Abstract
Osteoporosis (OP) is the most prevalent metabolic bone disease, characterized by the low bone mass and microarchitectural deterioration of bone tissue. Glucocorticoid (GC) clinically acts as one of the anti-inflammatory, immune-modulating, and therapeutic drugs, whereas the long-term use of GC may cause rapid bone resorption, followed by prolonged and profound suppression of bone formation, resulting in the GC-induced OP (GIOP). GIOP ranks the first among secondary OP and is a pivotal risk for fracture, as well as high disability rate and mortality, at both societal and personal levels, vital costs. Gut microbiota (GM), known as the “second gene pool” of human body, is highly correlated with maintaining the bone mass and bone quality, and the relation between GM and bone metabolism has gradually become a research hotspot. Herein, combined with recent studies and based on the cross-linking relationship between GM and OP, this review is aimed to discuss the potential mechanisms of GM and its metabolites on the OP, as well as the moderating effects of GC on GM, thereby providing an emerging thought for prevention and treatment of GIOP.
Keywords: Gut microbiota, Gut, Bone, Glucocorticoid-induced osteoporosis
Introduction
Osteoporosis (OP) is the most prevalent metabolic bone disease, characterized by low bone mass and microarchitectural deterioration of bone tissue [1, 2]. According to data provided by International Osteoporosis Foundation (IOF), more than 200 million people worldwide suffer from OP and there is an average fracture caused by OP every 3 s, and the incidence of hip fracture has been increasing in the world since 1990, which is expected to increase by 240% in women and 310% in men by 2050, and about 50% of osteoporotic fractures might occur again [3–6]. Glucocorticoid (GC) clinically acts as the anti-inflammatory, immune-modulating therapeutic drugs used to manage inflammatory diseases including inflammatory bowel disease (IBD), allergic conditions, bronchial asthma, rheumatoid arthritis (RA), ankylosing spondylitis, and chronic renal diseases, cancers, as well as severe corona virus disease 2019 (COVID-19) cases [7]. However, long-term application of GC may cause rapid bone resorption, followed by prolonged and profound suppression of bone formation, leading to an “imminent risk of fracture” [8–11]. More than 41.4% of patients developed the OP during the long-term treatment of GC [12]. A recent claim-based assessment on the costs of adverse events related to GC, established an incremental cost of $3201 per fracture at the cumulative doses greater than 1800 mg. GC is the cause of disability, mortality and, at both societal and personal level, significant costs [13, 14]. Though antiresorptive and anabolic drugs could help to reduce the OP due to GC, GC-induced OP (GIOP) ranks the first among secondary OP and is a significant risk for fractures [9, 10]. Thus, the in-depth studies of cross-linking relationship and development of corresponding targeted therapy strategies have become the key to the prevention and treatment of GIOP.
In general, GIOP is most common in drug-induced secondary OP [15]. The etiology of GIOP is mainly related to the increase of osteoblast apoptosis and osteoclast activity. Physiologically, cortisol secreted by the adrenal cortex is an essential hormone for the differentiation and functional regulation of osteoblastic lineage cells and osteoclastic lineage cells [16, 17]. However, excessive physiological dose of GC and its analogues might have apparent adverse influences on the development, growth, and metabolism of bone tissues [18]. Histologically, the osteoblast apoptosis and inhibition of osteoblast function could be observed, while the number of bone remodeling units increases, bone resorption lacunae increase, the bone formation is insufficient, and the bone trabecular thickness decreases, perforates, or disappears [19]. Additionally, different from primary OP, GIOP is mainly characterized by following three characteristics: (1) The influences of GC on the bone tissues are mainly characterized by the obvious activation of receptor activator for nuclear factor-κB ligand (RANKL) and enhanced bone resorption, but no corresponding increase in bone formation occurred during this period; (2) After a period of adaptation (approximately several weeks), the bone formation remains at a low level, while the bone resorption decreases; (3) The risk of fracture enhances rapidly, and in a dose-dependent manner after the application of GC [20–22].
In recent years, the relationship between GM and human diseases has been focused on. Gut microbiota (GM) is the community of microbes (bacteria, fungi, viruses, etc.) residing in the host gastrointestinal tract, also known as the “second gene pool” of the human body, and the number of genes in its genome is about 150 times as much as the total number of genes in human genome, approximately 10 trillion [23, 24]. Increasing researches have indicated a complex role of the gastrointestinal tract in keeping bone health through a “gut-bone” axis, in which the several mechanisms have been proposed [25–27]. Animal experiments have shown that GM can regulate bone mass by altering immune status, intestinal calcium absorption, and affecting osteoclasts-mediated bone resorption. Taken together, GM is highly correlated with maintaining bone mass and bone quality [28–30]. It would seem imperative to understand the mechanisms by which GM affects the development of GIOP to identify drug targets and design better therapies for this disease.
The relationship between GM and OP
GM are known as the “second gene pool” of the human body, encoding 150-fold more genes than human genome, and about 10 trillion bacteria colonizing in the human gastrointestinal tract interacts with the host [23, 28]. GM is referred to as a commensal, symbiotic, and pathogenic microorganism living in our intestines, playing an essential role in endocrine system, enteric nervous system, immune system, nutrient absorption and production, metabolic balance, gut–brain-bone axis, and resistance to pathogens at different period of ages [31–33]. Nowadays, the knowledge of the complex interaction between GM and health outcomes is a novel and rapidly expanding field [31]. In recent studies, it has been observed that GM alterations associated with reduced bone mineral density (BMD) in older adults [34, 35]. There is an increasing number of human and animal studies suggesting that GM can exert effects in skeletal system as it modulates gut permeability, hormonal secretion, and immune response, and stimulates calcium and vitamin D absorption [36].
Moreover, GM is a critical regulator of bone remodeling mediated by the osteoclasts with bone resorption function and the osteoblasts with bone formation function. Therein, bone strength refers to the ability of bones to respond to mechanical load, and GM may play a key role in the formation of differences in bone strength [37]. Long-term changes in GM during growth and development may not only result in the changes in bone mass, but also impair the biomechanical properties of bone [38]. Several studies have reported the alterations in the properties of bone collagen associated with bone fragility, such as changes in biochemical properties and protein structure [39, 40]. The supplementation of specific probiotics in OP-related mice models can improve BMD and enhance bone heterogeneity [41]. This is of great practical value, as most of the current anti-OP drug candidates (such as the bisphosphonate, calcitonin, cathepsin K inhibitors, and estrogen) may reduce the bone heterogeneity, and thereby enhancing the risk of bone fragility and fractures [42, 43]. Besides, Chen et al. [44] also observed that feeding GC-treated mice in the same nest with healthy mice, or transplanting the GM of healthy mice to the GC-treated mice could significantly reduce the decrease of bone mass, bone microstructure destruction, decreased angiogenesis, decrease in the number of osteoblasts and increase in apoptotic cells induced by GC.
From the aspect of clinical researches, Yang et al. [45] conducted the 16 S ribosomal RNA (16 S rRNA) sequencing analysis of the feces from 132 postmenopausal women, including patients with OP, patients with osteopenia and the individuals in the control group, and observed that the composition and diversity of GM in OP group and control group were significantly different, reflecting that the abundance of Fusicatenibacter, Lachnoclostridium, and Megamonas spp. was higher in the OP group. In a randomized, double-blind, placebo-controlled, multi-center trial, Jansson et al. [46] applied a mix of 3 kinds of Lactobacillus strains (Lactobacillus paracasei DSM 13434, Lactobacillus plantarum DSM 15312, and Lactobacillus plantarum DSM 15313) at a dose of 1 × 1010 CFU per day for the postmenopausal women, and it turned out that the supplementation of Lactobacillus strains reduced the bone loss in postmenopausal women compared with placebo, indicating the benefits of probiotics and prebiotics for bone metabolism. Morato-Martínez et al. [47] suggested that regular consumption of a dairy product to reconstitute enriched with bioactive nutrients significantly enhanced dietary intake of calcium and vitamin D, thus improving bone health-related markers in menopausal women at risk of OP without pharmacological treatment.
As for the animals’ studies, by constructing the rat models of postmenopausal OP induced by the ovariectomy (OVX), Li et al. [23] suggested that the metabolites of GM were effective regulators of osteoclast metabolism and bone homeostasis, and played a critical role in the prevention and treatment of metabolic syndrome. The treatment of Puerarin modulated the GM disorder to elicit the anti-OP effects in the OVX-induced rats by improving the bone micro-environment via regulating the levels of short-chain fatty acids (SCFAs) and repairing the intestinal mucosal integrity. In addition, Li et al. [9] indicated that tuna bone powder (TBP) stimulated bone formation via Wnt/β-catenin pathway activation and inhibited bone resorption via NF-κB pathway suppression to alleviate the GIOP. Meanwhile, GC significantly increased the transcription levels of tumor necrosis factor-α (TNF-α), interleukin (IL)-1, IL-6, and IL-17 in colon, whereas TBP treatment at high-dosage significantly decreased the transcription of all the above inflammatory cytokines, thus relieving systemic inflammation and intestinal integrity. Cheng et al. [32] showed that long-term alcohol consumption induced OP and affected the composition of GM, and alcohol can activate T lymphocytes directly or indirectly regulate the alterations of GM to produce the cytokines, and further activate osteoclasts. Moreover, the status of OP was more severe in old rats than young rats, which may be due to the higher diversity and stronger regulation ability of GM in young rats compared with old rats. Li et al. [48] revealed that the acceleration of senescence caused by the administration of D-galactosamine (D-gal) and NaNO2 to mice showed an osteoporotic bone phenotype, which was related to aggravated oxidative stress, activated Sirt6/NF-κB/CatK signaling, and disruption of GM. Remarkably, Fructus ligustri lucidi (FLL) aqueous extract might preserve the aging-related bone quality via inhibiting the above-mentioned signaling pathways [48]. In addition to this, Wang et al. [49] suggested that the feces from senile osteoporotic rats transferred to young rats could induce senile OP, indicating altered GM and impaired intestinal barrier contributed to the pathogenesis of OP. Zhang et al. [50] showed that fecal microbiota transplantation (FMT) ameliorated the bone loss in mice with OVX-induced OP through modulating the GM and metabolic function. To be specific, FMT prevented the OVX-induced bone loss by correcting the imbalance of GM, improving the level of SCFAs, optimizing the intestinal permeability, and inhibiting the release of pro-osteoclastogenic cytokines, which might be an option to serve as a promising candidate for the prevention and treatment of OP in the future.
In general, GM was closely associated with OP (the animals’ studies were shown in Table 1, and the population-based researches are shown in Table 2) [51–63]. Recent studies have underscored the emerging role of GM in regulating bone metabolism. The modification of GM could be a viable therapeutic strategy to regulate the bone metabolism under a variety of conditions that lead to bone loss and OP. Nonetheless, relevant studies on the relationship between GM and OP are still in the initial stage, and more researches are required to further clarify its in-depth mechanisms and explore effective treatments with less side effects.
Table 1.
