Abstract
Hospital samples collected in gel separator tubes are often submitted to forensic toxicology laboratories for analysis in impaired driving and death investigations. Drug adsorption to the gel separator material may lead to underestimation of the drug concentration present at the time of sample collection, potentially affecting the interpretation of analytical results. Using liquid chromatography--tandem mass spectrometry (LC--MS-MS), decreases in plasma concentration of 53 drugs and metabolites relevant to forensic toxicology casework were investigated in samples stored in BD Vacutainer® PSTTM tubes for up to 3 months. After storage for only 1 day, approximately 50% of the drugs and metabolites had significantly lower concentrations in plasma separation tubes (PSTs) compared to non-gel tubes (up to 27% lower). After storage for 3 months, approximately 75% of the drugs and metabolites had significantly lower concentrations in PSTs compared to non-gel tubes (up to 69% lower). Fentanyl, carfentanil, ketamine, diphenhydramine and several antidepressants were among the drugs most susceptible to adsorption. Central nervous system stimulants (e.g., methamphetamine and amphetamine) as well as naturally-occurring and semi-synthetic opioids (e.g., morphine, hydromorphone and oxycodone) were among the drugs least susceptible to adsorption and displayed only minimal relative decreases in concentration (if any) over the 3-month sample storage period. The potential for decreases in drug concentration due to adsorption of drugs to the gel material should be considered for toxicological interpretation based on the analysis of a sample collected in a gel separator tube.
Introduction
Blood collection tubes containing gel separators, such as BD Vacutainer® serum separation SSTTM and plasma separation PSTTM tubes (Becton Dickinson, Franklin Lakes, NJ, USA), are widely used to draw blood for medical purposes. Following collection of whole blood, these tubes are centrifuged to allow for physical separation of serum or plasma from the cellular components of whole blood. In certain investigations, including impaired driving and death investigations, samples collected in hospital in these tubes may be seized by police or coroners for toxicological analysis. In many of these cases, sample submission to a forensic laboratory and/or drug analysis by the forensic laboratory may not occur until weeks or months after sample collection.
Previous studies have examined the suitability of gel separator collection tubes for therapeutic drug monitoring (TDM) (1–11). A variety of drugs including antidepressants, antipsychotics, antiepileptics, benzodiazepines, antibiotics, asthma drugs and cardiac drugs have been investigated using both drug-spiked and authentic patient serum or plasma samples. Although some drugs such as theophylline and salicylate (1, 2) have been reported to be stable in samples stored in gel separator tubes, it has been reported that many drugs including phenytoin (1–3, 5, 10), lidocaine (1–3), carbamazepine (1, 2, 5, 10), various antidepressants (4, 7, 8), and some antipsychotics (7) may significantly decrease in concentration when samples are stored in gel separator tubes as compared to non-gel tubes. Numerous studies have attributed this decrease in drug concentration to drug adsorption to (or absorption by) the gel separator material during sample storage (1–11). In addition, Dasgupta et al. (1) directly demonstrated this effect by extracting drugs from the gel separator material.
The sample storage times investigated in the existing studies range from less than a couple hours (1, 4, 6, 7, 9, 10) up to a maximum of 7 days (5, 6, 8, 10). In several studies, significant decreases in drug concentration were reported within the first couple hours of sample storage in gel separator tubes. For example, Dasgupta et al. (1) reported a 14.4% decrease in the serum concentration of phenytoin after only 60 minutes of storage in a serum separation tube. Similarly, Wollmann et al. (7) reported significantly lower serum concentrations (up to 13% lower) for 14 out of 21 antidepressant drugs and metabolites in samples stored for approximately 2 hours in gel separator tubes as compared to non-gel tubes. When sample storage time is extended to several days, decreases in drug concentration of more than 40% have been reported for some drugs (1, 3, 7–9).
