Abstract
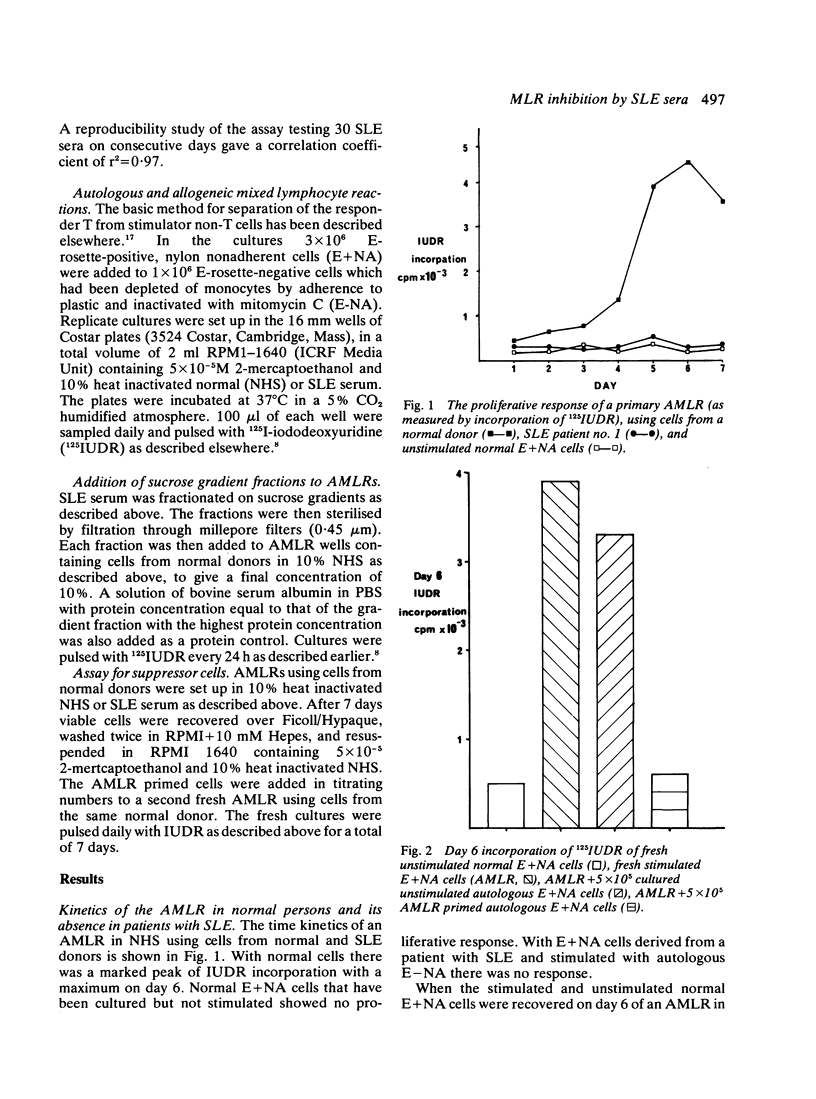
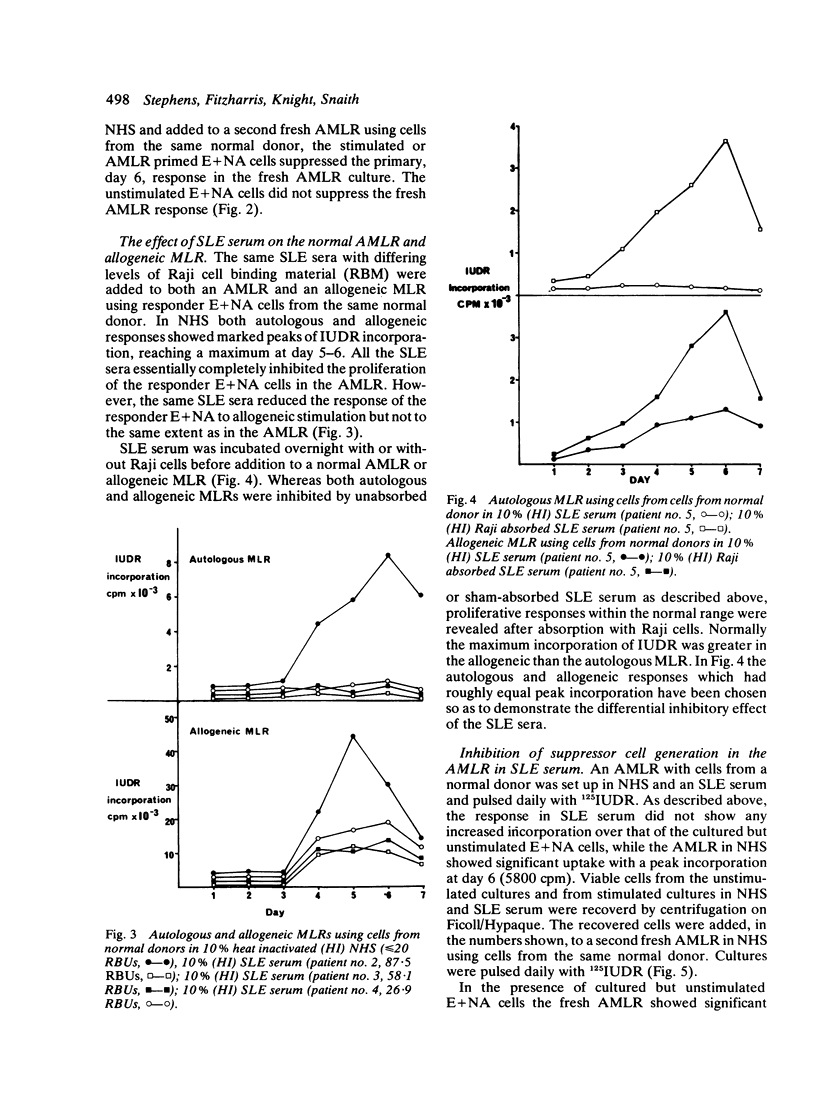
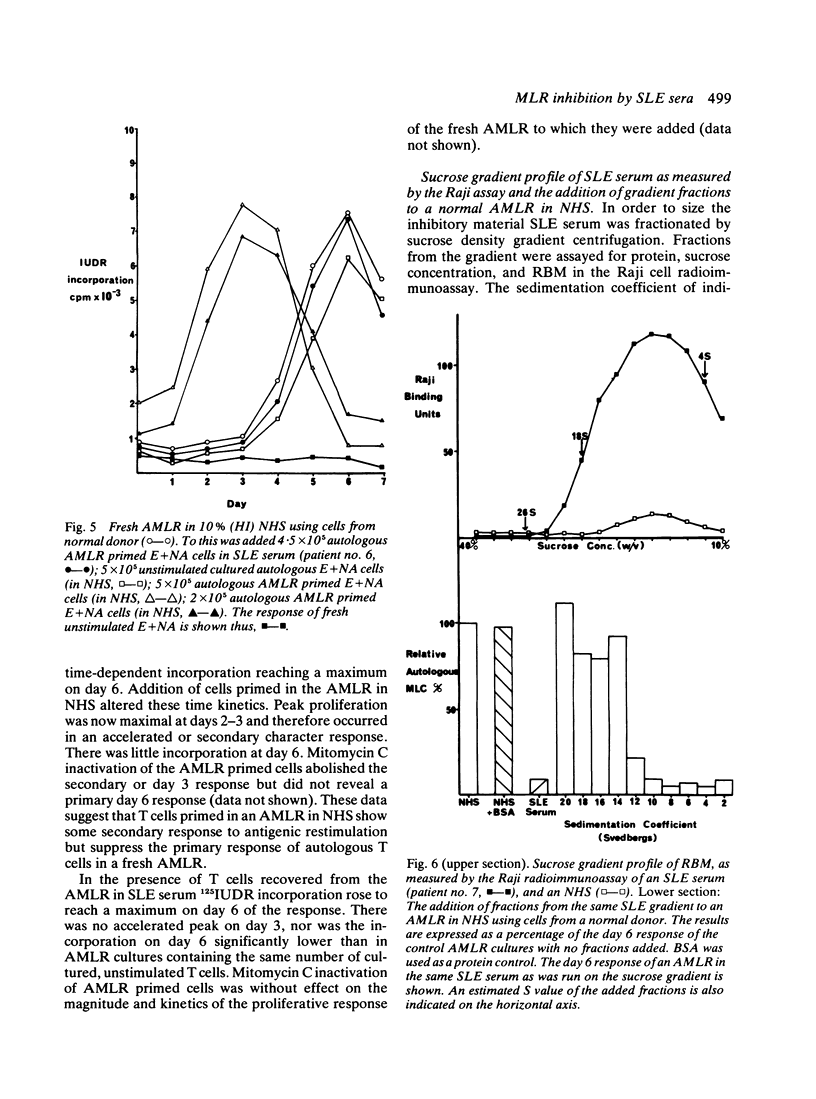
Serum from patients with systemic lupus erythematosus (SLE) prevents the proliferative response of normal T cells when stimulated by autologous or allogeneic non-T cells. The abrogation of proliferation in an autologous mixed lymphocyte reaction (AMLR) with SLE serum is associated with a lack of suppressor T cell generation. Fractionation of SLE sera on sucrose gradients reveals an 18-12 S peak of Raji cell binding material. Fractions with an S value of ≤12 S show inhibitory activity in an AMLR.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz D. A., Garrett M. A., Craig A. H. Serum effects of mitogenic reactivity in subjects with systemic lupus erythematosus, rheumatoid arthritis and scleroderma. Technical considerations and lack of correlation with anti-lymphocyte antibodies. Clin Exp Immunol. 1977 Jan;27(1):100–110. [PMC free article] [PubMed] [Google Scholar]
- Howie J. B., Helyer B. J. The immunology and pathology of NZB mice. Adv Immunol. 1968;9:215–266. doi: 10.1016/s0065-2776(08)60444-7. [DOI] [PubMed] [Google Scholar]
- Innes J. B., Kuntz M. M., Kim Y. T., Weksler M. E. Induction of suppressor activity in the autologous mixed lymphocyte reaction and in cultures with concanavalin A. J Clin Invest. 1979 Dec;64(6):1608–1613. doi: 10.1172/JCI109622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen L. W., Krakauer R. S., Steinberg A. D. Selective loss of suppressor cell function in New Zealand mice induced by NTA. J Immunol. 1977 Sep;119(3):830–830. [PubMed] [Google Scholar]
- Knight R. A., Fitzharris P. Separation of spontaneous-killing effector populations by target preference. Br J Cancer. 1980 Aug;42(2):243–251. doi: 10.1038/bjc.1980.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J., Barnett E. V., MacDonald N. S., Klinenberg J. R. Altered immunoglobulin metabolism in systemic lupus erythematosus and heumatoid arthritis. J Clin Invest. 