Abstract
Background
Post-COVID syndrome (PCS) is defined by symptom persistence accompanied by daily life impairment (DLI). The association of somatic symptom disorder (SSD) and symptoms with DLI after SARS-CoV-2 infection in the general population is unclear to date. The main objective of the study was to investigate the association of possible SSD, depression, anxiety, and participant-reported symptoms with DLI in a local population sample.
Methods
Anonymised cross-sectional study. A symptom questionnaire, including the scales Patient Health Questionnaire PHQ-15 (somatisation module), SSD-12 (psychological distress in SSD), PHQ-2 (depression), GAD-2 (anxiety), and FAS (fatigue assessment scale) was sent in 02/2022 to all adult residents of the district Bad Tölz-Wolfratshausen, Germany, who were registered for SARS-CoV-2-infection between 03/2020 and 11/2021 (8925 delivered). Associations between DLI, symptoms and scales were estimated using binary logistic regression models and network analysis.
Results
2828 questionnaires (31.7%) were complete. 1486 (52.5%) reported persistent symptoms, and 509 (18.0%) perceived DLI. DLI was strongest associated with self-reported fatigue (OR 7.86; 95%CI 5.63–10.97), dyspnea (3.93; 2.73–5.67), impaired concentration (3.05; 2.17–4.30), SSD-12 (4.36; 2.57–7.41), and PHQ-2 (2.48; 1.57–3.92). Self-reported fatigue showed the strongest correlation (rp = 0.248) and closest proximity to DLI in network analysis.
Conclusion
PCS appears as a complex clinical picture in which SSD might play an important role when DLI is present. The pychological burden might partly be explained by the persistent symptoms, which are difficult to treat up to now. Screening for SSD could help in differential diagnostic decision-making to ensure that patients receive appropriate psychosocial interventions for disease coping.
Keywords: SARS-CoV-2, Post-Covid syndrome, Somatic symptom disorder, Fatigue, Dyspnea, Concentration impairment
1. Introduction
The occurrence of SARS-CoV-2 resulted in a viral pandemic with significant health impacts on affected people and increased mortality, which also led to a concomitant global crisis. Although primarily a respiratory infection, COVID-19 appears to be a systemic disease in which almost all organ systems can be affected [1]. The course of COVID-19 infection is usually rather mild, especially at younger ages. However, the risk for hospitalization and mortality is rising with increasing age and concomitant comorbidity [2]. After overcoming the disease, a large proportion of those affected have recovered, but a considerable amount of patients suffer from persistent symptoms after the infection, often accompanied by daily life impairment (DLI). Accordingly, Long-COVID is defined as persistent symptoms more than four weeks after acute infection; and Post-COVID syndrome (PCS) is defined as symptom persistence of more than three months or first appearance after three months, with impact on everyday functioning [3,4]. Recent cohort studies reported varying prevalence numbers of PCS, ranging from 5% to 35% [5,6], depending in part on the type of PCS definition and sample population.
The most common PCS symptoms include fatigue/exhaustion, impaired concentration (“brain fog”), dyspnea, muscle pain, persistent olfactory and gustatory disturbances [[5], [6], [7], [8], [9], [10]]. Peter et al. found within their cohort study that the symptom groups fatigue and neurocognitive impairment contributed most to reduced health-related recovery and work ability, but that other symptoms such as anxiety, depression, and headache were also important [5]. On the one hand, specific single symptoms can be explained as organ-related dysfunction, such as dyspnea in the context of pulmonary residuals of COVID-19 infection [9]. On the other hand, the causes of many symptoms and their impact on daily life impairment are currently not well understood. Psychological mechanisms are therefore increasingly being considered for a better understanding of Long-COVID or PCS, respectively [11].
In this context, the question arises of an additionally existing somatic symptom disorder (SSD), i.e. a combination of suffering from physical complaints, regardless of their origin, with psychobehavioural characteristics of an excessive preoccupation with one's own physical symptoms [12]. Particularly regarding chronic fatigue, the origin of the symptoms is sometimes considered as a functional somatic syndrome [[13], [14], [15]]. Studies with highly selected patients from tertiary university hospital settings found clues to a high proportion of SSD in COVID-19 patients with neurological symptoms [16,17]. Increased somatisation scores were found in a small cohort study comparing 164 SARS-CoV-2 infected patients with 183 test-negative control subjects recruited in a university hospital testing laboratory [18]. Beyond that, several studies have described an association between PCS and anxiey, depression, and psychiatric disorders [[19], [20], [21]]. In-depth knowledge about the contribution of SSD to DLI could be important to support early identification of patients at risk, facilitate diagnostic decision-making and develop appropriate therapeutic support. However, the association of SSD and symptoms with DLI after SARS-CoV-2 infection in the general population is unclear to date. To address this knowledge gap, the main objective of the study was to investigate the association between possible SSD, depression, anxiety and participant-reported symptoms with DLI in a local population sample.
2. Methods
2.1. Study participants
The study was conducted as a cross-sectional survey. On February 07, 2022, an anonymous self-report questionnaire was sent by the local health authority of the Bavarian district Bad-Tölz/Wolfratshausen (total population = 128,212, as of December 2020) to all residents who tested positive for SARS-CoV-2 by polymerase chain reaction (PCR) test between March 1, 2020 and November 30, 2021. Exclusion criteria were patients under 18 years of age. The study was approved by the Ethics Committee of the Medical Faculty of the Technical University Munich.
2.2. Data collection and questionnaires
The questionnaire included psychometric questionnaires and a list of the most common PCS symptoms according to the German COVID-19 guideline [3]. Symptoms were surveyed that were present at the time of data collection and that were subjectively attributed by the participants to a SARS-CoV-2 infection. Accordingly, participants were asked ´Are you currently suffering from the symptoms listed below that you attribute to infection with the Corona virus?´. The time of infection was also queried, allowing calculation of symptom duration in months. Beyond that, age, sex, and BMI were inquired. Participants were asked to what extent they experienced DLI due to SARS-CoV-2 infection. The response options were ‘no limitation’, ‘persistent sick leave’, ‘retired due to COVID-19 infection’, ‘I cannot do my everyday tasks as well as before the infection (e.g. shopping, household)’, ‘I cannot be as active in my leisure activities and hobbies as before the infection’. Multiple answers could be given. Finally, participants were asked whether they were currently being treated in general or specialist practice in outpatient care or in a specialised COVID-19 outpatient clinic.
