Abstract
SARS-CoV-2 can be vertically transmitted from the mother to the fetus and the neonate. This transmission route is rare compared to the environmental or horizontal spread and therefore, the risk can be deemed inconsequential by some medical providers. However, severe, although just as rare, feto-neonatal consequences are possible: fetal demise, severe/critical neonatal COVID-19 and multi-inflammatory syndrome (MIS-N) have been described. Therefore, it is important for the clinicians to know the mechanism of vertical transmission, how to recognize this, and how to deal with neonatal COVID-19 and MIS-N. Our knowledge about this field has significantly increased in the last three years. This is a summary of the pathophysiology, diagnostics, and therapeutics of vertical SARS-CoV-2 transmission that clinicians apply in their clinical practice.
Keywords: COVID-19, Pregnancy, Infant, Vertical, Infection, Stillbirth, NICU
1. Background
Although initially denied when the worldwide attention was concentrated on the effect of pandemics in the general population, the vertical transmission of SARS-CoV-2 (i.e.: from the mother to the fetus or the neonate) eventually became evident. In 2020, two separate teams in Germany and United States detected SARS-CoV-2 in placental tissues at the maternal-fetal interface using electron microscopy [1,2]. This was not totally surprising as the placenta similar to the lung is an organ of gas exchange. In July of the same year, our team was able to demonstrate, for the first time, the transplacental passage of SARS-CoV-2 in a women affected by COVID-19 who gave birth to a critically ill baby requiring neonatal intensive care unit (NICU) admission [3]. This case had worldwide media coverage but was published several months after its occurrence, to allow completing multiple virological, immunological and pathological tests needed to confirm the transplacental transmission. In 2021, the World Health Organization (WHO) added SARS-CoV-2 to the list of vertically transmittable infectious agents, whose last example was the Zika virus [4]. We offer here a comprehensive update of the SARS-CoV-2 vertical transmission with focus on pathophysiology and clinical management.
2. Where and when does it happen? Defining the transmission routes
The term “vertical transmission” encompasses different types of transmission that may happen: 1) “In-utero” transmission during pregnancy through the placenta, infecting the amniotic fluid and subsequently entering the airways or the gastro-intestinal tract or the ear canal, 2) “Intrapartum” transmission at the moment of delivery, through the contact with the maternal genital mucosa and its secretions or with another maternal biological fluid (i.e., blood, fecal matter, urine etc.) and, 3) “Ascending” infection - during the pregnancy or the delivery as vaginal ascending infection with the virus passing from the vagina into the uterine cavity via the cervical mucosa. The first two ways have been described [4], while the third has not been demonstrated for SARS-CoV-2 as of this writing. It is a more complex scenario implying that SARS-CoV-2 would gain upstream access into the uterine cavity and then into the amniotic sac which may become compromised and ruptured [5,6]. Once the amniotic fluid is infected, the fetus would become infected as described above. The ascending infection is the most common route by which bacteria may invade the uterine cavity [5,6], but the relatively low prevalence of SARS-CoV-2 positivity in cervico-vaginal mucosa [[7], [8], [9], [10]] may prevent its occurrence. Patients with vaginal secretions positive for SARS CoV-2 are likely to present with a higher circulating viral load and may facilitate the transplacental infection [7,[11], [12], [13], [14]]. But even in presence of a mucosal infection, to create an ascending transmission, it should locally spread, trespass the mucosal immune defenses and gain access to the uterine cavity which is uncommon for viruses [15].
The first two scenarios have been defined as in utero and intrapartum transmission by the WHO [4]. The possibility of SARS-CoV-2 transmission via human milk has been essentially excluded as the virus has been inconsistently found in milk, and when present, a very low viral load was detected, although the capability to replicate has not been confirmed [10,16]. Conversely, antibodies have been detected in human milk, particularly after maternal immunization using mRNA vaccines [17]. Theoretically, the mother-to-child transmission through droplets or aerosol occurring in the first 72 h after delivery might also be considered “vertical”, but this has the same biological features of common environmental “horizontal” transmission, therefore, it will not be discussed here.
3. How often does it happen? The epidemiology
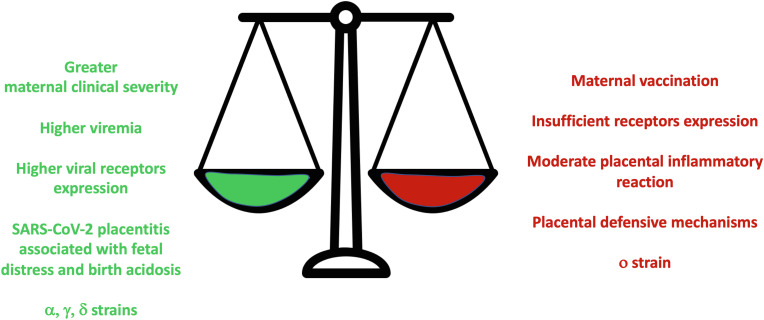
In the last 3 years, relevant data has been accumulated for both in utero and intrapartum transmissions. According to a meta-analysis published right after the first pandemic wave, these seem to represent approximately 40% and 60% of all vertical infections, respectively, which in their turn account for approximately 30% of all neonatal infections (i.e.: ∼70% of neonates are infected environmentally through the common route based on droplets and aerosol) [18]. In the beginning of pandemics the prevalence of vertical SARS-CoV-2 infection was estimated around 5% of positive pregnant women [19]. Recent data accumulated from a living meta-analysis had reduced the estimated prevalence at around 2% [20]. This clearly indicates that the vertical SARS-CoV-2 transmission does not represent a public health problem. Nonetheless a potential publication bias can be hypothesized so the prevalence may be relatively underestimated. Moreover, fetal and neonatal SARS-CoV-2 infections are classified with a variable level of likelihood using a dedicated clinical definition [21] or the WHO criteria [4] and this is needed because of the biological complexity of SARS-CoV-2 perinatal infection: the exact classification can only be obtained performing several tests on various matrices, which are not always available in all clinical laboratories [22]. In some, cases may go undiagnosed and, as an additional consequence, it is difficult to know the relative exact proportion of in utero and intrapartum transmissions, since not all tests that are needed to classify a case are systematically performed. Along these lines, COVID-19 is much less common in neonates than adults, although severe and life-threatening cases have been described. It is then important for obstetricians, pediatricians, and particularly for neonatologists, to be aware of this and to know how to recognize these cases and strategize management. Vertical SARS-CoV-2 transmission is significantly more likely when maternal COVID-19 is severe (OR: 2.4 (1.3–4.4), when mothers need critical care (OR: 3.5 (1.7–6.9) or die (OR: 14 (4.1–48) [20]. It is also known that some viral strains could theoretically influence the risk of vertical transmission by increasing the maternal disease severity, as this has been demonstrated for the alpha (α), gamma (γ) and delta (δ) variants [[23], [24], [25], [26]], while omicron (ο) strain causes less placental damage and might be associated with a reduced clinical severity and likelihood of vertical transmission [27]. These data were accumulated mainly in the pre-vaccination era such that immunization has changed the maternal disease severity and the risk of vertical transmission [28]. Further, it is unknown if the currently available antivirals may reduce this risk by reducing the circulating viral load and the maternal clinical severity. Some additional factors suggesting an increased risk of transplacental infection might be identified by the fetal monitoring close to delivery and will be discussed further. No link between vertical transmission and any other maternal characteristics has been demonstrated.
