Abstract
It has been reported that the toluene-degrading (xyl) genes from Pseudomonas putida plasmid pWW53 are able to translocate to broad-host-range drug resistance plasmid RP4, and pWW53-4 is one of the smallest RP4 derivatives (H. Keil, S. Keil, R. W. Pickup, and P. A. Williams, J. Bacteriol. 164:887–895, 1985). Our investigation of pWW53-4 in this study demonstrated that such a translocated region that is 39 kb long is a transposon. This mobile element, Tn4656, was classified as a class II transposon since its transposition occurred by a two-step process: transposase (TnpA)-mediated formation of the cointegrate and resolvase (TnpR)-mediated site-specific resolution of the cointegrate at the two copies of the res site. The Tn4656 TnpA and TnpR functions encoded in the rightmost 4-kb region were found to be exchangeable with those specified by other Tn1721-related class II transposons, including another toluene transposon, Tn4653. Sequence analysis of the transposition-related genes and sites of Tn4656 also supported the hypothesis that this transposon is closely related to the Tn1721-related transposons. The lower transposition frequency of Tn4656 has been suggested to be due to the unique nucleotide sequence of one of the terminal 39-bp inverted repeats.
A number of environmental bacterial strains that can use various kinds of xenobiotic compounds as sources of carbon and energy have been identified. Very similar catabolic gene clusters that are presumed to have common evolutionary origins are distributed on a variety of plasmids and chromosomes of phylogenetically divergent bacterial species (32, 35). Many of these catabolic gene clusters also undergo various types of rearrangements, including deletion, duplication, and fusion with other replicons (32, 34). In the last two decades, it has been demonstrated that some such gene clusters are located on transposons, explaining the rearrangements of catabolic gene clusters, as well as the wide dissemination and rapid evolution of the common catabolic pathways in the environment (31). Many of the catabolic transposons belong to the class I transposons in which some, but not all, of the catabolic genes for complete degradation of xenobiotic compounds are flanked by two copies of insertion sequences. In contrast, only three class II (Tn3-like) catabolic transposons have been identified in two self-transmissible and IncP-9 broad-host-range plasmids from two strains of Pseudomonas putida (Fig. 1) (27–30). Two of these transposons, Tn4651 (56 kb) and Tn4653 (70 kb), carry all the xylene- and toluene-degrading (xyl) genes from a 117-kb plasmid, pWW0, and the latter transposon includes the former (27, 28). The third transposon, Tn4655 (38 kb), carries all the naphthalene-degrading (nah) genes from an 83-kb plasmid, NAH7 (29). The terminal sequence structures of the three transposons have the properties commonly conserved in other class II transposons. The tnpA gene product (transposase) of Tn4651 or Tn4653 catalyzes formation of the cointegrate of the donor and target replicons connected by two directly repeated copies of the transposon, one at each junction. Tn4655 forms the cointegrate only in the presence of TnpA from Tn1721-related transposons, such as Tn4653 and Tn1722 (10, 29, 30). The tnpR gene product (revolvase) of Tn4655 or the tnpS and tnpT products of Tn4651 catalyze subsequent site-specific resolution of the cointegrate between the two copies of the resolution (res) site. However, Tn4653 and Tn4655 are defective because they lack the res site and the tnpA gene, respectively (29, 30). Our detailed analyses of transposons Tn4651, Tn4653, and Tn4655 and studies by other groups of workers have further revealed that (i) Tn4651 is a member of a novel subgroup of the class II transposons that includes mercury transposon Tn5041 and (ii) Tn4655 has a novel resolution system that is not functionally exchangeable with those of other class II transposons (Fig. 1) (12, 15, 29; Genka and Tsuda, unpublished results).
FIG. 1.
Structures of class II catabolic transposons and related transposons. See references 1, 7, 12, 16, 23, and 27 to 30 for details. Symbols and abbreviations: solid arrowhead, terminal IR; open arrowhead, IS1256 (21); open arrow, IS26 (23); solid circle, res site; solid half-circle, defective res site; A, tnpA; R, S, and T, genes for cointegrate resolution; nah, nah genes; xyl, xyl genes; M and M1, meta catabolic pathway operon; U, upper catabolic pathway operon; Hg, genes for resistance to mercuric ion; Sm, gene for resistance to Sm; Su, gene for resistance to sulfonamide; Tc, genes for resistance to Tc; Mcp, gene encoding methyl-accepting chemotaxis protein. The arrow above or below each gene or operon indicates the direction of transcription. Tn4651 and Tn4653 are located on pWW0, Tn4655 is located on NAH7, and Tn4656 is located on pWW53-4. The upper pathway operons encode the enzymes for conversion of toluene and xylenes to their respective carboxylic acids in the case of the TOL plasmids and the enzymes for conversion of naphthalene to salicylate in the case of NAH7. The meta pathway operons are involved in conversion of the final upper pathway products to catechol or its methyl derivatives and then to central metabolites. Tn4656 carries only one (M1) of the two meta pathway operons of pWW53. The regulatory genes xylS and xylR located downstream of the xyl meta pathway operon and the regulatory gene nahR located upstream of the nah meta pathway operon are not shown. Previous papers have clarified (i) the interchangeability of the TnpR functions among Tn21, Tn1722, and Tn4653, (ii) the identity of the amino acid sequences of the Tn4653 and Tn1722 resolvases, (iii) the interchangeability of the TnpA functions between Tn1722 and Tn4653, and (iv) the cointegration of Tn4655 by the Tn1722 or Tn4653 transposase (29, 30).
To date, workers have described a number of TOL plasmids which carry at least four xyl transcriptional units (the upper and meta pathway operons and the two regulatory genes, xylR and xylS) that are strongly homologous to those in pWW0 (4, 24, 34, 35). Many such TOL plasmids, however, differ from pWW0 in terms of basic plasmid functions, such as incompatibility and transmissibility, and in terms of copy number and the relative positions of the four xyl units. For example, pWW53 from P. putida MT53 is a nontransmissible 107-kb plasmid that does not belong to the IncP-9 group (13). This plasmid carries a single upper pathway operon, two highly homologous but distinguishable meta pathway operons, a single xylR gene, and three xylS-homologous genes (xylS1, xylS2, and xylS3), and the three operons with the same transcription direction are arranged in the order meta operon I-xylS1-xylR-upper operon-xylS3-meta operon II-xylS2 (2, 9, 13, 14, 20, 24). Keil et al. (13) reported that the xyl gene clusters of pWW53 could be inserted into a transmissible drug resistance plasmid, RP4. One of the smallest hybrid plasmids is pWW53-4, which carries the pWW53-derived fragment containing meta operon I-xylS1-xylR-upper operon. However, the order of the four xyl transcriptional units in pWW53-4 is different from the order in Tn4651, and the remaining xyl genes of pWW53 are not present in pWW53-4. It was expected that elucidation of the molecular mechanism of formation of pWW53-4 should provide some insight into the evolutionary mechanism that resulted in the present organization of the xyl gene clusters in pWW53. The transposability of the xyl gene cluster in pWW53-4 was investigated in this study, and the results indicated that the pWW53-derived xyl-containing region in pWW53-4 is a transposon belonging to the Tn1721-related transposon group.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The Escherichia coli strains used were DH1 (recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1) and HB101 (hsdS20 recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 supE44) (5). P. putida PaW611 is a PaW340 (Trp− Strr) derivative carrying pWW53-4 (13). The plasmids used are listed in Table 1. Routine cultivation of E. coli and P. putida cells was performed at 37 and 30°C, respectively. L broth (LB) was used as a liquid medium and was solidified with 1.5% agar to obtain LB agar. When required, antibiotics were added to the media at the following concentrations: ampicillin, 50 μg/ml; chloramphenicol, 30 μg/ml; kanamycin, 50 μg/ml; nalidixic acid, 20 μg/ml; spectinomycin, 40 μg/ml; streptomycin, 250 μg/ml; tetracycline, 10 μg/ml; and trimethoprim, 100 μg/ml.
