Abstract
Purpose
Methylene blue (MB) has been tested as a rescue therapy for patients with refractory septic shock. However, there is a lack of evidence on MB as an adjuvant therapy, its’ optimal timing, dosing and safety profile. We aimed to assess whether early adjunctive MB can reduce time to vasopressor discontinuation in patients with septic shock.
Methods
In this single-center randomized controlled trial, we assigned patients with septic shock according to Sepsis-3 criteria to MB or placebo. Primary outcome was time to vasopressor discontinuation at 28 days. Secondary outcomes included vasopressor-free days at 28 days, days on mechanical ventilator, length of stay in ICU and hospital, and mortality at 28 days.
Results
Among 91 randomized patients, forty-five were assigned to MB and 46 to placebo. The MB group had a shorter time to vasopressor discontinuation (69 h [IQR 59–83] vs 94 h [IQR 74–141]; p < 0.001), one more day of vasopressor-free days at day 28 (p = 0.008), a shorter ICU length of stay by 1.5 days (p = 0.039) and shorter hospital length of stay by 2.7 days (p = 0.027) compared to patients in the control group. Days on mechanical ventilator and mortality were similar. There were no serious adverse effects related to MB administration.
Conclusion
In patients with septic shock, MB initiated within 24 h reduced time to vasopressor discontinuation and increased vasopressor-free days at 28 days. It also reduced length of stay in ICU and hospital without adverse effects. Our study supports further research regarding MB in larger randomized clinical trials.
Trial registration ClinicalTrials.gov registration number NCT04446871, June 25, 2020, retrospectively registered.
Keywords: Methylene blue, Randomized controlled trial, Septic shock, Norepinephrine, Vasopressin, Catecholamine sparing
Background
Sepsis is a host’s dysregulated response to an infection, characterized by endothelial dysfunction leading to increased vascular permeability, abnormal nitric oxide (NO) metabolism, vasodilation, among other systemic derangements [1]. Along with antibiotics, the Surviving Sepsis Campaign (SSC) Guidelines recommend early fluid resuscitation as a cornerstone of management [2]; however, only half of the patients respond to fluid challenges [3] and their hemodynamic effects are transient, sometimes lasting as short as 10 min [4]; so, vasopressors are needed to improve organ perfusion. Norepinephrine is the first-choice [2], but high doses increase the risk of adverse effects such as tachyarrhythmia, myocardial dysfunction, peripheral ischemia, and even immunosuppression [5, 6]. Therefore, a combination of agents targeting different systems involved in blood pressure regulation and endothelial function has been recently proposed [7–9]. This “multimodal strategy” could help to restore tissue perfusion while decreasing the potential toxicity of single agents [10, 11].
Methylene blue (MB) is a specific inhibitor of the inducible nitric oxide synthase (iNOS) and its downstream enzyme soluble guanylate cyclase (sGC). Through its indirect pressor effects, it has been shown to restore vasoregulation in conditions of NO upregulation [12]. However, most of the clinical research has been performed in vasoplegia following cardiopulmonary bypass (CPB), another form of vasodilatory shock similar to sepsis [13]. Despite promising results of two small randomized controlled trials (RCT) with short follow-up (≤ 48 h) performed in patients with septic shock two decades ago [14, 15], the momentum to further research was shortly lost.
Based on large datasets, it is increasingly recognized that a higher exposure to catecholamine vasopressors is associated with an increased risk of multiple organ failure and death in septic shock [8, 16, 17]; thus, the time of norepinephrine requirement is a justified intermediate patient-centered outcome [18] in order to pave the way for adding catecholamine-sparing agents to a multimodal strategy [7, 19]. We designed this RCT to assess if early adjunctive MB administration could reduce the time to vasopressor discontinuation in patients with septic shock, as compared to placebo.
Materials and methods
Trial design and oversight
We conducted an investigator-initiated, parallel, double blinded, randomized controlled trial at an academic reference center in Mexico, in a medical-surgical intensive care unit (ICU). The study was approved by the institutional review board “Comité de Ética en Investigación Hospital Civil Fray Antonio Alcalde” (HCG/CEI-0252/17) and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. This trial was registered at clinicaltrials.gov (NCT04446871) after inclusion of 17 patients. Informed consent was obtained from all patients or decision makers.
Patients
Patients aged ≥ 18 years with septic shock as defined by Sepsis-3 criteria (highly suspected or confirmed infection, requiring norepinephrine to maintain a MAP ≥ 65 mmHg, and serum lactate > 2 mmol/L after adequate fluid resuscitation) [20] were assessed for eligibility.
Exclusion criteria included > 24 h since initiation of norepinephrine, pregnancy, high probability of death within 48 h, concurrent hemorrhagic, obstructive or hypovolemic shock, pending damage control surgery, major burn injury, personal or familiar history of glucose-6-phosphate dehydrogenase deficiency, allergy to methylene blue, phenothiazines, or food dyes, recent intake (4-weeks) of selective serotonin re-uptake inhibitors, and refusal of the patient or decision maker to participate. During the beginning of coronavirus disease (COVID-19) pandemic, data safety monitoring board did not allow us to recruit patients with this diagnosis, due to the unknown pathophysiologic underpinnings and the uncertainties about response to MB.
Randomization, intervention and measurements
After signing informed consent, patients were randomly assigned to receive MB using a predetermined randomization sequence prepared in sealed opaque envelopes. The sequence was generated by computer with a 1:1 allocation ratio, using permuted blocks with a size of 4. Critical care physicians were responsible for assignment of intervention. Patients, clinicians, investigators and outcome assessors were blinded to the treatment received.
Patients assigned to MB group received an intravenous (IV) infusion of 100 mg of MB in 500 ml of 0.9% sodium chloride solution over 6 h once daily for a total of 3 doses. Patients assigned to control group received the same dose of 500 ml of 0.9% sodium chloride without MB. In order to avoid visual identification of the infusion, all infusion bags and polyvinylchloride lines were prepared at central pharmacy with opaque envelopes.