4. How does it happen? The mechanisms
Mechanisms of SARS-CoV-2 vertical transmission are different if it occurs in utero or intrapartum. A complete molecular review of biological mechanisms is out of the scope of this manuscript, but we will focus on the main principles of the pathophysiological aspects. It is important to remember that, because of the complex biology of perinatal SARS-CoV-2 infections, the distinction between the two transmission routes can only be achieved with multiple tests on different samples, as described by WHO guidance [3]: these are schematically summarized in Table 1 . The data summarized below have been accumulated mainly in the early phase of the pandemic. As the immunization progressed worldwide, the cases of COVID-19 during pregnancy decreased and so were the vertical infections [28,29], as anti-Spike-IgG can easily pass the placenta in vaccinated pregnant women [17]. Therefore, it might be more difficult to further improve our knowledge on these mechanisms.
Table 1.
Summary table of the principle to distinguish the two main SARS-CoV-2 vertical transmission routes, according to the WHO definition [4].
| Maternal Infection | Evidence of in utero exposure | Viral persistence or immune response* | |
|---|---|---|---|
| In utero | Yes | Yes | Yes |
| Intrapartum | Yes* | No | Yes |
Note:
1. Maternal infection must be diagnosed according to standard WHO criteria. Maternal infection is intended to have occurred anytime during the pregnancy for in utero and in the time window from 30 days before to 2 days after the delivery, for intra partum transmission.
2. Evidence of in utero exposure is granted with several possible (virological, pathological or immunological) tests performed on placenta, amniotic fluid or cord blood. Viral persistence or immune response in the neonate is demonstrated with RT-PCR performed on several possible sites or with serology assay at different timepoints.
3. Both types of infection are classified in three level of likelihood (confirmed, possible and unlikely) according to the type of specimen and test used. The policy we suggest in the main text is compliant with this WHO guidance.
4. *For the diagnosis of in utero transmission with fetal demise, the viral persistence or immune response is replaced by the fetal tissue positivity (that might be associated with placenta or amniotic fluid positivity) obtained with RT-PCR or several possible pathology/immunology techniques.
5. More details regarding sampling, tests and their timing are freely available in the WHO definition for perinatal SARS-CoV-2 infections [4].
4.1. In utero transmission
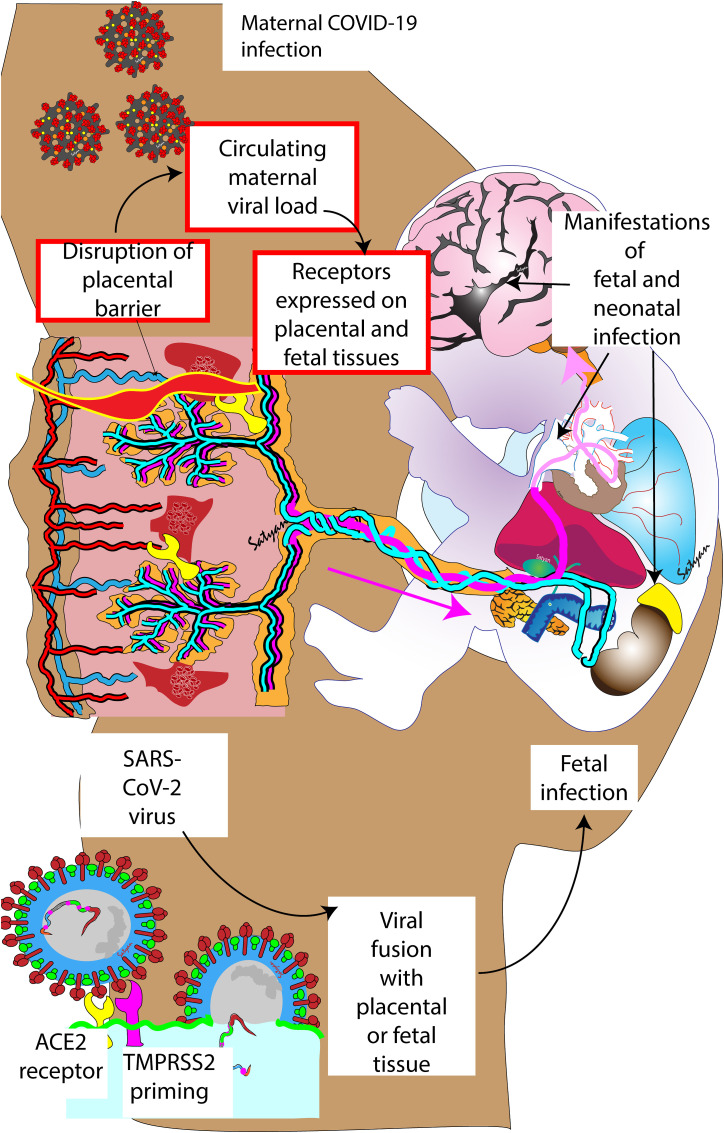
For SARS-CoV-2 to be transmitted transplacentally, a few things must occur (Fig. 1 ): 1) there should be a relevant circulating maternal viral load, although transiently; 2) at the same moment, viral receptors must be expressed on cellular membranes in placental and fetal tissues; 3) the placental barrier must be disrupted, and its defensive mechanisms must be overcome to allow the viral passage. All these conditions must occur at the same time and in a significant way to produce an active feto-neonatal infection, thus the need for this coincidence helps explaining the relative rarity of transplacental transmission. Maternal viremia has been reported during SARS-CoV-2 infection, but inconstantly and in relation with the clinical severity [[30], [31], [32]]. Angiotensin-converting enzyme-2 (ACE2) receptors are expressed in placental tissues throughout the pregnancy [33]. This expression is, however, variable depending on interindividual genetic variability, gestational age and comorbidities, such as pre-eclampsia [[34], [35], [36], [37]]. The presence of ACE2 receptors in fetal tissues was already known before the pandemics [38] and it has been confirmed thereafter, although it is not constant in all fetal tissues [39,40]. For the virus to invade the cells, these receptors are not sufficient though, since the Spike protein must be cleaved by transmembrane protease serine-2 (TMPRSS2) [41]. In fact, this is needed to expose a fusogenic peptide that promotes the fusion of the viral envelope with the host cell membrane [42]. Nonetheless, the factors controlling TMPRSS2 expression (beside fetal sex [43]) are not well known and may not coincide with those influencing ACE2 receptor, thus this complicates the situation. Finally, the existence of other pathways and viral receptors have been hypothesized: placental macrophages (Hofbauer's cells) and circulating fetal monocytes could carry the virus [44], but this mechanism is present, if at all, only in a minority of cases [45]; other receptors might be used by some viral strains and particularly by the omicron variant, but little is known about their placental presence and their possible role in the transplacental transmission [37,46,47].
Fig. 1.
Potential mechanisms factors influencing SARS-CoV-2 in utero (transplacental) transmission. Maternal viremia, disruption of placental barrier and presence of receptors in fetal and placental tissue (red boxes) should occur simultaneously for in-utero transmission to occur. The presence of angiotensin converting enzyme 2 (ACE2) receptors in fetal tissue and co-existence of transmembrane serine protease 2 (TMPRSS2) receptor that can prime the spike protein in the fetal tissue is necessary to enable viral fusion with fetal tissues. Copyright Satyan Lakshminrusimha.