TABLE 1.
Plasmids used in this study
| Plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| pBR322 | Apr Tc r, cloning vector | 33 |
| pRME1 | Apr Kmr, Kmr gene flanked by inverted repeats of pUC19-derived linkera | 11 |
| pUC4K | Apr Kmr, Kmr gene flanked by inverted repeats of a linkerb | 26 |
| pHP45Ω | Apr Sm/Spcr, Sm/Spcr gene flanked by inverted repeats of a linkerc | 8 |
| pUC18 | Apr, cloning vector | 36 |
| pUC19 | Apr, cloning vector | 36 |
| R388 | Tra+ Tpr Sur | 6 |
| pWW53-4 | Tra+ Apr Tcr Kmr Tol+, RP4::Tn4656d | 13 |
| pMT252 | Tcr, pACYC184Δ(ScaI-PvuII) | 27 |
| pMT258 | Cmr, pACYC184Δ(XbaI-SalI) | 27 |
| pMT266 | Apr Tcr, pBR322 derivative, unique EcoRI site of pBR322 is converted to EcoRI-SmaI-EcoRI sites | This study |
| pMT1209 | Cmr Apr, pMT258::Tn3 | 30 |
| pMT1214 | Cmr Apr, pMT258::Tn3ΔtnpA | This study |
| pMT1294 | Apr, pBR322 derivative carrying the Tn1722 tnpA gene | 30 |
| pMT1302 | Tcr Kmr, pMT252::Tn1722Δ(res-tnpR-tnpA), Kmr gene in the transposon | 30 |
| pMT1590 | Tcr Kmr, pMT252::Tn4653Δ(tnpR-tnpA), Kmr gene in the transposone | 30 |
| pMT1765 | Apr, pBR322 derivative carrying the Tn4653 tnpA gene | 30 |
| pMT2367 | Cmr Kmr, pMT258::Tn4655ΔtnpR, Kmr gene in the transposone | 29 |
| pMT2814 | Cmr, pMT258::Tn4656 | This study |
| pMT2827 | Apr, pMT266tet::Tn4656f | This study |
| pMT2875 | Cmr Kmr, pMT258::Tn4656-2875, pMT2814 derivative in which all the HindIII fragments of Tn4656 are replaced by the HindIII-flanked Kmr gene from pRME1g | This study |
| pMT2879 | Cmr Kmr, pMT258::Tn4656-2879, pMT2814 derivative in which all the KpnI fragments of Tn4656 are replaced by the KpnI-flanked Kmr gene from pRME1g | This study |
| pMT2890 | Cmr Kmr, pMT258::Tn4656-2890, pMT2814 derivative in which all the BamHI fragments of Tn4656 are replaced by the BamHI-flanked Kmr gene from pRME1g | This study |
| pMT2916 | Tra+ Tpr Sur Kmr, R388::Tn4656-2890, transposition of Tn4656-2890 from pMT2890 to R388 | This study |
| pMT2923 | Tcr Kmr, pMT252::Tn4656-2890, transposition of Tn4656-2890 from pMT2916 to pMT252g | This study |
| pMT2925 | Tcr, pMT252::Tn4656-2925, pMT2926 derivative lacking the KpnI-flanked Kmr geneh | This study |
| pMT2926 | Tcr Kmr, pMT252::Tn4656-2926, pMT2923 derivative in which the 0.25-kb BamHI-KpnI fragment and its adjacent Kmr gene are replaced by the KpnI-flanked Kmr gene in pMT2923h | This study |
| pMT2931 | Tcr Kmr, pMT252::Tn4656-2931, pMT2525 derivative with an insert of the pUC4K-derived, EcoRI-flanked Kmr gene at the leftmost EcoRI site of Tn4656-2925i | This study |
| pMT2932 | Tcr Kmr, pMT252::Tn4656-2932, pMT2525 derivative with an insert of the pUC4K-derived, EcoRI-flanked Kmr gene at the EcoRI site in the tnpR gene of Tn4656-2925i | This study |
| pMT2933 | Tcr Kmr, pMT252::Tn4656-2933, pMT2525 derivative with an insert of the pUC4K-derived, EcoRI-flanked Kmr gene at the EcoRI site in the right IR of Tn4656-2925i | This study |
| pMT2937 | Tcr Kmr, pMT252::Tn4656-2937, pMT2525 derivative with an insert of the pUC4K-derived, HincII-flanked Kmr gene at the StuI site in the tnpA gene of Tn4656-2925i | This study |
| pMT2939 | Tcr Kmr, pMT252::Tn4656-2939, pMT2526 derivative in which the DNA fragment between the SmaI site and the StuI site is replaced by the SmaI-derived Kmr gene from pRME1h | This study |
| pMT2944 | Tcr Kmr, pMT252::Tn4656-2944, pMT2526 derivative lacking the 1.1-kb PvuII fragment and its adjacent 0.86-kb PvuII fragmenti | This study |
| pMT2946 | Tcr Kmr, pMT252::Tn4656-2946, pMT2526 derivative lacking the 0.86-kb PvuII fragmenti | This study |
| pMT2947 | Tcr Kmr, pMT252::Tn4656-2947, pMT2526 derivative lacking the 1.1-kb PvuII fragmenti | This study |
| pMT2948 | Tcr Kmr, pMT252::Tn4656-2948, pMT2526 derivative lacking the 0.32-kb NcoI fragmenti | This study |
| pMT2978 | Tra+ Tpr Sur Tcr Kmr, Tn4656-2944-mediated cointegrate of R388 and pMT2944 constructed in the presence of the tnpA gene supplied from pMT3030j | This study |
| pMT3019 | Apr Kmr, pMT2827 derivative in which all the HindIII fragments of pMT2827 are replaced by the HindIII-flanked Kmr gene from pRME1k | This study |
| pMT3020 | Apr, pMT3019 derivative lacking all the KpnI fragments but possessing the rightmost res-tnpR-tnpA-right IR fragment of Tn4656k | This study |
| pMT3030 | Apr, pMT3020 derivative lacking the EcoRI fragment that contains the res site and most of the tnpR genek | This study |
| pMT3041 | Apr, pMT3020 derivative lacking the DNA fragment between the NruI site in the 5′ part of the tnpA gene and the NruI site in the pMT266 portionl | This study |
| pMT3042 | Apr, pMT3041 derivative lacking the 0.32-kb NcoI fragmentl | This study |
The HindIII sites in the Kmr gene cassette are located at the outermost positions.
