Abstract
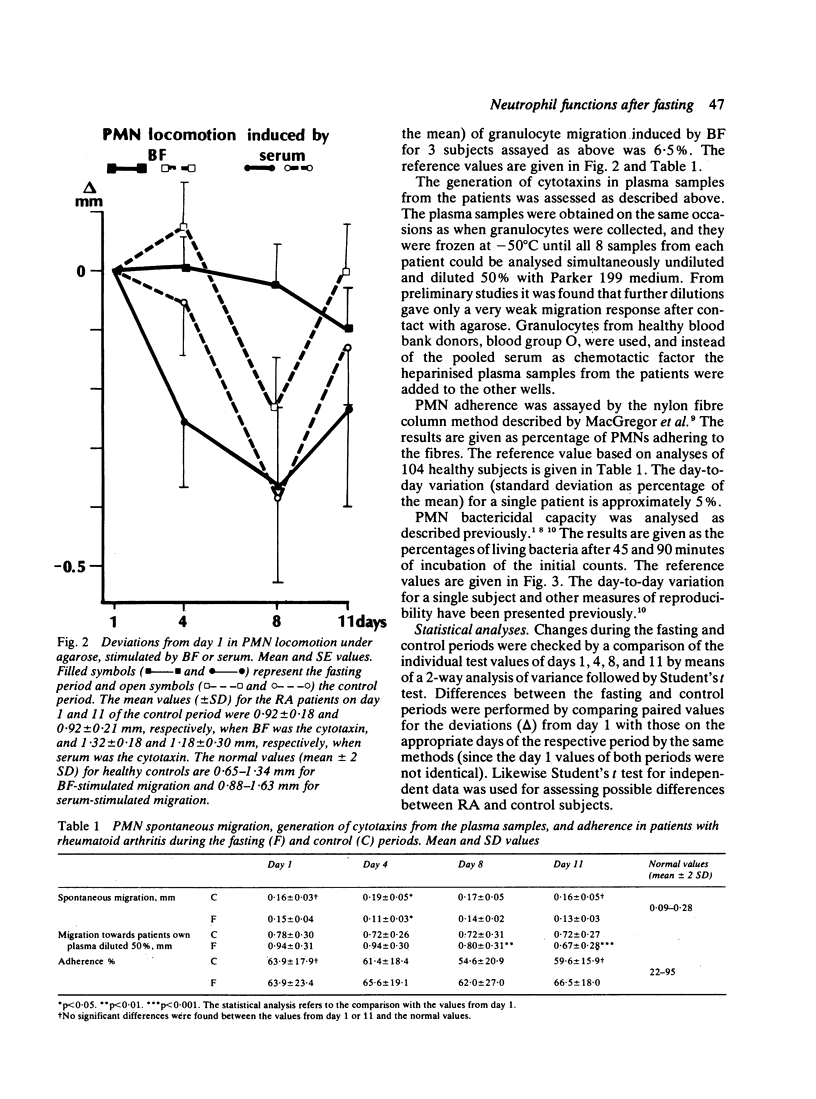
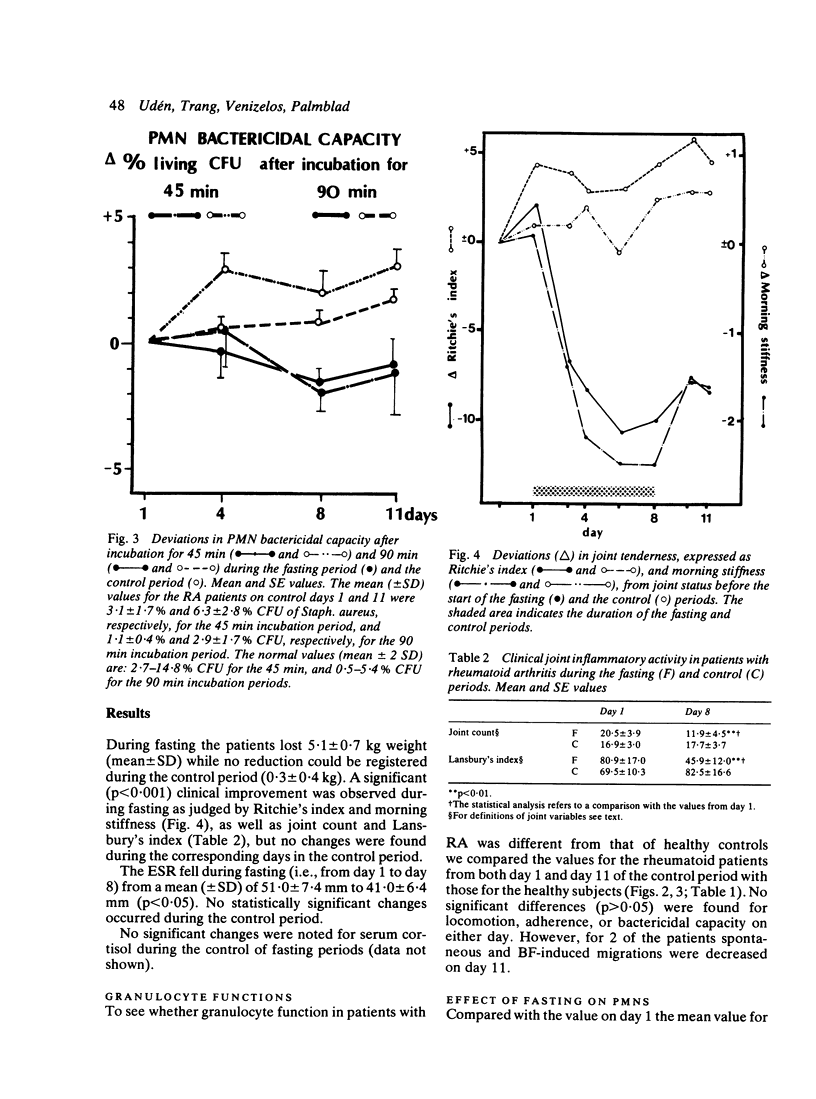
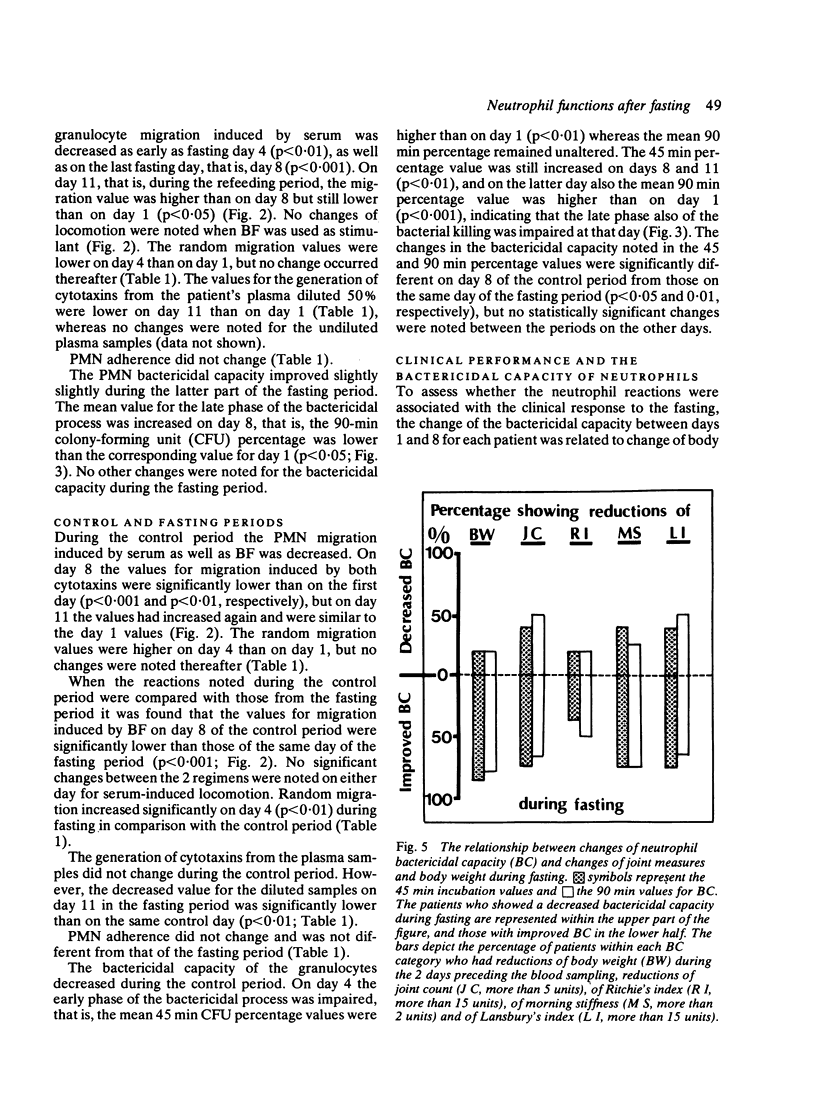
The effects of fasting for 7 days were investigated in 13 patients with rheumatoid arthritis (RA) in comparison with a control regimen in a cross-over trial. The effects of fasting on clinical performance and blood neutrophil functions were studied. During fasting, with a mean weight loss of 5.1 kg, clinical inflammation in the joints and the erythrocyte sedimentation rate (ESR) decreased. During the control period the joints either remained unchanged or deteriorated, and no change was observed in the body weight or the ESR. The locomotion of neutrophils under agarose, induced by a reference serum, decreased during the fasting period (p less than 0.001), but no change in their locomotion was induced by an Escherichia coli bacterial factor. During the control period, however, the locomotion induced by either stimulant was significantly decreased. Generation of migration-stimulating factors from the patients' plasma declined 3 days after the end of fasting (p less than 0.001). The adherence of the neutrophils to nylon fibres was unchanged during both periods. The bactericidal capacity improved during fasting, both in comparison with the initial value (p less than 0.005) and with the values from the control period (p less than 0.001). An association was found between improvement in inflammatory activity of the joints and enhancement of neutrophil bactericidal capacity. Fasting appears to improve the clinical status of patients with RA. This could partly be due to the observed changes in the functions of the neutrophils, since the latter contribute to the inflammatory joint reactions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afzelius B. A., Ewetz L., Palmblad J., Udén A. M., Venizelos N. Structure and function of neutrophil leukocytes from patients with the immotile-cilia syndrome. Acta Med Scand. 1980;208(3):145–154. doi: 10.1111/j.0954-6820.1980.tb01169.x. [DOI] [PubMed] [Google Scholar]