The presence of viremia and viral receptors may be less important than the third requirement, that is the disruption of the placental barrier. In fact, the histological or virological evidence of SARS-CoV-2 placental infection is more common than the feto-neonatal infection, thus local defenses must play a relevant role in preventing the contamination [48]. Recently we demonstrated, in a controlled study [49], that the placental viral load and the levels of ACE2 and TMPRSS2 placental expression do not significantly influence the transplacental transmission. Conversely, a strong placental inflammatory reaction is known to prevent the passage of many infectious agents to the fetus [50] and this reaction has been observed in many COVID-19 pregnant patients [51,52]. Following placental inflammation, post-entry pathways and autophagy can be activated to eliminate the virus from the already infected placental cells [53,54]. All these obstacles must be overcome in order to produce an active transplacental infection.
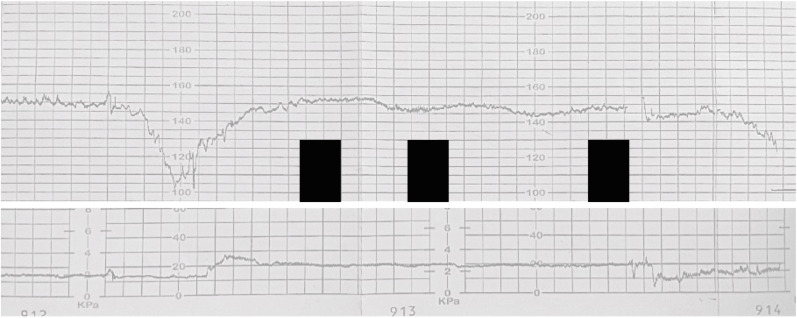
An excessive or dysregulated host inflammatory reaction can also play the opposite role and facilitate viral spread, as it is happens for acute respiratory distress syndrome and multi-organ failure observed in patients with critical COVID-19 [55]. A strong inflammatory reaction (typically characterized by diffuse chronic intervillositis, necrosis and massive fibrin perivillous deposits (>50% of the placenta)) has been seen in almost all cases of transplacental transmission and is now considered a typical “SARS-CoV-2 placentitis” [49]. This causes vascular fetal malperfusion and creates suitable anatomical environments for the passage of virions to the fetus. It is unclear if these anomalies may increase the risk of fetal growth restriction [56,57] (see below), however they are certainly associated with fetal distress (recognizable at the cardiotocogram, Fig. 2 ), stillbirths, birth acidosis and need for NICU admission [49,58]. The factors facilitating or preventing in utero SARS-CoV-2 transmission are summarized in Fig. 2.
Fig. 2.
Cardiotocogram (CTG) from an illustrative case of in utero (transplacental) transmission. Category III-CTG (absent baseline fetal heart rate variability and recurrent decelerations outside labor) recorded at 33 weeks' gestation (black boxes mask personal informations). Maternal history was marked by biochemical abnormalities during moderate COVID-19 (prolonged APTT, lymphocytopenia, transaminitis). A cesarean delivery was performed, and the neonate suffered mild perinatal asphyxia (5′ Apgar score = 5, cord pH = 7.25). The case fulfilled the WHO definition for confirmed in utero infection [4]: RT-PCR was positive on nasopharyngeal aspirate both at the birth and DOL4; RT-PCR was also positive on non-bronchoscopic bronchoalveolar lavage (DOL1) and in the placental tissue. Placenta showed the typical signature of SARS-CoV-2 placentitis (perivillous fibrin deposition, chronic histiocytic intervillitis and trophoblastic necrosis).
4.2. Intra partum transmission
Intrapartum transmission is a common way of contamination for several infectious agents during vaginal delivery and it can happen for SARS-CoV-2 as well. This transmission route is obviously possible only for assisted or unassisted vaginal deliveries. ACE2 receptors are upregulated in vaginal epithelium during the pregnancy, making the contamination theoretically possible [59], although we do not have any data about the vaginal TMPRSS2 expression and the prevalence of SARS-CoV-2 vaginal infection is relatively low [[7], [8], [9]]. The virus can reach the vaginal mucosa through systemic circulation and seminal or fecal contamination [7,60]. Personal hygiene measures may therefore be important, since the viral shedding in the stools is longer than in other bodily fluids [61]. Intrapartum might be more common than in utero contamination [18], but available data on this point are conflicting [62]. The actual proportion of intrapartum and in utero transmissions is difficult to clarify since there is a relevant degree of publication bias and many data come from the first pandemic waves when, at least in some settings, perinatal care might have been suboptimal and some tests may have been lacking. Furthermore, both in utero and intrapartum transmission requires several pre-existing factors (such vaginal delivery or disruption of placental defenses as discussed above) to occur and this complicates the estimation of their relative incidence.
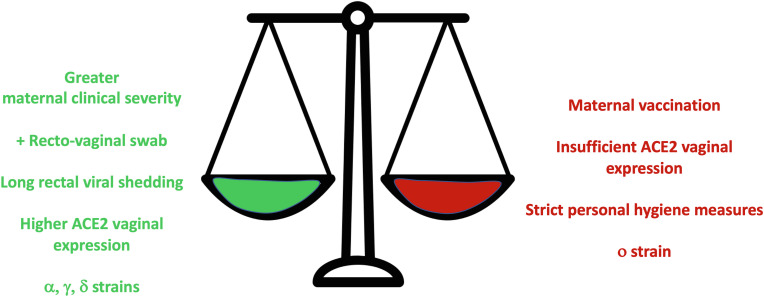
Interestingly, intrapartum infections have been described irrespective of the elimination of SARS-CoV-2 from maternal airways and despite the presence of circulating antibodies [63]. This is likely due to the persistent viral presence that may last longer in vaginal or rectal tissue than in upper airways [61]. To reduce the transmission risk, particular care should be provided during vaginal birth assistance by reducing fecal contamination [64]. Performing recto-vaginal swabs to all pregnant women affected by COVID-19 or infected by SARS-CoV-2 in the proximity of delivery is useful to classify an eventual vertical transmission [4] and is a common practice for other perinatal infections [65]. The positive results from these tests should not be used to decide about the delivery route. The delivery route choice should be based on common principles of obstetric care and case-by-case evaluation of multiple factors (see below). The factors facilitating or preventing intra partum SARS-CoV-2 transmission are summarized in Fig. 3 .
Fig. 3.
Factors increasing (green) and decreasing (red) the likelihood of SARS-CoV-2 in utero transmission. Factors have been listed according to the current available translational and clinical knowledge; see references in the text. The relative weight of each factor cannot be estimated.
5. What can we do to prevent and detect vertical SARS-COV-2 transmission?
There is not much to do to prevent the vertical transmission, beside promoting vaccination which has decreased the incidence by virtue of the reduction of maternal infection and its severity [28,29]. Strict hygiene measures should be adopted in the delivery room to prevent contamination from maternal biological fluids and the multidisciplinary team should be adequately trained to assist these deliveries [66].
During the first pandemic waves, approximately 10–22% of COVID-19 pregnant patients delivered vaginally [67,68] and this percentage is likely to be higher now. It is unclear if and how the vaginal delivery protects against the vertical transmission although some data suggest so [62,69,70]. It must be taken into account that cesarean section may be needed for reasons unrelated to COVID-19 and that the SARS-CoV-2 placentitis, which is strongly associated with the transplacental transmission, can present with fetal heart rate abnormalities, which may justify emergency cesarean delivery outside of labor [71]. Since many factors interplay on the occurrence of vertical transmission [72] and the choice of the delivery, a generalized recommendation cannot be provided.
