Abstract
Prior research suggests that flavors can influence the pharmacological effects of nicotine. We used commercially available JUUL pods to examine whether preferred menthol versus tobacco flavor increased the addictive potential of nicotine per se. This study recruited 15 regular JUUL e-cigarette users to complete a 2 × 2 factorial crossover trial using an entirely remote video format. Participants completed a sampling baseline session to identify preferred JUUL flavor (menthol vs. tobacco) followed by four counterbalanced experimental sessions separated by ≥48 hr: (a) low-nicotine dose (3% JUUL)/nonpreferred flavor; (b) low dose/preferred flavor; (c) high-nicotine dose (5% JUUL)/nonpreferred flavor; and (d) high dose/preferred flavor. In each experimental session, participants completed a puffing procedure followed by subjective ratings of e-cigarette liking and wanting (ELW), urges, and reinforcement using a JUUL pod purchase task. There was a dose-by-flavor interaction for average ELW (F = 4.58, p = .041) in which ELW was significantly greater for the preferred than the nonpreferred flavor at the low-nicotine dose but not the high-nicotine dose. There were also dose-by-flavor interactions for pre- to post-puffing change in overall urge to vape (F = 5.97, p = .021) and urge strength (F = 4.96, p = .049), with greater reductions in overall urge/strength for the preferred compared to the nonpreferred flavor at the low but not the high dose. We found no significant interaction effects for purchase task outcomes. Using a fully remote experimental puffing procedure, our findings suggest preferred flavors increase the rewarding effects most for lower nicotine e-cigarettes.
Keywords: e-cigarettes, JUUL, flavors, menthol, nicotine
Electronic cigarette (i.e., e-cigarette) use has increased substantially over the past decade in the United States (Cornelius et al., 2020; Stanton et al., 2020). Menthol and nontobacco e-cigarette flavors are popular (Ali et al., 2020; Zhu et al., 2014) and enhance the rewarding effects of vaping (Audrain-McGovern et al., 2016; Kroemer et al., 2018; Zare et al., 2018). Sweetness and coolness of flavors, in particular, have been shown to be associated with e-cigarette liking (Kim et al., 2016). Research on the interactive effects of flavors and nicotine on subjective reward is important to improve the understanding of e-cigarette abuse liability and addiction potential. Some prior laboratory studies have identified trends (Krishnan-Sarin et al., 2017; Rosbrook & Green, 2016) or significant effects (Baker et al., 2021), indicating that flavors increase the reinforcing properties of e-cigarettes at higher doses of nicotine. In contrast, others have found flavors increase the rewarding effects only in low- or no-nicotine e-cigarettes (DeVito et al., 2020; Leventhal et al., 2019; Pullicin et al., 2020). These discrepant findings could be related to variation in several important features of e-cigarettes used in these studies including nicotine dose, use of freebase nicotine versus nicotine salt, or available flavors.
The present study builds on prior research by examining the interactive effect of preferred flavor and nicotine concentration on subjective reward using the commercially available flavors and nicotine doses from JUUL, a popular e-cigarette on the U.S. market (Huang et al., 2019). Given prior findings that higher menthol concentrations appeared to enhance e-cigarettes’ rewarding effects at higher nicotine doses (Krishnan-Sarin et al., 2017), we hypothesized a flavor by nicotine interaction such that participants ‘ preferred flavor would increase the rewarding effects of high but not low-nicotine JUUL e-cigarettes.
Method
We recruited adult regular JUUL users to complete a 2 × 2 factorial crossover trial to examine the interactive effect of flavor preference and nicotine dose. All sessions were completed remotely using Zoom video calls (https://zoom.us/) due to COVID-19 restrictions on in-person interactions. Participants completed a baseline session to identify preferred JUUL flavor (menthol vs. tobacco) followed by four counterbalanced experimental sessions separated by ≥48 hr: (a) low-nicotine dose (3% JUUL)/nonpreferred flavor; (b) low dose/preferred flavor; (c) high-nicotine dose (5% JUUL)/nonpreferred flavor; and (d) high dose/preferred flavor. Each experimental session consisted of a puffing procedure followed by subjective ratings. Participants and experimenters were blind to nicotine dose but not flavor. Participants were provided JUUL pods via curbside pickup but used their own JUUL device for all sampling and puffing procedures. We report how we determined our sample size, and all manipulations, measures, and exclusions in this study. The study was approved by the host institutional review board and preregistered on ClinicalTrials.gov (NCT04696380).