Researches regarding the GM and OP at animals’ level
| Researchers | Sample | n | Group | Methods | Results | Conclusion/significance |
|---|---|---|---|---|---|---|
| Li et al. [34] | Male ICR mice | 40 |
(i) Normal n = 10 (ii) Aging n = 10 (iii) Aging + VE n = 10 (iv) Aging + FLL n = 10 |
Normal: 0.9% sterile saline Aging: D-gal (120 mg/kg/d) + NaNO2 (90 mg/kg/d) Aging + VE: D-gal + NaNO2 + VE (100 mg/kg/d; dissolved in 0.1% Tween 80) Aging + FLL: D-gal + NaNO2 + FLL (4.9 g/kg/d; dissolved in 0.1% Tween 80) |
FLL modulated GM composition in aging mice | The acceleration of senescence caused by administration of D-gal and NaNO2 to ICR mice exhibited an osteoporotic bone phenotype. This was associated with aggravated oxidative stress and activated Sirt6/NF-κB/CatK signaling and the disruption of GM, including increased levels of FMO3 and TMAO |
| Li et al. [9] | Female SD rats | 40 |
(i) Sham n = 10 (ii) OVX n = 10 (iii) OVX + P(L) n = 10 (iv) OVX + P(H) n = 10 |
OVX + P(L): OVX + puerarin (50 mg/kg/d) OVX + P(H): OVX + puerarin (100 mg/kg/d) |
Puerarin regulated the imbalance of GM in OVX rats | Puerarin treatment improved the bone microenvironment to inhibit OVX-induced OP via modulating SCFAs released by the GM and repairing intestinal mucosal integrity |
| Li et al. [23] | ICR mice | 48 |
(i) Control n = 8 (ii) Model n = 8 (iii) CC n = 8 (iv) LD n = 8 (v) MD n = 8 (vi) HD n = 8 |
Control: 0.9% sterile saline CC: CaCO3(570 mg/kg/d) LD: GC + TBP (500 mg/kg/d) MD: GC + TBP (1000 mg/kg/d) HD: GC + TBP (1500 mg/kg/d) |
TBP modulates the GM | TBP exerted anti-osteoporosis effects in GIOP mice. Beneficial effects also result from GM modulation, with enhanced SCFAs productions and anti-inflammatory bacterial abundance |
| Schepper et al. [10] | Male C57BL/6 J mice | 39 |
(i) Control n = 10 (ii) GC n = 9 (iii) ABX n = 10 (iv) ABX + GC n = 10 |
GC: 2.5 mg/kg/d ABX: ampicillin and neomycin, 160 and 80 mg/kg/day |
GC treatment alters fecal microbiota composition | Depletion of the GM prevents GC-induced trabecular bone loss |
| Liu et al. [2] | Female SD rats | 40 |
(i) Control n = 8 (ii) GC n = 8 (iii) L n = 8 (iv) M n = 8 (v) H n = 8 |
Control: saline (twice a week, 8 weeks) GC: dexamethasone (0.1 mg/100 g twice a week, 8 weeks) L: GC + APS (50 mg/kg) M: GC + APS (150 mg/kg) H: GC + APS (250 mg/kg) |
APS improved GC-induced OP in rats | Our studies discovered the structural and functional changes of GM in APS-improved OP, and inferred the potential key bacteria |
| Shen et al. [28] | Female SD rats | 40 |
(i) Control n = 8 (ii) OVX n = 8 (iii) OVX-E n = 8 (iv) OVX-CS n = 8 (v) OVX-CSCa n = 8 |
OVX: saline OVX-E: 17β-estradiol (100 μg/kg) OVX-CS: CS (150 mg/kg) OVX-CSCa: CSCa (150 mg/kg) |
GM structure change caused by CSCa intervention | CSCa, instead of CS, dietary intervention can alleviate the estrogen deficiency-induced OP, which probably involves the GM and metabolite profiles change in the faeces |
| Wang et al. [132] | Female SD rats | 32 |
(i) Control n = 16 (ii) FMT n = 16 |
Control: sterile saline oral gavage of 1 ml/rat three times a week FMT: fresh fecal samples of four 18-month-old female SD rats oral gavage of 1 ml/rat three times a week |
GM from senile rats increased bone turnover in 3‐month‐old rats | GM from senile osteoporotic rats could induce OP in young rats |
| Ma et al. [54] | Female SD rats | 8 |
(i) Control n = 4 (ii) OP n = 4 |
Control: 22-month-old rats OP: 6-month-old rats |
There was a significant reduction in alpha diversity and the F/B ratio in aged rats | Shifts in the GM contribute to senile osteoporosis through metabolic pathways and subsequent immune disorders |
| Tao et al. [63] | Female C57BL/6 mice | 28 |
(i) Sham n = 7 (ii) OVX n = 7 (iii) Low-UA n = 7 (iv) High-UA n = 7 |
Sham: 0.5% CMC-Na treatment OVX: OVX + 0.5% CMC-Na Low-UA: OVX + 10 mg/kg UA High-UA: OVX + 20 mg/kg UA |
UA reduced OVX-induced bone loss in mice | UA, a metabolite of the GM, ameliorated OVX-induced bone loss by suppressing osteoclast formation |
| Lan et al. [55] | Male C57BL/6 J mice | 40 |
(i) BC n = 8 (ii) MC n = 8 (iii) H n = 8 (iv) M n = 8 (v) L n = 8 |
BC: 0.9% NaCl MC: 0.9% NaCl + 2% DSS (1 week) + 1% DSS (1 week) H: BL-99 (1 × 1011 CFU/d/mouse) + 2% DSS (1 week) + 1% DSS (1 week) M: BL-99 (1 × 109 CFU/d/mouse) + 2%DSS (1 week) + 1% DSS (1 week) L: low-dose BL-99 (1 × 107 CFU/d/mouse) + 2% DSS (1 week) + 1% DSS (1 week) |
BL-99 administration is associated with changes in the composition of the GM | BL-99 administration was sufficient to prevent and alleviate DSS colitis-induced bone loss-associated inflammation and associated bone loss in mice |
| Cheng et al. [32] | SD rats | 12 |
(i) Control n = 6 (ii) Alcohol n = 6 |
Control: isocaloric liquid diet with maltose-dextran substituted for ethanol Alcohol: Bio-Serv Liquid Rat Diet LD82 containing 5.0% alcohol (35% alcohol-derived calories) which the dietary concentration of ethanol was increased from 0 to 35% |
A diversity of fecal bacterial flora in young and old rats increased significant after alcohol intake | Long-term alcohol consumption could affect the expression of bone metabolism regulating hormones, induce the activation of osteoclast differentiation and influence the composition of GM in rats |
| Liu et al. [19] | Male SD rats | 27 |
(i) Saline n = 9 (ii) Ethanol n = 9 (iii) Antibiotic n = 9 |
Saline: saline (10 ml/kg, 6 times/week, 1 time/d, 16 weeks) Ethanol: 20% ethanol (10 ml/kg, 6 times/week, 1 time/d, 16 weeks) Antibiotic: 1 g/L metronidazole and 0.2 g/L ciprofloxacin (10 ml/kg, 6 times/week, 1 time/d, 16 weeks) |
Compared with antibiotics application, ethanol-gavaged decreased the BMD in rats | Chronic heavy ethanol consumption causes osteoporosis and GM dysbiosis in rats, indicating that the regulation of the gut-bone axis might contribute to the ethanol-induced bone loss |
| Wang et al. [132] | Female C57BL6/J mice | 30 |
(i) Sham + Vehicle n = 10 (ii) OVX + Vehicle n = 10 (iii) OVX + P. histicola n = 10 |
Sham + Vehicle: plain culture medium OVX + Vehicle: plain culture medium OVX + P. histicola: 0.1 mL bacteria (1 × 109 CFU) every other day for 12 weeks |
P. histicola–treated OVX mice maintained a relatively higher bone volume than OVX controls | P. histicola could prevent estrogen deficiency-induced bone loss through the GM-bone axis |
| He et al. [30] | Female NTac: SD rats | 49 |
(i) DCa n = 7 (ii) DCa-La n = 7 (iii) DCa-In n = 7 (iv) CaC n = 7 (v) CaC-La n = 7 (vi) CaC-In n = 7 (vii) Control n = 7 |
CaC: OVX + Altromin C1031 added 0.5% calcium carbonate CaC-La: OVX + Altromin C1031 added 0.5% calcium carbonate + 0.5% lactose CaC-In: OVX + Altromin C1031 added 0.5% calcium carbonate + 5% inulin DCa: Altromin C1031 added 2% Capolac DCa-La: Altromin C1031 added 2% Capolac + 0.5% lactose DCa-In: Altromin C1031 added 2% Capolac + 5% inulin |
Rats fed with calcium-fortified diets have higher BMD, bone mineral content and femur mechanical strength, lower serum levels of bone markers, and lower expression of calcium absorption-related genes TRPV6, CaBP compared with control | Calcium modulates GM composition and function |
| Liu et al. [51] | Male Wistar rats | 18 |
(i) Control n = 6 (ii) Model n = 6 (iii) GSD n = 6 |
Control: saline Model: prednisolone (15 mg/kg twice a week) GSD: prednisolone (15 mg/kg twice a week) + GSD (30 g/kg/d) |
GM composition might be modified | GSD could play an anti-osteoporosis role by regulating these metabolic pathways |
| Li et al. [65] | Female C57BL/6 J mice | 20 |
(i) Sham n = 10 (ii) OVX n = 10 |
Sham: LGG or VSL#3 (1 × 109 CFU twice a week) OVX: OVX + LGG or VSL#3 (1 × 109 CFU twice a week) |
Feeding of LGG or VSL#3 increased bone formation in ovx mice | Supplementation of the normal flora of sex steroid-depleted mice with the commonly used probiotics LGG or VSL#3 significantly tightened intestinal barrier integrity and completely protected mice against sex steroid depletion-induced bone loss |
GM gut microbiota, OP osteoporosis, FLL Fructus Ligustri Lucidi, D-gal D-galactose, VE vitamin E, TMAO trimethylamine-N-oxide, NF-κB nuclear factor-kappa B, CatK cathepsin K, Sirt6 sirtuin 6, FMO3 flavin-containing monooxygenase 3, P(L) a low-dose puerarin, P(H) a high-dose puerarin, OVX ovariectomy, SCFAs short chain fatty acids, d day, SD Sprague–Dawley, TBP tuna bone powder, GIOP glucocorticoid-induced osteoporosis, CC CaCO3, LD low dose, MD medium dose, HD high dose, L low dose, M medium dose, H high dose, GCs glucocorticoids, ABX broad-spectrum antibiotics, APS astragalus polysaccharides, GS chondroitin sulfate, CSCa chondroitin sulfate calcium complex, E 17β-estradiol, FMT fecal microbiota transplantation, F/B firmicutes/bacteroidetes, UA Urolithin A, CMC-Na sodium carboxymethyl cellulose, BC blank control, MC model control, DSS dextran sodium sulfate, BMD bone mineral density, BL-99 Bifidobacterium lactis BL-99, TRPV6 transient receptor potential vanilloid type 6, CaBP calcium-binding protein, GSD gushudan
Table 2.