The linker contains EcoRI, BamHI, SalI, and PstI sites, and the EcoRI sites in the cassette are located at the outermost positions.
The linker contains EcoRI, BamHI, SmaI, and HindIII sites, and the EcoRI sites in the cassette are located at the outermost positions.
Tol+, utilization of toluene and xylenes.
The transposon is inserted between the SalI and NruI sites of pMT266 in the orientation so that the right end of Tn4656 (Fig. 1, 2, and 4) is proximal to the pMT266-derived NruI site.
See Fig. 2.
See Fig. 3.
See Fig. 4.
DNA manipulation and construction of plasmids.
Established procedures were used for preparation and manipulation of plasmid DNA, agarose gel electrophoresis, and transformation of E. coli cells (5).
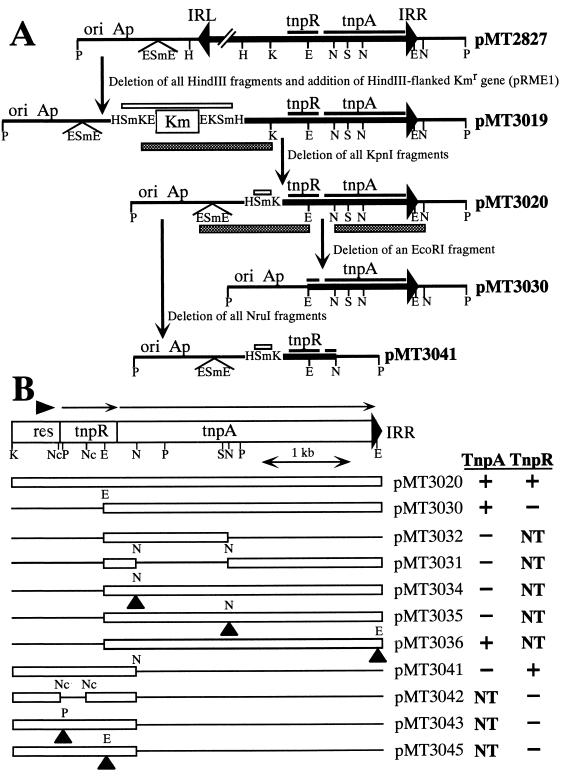
Removal of the EcoRV fragment from pMT1209 (pMT258::Tn3) (30) gave rise to pMT1214, which lacked the central part of the tnpA gene of Tn3. The EcoRI-flanked Ω fragment from pHP45Ω (8) was inserted into the EcoRI site of pBR322 (33). Subsequent excision of the Ω fragment by SmaI digestion led to construction of pMT266, in which the unique EcoRI site of pBR322 was converted to EcoRI-SmaI-EcoRI sites. pMT2890 (Fig. 2A) is a pMT258 derivative carrying Tn4656-2890, a Tn4656 derivative in which all the internal BamHI fragments are replaced by the BamHI-flanked Kmr gene from pRME1 (11). Tn4656-2890 was transposed to R388 to construct pMT2916 (Table 1), and pMT2916 was then used as the donor replicon to transpose Tn4656-2890 to pMT252. The resulting plasmid, pMT2923 (Fig. 2), was digested with KpnI, ligated, and used to transform DH1 to select Tcr Kmr clones. One such clone carried pMT2926 (pMT252::Tn4656-2926), in which the Tn4656 DNA fragment between the leftmost BamHI site and the rightmost KpnI site was replaced by the Kmr gene. Removal of the Kmr gene from pMT2926 by KpnI digestion generated pMT2925 (pMT252::Tn4656-2925) (Fig. 2 and 3). Removal of some restriction fragments from pMT2926 resulted in formation of pMT2939, pMT2944, pMT2946, pMT2947, and pMT2948, while insertion of the pUC4K-derived Kmr gene into some restriction sites in pMT2525 gave rise to pMT2931, pMT2932, pMT2933, and pMT2937 (Fig. 3). pMT2827 (pMT266tet::Tn4656) (Table 1) was completely digested with HindIII, and the plasmid portion was ligated with the HindIII-flanked Kmr gene from pRME1. Digestion of the resulting plasmid, pMT3019, with KpnI and subsequent self-ligation gave rise to pMT3020, which contained only the Tn4656 fragment located downstream of the rightmost KpnI site (Fig. 4). Removal of the EcoRI fragment containing the 5′ part of tnpR from pMT3020 generated pMT3030, while removal of the two NruI fragments containing most of the tnpA gene generated pMT3041 (Fig. 4). Partial NruI digestion of pMT3030 and subsequent ligation led to construction of pMT3031 and pMT3032, and removal of a unique NcoI fragment from pMT3041 gave rise to pMT3042. Insertion of the pUC4K-derived Kmr gene into a certain restriction site in pMT3030 or pMT3041 generated pMT3034, pMT3035, pMT3036, pMT3043, or pMT3045 (Fig. 4).
FIG. 2.
Structures of Tn4656 and its deletion derivatives. Abbreviations for restriction sites: B, BamHI; E, EcoRI; H, HindIII; K, KpnI, S, StuI; and Sm, SmaI. (A) Tn4656 and its large deletion derivatives. The location of the xyl genes is based on information from reference 13. A horizontal arrow indicates the direction of transcription of a gene or operon, and the vertical arrows indicate the outermost BamHI sites and the rightmost HindIII and KpnI sites. The res site located upstream of the tnpR gene is not shown for the sake of simplicity. The deleted fragment represented by a thin line was replaced by the pRME1-derived Kmr gene. Tn4656-2926 is loaded on pMT252, and the remaining three Tn4656 derivatives are loaded on pMT258. For details concerning construction of the plasmids, see Table 1 and Materials and Methods. The transposition frequency is expressed as the number of Kmr transconjugants per Tpr transconjugant. (B) Construction of the Tn4656-2890 derivatives. Abbreviations: IRL and IRR, left and right IRs, respectively. The derivatives are not drawn to scale. Construction of pMT2923 from pMT2890 via pMT2916 as an intermediate is described in Materials and Methods. The open and shaded boxes represent the pRME1-derived fragment and the fragment deleted in the descendant transposon, respectively. Note that only the relevant restriction sites derived from pRME1 are shown for the sake of simplicity. The restriction sites in parentheses are not digested by SmaI or StuI.