When the risk of vertical transmission is relevant (i.e.: in case of higher maternal clinical severity, high viral load, maternal viremia or cardiotocogram abnormalities suggesting fetal hypoxia, Fig. 2), we advise to send the placenta for virological and pathological analysis. Cord plasma and sterile amniotic fluid collection should also be taken and frozen: they can be used for later testing to provide a correct classification of the vertical transmission [4]. Placenta, amniotic fluid and plasma are therefore considered 2nd level tests, while the first (and often unique) test to be done is the real time-polymerase chain reaction (RT-PCR) on a neonatal nasopharyngeal sample, after having cleansed and dried the baby. It is advised to perform a nasopharyngeal lavage, rather than a swab, because it is easier due to the small size of newborn nostrils and because it ensures a higher sensitivity [73]. In our practice, we perform the test in every neonate born from a mother affected by COVID-19 in the last month before delivery: results are available with a quick turnaround time (approximately 1 hour). If the result is negative, there is no need for any further test, otherwise the aforementioned 2nd level tests will help to classify the case and nasopharyngeal lavage will have to be repeated after 24–48 h to rule out an exposure without active infection: readers are referred to the WHO definition for more details [4]. In sick neonates admitted to the NICU, other RT-PCR might eventually be done on different samples (i.e.: broncho-alveolar lavage, cerebro-spinal fluid etc …), depending on their conditions. The neonates should otherwise be assisted according to currently recommended practices and should be cleansed and dried as usual. This policy is compliant with the WHO definition and may provide the needed information to classify perinatal SARS-CoV-2 infections [4].
6. What can happen next? The feto-neonatal consequences
Vertically transmitted SARS-CoV-2 infections can have various consequences both for the fetus and the neonate. They are rare and generally mild, although life-threatening cases and deaths have been reported. The effects on neonates are globally better known than those on fetuses. We summarize here both focusing on practical clinical aspects.
6.1. Fetal consequences of vertical SARS-CoV-2 infection
SARS-CoV-2 does not cause malformations but fetal demises have been described both early [74,75] and late during the pregnancy [76,77]. Owing to its rarity, we are not yet able to identify a critical period when fetal SARS-CoV-2 infection would be more dangerous. Cases of fetal demise have been described only in small case series and we cannot exclude the existence of a publication bias. In fact, by meta-analyzing the published reports, we recently demonstrated that there is no clear relationship between the severity of maternal COVID-19 and fetal demise, while a relevant proportion (34%) of fetuses died without sufficient evidence of an actual fetal infection [76]. This suggests that at least some cases of fetal demise could be due to SARS-CoV-2 placentitis causing fetal hypoxia and hypoperfusion, without the transplacental viral passage. A relevant number (66%) of fetuses, however, presented a certain evidence of viral infection in various organs and tissues and this may be an additional mortality cause [76]. Consistently, we found that stillbirths/late miscarriages occurred approximately 6–13 days after the confirmation of SARS-CoV-2 infection or the beginning of maternal symptoms [76].
This problem may have been underestimated for the same reasons described above (lack of complete virological and pathological investigations). To address this, we performed an analysis of our internal database at Paris Saclay University, and the incidence of miscarriages (between 14- and 24-weeks’ gestation) since the beginning of pandemic (that is, from March to October 2020, 3.4%) was like that observed between March and October in 2019 (3.8%) and 2018 (3.7%, p = 0.862, χ2-test). This suggests that fetal demises induced by SARS-CoV-2 infection might need other still unknown cofactors. With the diffusion of maternal vaccination, these events are likely to become even more uncommon [29] (of note, amongst the meta-analyzed cases, none of the mothers experiencing fetal demise was vaccinated [76]).
The transplacental transmission of SARS-CoV-2 is associated with fetal distress, acidosis with need for neonatal resuscitation and NICU admission: this seems to be caused by the placental insufficiency induced by the SARS-CoV-2 placentitis [49]. The placental damage might theoretically cause intrauterine growth restriction as observed for other viral placental infections [78]. However, solid data coming from several studies negate an association between SARS-CoV-2 infection and fetal growth restriction at a population level. Intra-uterine growth restriction following SARS-CoV-2 placentitis may however occur in some particular circumstances, as some cases have been anecdotally reported [79,80]: these might have occurred because of the co-existence of other factors, such us the timing of viral infection in relation to the delivery or the pre-existing placental function.
6.2. Neonatal consequences of vertical infection
SARS-CoV-2 vertical infection can cause neonatal COVID-19. Neonates infected by SARS-CoV-2 are asymptomatic in approximately half of cases and those with clinical manifestations are usually not very ill: this might be due to the lower alveolar expression of viral receptors in neonates compared to adults, while they seem to be preferentially expressed in the upper airways [[81], [82], [83]]. Despite their clinical mildness, neonates can be important virus spreaders, since they still represent a significant proportion of the unvaccinated population [84]. Severe and critical cases of neonatal COVID-19 were also anecdotally reported with clinical features similar to those of adult patients [18,85,86]. The risk for NICU care remains low and difficult to be quantified but it has been estimated at 2% of all neonatal infections [87]; the case fatality rate has been estimated at 0.1% [88]. This creates logistic and epidemiological consequences, since these patients must be isolated to protect the NICU population, and particularly the preterm infants. Isolation should be done in negative pressure rooms (of which not all NICUs are provided), whereas, it is important not to use NICU beds to isolate SARS-CoV-2 positive-neonates not needing critical care [89]. As these infants are more likely doing well, isolation would cause more harm than benefit and is important to avoid it since neonatal emergencies may always happen and catching COVID-19 during the pregnancy increases the odds of prematurity and the general need for NICU admission [90,91]. As NICU beds are a valuable and often insufficient resource, it is pivotal to keep them for those who really need them [89].
We do not know yet if neonatal COVID-19 is more severe following in utero or intrapartum infection and if clinical severity is different between vertical- and environmental-acquired neonatal infections. The EPICENTRE registry will soon provide data on this matter [92]. As we know, the viral strain may influence the severity of neonatal COVID-19 as seen in neonatal infections by omicron variant which, although less frequent [27], were more severe, as their rate of hospitalization is equal in infants aged less than 6 months and in adults beyond 65 years [93].
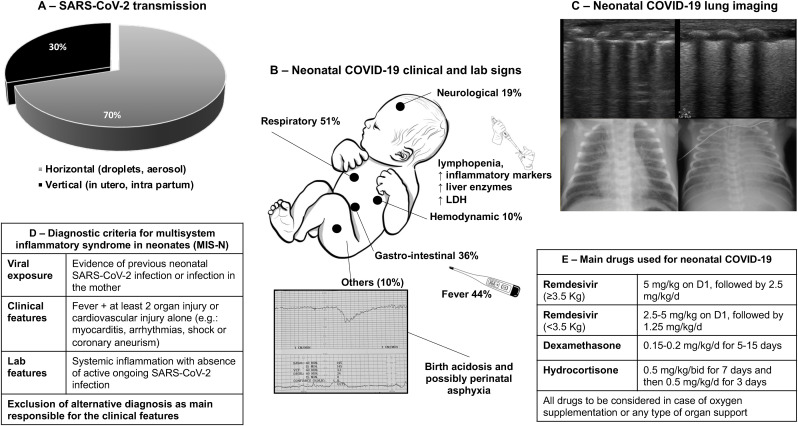
The distribution of neonatal COVID-19 signs is similar to that observed in adults, with a predominance of respiratory involvement but also neurological, cardiovascular and gastro-intestinal signs [18]. Beside neonatal COVID-19, neonatal inflammatory multi-system syndrome (MIS-N) can also occur after several weeks from SARS-CoV-2 infection, or in absence of neonatal infection, after a maternal one [94]. MIS-N is characterized by widespread inflammation leading to multiorgan injury and failure possibly linked to the presence of circulating anti-SARS-CoV-2 antibodies and autoantibodies against several antigens induced by the previous viral infection (in the neonate or the mother), while an active SARS-CoV-2 infection is lacking [95,96]. In some genetically susceptible patients, these antibodies would bind to several receptors causing hyperinflammatory state, macrophage activation and organ injury [97]. Neonatal COVID-19 and MIS-N can significantly share the clinical appearance and their difference is essentially represented by the presence or absence of an active SARS-CoV-2 infection. Fig. 5 summarizes the main clinical features of neonatal COVID-19, its laboratory and imaging abnormalities as well as the diagnostic criteria for MIS-N (see Fig. 5).