Participants
Participants were recruited between March 2021 and August 2021 from a sample of e-cigarette users who had previously screened ineligible for other Tobacco Centers of Regulatory Science trials at the University of Vermont and expressed interest in participating in other research. Of the 60 individuals who completed the screener, 25 met the following inclusion criteria: ≥21 years old; use of a JUUL device on ≥10 of the past 30 days; use of any e-cigarette with nicotine-containing e-liquid ≥4 days/week in the past 30 days; use of JUUL pods containing 5% nicotine; no plan to quit e-cigarettes in the next 30 days; not currently breastfeeding or planning to breastfeed in next 30 days; negative pregnancy test in females of reproductive potential; internet access for conducting video sessions; U.S. citizen; and a resident of Vermont. Participants of reproductive potential were mailed a pregnancy test to complete and self-report results through an online survey.
The sample size (N = 15) was determined based on feasibility considerations regarding recruitment and retention in the context of COVID-19 restrictions. Of those who met eligibility criteria, 19 consented and 16 were randomized. One randomized participant was discontinued from the study after the first experimental session due to symptoms of nicotine intoxication. Fifteen individuals completed all four experimental testing sessions and are included in the present study analyses (Supplemental Figure 1). Participants used their personal JUUL device for each of the vaping sessions.
Procedure
During the baseline remote video session, participants completed online surveys and sampled the two JUUL flavored pods commercially available at the time of recruitment—menthol and Virginia tobacco—both at the 3% dose, for 2 min each with 5 min between sampling each flavor. The sampling order was randomized. Participants were asked to select their preferred flavor between menthol and Virginia tobacco and then trained in the puffing protocol used in the experimental sessions (described below). Participants were instructed to abstain from all nicotine and tobacco products, nonnicotine-containing e-cigarettes, and tetrahydrocannabinol (THC)-containing products for 16 hr prior to each of the four counterbalanced conditions. Self-reported abstinence was assessed prior to each experimental session, and sessions were rescheduled if participants reported use in the prior 16 hr. Of note, the duration of required abstinence in similar prior studies has ranged from 2 hr (Leventhal et al., 2019; Pullicin et al., 2020) to ≥24 hr (Vargas-Rivera et al., 2021).
The puffing procedure, in this study, was adapted from procedures used in prior studies (Krishnan-Sarin et al., 2017; Vansickel et al., 2012) for our remote procedures. Each experimental session was conducted via Zoom and included three fixed puffing bouts and varied only in the JUUL pod used for that session. During each puffing bout, participants were asked to take 10 puffs with a 30-s interpuff interval. Puffing bouts were monitored by research personnel and separated by 10 min, during which participants completed ratings. Participants were permitted to stop the procedure or skip puffs to avoid adverse effects. We queried for adverse events at the end of the baseline and each of the four experimental sessions. Participants were reimbursed a total of $200 in online gift cards for participation.
Measures
Primary Outcomes
E-Cigarette Liking and Wanting.
Participants made subjective ratings of e-cigarette liking and wanting (ELW) after each puffing bout using a modified version of the Drug Effects Questionnaire (DEQ; Morean et al., 2013). Consistent with Krishnan-Sarin et al. (2017), we examined the mean of the following four items on a scale from 0 (not at all) to 100 (extremely): “I feel good e-cigarette effects,” “I want more of that e-cigarette I received,” “I feel the e-cigarette strength,” and “I like the e-cigarette effect.”
E-Cigarette Taste.
At the completion of each puffing bout, we assessed the extent to which participants liked or disliked the taste of each JUUL pod using a modified version of the Labeled Hedonic Scale (LHS; Kim et al., 2016; Lim et al., 2009), a bipolar category ratio scale ranging from −100 (most disliked taste imaginable) to 100 (most liked taste imaginable).
Urge to Use E-Cigarettes.
Using modified items from the mood and Physical Symptoms Scale (West & Hajek, 2004; West & Ussher, 2010), we assessed change in urges and strength of urges to use an e-cigarette as well as to smoke a tobacco cigarette on a 5-point scale. However, only one participant identified as a current smoker, so we report changes in e-cigarette urges only. Urges were assessed during each experimental session prior to and after each puffing bout.
JUUL Pod Purchase Task
To assess reinforcement from e-cigarettes, we modified an existing e-cigarette purchase task (Cassidy et al., 2020) to ask about JUUL pods at the end of each experimental session. The purchase task asks participants to make hypothetical purchases across a range of increasing prices to assess four indices of demand (i.e., reinforcing value) for a substance: intensity, breakpoint, maximum output (Omax), and maximum price (Pmax; Strickland et al., 2020). See the Supplemental Material for a full description of demand indices and analysis. The JUUL purchase task instructions stated that participants should “think about how they are feeling right now” and report how many JUUL pods they would buy for the week, rather than for the day, as one pod lasts more than a day for the majority of our participants (see Table 1). The instructions also specified that the available JUUL pod was their “study pod from today,” that they should imagine that they had no other access to sources of pods, and that they should purchase the number of pods they would actually use for the week and not save or stockpile them for later. The task had 18 price increments, from free up to $25.00 per pod; participants were also provided feedback on the price per four-pack of pods at different price points.