FIG. 3.
Localization of transposition-related genes and sites of Tn4656. The abbreviations for restriction sites are the same as those described in the legend to Fig. 2 except as follows: N, NruI; Nc, NcoI; and P, PvuII. The following symbols and other abbreviations are used in the Tn4656-2925 map: IRL and IRR, left and right IRs, respectively; arrow, direction of transcription; arrowhead, resolution site; thick vertical line, nucleotide sequence that connects the leftmost BamHI site and the rightmost KpnI site of Tn4656 (Fig. 2B). Plasmids pMT2939, pMT2944, pMT2946, pMT2947, and pMT2948 are deletion derivatives of pMT2926 (Fig. 2B), whereas pMT2931, pMT2932, pMT2933, and pMT2937 are pMT2925 derivatives with an insert of the Kmr gene from pUC4K. See Fig. 2B for construction of pMT2939. The open bars and thin lines in the transposons indicate the DNA fragments that are present and absent, respectively, and the open and solid triangles indicate the Kmr genes from pRME1 and pUC4K, respectively. +, transposon is able to cointegrate or resolve; −, transposon is not able to cointegrate or resolve; NA, not applicable. When a minitransposon was defective in either the cointegration function or the resolution function or both, complementation in the presence of pMT3020 (Fig. 4) was examined. The results obtained in the absence and in the presence of pMT3020 are shown before and after the slash, respectively.
FIG. 4.
pMT266 derivatives carrying tnpA and tnpR genes of Tn4656. The abbreviations for restriction sites are the same as those described in the legends to Fig. 2 and 3. (A) Construction of the pMT2827 derivatives. The derivatives are not drawn to scale. The thin line indicates the pMT266 portion. The open and shaded boxes represent the pRME1-derived fragment and the fragment deleted in the descendant plasmid, respectively. (B) Localization of tnpA and tnpR on pMT3020. Only the Tn4656 portion is shown. Plasmids pMT3031, pMT3032, pMT3034, pMT3035, and pMT3036 are derived from pMT3030, and plasmids pMT3042, pMT3043, and pMT3045 are derived from pMT3041. The thin lines and solid triangles in the pMT3020 derivatives represent the deleted fragment and the insert of the pUC4K-derived Kmr gene, respectively. Note that deletions in pMT3032, pMT3041, and their derivatives extend to the unique NruI site in the pMT266 portion. pMT3020 and its derivatives were examined to determine their ability to complement the defect in cointegration of Tn4656-2939 and the defects in resolution of the Tn4656-2944- and Tn4656-2948-mediated cointegrate (Fig. 3 and 5). For an explanation of the plus and minus signs see the legend to Fig. 3. NT, not tested.
Transposition assays.
Transposition of various transposon derivatives was investigated by performing mating-out experiments (27). A DH1 derivative harboring a transmissible and transposon-free plasmid, R388 (6), was transformed with a pACYC184-based plasmid containing an appropriate transposon derivative. The resulting transformant was employed as the donor to mate with HB101 on a membrane filter, and Smr transconjugants which also exhibited resistance to the marker specified by either the transposon or the pACYC184-based plasmid were selected. Such transconjugants were analyzed to determine their plasmid profiles. To complement the defect of the cointegration function of a transposon derivative in the pACYC184-based plasmid, a pBR322-based plasmid carrying a relevant tnpA gene was introduced into the donor strain described above. To characterize the defect in the cointegrate resolution function, the stable cointegrate of a pACYC184-based plasmid and R388 connected by the mutant transposon was transferred to the DH1 derivative that harbored a pBR322-based plasmid having a relevant resolvase gene. After overnight cultivation of the resulting strain in LB, the stability of the cointegrate was investigated by physical and genetical detection of the replicons resolved (27).
Nucleotide sequence analysis.
The DNA fragments cloned in pUC18 and pUC19 were sequenced with an ABI 373S automated DNA sequencer (Applied Biosystems Inc.) by using the protocols recommended by the manufacturer. A computer analysis of the sequences was performed with the software programs GENETYX 10 (SDC Inc., Tokyo, Japan) and BLAST 2.0 (National Institute of Genetics, Mishima, Japan).
Nucleotide sequence accession numbers.
The nucleotide sequences of Tn4656 described in this paper have been deposited in DDBJ/EMBL/GenBank under accession numbers AB052614 (left end), AB052615 (right end), AB052616 (res-tnpR), and AB062597 (res-tnpR-tnpA-right end).
RESULTS
Identification of Tn4656.
After transfer of pWW53-4 from PaW611 to E. coli, the transposability of the xyl genes was investigated by using pMT258 as a target replicon. Mating of DH1(pWW53-4)(pMT258) with HB101 gave rise to Cmr transconjugants at frequencies of approximately 5.0 × 10−4 transconjugant per recipient cell. All 100 transconjugants examined carried pMT258 derivatives with an insert of Tn1, an RP4-specified transposon that is nearly identical to Tn3 (25). To reduce the undesirable transposition of Tn1, pMT1214 (pMT258::Tn3ΔtnpA) was employed as the target replicon; we expected that this plasmid with Tn3 ends would be, due to transposition immunity (10, 25), much less available for insertion of the closely related transposon. Use of pMT1214 indeed led to a 100-fold decrease in the frequency of formation of the Cmr transconjugants. Although 95 to 98% of the transconjugants still contained the pMT1214::Tn1 plasmids, the remaining transconjugants contained pMT1214 derivatives carrying the insert consisting of a 39-kb fragment. Restriction analysis indicated that the insert carried the xyl gene clusters present in pWW53-4. This 39-kb fragment was designated Tn4656 because of its ability to retranspose into various sites on other replicons in a recA-independent manner.
Various internal fragments of Tn4656 in pMT252 and pMT258 were replaced by the pRME1-derived Kmr gene, and transposition of the resulting Tn4656 derivatives was examined by using R388 as the target replicon (Fig. 2). Deletion of the internal fragment of Tn4656 between the leftmost BamHI site and the rightmost KpnI site had no effect on transposition. Approximately 80 to 90% of the Kmr transconjugants obtained in the cross between DH1(pMT2926)(R388) and HB101 were sensitive to tetracycline and harbored only the R388::Tn4656-2926 plasmids. The remaining transconjugants showed resistance to tetracycline, and cleared lysate prepared from each transconjugant contained the three types of plasmids (pMT2926, R388::Tn4656-2926, and the cointegrate of the donor and target plasmids connected by two copies of the transposon), one at each junction. Such cointegrates were structurally unstable and efficiently resolved to the first two types of plasmids. This suggested that Tn4656 transposition occurred via formation of the cointegrate as the intermediate, and the results of the experiments described below supported this suggestion.