- Brandt L., Hedberg H. Impaired phagocytosis by peripheral blood granulocytes in systemic lupus erythematosus. Scand J Haematol. 1969;6(5):348–353. doi: 10.1111/j.1600-0609.1969.tb02420.x. [DOI] [PubMed] [Google Scholar]
- Camussi G., Emanuelli G., Ragni R. Interaction between immunocomplexes (ICs) and polymorphonuclear neutrophils (PMNs). Panminerva Med. 1978 Apr-Jun;20(2):103–105. [PubMed] [Google Scholar]
- Corberand J., Amigues H., de Larrard B., Pradere J. Neutrophil function in rheumatoid arthritis. Scand J Rheumatol. 1977;6(1):49–52. [PubMed] [Google Scholar]
- El-Ghobarey A. F., Mavrikakis M. E., Macleod M., Reynolds P. M., Capell H. A., Spencer D. G., Balint G., Mathieu J. P., McAllister T., Cooney A. Clinical and laboratory studies of levamisole in patients with rheumatoid arthritis. Q J Med. 1978 Jul;47(187):385–400. [PubMed] [Google Scholar]
- Holm G. Acute energy deprivation in man: effect on cell-mediated immunological reactions. Clin Exp Immunol. 1976 Aug;25(2):207–211. [PMC free article] [PubMed] [Google Scholar]
- Hällgren R., Håkansson L., Venge P. Kinetic studies of phagocytosis. I. The serum independent particle uptake by PMN from patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum. 1978 Jan-Feb;21(1):107–113. doi: 10.1002/art.1780210117. [DOI] [PubMed] [Google Scholar]
- Issekutz A. C., Lee K. Y., Biggar W. D. Neutrophil chemotaxis in two patients with recurrent staphylococcal skin infections and hyperimmunoglobulin E. J Lab Clin Med. 1978 Oct;92(4):640–647. [PubMed] [Google Scholar]