The Patient Health Questionnaire-15 (PHQ-15) assesses the presence and severity of common somatic symptoms (SSD A criterion) within the past 4 weeks using 15 items [22]. The Somatic Symptom Disorder - B Criteria Scale (SSD-12) is a reliable and valid questionnaire consisting of 12 items to assess the B criteria of somatoform disorders according to DSM-5 (psychological symptom burden in the context of somatic symptoms irrespective of their origin). Each of the three psychological subcriteria of SSD (cognitive, affective, and behavioral) is measured with four items, with all item scores ranging from 0 (never) to 4 (very frequently). The scores are summed to obtain a simple total score (which can vary between 0 and 48 points) [23]. The combination of PHQ-15 and SSD-12 effectively identifies individuals at risk for SSD. The configuration of PHQ ≥ 9 points and SSD ≥ 23 points was used for this purpose [24].
The Patient Health Questionnaire-4 (PHQ-4) is a two-dimensional measurement instrument for assessing depressiveness (PHQ-2) and anxiety (GAD-2) [25]. The PHQ-2 consists of two DSM-IV core diagnostic criteria for depressive disorders. The GAD-2 consists of the two core criteria for generalized anxiety disorder. The response options are “not at all,” “some days,” “more than half the days,” and “almost every day,” scored as 0, 1, 2, and 3, respectively. Scale scores of ≥3 have been recommended as cut-off values for the PHQ-2 and GAD-2 [26,27].
The Fatigue Assessment Scale (FAS) is a 10-item unidimensional questionnaire designed to assess the severity of fatigue. Five questions relate to physical fatigue and 5 questions (questions 3 and 6–9) relate to mental fatigue. Responses are mapped on a 5-point scale (from “1” = never to “5” = always); the sum score thus be 10–50 points. Scores of 10–21 indicate no fatigue, scores of 22–50 indicate mild-to-moderate fatigue, scores ≥35 indicate extreme fatigue [28,29].
2.3. Statistical analysis
DLI was dichotomized into ‘at least one impairment present’ (DLI group) versus ‘no impairment present’ (non-DLI group). Group differences regarding participants´ characteristics and psychometric scales were calculated using descriptive statistics and tested for statistical significance using Mann-Whitney U test and chi-square test, respectively. In addition, to assess possible differences in disease burden between symptom persistence and DLI, we compared patients with persistent symptoms with those without symptoms.
Univariate and multiple binary logistic regression models were used to estimate odds ratios (OR) of the participants' symptoms, psychometric questionnaire categorical scores (PHQ-15, SSD-12, PHQ-2, GAD-2), age, sex, body mass index (BMI), and time since infection as independent variables with respect to DLI as dependent variable. Collinearity of the independent variables was measured by variance inflation factors (VIF) [30,31]. In addition, stepwise backward variable selection based on Akaike's information criterion (AIC) was carried out to determine the most informative independent variables. Hypothesis tests were performed at exploratory two-sided 5% significance levels, and corresponding 95% confidence intervals (95% CI) are generated for effect sizes of interest.
In an additional analysis, pairwise partial correlations (rp) were computed to evaluate the independent relations between DLI, participants´ symptoms and the psychometric variables [32]. Variables with at least one correlation >0.2 were included in a network analysis, which was displayed in the two-dimensional space using Fruchterman-Reingold force-directed placement [33]. The variables of the network were clustered by optimizing weighted modularity, that is by defining clusters optimizing the tradeoff between (strong) within-cluster correlations and (weak) between-cluster correlations [34].
Ten-fold cross-validation stratified by DLI was used to quantify and compare the prognostic accuracy of a binary logistic regression model including the symptoms as independent variables to a respective model additionally including SSD-12. Receiver Operating Characteristics (ROC) curve analysis was performed to calculate the area under the curve (AUC) as performance measure [30,31]. Data entry and analysis were performed using SPSS 26.0 [IBM Corp., Armonk, NY] and R 4.0.3 [The R Foundation for Statistical Computing, Vienna, Austria] using the add-on packages corpcor [35] and igraph [36].