Analysis of the DNA regions necessary for transposition.
Tn4656-2925 and Tn4656-2926 in pMT252 were subjected to insertion and deletion mutagenesis (Fig. 3). The transposon derivatives having mutations in the rightmost 3-kb region (i.e., the transposon derivatives in pMT2933, pMT2937, pMT2939, pMT2644, pMT2946, and pMT2947) could not form cointegrates with R388; however, except for the defect of Tn4656-2933 in pMT2933, the defects were restored in the presence of pMT3020, a pMT266-based plasmid carrying the rightmost 4.1-kb fragment of Tn4656 (Fig. 4). Tn4656-2933, which had an insert of the Kmr gene at the rightmost EcoRI site, could not transpose even in the presence of pMT3020, indicating that there was a requirement in cis of this EcoRI site for cointegration (Fig. 3). Next, various derivatives of pMT3020 (Fig. 4) were examined to determine their ability to restore the cointegration defect of Tn4656-2939 in pMT2939 (Fig. 3), and a trans-acting cointegration (i.e., TnpA) activity was found in the rightmost 3.0-kb EcoRI fragment in pMT3030.
The cointegrates formed by the minitransposons in pMT2932, pMT2939, pMT2944, pMT2947, and pMT2948 were stable, and these Tn4656 derivatives had defects in the region between the KpnI site and the middle EcoRI site (Fig. 3). All of these stable cointegrates except that formed by Tn4656-2939 in pMT2929 resolved to the final transposition products in the presence of pMT3020 and its deletion derivative, pMT3041 (Fig. 3 to 5). This indicated that the 1.4-kb KpnI-NruI fragment in pMT3041 encodes a trans-acting resolution (i.e., TnpR) activity. The pMT3041 derivative lacking the 0.32-kb NcoI fragment (pMT3042) and the derivatives carrying an insert of a Kmr fragment at the PvuII and EcoRI sites (pMT3043 and pMT3045, respectively) did not have the TnpR activity (Fig. 4 and 5). The ability of pMT3041 to resolve the Tn4656-2944- and Tn4656-2948-mediated cointegrates and its inability to resolve the Tn4656-2939-mediated cointegrate also indicated that the cis-acting (res) site required for cointegrate resolution was located in the 0.52-kb KpnI-NcoI fragment (Fig. 3 and 4B).
FIG. 5.
Resolution of stable cointegrates. Plasmid pMT2978, a cointegrate formed by R388 and pMT2944 (= pMT252::Tn4656-2944), was transferred to DH1(pMT3041) and DH1(pMT3042). After overnight cultivation of the resulting strains in LB, the plasmids in the cleared lysate prepared from each strain were analyzed by electrophoresis in a 0.6% agarose gel. The figure is a negative of a photograph of the ethidium bromide-stained gel. Lane 1, lysate from DH1(pMT2978)(pMT3041); lane 2, lysate from DH1(pMT2978)(pMT3042). R388::Tn, R388::Tn4656-2944; Chr., chromosomal DNA.
Complementation of transposition functions by other transposons.
The genetic analysis described above clearly indicated that Tn4656 belongs to the class II transposons. We next investigated the exchangeability of the cointegration and resolution functions of Tn4656 with those of other class II transposons, including Tn3, Tn21, Tn1722, Tn4651, Tn4653, and Tn4655 (1, 7, 16, 29, 30). The Tn4656-specified resolution function could be efficiently exchanged with the resolution functions of Tn21, Tn1722, and Tn4653 but could not be exchanged at all with the resolution functions of Tn3, Tn4651, and Tn4655 (data not shown). The Tn4656-specified TnpA function could not be exchanged with the TnpA function of Tn3, Tn21, or Tn4651 (data not shown). The wild-type tnpA gene of Tn4656 could complement the tnpA defects of Tn4653, Tn1722, and Tn4655, while the tnpA defect of Tn4656 was complemented by the wild-type tnpA genes of Tn4653 and Tn1722 (Table 2). It was noteworthy that the wild-type tnpA genes of Tn4656, Tn4653, and Tn1722 complemented the tnpA mutations of Tn4653, Tn1722, and Tn4655 at frequencies more than 10-fold higher than the frequencies at which they complemented the tnpA mutation of Tn4656.
TABLE 2.
Complementation of tnpA mutantsa
| Donor plasmid (tnpA mutant of transposon) | Transposition frequency (relative transposition frequency) withb:
|
||
|---|---|---|---|
| pMT3030 (Tn4656) | pMT1765 (Tn4653) | pMT1294 (Tn1722) | |
| pMT2939 (Tn4656) | 2.0 × 10−6 (1.0) | 1.0 × 10−3 (8.3 × 10−2) | 1.1 × 10−2 (3.6 × 10−2) |
| pMT1590 (Tn4653) | 7.0 × 10−5 (3.5 × 10) | 1.2 × 10−2 (1.0) | 1.2 × 10−1 (4.0 × 10−1) |
| pMT1302 (Tn1722) | 3.0 × 10−5 (1.5 × 10) | 1.5 × 10−2 (1.3) | 3.0 × 10−1 (1.0) |
| pMT2367 (Tn4655) | 2.0 × 10−5 (1.0 × 10) | 3.0 × 10−2 (2.5) | 1.1 × 10−1 (3.6 × 10−1) |
The donor plasmid was a pACYC184-based plasmid with an insert of the transposon derivative lacking its res site and tnpA gene but possessing the Kmr gene, and the complementing plasmid was a pBR322- or pMT266-based plasmid carrying the tnpA gene. For detailed descriptions of the plasmids, see Fig. 3 and 4 and references 28 to 30. The donor and complementing plasmids were transformed into DH1(R388), and mating of the resulting strain with HB101 was performed to select the Kmr Smr transconjugants. The transconjugants contained the stable cointegrate of R388 with the donor plasmid connected by two copies of the mutant transposon.
The transposition frequency is expressed as the number of Kmr transconjugants per Tpr transconjugant. The relative transposition frequency obtained in a homologous combination of the wild-type and mutant tnpA genes was defined as 1.0. No Kmr transconjugants were obtained when pBR322 or pMT266 was used as the complementing plasmid.
Sequence analysis of Tn4656.
