concentrations (0–150 mM NaCl). Scale bar = 1 cm. CK, control (0 mM NaCl). Plant weight (E), root length (F), and lateral root number (G) of WT and MtEF1A1-OE seedlings grown on vertical plates with a range of salt concentrations (0–150 mM). Values are means ± SD of three biological replicates. Different lowercase letters between different OE lines of one treatment indicate significant differences (p < 0.05)
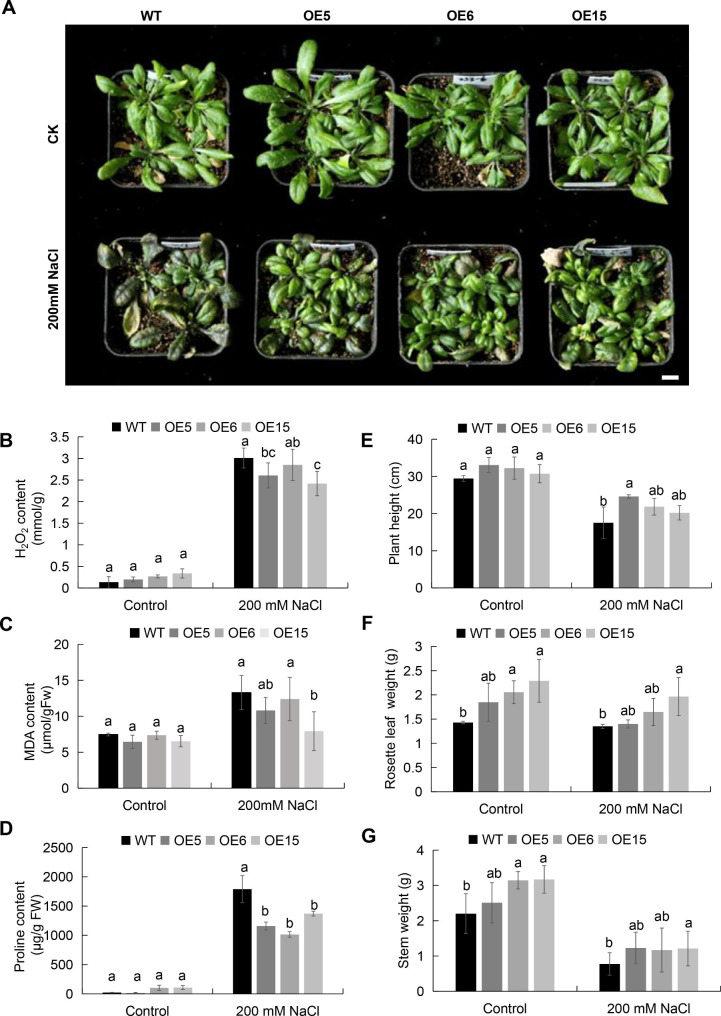
To further test the salt tolerance of Arabidopsis MtEF1A1-OE plants, three-week-old transgenic and WT plants grown in soil were treated with 0 or 200 mM NaCl for three weeks. There were no significant differences in leaf color between untreated WT and OE plants. However, with salt treatment, there were differences in growth and physiological indexes, with severe wilting and some chlorosis of WT rosette leaves (Fig. 4A). OE lines exhibited lower levels of H2O2 and MDA contents compared to the WT (Fig. 4B-C). Salt stress can lead to less accumulation of proline levels in the OE lines leaves (Fig. 4D). Specifically, OE15 seedlings accumulated 50% less MDA than WT seedlings, indicating that MtEF1A1 overexpression enhanced salt tolerance in Arabidopsis. To explore the effects of salt treatment on Arabidopsis growth, the rosette leaf fresh weight, plant height, and stem weight were measured (Fig. 4E-G). Under untreated growth conditions, MtEF1A1-OE plants had higher height and weight than WT plants, but the differences were not significant in plant height. In salt-treated plants, WT plants showed greater growth inhibition with respect to rosette leaf weight and stem weight than MtEF1A1-OE plants, whereas the aboveground weight of OE15 was significantly higher than in WT plants after salt treatment.
Fig. 4.
Salt tolerance analysis of three-week-old Arabidopsis seedlings grown in soil. (A) Phenotypes of three-week-old MtEF1A1-overexpression (OE) and wild-type (WT) Arabidopsis plants after salt treatment for 21 days. Scale bar = 1 cm. CK, control (0 mM NaCl). (B-D) Levels of H2O2, MDA, and proline in leaves. Plant height (E), rosette leaf weight (F), and stem weight (G) of WT and OE plants treated with 0 or 200 mM NaCl. Values are means ± SD of three biological replicates. Different lowercase letters between different OE lines of one treatment indicate significant differences (p < 0.05)
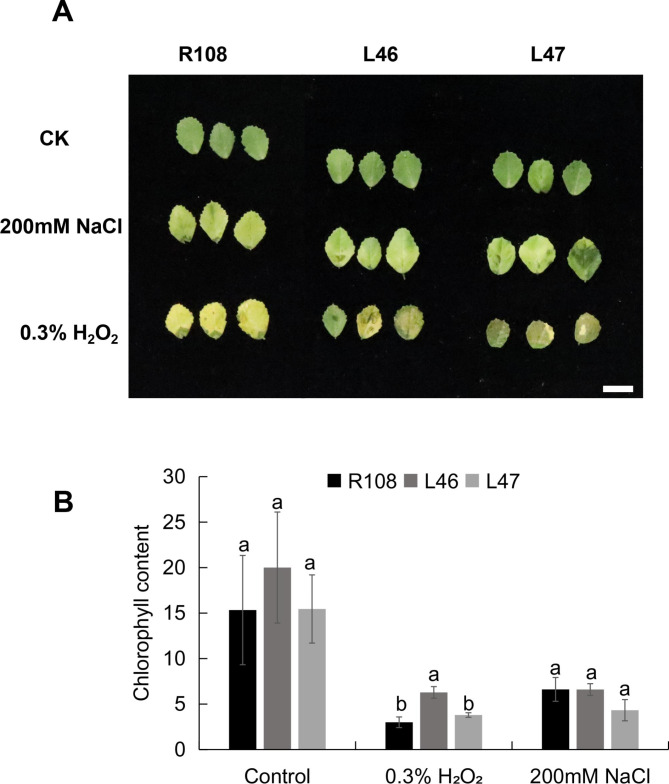
MtEF1A1 overexpression reduced chlorophyll degradation in transgenic M. truncatula leaves
Sixteen MtEF1A1-OE lines were next generated in M. truncatula, and two (L46 and L47) were selected for further study. The experiment was carried out using T2 individuals. M. truncatula R108 plants were used as the WT control. MtEF1A1 expression was 23–32 times higher in L46/L47 than in R108 plants (Additional file 2: Fig. S3B). In untreated plants, there was no significant difference in chlorophyll content between MtEF1A1-OE and R108 plants (Fig. 5A and B). After salt treatment, L46, L47, and R108 plants showed no significant difference in leaf color or chlorophyll content. After 0.3% H2O2 treatment, R108 leaves were clearly yellow, and L46/L47 leaves gradually turn dark green or yellowish (Fig. 5A). The chlorophyll content was significantly decreased compared with the MtEF1A1-OE line L46 (Fig. 5B). These results indicated that MtEF1A1 overexpression reduced toxic effect of ROS by H2O2 treatment of M. truncatula leaves in vivo.