In addition to TDM, potential adsorption of drugs to the gel separator material in collection tubes is also an important consideration in forensic toxicology. Analysis of serum/plasma samples collected in these tubes may underestimate drug concentrations present at the time of sample collection, potentially affecting the interpretation of analytical results. Application of the existing studies to forensic investigations (where samples may be stored for weeks or months prior to analysis) is limited by the short sample storage times investigated thus far. Furthermore, there is limited literature on the adsorption susceptibility of many of the psychoactive drugs commonly encountered in forensic toxicology casework. Opioids such as fentanyl and central nervous system stimulants such as methamphetamine and cocaine are among the most commonly encountered drugs in both impaired driving and death investigation casework (12–15). A recent study by Schrapp et al. (11) investigated the stability of 167 therapeutic drugs and drugs of abuse in samples stored in gel separator tubes for 4 hours as compared to storage in non-gel tubes. This study included many drugs relevant to forensic toxicology but was limited by the short sample storage time examined. As such, the aim of the present study was to investigate decreases in plasma concentration of 53 different drugs and metabolites relevant to forensic toxicology casework in samples stored for up to 3 months in gel separator tubes compared to non-gel tubes.
Materials and Methods
Standards and reagents
Certified reference drugs (Supplementary Table S1) and corresponding deuterated internal standards were obtained from Cerilliant (Round Rock, TX, USA). Working standard solutions for six calibrators, each containing all 53 analytes, were prepared by dilution of the stock solutions with acetonitrile. Additional working solutions for high and low positive controls, a low cut-off (LCO) and internal standard were also prepared in acetonitrile.
Liquid chromatography--mass spectrometry grade acetonitrile was obtained from Caledon Laboratory Chemicals (Halton Hills, ON, Canada). Formic acid (MS grade) and ammonium formate were obtained from Sigma-Aldrich (St. Louis, MO, USA). High purity deionized water was delivered by a Millipore Milli-Q® Reference water purification system (Molsheim, France).
Blood collection tubes and specimens
BD Vacutainer® PSTTM Gel and Lithium Heparin tubes (4.5 mL; 13 × 100 mm) and BD Vacutainer® Lithium Heparin (“control”) tubes (6.0 mL; 13 × 100 mm) were obtained from Becton Dickinson (Franklin Lakes, NJ, USA).
Drug-free (“blank”) human plasma was obtained from Interstate Blood Bank (Memphis, TN, USA) and screened via liquid chromatography--tandem mass spectrometry (LC--MS-MS) to confirm the absence of all analytes of interest. Lyphochek® drug-free (“blank”) lyophilized matrix was obtained from Bio-Rad Laboratories (Hercules, CA, USA).
Sample preparation and experimental setup
Blank human plasma was spiked with a standard solution containing all 53 analytes in order to obtain plasma drug concentrations that were approximately 60% of the highest calibrator (Supplementary Table S1). An aliquot of the drug-spiked human plasma was added (1.5 mL per tube) into 30 BD Vacutainer® PSTTM Gel and Lithium Heparin tubes (gel separator tubes) and 30 BD Vacutainer® Lithium Heparin tubes (control tubes); both tube types were prepared in triplicate for each of 10 different storage times. Following addition of the drug-spiked plasma, each tube was inverted 8 times and then centrifuged at 1,300 g for 10 minutes according to BD Vacutainer® recommendations. After centrifugation, an aliquot of plasma from each tube in one set (i.e., three gel separator tubes and three control tubes) was extracted within 1 hour of tube preparation and analyzed. The remaining nine sets of tubes (each consisting of three gel separator tubes and three control tubes) were stored at 4°C for the specified storage time (1 day, 3 days, 5 days, 1 week, 2 weeks, 3 weeks, 1 month, 2 months or 3 months). After the specified storage time, an aliquot of plasma from each tube in the set was extracted and analyzed.
Extraction procedure
On each extraction day, the six calibrators, LCO, high and low positive controls and a negative control were prepared in blank matrix. Specifically, Lyphochek® blank matrix (250 µL) was added to 25 µL of calibrator/LCO/positive control solution and 25 µL of internal standard solution and vortexed.
The drug-spiked plasma gel separator and control tubes were removed from the refrigerator approximately 30 minutes prior to extraction to allow them to reach room temperature. After equilibration to room temperature, the tubes were inverted 8 times before extraction. A 250 µL aliquot of drug-spiked plasma was then added to 25 µL of internal standard solution and vortexed.