Researches regarding the GM and OP at population-based level
| Researchers | Sample | n | Group | Methods | Results | Conclusion/significance |
|---|---|---|---|---|---|---|
| Takimoto et al. [57] | Postmenopausal Japanese women | 61 |
(i) Placebo n = 30 (ii) C-3102 n = 31 |
C-3102: Bacillus subtilis C-3102 (3.4 × 109 CFU/d) | C-3102 treatment resulted in a significant change in the relative abundance of 11 bacterial genera | C-3102 modulates host GM and inhibits bone resorption |
| Jansson et al. [46] | Swedish healthy postmenopausal women | 249 |
(i) Placebo n = 123 (ii) Lactobacillus n = 126 |
Lactobacillus: DSM 13,434 + DSM 15,312 + DSM 15,313 (1 × 1010 CFU/capsule/d) | Lactobacillus treatment reduced the LS-BMD loss compared with placebo | Probiotic treatment using a mix of three Lactobacillus strains naturally occurring in the human GM protects against LS-BMD loss in healthy postmenopausal women |
| Nilsson et al. [62] | Postmenopausal women with osteopenia | 90 |
(i) Placebo n = 45 (ii) LR6475 |
LR6475: Lactobacillus reuteri ATCC PTA6475 (1 × 1010 CFU/d) | L. reuteri 6475 reduced the loss of total vBMD compared to placebo | Daily supplementation with L. reuteri 6475 for 12 months reduced loss of tibia total vBMD in older women with low BMD |
| Lambert et al. [56] | Postmenopausal osteopenic women | 78 |
(i) Placebo n = 40 (ii) RCE n = 38 |
Placebo: placebo extract + CDM/d RCE: twice daily RCE (2 × 95 mL = 60 mg isoflavones/d total) + CDM/d |
A significant reduction in bone resorption marker plasma CTx concentration was found in the RCE group | RCE attenuate the BMD loss and improve the bone turnover |
| Morato-Martínez et al. [47] | Postmenopausal women with osteopenia | 78 |
(i) CG n = 39 (ii) EG n = 39 |
CG: a daily ration of the same product but without enrichment EG: one serving a day of the experimental product enriched with bioactive bone nutrients |
Consumption of an experimental dairy product resulted in a significant improvement in bone mass content and managed to mitigate the loss of BMD, and the intake of calcium and vitamin D improved significantly | Consumption of functional foods with a milk matrix enriched in nutrients essential for bone health could contribute to the primary prevention of OP and thus decrease the morbid mortality rates and the costs associated with the disease |
| Jafarnejad et al. [58] | Postmenopausal women with osteopenia | 50 |
(i) Probiotic n = 25 (ii) Control n = 25 |
Probiotic: gerilact capsules + 500 mg Ca + 200 IU vitamin D/d Control: placebo capsules + 500 mg Ca + 200 IU vitamin D/d |
Serum TNF-a, as a major pro-inflammatory cytokine, decreased significantly in the probiotic group compared to the placebo group | Multispecies probiotic supplementation among postmenopausal osteopenic women showed a possible role of OC, BALP, PTH, and TNF-a in suppressing bone resorption and bone turnover |
| Yang et al. [45] | Postmenopausal Chinese women with osteoporosis or osteopenia | 132 |
(i) OP (n = 34) (ii) OPe (n = 47) (iii) Control (n = 51) |
Enzyme-linked immunosorbent assay: The serum levels of IL-10, TNF-α, and LBP 16S rRNA gene V3-V4 region sequencing was performed to investigate the GM and VM |
It was noted that for GM, Romboutsia, unclassified_Mollicutes, and Weissella spp. were enriched in the control group, whereas the abundances of Fusicatenibacter, Lachnoclostridium, and Megamonas spp. were higher in the OP group | The results show that changes in BMD in postmenopausal women are associated with the changes in GM |
| Ozaki et al. (2020) | Postmenopausal Japanese women | 38 |
(i) high BMD n = 19 (ii) low BMD n = 19 Or (i) high vitamin K2 n = 10 (ii) low vitamin K2 n = 28 |
GM profiling: 16S rRNA gene sequencing Serum bone turnover markers: vitamin K fraction and TRACP-5b BMD: forearm dualenergy X-ray absorptiometry |
Rikenellaceae was significantly abundant in the low BMD group. Bacteroides and Sutterella were predominant in the high vitamin K2 group | Bacteroides and Rikenellaceae may be involved in bone metabolism and fracture risk |
| Das et al. [35] | Adult female and male subjects from Ireland | 181 |
(i) OPe n = 61 (ii) OP n = 60 (iii) normal BMD n = 60 |
Analysis of the 16S (V3-V4 region) amplicon dataset classified to the genus level was used to identify significantly differentially abundant taxa | A detailed study of microbiota associations with meta-data variables that included BMI, health status, diet and medication revealed that these meta-data explained 15–17% of the variance within the microbiota dataset | Reduced BMD in OPe and OP is associated with an altered microbiota |
| Xu et al. [59] | Chinese orthopedic inpatients | 96 |
(i) PO n = 48 (ii) Control n = 48 |
PO: Primary Osteoporosis patients Control: Healthy patients 16 s rDNA amplification sequencing |
The diversity of GM in PO patients was significantly higher than that in control group and there was significant difference in microbial composition in PO group | PO is related to the change of GM, especially the enriched Dialister and Faecalibacterium genera |
| Li et al. [34] | Healthy Chinese adults | 102 |
(i) Control n = 51 (ii) Low-BMD n = 51 |
16S rRNA gene sequencing | The low-BMD individuals had a smaller number of OTUs and bacterial taxa at each level | Several taxa with altered abundance and specific functional pathways were discovered in low-BMD individuals |
| Wei et al. [61] | Han Chinese adults older than 60 years or postmenopausal women | 108 |
(i) Control n = 64 (ii) OP n = 44 |
16S rRNA gene sequencing | Patients with OP had higher absolute and relative abundances of Bacteroidetes phylum, and Bacteroides and Eisenbergiella genera | GM compositions may contribute to the risk of OP |
| He et al. [30] | Postmenopausal Chinese women | 106 |
(i) OPe n = 33 (ii) OP n = 42 (iii) normal BMD n = 31 |
16S rRNA gene sequencing LC–MS-based metabolomics approach |
Decreased bacterial richness and diversity in PMOP | We described the disordered profiles of GM and fecal metabolomes in postmenopausal women with OPe and OP |
| Wang et al. [132] | Postmenopausal Chinese women | 42 |
(i) PMO n = 24 (ii) PMN n = 18 |
16S rRNA gene sequencing | GM in the PMO group featured a significantly decreased proportion of the genus Prevotella in comparison with that in the PMN group | The proportion of the genus Prevotella was notably higher in the PMN group, demonstrating its potential bone-protective effects on osteoporosis |
GM gut microbiota, OP osteoporosis, LS-BMD lumbar spine bone mineral density, C-3102 Bacillus subtilis C-3102, DSM 13434 L paracasei 8700:2, DSM 15312 L plantarum Heal 9, DSM 15313 L plantarum Heal 19, BMD bone mineral density, vBMD volumetric bone mineral density, L. reuteri 6475 Lactobacillus reuteri ATCCPTA 6475, RCE red clover extract, CDM vitamin and mineral tablets containing 1040 mg Ca, 487 mg Mg and 25 mg vitamin D, CG control group, EG experimental group, TRACP-5b tartrate-resistant acid phosphatase 5b, IL-10 interleukin-10, TNF-α tumor necrosis factor-α, LBP lipopolysaccharide-binding protein, BMI body mass index, PO primary osteoporosis, VM vaginal microbiota, PMOP postmenopausal osteoporosis, PMO postmenopausal women with osteoporosis, PMN postmenopausal women with normal bone mass, LC–MS-based liquid chromatography-mass spectrometry based
Relevant mechanisms of regulating OP through GM
Inflammatory regulation
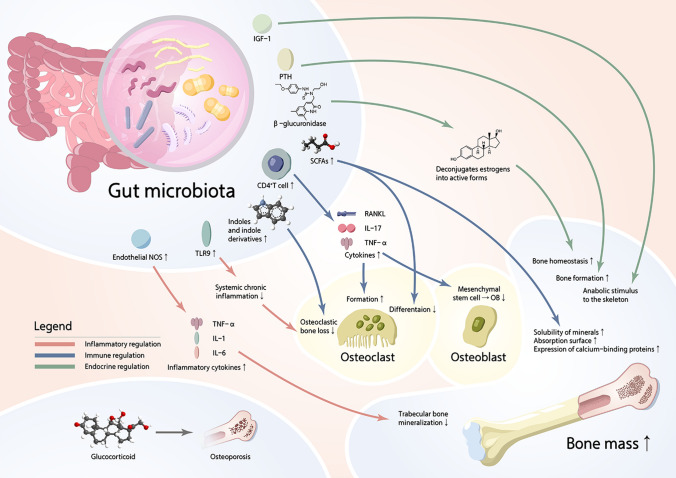
The regulatory role of inflammatory mediators in bone metabolism has been well explored [64]. In addition to osteoclasts and osteoblasts, T cells, B cells, hemopoietic cells, and osteocytes could play a role in inflammatory regulation and bone loss, which have also been shown to be the generators of RANKL [65]. Osteoclast-induced bone resorption is activated by oxidative and inflammatory cytokines such as nitric oxide (NO), IL-1β, IL6, IL-8, IL-18, IL-15, IL-17, IL-32, and TNF-α, many of which induce the osteoclastogenesis by upregulating the release of RANKL [66]. TNF-α inhibits the mesenchymal stromal cells (MSCs) to the transition of osteoblasts by regulating the expression of recombinant runt related transcription factor 2 (RUNX2), a master transcription factor that commits MSCs into osteogenic pathway. TNF-α targets the expression of Osterix, a key transcription necessary for osteoblast maturation [67, 68]. By contrast, bone resorption is down-regulated by anti-inflammatory cytokines, such as IL-3, IL-4, IL-10, IL-13, and transforming growth factor-β1 (TGF-β1) [69]. NO and anti-inflammatory cytokines affect the osteoblasts by increasing osteoprotegerin (OPG) and decreasing the production of RANKL, thus generating a high OPG to RANKL ratio that favors the inhibition of osteoclast differentiation [66]. Lipopolysaccharides (LPS), the important component of lipoproteins derived from gram-negative GM, is a strong stimulatory endotoxin triggering the inflammatory immune responses and influencing the health, which escalates the oxidative stress and inflammation, activating osteoclasts and bone resorption. In osteoblasts, LPS activates the NF-κB and inducible nitric oxide synthase (iNOS) [69, 70]. GM also promotes the endothelial NOS (eNOS) and increased the inflammatory cytokines, including the TNF-α, IL-1, and IL-6, repressing trabecular bone mineralization [69]. The estrogen-deficiency decreases intestinal permeability and represses estrogen receptor-mediated signaling pathways, such as GTP-binding protein Ras, Raf, IFN-γ, and mitogen-activated protein kinase (MAPK) in intestinal epithelial microenvironment [71]. Toll-like receptors (TLRs) play pivotal roles in inflammation and provide vital links between the immune and skeletal systems. According to Ding et al. [72], TLR9-deficiency causes osteoclastic bone loss via altering the composition of GM and inducing systemic chronic inflammation. In detail, Mucispirillum schaedleri and Parabacteroides distasonis presented significant enhancement in the TLR9−/− mice. GM could affect the inflammation state to regulate the bone mass, and the inflammation is also closely associated with bone metabolism [73]. Tousen et al. [74] observed that treatment with resistant starch can attenuated OVX-induced bone loss, which enhanced the abundance of Bifidobacterium in the feces, up-regulated the expression of anti-inflammatory cytokines in the colon, and reduced the expression of osteoclastogenic cytokines in the bone marrow of OVX-induced mice. Using the tilapia nilotica head lipids (THLs) on OVX-induced rats, Zhu et al. [75] demonstrated that the levels of pro-inflammatory cytokines had decreased notably after the intervention of THLs, which reduced the inflammation and prevented the bone resorption. Long-term high fat diet (HFD) resulted in the decreased bone mass, combined with GM dysbiosis, leaky gut, and systemic inflammation. Zhang et al. [76] revealed that the Fructooligosaccharides (FOS) and/or galactooligosaccharides (GOS) reversed high intestinal permeability and pro-inflammatory cytokines, alleviated intestinal and systemic low-grade inflammation induced by HFD, thus protecting against HFD-induced OP.
Immune regulation
A critical role of immune system is to facilitate the beneficial effects of symbiotic relationship while simultaneously preventing the invasion of the host by pathogenic organisms in GM [29]. It is currently clear that the interactions of immune system with GM have profound effects on the bone health [77, 78]. GM contributes to the maturation of the immune system in early life [77]. Villa et al. [79] reviewed that in the absence of the GM, the mucosal immune system was characterized by hypoplastic Peyer’s patches containing minimal germinal centers and a reduced number of IgA-producing plasma cells and lamina propria CD4 + T cells. In addition, the absence of GM results in immature systemic immunity with fewer and smaller germinal centers and reduced the number of CD4 + T cells in the spleen. GM modulates immune system through the production of molecules with immunomodulatory and anti-inflammatory function that are capable to influence the immune cells [77]. RANKL is considered as main cytokine in osteoclast differentiation which is produced by mesenchymal cells, osteoblasts, and osteocytes in the bone marrow [27]. During the process of inflammation, activated CD4 + T cells are also a source of RANKL, as well as other cytokines including IL-17 and TNF-α, which suppress the differentiation of MSCs into osteoblasts and stimulate osteoclastogenesis [80, 81]. Moreover, the relationship between GM and bone is also mediated by innate immunity through several receptors such as nucleotide-binding oligomerization domain proteins (NOD1 and NOD2) receptors and TLR5 [82]. NOD1 and NOD2 are ubiquitary intracellular sensors of the pathogen-associated molecular patterns (PAMPs), mainly expressed on the epithelial and immune cells, that bind bacterial peptidoglycans and activate the NF-κB pathway playing a key role in the effects of microbiota on bone [83]. The effects of GM on host immunity can be modulated by targeting Th17 cells and/or Tregs [84]. For instance, T- cell antigen receptor (TCR), specifically expressed by Th17 cells, could respond to antigens encoded by symbiotic segmented filamentous bacteria (SFB) [85]. SFB-mediated activation of TCR can induce Th17 cell expansion, which negatively affects skeletal maturation [86]. Moreover, Bifidobacteria and Streptococcus thermophilus can increase the concentration of TGF-β, regulate the differentiation of Tregs/Th17 cells, thereby indirectly regulating the immune responses [87]. Gut-derived bacterial metabolites regulate the distant organs, among which indole derivatives were among the first bacterial metabolites to be described to affect the intestinal immunity [88, 89]. The indoles and indole derivatives, such as indole-3-aldehyde (IAld), indole-3-acetic-acid (IAA), indole-3-propionic acid (IPA), indole-3-acetaldehyde (IAAld), and indoleacrylic acid, are the bioactive substances produced directly through the activity of tryptophanase in Escherichia coli and Lactobacillus spp [90]. In particular, SCFAs, the family of metabolites produced by GM, that has received the greatest attention for its capacity to diffuse to the distant organs and induce potent regulatory effects, which recently have been recognized as the pivotal regulators of bone resorption and bone formation [91]. SCFAs blunt the osteoclast differentiation, and the inhibition of histone deacetylase (HDAC) activity is one of the mechanisms whereby this occurs [92]. It has been verified that increased solubility of minerals caused by enriched SCFAs, promoted absorption surface, and enhanced expression of calcium-binding proteins are the underlying mechanisms of probiotics or prebiotics facilitating mineral utilization [93].