In our unit, fluid resuscitation of septic shock is guided by dynamic tests for prediction of volume responsiveness before any IV fluid load; the most common methods in order of frequency are aortic velocity–time integral change after passive leg raising (cut-off 10%), arterial pulse pressure variation (cut-off 13%), tidal volume challenge (cut-off 3.5%), and respiratory variation of carotid peak flow velocity (cut-off 14%) [21]. We defined adequate fluid resuscitation as at least 500 ml bolus of balanced crystalloid followed by negative volume responsiveness by at least 2 different methods.
In patients of both groups, adjunctive vasopressin was initiated at a dose of 0.03 IU/min if norepinephrine dose reached ≥ 0.25 mcg/kg/min; evaluation of volume responsiveness was repeated at least 3 times each day as long as vasopressors were needed. Hydrocortisone at 200 mg/day dose by continuous infusion is also a standard in our unit, and it is withheld within 6-h after discontinuation of all vasopressors without taper [22]. Nurse-led vasopressor tapering protocol consisted of norepinephrine titrated at 15–20-min intervals to maintain mean arterial pressure (MAP) between 65 and 75 mmHg until complete discontinuation, and vasopressin was progressively withdrawn by 0.005 UI/min each hour only after complete discontinuation of norepinephrine.
Recorded information at randomization included demographic, ventilatory and laboratory data, including diagnosis of acute respiratory distress syndrome (ARDS) defined according to Berlin criteria [23]. Norepinephrine dose was recorded at randomization, immediately after each dose of intervention drug, at 24- and 48-h post-treatment, for a total of 4 days. Comprehensive multiorgan point-of-care ultrasound was performed at enrollment and as needed by critical care physicians with > 8 years of experience in critical care ultrasound, who are all certified trainers of the WINFOCUS (World Interactive Network Focused on Critical Ultrasound, https://www.winfocus.org/) international training unit. Ejection fraction was calculated by Teichholz’s method. Methemoglobin capillary saturation was continuously monitored along the intervention timeframe by pulse co-oximetry (Masimo Rainbow Set, Irvine, CA, USA) and maximum daily values were recorded.
Outcomes
All patients were followed up until 28 days of enrollment, with time to vasopressor discontinuation as the primary outcome, defined as discontinuation of all vasopressors for at least 48 consecutive hours.
Secondary outcomes included vasopressor-free days at 28 days, all-cause mortality at 28 days, serum lactate levels, days on mechanical ventilator, length of stay in ICU and hospital; and the change in serum creatinine, bilirubin, aspartate/alanine aminotransferase, PaO2/FIO2 ratio and ejection fraction after intervention.
Sample size
According to data of a previous study at our settings [22], with an expected mean vasopressor duration of 97 ± 69 h (median 83 h), and considering a decrease of 24 h as clinically relevant, the calculated sample size was 88 patients for the trial to provide a statistical power of 80%, and α-error of 0.05. Assuming a minimal attrition rate, we aimed to recruit 92 patients in total (46 per group).
Statistical analysis
Numeric data are expressed as a percentage (%), using x2 or Fisher’s exact test for comparison as appropriate. According to Shapiro–Wilk test for normality, normally distributed data are expressed as means ± standard deviation, and skewed data are reported as medians with interquartile ranges 25th–75th (IQR). Comparison of continuous variables between groups was made with Student’s t or Mann–Whitney U test, as appropriate; repeated measures analysis of variance or Friedman’s test was used to compare variables at different points in time. A Kaplan–Meier curve was plotted for vasopressor discontinuation and analyzed with death as a competing risk event using Fine and Gray test with proportional hazards model. All p values were two-sided, and a value < 0.05 was considered as statistically significant. Outcomes were analyzed on an intention-to-treat basis. Statistical analysis and figures were performed with MedCalc (MedCalc Software Ltd Ostend, Belgium. Version 20.1), GraphPad Prism (version 9.3.1) and R version 4.2.2.
Results
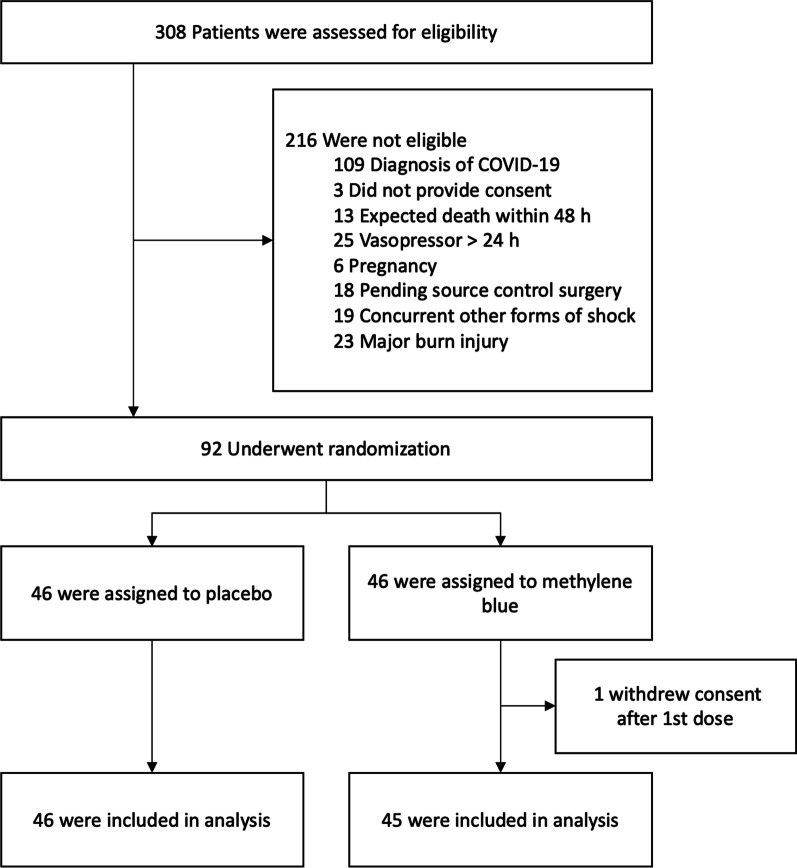
From March 16, 2017, to July 30, 2022, 308 patients were assessed for eligibility, of whom 92 underwent randomization (Fig. 1). One patient of the MB group withdrew consent after the first dose of intervention; therefore, 91 patients were included in the analysis, with 45 patients assigned to MB and 46 to control group. One patient in the control group died before receiving the 3rd dose; all other patients received the complete scheme. All patients received hydrocortisone. Median age was 46 years (IQR 35–55), sixty percent were men, and 46% presented acute kidney injury (AKI). The most common sources of sepsis were pulmonary (49.5%) and intra-abdominal (38.5%). All patients received antimicrobial treatment within 3 h of septic shock diagnosis. Most patients were on mechanical ventilation and received the assigned intervention after initial 6-h from shock identification. These and other baseline characteristics were similar in both groups (Table 1). The daily weight dose of MB in the intervention group was 1.2 mg/kg (IQR 1.1–1.4). No other vasopressors (phenylephrine, angiotensin II, epinephrine, midodrine) or inotropes (milrinone, dobutamine) were used.
