Abstract
Background
Multimodal treatment for patients with peritoneal metastases (PM) from colorectal cancer (CRC), including perioperative chemotherapy (CT) plus complete resection, is associated with prolonged survival. The oncologic impact of therapeutic delays is unknown.
Objective
The aim of this study was to assess the survival impact of delaying surgery and CT.
Methods
Medical records from the national BIG RENAPE network database of patients with complete cytoreductive (CC0–1) surgery of synchronous PM from CRC who received at least one neoadjuvant CT cycle plus one adjuvant CT cycle were retrospectively reviewed. The optimal interval between the end of neoadjuvant CT to surgery, surgery to adjuvant CT, and total interval without systemic CT were estimated using Contal and O’Quigley’s method plus restricted cubic spline methods.
Results
From 2007 to 2019, 227 patients were identified. After a median follow-up of 45.7 months, the median overall survival (OS) and progression-free survival (PFS) was 47.6 and 10.9 months, respectively. The best cut-off period was 42 days in the preoperative interval, no cut-off period was optimal in the postoperative interval, and the best cut-off period in the total interval without CT was 102 days. In multivariate analysis, age, biologic agent use, high peritoneal cancer index, primary T4 or N2 staging, and delay to surgery of more than 42 days (median OS 63 vs. 32.9 months; p = 0.032) were significantly associated with worse OS. Preoperative delay of surgery was also significantly associated with PFS, but only in univariate analysis.
Conclusion
In selected patients undergoing complete resection plus perioperative CT, a period of more than 6 weeks from completion of neoadjuvant CT to cytoreductive surgery was independently associated with worse OS.
In 2018, 1.8 million cases of colorectal cancer (CRC) were diagnosed worldwide,1 with peritoneal metastases (PM) being observed in 10% of these patients.2 Without surgical resection, PM are associated with a very poor prognosis, with a 5-year overall survival (OS) rate of 5%.3
In the PRODIGE 7 prospective randomized study, cytoreductive surgery (CRS) combined with systemic chemotherapy (CT) used in the pre- and postoperative periods offered long-term survival.4 The 5-year OS rate of 39% and median survival of 41 months has rarely been observed in previous studies.4 Systemic therapy has become a standard of care and oncologic societies recommend that patients with metastases from CRC consider systemic CT as the initial part of every treatment strategy.5
However, the optimal time from completion of neoadjuvant CT to CRS, and the time from CRS to initiation of adjuvant CT, is yet to be established and may affect the overall outcome. Experimental and clinical evidence support the hypothesis that this interval without CT is a window for the development of new metastases6 with a potentially harmful effect on OS.7 In current practice, resections were scheduled 4–5 weeks8 following completion of neoadjuvant CT, and adjuvant CT began 4–12 weeks after surgery. The timing of these sequential uses of CT was developed in an empirical fashion.
The delays in oncologic treatment significantly altered the prognosis in several different forms of cancer.9,10 For stage III colon cancer, the risk of death increases by 6%10 with a 4 week delay to surgery; the impact is more marked for delays to adjuvant CT, with an increased risk of death ranging from 6 to 100%.10–15 For stage IV colon cancer, few studies have evaluated the influence of delays to surgery on oncologic outcomes and no researchers have assessed the time delay to adjuvant CT or specifically evaluated patients with resectable PM.
For patients with liver metastases from CRC, an interval of more than 5 weeks between surgery and adjuvant CT was an independent predictor of decreased progression-free survival (PFS).8
The fight against the worldwide coronavirus disease 2019 (COVID-19) pandemic required extensive hospital resources, with staff having to make difficult decisions with regard to prioritization of care, resulting in delays in oncologic treatments such as elective surgery16 and systemic CT.17 Understanding the prognostic impact of treatment delays became crucial for guiding national policy making and organizing cancer services.18 One of the more current concerns is further improving the benefits of CT.
The main objective of this study was to (1) assess the survival impact of three time intervals without systemic CT: the preoperative interval (preI), corresponding to the time between completion of neoadjuvant CT and CRS; the postoperative interval (postI), corresponding to the time between CRS and the start of adjuvant CT; and the total interval (totalI) without CT, between completion of neoadjuvant CT and the start of adjuvant CT. The secondary objective of this study was to identify each component of the systemic CT regimen associated with changes in oncologic outcomes.
Patients and Methods
Data Source
BIG-RENAPE is a French National Network dedicated to peritoneal malignancy management and includes a clinical and biobank-based research database. This study was conducted in accordance with the Declaration of Helsinki and was approved by the appropriate Ethics Committees (CPP SUD-EST IV No. 2014-A01715-42, and CHU Angers No. 2021-104).
Patient Selection
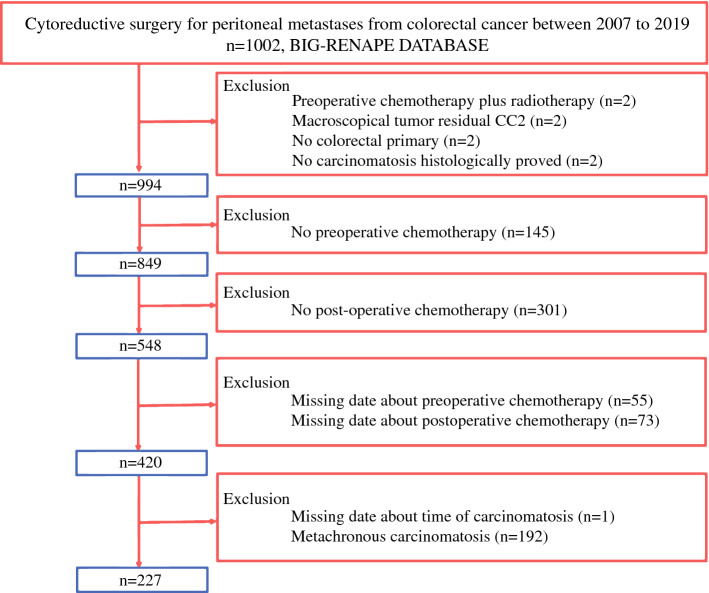
Patient selection is detailed in Fig. 1. All consecutive patients who underwent CRS from 2007 to 2019 were selected from the BIG RENAPE database. The inclusion criteria for the study included patients over the age of 18-years with pathologically confirmed PM from CRC, PM synchronous to primary, complete CRS (CC0–1) between 1 January 2007 and 31 December 2019, at least one cycle of neoadjuvant CT and one cycle of adjuvant CT, and timing of the neoadjuvant CT-surgery-adjuvant CT stated.