Endocrine regulation
Endocrine regulators of the energy metabolism, such as insulin and leptin, might influence the bone metabolism by changing substrate availability, interaction with bone cells or indirect signaling through central nervous system. Insulin-like growth factor 1 (IGF-1), a growth factor known to impact bone via endocrine and paracrine–autocrine mechanisms, also needs to be considered as a possible pathway for microbial impacts on bone [94]. Yan et al. [95] demonstrated that levels of IGF-1, a growth factor known to regulate skeletal formation, are dynamically modulated by changes in GM, thus modulating the anabolic stimulus to the skeleton. IGF-1 increases longitudinal femur development, and cartilage-specific deletion of receptor reveals that IGF-1 is required for growth plate maturation and secondary ossification center development [96]. Mice with a defined microbiota had greater IGF-1 levels than germ-free (GF) mice, indicating that GM can influence the levels of IGF-1, and IGF-1 has a significant impact on bone development and healthy maintenance [97, 98]. Reid et al. [99] revealed that leptin was a key regulator of energy intake and had also been shown to regulate bone metabolism and bone mass in the rodents and humans. Leptin receptor expression decreases as osteoblast differentiation advances, and leptin has a direct anabolic effect on the cells of osteoblast lineage. Moreover, leptin inhibition of osteoclast generation in the cultures of human peripheral blood mononuclear cells, and leptin increased the expression of OPG in cultures, suggesting that the inhibitory effect was mediated via the RANK/OPG system [100]. The endocrine regulation of bone cell activity, including that of leptin and insulin, might act as a sensor of substrate availability needed for the bone metabolism, suggesting that other endocrine factors related to energy availability may be involved in the regulation of bone cell activity [101]. Parathyroid hormone (PTH) is a calciotropic hormone critical for skeletal development. Similar to the butyrate, PTH stimulates the bone formation and induces the bone anabolism via Treg/Wnt10b/Wnt signaling pathway [102]. Using GM depletion by wide-spectrum antibiotics and GF female mice, Li et al. [65] showed that GM was required for PTH to stimulate bone formation and increase bone mass. GM depletion lowered the level of butyrate, a metabolite responsible for gut-bone communication, while the reestablishment of physiologic levels of butyrate restored PTH-induced anabolism [103]. Estrogen depletion observed in post-menopausal women adversely impacts the bone homeostasis, and one of the principal regulators of circulating estrogens is GM [104]. GM regulates estrogens through the secretion of β-glucuronidase, an enzyme that deconjugates estrogens into their active forms. When this process is impaired through, for example, lower diversity of GM, the decrease in deconjugation results in a reduction of circulating estrogens [105]. Excessive osteoclast formation and resorption are considered as the key pathological alterations in estrogen-deficiency induced OP [106]. Estrogen deprivation also increases intestinal permeability allowing the translocation of bacteria and increasing the number of antigens entering the epithelial mucosa what could lead to systemic inflammation [107]. In summary, there is a complicated and close link between GM and OP, and overall mechanisms of three patterns of regulation (inflammation, immune, endocrine) are shown in Fig. 1.
Fig. 1.
The overall mechanisms of the patterns of regulation (inflammation, immune, endocrine)
The effects of GC on GM
The effects on intestinal mucosal barrier
The gastrointestinal tract is the largest surface facing outside environment, being in direct contact with the commensal microbiota and antigens from the diets [108]. To ensure the homeostasis, the intestine acts as a permeable but selective barrier, absorbing nutrients and water to obtain energy and blocking the passage of antigens and bacteria to inner milieu. This critical property of the gut is known as intestinal barrier function (IBF). The regulation of paracellular permeability is exerted chiefly at the tight junction level [109]. The tight junctions are constituted by the transmembrane proteins (claudins, occludins, tricellulin and the junctional adhesion molecule) and peripheral membrane associated proteins that connect to the actin cytoskeleton (zonulae occludens (ZO) 1–3, AF-6, and cingulin) [110]. In most cases, the pathologically increased permeability, for instance, by dysregulated secretion of pro-inflammatory cytokines is associated with the modulation of tight junctions [111]. GC increased the intestinal permeability (barrier leaks) as evidenced by increased levels of endotoxin in the serum. More importantly, the treatment of high-molecular-weight polymer effectively prevented the GC-induced elevation of serum endotoxin and correspondingly prevented femoral trabecular bone loss induced by GC. Lactobacillus reuteri and chronic antibiotic treatments prevented GC-induced barrier leaks. Together, GC-altered barrier dysfunction is a key pathogenic event in GIOP [112].
The effects on GM-related metabolites
In this term, Qiu et al. [113] used gas chromatography to measure levels of SCFAs from 55 individuals, including patients with GC-induced obesity, and age- and gender-matched healthy controls. The result revealed that the overall content of SCFAs in GCs-induced obesity group tended to be lower than that of healthy control. Correspondingly, they observed that propionate and butyrate also decreased dramatically in that group. Zhang et al. [114] revealed that the treatment of prednisone altered the profile of fecal metabolites in the rats, and the changed fecal metabolites included SCFAs, fatty acids, amino acids, organic acids, benzenoids, and phenylpropanoic acids. Collectively, 11 down-regulated and 10 up-regulated metabolites were identified based on a volcano plot and a variable importance in projection analysis. In details, decreased metabolites included valeric acid, propanoic acid, isobutyric acid, isovaleric acid, caproic acid, hydrocinnamic acid, 2-phenylpropionate, phenylacetic acid, orthohydroxyphenylacetic acid, acetoacetic acid, and ethylmethylacetic acid. On the contrary, phenyllactic acid (PLA), hydroxyphenyllactic acid (OH-PLA), homovanillic acid, m-aminobenzoic acid, malonic acid, succinic acid, methylmalonic acid, 2-hydroxy-3methybutyric acid, L-tryptophan, and L-phenylalanine (PAH) increased significantly. Moreover, the pathway analysis suggested that differential metabolites were enriched in pathways, including the phenylalanine metabolism, butanoate metabolism, and propanoate metabolism. In the survey conducted by Marazzato et al. [115], the metabolomic analysis of SCFAs showed that early rheumatoid arthritis (ERA) patients presented significantly decreased propanoic acid levels at baseline compared to controls. Such differences persisted after standard administration (methotrexate plus GC) to the ERA group but the tendency to increase.
The effects of oxidative stress
Oxidative stress, which is induced by the excessive reactive oxygen species (ROS) production and/or an impaired antioxidant system, has been revealed as an underlying mechanism for loss of bone mass and quality [116]. ROS includes the superoxide anion (O2−), hydroxyl (HO.), singlet oxygen (1O2), and hydrogen peroxide (H2O2) [117]. They are highly reactive molecules formed upon incomplete reduction of oxygen during the aerobic metabolism [118]. GC has been reported to induce ROS generation, activating the PKCβ/p66shc/JNK signaling cascade, leading to apoptosis. In response to ROS, the activity of forkhead box O (FoxO) is spontaneously enhanced, along with inhibited Akt phosphorylation and attenuated osteoblastogenesis [119]. Supraphysiologic GC disturbs the balance between free radical generation and the scavenging activities of intracellular antioxidants, which results in oxidative stress [44]. There is currently enough evidence that oxidative stress and the development of OP go hand in hand. Excessive GC induces oxidative stress due to the production of enormous oxidants, leading to the apoptosis of osteoblasts [120]. The upregulation of GC could physiologically indicate increased stress levels, which may alter bone homeostasis, which induces osteoblast injury by triggering JNK phosphorylation mediated the production of ROS [121]. Overproduction of ROS also promotes lipid peroxidation leading to bone loss by inhibiting bone formation [122]. Rai et al. [121] delineated that compound 5e (a BMP2 secretagogue) activated the NRF2 signaling to counter the disturbed cellular redox homeostasis and escalate the osteoblast survival. On the other hand, 5e increased ALP, mineralization activity, and promoted osteoblast differentiation by activating WNT/β-catenin signaling in BMP2-dependent manner, which ameliorated the GC-induced oxidative stress in osteoblasts. Hua et al. [119] reviewed that connexin hemichannel opening was increased under oxidative stress conditions, which confered a cell protective role against the oxidative stress-induced cell death. Oxidative stress acts as a key contributor to the GIOP, and impairs osteocytic network and connexin gap junction communication [123, 124]. Lee et al. [125] reported that the elevated levels of oxidative stress may increase bone resorption by promoting osteoclastogenesis and inhibiting osteogenesis and ginkgolide B (GB), a small natural molecule from ginkgo biloba, possesses pharmacological activities by regulating the ROS in GIOP. In female mice with GIOP models, the oral gavage of GB significantly improved bone mass consistent with the increase in the OPG-to-RANKL ratio.
Potential approaches for prevention and treatment of GIOP from perspectives of GM and its metabolites
Recent researches underscore the emerging role of the GM and metabolites in the treatment of OP [126, 127]. Warmth enhances bacterial polyamine biosynthesis, resulting in higher total polyamine levels in vivo and spermidine supplementation can increase bone strength. The warmth exposure (34 ℃) protects against OVX-induced bone loss by increasing the trabecular bone volume, connectivity density, and thickness, leading to improved biomechanical bone strength in adult female, as well as in the young male mice [1]. Probiotics, which are microorganisms conferring a health benefit to the host when administered in adequate amounts, are present in fermented dairy products and some vegetable-derived food such as sauerkraut and kimchi [128, 129]. Modification of the GM by ingesting probiotics could be a viable therapeutic strategy to regulate bone metabolism under various conditions that result in the bone loss and OP [130, 131]. Prevotella histicola could prevent estrogen deficiency-induced bone loss via the GM-bone axis in postmenopausal women, which may serve as a therapeutic agent or target for OP [132]. The intervention of dietary chondroitin sulfate calcium complex (CSCa) has potentials to alleviate the OP and OP-related symptoms probably involving GM or metabolite profiles as demonstrated in rats [28]. Arecanut seed polyphenol-ameliorated OP by altering GM via lysozyme and immune system in estrogen deficiency rats [133]. FMT, which refers to the transplantation of GM from healthy donors to recipients with GM imbalance, so that the GM in recipients can be reshaped and play a normal function, and further prevent or treat the diseases related to the GM disorder. As a novel “organ transplantation” technique, it can be as a promising treatment option for the OP [50, 88]. Besides, exercise could enrich the abundance and diversity of GM, improve proportion of Firmicutes and Bacteroidetes, induce the proliferation of beneficial microbiota, and improve the function of intestinal mucosal barrier, thereby further modulating the bone metabolism [83, 134, 135].