Proteins were precipitated by adding 0.75 mL of acetonitrile, vortexing to mix and allowing to stand for 5 minutes. Following centrifugation (4,000 rpm for 20 minutes at 10°C), the supernatant was decanted into Phenomenex (Torrance, CA, USA) PhreeTM Phospholipid Removal Tabbed Tubes pre-inserted into 1.8 mL auto-sampler vials. The tubes were then centrifuged (2,000 rpm for 10 minutes at 10°C) and the auto-sampler vials were dried under nitrogen using a Biotage (Uppsala, Sweden) TurboVap® LV Concentration Workstation at 40°C and 4 psi. The residue was reconstituted with 1 mL reconstitution solvent (90:10 v/v 10 mM ammonium formate in 0.2% formic acid(aq): acetonitrile) and vortexed.
LC--MS-MS analysis
Extracts were analyzed using a Waters (Milford, MA, USA) ACQUITY Ultra Performance Liquid Chromatography UPLC® system paired with a SCIEX (Framingham, MA, USA) QTRAP® 5500 MS-MS operated in positive electrospray ionization (ESI) mode. Extracts (5 µL) were injected onto a conditioned Restek (Bellefonte, PA, USA) UltraTM Biphenyl 100 × 2.1 mm 3 µm analytical column with a Restek UltraTM Biphenyl 10 × 2.1 mm 3 µm guard column. Chromatographic separation occurred using a gradient method with a flow rate of 0.25 mL/min where mobile phase A was 10 mM ammonium formate in 0.2% formic acid(aq) and mobile phase B was 0.2% formic acid in acetonitrile. Data was acquired using SCIEX Analyst® 1.6.2 software in scheduled multiple reaction monitoring (MRM) mode with two transitions monitored for each analyte. The analytical method was previously validated for the quantitation of all included analytes.
Data analysis
Data was analyzed using SCIEX MultiQuantTM 3.0.3 processing software. The processing method utilized a quadratic curve fitting with 1/x weighting. Plasma drug concentrations were determined by comparison of the response ratio in the sample to the calibration curve. Mean drug concentration ± standard deviation (SD) was calculated for each analyte in each tube type after each storage time. Two-tailed, independent sample t-tests were used to compare mean drug concentrations for samples stored in PSTs to those for samples stored in control tubes after each storage time. A difference in mean drug concentration with a p-value < 0.05 was considered statistically significant.
To control for any decreases in drug concentration due to drug degradation and assess only decreases that were unique to the gel separator tube, mean plasma drug concentration in the PST was compared to mean plasma drug concentration in the control tube on a given day using the following formula:
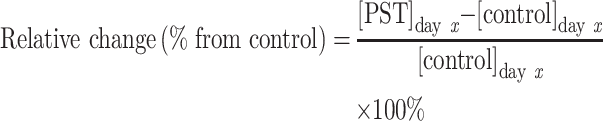 |
Results
Changes in plasma drug concentration during 3 months of refrigerated storage in PSTs and control tubes are shown in Figure 1 for six representative drugs. Plasma concentrations of fentanyl, methadone and diazepam were significantly lower in PSTs compared to control tubes whereas no major decline in the plasma concentrations of hydromorphone, methamphetamine and amphetamine were observed in PSTs relative to control tubes.
Figure 1.

Mean plasma concentration of representative drugs during 3 months of refrigerated storage in PSTs (n = 3) and control tubes (n = 3). Error bars show standard deviation.
Mean plasma concentrations for all 53 studied drugs and metabolites in samples stored in PSTs and control tubes are shown in Supplementary Table S2 for select storage times. Decreases in drug concentration in PSTs began immediately after tube preparation with approximately 50% of the studied drugs and metabolites already having significantly (p < 0.05) lower mean plasma concentrations in PSTs compared to control tubes within an hour after the spiked plasma was added into the tubes (2–17% lower). Drug concentrations continued to decrease over time and after 3 months of storage, approximately 75% of the studied drugs and metabolites had significantly lower mean concentrations in PSTs compared to control tubes (up to 69% lower).
Relative change (% from control) in plasma drug concentration for samples stored in PSTs compared to samples stored in control tubes for an equivalent length of time is shown in Figures 2 and 3 based on pharmacological drug classification. Since the relative change compares the drug concentration in gel and control tubes on a given day, it controls for drug degradation such that a calculated relative decrease in concentration can be attributed to drug adsorption. While the majority of drugs and metabolites had lower concentrations in PSTs than in control tubes (represented by a negative relative change or a “loss”), a few drugs which were susceptible to degradation and which decreased in concentration in both tube types had significantly higher concentrations in PSTs than in control tubes towards the end of the study (represented by a positive relative change). Data for drugs having a relative change greater than +10% after a given storage time was excluded from Figures 2 and 3 (denoted with an “X”), but is summarized below.