Fig. 1.
Patient selection process
The exclusion criteria included appendicular primary tumor, CC2 resection, preoperative radiotherapy, and prophylactic hyperthermic intraperitoneal chemotherapy (HIPEC; high risk of PM with Peritoneal Cancer Index [PCI] = 0 at surgical exploration).
Statistical Analysis
The first step was an overall descriptive analysis. Qualitative factors were described according to the frequency of their respective modalities, and continuous factors were described according to their mean ± standard deviation (SD). OS was defined as the time between the start date of adjuvant CT and the date of death from any cause (or censored at the date of the last news in life), while PFS was defined as the time between the start date of adjuvant CT and the date of progression or death (or censored at the date of the last update received in remission). OS of the overall population was described using Kaplan–Meier curves, and the median of the population follow-up was calculated using the inverse Kaplan–Meier method.
The second step was the univariate survival analysis. The univariate impact on OS of each of the studied factors was determined by the univariable Cox model.
For the delay variables, in order to facilitate their use in a prognostic score, we discretized them by looking for the best cut-off. Due to the multiple testing used to find the optimal cut-off point, we made an adjustment to the usual significance test to preserve the type I error rates. Several techniques can be considered at this point.
Contal and O’Quigley’s method19 uses the log-rank statistic (Q statistic) to categorize patients into high- or low-risk groups with regard to survival based on different thresholds for the predictor (i.e. delays). A Q statistic was computed for each threshold and the optimal threshold was selected, based on maximizing this Q statistic. The %FINDCUT SAS macro was used.
Cox models with restricted cubic splines (RCSs) were used to model the relationship between OS and perioperative delays.20 The number of knots for the splines was selected to minimize the AIC. The best model was then used to visualize inflection points in the least extreme data window. All models with splines were performed with the packages rms 6.2-0 using R software version 4.1.2 (2021-11-01) [‘Bird Hippie’].
The third step was the multivariate analysis to assess the independent prognostic impact of the factors studied. Variables significant at the 10% univariate level were entered into the multivariable Cox proportional hazards model and the adjusted hazard ratios [HRs] were calculated. Missing data for the variables selected for the multivariate analysis were imputed by multiple imputation if there was < 30% of missing data.
All analyses were performed with a final significance level set at 5% (two-sided formulation) using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA), Stata software version SE 17 (StataCorp LLC, College Station, TX, USA) and R software version 4.1.2 (2021-11-01) [‘Bird Hippie’].
Results
Patient Characteristics
Patient characteristics are in Table 1. Overall, 119 female patients (52.4%) and 108 male patients (47.6%) were included in this study, with a mean age of 58 years (SD 10.8).
Table 1.
Patient characteristics
| Characteristics | N = 227 |
|---|---|
| Mean age, years (±SD) | 58.3 ± 10.82 |
| Sex | |
| Female | 119 (52.4) |
| Male | 108 (47.6) |
| Mean body mass index (±SD) | 24.54 ± 5.09 |
| ASA score | |
| 1 | 55 (26.2) |
| 2 | 137 (65.2) |
| 3 | 17 (8.1) |
| 4 | 1 (0.5) |
| Missing data | 17 |
| Number of cytoreductive surgeries per patient | |
| 1 | 202 (89) |
| >1 | 25 (11) |
| Period | |
| 2007–2015 | 120 (52.9) |
| 2016–2020 | 107 (47.1) |
| Primary location | |
| Right colon | 80 (35.4) |
| Left colon | 134 (59.3) |
| Transverse colon | 8 (3.5) |
| Rectum | 4 (1.8) |
| Missing data | 1 |
| Primary T stage | |
| T1 | 1 (0.6) |
| T2 | 1 (0.6) |
| T3 | 48 (30.8) |
| T4 | 105 (67.3) |
| Tx | 1 (0.6) |
| Missing data | 71 |
| Primary N stage | |
| N0 | 34 (21.9) |
| N1 | 57 (36.8) |
| N2 | 64 (41.3) |
| Missing data | 72 |
Data are expressed as n (%) unless otherwise specified
SD standard deviation, ASA American society of anesthesiologists
The PM was a result of adenocarcinomas of the right colon (35.4%), transverse colon (3.5%), left colon (59.3%), and rectum (1.8%), and unknown in one patient. Overall, 180 patients (79.3%) were treated in one center (Lyon) and 47 (21.7%) were treated in the other eight centers.
Treatment
Operative and treatment characteristics are in Table 2. Preoperative systemic CT used two and three drug regimens in 90 (39.6%) and 125 (55.1%) patients, respectively, and included new targeted molecules in 96 (42.3%) cases.
Table 2.