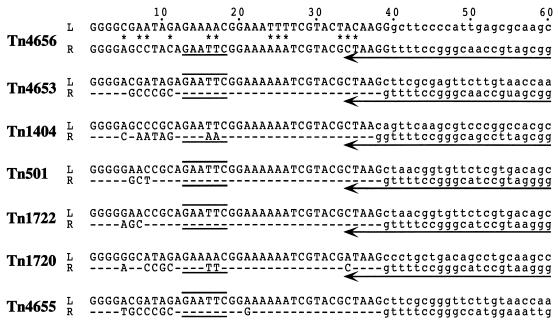
Analysis of the insertion sites of Tn4656 in pMT252 and pMT258 showed that insertion led to a 5-bp duplication of the target sequences (data not shown). The left and right terminal sequences of Tn4656 form 39-bp inverted repeats (IRs) with 12-bp mismatches and exhibit high levels of homology with the terminal sequences of the Tn1721-related transposons Tn4653, Tn1404, Tn501, Tn1722, Tn1720, and Tn4655 (Fig. 6) (1, 7, 10, 23, 29, 30). The sequence homology of the left extremity of Tn4656 with the left extremities of the Tn1721-related transposons was limited to the external 38-bp IR regions. In contrast, the rightmost 4,094-bp region of Tn4656 (AB062597) exhibited extensive sequence homology with the rightmost regions of the Tn1721-related transposons, and the 3,573-bp sequence of the right extremity of Tn4656 (the downstream region starting from base position 522 in Fig. 7) exhibited a very high level of homology (91%) with the sequences of the tnpR-tnpA-right IR regions of Tn501 and Tn1722 (1, 7). The right region of this sequence was occupied by a large open reading frame (ORF) that encoded a 988-amino-acid protein, and this ORF started with the canonical ATG codon and terminated in the right IR. The amino acid sequence of the predicted protein also exhibited 96% identity with the amino acid sequences of the Tn501 and Tn1722 TnpA proteins and 72% identity with the amino acid sequences of the TnpA proteins of the Tn21-related transposons Tn21 and Tn5036 (16, 37). A comparison of these sequence data and successful genetic complementation of the tnpA genes between Tn4656 and Tn1722 (see above) supported the hypothesis that the large ORF of Tn4656 is the tnpA gene.
FIG. 6.
Comparison of the ends of Tn4656-related transposons. The left (L) and right (R) ends of each transposon are defined as the ends distal and proximal, respectively, to the tnpA gene (Fig. 1). The Tn4656 sequences determined in this study are the leftmost 498-bp fragment (AB052614) and the rightmost 4,094-bp fragment (AB062597). The outermost 60 nucleotides of Tn4656 are shown together with the nucleotides of other related transposons (1, 7, 23, 29, 30). The nucleotides in the IRs are indicated by uppercase letters. Differences in the sequences of pairs of IRs in Tn4656 are indicated by asterisks, whereas sequence identities for pairs of IRs in each of the other transposons are indicated by dashes in the right IR. The arrows indicate the 3′ part of the tnpA gene, and the EcoRI site is indicated by lines.
FIG. 7.
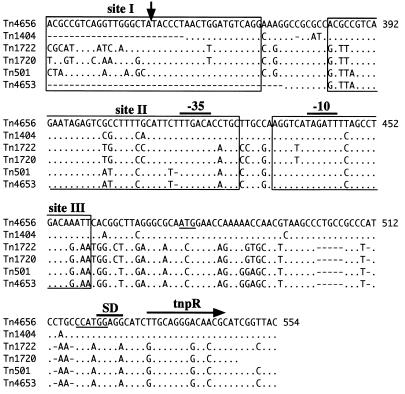
Comparison of res-containing regions of Tn4656-related transposons. Sequence data were obtained in this study (AB062597) and from references 1, 7, 16, 23, and 30. The numbers on the right are the base positions; the upstream KpnI site (Fig. 3) was defined as position 1. The dots represent nucleotides identical to nucleotides of Tn4656, whereas the dashes represent gaps that result in maximum matching. Three putative resolvase-binding sites are enclosed in boxes, and the putative crossover point based on the data for Tn1722 (22) is indicated by a vertical arrow. Note that Tn1404 and Tn4653 are defective in cointegrate resolution because of a lack of crossover points (23, 30). The predicted start of the Tn4656 tnpR gene is indicated by a horizontal arrow, and the predicted promoter and ribosome-binding (SD) sequences for the tnpR gene are overlined. The ATG codon of Tn4656 mentioned in the text and the NcoI site are underlined.
In the 4,094-bp sequence there were also two overlapping ORFs; these two ORFs started at nucleotide positions 477 (ATG) and 531 (TTG), and both terminated at the stop codon 6 bp upstream of the tnpA gene. The TTG start codon, but not the ATG codon, was preceded by a typical Shine-Dalgarno sequence (Fig. 7). It is very likely, but has not been proven, that the latter ORF, which gives rise to a protein with 186 amino acids, encodes the resolvase. The deduced amino acid sequence of the predicted resolvase exhibits extensive homology with the deduced amino acid sequences of the resolvases of the Tn1721-related transposons (Tn501, Tn1722, and Tn4653; 93 to 95% identity) (1, 7, 30) and the Tn21-related transposons (Tn21, Tn5036, and Tn5059; 81 to 83% identity) (16, 18, 37). At the DNA level, the Tn4656 tnpR gene is 87% identical to the tnpR genes of the former three transposons and 73 to 77% identical to the tnpR genes of the latter three transposons. The 5′ upstream regions of the tnpR genes of all of these related transposons except Tn4653 carry the res sites, and each res site is made up of the three resolvase-binding domains (sites I, II, and III) that contain the crossover point for cointegrate resolution and −35 and −10 sequences of the tnpR promoter, respectively (Fig. 7) (1, 7, 16, 22). Nucleotide sequences that are 128 bp long and are highly homologous to these three domains with appropriate spacers are located in the region approximately 70 bp upstream of the tnpR start codon of Tn4656. Although no additional biochemical experiments were carried out with respect to the Tn4656 res site, it is very probable based on the exchangeability of the resolution functions between Tn4656 and Tn1722 (see above) that the 128-bp sequence of Tn4656 has functional domains and a crossover point identical to those determined experimentally for the Tn1722 res site (22). The 330-bp sequence upstream of site I of Tn4656 exhibited no similarity to nucleotide sequences in the databases.
DISCUSSION
In this paper we describe a fourth class II catabolic transposon, Tn4656, residing in pWW53-4. Successful identification of this transposon depended on using transposition immunity, by which undesirable transposition of another pWW53-4-specified transposon, Tn1, was greatly suppressed. Structural and functional analyses of Tn4656 clearly demonstrated that this transposon is a member of the Tn1721-related transposon group (Fig. 1). However, the transposition frequency of Tn4656 was lower (<10-fold) than those of Tn1722 and Tn4653 (27, 28, 30). The lower frequency of transposition of Tn4656 is attributed to the sequences of its IRs and not to the sequence of the transposase because the Tn4656 transposase catalyzed cointegration of the tnpA mutants of Tn4653, Tn1722, and Tn4655 at frequencies that were more than 10-fold higher than the frequency of cointegration of the tnpA mutant of Tn4656 (Table 2). Although the nucleotides at positions 12 to 38 in the right IR of Tn4656 are essentially identical to those conserved in the IRs of other Tn1721-related transposons (Fig. 6) (1, 7, 10, 23, 29, 30), the conserved nucleotides are different at eight positions in the left IR of Tn4656. Except for the left IR of Tn4656, at least a five-base A stretch from position 22 to position 26 is conserved in the Tn1721-related transposons, and the heptanucleotide ACGNTAAG at positions 31 to 38 is conserved in the IRs of the class II transposons (10, 25). Mutational analyses of the 38-bp IRs of Tn3 and Tn1000 have indeed indicated that the base pair changes in the heptanucleotide lead to more-than-100-fold reductions in the cointegration frequencies (17, 19). Taking these facts into consideration, we suggest that either or both of the trinucleotides TTT (positions 24 to 26) and TAC (positions 33 to 35) in the left IR of Tn4656 are probably responsible for the relatively lower cointegration frequency of Tn4656, although no further experiments were carried out in this study.