Fig. 1.
Flowchart of participants
Table 1.
Baseline characteristics according to allocated group
| Characteristics | MB (n = 45) | Control (n = 46) |
|---|---|---|
| Age—years, median (IQR) | 46 (38–54) | 47 (31–60) |
| Female sex—no. (%) | 18 (40) | 18 (40) |
| Hypertension—no. (%) | 17 (38) | 18 (39) |
| Diabetes—no. (%) | 19 (42) | 17 (37) |
| Acute kidney injury—no. (%) | 22 (49) | 20 (44) |
| Infection source—no. (%) | ||
| Pneumonia—no. (%) | 22 (49) | 23 (50) |
| Intra-abdominal—no. (%) | 17 (38) | 18 (39) |
| Urinary tract—no. (%) | 4 (9) | 4 (9) |
| Other—no. (%) | 2 (4) | 1 (2) |
| Shock diagnosis to intervention—hours, mean (SD) | 8.3 ± 1.7 | 7.6 ± 2.3 |
| Positive fluid response at enrollment— no. (%) | 22 (49) | 20 (44) |
| Fluid load from shock diagnosis to intervention—ml/kg, mean (SD) | 24 ± 8.4 | 22 ± 9.6 |
| Heart rate, mean (SD) | 114 ± 9.9 | 115 ± 10.5 |
| Mean arterial pressure—mmHg, mean (SD) | 68 ± 4.5 | 67 ± 4.3 |
| Norepinephrine dose—mcg/kg/min), median (IQR) | 0.45 (0.27–0.68) | 0.37 (0.20–0.58) |
| Vasopressin use—no. (%) | 36 (80) | 34 (74) |
| Serum lactate—mmol/L, median (IQR) | 6.3 (4.8–7.4) | 5.0 (2.9–7.5) |
| Mechanical ventilation—no. (%) | 42 (93) | 38 (82) |
| ARDS—no. (%) | 36 (80) | 32 (70) |
| PaO2/FIO2 ratio (SD) | 190 ± 73 | 219 ± 79 |
| PEEP—cmH2O, IQR | 8 (6–8.5) | 7 (6–9) |
| Serum creatinine—mg/dL, median (IQR) | 1.5 (0.7–2.5) | 1.5 (0.8–2.1) |
| Serum bilirubin—mg/dL, median (IQR) | 1.4 (1.1–1.7) | 1.3 (0.8–1.6) |
| AST—mg/dL, median (IQR) | 113 (99–142) | 107 (75–163) |
| ALT—mg/dL, median (IQR) | 48 (41–78) | 39 (30–79) |
| Ejection fraction—%, median (IQR) | 61 (55–70) | 58 (54–66) |
| SOFA, median (IQR) | 10 (8–12) | 10 (8–12) |
| APACHE II, mean (SD) | 22.9 ± 4.4 | 22.4 ± 4.4 |
Plus–minus values are means ± standard deviation (SD); median with interquartile ranges (IQR) are in parentheses
MB methylene blue, ARDS acute respiratory distress syndrome, PEEP positive end-expiratory pressure, AST aspartate aminotransferase, ALT alanine aminotransferase, APACHE Acute Physiology and Chronic Health Evaluation
Primary outcome
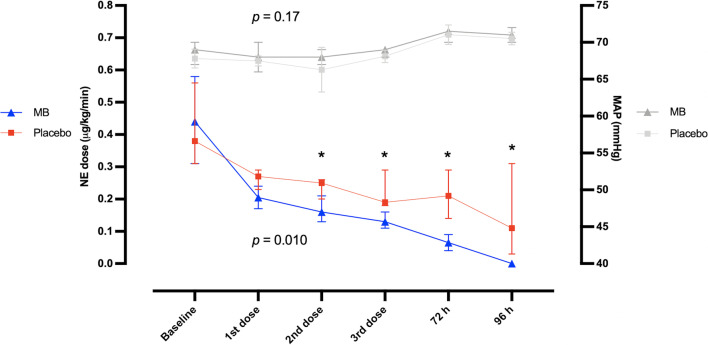
The time to vasopressor discontinuation was 69 h (IQR 59–83) in the MB group and 94 h (IQR 74–141) in the control group (median difference 29.4 [15.4–50.7]; p < 0.001). Norepinephrine dose requirement decreased more pronouncedly in the MB group as compared to placebo along the first 4 days (Fig. 2). A total of 5 of 45 (11%) patients in the MB group and 13 of 46 (28%) patients in the control group required re-initiation of NE within 48 h after discontinuation (p = 0.06).