Table 1:
Participant Characteristics
| Demographic information | |
|---|---|
| Age | M = 27.3 (SD = 7.2) |
| Sex, n (%) | |
| Female | 8 (53.3) |
| Male | 7 (46.7) |
| Race, n (%) | |
| White | 13 (86.7) |
| Black or African American | 1 (6.7) |
| More than one race | 1 (6.7) |
| Ethnicity (Hispanic), n (%) | |
| Yes | 3 (20.0) |
| No | 12 (80.0) |
| Marital status, n (%) | |
| Married/member of unmarried couple | 6 (40.0) |
| Never married | 9 (60.0) |
| Employment status, n (%) | |
| Employed full time | 6 (40.0) |
| Employed part time | 3 (20.0) |
| Homemaker/retired | 2 (13.3) |
| Student | 4 (26.7) |
| Education completed, nx1 (%) | |
| High school degree or GED | 2 (13.3) |
| Some college | 9 (60.0) |
| College graduate | 4 (26.7) |
| E-cigarette (EC) and tobacco cigarette (TC) use | |
|
| |
| Years since started using JUUL weekly | M = 2.5 (SD = 1.3) |
| Uses JUUL daily, n (%) | |
| Yes | 13 (86.7) |
| No | 2 (13.3) |
| Pods per day, n (%) | |
| Less than 1 pod | 10 (66.7) |
| 1 pod | 5 (33.3) |
| Uses ECs other than JUUL, n (%) | |
| Yes | 2 (13.3) |
| No | 13 (86.7) |
| Penn State Electronic Cigarette Dependence | M = 11.2 (SD = 3.9) |
| Index (0 = least to 10 = most) | |
| Baseline preferred JUUL flavor, n (%) | |
| Menthol | 12 (80.0) |
| Tobacco | 3 (20.0) |
| JUUL brand flavor used most | |
| Menthol | 10 (66.7) |
| Virginia tobacco | 3 (20.0) |
| Mint | 2 (13.3) |
| Smoked > 100 lifetime TCs, n (%) | |
| Yes | 10 (66.7) |
| No | 5 (33.3) |
| Current TC smoker, n (%) | |
| Yes | 1 (6.7) |
| No | 14 (93.3) |
Note. GED = General Education Development; EC = E-cigarette; TC = tobacco cigarette.
Secondary Outcomes
E-Cigarette Taste Intensity.
At the completion of each puffing bout, we assessed e-cigarette total taste intensity “right now” using a modified version of the general Labeled Magnitude Scale (gLMS; Bartoshuk et al., 2003; Green et al., 1993), a category ratio scale with seven semantic labels from 0 (no sensation) to 100 (strongest imaginable).
Stimulant Effects and Nicotine Withdrawal.
Consistent with Krishnan-Sarin et al. (2017), stimulant effects were assessed by averaging the two items “I feel energized” and “I feel high,” rated from 0 (not at all) to 100 (extremely) on the DEQ (Morean et al., 2013). Nicotine withdrawal was assessed using the same 0–100 scale, averaging the following items: “I feel sleepy,” “I feel angry,” “I feel irritable,” “I am having difficulty concentrating,” “I feel restless,” and “I feel hungry.” Stimulant effects and nicotine withdrawal were assessed after each puffing bout.
Statistical Analysis
For all outcomes, the main effects of dose and flavor preference, along with dose-by-flavor preference interactions, were evaluated using mixed-model repeated-measures analyses of variance. In a series of sensitivity analyses, we also tested the main effect of menthol versus tobacco flavor (regardless of preference) and a menthol versus tobacco by nicotine dose interaction for all outcomes. Significance level α was set a priori at 0.05 for all tests. Statistical analyses were conducted using the SAS statistical analysis software, Version 9.4 (SAS Institute, Inc., Cary, North Carolina, United States). Study data and the code used for analysis are available upon request.
Results
The mean age of participants was 27.5 years, approximately half were male, and 87% were daily JUUL users. During the sampling session, 12 participants reported a preference for menthol and three preferred tobacco flavor (Table 1).
E-Cigarette Liking and Wanting
There was a significant main effect of flavor for average ELW, F(1, 28) = 11.99, p = .002, with overall ELW greater for the preferred, M = 61.20 (SE = 4.52), than the nonpreferred flavor, M = 51.93 (SE = 4.52). There was also a significant dose-by-flavor interaction for average ELW, F(1, 28) = 4.58, p = .04 (Figure 1A). Contrary to our hypothesis, pairwise comparisons indicated ELW was greater for the preferred, M = 63.53 (SE = 4.96), than the nonpreferred flavor, M = 48.54, (SE = 4.96), at the low-nicotine dose, t(28) = −3.96, p = .001, but not at the high-nicotine dose, t(28) = −0.94, p = .36.