Catabolic transposons Tn4653 and Tn4655 belong to the Tn1721-related transposon group (Fig. 1). It has been proposed that these transposons became established after various kinds of unknown genetic rearrangements, even rearrangements in the transposition-related genes themselves (27–31). Tn4653 has a defect in its res site and contains another class II catabolic transposon, Tn4651, that is clearly distinct from the Tn1721-related transposons. It is thought that Tn4655 lost the res-tnpR-tnpA region of the Tn1721-related transposons and acquired the region encoding a new site-specific resolution system before establishment of the present structure. Therefore, Tn4653 and Tn4655 might be less suitable for investigating the putative molecular mechanisms of incorporation of the catabolic genes in the common ancestral transposon. Tn4656, in contrast, has the simple organization of the transposition-related genes, and use of this transposon might help clarify the diversification of the non-transposition-related genes in the Tn1721-related transposons.
Detailed characterization of the pWW53-specified xyl genes has been initiated by the pioneering work of Williams' group through translocation of the xyl genes of this plasmid to coresident plasmid RP4 in order to obtain RP4 derivatives carrying at least a set of xyl genes for complete degradation of toluene and xylenes (13). We are very interested in such RP4 derivatives for the following two reasons. The first is that the physical map of pWW53-4 reported by Keil et al. (13) appears, in our hands, not to cover the 4-kb DNA segment of Tn4656 that includes the res-tnpR-tnpA-IR fragment (our unpublished data). More detailed comparisons of physical maps of pWW53, pWW53-4, and Tn4656 should reveal the location of the 4-kb segment in the original host strain carrying pWW53 and should provide some clues for understanding the formation of Tn4656 from pWW53. The second reason is that the pWW53-derived insert in pWW53-4 has been reported to be the smallest fragment among the fragments translocated into RP4. We are also trying to detect various transposable regions of pWW53 by using a transposon-free and broad-host-range plasmid as a target replicon.
At a position downstream of xylS2, pWW53 carries a gene encoding a putative site-specific resolvase that exhibits high levels of homology with the enzymes specified by several plasmids and the class II transposons (3). The pWW53 resolvase (PWW53RES) gene is, however, clearly distinguishable from the Tn4656 tnpR gene in terms of the nucleotide sequence. These facts mean that PWW53RES is not involved in Tn4656 transposition at all. However, it would be interesting to know whether PWW53RES plays some role in formation of Tn4656 from pWW53, although the function of PWW53RES remains to be investigated.
For a long time the xyl operon organization of pWW53, meta operon I-upper operon-meta operon II, has been considered to be exceptional and unique. However, Sentchilo et al. (23) have recently reported that such an organization is not unusual, at least in the Pseudomonas TOL plasmids from Belarus. In spite of relatively minor structural diversity (i.e., insertion and deletion of small DNA fragments) in the xyl operons, these TOL plasmids have much more diversity, especially in the regions located outside the catabolic operons. The diversity includes differences in size, self-transmissibility, and incompatibility of the plasmids. Our identification of Tn4656 in pWW53 may also explain (as one possible mechanism) the structural diversity of the Belarus TOL plasmids.
ACKNOWLEDGMENTS
We are grateful to P. A. Williams, who kindly provided pWW53-4. We also thank M. Sota for determining nucleotide sequences.
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture, Japan.
REFERENCES
- 1.Allmeier H, Cresnar B, Greck M, Schmitt R. Complete nucleotide sequence of Tn1721: gene organization and a novel gene product with features of a chemotaxis protein. Gene. 1992;111:11–20. doi: 10.1016/0378-1119(92)90597-i. [DOI] [PubMed] [Google Scholar]
- 2.Assinder S J, de Marco P, Osborne D J, Poh C L, Shaw L E, Winson M K, Williams P A. A comparison of the multiple alleles of xylS carried by TOL plasmids pWW53 and pDK1 and its implications for their evolutionary relationship. J Gen Microbiol. 1993;139:557–568. doi: 10.1099/00221287-139-3-557. [DOI] [PubMed] [Google Scholar]
- 3.Assinder S J, de Marco P, Sayers J R, Shaw L E, Winson M K, Williams P A. Identical resolvases are encoded by Pseudomonas TOL plasmids pWW53 and pDK1. Nucleic Acids Res. 1992;20:5476. doi: 10.1093/nar/20.20.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assinder S J, Williams P A. The TOL plasmids: determinants of the catabolism of toluene and the xylenes. Adv Microb Physiol. 1990;31:1–69. doi: 10.1016/s0065-2911(08)60119-8. [DOI] [PubMed] [Google Scholar]
- 5.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1991. [Google Scholar]
- 6.Avila P, de la Cruz F. Physical and genetic mapping of the IncW plasmid R388. Plasmid. 1988;20:155–157. doi: 10.1016/0147-619x(88)90019-4. [DOI] [PubMed] [Google Scholar]
- 7.Brown N L, Winnie J N, Fritzinger D, Pridmore R D. The nucleotide sequence of the tnpA gene completes the sequence of the Pseudomonas transposon Tn501. Nucleic Acids Res. 1985;13:5657–5669. doi: 10.1093/nar/13.15.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 9.Gallegos M-T, Williams P A, Ramos J L. Transcriptional control of the multiple catabolic pathways encoded on the TOL plasmid pWW53 of Pseudomonas putida MT53. J Bacteriol. 1997;179:5024–5029. doi: 10.1128/jb.179.16.5024-5029.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grinsted J, de la Cruz F, Schmitt R. Tn21 subfamily of bacterial transposable elements. Plasmid. 1990;24:163–189. doi: 10.1016/0147-619x(90)90001-s. [DOI] [PubMed] [Google Scholar]