Figure 2.

Relative change (% from control) in opioid, benzodiazepine and stimulant drug and metabolite concentrations for plasma samples stored in PSTs compared to samples stored in control tubes for an equivalent length of time. * = statistically significant difference between concentration in PST and control (p < 0.05). nd = not detected. tr = traces (concentration < LOQ). X = data not included (relative change >+10%).
Figure 3.

Relative change (% from control) in antidepressant, antipsychotic and miscellaneous drug and metabolite concentrations for plasma samples stored in PSTs compared to samples stored in control tubes for an equivalent length of time. * = statistically significant difference between concentration in PST and control (p < 0.05). nd = not detected. tr = traces (concentration < LOQ). X = data not included (relative change >+10%).
Opioids
Of the 13 opioid drugs and metabolites studied, carfentanil, fentanyl, methadone and meperidine showed the largest relative decreases in concentration in PSTs. After storage for only 1 day, all had significant relative loss: carfentanil (−17%), methadone (−16%), fentanyl (−15%) and meperidine (−11%). Similarly, after storage for 1 month, all had a relative loss exceeding 30%: carfentanil (−53%), fentanyl (−51%), methadone (−42%) and meperidine (−32%). Fentanyl and carfentanil, in particular, were among the drugs most susceptible to adsorption in the entire study, both having a relative loss of −69% in PSTs after storage for 3 months. In contrast, plasma concentrations of morphine, hydromorphone, codeine, oxycodone and oxymorphone were less than 10% lower in PSTs compared to control tubes after all sample storage times studied. Hydrocodone had a relative loss of −12% after storage for 3 months; however, it was not statistically significant (Figure 2a).
During sample storage, plasma concentrations of 6-monoacetylmorphine (6-MAM) decreased and plasma concentrations of morphine increased in both PSTs and control tubes, presumably due to degradation of 6-MAM into morphine. After 3 months of storage, the plasma concentration of 6-MAM in PSTs was significantly higher than in control tubes (+16%).
Benzodiazepines
All 11 of the studied benzodiazepine drugs and metabolites had significantly lower concentrations in PSTs relative to control tubes after 1 week of storage with relative losses ranging from −5% (midazolam, oxazepam) to −20% (clonazepam). After storage for 1 month, five of the benzodiazepines had a relative loss in PSTs exceeding 20%: clonazepam (−38%), diazepam (−34%), N-desalkylflurazepam (−27%), temazepam (−22%) and nordiazepam (−21%). After 3 months, relative losses in PSTs ranged from −15% (oxazepam) to −59% (clonazepam) (Figure 2b).
Stimulants
Overall, the 8 stimulant drugs and metabolites studied showed minimal reductions in plasma concentration in PSTs relative to control tubes. The largest relative decreases among this drug class were observed at 1 week for cocaine (−16%) and mephedrone (−11%). Plasma concentrations of benzoylecgonine, methamphetamine, amphetamine, methylene-dioxymethamphetamine (MDMA), methylenedioxyamphetamine (MDA) and methylenedioxyethylamphetamine (MDEA) were less than 10% lower in PSTs compared to control tubes after all sample storage times studied and these relative losses were often not statistically significant (Figure 2c).
Plasma concentrations of cocaine and mephedrone decreased dramatically in both PSTs and control tubes during sample storage. Cocaine was not detectable in any of the tubes by 1 month and only traces of mephedrone (concentration less than the limit of quantitation (LOQ) of the method) remained in the tubes after 3 months of sample storage.
Antidepressants and antipsychotics
Many of the 14 studied antidepressant and antipsyhotic drugs and metabolites demonstrated large decreases in concentration in PSTs relative to control tubes. For example, after storage for only 1 week, half of the drugs/metabolites had a relative loss exceeding 20%: bupropion (−40%), citalopram (−29%), mirtazapine (−27%), amitriptyline (−23%), sertraline (−21%), nortriptyline (−20%) and fluoxetine (−20%). After storage for 3 months, half had a relative loss exceeding 60%, making them among the drugs most susceptible to adsorption in the study: mirtazapine (−69%), nortriptyline (−69%), sertraline (−62%), fluoxetine (−62%), amitriptyline (−61%), paroxetine (−61%) and citalopram (−60%) (Figure 3a).