Fig. 2.
Trends of mean arterial pressure and norepinephrine requirement along the first 4 days after recruitment. p values reflect the between subjects (groups) comparison test. * p < 0.05 between groups
Secondary outcomes
Patients in the MB group had 1.0 more days of vasopressor-free days at day 28 (p = 0.008). They had a lower cumulative fluid balance by 741 ml (CI95 293–1188; p = 0.001), a shorter ICU length of stay by 1.5 days (CI95 0.08–2.5; p = 0.039), and shorter hospital length of stay by 2.7 days (CI95 0.3–4.6; p = 0.027) compared to patients in the control group. At proportional hazards analysis, we found a hazard ratio for shock reversal of 2.7 for patients in the MB group at 28 days (CI95 1.5–5.0; p = 0.0007) (Fig. 3). Lactate levels within the first 3 days, days on mechanical ventilation and mortality at 28 days were similar (Table 2).
Fig. 3.
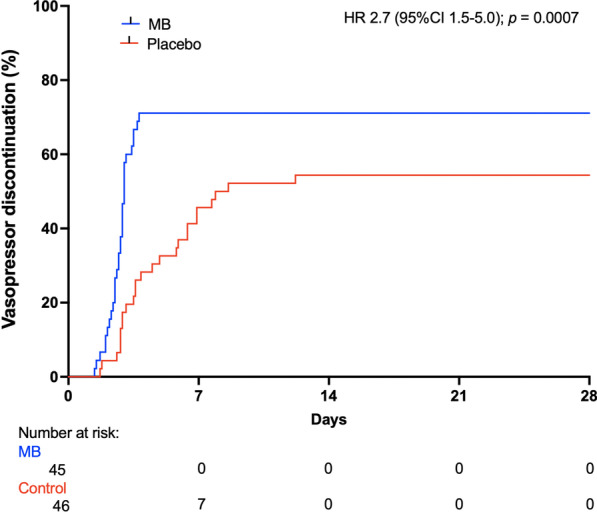
Kaplan–Meier plot of the cumulative incidence of vasopressor discontinuation. Adjusted hazard ratio with death as a competing risk analysis. MB methylene blue, HR hazard ratio
Table 2.
Outcomes according to allocated group
| Outcomes | MB (n = 45) | Control (n = 46) | Median difference (CI95) | p |
|---|---|---|---|---|
| Time to vasopressor discontinuation—hours, median (IQR) | 69 (59–83) | 94 (74–141) | 29.4 (15.4–50.7) | < 0.001 |
| Vasopressor-free days at 28 days, median (IQR) | 23.9 (0.0–24.8) | 19.5 (0.0–23.7) | 1.0 (0–4.1) | 0.008 |
| Cumulative fluid balance at 4 days, mean (SD) | 834 ± 1106 | 1575 ± 1040 | 741 (293–1188) | 0.001 |
| Days on mechanical ventilation, median (IQR) | 4.2 (3.3–5.1) | 5.1 (3.9–5.9) | 0.6 (− 0.06–1.2) | 0.075 |
| Serum lactate—mmol/L, median (IQR) | ||||
| 24 h | 2.9 (2.2–3.7) | 3.1 (2.6–6.0) | 0.5 (0.0–1.4) | 0.054 |
| 48 h | 1.7 (1.4–3.4) | 2.5 (1.1–3.3) | 0.3 (− 0.5–0.9) | 0.36 |
| 72 h | 1.4 (1.0–2.1) | 1.7 (0.5–3.1) | 0.0 (− 0.06–0.7) | 0.98 |
| ICU length of stay—days, median (IQR) | 6.6 (4.8–7.6) | 7.9 (5.0–10.0) | 1.5 (0.08–2.5) | 0.039 |
| Hospital length of stay—days, median (IQR) | 9.0 (6.3–9.3) | 10.5 (6.1–14.0) | 2.7 (0.3–4.6) | 0.027 |
| Relative Risk (CI95) | ||||
|---|---|---|---|---|
| Mortality at 28 days—no. (%) | 15/45 (33) | 21/46 (46) | 0.76 (0.55–1.05) | 0.23 |
Median with interquartile ranges are in parentheses
MB Methylene blue, CI confidence interval, ICU intensive care unit
Adverse effects
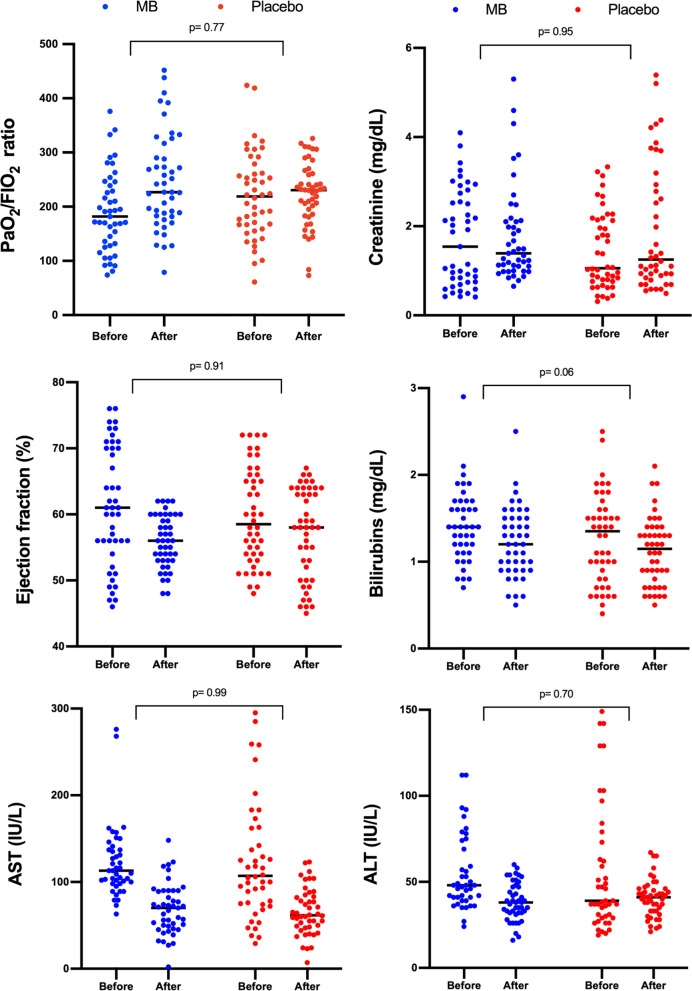
The most common adverse effect was green–blue discoloration of urine in 42 of 45 (93%) patients in the MB group. Maximum methemoglobin saturation was significantly higher in patients of the MB group (2.9% [IQR 2.2–3.3] vs 0.5% [IQR 0.4–0.7]; p < 0.001). Regarding other potential adverse effects, the change in ejection fraction, PaO2/FIO2, serum creatinine, bilirubins and liver aminotransferases were not different between groups after the intervention (Fig. 4).