Figure 1: Mean (SE) Primary Outcome Scores for JUUL Flavor Preference and Nicotine Dose.
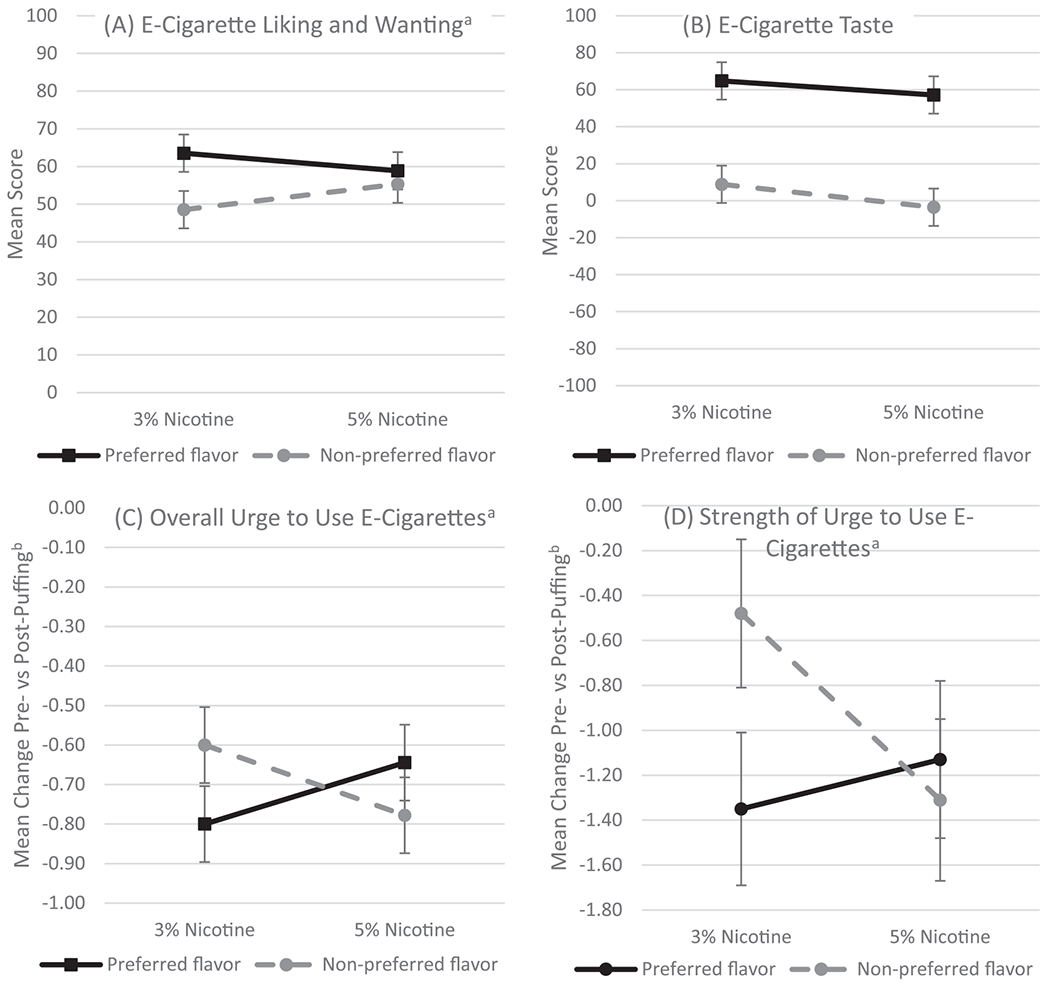
Note. SE = standard error.
a A significant flavor by nicotine dose interaction (p < .05). b Lower (i.e., more negative) change scores reflect a greater reduction in overall urges and strength of urges.
E-Cigarette Taste
There was a significant main effect of flavor, F(1, 14) = 37.23, p < .0001, with participants rating greater liking of their preferred flavor, M = 60.98 (SE = 8.63), compared to their nonpreferred flavor, M = 2.67 (SE = 8.63). For the modified LHS, we did not observe a significant dose-by-flavor interaction or main effect of dose (Figure 1B).
Change in Urge to Use E-Cigarettes
There was a significant dose-by-flavor interaction for pre-to post-puffing change in the urge to use e-cigarettes, F(1, 28) = 5.97, p = .02 (Figure 1C). Pairwise comparisons indicated a greater reduction in the presence of urges from pre- to post-puffing for the preferred, M = −0.80 (SE = 0.10), compared to the nonpreferred flavor, M −0.60 (SE = 0.1), at the low-nicotine dose, t(28) = 2.07, p = .048, but not the high dose, t(28) = −1.38, p = .18. Similarly, there was a significant dose-by-flavor interaction for the pre-to post-puffing change in strength of urges to use e-cigarettes, F(1, 10.4) = 4.96, p = .049 (Figure 1D). Pairwise comparisons indicated a greater reduction in the strength of urges from pre- to post-puffing for the preferred, M = −1.35 (SE = 0.33), compared to the nonpreferred flavor, M = −0.48 (SE = 0.34), at the low-nicotine dose, t(12.6) = 2.45, p = .03, but not the high-nicotine dose, t(8.58) = −0.59, p = .57. There were no main effects of dose or flavor.