3. Results
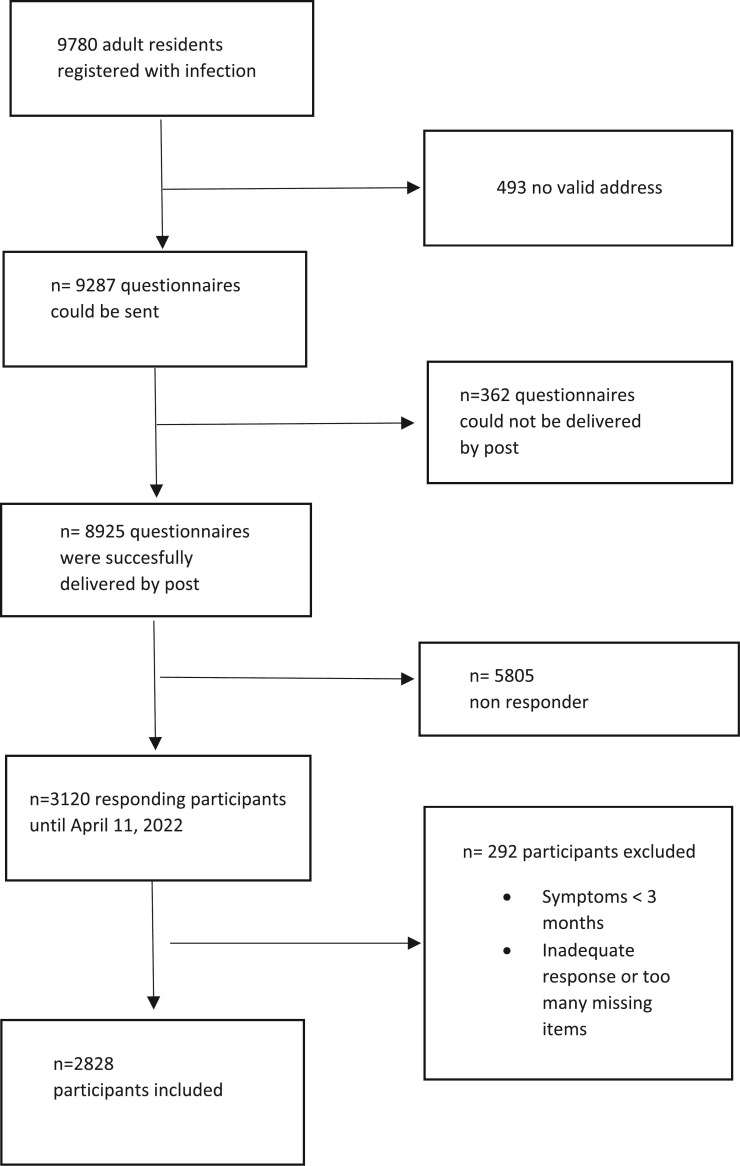
From March 1, 2020 to November 30, 2021, a total of 9780 adult residents of the district with SARS-CoV-2 infection were registered at the Bad Tölz Health Department; the mean age was 45.6 (standard deviation 18.5) years, and 4996 (51.1%) were female. 9287 questionnaires could be sent. 8925 questionnaires were successfully delivered in February 2022; 3120 (35.0%) questionnaires were received; 2828 (31.7%) questionaires were completely evaluable (Fig. 1 ). The mean age of participants was 47.3 (standard deviation 17.1) years, and 1523 (53.8%) were female. 1486 (52.5%) reported persistent symptoms for more than three months, and 509 (18.0%) perceived daily life impairment (DLI) (Table 1 ). The most frequently reported symptoms were fatigue (24.4%), changes in the sense of smell (20.5%) and taste (14.7%), impaired concentration (16.5%) and memory problems (15.2%). In patients with persistent symptoms and DLI, the most frequently reported symptoms were fatigue (79.5%), impaired concentration (53.2%), memory problems (47.6%), and dyspnea (47.8%). 11 participants without symptoms reported DLI, mainly because the symptom items did not match their complaint pattern. Within the group of symptomatic participants, 1183/1486 (79.6%) reported having remained without medical treatment. 116 (7.8%) were treated by a general practitioner only, 58 (3.9%) were treated by a specialist in outpatient care only, 93 (6.3%) were treated by a general practitioner and a specialist, and 9 (0.6%) were treated in a dedicated Post-COVID outpatient clinic (data not in table).
Fig. 1.
Flow chart of study.
Table 1.
Symptoms and questionnaires for somatic symptoms disorder, depression, anxiety, fatigue per group.
| All (N = 2828) |
PS+/DLI+ (N = 498) |
PS-/DLI+ (N = 11) |
PS+/DLI- (N = 988) |
PS-/DLI- (N = 1331) |
|
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Baseline characteristics | |||||
| Female (42 missing) | 1523 (53.8) | 275 (56.1) | 4 (36.4) | 567 (58.4) | 677 (51.5) |
| Age in years (12 missing) [mean ± sd)] | 47.3 ± 17.1 | 53.6 ± 16.6 | 50.9 ± 17.6 | 47.0 ± 17.1 | 45.0 ± 16.6 |
| BMI (44 missing) [mean ± sd)] | 25.7 ± 4.8 | 27.1 ± 5.1 | 29.3 ± 12.2 | 25.7 ± 4.8 | 25.5 ± 4.5 |
| Months since infection (0 missing) [mean ± sd)] | 9.2 ± 6.0 | 11.2 ± 6.4 | 10.4 ± 7.0 | 9.2 ± 5.9 | 8.4 ± 5.6 |
| Daily life impairment (0 missing)⁎ | 509 (18.0) | 498 (100) | 11 (100) | 0 | 0 |
| - pension due to COVID-19 | 4 (0.1) | 4 (0.8) | 0 | 0 | 0 |
| - sick leave | 38 (1.3) | 37 (7.4) | 1 (9.1) | 0 | 0 |
| - daily activities | 255 (9.0) | 250 (50.2) | 5 (45.5) | 0 | 0 |
| - leisure time only | 212 (7.5) | 207 (41.5) | 5 (45.5) | 0 | 0 |
| Self-reported symptoms (0 missing)⁎ | 1486 (52.5) | 498 (100) | 11 (0) | 988 (100) | 0 |
| Fatigue | 691 (24.4) | 396 (79.5) | 0 | 295 (29.9) | 0 |
| Distorted sense of smell | 579 (20.5) | 133 (33.3) | 0 | 413 (41.8) | 0 |
| Impaired concentration | 466 (16.5) | 265 (53.2) | 0 | 201 (20.3) | 0 |
| Distorted sense of taste | 415 (14.7) | 151 (30.3) | 0 | 300 (30.4) | 0 |
| Problems with memory | 429 (15.2) | 237 (47.6) | 0 | 192 (19.4) | 0 |
| Sleep disturbance | 406 (14.4) | 221 (44.4) | 0 | 185 (18.7) | 0 |
| Headache | 361 (12.8) | 173 (34.7) | 0 | 188 (19.0) | 0 |
| Muscular pain | 333 (11.8) | 208 (41.8) | 0 | 147 (14.9) | 0 |
| Dyspnea | 365 (12.9) | 238 (47.8) | 0 | 127 (12.9) | 0 |
| Cough | 282 (10.0) | 127 (25.5) | 0 | 155 (15.7) | 0 |