Operative and treatment characteristics
| Characteristics | N = 227 |
|---|---|
| Number of chemotherapy lines | |
| 1 | 207 (91.2) |
| >1 | 20 (8.8) |
| Neoadjuvant chemotherapy regimen | |
| Monotherapy (capecitabine/5-fluorouracil) | 2 (0.9) |
| Doublet (FOLFIRI/FOLFOX) | 90 (39.6) |
| Triplet (FOLFIRI+biologic agent/FOLFOX+biologic agent/FOLFIRINOX) | 125 (55.1) |
| Quadruplet (FOLFIRINOX+biologic agent) | 10 (4.4) |
| Neoadjuvant biologic agent, | |
| None | 96 (42.3) |
| EGFR antibody (cetuximab/panitumumab) | 39 (17.2) |
| VEGF antibody (bevacizumab) | 57 (25.1) |
| Mean number of neoadjuvant chemotherapy cycles (SD) | 6.02 ± 3.01 |
| Mean Peritoneal Cancer Index (SD) | 8.79 ± 7.31 |
| Completeness of cytoreductive surgery | |
| CC0 | 215 (95.1) |
| CC1 | 11 (4.9) |
| Missing data | 1 |
| HIPEC | |
| No | 197 (86.8) |
| Yes | 30 (13.2) |
| Primary tumor differentiation | |
| Good | 35 (15.4) |
| Moderate | 92 (40.5) |
| Low | 25 (11) |
| Missing data | 6 |
| KRAS status | |
| Wild-type | 110 (60.4) |
| Mutated | 72 (39.6) |
| Missing data | 45 |
| Postoperative morbidity at 90 days (grade III–IV) | |
| No | 141 (62.1) |
| Yes | 86 (37.9) |
| Mean number of days of hospitalization (SD) | 17.43 ± 9.65 |
| Adjuvant chemotherapy protocol | |
| Monotherapy (capecitabine/5-fluorouracil) | 15 (6.8) |
| Doublet (FOLFIRI/FOLFOX) | 152 (69.1) |
| Triplet (FOLFIRI+biologic agent/FOLFOX+biologic agent/FOLFIRINOX) | 46 (20.9) |
| Quadruplet (FOLFIRINOX+biologic agent) | 7 (3.1) |
| Missing data | 7 |
| Adjuvant biological agent | |
| None | 173 (76.2) |
| EGFR antibody | 15 (6.6) |
| VEGF antibody | 35 (15.4) |
| Unknown | 4 |
| Mean number of adjuvant chemotherapy cycles (SD) | 5.96 ± 2.06 |
| Mean number of preoperative days without systemic chemotherapy (SD) | 37.63 ± 17.53 |
| Mean number of postoperative days without systemic chemotherapy (SD) | 63.16 ± 19.19 |
| Mean number of total perioperative days without systemic chemotherapy (SD) | 100.79 ± 26.02 |
| Total number of perioperative chemotherapy cycles (SD) | 11.98 ± 2.9 |
Data are expressed as n (%) unless otherwise specified
SD standard deviation, EGFR epidermal growth factor receptor, VEGF vascular endothelial growth factor, HIPEC hyperthermic intraperitoneal chemotherapy
On completion of the best surgical effort with CRS, 215 patients (95.1%) had a CC-0 resection and 11 patients (4.9%) had a CC-1 resection. Overall, 197 (86.8%) patients had undergone HIPEC, with many variations in exposure techniques (i.e., open or closed wall), duration (30–90 min), or drugs (oxaliplatin/oxaliplatin plus irinotecan/mitomycin); 30 (13.2%) patients received no intraperitoneal CT.
Postoperative systemic CT used two and three drug regimens in 152 (69.1%) and 46 (20.9%) patients, respectively, and included new targeted molecules in 50 (22.4%) cases. The mean number of CT cycles was 6.02 ± 3.01 and 5.96 ± 2.06 in the neoadjuvant and adjuvant periods, respectively. The rate of postoperative morbidity grades III/IV at 90 days was 37.9%. Postoperative deaths were excluded from analysis of those patients because they never received adjuvant CT.
Cut-Off Interval
The mean intervals for preI, postI, and totalI were 37.63 (±17.53), 63.16 (±19.19), and 100.79 (±26.02) days, respectively.
For preI, the RCS function found that the optimal cut-off time was between 38 and 47 days (5–7 weeks) for obtaining the best OS discrimination. The Contal and O’Quigley method located the optimal cut-off in a time period of between 41 and 47 days (6–7 weeks). The Contal and O’Quigly method calculations maximized abs (SK) of 15.96 at preI (43 days; within the first 6 weeks vs. more than 6 weeks), with a Contal and O’Quigley adjusted p value of 0.0073 and a false discovery rate p value of 0.0009. The best cut-off was thus also 42 days.
For postI, the RCS function found no inflection points and the Contal and O’Quigley method found no significant cut-off points among the 22 levels tested.
For TotalI, the RCS function defined an optimal TotalI of between 100 and 116 days (14–16 weeks) for obtaining the best OS. The Contal and O’Quigley method located the optimal cut-off at 102 days, with abs (SK) = 13.52 and adjusted p = 0.035.
Survival
With a median follow-up of 45.7 months (95% confidence interval [CI] 37.7–50.6), the median PFS and OS was 10.9 and 47.6 months, respectively. The PFS rates at 1, 3, and 5 years were 44.3%, 23.3%, and 23.3%, respectively, while the OS rates at 1, 3, and 5 years were 87.8%, 60.9%, and 42.1%, respectively.