Fig. 4.
Comparison of the change in monitoring values before initiation and after the last dose of intervention. p values reflect the between subjects (groups) comparison test. MB methylene blue, AST aspartate aminotransferase, ALT alanine aminotransferase
Discussion
In this single-center RCT, we found that adjunctive MB administered within 24 h of septic shock diagnosis reduced time to vasopressor discontinuation, and importantly, no severe adverse effects were detected. The clinical implications of our results might shift the current understanding of MB as a rescue therapy [24] to an adjunctive one at earlier stages of the disease. Due to its safety profile, wider availability and lower cost than other catecholamine-sparing agents [25, 26], MB could emerge as a viable therapy within a multimodal strategy to maintain MAP and improve tissue perfusion, while decreasing the risk of high-dose vasopressors [9, 27–29].
To our knowledge, this is the largest RCT comparing MB vs placebo in patients with septic shock. In the study by Kirov et al. in 2001, they randomized 20 patients with septic shock to placebo or a bolus injection of 2 mg/kg of MB, followed by a continuous 4-h infusion to a total dose of 5.75 mg/kg, and followed patients up to 24 h. Although more patients died in the control group (7 vs 3) and duration of vasopressor support was shorter in MB group (71 h vs 93 h), these differences were not significant [14]. In 2002, Memis et al. randomized 30 patients with septic shock to placebo or an infusion of MB at 3 mg/kg over 6 h, with a follow-up of 48 h. They found no differences in cytokine levels or any other relevant outcome; nonetheless, they reported a significant but transient increase in MAP in patients of MB group. Shortly after publication of these 2 promising studies, a large phase III multicenter RCT which included 797 patients with septic shock was published. López et al. randomized patients to placebo or the NOS inhibitor N(G)-methyl-L-arginine hydrochloride (546C88). The study was terminated early due to a significant increase by 10% in mortality of cardiovascular cause at 28 days [30]. This seems to have halted the enthusiasm in research about NO inhibition; however, it is important to note that this molecule is non-selective, inhibiting both inducible and constitutive isoforms of NOS (unlike MB which selectively inhibits the inducible form). Sparing the constitutive isoform is important to maintain homeostasis even in sepsis; for instance, to improve microcirculatory flow, increase blood flow to ischemic areas, scavenge oxygen-free radicals, and enhance microbial killing by macrophages [31]. It has been postulated that a global inhibition of NO is associated with detrimental effects in sepsis that can result in accelerated organ damage [32]; thus, a more downstream inhibition within the NO cascade as provided by MB could be a better approach. In line with these assumptions, MB was associated with a reduction in mortality in a recent systematic review and meta-analysis pooling observational and RCT studies [33].
The most common use of MB for septic shock is reported as single infusions due to extrapolation from the predominant literature on CPB [14, 15, 34–39]; but unlike sepsis, the systemic inflammatory insult of CPB which occurs in up to 50% of the patients is limited to a few hours [13]. By contrast, the duration of the inflammatory insult in septic shock is less predictable, and we know that the expected duration of vasopressor requirement is of 2–3 days even in optimal conditions as in recent clinical trials, [40]. This is the reason we decided to administer MB in repeated doses. Considering that NE requirement was significantly lower in MB than in control group only after the second dose (Fig. 2), we believe our positive findings were in part due to this approach, which provided a longer timeframe of iNOS and sGC inhibition. Data from a recent large retrospective study support this assumption; Sari-Yavuz et al. studied different modes of administration of MB in critically-ill patients with shock, and they reported that the length of inhibition of the NO pathway could influence the response to MB, rather than the cumulative dose [41].
Regarding potential adverse effects, two prior small non-randomized studies suggested that MB could induce worsening of oxygenation due to pulmonary vasoconstriction. Gachot et al. reported a significant decrease of PaO2/FIO2 ratio from 229 to 162 after a 3 mg/kg bolus of MB over 10 min [35], and Weingartner et al. found a decrease of PaO2/FIO2 ratio from 168 to 132 after a dose of 4 mg/kg over 4 h [38]; however, these detrimental effects were transient and not confirmed by other RCTs including ours, as most of our patients had ARDS diagnosis and the change in PaO2/FIO2 ratio and days of mechanical ventilation were similar between groups after treatment. Moreover, it is known that unlike the beneficial effects, the toxic profile of MB is dose related, so the rate/dose used in those non-randomized studies could have been unsafe. A dose of 1 mg/kg has been shown to be enough to improve MAP, left ventricular function and tissue oxygenation in human septic shock, while ≥ 7 mg/kg might compromise splanchnic perfusion [42] and adverse effects are rarely present under 2 mg/kg [43]. Besides, continuous prolonged infusion of MB may lead to higher cumulative doses, which might be toxic and result in methemoglobinemia [15, 42]; this is the pragmatic reason why we decided to administer a fixed dose of 100 mg vials (available presentation at our institution) assuming that most patients would receive at least 1 mg/kg while avoiding high cumulative doses. This schedule resulted in a total cumulative dose of 3.6 mg/kg over 54 h, which was sufficient to reduce vasopressor support with no detrimental effects in pulmonary, kidney, cardiac or liver function. Although methemoglobin levels were significantly higher in patients of MB, this elevation was far from the clinically relevant threshold of 10% [44].