- Kemp A. S., Roberts-Thomson P., Neoh S. H., Brown S. Inhibition of neutrophil migration by sera from patients with rheumatoid arthritis. Clin Exp Immunol. 1979 Jun;36(3):423–429. [PMC free article] [PubMed] [Google Scholar]
- LANSBURY J. Report of a three-year study on the systemic and articular indexes in rheumatoid arthritis; theoretic and clinical considerations. Arthritis Rheum. 1958 Dec;1(6):505–522. doi: 10.1002/art.1780010604. [DOI] [PubMed] [Google Scholar]
- MacGregor R. R., Spagnuolo P. J., Lentnek A. L. Inhibition of granulocyte adherence by ethanol, prednisone, and aspirin, measured with an assay system. N Engl J Med. 1974 Sep 26;291(13):642–646. doi: 10.1056/NEJM197409262911302. [DOI] [PubMed] [Google Scholar]
- Mowat A. G., Baum J. Chemotaxis of polymorphonuclear leukocytes from patients with rheumatoid arthritis. J Clin Invest. 1971 Dec;50(12):2541–2549. doi: 10.1172/JCI106754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmblad J., Cantell K., Holm G., Norberg R., Strander H., Sunblad L. Acute energy deprivation in man: effect on serum immunoglobulins antibody response, complement factors 3 and 4, acute phase reactants and interferon-producing capacity of blood lymphocytes. Clin Exp Immunol. 1977 Oct;30(1):50–55. [PMC free article] [PubMed] [Google Scholar]
- Palmblad J., Engstedt L. Activation of the bactericidal capacity of blood granulocytes. Evaluation of a new method and the effect of levamisole. Acta Pathol Microbiol Scand C. 1979 Dec;87(6):357–364. [PubMed] [Google Scholar]
- Palmblad J. Fasting (acute energy deprivation) in man: effect on polymorphonuclear granulocyte functions, plasma iron and serum transferrin. Scand J Haematol. 1976 Sep;17(3):217–226. doi: 10.1111/j.1600-0609.1976.tb01178.x. [DOI] [PubMed] [Google Scholar]
- Palmblad J., Fohlin L., Lundström M. Anorexia nervosa and polymorphonuclear (PMN) granulocyte reactions. Scand J Haematol. 1977 Oct;19(4):334–342. doi: 10.1111/j.1600-0609.1977.tb01483.x. [DOI] [PubMed] [Google Scholar]