Fig. 5.
Leaf phenotypes of wild-type (WT) and MtEF1A1-overexpression (OE) M. truncatula lines treated with salt or H2O2. (A) Leaf phenotypes of WT (R108) and MtEF1A1-OE plants (L46 and L47). Plants were treated with 200 mM salt or 0.3% H2O2. Scale bar = 1 cm. CK, control (0 mM NaCl). (B) Leaf chlorophyll content in R108, L46 and L47 plants after salt or H2O2 treatment. Values are means ± SD of three biological replicates. Different lowercase letters between different OE lines of one treatment indicate significant differences within each treatment (p < 0.05)
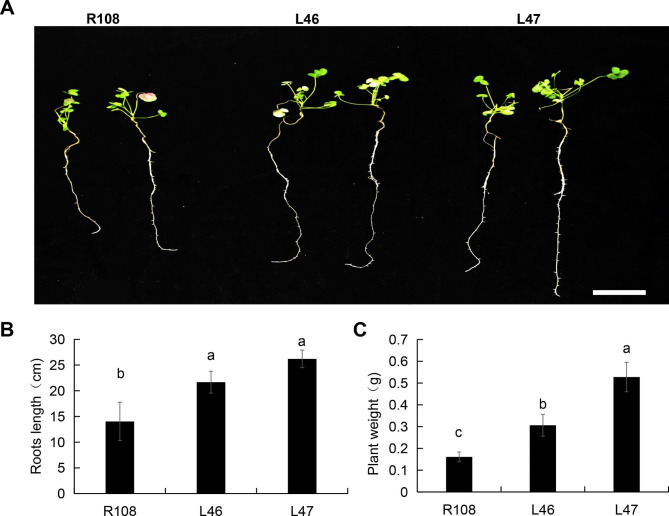
MtEF1A1 overexpression improved salt tolerance in transgenic M. truncatula
M.truncatula plants (R108 and EF1A1-OE lines) were cultured in 1/2 Hoagland nutrient solution medium for three weeks. Mature seedlings were collected and treated with 100 mM NaCl for two weeks. R108 plants gradually wilted and collapsed, and the abaxial surfaces of some leaves began to turn purple (Fig. 6A). L46 and L47 had longer roots than R108, and the difference was statistically significant (Fig. 6B). L46 and L47 also had significantly higher plant weight than R108 (Fig. 6C).
Fig. 6.
Salt stress analysis in three-week-old M. truncatula plants. (A) Images of wild-type R108 and MtEF1A1-overexpression (OE) plants (L46 and L47). Plants were cultured in 1/2 Hoagland nutrient solution with 100 mM NaCl for two weeks. Scale bar = 5 cm. (B-C) Roots length (B) and plant weight (C) of R108, L46 and L47 plants after salt stress. Values are means ± SD of three biological replicates. Different lowercase letters indicate significant differences (p < 0.05)
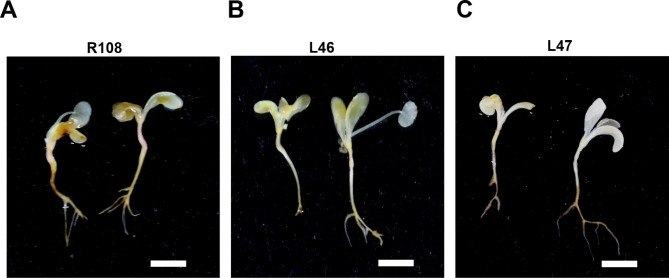
To explore the effect of MtEF1A1 overexpression on salt stress tolerance at the seedling stage of M. truncatula, plants treated with salt for 10 d were stained with DAB to detect accumulation of H2O2. Observation of whole seedlings stained with DAB showed that R108 had a much larger stained area and stained darker than L46 and L47 plants, indicating higher levels of ROS in R108 (Fig. 7).
Fig. 7.
DAB staining assay in M. truncatula seedings treated with salt. (A) Wild-type (R108) and (B, C) MtEF1A1-overexpression (L46 and L47) seedlings were cultured in 1/2 MS medium with 150 mM NaCl. Scale bar = 1 cm
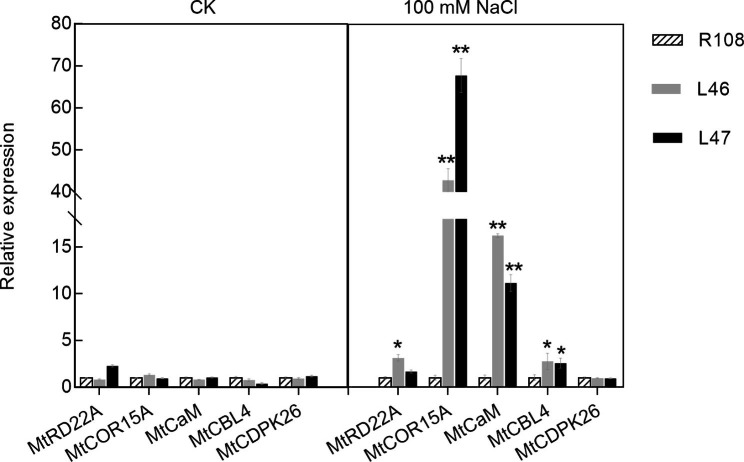
Effects of MtEF1A1 overexpression on abiotic stress-responsive gene expression
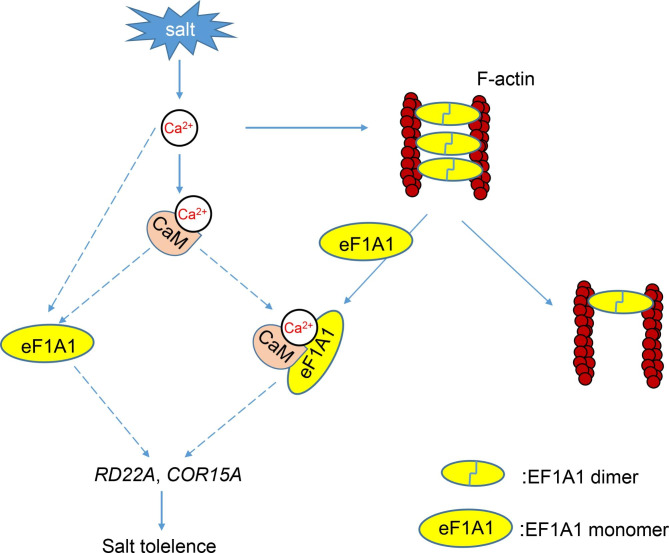
To determine a possible mechanism for MtEF1A1 functioning in the salt stress response, expression levels of known stress-responsive genes were analyzed in R108 and MtEF1A1-OE M. truncatula plants after salt treatment. BURP domain protein RD22 (MtRD22A), Cold-regulated 15 A (MtCOR15A), Calmodulin (MtCaM) and Calcineurin B-like proteins 4 (MtCBL4) were upregulated in MtEF1A1-OE plants compared to R108, and MtCOR15A and MtCaM genes were significantly induced after salt treatment. In untreated M. truncatula (CK), there were no significant differences in these genes (Fig. 8). We therefore speculate that MtEF1A1 may be involved in Ca2+-dependent signaling pathways that are induced by salt stress (Fig. 9). CaM participates in Ca2+-dependent signaling pathways associated with stress and promotes dimerization of target proteins through direct binding. However, there was no significant difference in the expression of Calcium-dependent protein 26 (MtCDPK26) between the R108 and MtEF1A1-OE lines (Fig. 8).