Fig. 5.
Main clinical features, laboratory and imaging abnormalities of neonatal COVID-19, as well as diagnostic criteria for MIS-N (adapted from Ref. [96]). Panel A shows the distribution of SARS-CoV-2 transmission routes: black and grey areas indicate vertical and horizontal transmission, respectively. Panel B reports the clinical and laboratory signs observed in neonates with clinically evident SARS-CoV-2 infection (at least one clinical sign develops in approximately 50% of neonatal infections; multiple signs are possible in a patient), as reported by Ref. [18]. Panel C illustrates lung ultrasound and conventional radiology findings in neonates with respiratory involvement: images on the left- and on the right-side show a typical interstitial and alveolar pattern, respectively: the interstitial is more common, but it may evolve in alveolar pattern; neonatal ARDS [106] has been diagnosed in the rare severest cases. Panel D reports the diagnostic criteria for multisystem inflammatory syndrome in neonates (MIS-N), as proposed by Ref. [94]. Panel E summarizes drug doses for severe/critical neonatal COVID-19 and MIS-N treatment. Remdesivir (VEKLURY®, Gilead, Foster City-CA, USA) has been approved by FDA and EMA for the treatment of neonates weighting at least 3 Kg; same case reports have described its use, at lower doses, also for smaller neonates [96]. Dexamethasone and hydrocortisone are the two steroids used in case series of neonatal COVID-19 and MIS-N have also been used together with intravenous immunoglobulins-G with classical dosing.
Some evidence suggests that prenatal exposure to SARS-CoV-2 may be associated with long-term neurodevelopmental sequelae in infants up to 12 months of age [98,99]. This does not seem related to the vertical transmission as the incidence of these sequelae is higher than that of feto-neonatal infection. As worrisome as it can be, this finding requires further studies to understand the cause-effect link and exclude any confounding effect. An in-depth discussion of long-term consequences of neonatal COVID-19 is presented in a different manuscript in this issue.
There are no dedicated criteria to classify the severity of neonatal COVID-19. By modifying the WHO criteria for the adult population, we advise to classify neonatal cases as follows [100].
-
•
Mild: only minor clinical signs (e.g.: conjunctivitis, runny nose, rash)
-
•
Moderate: fever, mild feeding difficulties, mild diarrhea
-
•
Severe: signs requiring hospitalization (e.g.: need for oxygen therapy, dehydration needing intravenous line, neurological signs needing monitoring)
-
•
Critical: respiratory, hemodynamic, kidney or liver failure needing vital support; seizure or coma.
Treatment of neonatal COVID-19 shall be based on supportive care, including temperature control with paracetamol (acetaminophen), upper airway cleansing and humidification, attentive hydration, and nutrition for mild-to-moderate cases. Breastfeeding should be encouraged whenever possible according to maternal and infant conditions. Moderate cases may require hospitalization for clinical observation, while severe-to-critical cases require vital function monitoring in NICU. This should be provided according to usual protocols and the best available evidence [[101], [102], [103]]. We advise to use negative pressure rooms and place a high-capacity HEPA filter on the expiratory limb of ventilatory circuits and other common measures to reduce nosocomial infections [104]. Clear protocols for personnel protection, isolation/deisolation and resource optimization should be available in each center [105].
Drugs that have been used to treat severe/critical neonatal COVID-19 are essentially remdesivir (VEKLURY®, Gilead, Foster City-CA, USA), which is FDA- and EMA-approved for neonates weighting at least 3 Kg, and steroids (Fig. 4). Remdesivir has also been anecdotally used, at lower dose, in smaller neonates [96]. It is strongly recommended to consult with an expert neonatal/pediatric intensivist and/or a pediatric infectious disease specialist to treat these cases, as this may require expertise in both advanced critical care and COVID-19.
Fig. 4.
Factors increasing (green) and decreasing (red) the likelihood of SARS-CoV-2 intra partum transmission. Factors have been listed according to the current available translational and clinical knowledge; see references in the text. The relative weight of each factor cannot be estimated.
Declaration of competing interest
None.
Practice points
-
•
Vertical transmission of SARS-CoV-2 happens in 2% of infected pregnant women and consist in either in utero or intrapartum transmission.
-
•
Specific WHO criteria should be followed for the diagnosis of SARS-CoV-2 vertical transmission, and these requires multiple tests.
-
•
Vertical acquired transmission represent 30% of all neonatal SARS-CoV-2 infections and most of the time they are silent or mild although severe consequences have been described.
-
•
Severe disease may result in fetal demise, neonatal COVID-19 and MIS-N. Neonates infected by SARS-CoV-2 require critical care in approximately 2% and the case fatality rate is approximately 0.1%.
-
•
Intravenous remdesivir and steroids can be used to treat severe/critical neonatal COVID-19 and MIS-N.
Research directions
-
•
The role of the type of transmission on clinical severity and outcomes.
-
•
Pathobiological mechanisms of MIS-N.
-
•
The effect of delivery route on the risk of viral transmission.
-
•
The long-term effect of maternal and neonatal SARS-CoV-2 infections.
Acknowledgements
This work received no funding.
Authors are grateful to their medical and paramedical team members who allowed thoroughly investigations on perinatal SARS-CoV-2 infections during the hardest moment of pandemics.
References
most relevant references are indicated by *
- 1.Hosier H., Farhadian S.F., Morotti R.A., et al. SARS–CoV-2 infection of the placenta. J Clin Invest. 2020;130:4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Algarroba G.N., Rekawek P., Vahanian S.A., et al. Visualization of SARS-CoV-2 virus invading the human placenta using electron microscopy. Am J Obstet Gynecol. 2020;223:275–278. doi: 10.1016/j.ajog.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vivanti A.J., Vauloup-Fellous C., Prevot S., et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11(1):3572. doi: 10.1038/s41467-020-17436-6. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. * https://www.who.int/publications/i/item/WHO-2019-nCoV-mother-to-child-transmission-2021.1. accessed on.