Limitations
Some limitations should be acknowledged. First, this was a single-center study. As a one of the largest reference centers in Mexico, it is common that our patients have a relatively short ICU/hospital stance compared to other larger multicenter trials [40]; as long as there is no need for strictly needed care, persistent organ failure or pending surgery/procedure, patients are discharged as rapidly as possible and followed up through outpatient visits. Thus, a different effect of MB on length of stay in other settings cannot be ruled out. Second, most patients were managed in other departments at the moment of septic shock identification before admission to ICU, the lack of trained healthcare staff and resources (ultrasound, invasive monitoring) might have led to sub-optimal resuscitation and worse patients’ status, as shown by the relatively higher doses of norepinephrine compared to other larger RCTs of patients with septic shock [45]. Therefore, although the time from enrollment to intervention was negligible, an improved response to MB at an earlier phase of the disease cannot be ruled out, as it has been suggested that MB could be more effective within an early “window of opportunity” [24]. Third, this trial took many years to complete as the COVID-19 pandemic slowed down the recruitment rate, and the SSC guidelines were updated while this study was still ongoing [2]. However, it is worth to note that the changes were few, and the resuscitation protocol in our study was still consistent with the new guidelines. For instance, we did not aim for a dose of 30 ml/kg of IV crystalloids at initial resuscitation, and this recommendation was downgraded; we also used IV corticosteroids in all patients while this recommendation was upgraded [2]. Fourth, we did not measure cytokine or nitrate/nitrite serum levels to confirm the mechanism of the effects of MB, but this should not distract from the pragmatic finding that MB reduced vasopressor duration, and ICU and hospital length of stay. Fifth, as we excluded patients with COVID-19, the benefit of MB still needs to be confirmed for this subgroup in future RCTs. Sixth, despite the effort to maintain blinding regarding allocation, the high incidence of urine discoloration and continuous assessment of methemoglobin levels through co-oximetry could have led to identification of group assignment, therefore, a biased adjustment of vasopressors by clinicians cannot be ruled-out. Nonetheless, the similar MAP during the first 4 days (Fig. 2) and less fluid administration suggests that if present, this bias was minimal. Lastly, the difference in mortality trends between groups should be interpreted cautiously, as this study was underpowered to draw any conclusion on this outcome, and larger studies should confirm these results.
Conclusions
In conclusion, early adjunctive MB administration reduced vasopressor duration, cumulative fluid balance, and ICU and hospital length of stay among patients with septic shock, as compared with placebo. There were no severe adverse effects related to its use. Our results support the continuous research of MB as an early adjunctive therapy in patients with septic shock to confirm the potential benefit in larger multicenter randomized clinical trials.
Acknowledgements
We thank the nurse and medical staff members of the critical care unit at Hospital Civil Fray Antonio Alcalde, and the patients and relatives for their participation. We also thank Dr. Luis Enrique Colunga Lozano, MD, MsC, (Department of Health Research Methods, Evidence and Impact. McMaster University. Hamilton, Ontario, Canada/ Department of intensive care medicine, Hospital Civil de Guadalajara “Dr. Juan I. Menchaca”. Guadalajara, Jalisco, México) for performing the Fine and Gray model with proportional hazards.
Abbreviations
- SCC
Surviving sepsis campaign
- MB
Methylene blue
- sGC
Soluble guanylate cyclase
- iNOS
Inducible nitric oxide synthase
- NO
Nitric oxide
- CPB
Cardiopulmonary bypass
- RCT
Randomized controlled trial
- ICU
Intensive care unit
- IV
Intravenous
- COVID-19
Coronavirus disease
- MAP
Mean arterial pressure
- APACHE
Acute physiology and chronic health evaluation
- WINFOCUS
World interactive network focused on critical ultrasound
- IQR
Interquartile range
- ROC
Receiver operating characteristic
- AKI
Acute kidney injury
- ARDS
Acute respiratory distress syndrome
- PEEP
Positive end-expiratory pressure
- CI95
Confidence interval
- HR
Hazard ratio
Author contributions
MIE designed the study. MIE, PAG, URJ, IOM, JALP, QCP, JCMM and GAA conducted the study. MIE and GAA supervised the study. MIE and LSP provided critical consultancy on the study implementation. MIE analyzed the data. MIE, LSP, EK and GH interpreted the data. MIE and JALP had full access to the data. MIE, LSP, EK and GH drafted the manuscript. All authors reviewed the manuscript for important intellectual content and approved the final version.
Funding
This trial did not receive any funding support.