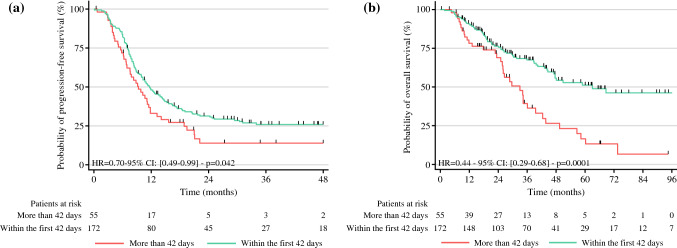
A long preI (> 42 days) significantly decreased the median PFS: 11.6 versus 9.3 months (p = 0.042) [Fig. 2]
Fig. 2.
a Progression-free survival and b overall survival among patients with a preoperative interval between completion of neoadjuvant chemotherapy and surgery of more or less than 42 days. HR hazard ratio, CI confidence interval
In the univariate analysis (Table 3), primary N2 status, neoadjuvant biologic agent use, neoadjuvant bevacizumab use, preI > 42 days, high PCI, no HIPEC use, and low number of adjuvant CT cycles significantly decreased PFS. In multivariate analysis (Table 4), no HIPEC use, high PCI, neoadjuvant biologic agent use, and primary N2 status also significantly decreased PFS.
Table 3.
Univariable analysis for progression-free and overall survival of 227 patients treated with cytoreductive surgery combined with perioperative chemotherapy for synchronous peritoneal metastases (stratified by center)
| Variable | Progression-free survival | Overall survival | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | 0.99 (0.98–1.01) | 0.184 | 0.98 (0.96–0.99) | 0.025 |
| Sex | ||||
| Female | 1 | 1 | ||
| Male | 1.17 (0.85–1.59) | 0.332 | 1.00 (0.67–1.50) | 0.985 |
| Body mass index | 1.01 (0.98–1.04) | 0.602 | 0.99 (0.95–1.03) | 0.550 |
| ASA | ||||
| 1 | 1.00 | 1.00 | ||
| 2 | 1.10 (0.76–1.60) | 0.597 | 1.02 (0.65–1.61) | 0.935 |
| 3/4 | 0.68 (0.33–1.37) | 0.280 | 0.63 (0.22–1.81) | 0.368 |
| Number of CRSs | ||||
| 1 | 1.00 | 1.00 | ||
| >1 | 1.28 (0.80–2.06) | 0.303 | 1.30 (0.73–2.32) | 0.369 |
| Period | ||||
| 2007–2015 | 1.00 | 1.00 | ||
| 2016–2020 | 0.74 (0.54–1.01) | 0.060 | 0.78 (0.49–1.22) | 0.275 |
| Primary tumor location | ||||
| Right colon | 1.00 | 1.00 | ||
|
Left colon T. colon Rectum |
0.82 (0.60–1.13) 0.56 (0.20–1.53) 0.72 (0.17–2.94) |
0.232 0.257 0.644 |
0.72 (0.48–1.09) 0.60 (0.15–2.49) 1.22 (0.29–5.09) |
0.122 0.485 0.783 |
| Primary T stage | ||||
| <4 | 1.00 | 1.00 | ||
| ≥4 | 1.30 (0.87–1.93) | 0.198 | 1.57 (0.92–2.68) | 0.095 |
| Primary N stage | ||||
| N0 | 1.00 | 1.00 | ||
| N1 | 1.38 (0.88–2.17) | 0.159 | 1.25 (0.66–2.36) | 0.497 |
| N2 | 1.94 (1.21–3.11) | 0.006 | 1.93 (1.01–3.70) | 0.048 |
| Number of neoadjuvant CT lines | ||||
| 1 | 1.00 | 1.00 | ||
| >1 | 1.32 (0.78–2.23) | 0.300 | 1.24 (0.59–2.57) | 0.573 |
| Neoadjuvant CT regimen | ||||
| Monotherapy/doublet | 1.00 | 1.00 | ||
| Triplet/quadruplet | 1.28 (0.93–1.76) | 0.127 | 1.37 (0.90–2.07) | 0.143 |
| Neoadjuvant biologic agent | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.69 (1.25–2.30) | 0.001 | 1.63 (1.10–2.43) | 0.016 |
| Neoadjuvant bevacizumab agent | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.77 (1.27–2.47) | 0.001 | 1.58 (1.04–2.40) | 0.030 |
| No. of neoadjuvant CT cycles | 1.02 (0.98–1.07) | 0.289 | 1.01 (0.95–1.08) | 0.649 |
| Preoperative interval without CT | ||||
| >42 days | 1.00 | 1.00 | ||
| ≤42 days | 0.70 (0.49–0.99) | 0.042 | 0.44 (0.29–0.68) | < 0.001 |
| Peritoneal Cancer Index | 1.03 (1.01–1.05) | 0.001 | 1.05 (1.03–1.08) | < 0.0001 |
| Completeness of cytoreductive surgery | ||||
| CC0 | 1.00 | 1.00 | ||
| CC1 | 1.01 (0.49–2.06) | 0.984 | 1.40 (0.61–3.23) | 0.428 |
| HIPEC | ||||
| No | 1.00 | 1 | ||
| Yes | 0.61 (0.40–0.92) | 0.018 | 0.75 (0.44–1.27) | 0.285 |
| KRAS status | ||||
| Wild-type | 1.00 | 1.00 | ||
| Mutated | 1.06 (0.75–1.50) | 0.729 | 0.78 (0.50–1.24) | 0.294 |
| Postoperative morbidity at 90 days (grade III–IV) | ||||
| No | 1.00 | 1.00 | ||
| Yes | 0.94 (0.70–1.31) | 0.694 | 1.21 (0.79–1.87) | 0.376 |
| Number of days of hospitalization | 1.01 (0.99–1.03) | 0.103 | 1.02 (1.01–1.04) | 0.016 |
| Adjuvant CT protocol | ||||
| Mono/doublet | 1.00 | 1.00 | ||
| Triplet/quadruplet | 1.19 (0.77–1.84) | 0.438 | 1.19 (0.77–1.84) | 0.438 |
| Adjuvant biologic agent | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.38 (0.97–1.95) | 0.074 | 1.13 (0.72–1.78) | 0.588 |
| Number of adjuvant CT cycles | 0.91 (0.84–0.98) | 0.017 | 0.95 (0.86–1.05) | 0.350 |
| Total number of perioperative CT cycles | 0.98 (0.93–1.04) | 0.516 | 0.99 (0.92–1.06) | 0.701 |
Significant results are in bold
ASA American society of anesthesiologists, CRS cytoreductive surgery, T colon transverse colon, CT chemotherapy, HIPEC hyperthermic intraperitoneal chemotherapy
Table 4.