| Vertigo | 271 (9.6) | 160 (32.1) | 0 | 111 (11.2) | 0 |
| Palpitations | 266 (9.4) | 156 (31.3) | 0 | 110 (11.1) | 0 |
| Chest pain | 192 (6.8) | 115 (23.1) | 0 | 77 (7.8) | 0 |
| Depression | 159 (5.6) | 109 (24.7) | 0 | 50 (5.4) | 0 |
| Anxiety | 150 (5.3) | 100 (22.1) | 0 | 50 (5.4) | 0 |
| Tinnitus | 139 (4.9) | 67 (13.5) | 0 | 72 (7.3) | 0 |
| Loss of appetite | 133 (4.7) | 67 (13.5) | 0 | 66 (6.7) | 0 |
| Weight loss | 128 (4.5) | 67 (13.5) | 0 | 61 (6.2) | 0 |
| Skin rashes | 90 (3.2) | 44 (8.8) | 0 | 46 (4.7) | 0 |
| Questionnaires | |||||
| PHQ-15 ≥ 9 (114 missing) | 416 (14.7) | 251 (56.0) | 0 | 134 (14.2%) | 31 (2.3%) |
| SSD-12 ≥ 23 (64 missing) | 210 (7.4) | 152 (32.0) | 0 | 37 (3.8%) | 21 (1.6%) |
| PHQ-15 ≥ 9 and SSD-12 ≥ 23 (152 missing) | 136 (4.8) | 113 (25.6) | 0 | 20 (2.1%) | 3 (0.2%) |
| PHQ-2 ≥ 3 (65 missing) | 271 (9.6) | 158 (33.0) | 0 | 70 (7.2%) | 43 (3.2%) |
| GAD-2 ≥ 3 (65 missing) | 216 (7.6) | 124 (25.8) | 0 | 63 (6.5%) | 29 (2.2%) |
| FAS = 22 to 34 (47 missing) FAS ≥ 35 |
674 (23.8) 136 (4.8) |
267 (55.7) 108 (22.5) |
4 (36.4%) 0 |
273 (28.1%) 24 (2.5%) |
130 (9.9%) 4 (0.3%) |
PS-/DLI- Participants without current, persistent symptoms and without daily life impairment.
PS-/DLI + Participants without current, persistent symptoms but with daily life impairment.
PS+/DLI- Paticipants with current, persistent symptoms but without daily life impairment.
PS+/DLI + Paticipants with current, persistent symptoms and daily life impairment.
PHQ-15, Patient Health Questionnaire-15; SSD-12, Somatic Symptom Disorder-B Criteria Scale; PHQ-2, Patient Health Questionnaire-2, GAD-2, Generalized Anxiety Disorder-2 questionnaire; FAS, Fatigue Assessment Scale.
multiple answer options possible.
416 (14.7%) participants showed PHQ-15 sum scores ≥9, and 210 (7.4%) SSD-12 sum scores ≥23, and 136 (4.8%) scored above cut-off values in both questionnaires (Table 1). 271 (9.6%) participants showed PHQ-2 ≥ 3, and 216 (7.6%) GAD-2 ≥ 3. Mild-to-moderate and severe fatigue according to the FAS occured in 674 (23.8%) and 136 (4.8%) participants, respectively. The proportion of participants scoring above critical cut-values for PHQ-15, SSD-12, PHQ-2 and GAD-2 ranged between 25.8% and 56.0% among participants with both persistent symptoms and DLI, between 2.1% to 14.2% among those with persistent symptoms but without DLI, and between 0.2% and 3.2% among participants without persistent symptoms and also without DLI. Regarding the Fatigue Assessment Scale, it was noticeable that patients with persistent symptoms and DLI scored in 78.2% above the critical cut-value of the FAS questionnaire.
The univariate regression model showed strikingly high unconditional ORs for prediction of DLI, whereas the conditional ORs of the multiple regression model were significantly reduced (Table 2 ). There was a moderate collinearity among the independent variables with respective VIF ranging from 1.07 to 2.19. The regression analysis with stepwise backward variable selection is depicted in Table 3 . It showed that DLI was strongest associated with self-reported fatigue (OR 7.86; 95%CI 5.63–10.97), dyspnea (3.93; 2.73–5.67), impaired concentation (3.05; 2.17–4.30), SSD-12 (4.36; 2.57–7.41), and PHQ-2 (2.48; 1.57–3.92). PHQ-15, FAS, GAD-2, self-rated depression, and self-rated anxiety were excluded by variable selection.
Table 2.
Predictive odds ratios for daily life impairment (2828 completers); univariate model and multiple model (inclusion model).