Demand for JUUL Pods
There was a significant main effect of flavor for the Pmax index, F(1, 12.3) = 4.76, p = .049, indicating that the price at which expenditure was maximized was greater for participants’ preferred (M = 6.63, SE = 0.97) than nonpreferred (M = 5.12, SE = 0.74) flavor. There were no other significant main effects or significant interactions for the demand indices.
Taste Intensity, Stimulant Effects, and Withdrawal
There were no significant main effects or significant interactions for total taste intensity, stimulant effects, or withdrawal.
Sensitivity Analysis
There was a significant main effect of test flavor for ELW mean, F(1, 28) = 4.71, p = .04, such that ELW mean was greater for menthol (M = 59.89, SE = 4.58) than tobacco (M = 53.24, SE = 4.58) flavoring when flavor preference was ignored. Similarly, we found a significant main effect of test flavor for LMS, F(1, 14) = 6.17, p = .03, with mean taste intensity greater for menthol (M = 47.03, SE = 5.99) than tobacco (M = 35.60, SE = 5.99) and a significant main effect of test flavor for breakpoint, F(1, 12.5) = 4.71, p = .049, using the purchase task such that the mean price at which participants indicated they would stop purchasing JUUL pods was greater for menthol (M = 9.17, SE = 1.27) than tobacco (M = 7.47, SE = 1.05) flavored pods. We found no other significant main effects for menthol versus tobacco. We found a marginally significant interaction for demand elasticity, F(1, 22.1) = 4.09, p = .06, indicating slightly greater elasticity at the low-nicotine dose (M = 0.004, SE = 0.001) compared to the high dose (M = 0.003, SE = 0.001) for the tobacco, t(21.9) = 2.06, p = .05, but not the menthol, t(22.3) = −0.83, p = .42, flavored pods. There were no significant interactions when flavor preference was ignored (other p values = .10–.97).
Discussion
This study expanded on prior laboratory research (Krishnan-Sarin et al., 2017) by testing interactions between nicotine dose and flavor in commercially available JUUL pods using a novel remote methodology. Contrary to our hypothesis, we found an interaction in which liking and wanting as well as the reduction in urges to use e-cigarettes were greater for the preferred flavor at the low but not the high-nicotine dose. As expected, there was a main effect for flavor where participants liked the taste of their preferred flavor more than their nonpreferred flavor, but we did not observe a nicotine dose-by-flavor interaction. Further, we found no interactive effects for e-cigarette demand, acute stimulant effects, or nicotine withdrawal.
Differences in findings between this study and prior research could be due to a number of methodological differences, including that we used JUUL e-cigarettes containing nicotine salts while others used freebase nicotine e-liquids (Baker et al., 2021; Krishnan-Sarin et al., 2017; Rosbrook & Green, 2016). JUUL delivers more nicotine than older generation e-liquids (Goldenson et al., 2020; Hajek et al., 2020) and thus, the low (3%) nicotine JUUL used in our study may actually be more similar to the high-nicotine e-liquids used in some prior research (Baker et al., 2021; Krishnan-Sarin et al., 2017). Our findings are consistent with prior laboratory studies that found flavors increased the rewarding effects of JUUL (Pacek et al., 2021; Vargas-Rivera et al., 2021). However, we know of no prior within-subject studies that have tested the interactive effects of different flavors and nicotine doses using JUUL pods.
Our findings provide support for the hypothesis that preferred e-cigarette flavors mask the acute aversive qualities of nicotine (i.e., increasing appeal) more at lower rather than higher nicotine doses (Patten & De Biasi, 2020). This is consistent with prior findings that nicotine elicits irritation and thus flavors’ influence on e-cigarette liking is diminished with greater nicotine doses (Pullicin et al., 2020). However, other research on flavors suggests that well-liked flavors may simply increase the pleasantness of electronic nicotine delivery systems (ENDS) rather than mask the aversiveness of nicotine per se (Baker et al., 2021). It should be noted that all participants in the present study had experience using 5% JUUL pods at baseline and thus were not naïve to high-nicotine e-cigarettes, which could have influenced our findings. If preferred flavors do indeed enhance the experience and acceptability of lower nicotine content e-liquid, this could facilitate nicotine reduction as a strategy for individuals who intend to taper or quit e-cigarettes, as proposed by Baker et al. (2021).