Fig. 8.
Expression of stress-related genes of three-week-old M. truncatula MtEF1A1-overexpressing plants (L46 and L47) and wild-type plants (R108) in 1/2 Hoagland medium (CK) and 100 mM NaCl for two weeks. R108 was used as the reference to calculate relative expression levels in the overexpression lines. Values are means ± SD of three biological replicates. Different lowercase letters between different OE lines of stress-related genes indicate significant differences (p < 0.05). ** indicates a significant difference at p < 0.01, * indicates a significant difference at p < 0.05
Fig. 9.
Proposed model of the regulatory mechanism of MtEF1A1 in response to salt stress. Salt stress induces the expression of MtEF1A1 through Ca2+ signaling. MtEF1A1 promotes expression of calmodulin (CaM), which binds Ca2+ to promote the expression of downstream genes, MtEF1A1, or the eEF1A1/Ca2+/CaM complex
Discussion
Elongation is the most important step in translation and is completed by highly conserved eukaryotic EF genes. EF1A protein has chaperone activity and can function in protein renaturation and structural changes [32]. The EF1A plastid protein is encoded by the nuclear genome, synthesized in the cytoplasm, then targeted to the plastid. EF1A is also found in mitochondria, which can play a role in protein synthesis and translation by catalyzing the extension of amino acid chains on the ribosome [33]. The sequence of MtEF1A1 (MTR_6g021805) was obtained from Ensembl (http://plants.ensembl.org/index.html), and homology comparison on the TAIR website (https://www.arabidopsis.org/cgi-bin/Blast/) yielded the four Arabidopsis genes with the highest similarity (96% amino acid sequence identity): AtEF1A1 (AT1G07920), AtEF1A2 (AT1G07930), AtEF1A3 (AT1G07940), and AtEF1A4 (AT5G60390). EF1A plays a role in protein synthesis, and it is often considered a housekeeping gene due to its conserved structure between different species. However, plant EF1A proteins are always encoded by multiple genes, and EF1A expression is not consistent between organs and developmental stages. In tomato and apple, EF1A is expressed at higher levels in young leaves than in mature leaves [34, 35]. EF1A is more highly expressed in the stems and roots of Chinese jujube (Ziziphus jujube) and cotton (Gossypium spp.) compared to the flowers [36, 37], but the opposite is true in banana (Musa paradisiaca) [38]. EF1A is expressed at higher levels in tomato roots than stems [39], consistent with the expression of MtEF1A1 in M. truncatula (Fig. 1C).
The growth status of plants is shown by related characteristics indicators such as plant height, plant weight, root length and lateral root number, which can be inhibited under stress conditions. At the same time, stress can affect indicators of plant growth at different stages, such as germination rate and survival rate. Studies have shown involvement of EF1A in abiotic and biotic stress responses in plants, potentially allowing for crop improvement via manipulation of EF1A [33]. In the present study, Arabidopsis plants overexpressing MtEF1A1 showed that there was no significant difference in terms of germination rate compared with WT plants when they were grown on 1/2 MS medium; MtEF1A1-OE lines did have a significantly higher germination rate than WT when the seedlings were exposed to salt stress (Fig. 2). This indicated that overexpression of MtEF1A1 could improve germination rate to some extent. WT Arabidopsis plants were more sensitive to salt stress than OE lines; the germination rates of OE5, OE6, and OE15 plants were higher than the WT (Fig. 2A and D). In Arabidopsis plants grown in soil, those overexpressing MtEF1A1 had higher weight than WT plants, with or without salt treatment (Fig. 4A); there was also a significant difference in the stem weight of MtEF1A1-OE plants (Fig. 4G). Based on these results, MtEF1A1 may be involved in pathways related to plant salt stress responses, but may also improve growth and development of Arabidopsis. It has previously been shown that there is crosstalk pathways related to salt stress responses and plant development [40]. The content of MDA, H2O2, proline and chlorophyll in plant leaf cells is an important physiological index to evaluate plant stress resistance, which can essentially explore the physiological mechanism of plants. In soybean, the contents of H2O2, O2− and MDA decreased, while the content of proline increased in GmEF4 overexpressing plants after stress treatment [29]. H2O2 is an important reactive oxygen molecule. In this study, the OE lines maintained lower levels of H2O2 and the result showed that OE lines could decrease the membrane lipid peroxidation damage caused by stress (Fig. 4B) [29]. The MDA content is known as a physiological marker of lipid peroxidation rate in plant, and is correlated with oxidative stress [41]. MDA was lower in OE lines indicating that the plasma membrane of OE lines was less influenced and lipid peroxidation was reduced (Fig. 4C). Proline, a stress response factor, did not always accumulate significantly in plants under stress, and the proline content of OE lines was lower than WT, which may be that the proline accumulation at this point appears to be a response to injury rather than an alleviator, and MtEF1A-overexpression Arabidopsis lines were less stimulated by the stress than WT [42].