Currently, the individuals are trying to treat GIOP from the perspectives of GM and its metabolites. Current therapies for OP treatment by inducing osteoblast activity (such as parathyroid hormone treatment), or inhibiting osteoclast function (such as treatment of bisphosphonates), have certain limitations [136, 137]. Due to complex compositions of bioactive substances in natural products and its possible synergistic effects, natural product-derived alternatives for OP therapy are becoming more and more popular. Li et al. [9] reported that TBP alleviates GIOP via the coregulation of NF-κB and Wnt/β-Catenin signaling pathways and the modulation of composition of GM and metabolism, enhancing the abundance of anti-inflammatory bacteria and SCFAs producers. The GC treatment increased the abundance of Bacteroidetes and Proteobacteria and decreased Firmicutes, whereas TBP aggravated the alterations in Bacilli and Clostridia induced by GC and restored the abundance of Actinobacteria. In terms of effects of TBP on the production of SCFAs in feces, the contents of acetic, propionic, and n-butyric acids in feces were reduced in the model group and increased dose dependently after treatments with different dosages of TBP. Liu et al. [2] investigated APS-modified GM and the potential key bacteria to alleviate OP, as well as its relationship with improved OP. They demonstrated the anti-osteoporotic function of APS with restored BMD and repaired bone microarchitecture in a dexamethasone-induced OP rat model. In APS-treated rats, analysis on the bacterial community revealed that the structure of GM was dramatically altered by APS, and the bacteria (c_Bacteroidia, p_Bacteroidetes, and g_Allpprevotella) could serve as biomarkers for APS-improved OP. In addition to this result, five genera (uncultured_bacterium_f_Ruminococcaceae, Alloprevotella, Ruminococcaceae_UCG-014, Blautia and Lactobacillus) were inferred as the key bacteria in APS-improved OP. Researches of GM change accordingly in various food and nutritional conditions which provide a basic knowledge for the future investigations of how interactions between the food components and GM may influence or even determine human health and disease [138–140]. Accordingly, Yun et al. [141] reported that bovine colostrum-derived exosomes (BCE) can delay the progress of GIOP and change the GM in GIOP mice. Osteoporotic mice fed a high BCE exhibited higher BMD and the percent of bone volume than the osteoporotic mice not fed BCE, indicating that BCE ingestion significantly prevented GIOP. Based on the heat map, the relative abundance of Lactobacillus and Bacteroides in PDS group was decreased more than that in the Sham group, but that decrease was significantly reversed by the intake of BCE. Moreover, GIOP increased Firmicutes and Deferribacteres, whereas Bacteroidetes were decreased. The increased Deferribacteres and Bacteroidetes in PDS group was significantly decreased by BCE. Schepper et al. [10] conducted an experiment with adult C57BL/6 J male mice, revealing that probiotic Lactobacillus reuteri 6475 supplementation prevents GC-induced bone loss. Moreover, the composition of GM was significantly different between the groups when examined at the level of OTUs, and the GC-induced bone loss was transferable by FMT. Wang et al. [142] presented that GM diversely changed in GIOP rats and Daphnetin treatment ameliorated this disorder effectively. PCoA plot indicated that the main components of GM in DEX group differed from the control group, while H-Daph treatment narrowed the difference between the GIOP rats and control rats. In addition, the expressions of Clostridiales_vadin_BB60_group and Peptostreptococcaceae were all up-regulated, while the expression of Bacteroidales_RF16_group was down-regulated in GIOP rats, while the treatment of H-Daph restored the expressions of these bacterium. Pan et al. [143] systematically analyzed metabonomic characteristics of GIOP rats and elucidated the therapeutic effects of Epimedium (a common kind of traditional Chinese medicine), using a 1H NMR-based metabonomic approach in conjunction with multivariate data analysis. They observed that GC led to the metabolic disorders of GM and an increase on phenylalanine. Compared with GIOP rats, the concentration of phenylacetylglycine (a metabolic product of phenylalanine from the GM) decreased in the Epimedium group, indicating that Epimedium had effects on metabolism of phenylalanine and improved the status of GM.
Conclusions and perspectives
GC is widely and successfully used in a variety of inflammatory conditions as an immunosuppressive agent, despite the long-term or high-dose use of GC is associated with detrimental effects on bone, leading to GIOP and increased fracture risk. With the increasing understanding of GM-host interactions in recent years, the involvement of GM has become an ingenious and non-negligible way to regulate the host health. The apparent association between the GM and bone metabolic processes suggests that the characterization and identification of GM features may have great clinical potential. Based on the proven regulatory effects of GC on GM, as well as GM on OP, the pivotal contribution of GM in maintaining the balance between the bone formation and bone absorption in the GIOP has become a research hotpot. Amount of clinical and animal researches indicated the beneficial effects of probiotics, prebiotics, traditional Chinese medicines, and bioactive substances derived from natural products on the treatment of GIOP. On this basis, we should also focus on whether GM plays a modulatory role in primary OP and GIOP via the same mechanisms, and how GM and its metabolites affect primary OP and GIOP through different ways and mechanisms in the future researches. Over and above, there are still challenges in translating the applications of GM from the animal studies to clinical practice, including the safety, efficacy, duration of use, and methods for making rational use in humans. Nonetheless, it is reasonable to realize that with further research development in the future, GM and its metabolites may become an important target for regulating the bone metabolism, which is expected to provide a novel approach for the prevention and clinical treatment of GIOP.
Acknowledgements
Not applicable.
Author contributions
RXZ and YWZ wrote the manuscript. RXZ, YWZ, MMC and CHL participated in the creation of tables and figure. YJL and YFR provided intellectual contributions. YJL and YFR supervised the project and undertook the financial support of project. All authors read and agreed to the final manuscript.
Funding
This current work was supported by grants from Winfast Charity Foundation Project (YL20220525); and Jiangsu Elderly Health Research Project, Key Project of Elderly Health Research Project (LKZ2022010); Open Project of National Key Professional Base for Standardized Training of Resident Physicians in Zhongda Hospital Affiliated to Southeast University (ZDZYJD-QK-2022-7).
Declarations
Conflict of interest
All the authors declare that they have no potential conflict of interests, nor biomedical financial interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rui-Xin Zhou and Yuan-Wei Zhang have contributed equally to this work.
References
- 1.Chevalier C, Kieser S, Çolakoğlu M, Hadadi N, Brun J, Rigo D, Suárez-Zamorano N, Spiljar M, Fabbiano S, Busse B, Ivanišević J, Macpherson A, Bonnet N, Trajkovski M. Warmth prevents bone loss through the gut microbiota. Cell Metab. 2020;32:575–590.e7. doi: 10.1016/j.cmet.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J, Liu J, Liu L, Zhang G, Zhou A, Peng X. The gut microbiota alteration and the key bacteria in Astragalus polysaccharides (APS)-improved osteoporosis. Food Res Int. 2020;138:109811. doi: 10.1016/j.foodres.2020.109811. [DOI] [PubMed] [Google Scholar]
- 3.Zhang YW, Lu PP, Li YJ, Wang H, Zhao YK, Chen H, Rui YF. Short report: relationship between self-reported sleep characteristics and falls-associated fractures in elderly individuals: a population-based study. Psychol Health Med. 2022 doi: 10.1080/13548506.2022.2119482. [DOI] [PubMed] [Google Scholar]
- 4.Zhang YW, Lu PP, Li YJ, Dai GC, Chen MH, Zhao YK, Cao MM, Rui YF. Prevalence, characteristics, and associated risk factors of the elderly with hip fractures: a cross-sectional analysis of NHANES 2005–2010. Clin Interv Aging. 2021;16:177–185. doi: 10.2147/CIA.S291071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang YW, Lu PP, Li YJ, Dai GC, Cao MM, Xie T, Zhang C, Shi L, Rui YF. Low dietary choline intake is associated with the risk of osteoporosis in elderly individuals: a population-based study. Food Funct. 2021;12:6442–6451. doi: 10.1039/D1FO00825K. [DOI] [PubMed] [Google Scholar]
- 6.Zhang YW, Cao MM, Li YJ, Dai GC, Lu PP, Zhang M, Bai LY, Chen XX, Shi L, Zhang C, Rui YF. Dietary protein intake in relation to the risk of osteoporosis in middle-aged and older individuals: a cross-sectional study. J Nutr Health Aging. 2022;26:252–258. doi: 10.1007/s12603-022-1748-1. [DOI] [PubMed] [Google Scholar]
- 7.Messina OD, Vidal LF, Wilman MV, Bultink IEM, Raterman HG, Lems W. Management of glucocorticoid-induced osteoporosis. Aging Clin Exp Res. 2021;33:793–804. doi: 10.1007/s40520-021-01823-0. [DOI] [PubMed] [Google Scholar]
- 8.Adami G, Saag KG. Glucocorticoid-induced osteoporosis: 2019 concise clinical review. Osteoporos Int. 2019;30:1145–1156. doi: 10.1007/s00198-019-04906-x. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Yang M, Lu C, Han J, Tang S, Zhou J, Li Y, Ming T, Wang ZJ, Su X. Tuna bone powder alleviates glucocorticoid-induced osteoporosis via coregulation of the NF-κB and Wnt/β-catenin signaling pathways and modulation of gut microbiota composition and metabolism. Mol Nutr Food Res. 2020;64:e1900861. doi: 10.1002/mnfr.201900861. [DOI] [PubMed] [Google Scholar]
- 10.Schepper JD, Collins F, Rios-Arce ND, Kang HJ, Schaefer L, Gardinier JD, Raghuvanshi R, Quinn RA, Britton R, Parameswaran N, McCabe LR. Involvement of the gut microbiota and barrier function in glucocorticoid-induced osteoporosis. J Bone Miner Res. 2020;35:801–820. doi: 10.1002/jbmr.3947. [DOI] [PubMed] [Google Scholar]
- 11.Tena-Garitaonaindia M, Arredondo-Amador M, Mascaraque C, Asensio M, Marin JJG, Martínez-Augustin O, Sánchez de Medina F. Modulation of intestinal barrier function by glucocorticoids: lessons from preclinical models. Pharmacol Res. 2022;177:106056. doi: 10.1016/j.phrs.2022.106056. [DOI] [PubMed] [Google Scholar]
- 12.Zayny A, Almokhtar M, Wikvall K, Ljunggren Ö, Ubhayasekera K, Bergquist J, Kibar P, Norlin M. Effects of glucocorticoids on vitamin D(3)-metabolizing 24-hydroxylase (CYP24A1) in Saos-2 cells and primary human osteoblasts. Mol Cell Endocrinol. 2019;496:110525. doi: 10.1016/j.mce.2019.110525. [DOI] [PubMed] [Google Scholar]
- 13.Adami G, Saag KG. Glucocorticoid-induced osteoporosis update. Curr Opin Rheumatol. 2019;31:388–393. doi: 10.1097/BOR.0000000000000608. [DOI] [PubMed] [Google Scholar]
- 14.Best JH, Kong AM, Lenhart GM, Sarsour K, Stott-Miller M, Hwang Y. Association between glucocorticoid exposure and healthcare expenditures for potential glucocorticoid-related adverse events in patients with rheumatoid arthritis. J Rheumatol. 2018;45:320–328. doi: 10.3899/jrheum.170418. [DOI] [PubMed] [Google Scholar]