Multivariable analysis for progression-free and overall survival of 227 patients treated with cytoreductive surgery combined with perioperative chemotherapy for synchronous peritoneal metastases (stratified by center)
| Variable | Progression-free survival | Overall survival | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | 0.97 (0.95–0.99) | 0.001 | ||
| Primary T stage | ||||
| <4 | 1.00 | |||
| ≥4 | 1.60 (0.96–2.66) | 0.071 | ||
| Primary N stage | ||||
| N0 | 1.00 | 1.00 | ||
| N1 | 1.24 (0.76–2.01) | 0.384 | 1.36 (0.65–2.82) | 0.409 |
| N2 | 1.73 (1.05–2.86) | 0.031 | 2.08 (1.01–4.32) | 0.049 |
| Neoadjuvant biologic agent | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.81 (1.03–3.19) | 0.040 | 2.16 (1.03–4.53) | 0.042 |
| Preoperative interval without CT | ||||
| >42 days | 1.00 | 1.00 | ||
| ≤42 days | 0.79 (0.54–1.16) | 0.238 | 0.36 (0.22–0.59) | <0.001 |
| Peritoneal Cancer Index | 1.04 (1.01–1.07) | 0.002 | 1.05 (1.02–1.09) | 0.001 |
| HIPEC | ||||
| No | 1.00 | |||
| Yes | 0.62 (0.39–0.98) | 0.040 | ||
Significant results are in bold
CT chemotherapy, HIPEC hyperthermic intraperitoneal chemotherapy
In the univariate analysis (Table 3), sex, body mass index, histologic grade, primary location, and number of CRSs did not have a prognostic impact on OS. In contrast, advanced age, an extended carcinomatosis (i.e., PCI; p < 0.001), primary T4 and primary N2 (p < 0.05) stages had significantly negative prognostic impacts. Of the therapeutic factors, neoadjuvant setting, adjuvant setting, number of cycles, use of bevacizumab, number of drugs, biological agent used, or hyperthermic intraperitoneal CT use had no prognostic impact. Neoadjuvant biologic agent use (p < 0.05) and the timing between neoadjuvant CT completion and surgery (p < 0.01) were prognostic factors. A long preI (> 42 days) significantly decreased the median OS: 63 versus 32.9 months (p < 0.01) [Fig. 2].
A totalI of less or more than 102 days had a median OS of 50.8 months versus 36 months (p = 0.016) [Fig. 3], respectively. PostI had no influence on the risk of death.
Fig. 3.
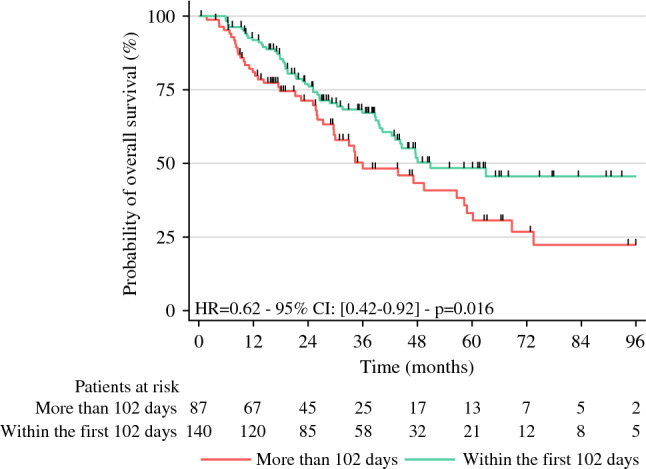
Overall survival among patients with a perioperative interval between completion of neoadjuvant chemotherapy and the start of adjuvant chemotherapy of more or less than 102 days. HR hazard ratio, CI confidence interval
In multivariate analysis (Table 4), age, primary T4, primary N2 stages, biologic agent use, high PCI, and preI > 42 days significantly decreased OS.
Discussion
Our study is the first to investigate whether the different time intervals between the completion of neoadjuvant CT and surgery, and after surgery and the start of adjuvant CT, has any prognostic relevance in patients with advanced PM from CRC. Although no phase III trials have shown any survival advantage for perioperative systemic CT in patients with colorectal PM, systemic CT is an important component in the multimodal treatment of patients with PM4,21 and is accepted as a standard of care.5
In our selected population of patients with colorectal PM with ambitious multidisciplinary treatment, including optimal resection and systematic pre- and postoperative CT, the median OS was 47.6 months. This promising survival is in line with the OS of 41 months reported in the PRODIGE 7 randomized study;4 this phase III trial used similar multimodal treatments. These long survivals were rarely observed in previous studies, in which survival ranged from 33 to 38 months.22–24 This complex sequential treatment is challenging in health care organizations and there is a need to understand the impact on survival of each component to use them to their fullest potential.