Menthol was selected as the preferred flavor by most (80%) participants in our study, in line with prior research documenting the association with “coolness” and e-cigarette liking (Kim et al., 2016). Consistent with research on the role of menthol in cigarettes (Ahijevych & Garrett, 2004), our findings partially support prior e-cigarette findings that menthol’s “smooth” sensation may suppress nicotine’s aversive qualities and increase e-cigarette appeal (DeVito et al., 2020; Leventhal et al., 2020). However, we found no significant interactions between test flavor (menthol vs. tobacco) and nicotine dose in sensitivity analyses that ignored participants’ flavor preferences. Thus, future research is needed to determine the extent to which flavor or individuals’ flavor preference is responsible for the increase in rewarding effects of e-cigarettes.
Strengths of our study include the use of a within-participant design, high-participant retention, examination of dose responsivity, use of a popular pod-based e-cigarette (JUUL), and use of multiple tests of reinforcement. Further, this study demonstrates the feasibility of controlled behavioral laboratory procedures using a fully remote methodology. Limitations of the study include our small sample size, the inclusion of only two flavors and two nicotine doses, and omission of baseline measures of stimulant and withdrawal effects as well as biochemical verification (e.g., urine cotinine) of regular e-cigarette use. Participants used their own JUUL devices, which could have varied in power output, and were limited to select between the only commercially available JUUL flavors (tobacco or menthol) as their preferred flavor, which may have affected the results. However, both of these factors also increase the external validity of the study.
Conclusion
Using a remote experimental puffing procedure, our findings suggest a preferred flavor increases the rewarding effects most for lower nicotine e-cigarettes. Future research using pod-based e-cigarettes in a greater variety of flavors is needed to further inform the role of flavor preference in abuse liability of e-cigarettes at various nicotine doses. Research is also needed to examine the extent to which these e-cigarette findings extend to preference for menthol flavoring in very low versus normal nicotine content cigarettes.
Supplementary Material
Public Health Significance.
Our study found greater e-cigarette liking and wanting and greater reductions in urges to use e-cigarettes for the preferred flavor at the low but not the high-nicotine dose. These findings suggest that preferred e-cigarette flavors may heighten the rewarding effects more for e-cigarettes with lower nicotine content.
Acknowledgments
The authors thank Shelly Naud and Shannon O’Connor for their help preparing files for this article. The authors were supported by Grants U54DA036114 (Catherine Peasley-Miklus, John R. Hughes, Elias M. Klemperer, Andrea C. Villanti), U54DA036151 (Suchitra Krishnan-Sarin) from the National Institute on Drug Abuse (NIDA) and Food and Drug Administration (FDA) Center for Tobacco Products (CTP), and T32 DA007242 (Marc Jerome P. Feinstein) from the NIDA as well as P20GM103644 from National Institute of General Medical Sciences (Elias M. Klemperer).
Elias M. Klemperer, Rachel N. Cassidy, Marc Jerome P. Feinstein, Andrea C. Villanti, Letizia A. Mosca, Suchitra Krishnan-Sarin, Alan Su, and Michael J. DeSarno have nothing to disclose. Catherine Peasley-Miklus’s spouse is employed by Perrigo, which markets consumer smoking cessation products. John R. Hughes has received consulting and speaking fees from several companies that develop or market pharmacological and behavioral treatments for smoking cessation or harm reduction and from several nonprofit organizations that promote tobacco control. He also consults for Swedish Match on their harm reduction products.
Catherine Peasley-Miklus played a lead role in project administration, supervision, and writing of original draft, a supporting role in investigation and methodology, and an equal role in writing of review and editing. Elias M. Klemperer played a supporting role in methodology and supervision, and an equal role in writing of original draft and writing of review and editing. John R. Hughes played a lead role in conceptualization and funding acquisition, a supporting role in supervision, writing of original draft, and writing of review and editing, and an equal role in methodology. Andrea C. Villanti played a supporting role in conceptualization, methodology, and writing of review and editing. Suchitra Krishnan-Sarin played a supporting role in conceptualization, methodology, and writing of review and editing. Michael J. DeSarno played a lead role in data curation and formal analysis, and a supporting role in writing of review and editing. Letizia A. Mosca played a supporting role in investigation and writing of review and editing. Alan Su played a supporting role in investigation and writing of review and editing. Rachel N. Cassidy played a supporting role in methodology and writing of review and editing. Marc Jerome P. Feinstein played a supporting role in investigation and writing of review and editing.
Footnotes
Supplemental materials: https://doi.org/10.1037/pha0000591.supp
This study’s design and hypotheses were preregistered on ClinicalTrials.gov (NCT04696380) and can be found at https://clinicaltrials.gov/ct2/show/NCT04696380. Data from primary outcome measures were presented in abstract and digital poster format as part of the 2022 annual meeting of the Society for Research on Nicotine and Tobacco held in Baltimore, MD in March 2022. The data reported in this article have not been made publicly available. The data and code used for analysis are available upon request.