- 11.Harayama S, Leppik R A, Rekik M, Mermod N, Lehrbach P R, Reineke W, Timmis K N. Gene order of the TOL catabolic plasmid upper pathway operon and oxidation of both toluene and benzyl alcohol by the xylA product. J Bacteriol. 1986;167:455–461. doi: 10.1128/jb.167.2.455-461.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hõrak R, Kivisaar M. Expression of the transposase gene tnpA of Tn4652 is positively affected by integration host factor. J Bacteriol. 1998;180:2822–2829. doi: 10.1128/jb.180.11.2822-2829.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keil H, Keil S, Pickup R W, Williams P A. Evolutionary conservation of genes coding for meta pathway enzymes within TOL plasmids pWW0 and pWW53. J Bacteriol. 1985;164:887–895. doi: 10.1128/jb.164.2.887-895.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keil H, Saint C M, Williams P A. Gene organization of the first catabolic operon of TOL plasmid pWW53: production of indigo by the xylA gene product. J Bacteriol. 1987;169:764–770. doi: 10.1128/jb.169.2.764-770.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kholodii G Y, Yurieva O V, Gorlenko Z M, Mindlin S Z, Bass I A, Lomovskaya O L, Kopteva A V, Nikiforov V G. Tn5041: a chimeric mercury resistance transposon closely related to the toluene degradative transposon Tn4651. Microbiology. 1997;143:2549–2556. doi: 10.1099/00221287-143-8-2549. [DOI] [PubMed] [Google Scholar]
- 16.Liebert C A, Hall R M, Summers A O. Transposon Tn21, a flagship of the floating genome. Microbiol Mol Biol Rev. 1999;63:507–522. doi: 10.1128/mmbr.63.3.507-522.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.May E W, Grindley N D F G. A functional analysis of the inverted repeats of the γδ transposable element. J Mol Biol. 1995;247:578–587. doi: 10.1006/jmbi.1995.0164. [DOI] [PubMed] [Google Scholar]
- 18.Minakhina S, Kholodii G, Mindlin S, Yurieva O, Nikiforov V. Tn5053 family transposons are res site hunters sensing plasmidal res sites occupied by cognate resolvases. Mol Microbiol. 1999;33:1059–1068. doi: 10.1046/j.1365-2958.1999.01548.x. [DOI] [PubMed] [Google Scholar]
- 19.Nissley D V, Lindh F G, Fennewald M A. Mutational analysis of the inverted repeats of Tn3. J Mol Biol. 1990;213:671–676. doi: 10.1016/S0022-2836(05)80254-2. [DOI] [PubMed] [Google Scholar]
- 20.Osborne D J, Pickup R W, Williams P A. The presence of two complete homologous meta pathway operons on TOL plasmid pWW53. J Gen Microbiol. 1988;134:2965–2975. doi: 10.1099/00221287-134-11-2965. [DOI] [PubMed] [Google Scholar]
- 21.Reddy B R, Shaw L E, Sayers J R, Williams P A. Two identical copies of IS1246, a 1275 base pair sequence related to other bacterial insertion sequences, enclose the xyl genes on TOL plasmid pWW0. Microbiology. 1994;140:2305–2307. doi: 10.1099/13500872-140-9-2305. [DOI] [PubMed] [Google Scholar]
- 22.Rogowsky P, Halford S E, Schmitt R. Definition of three resolvase binding sites at the res loci of Tn21 and Tn1721. EMBO J. 1985;4:2135–2141. doi: 10.1002/j.1460-2075.1985.tb03904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnabel E L, Jones A L. Distribution of tetracycline resistance genes and transposons among phylloplane bacteria in Michigan apple orchards. Appl Environ Microbiol. 1999;65:4898–4907. doi: 10.1128/aem.65.11.4898-4907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sentchilo V S, Perebituk A N, Zehnder A J B, van der Meer J R. Molecular diversity of plasmids bearing genes that encode toluene and xylene metabolism in Pseudomonas strains isolated from different contaminated sites in Belarus. Appl Environ Microbiol. 2000;66:2842–2852. doi: 10.1128/aem.66.7.2842-2852.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheratt D. Tn3 and related transposable elements: site-specific recombination and transposition. In: Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C.: American Society for Microbiology; 1989. pp. 163–184. [Google Scholar]
- 26.Taylor L A, Rose R E. A correction in the nucleotide sequence of the Tn903 kanamycin resistance determinant in pUC4K. Nucleic Acids Res. 1988;16:358. doi: 10.1093/nar/16.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuda M, Iino T. Genetic analysis of a transposon carrying toluene degrading genes on a TOL plasmid pWW0. Mol Gen Genet. 1987;210:270–276. doi: 10.1007/BF00325693. [DOI] [PubMed] [Google Scholar]
- 28.Tsuda M, Iino T. Identification and characterization of Tn4653, a transposon covering the toluene transposon Tn4651 on TOL plasmid pWW0. Mol Gen Genet. 1988;213:72–77. doi: 10.1007/BF00333400. [DOI] [PubMed] [Google Scholar]
- 29.Tsuda M, Iino T. Naphthalene degrading genes on plasmid NAH7 are on a defective transposon. Mol Gen Genet. 1990;223:33–39. doi: 10.1007/BF00315794. [DOI] [PubMed] [Google Scholar]
- 30.Tsuda M, Minegishi K-I, Iino T. Toluene transposons Tn4651 and Tn4653 are class II transposons. J Bacteriol. 1989;171:1386–1393. doi: 10.1128/jb.171.3.1386-1393.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuda M, Tan H M, Nishi A, Furukawa K. Mobile catabolic genes in bacteria. J Biosci Bioeng. 1999;87:401–410. doi: 10.1016/s1389-1723(99)80086-3. [DOI] [PubMed] [Google Scholar]
- 32.van der Meer J R, de Vos W M, Harayama S, Zehnder A J B. Molecular mechanisms of genetic adaptation to xenobiotic compounds. Microbiol Rev. 1992;56:677–694. doi: 10.1128/mr.56.4.677-694.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson N. A new revision of the sequence of plasmid pBR322. Gene. 1988;70:399–403. doi: 10.1016/0378-1119(88)90212-0. [DOI] [PubMed] [Google Scholar]
- 34.Williams P A, Assinder S J, de Marco P, O'Donnell K J, Poh C L, Shaw L E, Winson M K. Catabolic gene duplications in TOL plasmids. In: Galli E, Silver S, Witholt B, editors. Pseudomonas: molecular biology and biotechnology. Washington, D.C.: American Society for Microbiology; 1992. pp. 341–352. [Google Scholar]
- 35.Williams P A, Sayers J R. The evolution of pathways for aromatic hydrocarbon oxidation in Pseudomonas. Biodegradation. 1994;5:195–217. doi: 10.1007/BF00696460. [DOI] [PubMed] [Google Scholar]
- 36.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 37.Yurieva O, Kholodii G, Minakhin L, Gorlenko Z, Kalyaeva E, Mindlin S, Nikiforov V. Intercontinental spread of promiscuous mercury-resistance transposons in environmental bacteria. Mol Microbiol. 1997;24:321–329. doi: 10.1046/j.1365-2958.1997.3261688.x. [DOI] [PubMed] [Google Scholar]