The CT regimen used in our study was the most effective in current modern practice and was an intensified CT. A triplet regimen was used in more than 60% of cases and targeted therapy was used in more than 50% of cases. Among the characteristics of the pre- or postoperative CT protocols, only the biologic agents used were associated with OS. Surprisingly, biologic agent use was associated with worse outcomes, which could be explained by the fact that patients administered a preoperative biologic agent may have also had more disease burden and/or more aggressive disease, which would explain their course. In the literature, the most evident advantage of intensified CT is the improved resectability of liver metastases,5 but there is no clear impact on OS, especially for resectable disease. For resectable PM, only one retrospective study showed a survival benefit when using neoadjuvant bevacizumab.25 No single regimen or drug has sufficient efficacy to be recommended in all situations. The choice of CT regimen depends on many biomarkers and prognostics factors,5 with some difficulties encountered in standardizing practices.
In this large population of patients with PM from CRC, with complete CRS plus perioperative CT, we observed that the best cut-off interval for totalI was 102 days. This interval was long in comparison with patients with PM from ovarian cancer. The study by Lee et al.26 found better OS and PFS with a totalI of ≤ 42 days, a finding that may be explained by a long preI and postI. Unlike PM from ovarian cancer, the chemosensitivity of PM from CRC is moderate. After 5–6 cycles of neoadjuvant CT, pathological complete response was 9% and 23% for PM from CRC and ovarian cancer, respectively.31,32 This modest chemosensitivity of PM from CRC might explain clinicians’ preferences for using intensified regimens with more drugs and cycles, leading to more adverse effects.
A long totalI of more than 102 days was associated with unfavorable median OS (50.8 vs. 36 months; p = 0.016); however, our multivariate analysis evaluating preI and postI separately showed that a delay in surgery after neoadjuvant CT was the only interval associated with worse OS. In the literature, there was no strong evidence for the efficacy of neoadjuvant CT before CRS. Four studies demonstrated a significant improvement in OS, while another three studies showed no benefit or a negative association.27 None of these studies mentioned the timing between neoadjuvant CT and CRS.
Our results indicated that completion of neoadjuvant CT < 42 days after CRS significantly improved OS and PFS in univariate analysis. In multivariate analysis, benefit on OS remained significant. During follow-up, a short preI of < 42 days strongly decreased the risk of death by 63%. To our knowledge, there are no studies reporting on the optimal time intervals for perioperative CT for PM from CRC. The optimum timing for surgery post neoadjuvant CT is a recurrent question in many oncological surgical specialties, and our results are in accordance with findings from other metastatic carcinomas. In the study by Chen et al.8 a preI of more than 5 weeks was significantly associated with a worse PFS in patients with liver metastases from CRC. Finding a cut-off for the preI between neoadjuvant CT and surgery is consistent with the physiology-based hypotheses of tumor growth—the smaller the tumor, the higher the cell growth fraction. The multiple small foci of carcinomatosis tend to grow rapidly over time; the kinetics are not linear but exponential.28 The effect of an anticancer drug on tumor weight is typically delayed and smoothes the growth curve, but in most cases the kinetic growth becomes exponential again several days after stopping CT.28 These findings are an important rationale for removing tumors as fast as possible after completing neoadjuvant CT because the prognosis for PM depends highly on the extent of the disease.4,23,24
Several factors can influence time to treatment, such as CT adverse effects or postoperative complications. A recent factor was the worldwide COVID-19 pandemic, leading to major alterations in health system performances. This pandemic was significantly associated with no or delayed scheduled oncological surgery, especially in advanced cancer.29 Patients undergoing CRS required intensive perioperative care, in competition with patients with severe COVID-19 infections. Populations with advanced cancer such as PM were vulnerable to a health system with low resilience and an exacerbated scarcity of resources. Our study demonstrated a significant oncologic impact with worse OS in cases of delayed surgery after neoadjuvant CT. This requires long-term investment to manage public health emergencies without major disruption to elective care and operational cancer planning. Fast and adaptative reorganization of health systems to allow them to deal with external system shocks are essential for protecting the capacity for elective oncologic surgery.
In our study, postI was not associated with OS. The survival benefit of postoperative CT after CRS for PM from CRC is inconsistent in the literature. We hypothesized that our selection of patients who underwent optimal and complete CRS limited the advantages of postoperative CT. For patients with PM from ovarian cancer, Hofstetter et al.30 showed that delayed postoperative CT compromised OS, but only in patients with suboptimal debulking. Similarly, the review of the literature by Waite et al.27 on PM from CRC showed that adjuvant CT improved OS, especially for patients with incomplete CRS.
Moreover, all patients received neoadjuvant CT in our study. Neoadjuvant CT could limit the benefit of adjuvant CT. European Society for Medical Oncology (ESMO) guidelines recommend adjuvant CT, particularly for patients who did not receive neoadjuvant CT.5
In multivariate analysis, extended carcinoma (PCI) and primary staging (T and N status) were significantly associated with OS. In the literature, these factors are well-known for having a strong impact on survival;23,24 they mark the aggressiveness and tumor load of the disease. Despite the selection of patients who systematically received neoadjuvant CT, the PCI and T and N factors remained significantly associated with OS. This finding suggests that tumor shrinkage or the biological behavior of tumors controlled by neoadjuvant CT was not as significant as expected.