- 15.Soen S, Kaku M, Okubo N, Onishi Y, Saito K, Kobayashi M. Fracture risk associated with glucocorticoid-induced osteoporosis in Japan. J Bone Miner Metab. 2022;40:636–647. doi: 10.1007/s00774-022-01325-7. [DOI] [PubMed] [Google Scholar]
- 16.Tripathi AK, Rai D, Kothari P, Kushwaha P, Sashidhara KV, Trivedi R. Benzofuran pyran hybrid prevents glucocorticoid induced osteoporosis in mice via modulation of canonical Wnt/β-catenin signaling. Apoptosis. 2022;27:90–111. doi: 10.1007/s10495-021-01702-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahata M, Shimizu T, Yamada S, Yamamoto T, Hasegawa T, Fujita R, Kobayashi H, Endo T, Koike Y, Amizuka N, Todoh M, Okumura JI, Kajino T, Iwasaki N. Bone biopsy findings in patients receiving long-term bisphosphonate therapy for glucocorticoid-induced osteoporosis. J Bone Miner Metab. 2022;40:613–622. doi: 10.1007/s00774-022-01323-9. [DOI] [PubMed] [Google Scholar]
- 18.de Vasconcelos RF, Costa V, Araujo B, Maia TAC, Dias R, Vasconcelos L, Silveira H, Carneiro B, Thiers D, Costa FWG, Kurita L, Ayala A, Leitão R, Pereira KMA, Gondim DV, Goes P. Milk kefir therapy improves the skeletal response to resistance exercise in rats submitted to glucocorticoid-induced osteoporosis. Exp Gerontol. 2022;167:111921. doi: 10.1016/j.exger.2022.111921. [DOI] [PubMed] [Google Scholar]
- 19.Liu P, Gao Y, Luo P, Yu H, Guo S, Liu F, Gao J, Xu J, Wang S, Zhang C. Glucocorticoid-induced expansion of classical monocytes contributes to bone loss. Exp Mol Med. 2022;54:765–776. doi: 10.1038/s12276-022-00764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cabrera D, Kruger M, Wolber FM, Roy NC, Fraser K. Effects of short- and long-term glucocorticoid-induced osteoporosis on plasma metabolome and lipidome of ovariectomized sheep. BMC Musculoskelet Disord. 2020;21:349. doi: 10.1186/s12891-020-03362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato D, Takahata M, Ota M, Fukuda C, Hasegawa T, Yamamoto T, Amizuka N, Tsuda E, Okada A, Hiruma Y, Fujita R, Iwasaki N. Siglec-15-targeting therapy protects against glucocorticoid-induced osteoporosis of growing skeleton in juvenile rats. Bone. 2020;135:115331. doi: 10.1016/j.bone.2020.115331. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka I, Tanaka Y, Soen S, Oshima H. Efficacy of once-weekly teriparatide in patients with glucocorticoid-induced osteoporosis: the TOWER-GO study. J Bone Miner Metab. 2021;39:446–455. doi: 10.1007/s00774-020-01171-5. [DOI] [PubMed] [Google Scholar]
- 23.Li B, Liu M, Wang Y, Gong S, Yao W, Li W, Gao H, Wei M. Puerarin improves the bone micro-environment to inhibit OVX-induced osteoporosis via modulating SCFAs released by the gut microbiota and repairing intestinal mucosal integrity. Biomed Pharmacother. 2020;132:110923. doi: 10.1016/j.biopha.2020.110923. [DOI] [PubMed] [Google Scholar]
- 24.Liu JH, Chen CY, Liu ZZ, Luo ZW, Rao SS, et al. Extracellular vesicles from child gut microbiota enter into bone to preserve bone mass and strength. Adv Sci (Weinh) 2021;8:2004831. doi: 10.1002/advs.202004831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen MS, Frost M. Alliances of the gut and bone axis. Semin Cell Dev Biol. 2022;123:74–81. doi: 10.1016/j.semcdb.2021.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Zaiss MM, Jones RM, Schett G, Pacifici R. The gut-bone axis: how bacterial metabolites bridge the distance. J Clin Invest. 2019;129:3018–3028. doi: 10.1172/JCI128521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang YW, Li YJ, Lu PP, Dai GC, Chen XX, Rui YF. The modulatory effect and implication of gut microbiota on osteoporosis: from the perspective of “brain-gut-bone” axis. Food Funct. 2021;12:5703–5718. doi: 10.1039/D0FO03468A. [DOI] [PubMed] [Google Scholar]
- 28.Shen Q, Zhang C, Qin X, Zhang H, Zhang Z, Richel A. Modulation of gut microbiota by chondroitin sulfate calcium complex during alleviation of osteoporosis in ovariectomized rats. Carbohydr Polym. 2021;266:118099. doi: 10.1016/j.carbpol.2021.118099. [DOI] [PubMed] [Google Scholar]
- 29.Lorenzo J. From the gut to bone: connecting the gut microbiota with Th17 T lymphocytes and postmenopausal osteoporosis. J Clin Invest. 2021 doi: 10.1172/JCI146619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He W, Xie Z, Thøgersen R, Rasmussen MK, Zachariassen LF, Jørgensen NR, Nørgaard JV, Andersen HJ, Nielsen DS, Hansen AK, Bertram HC. Effects of calcium source, inulin, and lactose on gut-bone associations in an ovarierectomized rat model. Mol Nutr Food Res. 2022;66:e2100883. doi: 10.1002/mnfr.202100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Behera J, Ison J, Tyagi SC, Tyagi N. The role of gut microbiota in bone homeostasis. Bone. 2020;135:115317. doi: 10.1016/j.bone.2020.115317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng M, Tan B, Wu X, Liao F, Wang F, Huang Z. Gut microbiota is involved in alcohol-induced osteoporosis in young and old rats through immune regulation. Front Cell Infect Microbiol. 2021;11:636231. doi: 10.3389/fcimb.2021.636231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Wang X, Zhang C, Liu Z, Li C, Ren Z. Gut microbiota and bone diseases: a growing partnership. Front Microbiol. 2022;13:877776. doi: 10.3389/fmicb.2022.877776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C, Huang Q, Yang R, Dai Y, Zeng Y, Tao L, Li X, Zeng J, Wang Q. Gut microbiota composition and bone mineral loss-epidemiologic evidence from individuals in Wuhan, China. Osteoporos Int. 2019;30:1003–1013. doi: 10.1007/s00198-019-04855-5. [DOI] [PubMed] [Google Scholar]
- 35.Das M, Cronin O, Keohane DM, Cormac EM, Nugent H, Nugent M, Molloy C, O’Toole PW, Shanahan F, Molloy MG, Jeffery IB. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatol (Oxford) 2019;58:2295–2304. doi: 10.1093/rheumatology/kez302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Locantore P, Del Gatto V, Gelli S, Paragliola RM, Pontecorvi A. The interplay between immune system and microbiota in osteoporosis. Mediat Inflamm. 2020;2020:3686749. doi: 10.1155/2020/3686749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orwoll ES, Parimi N, Wiedrick J, Lapidus J, Napoli N, Wilkinson JE, Huttenhower C, Langsetmo L, Kiel DP. Analysis of the associations between the human fecal microbiome and bone density, structure, and strength: the osteoporotic fractures in men (MrOS) Cohort. J Bone Miner Res. 2022;37:597–607. doi: 10.1002/jbmr.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez CJ. Bone mechanical function and the gut microbiota. Adv Exp Med Biol. 2017;1033:249–270. doi: 10.1007/978-3-319-66653-2_12. [DOI] [PubMed] [Google Scholar]
- 39.Guss JD, Horsfield MW, Fontenele FF, Sandoval TN, Luna M, Apoorva F, Lima SF, Bicalho RC, Singh A, Ley RE, van der Meulen MC, Goldring SR, Hernandez CJ. Alterations to the gut microbiome impair bone strength and tissue material properties. J Bone Miner Res. 2017;32:1343–1353. doi: 10.1002/jbmr.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luna M, Guss JD, Vasquez-Bolanos LS, Castaneda M, Rojas MV, Strong JM, Alabi DA, Dornevil SD, Nixon JC, Taylor EA, Donnelly E, Fu X, Shea MK, Booth SL, Bicalho R, Hernandez CJ. Components of the gut microbiome that influence bone tissue-level strength. J Bone Miner Res. 2021;36:1823–1834. doi: 10.1002/jbmr.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu H, Gu R, Li W, Zhou W, Cong Z, Xue J, Liu Y, Wei Q, Zhou Y. Lactobacillus rhamnosus GG attenuates tenofovir disoproxil fumarate-induced bone loss in male mice via gut-microbiota-dependent anti-inflammation. Ther Adv Chronic Dis. 2019;10:2040622319860653. doi: 10.1177/2040622319860653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang P, Li Y, Zhuang H, Yu H, Cai S, Xu H, Chen Z, Lin J, Yao X. Influence of bone densitometry on the anti-osteoporosis treatment after fragility hip fracture. Aging Clin Exp Res. 2019;31:1525–1529. doi: 10.1007/s40520-018-1094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou J, Qin MZ, Liu Q, Liu JP. Investigation and analysis of osteoporosis, falls, and fragility fractures in elderly people in the Beijing area: a study on the bone health status of elderly people ≥ 80 years old with life self-care. Arch Osteoporos. 2017;12:108. doi: 10.1007/s11657-017-0408-2. [DOI] [PubMed] [Google Scholar]
- 44.Chen CY, Rao SS, Yue T, Tan YJ, Yin H, Chen LJ, Luo MJ, Wang Z, Wang YY, Hong CG, Qian YX, He ZH, Liu JH, Yang F, Huang FY, Tang SY, Xie H. Glucocorticoid-induced loss of beneficial gut bacterial extracellular vesicles is associated with the pathogenesis of osteonecrosis. Sci Adv. 2022;8:e8335. doi: 10.1126/sciadv.abg8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang X, Chang T, Yuan Q, Wei W, Wang P, Song X, Yuan H. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244. doi: 10.3389/fimmu.2022.930244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jansson P-A, Curiac D, Lazou Ahrén I, Hansson F, Martinsson Niskanen T, Sjögren K, Ohlsson C. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019;1:e154–e162. doi: 10.1016/S2665-9913(19)30068-2. [DOI] [PubMed] [Google Scholar]
- 47.Morato-Martínez M, López-Plaza B, Santurino C, Palma-Milla S, Gómez-Candela C. A dairy product to reconstitute enriched with bioactive nutrients stops bone loss in high-risk menopausal women without pharmacological treatment. Nutrients. 2020;12:2203. doi: 10.3390/nu12082203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L, Chen B, Zhu R, Li R, Tian Y, Liu C, Jia Q, Wang L, Tang J, Zhao D, Mo F, Liu Y, Li Y, Orekhov AN, Brömme D, Zhang D, Gao S. Fructus Ligustri Lucidi preserves bone quality through the regulation of gut microbiota diversity, oxidative stress, TMAO and Sirt6 levels in aging mice. Aging (Albany NY) 2019;11:9348–9368. doi: 10.18632/aging.102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang N, Ma S, Fu L. Gut microbiota dysbiosis as one cause of osteoporosis by impairing intestinal barrier function. Calcif Tissue Int. 2022;110:225–235. doi: 10.1007/s00223-021-00911-7. [DOI] [PubMed] [Google Scholar]
- 50.Zhang YW, Cao MM, Li YJ, Lu PP, Dai GC, Zhang M, Wang H, Rui YF. Fecal microbiota transplantation ameliorates bone loss in mice with ovariectomy-induced osteoporosis via modulating gut microbiota and metabolic function. J Orthop Translat. 2022;37:46–60. doi: 10.1016/j.jot.2022.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu S, Yuan X, Ma C, Zhao J, Xiong Z. (1)H-NMR-based urinary metabolomic analysis for the preventive effects of gushudan on glucocorticoid-induced osteoporosis rats. Anal Biochem. 2020;610:113992. doi: 10.1016/j.ab.2020.113992. [DOI] [PubMed] [Google Scholar]
- 52.Liu Z, Xu X, Shen Y, Hao Y, Cui W, Li W, Zhang X, Lv H, Li X, Hou Y, Zhang X. Altered gut microbiota and metabolites profile are associated with reduced bone metabolism in ethanol-induced osteoporosis. Cell Prolif. 2022;55:e13245. doi: 10.1111/cpr.13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozaki D, Kubota R, Maeno T, Abdelhakim M, Hitosugi N. Association between gut microbiota, bone metabolism, and fracture risk in postmenopausal Japanese women. Osteoporos Int. 2021;32:145–156. doi: 10.1007/s00198-020-05728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma S, Qin J, Hao Y, Fu L. Association of gut microbiota composition and function with an aged rat model of senile osteoporosis using 16S rRNA and metagenomic sequencing analysis. Aging (Albany NY) 2020;12:10795–10808. doi: 10.18632/aging.103293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lan H, Liu WH, Zheng H, Feng H, Zhao W, Hung WL, Li H. Bifidobacterium lactis BL-99 protects mice with osteoporosis caused by colitis via gut inflammation and gut microbiota regulation. Food Funct. 2022;13:1482–1494. doi: 10.1039/D1FO02218K. [DOI] [PubMed] [Google Scholar]