- Palmblad J., Levi L., Burger A., Melander A., Westgren U., von Schenck H., Skude G. Effects of total energy withdrawal (fasting) on thelevels of growth hormone, thyrotropin, cortisol, adrenaline, noradrenaline, T4, T3, and rT3 in healthy males. Acta Med Scand. 1977 Jan;201(1-2):15–22. doi: 10.1111/j.0954-6820.1977.tb15648.x. [DOI] [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Ritchie D. M., Boyle J. A., McInnes J. M., Jasani M. K., Dalakos T. G., Grieveson P., Buchanan W. W. Clinical studies with an articular index for the assessment of joint tenderness in patients with rheumatoid arthritis. Q J Med. 1968 Jul;37(147):393–406. [PubMed] [Google Scholar]
- Roberts-Thomson P. J., Hazleman B. L., Barnett I. G., MacLennan I. C., Mowat A. G. Factors relating to circulating immune complexes in rheumatoid arthirits. Ann Rheum Dis. 1976 Aug;35(4):314–320. doi: 10.1136/ard.35.4.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showell H. J., Williams D., Becker E. L., Naccache P. H., Sha'afi R. Desensitization and deactivation of the secretory responsiveness of rabbit neutrophils induced by the chemotactic peptide, formyl-methionyl-leucyl-phenylalanine. J Reticuloendothel Soc. 1979 Feb;25(2):139–150. [PubMed] [Google Scholar]
- Spilberg I., Mandell B., Hoffstein S. A proposed model for chemotactic deactivation: evidence for microtubule modulation of polymorphonuclear leukocyte chemotaxis. J Lab Clin Med. 1979 Aug;94(2):361–369. [PubMed] [Google Scholar]
- Walker J. R., Smith M. J., James D. W. A comparison of two in vitro methods for studying a defect in leucocyte movement in rheumatoid arthritis. Int Arch Allergy Appl Immunol. 1979;59(3):343–348. doi: 10.1159/000232279. [DOI] [PubMed] [Google Scholar]