This study has some limitations inherent to the retrospective nature of the study. Some data were missing. The study lacked information about physical status, nutritional status, economic level, or causes of therapeutics delays, all of which can potentially confound bias in the prognostic analysis of intervals. Patients with major postoperative complications who become too frail to receive adjuvant CT were not included in this study. The OS of our study reflected a select population able to receive CT and not survival of a population in intention-to-treat analysis.
Despite these limitations, the major strengths of this study are that it was based on a prospective database, using multicentric institution experience from expert centers on a homogeneous population who received modern CT, with an optimal statistical method for identifying the best timing. Moreover, this is the largest series on this population with multimodal treatment.
Conclusion
In selected patients undergoing complete resection plus perioperative CT, a time interval of more than 42 days from the completion of neoadjuvant CT to cytoreductive surgery was independently associated with worse OS. Optimal planning of surgery is mandatory with a short preI without systemic CT.
Acknowledgment
To member of BIG RENAPE collaborators. Catherine Arvieux M.D., Ph.D., (Hôpital d’instruction des Armées Bégin, Saint-Mandé), Cécile Brigand M.D. (Hôpitaux Universitaires de Hautepierre, Starsbourg), Ph.D., Jean-Baptiste Delhorme (Hôpitaux Universitaires de Hautepierre, Starsbourg), M.D. Ph.D., Diane Goere (Hôpital Saint-louis, AP-HP, Paris), M.D., Ph.D., Antoine Mariani, M.D. (Hôpital Européen Georges Pompidou, Paris) Marc Pocard M.D., Ph;D., (Hôpitaux Universitaires Pitié Salpêtrière, AP-HP, Paris), François Quenet, M.D., (Institut de cancérologie de montpellier Val D’Aurrelle, Montpellier), Olivia Sgarbura, M.D., Ph.D., (Institut de Cancérologie de Montpellier Val D’Aurrelle, Montpellier) Isabelle Sourrouille., M.D., Ph.D., (Gustave Roussy Cancer Campus, Villejuif, grand Paris) Abdelkader Taibi., M.D., Ph.D. (Centre Hospitalier Universitaire Dupuytren, Limoges).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Frédéric Dumont, Email: fredericdumont3108@yahoo.fr, Email: frederic.dumont@ico.unicancer.fr.
BIG-RENAPE Working Groups:
Catherine Arvieux, Cécile Brigand, Jean-Baptiste Delhorme, Diane Goere, Antoine Mariani, Marc Pocard, François Quenet, Olivia Sgarbura, Isabelle Sourrouille, and Abdelkader Taibi
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Quere P, Facy O, Manfredi S, Jooste V, Faivre J, Lepage C, et al. Epidemiology, management, and survival of peritoneal carcinomatosis from colorectal cancer: a population-based study. Dis Colon Rectum. 2015;58(8):743–752. doi: 10.1097/DCR.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 3.Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol. 2012;30(3):263–267. doi: 10.1200/JCO.2011.37.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quenet F, Elias DM, Roca L, Goere D, Ghouti L, Pocard M, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):256–266. doi: 10.1016/S1470-2045(20)30599-4. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 6.Kaifi JT, Kunkel M, Dicker DT, Joude J, Allen JE, Das A, et al. Circulating tumor cell levels are elevated in colorectal cancer patients with high tumor burden in the liver. Cancer Biol Therapy. 2015;16(5):690–698. doi: 10.1080/15384047.2015.1026508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabel L, Proudhon C, Gortais H, Loirat D, Coussy F, Pierga J-Y, et al. Circulating tumor cells: clinical validity and utility. Int J Clin Oncol. 2017;22(3):421–430. doi: 10.1007/s10147-017-1105-2. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Mao R, Zhao J, Bi X, Li Z, Huang Z, et al. From the completion of neoadjuvant chemotherapy to surgery for colorectal cancer liver metastasis: What is the optimal timing? Cancer Med. 2020;9(21):7849–7862. doi: 10.1002/cam4.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrelli Z, Ghidini G, Turati P, et al. Timing of adjuvant chemotherapy and survival in colorectal, gastric, and pancreatic cancer. A systematic review and meta-analysis. Cancers. 2019;11(4):550. doi: 10.3390/cancers11040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanna T, King W, Thibodeau S, Jalink M, Paulin G, Harvey-Jones E, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;4(371):m4087. doi: 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayraktar UD, Chen E, Bayraktar S, Sands LR, Marchetti F, Montero AJ, et al. Does delay of adjuvant chemotherapy impact survival in patients with resected stage II and III colon adenocarcinoma? Cancer. 2011;117(11):2364–2370. doi: 10.1002/cncr.25720. [DOI] [PubMed] [Google Scholar]
- 12.Turner MC, Farrow NE, Rhodin KE, Sun Z, Adam MA, Mantyh CR, et al. Delay in adjuvant chemotherapy and survival advantage in stage III colon cancer. J Am College Surg. 2018;226(4):670–678. doi: 10.1016/j.jamcollsurg.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 13.Lima ISF, Yasui Y, Scarfe A, Winget M. Association between receipt and timing of adjuvant chemotherapy and survival for patients with stage III colon cancer in Alberta, Canada. Cancer. 2011;117(16):3833–3840. doi: 10.1002/cncr.25954. [DOI] [PubMed] [Google Scholar]