| Univariate Model |
Multiple Model |
|||
|---|---|---|---|---|
| OR (95%CI) | p-value | OR (95%CI) | p-value | |
| SSD-12 | 17.39 (12.59–24.02) | <0.001 | 2.38 (1.26–4.49) | 0.008 |
| PHQ-15 | 15.29 (11.99–19.48) | <0.001 | 1.05 (0.65–1.72) | 0.832 |
| Fatigue (FAS) mild – moderate * | 11.27 (8.81–14.41) | <0.001 | 3.31 (2.21–4.95) | <0.001 |
| Fatigue (FAS) severe * | 64.62 (40.90–102.14) | <0.001 | 5.12 (2.11–12.42) | <0.001 |
| Depression (PHQ-2) | 9.16 (7.01–11.97) | <0.001 | 1.74 (0.97–3.12) | 0.062 |
| Anxiety (GAD-2) | 8.04 (6.00–10.76) | <0.001 | 0.80 (0.43–1.63) | 0.594 |
| Fatigue | 28.87 (22.29–37.39) | <0.001 | 5.98 (4.04–8.85) | <0.001 |
| Dyspnea | 17.00 (13.19–21.92) | <0.001 | 3.49 (2.25–5.44) | <0.001 |
| Concentration disturbance | 13.10 (10.38–16.53) | <0.001 | 1.91 (1.18–3.11) | 0.009 |
| Memory distorbance | 10.66 (8.45–13.45) | <0.001 | 1.42 (0.87–2.31) | 0.164 |
| Sleep disorder | 9.76 (7.72–12.34) | <0.001 | 1.89 (1.23–2.90) | 0.004 |
| Cough | 5.23 (4.03–6.80) | <0.001 | 0.60 (0.35–1.04) | 0.069 |
| Muscle pain | 11.18 (8.74–14.32) | <0.001 | 1.69 (1.06–2.69) | 0.028 |
| Chest pain | 9.39 (6.89–12.80) | <0.001 | 1.61 (0.88–2.92) | 0.121 |
| Palpitation | 9.90 (7.54–12.98) | <0.001 | 1.37 (0.80–2.35) | 0.259 |
| Vertigo | 10.11 (7.72–13.24) | <0.001 | 1.37 (0.82–2.27) | 0.238 |
| Tinnitus | 5.27 (3.71–7.48) | <0.001 | 0.96 (0.49–1.89) | 0.909 |
| Loss of appetite | 5.67 (3.97–8.09) | <0.001 | 1.22 (0.55–2.70) | 0.633 |
| Weight loss | 6.16 (4.29–8.86) | <0.001 | 1.37 (0.64–2.95) | 0.420 |
| Headache | 6.46 (5.09–8.22) | <0.001 | 1.01 (0.65–1.76) | 0.804 |
| Altered smell | 2.49 (2.01–3.10) | <0.001 | 1.23 (0.70–2.16) | 0.469 |
| Altered taste | 3.14 (2.45–3.95) | <0.001 | 1.16 (0.62–2.14) | 0.643 |
| Scin rash | 5.09 (3.32–7.79) | <0.001 | 1.03 (0.48–2.21) | 0.935 |
| Months since infection | 1.07 (1.05–1.08) | <0.001 | 1.06 (1.03–1.09) | <0.001 |
| Age | 1.03 (1.02–1.03) | <0.001 | 1.02 (1.01–1.03) | <0.001 |
| Male sex | 0.95 (0.78–1.16) | 0.659 | 1.44 (0.99–2.08) | 0.054 |
| Body mass index | 1.07 (1.05–1.09) | <0.001 | 1.02 (0.99–1.06) | 0.248 |
PHQ-15, Patient Health Questionnaire-15; SSD-12, Somatic Symptom Disorder-B Criteria Scale; PHQ-2, Patient Health Questionnaire-2, GAD-2, Generalized Anxiety Disorder-2 questionnaire; FAS, Fatigue Assessment Scale.
Table 3.
Predictive odds ratios for daily life impairment (2828 completers) – multiple model with variable selection due to Aikaike information criteria.
| Multiple Model |
||
|---|---|---|
| Questionnaire / Symptom | OR (95% CI) | p-value |
| SSD-12 | 4.36 (2.57–7.41) | <0,001 |
| Depression (PHQ-2) | 2.48 (1.57–3.92) | <0,001 |
| Fatigue | 7.86 (5.63–10.97) | <0,001 |
| Dyspnea | 3.93 (2.73–5.67) | <0,001 |
| Concentration disturbance | 3.05 (2.17–4.30) | <0,001 |
| Sleep disorder | 2.08 (1.45–3.00) | <0,001 |
| Muscle pain | 2.18 (1.49–3.20) | <0,001 |
| Months since infection | 1.06 (1.03–1.08) | <0,001 |
| Age | 1.02 (1.01–1.03) | <0,001 |
SSD-12, Somatic Symptom Disorder-B Criteria Scale; PHQ-2, Patient Health Questionnaire-2.
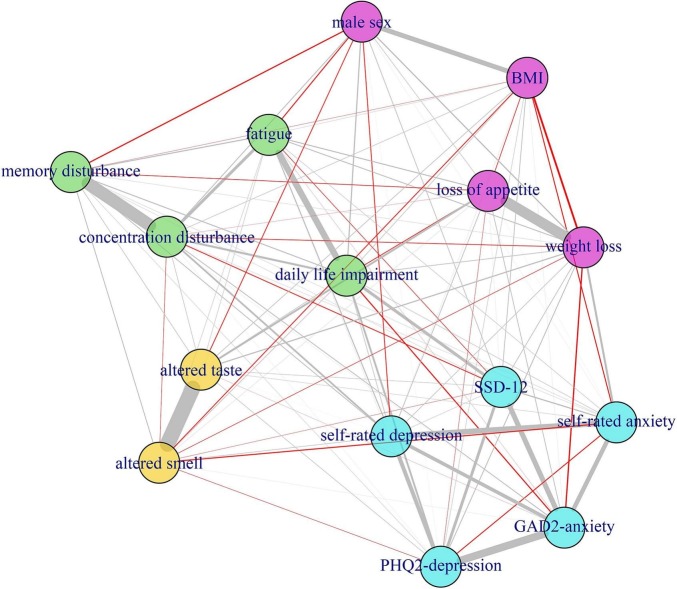
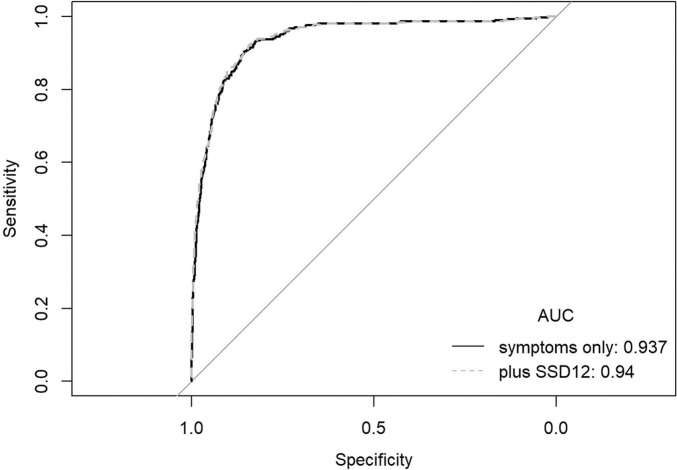
Fig. 2 illustrates the strength of bivariate partial correlations and the respective clustering of DLI, symptoms and psychometric variables. Four clusters emerged: a cluster comprising DLI and self-rated symptoms fatigue, concentration and memory disturbance (green colour); a psychological cluster (including self-rated anxiety, self-rated depression, PHQ-2, GAD-2, and SSD-12 (light blue)); a sensory cluster comprising altered smell and taste (yellow); and a weight associated cluster comprising weight loss, loss of appetite, body mass index, and male sex (violet). Self-reported fatigue showed the strongest correlation (rp = 0.248) and closest proximity to DLI. The second strongest but overall weak correlation with DLI was observed for SSD-12 (rp = 0.153), which clustered with PHQ-2, GAD-2, self-reported anxiety and depression. However, many of the relations are spurious and there are only few and moderate to weak partial relations to DLI. Finally, the ROC analysis showed only a minor additive value of the SSD-12 for the prediction of DLI, as the cross-validated AUC of the model using the symptoms as independent variables was 0.937, and increased to 0.940 when SSD-12 was additionally included (Fig. 3 ).