Availability of data and materials
After publication, de-identified data will be available for sharing to researchers who provide a methodologically sound and ethically approved proposal, after approval of data sharing agreement and for research purposes only.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board “Comité de Ética en Investigación, Hospital Civil Fray Antonio Alcalde” (HCG/CEI-0252/17) and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guadalupe Aguirre-Avalos and Glenn Hernández contributed equally to this work as co-senior authors
References
- 1.Ince C, Mayeux PR, Nguyen T, Gomez H, Kellum JA, Ospina-Tascón GA, Hernandez G, Murray P, De Backer D. ADQI XIV workgroup THE ENDOTHELIUM IN SEPSIS. Shock. 2016;45:259–270. doi: 10.1097/SHK.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–1247. doi: 10.1007/s00134-021-06506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentzer P, Griesdale DE, Boyd J, MacLean K, Sirounis D, Ayas NT. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA. 2016;316:1298–1309. doi: 10.1001/jama.2016.12310. [DOI] [PubMed] [Google Scholar]
- 4.Aya HD, Ster IC, Fletcher N, Grounds RM, Rhodes A, Cecconi M. Pharmacodynamic analysis of a fluid challenge. Crit Care Med. 2016;44:880–891. doi: 10.1097/CCM.0000000000001517. [DOI] [PubMed] [Google Scholar]
- 5.Stolk RF, van der Pasch E, Naumann F, Schouwstra J, Bressers S, van Herwaarden AE, et al. Norepinephrine dysregulates the immune response and compromises host defense during sepsis. Am J Respir Crit Care Med. 2020;202:830–842. doi: 10.1164/rccm.202002-0339OC. [DOI] [PubMed] [Google Scholar]
- 6.Stolk RF, Kox M, Pickkers P. Noradrenaline drives immunosuppression in sepsis: clinical consequences. Intensive Care Med. 2020;46:1246–1248. doi: 10.1007/s00134-020-06025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leone M, Einav S, Antonucci E, Depret F, Lakbar I, Martin-Loeches I, et al. Multimodal strategy to counteract vasodilation in septic shock. Anaesth Crit Care Pain Med. 2023;42:101193. doi: 10.1016/j.accpm.2023.101193. [DOI] [PubMed] [Google Scholar]
- 8.Wieruszewski PM, Khanna AK. Early multimodal vasopressors-are we ready for it? Crit Care Med. 2022;50:705–708. doi: 10.1097/CCM.0000000000005344. [DOI] [PubMed] [Google Scholar]
- 9.Wieruszewski PM, Khanna AK. Vasopressor choice and timing in vasodilatory shock. Crit Care. 2022;26:76. doi: 10.1186/s13054-022-03911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin C, Medam S, Antonini F, Alingrin J, Haddam M, Hammad E, et al. Norepinephrine: not too much, too long. Shock. 2015;44:305–309. doi: 10.1097/SHK.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 11.Auchet T, Regnier MA, Girerd N, Levy B. Outcome of patients with septic shock and high-dose vasopressor therapy. Ann Intensive Care. 2017;7:43. doi: 10.1186/s13613-017-0261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puntillo F, Giglio M, Pasqualucci A, Brienza N, Paladini A, Varrassi G. Vasopressor-sparing action of methylene blue in severe sepsis and shock: a narrative review. Adv Ther. 2020;37:3692–3706. doi: 10.1007/s12325-020-01422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busse LW, Barker N, Petersen C. Vasoplegic syndrome following cardiothoracic surgery-review of pathophysiology and update of treatment options. Crit Care. 2020;24:36. doi: 10.1186/s13054-020-2743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirov MY, Evgenov OV, Evgenov NV, Egorina EM, Sovershaev MA, Sveinbjørnsson B, et al. Infusion of methylene blue in human septic shock: a pilot, randomized, controlled study. Crit Care Med. 2001;29:1860–1867. doi: 10.1097/00003246-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Memis D, Karamanlioglu B, Yuksel M, Gemlik I, Pamukcu Z. The influence of methylene blue infusion on cytokine levels during severe sepsis. Anaesth Intensive Care. 2002;30:755–762. doi: 10.1177/0310057X0203000606. [DOI] [PubMed] [Google Scholar]
- 16.Sacha GL, Lam SW, Wang L, Duggal A, Reddy AJ, Bauer SR. Association of catecholamine dose, lactate, and shock duration at vasopressin initiation with mortality in patients with septic shock. Crit Care Med. 2022;50:614–623. doi: 10.1097/CCM.0000000000005317. [DOI] [PubMed] [Google Scholar]
- 17.Richards-Belle A, Hylands M, Muttalib F, Taran S, Rochwerg B, Day A, et al. Lower versus higher exposure to vasopressor therapy in vasodilatory hypotension: a systematic review with meta-analysis. Crit Care Med. 2023;51:254–266. doi: 10.1097/CCM.0000000000005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ospina-Tascón GA, Büchele GL, Vincent JL. Multicenter, randomized, controlled trials evaluating mortality in intensive care: Doomed to fail? Crit Care Med. 2008;36:1311–1322. doi: 10.1097/CCM.0b013e318168ea3e. [DOI] [PubMed] [Google Scholar]
- 19.Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, et al. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med. 2017;377:419–430. doi: 10.1056/NEJMoa1704154. [DOI] [PubMed] [Google Scholar]
- 20.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibarra-Estrada MÁ, López-Pulgarín JA, Mijangos-Méndez JC, Díaz-Gómez JL, Aguirre-Avalos G. Respiratory variation in carotid peak systolic velocity predicts volume responsiveness in mechanically ventilated patients with septic shock: a prospective cohort study. Crit Ultrasound J. 2015;7:29. doi: 10.1186/s13089-015-0029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibarra-Estrada MA, Chávez-Peña Q, Reynoso-Estrella CI, Rios-Zermeño J, Aguilera-González PE, García-Soto MA, et al. Timing, method and discontinuation of hydrocortisone administration for septic shock patients. World J Crit Care Med. 2017;6:65–73. doi: 10.5492/wjccm.v6.i1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33. [DOI] [PubMed]