- 56.Lambert MNT, Thybo CB, Lykkeboe S, Rasmussen LM, Frette X, Christensen LP, Jeppesen PB. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: a randomized controlled trial. Am J Clin Nutr. 2017;106:909–920. doi: 10.3945/ajcn.117.153353. [DOI] [PubMed] [Google Scholar]
- 57.Takimoto T, Hatanaka M, Hoshino T, Takara T, Tanaka K, Shimizu A, Morita H, Nakamura T. Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: a randomized, placebo-controlled, double-blind clinical trial. Biosci Microbiota Food Health. 2018;37:87–96. doi: 10.12938/bmfh.18-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jafarnejad S, Djafarian K, Fazeli MR, Yekaninejad MS, Rostamian A, Keshavarz SA. Effects of a multispecies probiotic supplement on bone health in osteopenic postmenopausal women: a randomized, double-blind, controlled trial. J Am Coll Nutr. 2017;36:497–506. doi: 10.1080/07315724.2017.1318724. [DOI] [PubMed] [Google Scholar]
- 59.Xu Z, Xie Z, Sun J, Huang S, Chen Y, Li C, Sun X, Xia B, Tian L, Guo C, Li F, Pi G. Gut microbiome reveals specific dysbiosis in primary osteoporosis. Front Cell Infect Microbiol. 2020;10:160. doi: 10.3389/fcimb.2020.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He J, Xu S, Zhang B, Xiao C, Chen Z, Si F, Fu J, Lin X, Zheng G, Yu G, Chen J. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging (Albany NY) 2020;12:8583–8604. doi: 10.18632/aging.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei M, Li C, Dai Y, Zhou H, Cui Y, Zeng Y, Huang Q, Wang Q. High-throughput absolute quantification sequencing revealed osteoporosis-related gut microbiota alterations in Han Chinese elderly. Front Cell Infect Microbiol. 2021;11:630372. doi: 10.3389/fcimb.2021.630372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nilsson AG, Sundh D, Bäckhed F, Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med. 2018;284:307–317. doi: 10.1111/joim.12805. [DOI] [PubMed] [Google Scholar]
- 63.Tao H, Li W, Zhang W, Yang C, Zhang C, Liang X, Yin J, Bai J, Ge G, Zhang H, Yang X, Li H, Xu Y, Hao Y, Liu Y, Geng D. Urolithin A suppresses RANKL-induced osteoclastogenesis and postmenopausal osteoporosis by, suppresses inflammation and downstream NF-κB activated pyroptosis pathways. Pharmacol Res. 2021;174:105967. doi: 10.1016/j.phrs.2021.105967. [DOI] [PubMed] [Google Scholar]
- 64.Iqbal J, Yuen T, Sun L, Zaidi M. From the gut to the strut: where inflammation reigns, bone abstains. J Clin Invest. 2016;126:2045–2048. doi: 10.1172/JCI87430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li JY, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, Darby TM, Weitzmann MN, Mulle JG, Gewirtz AT, Jones RM, Pacifici R. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126:2049–2063. doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Damani JJ, De Souza MJ, VanEvery HL, Strock NCA, Rogers CJ. The role of prunes in modulating inflammatory pathways to improve bone health in postmenopausal women. Adv Nutr. 2022;13:1476–1492. doi: 10.1093/advances/nmab162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maruyama M, Rhee C, Utsunomiya T, Zhang N, Ueno M, Yao Z, Goodman SB. Modulation of the inflammatory response and bone healing. Front Endocrinol (Lausanne) 2020;11:386. doi: 10.3389/fendo.2020.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu D, Cline-Smith A, Shashkova E, Perla A, Katyal A, Aurora R. T-Cell mediated inflammation in postmenopausal osteoporosis. Front Immunol. 2021;12:687551. doi: 10.3389/fimmu.2021.687551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lian WS, Wang FS, Chen YS, Tsai MH, Chao HR, Jahr H, Wu RW, Ko JY. Gut microbiota ecosystem governance of host inflammation, mitochondrial respiration and skeletal homeostasis. Biomedicines. 2022;10:860. doi: 10.3390/biomedicines10040860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jia X, Yang R, Li J, Zhao L, Zhou X, Xu X. Gut-bone axis: a non-negligible contributor to periodontitis. Front Cell Infect Microbiol. 2021;11:752708. doi: 10.3389/fcimb.2021.752708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He S, Li H, Yu Z, Zhang F, Liang S, Liu H, Chen H, Lü M. The gut microbiome and sex hormone-related diseases. Front Microbiol. 2021;12:711137. doi: 10.3389/fmicb.2021.711137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ding P, Tan Q, Wei Z, Chen Q, Wang C, Qi L, Wen L, Zhang C, Yao C. Toll-like receptor 9 deficiency induces osteoclastic bone loss via gut microbiota-associated systemic chronic inflammation. Bone Res. 2022;10:42. doi: 10.1038/s41413-022-00210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Livshits G, Kalinkovich A. Targeting chronic inflammation as a potential adjuvant therapy for osteoporosis. Life Sci. 2022;306:120847. doi: 10.1016/j.lfs.2022.120847. [DOI] [PubMed] [Google Scholar]
- 74.Tousen Y, Matsumoto Y, Nagahata Y, Kobayashi I, Inoue M, Ishimi Y. Resistant starch attenuates bone loss in ovariectomised mice by regulating the intestinal microbiota and bone-marrow inflammation. Nutrients. 2019;11:297. doi: 10.3390/nu11020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu Y, Liu S, Mei F, Zhao M, Xia G, Shen X. Tilapia nilotica head lipids improved bone loss by regulating inflammation and serum metabolism through gut microbiota in ovariectomized rats. Front Nutr. 2021;8:792793. doi: 10.3389/fnut.2021.792793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Z, Lin T, Meng Y, Hu M, Shu L, Jiang H, Gao R, Ma J, Wang C, Zhou X. FOS/GOS attenuates high-fat diet induced bone loss via reversing microbiota dysbiosis, high intestinal permeability and systemic inflammation in mice. Metabolism. 2021;119:154767. doi: 10.1016/j.metabol.2021.154767. [DOI] [PubMed] [Google Scholar]
- 77.D'Amelio P, Sassi F. Gut microbiota, immune system, and bone. Calcif Tissue Int. 2018;102:415–425. doi: 10.1007/s00223-017-0331-y. [DOI] [PubMed] [Google Scholar]
- 78.Yan J, Charles JF. Gut microbiome and bone: to build, destroy, or both? Curr Osteoporos Rep. 2017;15:376–384. doi: 10.1007/s11914-017-0382-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Villa CR, Ward WE, Comelli EM. Gut microbiota-bone axis. Crit Rev Food Sci Nutr. 2017;57:1664–1672. doi: 10.1080/10408398.2015.1010034. [DOI] [PubMed] [Google Scholar]
- 80.Chen YC, Greenbaum J, Shen H, Deng HW. Association between gut microbiota and bone health: potential mechanisms and prospective. J Clin Endocrinol Metab. 2017;102:3635–3646. doi: 10.1210/jc.2017-00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ibáñez L, Rouleau M, Wakkach A, Blin-Wakkach C. Gut microbiome and bone. Joint Bone Spine. 2019;86:43–47. doi: 10.1016/j.jbspin.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 82.Ohlsson C, Nigro G, Boneca IG, Bäckhed F, Sansonetti P, Sjögren K. Regulation of bone mass by the gut microbiota is dependent on NOD1 and NOD2 signaling. Cell Immunol. 2017;317:55–58. doi: 10.1016/j.cellimm.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 83.Zhang YW, Cao MM, Li YJ, Chen XX, Yu Q, Rui YF. A narrative review of the moderating effects and repercussion of exercise intervention on osteoporosis: ingenious involvement of gut microbiota and its metabolites. J Transl Med. 2022;20:490. doi: 10.1186/s12967-022-03700-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li L, Rao S, Cheng Y, Zhuo X, Deng C, Xu N, Zhang H, Yang L. Microbial osteoporosis: the interplay between the gut microbiota and bones via host metabolism and immunity. Microbiologyopen. 2019;8:e00810. doi: 10.1002/mbo3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shirakashi M, Maruya M, Hirota K, Tsuruyama T, Matsuo T, Watanabe R, Murata K, Tanaka M, Ito H, Yoshifuji H, Ohmura K, Elewaut D, Sakaguchi S, Fagarasan S, Mimori T, Hashimoto M. Effect of impaired T cell receptor signaling on the gut microbiota in a mouse model of systemic autoimmunity. Arthr Rheumatol. 2022;74:641–653. doi: 10.1002/art.42016. [DOI] [PubMed] [Google Scholar]
- 86.Tyagi AM, Darby TM, Hsu E, Yu M, Pal S, Dar H, Li JY, Adams J, Jones RM, Pacifici R. The gut microbiota is a transmissible determinant of skeletal maturation. Elife. 2021 doi: 10.7554/eLife.64237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu L, Chen X, Liu Y, Yu X. Gut microbiota and bone metabolism. Faseb J. 2021;35:e21740. doi: 10.1096/fj.202100451R. [DOI] [PubMed] [Google Scholar]
- 88.Zhang YW, Cao MM, Li YJ, Zhang RL, Wu MT, Yu Q, Rui YF. Fecal microbiota transplantation as a promising treatment option for osteoporosis. J Bone Miner Metab. 2022;40(6):874–889. doi: 10.1007/s00774-022-01375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schiering C, Wincent E, Metidji A, Iseppon A, Li Y, Potocnik AJ, Omenetti S, Henderson CJ, Wolf CR, Nebert DW, Stockinger B. Feedback control of AHR signalling regulates intestinal immunity. Nature. 2017;542:242–245. doi: 10.1038/nature21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y, Lu L, Xie Y, Chen X, Tian L, Liang Y, Li H, Zhang J, Liu Y, Yu X. Interleukin-6 knockout inhibits senescence of bone mesenchymal stem cells in high-fat diet-induced bone loss. Front Endocrinol (Lausanne) 2020;11:622950. doi: 10.3389/fendo.2020.622950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Knudsen JK, Leutscher P, Sørensen S. Gut microbiota in bone health and diabetes. Curr Osteoporos Rep. 2021;19:462–479. doi: 10.1007/s11914-020-00629-9. [DOI] [PubMed] [Google Scholar]
- 92.de Sire A, de Sire R, Curci C, Castiglione F, Wahli W. Role of dietary supplements and probiotics in modulating microbiota and bone health: the gut-bone axis. Cells. 2022;11:743. doi: 10.3390/cells11040743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tu Y, Yang R, Xu X, Zhou X. The microbiota-gut-bone axis and bone health. J Leukoc Biol. 2021;110:525–537. doi: 10.1002/JLB.3MR0321-755R. [DOI] [PubMed] [Google Scholar]
- 94.Yan J, Charles JF. Gut microbiota and IGF-1. Calcif Tissue Int. 2018;102:406–414. doi: 10.1007/s00223-018-0395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yan J, Takakura A, Zandi-Nejad K, Charles JF. Mechanisms of gut microbiota-mediated bone remodeling. Gut Microbes. 2018;9:84–92. doi: 10.1080/19490976.2017.1371893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y, Menendez A, Fong C, ElAlieh HZ, Kubota T, Long R, Bikle DD. IGF-I signaling in Osterix-expressing cells regulates secondary ossification center formation, growth plate maturation, and metaphyseal formation during postnatal bone development. J Bone Miner Res. 2015;30:2239–2248. doi: 10.1002/jbmr.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pacifici R. Bone remodeling and the microbiome. Cold Spring Harb Perspect Med. 2018;8:a031203. doi: 10.1101/cshperspect.a031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seely KD, Kotelko CA, Douglas H, Bealer B, Brooks AE. The human gut microbiota: a key mediator of osteoporosis and osteogenesis. Int J Mol Sci. 2021;22:9452. doi: 10.3390/ijms22179452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reid IR, Baldock PA, Cornish J. Effects of leptin on the skeleton. Endocr Rev. 2018;39:938–959. doi: 10.1210/er.2017-00226. [DOI] [PubMed] [Google Scholar]