Fig. 2.
Network analysis – grey lines, positive correlations; red lines negative correlations. Thickness of the lines correlates with the strength of association.
Fig. 3.
Receiver Operating Characteristics (ROC) curve analysis to compare the area under the curve (AUC) of ´symptoms only´ with ´symptoms and SSD-12′ (SSD 12, Somatic Symptom Disorder-B Criteria Scale).
4. Discussion
The population-based survey found that 18.0% of participants suffered from DLI, and 52.5% of participants had long-lasting symptoms more than three months after SARS CoV-2 infection. DLI was mainly related to self-reported fatigue, though excessive preoccupation with one's bodily symptoms (SSD-12), dyspnea, impaired concentration, and depression (PHQ-2) additionally contributed.
Recently, a large population-based study showed that the symptom groups fatigue and neurocognitive impairment contribute most to reduced health recovery, while chest symptoms, including shortness of breath, follow in third place [5]. The global dominance of these symptoms was also demonstrated by systematic reviews with a meta-analysis [9,37], and in outpatient care by routine data analysis [7]. Our survey now provides evidence on the extent to which SSD is of importance for DLI after SARS-CoV-2 infection in the normal population. 25.6% of participants with persistent symptoms and DLI fulfilled the criteria of possible SSD, as assessed with SSD-12 and PHQ-15, compared to 2.1% without DLI. The univariate regression analysis confirmed these strong associations. However, the multiple regression model exhibited a moderate collinearity of the variables. Correspondingly, the network analysis uncovers the rather weak correlations between the variables and DLI. It illustrates in depth the relation of screening questionnaire results to participant-reported symptoms and shows that the subjectively perceived fatigue dominates the relation to DLI. This might serve as a hint that an excessive preoccupation with one's bodily symptoms in terms of B criterion of SSD, as assessed by the SSD-12, could be partly explained by the persistent and subjectively highly disturbing symptoms in the new disease pattern of PCS itself, especially as these are difficult to treat. Fitting to this, the ROC analysis to predict DLI showed almost no increase in AUC when SSD-12 was additionally combined with the symptoms. Noticeably, the scales GAD-2 and PHQ-15 were eliminated by variable selection in the regression analysis and were also not relevant in the network analysis. In the case of PHQ-15, this is obvious, as it is a list of symptoms, so that the additional explanatory value is redundant with regard to DLI; but anxiety also does not seem to have any significant meaning for a better understanding of the illness experience.
Our results might contribute to the discussion that Post-COVID research offers the opportunity to improve the understanding of the post-viral illness experience and its relationship to functional syndromes [11,13]. Up to now, diagnostic and therapeutic decision making regarding PCS might have been guided by the major disease clusters of the cardiovascular [38] and pulmonary systems [39] as well as the nervous system [40] that are affected by COVID-19. Based on our results, it seems reasonable to also consider certain aspects of SSD. Our findings suggest that a relevant proportion of patients with PCS, as defined by persistent symptoms and DLI, might suffer from SSD. These patients at risk should be identified early to ensure optimal supportive treatment. On the other hand, based on our findings, it seems likley that the majority of individuals in the general population meeting the criteria of PCS do not suffer from SSD. Particularly, among persons with persistent symptoms but without DLI the psychological burden of symptoms as assessed by the SSD-12 questionnaire seems very limited in the overwhelming majority. The comparatively low prevalence of SSD in our survey could also be an indication that the new diagnosis according to DSM-5 would not lead to an overestimation of SSD, as has already been considered [12,41]. Accordingly, the particular usefulness of the SSD-12 for a better understanding of the clinical picture could be given above all to those persons who are affected by infections or disease states.
It seems disconcerting that a significant proportion of patients still suffer from Post-COVID symptoms and DLI after SARS-CoV2 infection; and apparently patients with DLI also had a considerably higher symptom burden. With a response rate of 31.7% and assuming that residents without symptoms are less likely to respond, the incidence of persistent symptoms could be at least 15% (1486/9780) and the incidence of DLI 5% (509/9780), which fits well with previous studies [5,7,37]. However, 80.7% of the respondents with persistent symptoms were not under medical treatment, and only a very small proportion was treated in a Post-COVID outpatient clinic. It is remarkable that most of the participants were treated in outpatient care by general practitioners and specialists in private practice. In this regard, outpatient care is faced with the challenge of developing concepts that effectively support patients in coping with the most common physical and psychological symptoms.