1970 Apr;49(4):708–715. doi: 10.1172/JCI106283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opelz G., Kiuchi M., Takasugi M., Terasaki P. I. Autologous stimulation of human lymphocyte subpopulation. J Exp Med. 1975 Nov 1;142(5):1327–1333. doi: 10.1084/jem.142.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton T. A., Crowther M. E., Hay F. C., Nineham L. J. Immune complexes in ovarian cancer. Lancet. 1978 Jul 8;2(8080):72–73. doi: 10.1016/s0140-6736(78)91383-1. [DOI] [PubMed] [Google Scholar]
- Revillard J. P., Vincent C., Rivera S. Anti-beta2-microglobulin lymphocytotoxic autoantibodies in systemic lupus erythematosus. J Immunol. 1979 Feb;122(2):614–618. [PubMed] [Google Scholar]
- Sakane T., Green I. Specificity and suppressor function of human T cells responsive to autologous non-T cells. J Immunol. 1979 Aug;123(2):584–589. [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Failure of autologous mixed lymphocyte reactions between T and non-T cells in patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3464–3468. doi: 10.1073/pnas.75.7.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Studies of immune functions of patients with systemic lupus erythematosus. I. Dysfunction of suppressor T-cell activity related to impaired generation of, rather than response to, suppressor cells. Arthritis Rheum. 1978 Jul-Aug;21(6):657–664. doi: 10.1002/art.1780210608. [DOI] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Studies of immune functions of patients with systemic lupus erythematosus. I. Dysfunction of suppressor T-cell activity related to impaired generation of, rather than response to, suppressor cells. Arthritis Rheum. 1978 Jul-Aug;21(6):657–664. doi: 10.1002/art.1780210608. [DOI] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Reeves J. P., Green I. Studies of immune functions of patients with systemic lupus erythematosus. Complement-dependent immunoglobulin M anti-thymus-derived cell antibodies preferentially inactivate suppressor cells. J Clin Invest. 