References
- Ahijevych K, & Garrett BE (2004). Menthol pharmacology and its potential impact on cigarette smoking behavior. Nicotine & Tobacco Research, 6(Suppl. 1), S17–S28. 10.1080/14622200310001649469 [DOI] [PubMed] [Google Scholar]
- Ali FRM, Diaz MC, Vallone D, Tynan MA, Cordova J, Seaman EL, Trivers KF, Schillo BA, Talley B, & King BA (2020). E-cigarette unit sales, by product and flavor type—United States, 2014—2020. Morbidity and Mortality Weekly Report, 69(37), 1313–1318. 10.15585/mmwr.mm6937e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Strasser AA, & Wileyto EP (2016). The impact of flavoring on the rewarding and reinforcing value of e-cigarettes with nicotine among young adult smokers. Drug and Alcohol Dependence, 166, 263–267. 10.1016/j.drugalcdep.2016.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AN, Bakke AJ, Branstetter SA, & Hayes JE (2021). Harsh and sweet sensations predict acute liking of electronic cigarettes, but flavor does not affect acute nicotine intake: A pilot laboratory study in men. Nicotine & Tobacco Research, 23(4), 687–693. 10.1093/ntr/ntaa209 [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Fast K, Green BG, Prutkin J, & Snyder DJ (2003). Labeled scales (e.g., category, Likert, VAS) and invalid across-group comparisons: What we have learned from genetic variation in taste. Food Quality and Preference, 14(2), 125–138. 10.1016/S0950-3293(02)00077-0 [DOI] [Google Scholar]
- Cassidy RN, Long V, Tidey JW, & Colby SM (2020). Validation of an E-cigarette purchase task in advanced generation device users. Nicotine & Tobacco Research, 22(10), 1851–1859. 10.1093/ntr/ntaa060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius ME, Wang TW, Jamal A, Loretan CG, & Neff LJ (2020). Tobacco product use among adults—United States, 2019. Morbidity and Mortality Weekly Report, 69(46), 1736–1742. 10.15585/mmwr.mm6946a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Jensen KP, O’Malley SS, Gueorguieva R, Krishnan-Sarin S, Valentine G, Jatlow PI, & Sofuoglu M (2020). Modulation of “protective” nicotine perception and use profile by flavorants: Preliminary findings in e-cigarettes. Nicotine & Tobacco Research, 22(5), 771–781. 10.1093/ntr/ntz057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenson NI, Buchhalter AR, Augustson EM, Rubinstein ML, & Henningfield JE (2020). Abuse liability assessment of the JUUL system in four flavors relative to combustible cigarette, nicotine gum and a comparator electronic nicotine delivery system among adult smokers. Drug and Alcohol Dependence, 217, Article 108395. 10.1016/j.drugalcdep.2020.108395 [DOI] [PubMed] [Google Scholar]
- Green BG, Shaffer GS, & Gilmore MM (1993). Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chemical Senses, 18(6), 683–702. 10.1093/chemse/18.6.683 [DOI] [Google Scholar]
- Hajek P, Pittaccio K, Pesola F, Myers Smith K, Phillips-Waller A, & Przulj D (2020). Nicotine delivery and users’ reactions to JUUL compared with cigarettes and other e-cigarette products. Addiction, 115(6), 1141–1148. 10.1111/add.14936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Duan Z, Kwok J, Binns S, Vera LE, Kim Y, Szczypka G, & Emery SL (2019). Vaping versus JUULing: How the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tobacco Control, 28(2), 146–151. 10.1136/tobaccocontrol-2018-054382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, & Silberberg A (2008). Economic demand and essential value. Psychological Review, 115(1), 186–198. 10.1037/0033-295X.115.1.186 [DOI] [PubMed] [Google Scholar]
- Kim H, Lim J, Buehler SS, Brinkman MC, Johnson NM, Wilson L, Cross KS, & Clark PI (2016). Role of sweet and other flavours in liking and disliking of electronic cigarettes. Tobacco Control, 25(Suppl. 2), ii55–ii61. 10.1136/tobaccocontrol-2016-053221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Franck CT, Stein JS, & Bickel WK (2015). A modified exponential behavioral economic demand model to better describe consumption data. Experimental and Clinical Psychopharmacology, 23(6), 504–512. 10.1037/pha0000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Green BG, Kong G, Cavallo DA, Jatlow P, Gueorguieva R, Buta E, & O’Malley SS (2017). Studying the interactive effects of menthol and nicotine among youth: An examination using e-cigarettes. Drug and Alcohol Dependence, 180, 193–199. 10.1016/j.drugalcdep.2017.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer NB, Veldhuizen MG, Delvy R, Patel BP, O’Malley SS, & Small DM (2018). Sweet taste potentiates the reinforcing effects of e-cigarettes. European Neuropsychopharmacology, 28(10), 1089–1102. 10.1016/j.euroneuro.2018.07.102 [DOI] [PubMed] [Google Scholar]