- 5.Chan M.Y., Smith M.A. Comprehensive toxicology. Elsevier; Amsterdam (Netherlands): 2018. Infections in pregnancy; pp. 232–249. [DOI] [Google Scholar]
- 6.Redline R.W. Inflammatory response in acute chorioamnionitis. Semin Fetal Neonatal Med. 2012;17:20–25. doi: 10.1016/j.siny.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Milbak J., Holten V.M.F., Axelsson P.B., et al. A prospective cohort study of confirmed severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection during pregnancy evaluating SARS‐CoV‐2 antibodies in maternal and umbilical cord blood and SARS‐CoV‐2 in vaginal swabs. Acta Obstet Gynecol Scand. 2021;100:2268–2277. doi: 10.1111/aogs.14274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenizia C., Saulle I., Di Giminiani M., et al. Unlikely SARS-CoV-2 transmission during vaginal delivery. Reprod Sci. 2021;28:2939–2941. doi: 10.1007/s43032-021-00681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barber E., Kovo M., Leytes S., et al. Evaluation of SARS-CoV-2 in the vaginal secretions of women with COVID-19: a prospective study. J Clin Med. 2021;10(12):2735. doi: 10.3390/jcm10122735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Picone O., Vivanti A., Sibiude J., et al. SARS-COV-2 excretion and maternal-fetal transmission: virological data of French prospective multi-center cohort study COVIPREG during the first wave. J Gynecol Obstet Human Reprod. 2023 doi: 10.1016/j.jogoh.2023.102547. Published online in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu D., Zhang Y., Chen D., et al. Evaluation of the presence of SARS-CoV-2 in vaginal and anal swabs of women with omicron variants of SARS-CoV-2 infection. Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.1035359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atarod Z., Zamaniyan M., Moosazadeh M., Valadan R., Soleimanirad S.M., Gordani N. Investigation of vaginal and rectal swabs of women infected with COVID-19 in two hospitals covered by Mazandaran University of Medical Sciences, 2020. J Obstet Gynaecol. 2022;42(6):2225–2229. doi: 10.1080/01443615.2022.2036966. [DOI] [PubMed] [Google Scholar]
- 13.Khoiwal K., Kalita D., Kumari R., et al. Presence of SARS‐COV ‐2 in the lower genital tract of women with active COVID ‐19 infection: a prospective study. Int J Gynaecol Obstet. 2022;157:744–747. doi: 10.1002/ijgo.14153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal M., Basumatary S., Bhusan D., Pati B.K. Detection of severe acute respiratory syndrome corona virus 2 in cervico-vaginal secretion of COVID-19-affected female: a prospective observational study from India. SAGE Open Med. 2021;9 doi: 10.1177/20503121211022993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Megli C.J., Coyne C.B. Infections at the maternal–fetal interface: an overview of pathogenesis and defence. Nat Rev Microbiol. 2022;20:67–82. doi: 10.1038/s41579-021-00610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centeno‐Tablante E., Medina‐Rivera M., Finkelstein J.L., et al. Transmission of SARS‐CoV‐2 through breast milk and breastfeeding: a living systematic review. Ann NY Acad Sci. 2021;1484:32–54. doi: 10.1111/nyas.14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray K.J., Bordt E.A., Atyeo C., et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225:303.e1–303.e17. doi: 10.1016/j.ajog.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raschetti R., Vivanti A.J., Vauloup-Fellous C., Loi B., Benachi A., De Luca D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-18982-9. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotlyar A.M., Grechukhina O., Chen A., et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224(1):35–53.e3. doi: 10.1016/j.ajog.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allotey J., Chatterjee S., Kew T., et al. SARS-CoV-2 positivity in offspring and timing of mother-to-child transmission: living systematic review and meta-analysis. BMJ. 2022 Mar 16;376 doi: 10.1136/bmj-2021-067696. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah P.S., Diambomba Y., Acharya G., Morris S.K., Bitnun A. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand. 2020;99:565–568. doi: 10.1111/aogs.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz D.A., Morotti D., Beigi B., Moshfegh F., Zafaranloo N., Patanè L. Confirming vertical fetal infection with COVID-19: neonatal and pathology criteria for early onset and transplacental transmission of SARS-CoV-2 from infected pregnant mothers. Arch Pathol Lab Med. 2020;144(12):1451–1456. doi: 10.5858/arpa.2020-0442-SA. [DOI] [PubMed] [Google Scholar]
- 23.Mosnino E., Bernardes L.S., Mattern J., et al. Impact of SARS-CoV-2 alpha and gamma variants among symptomatic pregnant women: a two-center retrospective cohort study between France and Brazil. J Clin Med. 2022;11:2663. doi: 10.3390/jcm11092663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donati S., Corsi E., Maraschini A., et al. SARS‐CoV‐2 infection among hospitalised pregnant women and impact of different viral strains on COVID‐19 severity in Italy: a national prospective population‐based cohort study. BJOG. 2022;129:221–231. doi: 10.1111/1471-0528.16980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eid J., Abdelwahab M., Caplan M., et al. Increasing oxygen requirements and disease severity in pregnant individuals with the SARS-CoV-2 Delta variant. Am J Obstet Gynecol MFM. 2022;4(3) doi: 10.1016/j.ajogmf.2022.100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adhikari E.H., MacDonald L., SoRelle J.A., Morse J., Pruszynski J., Spong C.Y. COVID-19 cases and disease severity in pregnancy and neonatal positivity associated with delta (B.1.617.2) and omicron (B.1.1.529) variant predominance. JAMA. 2022;327:1500–1502. doi: 10.1001/jama.2022.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kienast P., Prayer D., Binder J., et al. SARS-CoV-2 variant-related abnormalities detected by prenatal MRI: a prospective case–control study. Lancet Reg Health Eur. 2023 Jan 21 doi: 10.1016/j.lanepe.2023.100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlsen E.Ø., Magnus M.C., Oakley L., et al. Association of COVID-19 vaccination during pregnancy with incidence of SARS-CoV-2 infection in infants. JAMA Intern Med. 2022;182(8):825–831. doi: 10.1001/jamainternmed.2022.2442. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hui L., Marzan M.B., Rolnik D.L., et al. Reductions in stillbirths and preterm birth in COVID-19-vaccinated women: a multicenter cohort study of vaccination uptake and perinatal outcomes. Am J Obstet Gynecol. 2022;S0002–9378(22) doi: 10.1016/j.ajog.2022.10.040. * 00882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng S., Fan J., Yu F., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Järhult J.D., Hultström M., Bergqvist A., Frithiof R., Lipcsey M. The impact of viremia on organ failure, biomarkers and mortality in a Swedish cohort of critically ill COVID-19 patients. Sci Rep. 2021;11:7163. doi: 10.1038/s41598-021-86500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y., Schneider A.M., Mehta A., et al. SARS-CoV-2 viremia is associated with distinct proteomic pathways and predicts COVID-19 outcomes. J Clin Invest. 2021;131 doi: 10.1172/JCI148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gengler C., Dubruc E., Favre G., Greub G., Leval L de, Baud D. SARS-CoV-2 ACE-Receptor detection in the placenta throughout pregnancy. Clin Microbiol Infect. 2021;27(3):489–490. doi: 10.1016/j.cmi.2020.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taglauer E., Benarroch Y., Rop K., et al. Consistent localization of SARS-CoV-2 spike glycoprotein and ACE2 over TMPRSS2 predominance in placental villi of 15 COVID-19 positive maternal-fetal dyads. Placenta. 2020;100:69–74. doi: 10.1016/j.placenta.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komine-Aizawa S., Takada K., Hayakawa S. Placental barrier against COVID-19. Placenta. 2020;99:45–49. doi: 10.1016/j.placenta.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui D., Liu Y., Jiang X., et al. Single‐cell RNA expression profiling of SARS‐CoV‐2‐related ACE2 and TMPRSS2 in human trophectoderm and placenta. Ultrasound Obstet Gynecol. 