Similar results were observed in M. truncatula; MtEF1A1 overexpression improved salt tolerance. To verify the salt tolerance of three-week-old M. truncatula plants, we examined the responses of WT and MtEF1A1-OE plants to salt stress during seedling growth (Fig. 6). Under salt stress conditions, MtEF1A1-OE lines had longer roots and higher fresh weight compared with the WT plants significantly (Fig. 6A-C). However, the difference of root length was not significant between the two OE lines, but there was difference in plant weight (Fig. 6B and C). This may have been a result of the low contribution of root weight to the total fresh weight. The influence of MtEF1A1 overexpression on aboveground tissue weight compared to underground biomass requires further study in M. truncatula. In M. truncatula grown in soil for 60 d, those overexpressing MtEF1A1 isolated leaves had increased tolerance of H2O2 treatment, and the chlorophyll content was significantly higher in the leaves of one MtEF1A1-OE line than in WT plants. However, when isolated leaves were treated with 200 mM NaCl solution, there was no difference in chlorophyll content between MtEF1A1-OE and WT leaves (Fig. 5). This may be due to the faster process of ROS accumulation after H2O2 treatment. Meanwhile, salt treatment of the whole Arabidopsis also affected the H2O2 content of the plant, which EF1A overexpression can alleviate (Fig. 4B). DAB staining in seedlings showed that the MtEF1A1-OE lines had lower ROS accumulation in response to salt stress compared to the WT, and that the WT showed generally higher ROS accumulation in the roots. This may be due to the high expression level of EF1A in the roots of M. truncatula, so the root damage degree of EF1A-overexpression lines was lower than that of WT under salt stress (Fig. 7). All above experiments of stress treatment in isolated leaves and DAB staining test speculated that overexpression of MtEF1A1 may enhance plant salt tolerance by inhibiting the accumulation of ROS.
Regulation of salt stress involves the salt sensitive (SOS) pathway, which regulates a range of proteins, including kinases, ion transporters [43], members of the calcium-dependent protein kinase (CDPK) pathway [44], and those in the mitogen-activated protein kinase (MAPK) pathway [45]. Ca2+ signaling usually functions as a secondary messenger in response to external stimuli. CaM, CAM-like protein (CML), and CDPKs regulate plant resistance by binding to downstream effector proteins. For example, AtCaM7 acts as a transcription factor in direct response to light stress [46]. AtTGA3 transcription factor can directly bind to the AtCaM3 promoter region and promote downstream stress response [47]. Ca2+ signal-dependent CaM binding usually causes structural changes in the target proteins or promotes their expression [48–50]. MtCDPK26 and AtCPK6 are both CDPKs. AtCPK6 and other CDPKs are activated by Ca2+ signals. AtCPK6 overexpression promotes salt stress tolerance in Arabidopsis [51, 52]. Calcineurin B-like proteins (CBLs) act as Ca2+ sensors; their targets, CBL-interacting protein kinases (CIPKs), function in plant signal regulation. Salt signaling promotes Ca2+ accumulation, which activates CBL4/SOS3 and CBL10 Ca2+ sensors to interact with CIPK24/SOS2 and participate in ion transport with SOS1 [53]. AtRD22 and AtCOR15A can participate in abscisic acid (ABA)-dependent pathways, and are upregulated by a variety of abiotic stressors. AtCOR15A has DRE and other abiotic stress-associated motifs in the promoter and participates in the Arabidopsis CBF-related cold resistance pathway [54]. AtRD22 is believed to be primarily regulated by activation of AtMYB2/AtMYC2 expression [55]. In the present study, we found that CaM expression was significantly increased in response to salt treatment of EF1A overexpression in M. truncatula. However, there were no significant differences in expression of MtCDPK26, suggesting that MtEF1A1 may interact with MtCaM but that it does not directly participate in regulation of CDPK pathway.
Previous studies showed that EF1A genes may be involved in transcription or post-transcriptional regulation in response to salt stress [25]. As a multifunctional protein, EF1A is also highly sensitive to changes in Ca2+ and pH, and may be an important downstream target of Ca2+ and lipid-related signal transduction pathways, causing changes in cytoskeleton structure and protein synthesis [56]. EF1A may not only participate in the common salt response pathway, but may also function at the protein level. EF1A has been shown to interact directly with tubulin, calmodulin, and filamentous actin (F-actin) in vitro [57]. EF1A has two forms, a monomer and a dimer. Under normal circumstances, the EF1A dimer bundles into F-actin to form a binding protein. In the presence of salt or high levels of Ca2+, the EF1A/Ca2+/CaM complex binds to F-actin, and EF1A in the dimer form reverts to the monomeric state [58, 59]. The eEF1A/Ca2+/CaM complex is speculated to regulate the downstream salt response pathway either directly or indirectly (Fig. 9). Protein hydrolysis can rapidly reduce the functional activity of EF1A. Treatment with phospholipase C and D leads to the reduction of amount of [14 C] ethanolamine-labeled eEF1A in the carrot microsomal fraction, and EF1A content can be quantified by PGE modification [56]. Studying the dynamic changes of MtEF1A1 levels in response to stress treatment is expected to provide novel insights into the salt-response pathway of EF1A.
Conclusion
Elongation factors are indispensable in protein synthesis. Overexpression of the elongation factor MtEF1A1 in Arabidopsis enhanced salt resistance, increasing the seed germination rate and decreasing levels of H2O2, MDA, and proline in the leaves of MtEF1A1-OE plants compared to the WT. In M. truncatula overexpressing MtEF1A1, leaf chlorophyll level was higher than in WT leaves after treatment with H2O2. Root damage was more severe in WT seedlings and DAB staining was darker, indicating higher ROS levels. The MtEF1A1 of M. truncatula plants were resistant to salt stress and showed higher expression of the Ca2+-signaling-related genes MtCaM and MtCBL4, and the salt-induced genes MtRD22A and MtCOR15A. This study shows that overexpression of MtEF1A1 increased salt stress tolerance in Arabidopsis and M. truncatula, indicating that MtEF1A1 plays a positive regulatory role in the salt stress response.
Materials and methods
Plant materials and growing conditions
Arabidopsis Col-0 used in this study was provided by our lab. Seeds were stratified for 2 d under dark conditions at 4 °C, then cultured on 1/2 MS medium. After 7 d, the seedlings were transferred to petri dishes in incubator under a 16/8 h light/dark photoperiod at 22/18°C, or transferred to soil prior to salt treatments in a greenhouse (26/22°C, 16/8 h light/dark photoperiod).
For M. truncatula experiments, ecotype R108 provided by our lab was used as the WT. Seeds were surface-sterilized and grown on 1/2 MS medium at 22/18 ℃ under a 16/8 h light/dark cycle in a growth chamber with 60–70% relative humidity. M. truncatula seeds germinated on wet filter paper were transferred to 1/2 Hoagland’s solution and grown in a growth chamber, or transferred into soil (1:1 vermiculite:sand) and grown in a greenhouse at 26/22°C under a 16/8 h light/dark cycle.
Cloning MtEF1A1 and bioinformatics analyses
A GTP-binding factor Tu family protein gene (MTR_6g021805) from M. truncatula was cloned in our study. BLASTP results showed that MTR_6g021805 shared high similarity (96%) and a conserved functional domain with AtEF1A1 (AT1G07920); MTR_6g021805 was therefore designated MtEF1A1. Primer Premier 5.0 was used to design primers to clone the CDS of MtEF1A1 (Additional file 1: Table S1).