1979 May;63(5):954–965. doi: 10.1172/JCI109396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Reeves J. P., Green I. Studies of immune functions of patients with systemic lupus erythematosus. T-cell subsets and antibodies to T-cell subsets. J Clin Invest. 1979 Nov;64(5):1260–1269. doi: 10.1172/JCI109581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Reeves J. P., Green I. Studies of immune functions of patients with systemic lupus erythematosus. T-cell subsets and antibodies to T-cell subsets. J Clin Invest. 1979 Nov;64(5):1260–1269. doi: 10.1172/JCI109581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopelitis E., Biundo J. J., Jr, Alspaugh M. A. Anti-SS-A antibody and other antinuclear antibodies in systemic lupus erythematosus. Arthritis Rheum. 1980 Mar;23(3):287–293. doi: 10.1002/art.1780230304. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Knowlton R. P. Activation of suppressor T cells in human autologous mixed lymphocyte culture. J Immunol. 1979 Jul;123(1):419–422. [PubMed] [Google Scholar]
- Taurog J. D., Raveche E. S., Smathers P. A., Glimcher L. H., Huston D. P., Hansen C. T., Steinberg A. D. T cell abnormalities in NZB mice occur independently of autoantibody production. J Exp Med. 1981 Feb 1;153(2):221–234. doi: 10.1084/jem.153.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Wilson C. B., Bokisch V. A., Dixon F. J. Binding of soluble immune complexes to human lymphoblastoid cells. II. Use of Raji cells to detect circulating immune complexes in animal and human sera. J Exp Med. 1974 Nov 1;140(5):1230–1244. doi: 10.1084/jem.140.5.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Wilson C. B., Dixon F. J. The Raji cell radioimmune assay for detecting immune complexes in human sera. J Clin Invest. 1976 Jan;57(1):169–182. doi: 10.1172/JCI108257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan J. H., Chihara T. Lymphocyte function in rheumatic disorders. Arch Intern Med. 1975 Oct;135(10):1324–1328. [PubMed] [Google Scholar]