- 100.Pacifici R. Role of gut microbiota in the skeletal response to PTH. J Clin Endocrinol Metab. 2021;106:636–645. doi: 10.1210/clinem/dgaa895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kermgard E, Chawla NK, Wesseling-Perry K. Gut microbiome, parathyroid hormone, and bone. Curr Opin Nephrol Hypertens. 2021;30:418–423. doi: 10.1097/MNH.0000000000000714. [DOI] [PubMed] [Google Scholar]
- 102.Yu M, Malik Tyagi A, Li JY, Adams J, Denning TL, Weitzmann MN, Jones RM, Pacifici R. PTH induces bone loss via microbial-dependent expansion of intestinal TNF(+) T cells and Th17 cells. Nat Commun. 2020;11:468. doi: 10.1038/s41467-019-14148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li JY, Yu M, Pal S, Tyagi AM, Dar H, Adams J, Weitzmann MN, Jones RM, Pacifici R. Parathyroid hormone-dependent bone formation requires butyrate production by intestinal microbiota. J Clin Invest. 2020;130:1767–1781. doi: 10.1172/JCI133473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang YW, Cao MM, Li YJ, Dai GC, Lu PP, Zhang M, Bai LY, Chen XX, Zhang C, Shi L, Rui YF. The regulative effect and repercussion of probiotics and prebiotics on osteoporosis: involvement of brain-gut-bone axis. Crit Rev Food Sci Nutr. 2022 doi: 10.1080/104083982047005. [DOI] [PubMed] [Google Scholar]
- 105.Bhardwaj A, Sapra L, Tiwari A, Mishra PK, Sharma S, Srivastava RK. “Osteomicrobiology”: the nexus between bone and bugs. Front Microbiol. 2021;12:812466. doi: 10.3389/fmicb.2021.812466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu M, Pal S, Paterson CW, Li JY, Tyagi AM, Adams J, Coopersmith CM, Weitzmann MN, Pacifici R. Ovariectomy induces bone loss via microbial-dependent trafficking of intestinal TNF+ T cells and Th17 cells. J Clin Invest. 2021 doi: 10.1172/JCI143137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li R, Boer CG, Oei L, Medina-Gomez C. The gut microbiome: a new frontier in musculoskeletal research. Curr Osteoporos Rep. 2021;19:347–357. doi: 10.1007/s11914-021-00675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zheng G, Victor Fon G, Meixner W, Creekmore A, Zong Y, Michael KD, Colacino J, Dedhia PH, Hong S, Wiley JW. Chronic stress and intestinal barrier dysfunction: glucocorticoid receptor and transcription repressor HES1 regulate tight junction protein Claudin-1 promoter. Sci Rep. 2017;7:4502. doi: 10.1038/s41598-017-04755-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee YM, Ayres JS. Decoding the intestinal epithelium cell by cell. Nat Immunol. 2018;19:7–9. doi: 10.1038/s41590-017-0011-0. [DOI] [PubMed] [Google Scholar]
- 111.Aranda CJ, Arredondo-Amador M, Ocón B, Lavín JL, Aransay AM, Martínez-Augustin O, de Medina FS. Intestinal epithelial deletion of the glucocorticoid receptor NR3C1 alters expression of inflammatory mediators and barrier function. Faseb j. 2019;33:14067–14082. doi: 10.1096/fj.201900404RR. [DOI] [PubMed] [Google Scholar]
- 112.Shukla PK, Meena AS, Pierre JF, Rao R. Central role of intestinal epithelial glucocorticoid receptor in alcohol- and corticosterone-induced gut permeability and systemic response. Faseb J. 2022;36:e22061. doi: 10.1096/fj.202101424R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Qiu D, Xia Z, Deng J, Jiao X, Liu L, Li J. Glucorticoid-induced obesity individuals have distinct signatures of the gut microbiome. BioFactors. 2019;45:892–901. doi: 10.1002/biof.1565. [DOI] [PubMed] [Google Scholar]
- 114.Zhang J, Feng D, Law HK, Wu Y, Zhu GH, Huang WY, Kang Y. Integrative analysis of gut microbiota and fecal metabolites in rats after prednisone treatment. Microbiol Spectr. 2021;9:e0065021. doi: 10.1128/Spectrum.00650-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marazzato M, Iannuccelli C, Guzzo MP, Nencioni L, Lucchino B, Radocchia G, Gioia C, Bonfiglio G, Neroni B, Guerrieri F, Pantanella F, Garzoli S, Vomero M, Barbati C, Di Franco M, Schippa S. Gut microbiota structure and metabolites, before and after treatment in early rheumatoid arthritis patients: a pilot study. Front Med (Lausanne) 2022;9:921675. doi: 10.3389/fmed.2022.921675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang N, Sun H, Xue Y, Zhang W, Wang H, Tao H, Liang X, Li M, Xu Y, Chen L, Zhang L, Huang L, Geng D. Inhibition of MAGL activates the Keap1/Nrf2 pathway to attenuate glucocorticoid-induced osteonecrosis of the femoral head. Clin Transl Med. 2021;11:e447. doi: 10.1002/ctm2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen K, Qiu P, Yuan Y, Zheng L, He J, Wang C, Guo Q, Kenny J, Liu Q, Zhao J, Chen J, Tickner J, Fan S, Lin X, Xu J. Pseurotin A inhibits osteoclastogenesis and prevents ovariectomized-induced bone loss by suppressing reactive oxygen species. Theranostics. 2019;9:1634–1650. doi: 10.7150/thno.30206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin J, Epel E. Stress and telomere shortening: insights from cellular mechanisms. Ageing Res Rev. 2022;73:101507. doi: 10.1016/j.arr.2021.101507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hua R, Zhang J, Riquelme MA, Jiang JX. Connexin gap junctions and hemichannels link oxidative stress to skeletal physiology and pathology. Curr Osteoporos Rep. 2021;19:66–74. doi: 10.1007/s11914-020-00645-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Suarez-Bregua P, Guerreiro PM, Rotllant J. Stress, glucocorticoids and bone: a review from mammals and fish. Front Endocrinol (Lausanne) 2018;9:526. doi: 10.3389/fendo.2018.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rai D, Tripathi AK, Sardar A, Pandey AR, Sinha S, Chutani K, Dhaniya G, Kothari P, Sashidhara KV, Trivedi R. A novel BMP2 secretagogue ameliorates glucocorticoid induced oxidative stress in osteoblasts by activating NRF2 dependent survival while promoting Wnt/β-catenin mediated osteogenesis. Free Radic Biol Med. 2022;190:124–147. doi: 10.1016/j.freeradbiomed.2022.08.007. [DOI] [PubMed] [Google Scholar]
- 122.Han D, Gu X, Gao J, Wang Z, Liu G, Barkema HW, Han B. Chlorogenic acid promotes the Nrf2/HO-1 anti-oxidative pathway by activating p21(Waf1/Cip1) to resist dexamethasone-induced apoptosis in osteoblastic cells. Free Radic Biol Med. 2019;137:1–12. doi: 10.1016/j.freeradbiomed.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 123.Riquelme MA, Cardenas ER, Xu H, Jiang JX. The role of connexin channels in the response of mechanical loading and unloading of bone. Int J Mol Sci. 2020;21:1146. doi: 10.3390/ijms21031146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kar R, Riquelme MA, Werner S, Jiang JX. Connexin 43 channels protect osteocytes against oxidative stress-induced cell death. J Bone Miner Res. 2013;28:1611–1621. doi: 10.1002/jbmr.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee CW, Lin HC, Wang BY, Wang AY, Shin RL, Cheung SYL, Lee OK. Ginkgolide B monotherapy reverses osteoporosis by regulating oxidative stress-mediated bone homeostasis. Free Radic Biol Med. 2021;168:234–246. doi: 10.1016/j.freeradbiomed.2021.03.008. [DOI] [PubMed] [Google Scholar]
- 126.Rizzoli R, Biver E. Are probiotics the new calcium and vitamin D for bone health? Curr Osteoporos Rep. 2020;18:273–284. doi: 10.1007/s11914-020-00591-6. [DOI] [PubMed] [Google Scholar]
- 127.Polzonetti V, Pucciarelli S, Vincenzetti S, Polidori P. Dietary intake of vitamin D from dairy products reduces the risk of osteoporosis. Nutrients. 2020;12:1743. doi: 10.3390/nu12061743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rizzoli R. Nutritional influence on bone: role of gut microbiota. Aging Clin Exp Res. 2019;31:743–751. doi: 10.1007/s40520-019-01131-8. [DOI] [PubMed] [Google Scholar]
- 129.Rizzoli R, Biver E, Brennan-Speranza TC. Nutritional intake and bone health. Lancet Diabetes Endocrinol. 2021;9:606–621. doi: 10.1016/S2213-8587(21)00119-4. [DOI] [PubMed] [Google Scholar]
- 130.Huidrom S, Beg MA, Masood T. Post-menopausal osteoporosis and probiotics. Curr Drug Targets. 2021;22:816–822. doi: 10.2174/18735592MTEwrOTUbx. [DOI] [PubMed] [Google Scholar]
- 131.Collins FL, Rios-Arce ND, Schepper JD, Parameswaran N, McCabe LR. The potential of probiotics as a therapy for osteoporosis. Microbiol Spectr. 2017 doi: 10.1128/microbiolspec.BAD-0015-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang Z, Chen K, Wu C, Chen J, Pan H, Liu Y, Wu P, Yuan J, Huang F, Lang J, Du J, Xu J, Jin K, Chen L. An emerging role of Prevotella histicola on estrogen deficiency-induced bone loss through the gut microbiota-bone axis in postmenopausal women and in ovariectomized mice. Am J Clin Nutr. 2021;114:1304–1313. doi: 10.1093/ajcn/nqab194. [DOI] [PubMed] [Google Scholar]
- 133.Mei F, Meng K, Gu Z, Yun Y, Zhang W, Zhang C, Zhong Q, Pan F, Shen X, Xia G, Chen H. Arecanut (Areca catechu L.) seed polyphenol-ameliorated osteoporosis by altering gut microbiome via LYZ and the immune system in estrogen-deficient rats. J Agric Food Chem. 2021;69:246–258. doi: 10.1021/acs.jafc.0c06671. [DOI] [PubMed] [Google Scholar]
- 134.Chow TH, Lee BY, Ang ABF, Cheung VYK, Ho MMC, Takemura S. The effect of Chinese martial arts Tai Chi Chuan on prevention of osteoporosis: a systematic review. J Orthop Translat. 2018;12:74–84. doi: 10.1016/j.jot.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhu Y, Huang Z, Wang Y, Xu W, Chen H, Xu J, Luo S, Zhang Y, Zhao D, Hu J. The efficacy and safety of denosumab in postmenopausal women with osteoporosis previously treated with bisphosphonates: a review. J Orthop Translat. 2020;22:7–13. doi: 10.1016/j.jot.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Reid IR, Billington EO. Drug therapy for osteoporosis in older adults. Lancet. 2022;399:1080–1092. doi: 10.1016/S0140-6736(21)02646-5. [DOI] [PubMed] [Google Scholar]
- 137.Anagnostis P, Gkekas NK, Potoupnis M, Kenanidis E, Tsiridis E, Goulis DG. New therapeutic targets for osteoporosis. Maturitas. 2019;120:1–6. doi: 10.1016/j.maturitas.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 138.Makki K, Deehan EC, Walter J, Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23:705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 139.Danneskiold-Samsøe NB, de Freitas D, Queiroz Barros H, Santos R, Bicas JL, Cazarin CBB, Madsen L, Kristiansen K, Pastore GM, Brix S, Maróstica Júnior MR. Interplay between food and gut microbiota in health and disease. Food Res Int. 2019;115:23–31. doi: 10.1016/j.foodres.2018.07.043. [DOI] [PubMed] [Google Scholar]
- 140.Ercolini D, Fogliano V. Food design to feed the human gut microbiota. J Agric Food Chem. 2018;66:3754–3758. doi: 10.1021/acs.jafc.8b00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yun B, Maburutse BE, Kang M, Park MR, Park DJ, Kim Y, Oh S. Short communication: dietary bovine milk-derived exosomes improve bone health in an osteoporosis-induced mouse model. J Dairy Sci. 2020;103:7752–7760. doi: 10.3168/jds.2019-17501. [DOI] [PubMed] [Google Scholar]
- 142.Wang Y, Chen J, Chen J, Dong C, Yan X, Zhu Z, Lu P, Song Z, Liu H, Chen S. Daphnetin ameliorates glucocorticoid-induced osteoporosis via activation of Wnt/GSK-3β/β-catenin signaling. Toxicol Appl Pharmacol. 2020;409:115333. doi: 10.1016/j.taap.2020.115333. [DOI] [PubMed] [Google Scholar]
- 143.Pan S, Chen A, Han Z, Wang Y, Lu X, Yang Y. (1)H NMR-based metabonomic study on the effects of epimedium on glucocorticoid-induced osteoporosis. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1038:118–126. doi: 10.1016/j.jchromb.2016.10.015. [DOI] [PubMed] [Google Scholar]