- 24.Evora PR. Methylene blue does not have to be considered only as rescue therapy for distributive shock. J Med Toxicol. 2013;9:426. doi: 10.1007/s13181-013-0333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busse LW, Nicholson G, Nordyke RJ, Lee CH, Zeng F, Albertson TE. Angiotensin II for the treatment of distributive shock in the intensive care unit: a US cost-effectiveness analysis. Int J Technol Assess Health Care. 2020;36:145–151. doi: 10.1017/S0266462320000082. [DOI] [PubMed] [Google Scholar]
- 26.Farina N, Bixby A, Alaniz C. Angiotensin II brings more questions than answers. P T. 2018;43:685–687. [PMC free article] [PubMed] [Google Scholar]
- 27.Venkatesh B, Khanna AK, Cohen J. Less is more: catecholamine-sparing strategies in septic shock. Intensive Care Med. 2019;45:1810–1812. doi: 10.1007/s00134-019-05770-3. [DOI] [PubMed] [Google Scholar]
- 28.Kotani Y, Di Gioia A, Landoni G, Belletti A, Khanna AK. An updated "norepinephrine equivalent" score in intensive care as a marker of shock severity. Crit Care. 2023;27:29. doi: 10.1186/s13054-023-04322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bakker J, Kattan E, Annane D, Castro R, Cecconi M, et al. Current practice and evolving concepts in septic shock resuscitation. Intensive Care Med. 2022;48:148–163. doi: 10.1007/s00134-021-06595-9. [DOI] [PubMed] [Google Scholar]
- 30.López A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32:21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 31.Hosseinian L, Weiner M, Levin MA, Fischer GW. Methylene blue: Magic bullet for vasoplegia? Anesth Analg. 2016;122:194–201. doi: 10.1213/ANE.0000000000001045. [DOI] [PubMed] [Google Scholar]
- 32.Singh J, Lee Y, Kellum JA. A new perspective on NO pathway in sepsis and ADMA lowering as a potential therapeutic approach. Crit Care. 2022;26:246. doi: 10.1186/s13054-022-04075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao CC, Zhai YJ, Hu ZJ, Huo Y, Li ZQ, Zhu GJ. Efficacy and safety of methylene blue in patients with vasodilatory shock: a systematic review and meta-analysis. Front Med (Lausanne) 2022;9:950596. doi: 10.3389/fmed.2022.950596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evora PR, Alves Junior L, Ferreira CA, Menardi AC, Bassetto S, Rodrigues AJ. Twenty years of vasoplegic syndrome treatment in heart surgery. Methylene blue revised. Rev Bras Cir Cardiovasc. 2015;30:84–92. [DOI] [PMC free article] [PubMed]
- 35.Gachot B, Bedos JP, Veber B, Wolff M, Regnier B. Short-term effects of methylene blue on hemodynamics and gas exchange in humans with septic shock. Intensive Care Med. 1995;21:1027–1031. doi: 10.1007/BF01700666. [DOI] [PubMed] [Google Scholar]
- 36.Park BK, Shim TS, Lim CM, Lee SD, Kim WS, Kim DS, et al. The effects of methylene blue on hemodynamic parameters and cytokine levels in refractory septic shock. Korean J Intern Med. 2005;20:123–128. doi: 10.3904/kjim.2005.20.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donati A, Conti G, Loggi S, Münch C, Coltrinari R, Pelaia P. Does methylene blue administration to septic shock patients affect vascular permeability and blood volume? Crit Care Med. 2002;30:2271–2277. doi: 10.1097/00003246-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Weingartner R, Oliveira E, Oliveira ES, Sant'Anna UL, Oliveira RP, Azambuja LA, et al. Blockade of the action of nitric oxide in human septic shock increases systemic vascular resistance and has detrimental effects on pulmonary function after a short infusion of methylene blue. Braz J Med Biol Res. 1999;32:1505–1513. doi: 10.1590/s0100-879x1999001200009. [DOI] [PubMed] [Google Scholar]
- 39.Andresen M, Dougnac A, Díaz O, Hernández G, Castillo L, Bugedo G. Use of methylene blue in patients with refractory septic shock: impact on hemodynamics and gas exchange. J Crit Care. 1998;13:164–168. doi: 10.1016/s0883-9441(98)90001-6. [DOI] [PubMed] [Google Scholar]
- 40.Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, ADRENAL Trial Investigators and the Australian–New Zealand Intensive Care Society Clinical Trials Group. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N Engl J Med. 2018;378:797–808. [DOI] [PubMed]
- 41.Sari-Yavuz S, Heck-Swain KL, Keller M, Magunia H, Feng YS, Haeberle HA. Methylene blue dosing strategies in critically ill adults with shock-A retrospective cohort study. Front Med (Lausanne) 2022;9:1014276. doi: 10.3389/fmed.2022.1014276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Juffermans NP, Vervloet MG, Daemen-Gubbels CR, Binnekade JM, de Jong M, Groeneveld AB. A dose-finding study of methylene blue to inhibit nitric oxide actions in the hemodynamics of human septic shock. Nitric Oxide. 2010;22:275–280. doi: 10.1016/j.niox.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Leyh RG, Kofidis T, Strüber M, Fischer S, Knobloch K, Wachsmann B, et al. Methylene blue: The drug of choice for catecholamine-refractory vasoplegia after cardiopulmonary bypass? J Thorac Cardiovasc Surg. 2003;125:1426–1431. doi: 10.1016/s0022-5223(02)73284-4. [DOI] [PubMed] [Google Scholar]
- 44.Cefalu JN, Joshi TV, Spalitta MJ, Kadi CJ, Diaz JH, Eskander JP, et al. Methemoglobinemia in the operating room and intensive care unit: early recognition, pathophysiology, and management. Adv Ther. 2020;37:1714–1723. doi: 10.1007/s12325-020-01282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hernández G, Ospina-Tascón GA, Damiani LP, Estenssoro E, Dubin A, Hurtado J, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the ANDROMEDA-SHOCK randomized clinical trial. JAMA. 2019;321:654–664. doi: 10.1001/jama.2019.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
After publication, de-identified data will be available for sharing to researchers who provide a methodologically sound and ethically approved proposal, after approval of data sharing agreement and for research purposes only.