2021;57:248–256. doi: 10.1002/uog.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashary N., Bhide A., Chakraborty P., et al. Single-cell RNA-seq identifies cell subsets in human placenta that highly expresses factors driving pathogenesis of SARS-CoV-2. Front Cell Dev Biol. 2020;8:783. doi: 10.3389/fcell.2020.00783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vento-Tormo R., Efremova M., Botting R.A., et al. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature. 2018;563(7731):347–353. doi: 10.1038/s41586-018-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li M., Chen L., Zhang J., Xiong C., Li X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beesley M., Davidson J., Panariello F., et al. COVID‐19 and vertical transmission: assessing the expression of ACE2/TMPRSS2 in the human fetus and placenta to assess the risk of SARS‐CoV‐2 infection. BJOG. 2022;129:256–266. doi: 10.1111/1471-0528.16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.HCA Lung Biological Network. Sungnak W., Huang N., et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shook L.L., Bordt E.A., Meinsohn M.C., et al. Placental expression of ACE2 and TMPRSS2 in maternal severe acute respiratory syndrome coronavirus 2 infection: are placental defenses mediated by fetal sex? J Infect Dis. 2021;224(Supplement_6):S647–S659. doi: 10.1093/infdis/jiab335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Facchetti F., Bugatti M., Drera E., et al. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of placenta. EBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz D.A., Baldewijns M., Benachi A., et al. Hofbauer cells and coronavirus disease 2019 (COVID-19) in pregnancy: molecular pathology analysis of villous macrophages, endothelial cells, and placental findings from 22 placentas infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with and without fetal transmission. Arch Pathol Lab Med. 2021;145:1328–1340. [Google Scholar]
- 46.Peacock T.P., Brown J.C., Zhou J., et al. The SARS-CoV-2 variant, omicron, shows rapid replication in human primary nasal epithelial cultures and efficiently uses the endosomal route of entry. bioRxiv. 2021;12(31) doi: 10.1101/2021.12.31.474653. [preprint] [DOI] [Google Scholar]
- 47.Willett B.J., Grove J., MacLean O.A., et al. SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway. Nat Microbiol. 2022;7:1161–1179. doi: 10.1038/s41564-022-01143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colson A., Depoix C.L., Dessilly G., et al. Clinical and in vitro evidence against placenta infection at term by severe acute respiratory syndrome coronavirus 2. Am J Pathol. 2021;191:1610–1623. doi: 10.1016/j.ajpath.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vivanti A.J., Vauloup-Fellous C., Escourrou G., et al. Factors associated with SARS-CoV-2 transplacental transmission. Am J Obstet Gynecol. 2022;227:541–543.e11. doi: 10.1016/j.ajog.2022.05.015. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delorme-Axford E., Sadovsky Y., Coyne C.B. The placenta as a barrier to viral infections. Annu Rev Virol. 2014;1:133–146. doi: 10.1146/annurev-virology-031413-085524. [DOI] [PubMed] [Google Scholar]
- 51.Sharps M.C., Hayes D.J.L., Lee S., et al. A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection. Placenta. 2020;101:13–29. doi: 10.1016/j.placenta.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patanè L., Morotti D., Giunta M.R., et al. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019–positive mothers and neonates at birth. Am J Obstet Gynecol MFM. 2020;2(3) doi: 10.1016/j.ajogmf.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh S., Dellibovi-Ragheb T.A., Kerviel A., et al. β-Coronaviruses use lysosomes for egress instead of the biosynthetic secretory pathway. Cell. 2020;183(6):1520–1535.e14. doi: 10.1016/j.cell.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robbins J.R., Bakardjiev A.I. Pathogens and the placental fortress. Curr Opin Microbiol. 2012;15:36–43. doi: 10.1016/j.mib.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Narang K., Miller M., Trinidad C., et al. Impact of asymptomatic and mild COVID-19 infection on fetal growth during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2023;281:63–67. doi: 10.1016/j.ejogrb.2022.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smithgall M.C., Murphy E.A., Rand S., et al. Placental pathology, neonatal birth weight, and Apgar score in acute and distant SARS-CoV-2 infection. J Clin Transl Res. 2022;8:351–359. [PMC free article] [PubMed] [Google Scholar]
- 58.Schwartz D.A., Avvad-Portari E., Babál P., et al. Placental tissue destruction and insufficiency from COVID-19 causes stillbirth and neonatal death from hypoxic-ischemic injury. Arch Pathol Lab Med. 2022;146:660–676. doi: 10.5858/arpa.2022-0029-SA. * [DOI] [PubMed] [Google Scholar]
- 59.Narang K., Enninga E.A.L., Gunaratne M.D.S.K., et al. SARS-CoV-2 infection and COVID-19 during pregnancy: a multidisciplinary review. Mayo Clin Proc. 2020;95:1750–1765. doi: 10.1016/j.mayocp.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li D., Jin M., Bao P., Zhao W., Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8292. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cevik M., Tate M., Lloyd O., Maraolo A.E., Schafers J., Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2(1):e13–e22. doi: 10.1016/S2666-5247(20)30172-5. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferrazzi E., Frigerio L., Savasi V., et al. Vaginal delivery in SARS‐CoV‐2‐infected pregnant women in Northern Italy: a retrospective analysis. BJOG. 2020;127:1116–1121. doi: 10.1111/1471-0528.16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hascoët J.M., Jellimann J.M., Hartard C., et al. Case series of COVID-19 asymptomatic newborns with possible intrapartum transmission of SARS-CoV-2. Front Pediatr. 2020;8 doi: 10.3389/fped.2020.568979. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carosso A., Cosma S., Serafini P., Benedetto C., Mahmood T. How to reduce the potential risk of vertical transmission of SARS-CoV-2 during vaginal delivery? Eur J Obstet Gynecol Reprod Biol. 2020;250:246–249. doi: 10.1016/j.ejogrb.2020.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.American College of Obstetrics and Gynecology Committee opinion No. 797: prevention of group B streptococcal early-onset disease in newborns: correction. Obstet Gynecol. 2020;135:978–979. doi: 10.1097/AOG.0000000000003824. [DOI] [PubMed] [Google Scholar]
- 66.Wang J., Dong W. COVID-19: the possibility, ways, mechanisms, and interruptions of mother-to-child transmission. Arch Gynecol Obstet. 2022 Jun 4:1–10. doi: 10.1007/s00404-022-06639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang Z., Liu Y. Vertical transmission of severe acute respiratory syndrome coronavirus 2: a systematic review. Am J Perinatol. 2020;37:1055–1060. doi: 10.1055/s-0040-1712161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Juan J., Gil M.M., Rong Z., Zhang Y., Yang H., Poon L.C. Effect of coronavirus disease 2019 (COVID‐19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. 2020;56:15–27. doi: 10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lopian M., Kashani-Ligumsky L., Czeiger S., et al. Safety of vaginal delivery in women infected with COVID-19. Pediatr Neonatol. 2021;62:90–96. doi: 10.1016/j.pedneo.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rottenstreich A., Tsur A., Braverman N., et al. Vaginal delivery in SARS-CoV-2-infected pregnant women in Israel: a multicenter prospective analysis. Arch Gynecol Obstet. 2021;303:1401–1405. doi: 10.1007/s00404-020-05854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vivanti A.J., De Luca D., Benachi A. Cesarean delivery and neonatal SARS-CoV-2 infections: beware of hasty shortcuts. Am J Obstet Gynecol. 2022;S0002–9378(22):824–829. doi: 10.1016/j.ajog.2022.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Angelidou A., Sullivan K., Melvin P.R., et al. Association of maternal perinatal SARS-CoV-2 infection with neonatal outcomes during the COVID-19 pandemic in Massachusetts. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Macfarlane P. RSV testing in bronchiolitis: which nasal sampling method is best? Arch Dis Child. 2005;90:634–635. doi: 10.1136/adc.2004.065144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shende P., Gaikwad P., Gandhewar M., et al. Persistence of SARS-CoV-2 in the first trimester placenta leading to transplacental transmission and fetal demise from an asymptomatic mother. Hum Reprod. 2021;36:899–906. doi: 10.1093/humrep/deaa367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baud D., Greub G., Favre G., et al. Second-Trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA. 2020;323:2198. doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alcover N., Regiroli G., Benachi A., Vauloup-fellous C., Vivanti A.J., De luca D. Systematic review and synthesis of stillbirths and late miscarriages following SARS-CoV-2 infections. Am J Obstet Gynecol. 2023 Jan 24 doi: 10.1016/j.ajog.2023.01.019. * S0002-9378(23)00026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Contro E., deSouza R., Bhide A. Chronic intervillositis of the placenta: a systematic review. Placenta. 2010;31:1106–1110. doi: 10.1016/j.placenta.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 79.Moltner S., de Vrijer B., Banner H. Placental infarction and intrauterine growth restriction following SARS-CoV-2 infection. Arch Gynecol Obstet. 2021;304:1621–1622. doi: 10.1007/s00404-021-06176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bouachba A., Allias F., Nadaud B., et al. Placental lesions and SARS-Cov-2 infection: diffuse placenta damage associated to poor fetal outcome. Placenta. 2021;112:97–104. doi: 10.1016/j.placenta.2021.07.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao D., Chen X., Han D., Zhong J., Zhang S., Yang C. Pulmonary ACE2 expression in neonatal and adult rats. FEBS Open Bio. 2021;11:2266–2272. doi: 10.1002/2211-5463.13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bunyavanich S., Do A., Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323:2427. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heinonen S., Helve O., Andersson S., Janér C., Süvari L., Kaskinen A. Nasal expression of SARS-CoV-2 entry receptors in newborns. Arch Dis Child Fetal Neonatal Ed. 2022;107:95–97. doi: 10.1136/archdischild-2020-321334. [DOI] [PubMed] [Google Scholar]
- 84.Paul L.A., Daneman N., Schwartz K.L., et al. Association of age and pediatric household transmission of SARS-CoV-2 infection. JAMA Pediatr. 2021;175:1151. doi: 10.1001/jamapediatrics.2021.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marsico C., Capretti M.G., Aceti A., et al. Severe neonatal COVID‐19: challenges in management and therapeutic approach. J Med Virol. 2022;94:1701–1706. doi: 10.1002/jmv.27472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frauenfelder C., Brierley J., Whittaker E., Perucca G., Bamford A. Infant with SARS-CoV-2 infection causing severe lung disease treated with remdesivir. Pediatrics. 2020;146(3) doi: 10.1542/peds.2020-1701. [DOI] [PubMed] [Google Scholar]
- 87.Di Toro F., Gjoka M., Di Lorenzo G., et al. Impact of COVID-19 on maternal and neonatal outcomes: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:36–46. doi: 10.1016/j.cmi.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Di Nardo M., van Leeuwen G., Loreti A., et al. A literature review of 2019 novel coronavirus (SARS-CoV2) infection in neonates and children. Pediatr Res. 2021;89:1101–1108. doi: 10.1038/s41390-020-1065-5. [DOI] [PubMed] [Google Scholar]
- 89.De Luca D. Managing neonates with respiratory failure due to SARS-CoV-2. Lancet Child Adolesc Health. 2020;4(4):e8. doi: 10.1016/S2352-4642(20)30073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Badr D.A., Picone O., Bevilacqua E., et al. Severe acute respiratory syndrome coronavirus 2 and pregnancy outcomes according to gestational age at time of infection. Emerg Infect Dis. 2021;27:2535–2543. doi: 10.3201/eid2710.211394. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Metz T.D., Clifton R.G., Hughes B.L., et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2021;137:571–580. doi: 10.1097/AOG.0000000000004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Luca D., Rava L., Nadel S., et al. The EPICENTRE (ESPNIC Covid pEdiatric Neonatal Registry) initiative: background and protocol for the international SARS-CoV-2 infections registry. Eur J Pediatr. 2020;179:1271–1278. doi: 10.1007/s00431-020-03690-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hamid S., Woodworth K., Pham H., et al. COVID-19–Associated hospitalizations among U.S. Infants aged <6 Months — COVID-NET, 13 states, june 2021–August 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1442–1448. doi: 10.15585/mmwr.mm7145a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.More K., Aiyer S., Goti A., et al. Multisystem inflammatory syndrome in neonates (MIS-N) associated with SARS-CoV2 infection: a case series. Eur J Pediatr. 2022;181:1883–1898. doi: 10.1007/s00431-022-04377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Consiglio C.R., Cotugno N., Sardh F., et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183:968–981.e7. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Luca D., Vauloup-Fellous C., Benachi A., Masturzo B., Manzoni P., Vivanti A. The essentials about neonatal severe acute respiratory syndrome coronavirus 2 infection and coronavirus disease: a narrative review. Am J Perinatol. 2022;39(S 01):S18–S22. doi: 10.1055/s-0042-1758487. * [DOI] [PubMed] [Google Scholar]
- 97.Nakra N., Blumberg D., Herrera-Guerra A., Lakshminrusimha S. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children. 2020;7:69. doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aldrete-Cortez V., Bobadilla L., Tafoya S.A., et al. Infants prenatally exposed to SARS-CoV-2 show the absence of fidgety movements and are at higher risk for neurological disorders: a comparative study. PLoS One. 2022;17(5) doi: 10.1371/journal.pone.0267575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Edlow A.G., Castro V.M., Shook L.L., Kaimal A.J., Perlis R.H. Neurodevelopmental outcomes at 1 Year in infants of mothers who tested positive for SARS-CoV-2 during pregnancy. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.15787. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marshall J.C., Murthy S., Diaz J., et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kneyber M.C.J., de Luca D., Calderini E., Jarreau P.H., Javouhey E., Lopez-Herce J., et al. On behalf of the section respiratory failure of the European society for paediatric and neonatal intensive care. Recommendations for mechanical ventilation of critically ill children from the paediatric mechanical ventilation consensus conference (PEMVECC) Intensive Care Med. 2017;43:1764–1780. doi: 10.1007/s00134-017-4920-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weiss S.L., Peters M.J., Alhazzani W., et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020;46:10–67. doi: 10.1007/s00134-019-05878-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morin L., Ray S., Wilson C., Remy S., Benissa M.R., Jansen N.J.G., et al. On behalf of the ESPNIC refractory septic shock definition taskforce. Refractory septic shock in children: a European society of paediatric and neonatal intensive care definition. Intensive Care Med. 2016;42:1948–1957. doi: 10.1007/s00134-016-4574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Manzoni P., De Luca D., Stronati M., et al. Prevention of nosocomial infections in neonatal intensive care units. Am J Perinatol. 2013;30:81–88. doi: 10.1055/s-0032-1333131. [DOI] [PubMed] [Google Scholar]
- 105.Chidini G., Villa C., Calderini E., Marchisio P., De Luca D. SARS-CoV-2 infection in a pediatric department in milan: a logistic rather than a clinical emergency. Pediatr Infect Dis J. 2020;39:e79–e80. doi: 10.1097/INF.0000000000002687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.De Luca D., van Kaam A.H., Tingay D.G., et al. The Montreux definition of neonatal ARDS: biological and clinical background behind the description of a new entity. Lancet Respir Med. 2017;5:657–666. doi: 10.1016/S2213-2600(17)30214-X. [DOI] [PubMed] [Google Scholar]