Plasma concentrations of bupropion and olanzapine decreased dramatically in both PSTs and control tubes during sample storage. Bupropion was not detectable in any of the tubes by 2 months and olanzapine was not detectable in any of the tubes by 3 months. After 1 month of sample storage, the plasma concentration of olanzapine in PSTs was significantly higher than in control tubes (+129%).
Miscellaneous
With the exception of zopiclone, the other six miscellaneous drugs and metabolites displayed significantly lower plasma concentrations in PSTs relative to control tubes. All had a relative loss greater than 15% after 1 week, greater than 25% after 1 month and greater than 40% after 3 months. Ketamine, diphenhydramine, and cyclobenzaprine had the largest relative losses among this group (−69%, −64% and −62%, respectively, after 3 months) (Figure 3b).
Zopiclone did not appear to be susceptible to adsorption but decreased dramatically in concentration in both PSTs and control tubes during sample storage. It was not detectable in any of the tubes by 2 months.
Discussion
The results of this study demonstrated that many of the drugs and metabolites studied are susceptible to decreases in concentration due to drug adsorption to the gel separator in collection tubes during sample storage. The results also confirmed that the magnitude of the loss is both drug- and time-dependent. While most of the studied drugs and metabolites exhibited significant decreases in plasma concentration as a function of storage time in PSTs relative to non-gel control tubes, there were some notable exceptions. For example, stimulant and some opioid drugs displayed minimal relative decreases in concentration over 3 months of sample storage. Nevertheless, for those drugs and metabolites that were susceptible to adsorption, the most rapid decline in concentration was generally observed in the first week of sample storage. This may suggest that gel saturation or a gel/plasma equilibrium is developed over time. Significant decreases in drug concentration occurring in samples stored in gel separator tubes have been previously reported even within the first hour after sample preparation (1). This was also observed in the current study where approximately 50% of the analytes already had statistically significant lower plasma concentrations in PSTs relative to control tubes within an hour after the plasma was added into the tubes (up to 17% lower).
Existing studies generally focus on drugs involved in TDM. Data is limited for most of the psychoactive drugs in this study; however, there are several exceptions. Many of the antidepressant drugs included here have been previously examined (4, 7, 8) and gel separator tubes were found to be unsuitable for TDM of these drugs due to significant decreases in drug concentration. In addition, Schrapp et al. (11) previously tested 39 of the 53 drugs and metabolites included here. The results of the current study generally support their findings; however, direct comparisons are difficult due to differences in the data collected. For example, Schrapp et al. (11) stored samples in gel separator tubes for only 4 hours and used a concentration of 20 ng/mL for each analyte. The current study examined considerably longer storage times and used concentrations that were significantly higher for the majority of drugs/metabolites tested.
Several drugs, including cocaine, bupropion, olanzapine, zopiclone and mephedrone, decreased rapidly in concentration in both PSTs and control tubes, making susceptibility to adsorption difficult to assess. For instance, cocaine was not detectable in any of the control tubes or PSTs by 1 month, bupropion and zopiclone by 2 months and olanzapine by 3 months. This confirms earlier observations that these drugs are unstable and susceptible to degradation during sample storage (16–20). Although the relative percent change values presented in this study control for drug degradation for the purposes of examining drug adsorption, the potential for decreases in drug concentration due to drug degradation must also be considered when providing a toxicological interpretation. Interestingly, as degradation progressed and the concentrations of these drugs declined, some (e.g., olanzapine at 3 weeks and 1 month, zopiclone at 2 weeks and 3 weeks, 6-MAM at 3 months) displayed significantly higher plasma concentrations in PSTs compared to control tubes. Further investigation would be required to determine the mechanism for this unexpected observation.