5. Strengths and limitations
A strength was the mailing to all residents of the administrative district with PCR-verified confirmation of SARS-CoV-2 infection. Despite the comparatively high response rate, it is reasonable to assume that participants with persistent symptoms are more likely to respond. The participants were slightly older and more female than all infected persons in the district during this period. While the impact of age is inconsistent, female sex was identified as a risk factor for Long-COVID or PSC, respectively [42,43]. In terms of generalisability, the prevalence of symptom persistence and DLI in our study might therefore tend to be overestimated. Certainly, it should be noted that the cross-sectional design of the study does not allow any conclusions to be drawn about the longitudinal course of the disease. Another limitation is the use of questionnaires to diagnose psychological comorbidity. Ideally, the diagnosis would have to be validated by a structured clinical interview, especially since the diagnosis of SSD no longer differentiates between organically explained and unexplained complaints. This was not possible due to the completely anonymized survey and the size of the population-based study sample. Another limitation is the lack of a control group, so that the causality of symptom development remains unclear. However, there is increasing evidence that the symptoms fatigue, dyspnea, impaired concentration are quite characteristic for SARS-CoV-2 infection [5,7,37]. Furthermore, the absence of a control group would not affect our results regarding the association between increased SSD in participants with DLI compared to participants without DLI. In addition, the study participants were probably infected with different virus strains (wild-type, Alpha, Delta). However, the Omicron variant, which is known to cause less severe disease, was documented in South Africa at the end of the study, but not in Germany. Finally, we could not ask about vaccination status. Since at that time the vaccination discussion in the population was very controversial, we abstained from this question to avoid a non-response out of protest here. In this respect, the impact of vaccination on symptom presentation and DLI remains unclear. The latter two aspects would not affect our findings that patients with DLI suffer from increased psychological symptom burden.
6. Conclusion
PCS appears as a complex clinical picture in which SSD might play an important role when DLI is present. An excessive preoccupation with one's bodily symptoms in terms of B criterion of SSD, as assessed by the SSD-12, might be partly explained by the persistent unexplained and subjectively highly disturbing symptoms in the new disease pattern of PCS itself, especially as these are difficult to treat. Screening for SSD could help in differential diagnostic decision-making to ensure that patients receive appropriate psychosocial interventions to help them cope with the disease.
Ethics and dissemination
The study was approved by the Ethical Committee of the Technical University of Munich (Reference number 615/21 S-NP). Written, informed consent to participate will be obtained from all participants.
Authors´ contributions
AS, KL, JL, AH conceived the study. AH performed the statistical analysis. LH implemented and managed the study, and was responsible for the data entry. AG, PH, HS helped with interpretation of the data. AS drafted the first version of the manuscript. All authors helped with writing. All authors revised it critically for important intellectual content and approved the final version. AS is the full guarantor of the study and had full access to the data. AS and AH verified the data.
All authors confirm that they had full access to all the data in the study and accept responsibility to submit for publication to Journal of Psychosomatic Research.
Funding
There was no external funding for the study.
Declaration of Competing Interest
There are no conflicts of interest.
Acknowledgements
We would like to express our sincere thanks to the District Administrator of the Bad Tölz-Wolfratshausen district, Mr. Josef Niedermaier, and the staff at the local health authority, Dr. Julia Trempetic and Dr. Stephan Gebrande, and the district office, who supported us in conducting the study.
Data availability
Data are available from the authors upon reasonable request.
References
- 1.Crook H., Raza S., Nowell J., Young M., Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374 doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 2.Clift A.K., Coupland C.A.C., Keogh R.H., Diaz-Ordaz K., Williamson E., et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371 doi: 10.1136/bmj.m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koczulla A.R., Ankermann T., Behrends U., Berlit P., Böing S., Brinkmann F., et al. S1 guideline post-COVID/long-COVID. Pneumologie. 2021;75:869–900. doi: 10.1055/a-1551-9734. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus. 2021. https://www.who.int/publications-detailredirect/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 [Accessed January 25, 2023]
- 5.Peter R.S., Nieters A., Kräusslich H.G., Brockmann S.O., Göpel S., Kindle G., et al. Post-acute sequelae of covid-19 six to 12 months after infection: population based study. BMJ. 2022;379 doi: 10.1136/bmj-2022-071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnachie E., Hapfelmeier A., Linde K., Tauscher M., Gerlach R., Greissel A., et al. Incidence of post-COVID syndrome and associated symptoms in outpatient care in Bavaria, Germany: a retrospective cohort study using routinely collected claims data. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2022-064979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.T. Bahmer, C. Borzikowsky, W. Lieb, A. Horn, L. Krist, J. Fricke, et al., NAPKON study group. Severity, predictors and clinical correlates of Post-COVID syndrome (PCS) in Germany: A prospective, multi-centre, population-based cohort study, EClin. Med. 51 (2022) 101549, doi: 10.1016/j.eclinm.2022.101549. [DOI] [PMC free article] [PubMed]
- 9.O’Mahoney L.L., Routen A., Gillies C., Ekezie W., Welford A., Zhang A., et al. The prevalence and long-term health effects of long Covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. EClinicalMedicine. 2022;55 doi: 10.1016/j.eclinm.2022.101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wensink M., Schaap G., Ten Klooster P.M., Doggen C.J.M., van der Palen J., Vonkeman H.E., Bode C. Physical and mental fatigue in post-COVID syndrome and their associations over time: a small-sample ESM-study to explore fatigue, quality of sleep and behaviours. J. Psychosom. Res. 2023;164 doi: 10.1016/j.jpsychores.2022.111084. [DOI] [PubMed] [Google Scholar]