- 14.Cheung WY, Neville BA, Earle CC. Etiology of delays in the initiation of adjuvant chemotherapy and their impact on outcomes for stage II and III rectal cancer. Dis Colon Rectum. 2009;52(6):1054–1064. doi: 10.1007/DCR.0b013e3181a51173. [DOI] [PubMed] [Google Scholar]
- 15.Hershman D, Hall MJ, Wang X, Jacobson JS, McBride R, Grann VR, et al. Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer. Cancer. 2006;107(11):2581–2588. doi: 10.1002/cncr.22316. [DOI] [PubMed] [Google Scholar]
- 16.Nunoo-Mensah JW, Rizk M, Caushaj PF, Giordano P, Fortunato R, Dulskas A, et al. COVID-19 and the global impact on colorectal practice and surgery. Clin Colorectal Cancer. 2020;19(3):178–190.e1. doi: 10.1016/j.clcc.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang M, Daniels B, Aslam M, Schaffer A, Pearson S-A. Changes in systemic cancer therapy in Australia during the COVID-19 pandemic: a population-based study. Lancet Reg Health Western Pac. 2021;14:100226. doi: 10.1016/j.lanwpc.2021.100226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glehen O, Kepenekian V, Bouché O, Gladieff L, Honore C, Abba J, et al. Treatment of primary and metastatic peritoneal tumors in the Covid-19 pandemic. Proposals for prioritization from the RENAPE and BIG-RENAPE groups. J Visc Surgery. 2020;157(3):S25–S31. doi: 10.1016/j.jviscsurg.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Contal C, O’Quigley J. An application of change point methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999;30(3):253–270. doi: 10.1016/S0167-9473(98)00096-6. [DOI] [Google Scholar]
- 20.Ranney DN, Mulvihill MS, Yerokun BA, Fitch Z, Sun Z, Yang C-F, et al. Surgical resection after neoadjuvant chemoradiation for oesophageal adenocarcinoma: What is the optimal timing? Eur J Cardio-Thorac Surgery. 2017;52(3):543–551. doi: 10.1093/ejcts/ezx132. [DOI] [PubMed] [Google Scholar]
- 21.van Eden WJ, Kok NF, Jóźwiak K, Lahaye ML, Beets GL, van Leerdam ME, et al. Timing of systemic chemotherapy in patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Dis Colon Rectum. 2017;60(5):477–487. doi: 10.1097/DCR.0000000000000774. [DOI] [PubMed] [Google Scholar]
- 22.Devilee RA, Simkens GA, van Oudheusden TR, Rutten HJ, Creemers GJ, ten Tije AJ, et al. Increased survival of patients with synchronous colorectal peritoneal metastases receiving preoperative chemotherapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2016;23(9):2841–2848. doi: 10.1245/s10434-016-5214-3. [DOI] [PubMed] [Google Scholar]
- 23.Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22(16):3284–3292. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Elias D, Gilly F, Boutitie F, Quenet F, Bereder J-M, Mansvelt B, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28(1):63–68. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 25.Ceelen W, Van Nieuwenhove Y, Putte DV, Pattyn P. Neoadjuvant chemotherapy with bevacizumab may improve outcome after cytoreduction and hyperthermic intraperitoneal chemoperfusion (HIPEC) for colorectal carcinomatosis. Ann Surg Oncol. 2014;21(9):3023–3028. doi: 10.1245/s10434-014-3713-7. [DOI] [PubMed] [Google Scholar]
- 26.Lee YJ, Chung YS, Lee J-Y, Nam EJ, Kim SW, Kim S, et al. Impact of the time interval from completion of neoadjuvant chemotherapy to initiation of postoperative adjuvant chemotherapy on the survival of patients with advanced ovarian cancer. Gynecol Oncol. 2018;148(1):62–67. doi: 10.1016/j.ygyno.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Waite K, Youssef H. The role of neoadjuvant and adjuvant systemic chemotherapy with cytoreductive surgery and heated intraperitoneal chemotherapy for colorectal peritoneal metastases: a systematic review. Ann Surg Oncol. 2017;24(3):705–720. doi: 10.1245/s10434-016-5712-3. [DOI] [PubMed] [Google Scholar]
- 28.Simeoni M, Magni P, Cammia C, De Nicolao G, Croci V, Pesenti E, et al. Predictive pharmacokinetic-pharmacodynamic modeling of tumor growth kinetics in xenograft models after administration of anticancer agents. Cancer Res. 2004;64(3):1094–1101. doi: 10.1158/0008-5472.CAN-03-2524. [DOI] [PubMed] [Google Scholar]
- 29.Glasbey J, Ademuyiwa A, Adisa A, AlAmeer E, Arnaud AP, Ayasra F, et al. Effect of COVID-19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: an international, prospective, cohort study. Lancet Oncol. 2021;22(11):1507–1517. doi: 10.1016/S1470-2045(21)00493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofstetter G, Concin N, Braicu I, Chekerov R, Sehouli J, Cadron I, et al. The time interval from surgery to start of chemotherapy significantly impacts prognosis in patients with advanced serous ovarian carcinoma—analysis of patient data in the prospective OVCAD study. Gynecol Oncol. 2013;131(1):15–20. doi: 10.1016/j.ygyno.2013.07.086. [DOI] [PubMed] [Google Scholar]
- 31.Passot G, You B, Boschetti G, Fontaine J, Isaac S, Decullier E, et al. Pathological response to neoadjuvant chemotherapy: a new prognosis tool for the curative management of peritoneal colorectal carcinomatosis. Ann Surg Oncol. 2014;21(8):2608–2614. doi: 10.1245/s10434-014-3647-0. [DOI] [PubMed] [Google Scholar]
- 32.Petrillo M, Zannoni GF, Tortorella L, Pedone Anchora L, Salutari V, Ercoli A, et al. Prognostic role and predictors of complete pathologic response to neoadjuvant chemotherapy in primary unresectable ovarian cancer. Am J Obstet Gynecol. 2014;211(6):632.e1–632.e8. doi: 10.1016/j.ajog.2014.06.034. [DOI] [PubMed] [Google Scholar]