- Leventhal A, Cho J, Barrington-Trimis J, Pang R, Schiff S, & Kirkpatrick M (2020). Sensory attributes of e-cigarette flavours and nicotine as mediators of interproduct differences in appeal among young adults. Tobacco Control, 29(6), 679–686. 10.1136/tobaccocontrol-2019-055172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Goldenson NI, Barrington-Trimis JL, Pang RD, & Kirkpatrick MG (2019). Effects of non-tobacco flavors and nicotine on e-cigarette product appeal among young adult never, former, and current smokers. Drug and Alcohol Dependence, 203, 99–106. 10.1016/j.drugalcdep.2019.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Wood A, & Green BG (2009). Derivation and evaluation of a labeled hedonic scale. Chemical Senses, 34(9), 739–751. 10.1093/chemse/bjp054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, & O’Malley SS (2013). The drug effects questionnaire: Psychometric support across three drug types. Psychopharmacology, 227(1), 177–192. 10.1007/s00213-012-2954-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Kozink RV, Carson CE, & McClernon FJ (2021). Appeal, subjective effects, and relative reinforcing effects of JUUL that vary in flavor and nicotine content. Experimental and Clinical Psychopharmacology, 29(3), 279–287. 10.1037/pha0000481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten T, & De Biasi M (2020). History repeats itself: Role of characterizing flavors on nicotine use and abuse. Neuropharmacology, 177, Article 108162. 10.1016/j.neuropharm.2020.108162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullicin AJ, Kim H, Brinkman MC, Buehler SS, Clark PI, & Lim J (2020). Impacts of nicotine and flavoring on the sensory perception of e-cigarette aerosol. Nicotine & Tobacco Research, 22(5), 806–813. 10.1093/ntr/ntz058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbrook K, & Green BG (2016). Sensory effects of menthol and nicotine in an E-cigarette. Nicotine & Tobacco Research,, 18(7), 1588–1595. 10.1093/ntr/ntw019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton CA, Sharma E, Edwards KC, Halenar MJ, Taylor KA, Kasza KA, Day H, Anic G, Gardner LD, Hammad HT, Bansal-Travers M, Limpert J, Borek N, Kimmel HL, Compton WM, & Hyland A (2020). Longitudinal transitions of exclusive and polytobacco electronic nicotine delivery systems (ENDS) use among youth, young adults and adults in the USA: Findings from the PATH Study Waves 1–3 (2013–2016). Tobacco Control, 29(Suppl. 3), s147–s154. 10.1136/tobaccocontrol-2019-055574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Koffarnus MN, Snider SE, Quisenberry AJ, & Bickel WK (2015). Identification and management of nonsystematic purchase task data: Toward best practice. Experimental and Clinical Psychopharmacology, 23(5), 377–386. 10.1037/pha0000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland JC, Campbell EM, Lile JA, & Stoops WW (2020). Utilizing the commodity purchase task to evaluate behavioral economic demand for illicit substances: A review and meta-analysis. Addiction, 115(3), 393–406. 10.1111/add.14792 [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Weaver MF, & Eissenberg T (2012). Clinical laboratory assessment of the abuse liability of an electronic cigarette. Addiction, 107(8), 1493–1500. 10.1111/j.1360-0443.2012.03791.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Rivera M, Ebrahimi Kalan M, Ward-Peterson M, Osibogun O, Li W, Brown D, Eissenberg T, & Maziak W (2021). Effect of flavour manipulation on ENDS (JUUL) users’ experiences, puffing behaviour and nicotine exposure among US college students. Tobacco Control, 30(4), 399–404. 10.1136/tobaccocontrol-2019-055551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, & Hajek P (2004). Evaluation of the mood and physical symptoms scale (MPSS) to assess cigarette withdrawal. Psychopharmacology (Berl), 177(1–2), 195–199. 10.1007/s00213-004-1923-6 [DOI] [PubMed] [Google Scholar]
- West R, & Ussher M (2010). Is the ten-item Questionnaire of Smoking Urges (QSU-brief) more sensitive to abstinence than shorter craving measures? Psychopharmacology (Berl), 208(3), 427–432. 10.1007/s00213-009-1742-x [DOI] [PubMed] [Google Scholar]
- Zare S, Nemati M, & Zheng Y (2018). A systematic review of consumer preference for e-cigarette attributes: Flavor, nicotine strength, and type. PLOS ONE, 13(3), Article e0194145. 10.1371/journal.pone.0194145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S-H, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, & Lee M (2014). Four hundred and sixty brands of e-cigarettes and counting: Implications for product regulation. Tobacco Control, 23(Suppl. 3), iii3–iii9. 10.1136/tobaccocontrol-2014-051670 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