The physicochemical properties of a drug can influence the degree to which it is adsorbed to the gel separator. Lipophilic drugs have been shown to be more prone to adsorption to the lipophilic polymer-based gel separator and thus are most affected by storage in gel separator tubes (7, 8, 11). While the primary focus of the current study was not to correlate drug lipophilicity with susceptibility to adsorption, the results appear to support this conclusion. For instance, the degree of adsorption of opioid drugs appeared to be dependent upon molecular structure and lipophilicity. Opioid drugs are classified as either naturally-occurring, semi-synthetic or synthetic. Semi-synthetic opioids such as hydromorphone, hydrocodone, oxycodone and oxymorphone are derived from, and are structurally similar to, naturally-occurring opiates such as morphine and codeine. Synthetic opioids such as fentanyl, carfentanil, methadone, meperidine and tramadol are structurally dissimilar to naturally-occurring opiates and have been reported to be substantially more lipophilic (21, 22). The relationship between opioid lipophilicity/molecular structure and the degree of adsorption is demonstrated in Figure 2a where the synthetic opioids showed the most significant adsorption. In comparison, all naturally-occurring and semi-synthetic opioids had plasma concentrations that were less than 12% lower in PSTs compared to control tubes, even after storage for 3 months.
This study investigated only one sample storage temperature (4°C), plasma volume (1.5 mL) and plasma concentration of each analyte. Future research directed at exploring these variables could be beneficial. Refrigerated storage is commonly used in forensic laboratories for short-term storage of samples until analyses are complete; however, other storage conditions may be encountered at some laboratories, at the hospital and/or police service where the samples are stored prior to submission and during transport to the laboratory. A plasma volume of 1.5 mL was selected for this study because it is representative of the sample volumes often received for analysis in forensic toxicology casework. Nevertheless, smaller sample volumes are also commonly received (sometimes less than 500 µL). Previous studies have demonstrated that the magnitude of decreases in drug concentration attributed to adsorption are dependent upon the volume of sample in the tube (1, 3, 9). Dasgupta et al. (1) found that decreases in drug concentration were most pronounced when small volumes (less than 500 µL) were stored in gel separator tubes. For example, the mean concentration of phenytoin declined by 64.5% and 10.7% when 200 µL and 1.5 mL aliquots of serum, respectively, were stored in serum separation tubes for 48 hours (1). As such, decreases in drug concentration may be greater than observed in the current study if the volume of sample in the tube is less than 1.5 mL.
Given that only one plasma concentration of each drug/metabolite was investigated in this study, it is unknown whether the magnitude of the relative reduction in drug concentration would differ for other plasma concentrations. Concentrations were selected such that they were on the upper half of the calibration curve. For some drugs, the starting concentration exceeded a typical therapeutic range; however, in impaired driving and death investigation cases, samples with elevated drug concentrations are commonly encountered. Other studies have examined the effect of drug concentration on the magnitude of adsorption and the results have been mixed. Quattrocchi et al. (3) found that the percent decrease in drug concentration remained relatively consistent regardless of the initial concentration. In comparison, Steuer et al. (8) found that the relative decrease in concentration was larger at lower drug concentrations and attributed this difference to a saturable mechanism of adsorption to the gel separator material.
The current study used drug-spiked human plasma samples rather than authentic samples taken from individuals who had administered the drugs of interest. The use of drug-spiked samples enabled consistent and controlled evaluation of the behaviour of all 53 analytes. Existing studies use drug-spiked samples, authentic patient samples or both. In one study that used both drug-spiked and authentic patient samples, the magnitude of adsorption was reported to be greater in drug-spiked samples (4). The authors attributed this difference to drugs not being highly bound to serum proteins in spiked samples (4). In contrast, another study that used both drug-spiked and authentic patient samples reported that drug concentrations in both sample types were affected by adsorption to a similar degree (7). The extent of protein binding can affect the magnitude of adsorption. For example, significantly greater adsorption was observed when drug-spiked protein-free serum ultrafiltrate was stored in serum separation tubes as compared to when drug-spiked normal serum was stored in the same tube type (1). Similarly, significantly greater adsorption was observed when drug-spiked phosphate buffer was stored in serum separation tubes as compared to when drug-spiked serum was stored in the same tube type (5). Given this information, it may be worthwhile to also evaluate the magnitude of adsorption occurring during storage of authentic samples taken from individuals who have administered the drugs of interest in the current study.