ProtParam on ExPASy (https://web.expasy.org/protparam/) was used to predict the isoelectric point and molecular weight of MtEF1A1; Prosite (https://prosite.expasy.org/) and Protscale were used to analyze the protein domains, conserved structures, and protein hydrophobicity (https://web.expasy.org/protscale/) [60]. SOPMA (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page = npsa_sopma.html) was used to analyze secondary protein structure [61]. Tertiary protein structure was analyzed with SWISS-MODEL (https://swissmodel.expasy.org/interactive) [62]. Protein transmembrane structure and signal peptide predictions were performed with Phobius (http://phobius.sbc.su.se/) [63]. Protein subcellular localization was predicted with Cell-PLoc 2.0 (http://phobius.sbc.su.se/) [64].
MtEF1A1 sequences and EF1A homologs in Arabidopsis, soybean, and rice were obtained from ensemblplant and aligned using DNAMAN software to determine the functional domains. The evolutionary relationships between EF1A genes were determined with ITOL online software. Clustal Omega was used to perform multiple sequence alignment of M. truncatula EF1A proteins. Motifs in the M. truncatula EF family were identified with MEME (http://meme-suite.org/).
MtEF1A1 gene expression
Total RNA was extracted using the Eastep Total RNA Extraction Kit (Promega, Beijing, China), and cDNA was synthesized using the PrimeScript™ RT Reagent Kit with gDNA Eraser (Takara, Shiga, Japan). cDNA was diluted to 100 ng/µL. qRT-PCR was performed using TB Green® Premix Ex Taq™ II (Tli RNaseH Plus) (Takara, Shiga, Japan) on the CFX384 Touch Real-time PCR Detection System (Bio-Rad, Hercules, CA, USA). Each 20 µL qRT-PCR reaction contained 10 µL 2x SYBR Premix Ex Taq mix, 0.2 mM of each primer, and 2 µL cDNA. Primers for each gene are shown in Table S1. MtActin and AtActin were used as the internal reference genes for M. truncatula and Arabidopsis samples, respectively. Relative gene expression was calculated using the 2−ΔΔCt method [65]. There were three technical replicates for each experiment.
Transformation of Arabidopsis and M. truncatula
The CDS of MtEF1A1 was inserted into the plant transformation vector pCAMBIA3301 driven by the CaMV 35 S promoter. The 35 S::MtEF1A1-GUS expression plasmid was transferred into Agrobacterium tumefaciens strain GV3101. Arabidopsis plants were then transformed using the floral dip method [66]. The seeds of transformed plants were cultured on 1/2 MS medium and inoculated with 4 mg/L phosphinothricin (PPT) (Sigma-Aldrich, St Louis, MO, USA) for 7 d. Homozygous T3 plants were obtained for use in further experiments. Agrobacterium strain EHA105 with an overexpression vector was used to infect M. truncatula using the leaf infection method [67]. Well-developed transgenic M. truncatula seedlings screened with 2 mg/L PPT were transplanted into soil, identified with gene-specific primers, and T0 seeds were collected. Homozygous T2 plants were used for subsequent experiments.
Salt tolerance analyses in Arabidopsis
Transgenic and WT Arabidopsis seeds were disinfected, then incubated for 7 d on 1/2 MS medium with 0, 100, 125, or 150 mM NaCl. Germination rates were recorded for each treatment. Transgenic and WT Arabidopsis seedlings were grown in 1/2 MS medium for 7 d, then transferred to vertical petri dishes containing 1/2 MS with 0, 100, 125, or 150 mM NaCl for 15 d. The fresh weight, root length, and lateral root number were then measured. Three-week-old Arabidopsis seedlings grown in soil under cool-white fluorescent lights were treated with 0 or 200 mM NaCl solution every 3 d for a period of 21 d. Plant phenotypes were recorded after stems were removed and rosette leaves were collected, immediately frozen in liquid nitrogen, and stored at 80 °C prior to further analysis. There were three replicates for each treatment.
Salt tolerance analyses of M. truncatula
Four-week-old WT M. truncatula (R108) seedlings grown in 1/2 Hoagland medium were incubated in 200 mM NaCl. Roots, stems, and leaves were collected at 0, 1, 2, 8, 12, and 24 h. The experiment was repeated three times.
MtEF1A1-OE and WT plants were transplanted to 1/2 Hoagland nutrient solution for 3 weeks, then treated with 100 mM NaCl on 1/2 Hoagland’s solution for 2 weeks. The nutrient solution was changed every 5 d. Phenotypes were observed and the fresh weight and root length were measured. For M. truncatula leaf treatments, 60-day-old seedings grown in soil were treated with 30 mL distilled water, 200 mM NaCl, or 0.3% H2O2. After 5 d of treatment, leaf phenotypes were recorded. Chlorophyll levels were measured using the ethanol immersion method as described by Emel Ergun [68].
Physiological indices quantification and DAB staining
H2O2, malondialdehyde (MDA), and proline levels were measured in the leaves of transgenic and WT Arabidopsis seedlings using the Hydrogen peroxide determination kit (Jiancheng, Nanjing, China), the Micro Malondialdehyde (MDA) Assay Kit (Solarbio, Beijing, China), and the Proline (PRO) Content Detection Kit (Solarbio, Beijing, China), respectively. M. truncatula seeds (WT and MtEF1A1-OE lines) were grown on 1/2 MS medium for 2 d, then transferred to vertical plates on 1/2 MS supplemented with 150 mM NaCl. Seedlings were collected after 10 d and stained with 3,3’-dia-minobenzidine (DAB, Solarbio, Beijing, China). The stain (1 mg/ml) was placed in conical flasks containing seedlings; leaves were lowered below the liquid surface with vacuuming, then incubated in the dark for 5 h. After staining, fixative solution (3:1:1 ethanol:lactic acid:glycerol) was added and samples were boiled in water for 5 min. After cooling, leaves were rinsed with ethanol several times. Samples were observed with a Stemi microscope (ZEISS, Germany) and images were captured.
Statistical analysis
All experiments were conducted with three biological replicates. Data were analyzed using analysis of variance (ANOVA) with post-hoc Duncan’s multiple range test for multiple comparisons.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all colleagues in our laboratory for providing useful discussions and technical assistance. We are very grateful to the editor and reviewers for critically evaluating the manuscript and providing constructive comments for its improvement.
Abbreviations
- MDA
Malondialdehyde
- ROS
Reactive oxygen species
- CaM
Calmodulin
- EF
Elongation factors
- GEF
Guanine nucleotide exchange factor
- GST
Glutathione transferase
- eEF2K
Extension factor-2 kinase
- PPT
Phosphinothricin
- DAB
3,3’-dia-minobenzidine
- RD22A
BURP domain protein RD22
- COR15A
Cold-regulated 15 A
- CBL4
Calcineurin B-like proteins 4
- CDPK26
Calcium-dependent protein 26
- MAPK
Mitogen-activated protein kinase
- CIPKs
CBL-interacting protein kinases
- F-actin
Filamentous actin.