- 11.Lemogne C., Gouraud C., Pitron V., Ranque B. Why the hypothesis of psychological mechanisms in long COVID is worth considering. J. Psychosom. Res. 2023;165 doi: 10.1016/j.jpsychores.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Löwe B., Levenson J., Depping M., Hüsing P., Kohlmann S., Lehmann M., et al. Somatic symptom disorder: a scoping review on the empirical evidence of a new diagnosis. Psychol. Med. 2022;52:632–648. doi: 10.1017/S0033291721004177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson S.L., Menkes D.B. Long covid: reshaping conversations about medically unexplained symptoms. BMJ. 2021;374 doi: 10.1136/bmj.n1859. [DOI] [PubMed] [Google Scholar]
- 14.Newman M. Chronic fatigue syndrome and long covid: moving beyond the controversy. BMJ. 2021;373 doi: 10.1136/bmj.n1559. [DOI] [PubMed] [Google Scholar]
- 15.Verveen A., Müller F., Lloyd A., Moss-Morris R., Omland T., Penninx B., et al. A research agenda for post-COVID-19 fatigue. J. Psychosom. Res. 2022;154 doi: 10.1016/j.jpsychores.2022.110726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kachaner A., Lemogne C., Dave J., Ranque B., de Broucker T., Meppiel E. Somatic symptom disorder in patients with post-COVID-19 neurological symptoms: a preliminary report from the somatic study (somatic symptom disorder triggered by COVID-19) J. Neurol. Neurosurg. Psychiatry. 2022 doi: 10.1136/jnnp-2021-327899. jnnp-2021-327899. [DOI] [PubMed] [Google Scholar]
- 17.Fleischer M., Szepanowski F., Tovar M., Herchert K., Dinse H., Schweda A., et al. Post-COVID-19 syndrome is rarely associated with damage of the nervous system: findings from a prospective observational cohort study in 171 patients. Neurol. Ther. 2022;11:1637–1657. doi: 10.1007/s40120-022-00395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noviello D., Costantino A., Muscatello A., Bandera A., Consonni D., Vecchi M., Basilisco G. Functional gastrointestinal and somatoform symptoms five months after SARS-CoV-2 infection: a controlled cohort study. Neurogastroenterol. Motil. 2022;34 doi: 10.1111/nmo.14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shanbehzadeh S., Tavahomi M., Zanjari N., Ebrahimi-Takamjani I., Amiri-Arimi S. Physical and mental health complications post-COVID-19: scoping review. J. Psychosom. Res. 2021;147 doi: 10.1016/j.jpsychores.2021.110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renaud-Charest O., Lui L.M.W., Eskander S., et al. Onset and frequency of depression in post-COVID-19 syndrome: a systematic review. J. Psychiatr. Res. 2021;144:129–137. doi: 10.1016/j.jpsychires.2021.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taquet M., Sillett R., Zhu L., Mendel J., Camplisson I., Dercon Q., Harrison P.J. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients, lancet. Psychiatry. 2022;9:815–827. doi: 10.1016/S2215-0366(22)00260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom. Med. 2002;64:258–266. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Toussaint A., Murray A.M., Voigt K., et al. Development and validation of the somatic symptom disorder-B criteria scale (SSD-12) Psychosom. Med. 2016;78:5–12. doi: 10.1097/PSY.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 24.Toussaint A., Husing P., Kohlmann S., Löwe B. Detecting DSM-5 somatic symptom disorder: criterion validity of the patient health Questionnaire-15 (PHQ-15) and the somatic symptom Scale-8 (SSS-8) in combination with the somatic symptom disorder - B criteria scale (SSD-12) Psychol. Med. 2020;50:324–333. doi: 10.1017/S003329171900014X. [DOI] [PubMed] [Google Scholar]
- 25.Löwe B., Wahl I., Rose M., Spitzer C., Glaesmer H., Wingenfeld K., et al. A 4-item measure of depression and anxiety: validation and standardization of the patient health Questionnaire-4 (PHQ-4) in the general population. J. Affect. Disord. 2010;122:86–95. doi: 10.1016/j.jad.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Kroenke K., Spitzer R.L., Williams J.B.W. The patient health Questionnaire-2 - validity of a two-item depression screener. Med. Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 27.Löwe B., Kroenke K., Gräfe K. Detecting and monitoring depression with a two-item questionnaire (PHQ-2) J. Psychosom. Res. 2005;58:163–171. doi: 10.1016/j.jpsychores.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 28.De Vries J., Michielsen H., Van Heck G.L., Drent M. Measuring fatigue in sarcoidosis: the fatigue assessment scale (FAS) Br. J. Health Psychol. 2004;9:279–291. doi: 10.1348/1359107041557048. [DOI] [PubMed] [Google Scholar]
- 29.Michielsen H.J., De Vries J., Van Heck G.L. Psychometric qualities of a brief self-rated fatigue measure: The fatigue assessment scale. J. Psychosom. Res. 2003;54:345–352. doi: 10.1016/s0022-3999(02)00392-6. [DOI] [PubMed] [Google Scholar]
- 30.Harrell F.E. second ed. Springer; New York: 2015. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. [Google Scholar]
- 31.Steyerberg E.W. Springer; New York: 2009. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. [Google Scholar]
- 32.Whittaker J. John Wiley; Chichester: 1990. Graphical Models in Applied Multivariate Statistics. [Google Scholar]
- 33.Fruchterman T.M.J., Reingold E.M. Graph drawing by force-directed placement. Soft. Pract. Exp. 1991;21:1129–1164. doi: 10.1002/spe.4380211102. [DOI] [Google Scholar]
- 34.Brandes U., Delling D., Gaertler M., et al. On modularity clustering, IEEE. Transactions. Knowledge. Data. Engineering. 2008;20:172–188. doi: 10.1109/TKDE.2007.190689. [DOI] [Google Scholar]
- 35.Schafer J., Opgen-Rhein R., Zuber V., Ahdesmaki M., Duarte Silva A.P., Strimmer K. Corpcor: Efficient Estimation of Covariance and (Partial) Correlation. R package version 1.6.10. 2021. https://CRAN.R-project.org/package=corpcor
- 36.Csardi G., Nepusz T. The Igraph Software Package for Complex Network Research, InterJournal, Complex Systems 1695. 2006. https://igraph.org [Accessed January 25, 2023]
- 37.Global Burden of Disease Long COVID Collaborators Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. 2022;328 doi: 10.1001/jama.2022.18931. 604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 40.Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., McCarthy P., et al. SARS-CoV-2 is associated with changes in brain structure in UK biobank. Nature. 2022;604:697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frances A., The A. New somatic symptom disorder in DSM-5 risks mislabeling many people as mentally ill. BMJ. 2013;346 doi: 10.1136/bmj.f1580. [DOI] [PubMed] [Google Scholar]
- 42.Thompson E.J., Williams D.M., Walker A.J., Mitchell R.E., Niedzwiedz C.L., et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat. Commun. 2022;13:3528. doi: 10.1038/s41467-022-30836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subramanian A., Nirantharakumar K., Hughes S., Myles P., Williams T., Gokhale K.M., et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 2022;28:1706–1714. doi: 10.1038/s41591-022-01909-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the authors upon reasonable request.