Despite these limitations, this study has important implications for forensic toxicology casework. It demonstrates that drug analysis of samples collected in gel separator tubes may not be preferrable if samples collected in other tube types are also available for analysis. Prompt analysis of samples collected in gel separator tubes is important; however, this can prove difficult as samples may not be submitted to the forensic laboratory until weeks or months after sample collection. Further, for some drugs, the results of a second confirmatory drug quantitation may be lower than the results of the initial drug quantitation if an appreciable amount of time has elapsed between the two analyses. When interpreting analytical findings, this study demonstrates that, for some drugs, concentrations determined in samples stored in gel separator tubes may significantly underestimate the concentration at the time of sample collection. Toxicologically significant drug concentrations present at the time of sample collection may decrease to a concentration that may be regarded as insignificant upon analysis. Additionally, drugs present at the time of sample collection may no longer be detectable at the time of analysis. The potential for decreases in drug concentration due to adsorption of drugs to the gel material should be considered (along with other factors such as drug degradation) in any toxicological interpretation that is based on the analysis of a sample collected in a gel separator tube.
Conclusion
Plasma concentrations of many of the studied drugs and metabolites were significantly lower in samples stored in gel separator tubes compared to non-gel control tubes. Concentration decreases due to adsorption were both drug- and time-dependent. Fentanyl, carfentanil, ketamine, diphenhydramine and several antidepressants were among the drugs most susceptible to adsorption during extended sample storage of up to 3 months. Central nervous system stimulants as well as naturally-occurring and semi-synthetic opioids were among the drugs least susceptible to adsorption and displayed only minimal relative decreases in concentration (if any) over the 3-month sample storage period.
Supplementary Material
Acknowledgments
Betty Chow, Rachelle Wallage and Patricia Solbeck are acknowledged for their review of the manuscript and for thoughtful discussions throughout the process.
Contributor Information
Cara L Shepard, Toxicology Section, Centre of Forensic Sciences, 25 Morton Shulman Avenue, Toronto, ON M3M 0B1, Canada.
Liora Bliumkin, Laboratory Services Section, Centre of Forensic Sciences, 25 Morton Shulman Avenue, Toronto, ON M3M 0B1, Canada.
Supplementary Data
Supplementary Data is available at Journal of Analytical Toxicology online.
Funding
No funding received for this work.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. Dasgupta A., Dean R., Saldana S., Kinnaman G., McLawhon R.W. (1994) Absorption of therapeutic drugs by barrier gels in serum separator blood collection tubes. Volume- and time-dependent reduction in total and free drug concentrations. American Journal of Clinical Pathology, 101, 456–461. [DOI] [PubMed] [Google Scholar]
- 2. Dasgupta A., Blackwell W., Bard D. (1996) Stability of therapeutic drug measurement in specimens collected in Vacutainer plastic blood-collection tubes. Therapeutic Drug Monitoring, 18, 306–309. [DOI] [PubMed] [Google Scholar]
- 3. Quattrocchi F., Karnes H.T., Robinson J.D., Hendeles L. (1983) Effect of serum separator blood collection tubes on drug concentrations. Therapeutic Drug Monitoring, 5, 359–362. [DOI] [PubMed] [Google Scholar]
- 4. Karppi J., Akerman K.K., Parviainen M. (2000) Suitability of collection tubes with separator gels for collecting and storing blood samples for therapeutic drug monitoring (TDM). Clinical Chemistry and Laboratory Medicine, 38, 313–320. [DOI] [PubMed] [Google Scholar]
- 5. Bergqvist Y., Eckerbom S., Funding L. (1984) Effect of use of gel-barrier sampling tubes on determination of some antiepileptic drugs in serum. Clinical Chemistry, 30, 465–466. [PubMed] [Google Scholar]