Authors’ Contribution
Ruicai Long and Qingchuan Yang conceived and designed the experiments; Lei Xu performed the experiments and wrote paper; Lixia Zhang and Yajiao Liu contributed to data analyzed; Bilig Sod, Mingna Li, Tianhui Yang and Ting Gao contributed with valuable discussions; Ruicai Long reviewed and edited the paper. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 32071865), Key Projects in Science and Technology of Inner Mongolia (Grant No. 2021ZD0031), Key research and development project of Ningxia Hui Autonomous Region (Grant No. 2022BBF02029), China Agriculture Research System of MOF and MARA (Grant No. CARS-34). The funding body played no role in the design of the study, the collection, analysis and interpretation of the data or the writing of the manuscript.
Data Availability
The genomic data of M. truncatula in the article can be downloaded from Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html). Protein sequences of plant species includes Arabidopsis thaliana, Glycine max, and Oryza sativa were downloaded from EnsemblPlants database (http://plants.ensembl.org/index.html).
Declaration of legitimacy
In this study, we comply with the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora. The procedures complied to the relevant institutional, national, and international guidelines and legislation.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing Interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Browning KS. The plant translational apparatus. Plant Mol Biol. 1996;32(1–2):107–44. doi: 10.1007/BF00039380. [DOI] [PubMed] [Google Scholar]
- 2.Browning KS, Humphreys J, Hobbs W, Smith GB, Ravel JM. Determination of the amounts of the protein synthesis initiation and elongation factors in wheat germ. J Biol Chem. 1990;265(29):17967. doi: 10.1016/S0021-9258(18)38258-9. [DOI] [PubMed] [Google Scholar]
- 3.Pittman YR, Valente L, Jeppesen MG, Andersen GR, Patel S, Kinzy TG. Mg2+ and a key lysine modulate exchange activity of eukaryotic translation elongation factor 1B alpha. J Biol Chem. 2006;281(28):19457–68. doi: 10.1074/jbc.M601076200. [DOI] [PubMed] [Google Scholar]
- 4.Nadal ED, Fadden RP, Ruiz A, Timothy H, Ariño J. A role for the Ppz Ser/Thr protein phosphatases in the regulation of translation elongation factor 1Bα. J Biol Chem. 2001;276(18):14829–34. doi: 10.1074/jbc.M010824200. [DOI] [PubMed] [Google Scholar]
- 5.Hossain Z, Amyot L, McGarvey B, Gruber M, JungJ, Hannoufa A. The translation elongation factor eEF-1Bβ1 is involved in cell wall biosynthesis and plant development in Arabidopsis thaliana. PLoS ONE. 2012;7(1):e30425. doi: 10.1371/journal.pone.0030425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bchini R, Girardet JM, Sormani R, Gelhaye E, Rouhier MM. Oxidized glutathione promotes association between eukaryotic translation elongation factor 1Bγ and Ure2p glutathione transferase from Phanerochaete chrysosporium. FEBS J. 2021;288(9):2956–69. [DOI] [PubMed]
- 7.Chen Z, Gopalakrishnan SM, Bui MH, Soni NB, Warrior U, Johnson EF, Donnelly JB, Glaser KB. 1-Benzyl-3-cetyl-2-methylimidazolium iodide (NH125) induces phosphorylation of eukaryotic elongation factor-2 (eEF2): a cautionary note on the anticancer mechanism of an eEF2 kinase inhibitor. J Biol Chem. 2011;286(51):43951–8. doi: 10.1074/jbc.M111.301291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gocińska K, Ghahe SS, Domogaa S, Topf U. Eukaryotic elongation factor 3 protects Saccharomyces cerevisiae yeast from oxidative stress. Genes. 2020;11(12). [DOI] [PMC free article] [PubMed]
- 9.Axelos M, Bardet C, Liboz T, Thai AL, Curie C, Lescure B. The gene family encoding the Arabidopsis thaliana translation elongation factor EF-1α: molecular cloning, characterization and expression. Mol Gen Genet Mgg. 1989;219(1–2):106–12. doi: 10.1007/BF00261164. [DOI] [PubMed] [Google Scholar]
- 10.Maurer F, Muron M, Stutz E. The tuf gene family of soybean: structure and differential transcription. Plant Sci. 1996;117(1–2):83–93. doi: 10.1016/0168-9452(96)04401-9. [DOI] [Google Scholar]
- 11.Thornton S, Anand N, Purcell D, Lee J. Not just for housekeeping: protein initiation and elongation factors in cell growth and tumorigenesis. J Mol Med. 2003;81(9):536–48. doi: 10.1007/s00109-003-0461-8. [DOI] [PubMed] [Google Scholar]
- 12.Tominaga R, Iwata M, Sano R, Inoue K, Okada K, Wada T. Arabidopsis CAPRICE-LIKE MYB 3 (CPL3) controls endoreduplication and flowering development in addition to trichome and root hair formation. Development. 2008;135(7):1335–45. doi: 10.1242/dev.017947. [DOI] [PubMed] [Google Scholar]
- 13.Wang HB, Wang JJ, Jiang JF, Chen SM, Guan ZY, Liao Y, Chen FD. Reference genes for normalizing transcription in diploid and tetraploid Arabidopsis. Sci Rep. 2014;4:6781. doi: 10.1038/srep06781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng YL, Wang YS, Gu JD. Identification of suitable reference genes in mangrove Aegiceras corniculatum under abiotic stresses. Ecotoxicology. 2015;24(7):1714–21. doi: 10.1007/s10646-015-1487-8. [DOI] [PubMed] [Google Scholar]
- 15.Migocka M, Papierniak A. Identification of suitable reference genes for studying gene expression in cucumber plants subjected to abiotic stress and growth regulators. Mol Breeding. 2011;28(3):343–57. doi: 10.1007/s11032-010-9487-0. [DOI] [Google Scholar]
- 16.Qi J, Yu S, Zhang F, Shen X, Zhao X, Yu Y, Zhang D. Reference gene selection for real-time quantitative polymerase chain reaction of mRNA transcript levels in chinese cabbage ( Brassica rapa L. ssp. pekinensis) Plant Mol Biology Report. 2010;28(4):597–604. doi: 10.1007/s11105-010-0185-1. [DOI] [Google Scholar]
- 17.Dunn MA, Morris A, Jack PL, Hughes MA. A low-temperature-responsive translation elongation factor 1 alpha from barley (Hordeum vulgare L.) Plant Mol Biol. 1993;23(1):221–5. doi: 10.1007/BF00021434. [DOI] [PubMed] [Google Scholar]
- 18.Berberich T, Sugawara K, Harada M, Kusano T. Molecular cloning, characterization and expression of an elongation factor 1α gene in maize. Plant Mol Biol. 1995;29(3):611–5. doi: 10.1007/BF00020988. [DOI] [PubMed] [Google Scholar]
- 19.Bhadula SK, Elthon TE, Habben JE, Helentjaris TG, Jiao SP, Ristic Z. Heat-stress induced synthesis of chloroplast protein synthesis elongation factor (EF-Tu) in a heat-tolerant maize line. Planta. 2001;212(3):359–66. doi: 10.1007/s004250000416. [DOI] [PubMed] [Google Scholar]
- 20.Bukovnik U, Fu JM, Bennett M, Prasad PVV, Ristic Z. Heat tolerance and expression of protein synthesis elongation factors, EF-Tu and EF-1α, in spring wheat. Funct Plant Biol. 2009;36(3):234–41. doi: 10.1071/FP08266. [DOI] [PubMed] [Google Scholar]
- 21.Young CC, Bernlohr RW. Elongation factor Tu is methylated in response to nutrient deprivation in Escherichia coli. J Bacteriol. 1991;173(10):3096–100. doi: 10.1128/jb.173.10.3096-3100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Momčilović I, Pantelić D, Zdravković KS, Oljača J, Rudić J, Fu JM. Heat-induced accumulation of protein synthesis elongation factor 1A implies an important role in heat tolerance in potato. Planta. 2016;244(3):1–9. doi: 10.1007/s00425-016-2534-2. [DOI] [PubMed] [Google Scholar]
- 23.Yamada A, Nozaki A, Sano E, Mimura T, Ozeki Y. Expression of mangrove eEF1A enhances tolerance to salt and osmotic stress in escherichia coli. Plant Biotechnol. 2003;20(1):81–5. doi: 10.5511/plantbiotechnology.20.81. [DOI] [Google Scholar]
- 24.Shin D, Moon SJ, Sang RP, Kim BG, Byun MO. Elongation factor 1α from A. thaliana functions as molecular chaperone and confers resistance to salt stress in yeast and plants. Plant Sci. 2009;177(3):156–60. doi: 10.1016/j.plantsci.2009.05.003. [DOI] [Google Scholar]
- 25.Kuang L, Shen Q, Wu L, Yu J, Fu L, Wu D, Zhang G. Identification of microRNAs responding to salt stress in barley by high-throughput sequencing and degradome analysis. Environ Exp Bot. 2019;160:59–70. doi: 10.1016/j.envexpbot.2019.01.006. [DOI] [Google Scholar]
- 26.Fricke W, Peters WS. The biophysics of leaf growth in salt-stressed barley. A study at the cell level. Plant Physiol. 2002;129(1):374–88. doi: 10.1104/pp.001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akhtar SS, Andersen MN, Liu F. Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric Water Manage. 2015;158:61–8. doi: 10.1016/j.agwat.2015.04.010. [DOI] [Google Scholar]
- 28.Ashraf M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv. 2009;27:84–93. doi: 10.1016/j.biotechadv.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Gao Y, Ma J, Zheng JC, Chen J, Chen M, Zhou YB, Fu JD, Xu ZS, Ma YZ. The elongation factor GmEF4 is involved in the response to drought and salt tolerance in soybean. Int J Mol Sci. 2019;19(12):3001. doi: 10.3390/ijms20123001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long RC, Gao YL, Sun H, Zhang TJ, Li X, Li MN, Sun Y, Kang JM, Wang Z, Ding W, Yang QC. Quantitative proteomic analysis using iTRAQ to identify salt-responsive proteins during the germination stage of two Medicago species. Sci Rep. 2018;8(1):9553. doi: 10.1038/s41598-018-27935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long RC, Sun H, Cao C, Zhang TJ, Kang JM, Wang Z, Li MN, Gao YL, Li X, Yang QC. Identification of alkali-responsive proteins from early seedling stage of two contrasting Medicago species by iTRAQ-based quantitative proteomic analysis. Environ Experimental Bot. 2019;157:26–34. doi: 10.1016/j.envexpbot.2018.09.021. [DOI] [Google Scholar]
- 32.Lukash TO, Turkovskaya GV, Negrutskii BS, Elskaya AV. Renaturation of phenylalanyl-tRNA synlhetase by translation elongation factor eEF1A. Biopolymers & Cell. 2003;19(4):350–4. doi: 10.7124/bc.000666. [DOI] [Google Scholar]
- 33.Fu J, Momčilović I, Vara Prasad PV. Roles of Protein Synthesis Elongation Factor EF-Tu in Heat Tolerance in Plants. J Bot. 2012;835836:1–8.
- 34.Pokalsky AR, Hiatt WR, Ridge N, Rasmussen R, Shewmaker CK. Structure and expression of elongation factor 1 alpha in tomato. Nucleic Acids Res. 1989;17(12):4661–73. doi: 10.1093/nar/17.12.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watillon B, Kettmann R, Boxus P, Burny A. Elongation factor 1α (EF-1α) transcript levels are developmentally and environmentally regulated in apple plants. Physiol Plant. 2010;104(1):1–9. doi: 10.1034/j.1399-3054.1998.1040101.x. [DOI] [Google Scholar]
- 36.Sun HF, Meng YP, Cui GM, Cao QF, Li J, Liang AH. Selection of housekeeping genes for gene expression studies on the development of fruit bearing shoots in chinese jujube (Ziziphus jujube Mill.) Mol Biol Rep. 2009;36(8):2183. doi: 10.1007/s11033-008-9433-y. [DOI] [PubMed] [Google Scholar]
- 37.Sun B, Sun GQ, Meng ZG, Zhang R, Guo SD, Institute B, Sciences C. A novel constitutive promoter and its downstream 5′ UTR derived from cotton (Gossypium spp.) drive high-level gene expression in stem and leaf tissues. J Integr Agric. 2016;4(15):755–62. doi: 10.1016/S2095-3119(15)61054-1. [DOI] [Google Scholar]
- 38.Liu JH, Xu BY, Chen DZ, Zhang JB, Jia CH, Jin ZQ. Isolation and functional analysis of a elongation factor 1A ( MaEF1A ) gene from banana. J Fruit Sci. 2011;28(5):825–30. [Google Scholar]
- 39.Girardi CL, Bermudez K, Bernadac A, Chavez A, Zouine M, Miranda FG, Bouzayen M, Pech JC, Latché A. The mitochondrial elongation factor LeEF-Tsmt is regulated during tomato fruit ripening and upon wounding and ethylene treatment. Postharvest Biol Technol. 2006;42(1):1–7. doi: 10.1016/j.postharvbio.2006.06.002. [DOI] [Google Scholar]
- 40.Cha JY, Kim J, Kim TS, Zeng Q, Wang L, Lee SY, Kim WY, Somerset DE. Gigantea is a co-chaperone which facilitates maturation of ZEITLUPE in the Arabidopsis circadian clock. Nat Commun. 2017;8(1):3. doi: 10.1038/s41467-016-0014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heidari P, Amerian MR, Barcaccia G. Hormone Profiles and Antioxidant Activity of Cultivated and Wild Tomato Seedlings under Low-Temperature Stress. Agronomy. 2021;(6).
- 42.Moftah AE. Salinity effects on growth, ion distribution, carbohydrate metabolism, and photosynthetic rate in soybean cultivars differing in their tolerance to salinity stress. University of Georgia. 1988.