- 6. Bush V., Blennerhasset J., Wells A., Dasgupta A. (2001) Stability of therapeutic drugs in serum collected in Vacutainer serum separator tubes containing a new gel (SST II). Therapeutic Drug Monitoring, 23, 259–262. [DOI] [PubMed] [Google Scholar]
- 7. Wollmann B.M., Lunde H.A., Stoten L.K., Lunder N., Molden E. (2019) Substantial differences in serum concentrations of psychoactive drugs measured in samples stored for 2 days or more on standard serum tubes versus serum tubes containing gel separators. Therapeutic Drug Monitoring, 41, 396–400. [DOI] [PubMed] [Google Scholar]
- 8. Steuer C., Huber A.R., Bernasconi L. (2016) Where clinical chemistry meets medicinal chemistry. Systematic analysis of physico-chemical properties predicts stability of common used drugs in gel separator serum tubes. Clinica Chimica Acta, 462, 23–27. [DOI] [PubMed] [Google Scholar]
- 9. Hegstad S., Spigset O., Helland A. (2020) Stability of 21 antihypertensive drugs in serum collected in standard (nongel) serum tubes versus tubes containing a gel separator. Therapeutic Drug Monitoring, 42, 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yan R., Colantonio D., Wong P.-Y., Chen Y. (2014) Suitability of Becton Dickinson Vacutainer rapid serum tube for collecting and storing blood samples for antibiotic and anticonvulsant drug monitoring. Journal of Clinical Pathology, 67, 807–810. [DOI] [PubMed] [Google Scholar]
- 11. Schrapp A., Mory C., Duflot T., Pereira T., Imbert L., Lamoureux F. (2019) The right blood collection tube for therapeutic drug monitoring and toxicology screening procedures: standard tubes, gel or mechanical separator? Clinica Chimica Acta, 488, 196–201. [DOI] [PubMed] [Google Scholar]
- 12. Rohrig T.P., Nash E., Osawa K.A., Shan X., Scarneo C., Youso K.B., et al. (2021) Fentanyl and driving impairment. Journal of Analytical Toxicology, 45, 389–396. [DOI] [PubMed] [Google Scholar]
- 13. Chan-Hosokawa A., Bierly J.J. (2021) 11-year study of fentanyl in driving under the influence of drugs casework. Journal of Analytical Toxicology. 10.1093/jat/bkab049. [DOI] [PubMed] [Google Scholar]
- 14. Vaillancourt L., Viel E., Dombrowski C., Desharnais B., Mireault P. (2021) Drugs and driving prior to cannabis legalization: a 5-year review from DECP (DRE) cases in the province of Quebec, Canada. Accident Analysis and Prevention, 149, 105832. [DOI] [PubMed] [Google Scholar]
- 15. O’Donnell J., Gladden R.M., Mattson C.L., Hunter C.T., Davis N.L. (2020) Vital signs: characteristics of drug overdose deaths involving opioids and stimulants – 24 states and the District of Columbia, January-June 2019. Morbidity and Mortality Weekly Report, 69, 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Isenschmid D.S., Levine B.S., Caplan Y.H. (1989) A comprehensive study of the stability of cocaine and its metabolites. Journal of Analytical Toxicology, 13, 250–256. [DOI] [PubMed] [Google Scholar]
- 17. Laizure S.C., DeVane C.L. (1985) Stability of bupropion and its major metabolites in human plasma. Therapeutic Drug Monitoring, 7, 447–450. [DOI] [PubMed] [Google Scholar]
- 18. Olesen O.V., Linnet K. (1998) Determination of olanzapine in serum by high-performance liquid chromatography using ultraviolet detection considering the easy oxidability of the compound and the presence of other psychotropic drugs. Journal of Chromatography B, 714, 309–315. [DOI] [PubMed] [Google Scholar]
- 19. Nilsson G.H., Kugelberg F.C., Kronstrand R., Ahlner J. (2010) Stability tests of zopiclone in whole blood. Forensic Science International, 200, 130–135. [DOI] [PubMed] [Google Scholar]
- 20. Johnson R.D., Botch-Jones S.R. (2013) The stability of four designer drugs: MDPV, mephedrone, BZP and TFMPP in three biological matrices under various storage conditions. Journal of Analytical Toxicology, 37, 51–55. [DOI] [PubMed] [Google Scholar]
- 21. Roy S.D., Flynn G.L. (1988) Solubility and related physicochemical properties of narcotic analgesics. Pharmaceutical Research, 5, 580–586. [DOI] [PubMed] [Google Scholar]
- 22. Concheiro M., Chesser R., Pardi J., Cooper G. (2018) Postmortem toxicology of new synthetic opioids. Frontiers in Pharmacology, 9, 1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.