- 43.Mahajan S, Pandey GK, Tuteja N. Calcium- and salt-stress signaling in plants: shedding light on SOS pathway. Arch Biochem Biophys. 2008;471(2):146–58. doi: 10.1016/j.abb.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Asano T, Hayashi N, Kobayashi M, Aoki N, Miyao A, Mitsuhara I, Ichikawa H, Komatsu S, Hirochika H, Kikuchi S, Ohsugi R. A rice calcium-dependent protein kinase OsCPK12 oppositely modulates salt-stress tolerance and blast disease resistance. Plant J. 2012;69(1):26–36. [DOI] [PubMed]
- 45.Mehlmer N, Wurzinger B, Stael S. The Ca2+-dependent protein kinase CPK3 is required for MAPK-independent salt-stress acclimation in Arabidopsis. Plant J. 2010;63(3):484–98. doi: 10.1111/j.1365-313X.2010.04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abbas N, Maurya JP, Senapati D, Gangappa SN, Chattopadhyay S. Arabidopsis CAM7 and HY5 physically interact and directly bind to the HY5 promoter to regulate its expression and thereby promote photomorphogenesis. Plant Cell. 2014;26(3):1036. doi: 10.1105/tpc.113.122515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szymanski DB, Liao B, Zielinski RE. Calmodulin isoforms differentially enhance the binding of cauliflower nuclear proteins and recombinant TGA3 to a region derived from the Arabidopsis Cam-3 promoter. Plant Cell. 1996;8:1069–77. doi: 10.1105/tpc.8.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schumacher MA, Rivard AF, Bchinger HP, Adelman JP. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 2001;410(6832):1120–4. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- 49.Drum CL, Yan SZ, Bard J, Shen YQ, Lu D, Soelaiman S, Grabarek Z, Bohm A, Tang WJ. Structural basis for the activation of anthrax adenylyl cyclase exotoxin by calmodulin. Nature. 2002;415(6870):396. doi: 10.1038/415396a. [DOI] [PubMed] [Google Scholar]
- 50.Gut H, Dominici P, Pilati S, Astegno A, Petoukhov MV, Svergun DI, Grütter MG, Capitani G. A common structural basis for pH and calmodulin-mediated regulation in plant glutamate decarboxylase. J Mol Biol. 2009;392:334–51. doi: 10.1016/j.jmb.2009.06.080. [DOI] [PubMed] [Google Scholar]
- 51.Xu J, Tian YS, Peng RH, Xiong AS, Zhu B, Jin XF, Gao F, Fu XY, Hou XL, Yao QH. AtCPK6, a functionally redundant and positive regulator involved in salt/drought stress tolerance in Arabidopsis. Planta. 2010;231:1251–60. doi: 10.1007/s00425-010-1122-0. [DOI] [PubMed] [Google Scholar]
- 52.Mori IC, Yoshiyuki M, Yang Y, Shintaro M, Wang YF, Shannon A, Hervé T, Alonso JM, Harper JF, Ecker JR. CDPKs CPK6 and CPK3 function in ABA regulation of Guard Cell S-Type Anion- and Ca 2+ - permeable channels and Stomatal Closure. PLoS Biol. 2006;4(10):e327. doi: 10.1371/journal.pbio.0040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheng L. The CBL-CIPK network in plant calcium signaling. Trends Plant Sci. 2009;14(1):37–42. doi: 10.1016/j.tplants.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Cheng H, Cai HB, Fu HT, An ZW. Functional characterization of Hevea brasiliensisCRT/DRE binding factor 1 gene revealed regulation potential in the CBF pathway of tropical perennial tree. Plos One. 2015;10(9):e0137634. [DOI] [PMC free article] [PubMed]
- 55.Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi SK. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell. 2003;15(1):63–78. doi: 10.1105/tpc.006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ransom-Hodgkins WD, Brglez I, Wang X, Boss WF. Calcium-regulated proteolysis of eEF1A. Plant Physiol. 2000;122(3):957–65. doi: 10.1104/pp.122.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun D, Ji X, Jia Y, Huo D, Si S, Zeng L, Zhang Y, Niu L. LreEF1A4, a translation elongation factor from Lilium regale, is pivotal for cucumber mosaic virus and tobacco rattle virus infections and tolerance to salt and drought. Int J of Mol Sci. 2020;21(6):2083. [DOI] [PMC free article] [PubMed]
- 58.Bunai F, Ando K, Ueno H, Numata O. Tetrahymena eukaryotic translation elongation factor 1A (eEF1A) bundles filamentous actin through dimer formation. J BioChem. 2006;140(3):393–9. doi: 10.1093/jb/mvj169. [DOI] [PubMed] [Google Scholar]
- 59.Kurasawa Y, Watanabe Y, Numata O. Characterization of F-Actin bundling activity of tetrahymena elongation factor 1α investigated with rabbit skeletal muscle actin. Zoolog Sci. 1996;13(3):371. doi: 10.2108/zsj.13.371. [DOI] [PubMed] [Google Scholar]
- 60.Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31(13):3784–8. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Geourjon C, Deléage G. SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Bioinformatics. 1995;11(6):681–4. doi: 10.1093/bioinformatics/11.6.681. [DOI] [PubMed] [Google Scholar]
- 62.Torsten S, Jürgen K, Nicolas G, Peitsch MC. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 2003;31(13):3381–5. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lukas K, Anders K, Sonnhammer ELL. Advantages of combined transmembrane topology and signal peptide prediction—the Phobius web server. Nucleic Acids Res. 2007;35(Web Server):W429–32. doi: 10.1093/nar/gkm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chou KC, Shen HB. Cell-PLoc 2.0: an improved package of web-servers for predicting subcellular localization of proteins in various organisms. Nat Sci. 2010;2(10):1090. doi: 10.1038/nprot.2007.494. [DOI] [PubMed] [Google Scholar]
- 65.Schefe JH, Lehmann KE, Buschmann IR, Unger T, Kaiser HF. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression’s C T difference” formula. J Mol Med (Berl). 2006;84(11):901–10. [DOI] [PubMed]
- 66.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobactrium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–43. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 67.Chabaud M, Ratet P, Araújo S, Duque S. Medicago truncatula handbook: Agrobacterium tumefaciens-mediated transformation and in vitro plant regeneration of M. truncatula. 2007;1–3.
- 68.Ergun E, Demirata B, Gumus G. Simultaneous determination of chlorophyll a and chlorophyll b by synchronous fluorimetry. Anal Bioanalytical Chem. 2004;379(5–6):803–11. doi: 10.1007/s00216-004-2637-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genomic data of M. truncatula in the article can be downloaded from Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html). Protein sequences of plant species includes Arabidopsis thaliana, Glycine max, and Oryza sativa were downloaded from EnsemblPlants database (http://plants.ensembl.org/index.html).