Abstract
We report a high rate of seropositivity against SARS-CoV-2 in wild felines in India. Seropositivity was determined by microneutralization and plaque reduction neutralization assays in captive Asiatic lions, leopards, and Bengal tigers. The rate of seropositivity was positively correlated with that of the incidence in humans, suggesting the occurrence of large spillover events.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00705-023-05735-4.
A novel coronavirus emerged in Wuhan province of China during early 2020, which was later named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This virus went on to cause a global pandemic that lasted almost 2 years with over 530 million confirmed cases and 6 million deaths worldwide [WHO].
India experienced huge losses, with the second largest number of total cases and deaths among all the countries worldwide [WHO]. There is still a threat of emergence of another variant of the virus. SARS-CoV-2 shares 96.2% overall genome sequence identity of with a bat coronavirus (BatCoV RaTG13), indicating a possible origin from bats [1]. Although SARS-CoV-2 also shares a high degree of sequence similarity with pangolin coronavirus, phylogenetic analysis does not support the hypothesis that SARS-CoV-2 originated from pangolin coronavirus [2]. SARS-CoV-2 uses angiotensin converting enzyme 2 (ACE2) as a receptor for entry into cells, suggesting a broad host range [1, 3, 4]. The ACE2 gene is highly conserved, with a high degree of amino acid sequence similarity among different species of mammals [3]. Cat and tiger ACE2 orthologs differ from the human ACE2 by only 14.2 and 13.8%, respectively. It has also been shown that both of these orthologs can form stable interactions with the receptor-binding domain (RBD) of the spike protein of SARS-CoV-2 [4]. The first case of natural infection of a domestic cat with SARS-CoV-2 was reported in Belgium in March 2020 [5]. Since then, there have been many other reports of SARS-CoV-2 infections in domestic cats [6, 7] as well as in wild feline species throughout the world [8, 9].
India is a country with a rich natural heritage and a high level of biodiversity. The country is home to the largest number of wild feline species in the world. Due to continuous deforestation and an expanding human population, there are increasing interactions between wild animals and humans in India. With over 43 million cases of COVID-19, there are many opportunities for spillover of SARS-CoV-2 from humans to animals. Despite this, only four incidences of natural infection of Asiatic lions (Panthera leo persica) with SARS-CoV-2 have been documented, and all of them were in captive animals in zoos or parks [10, 11]. Only one incidence was reported in a free-ranging leopard (Panthera pardus fusca) in India [12]. The low incidence in wild and domestic felines could be due to limited testing and the lack of major clinical signs [13], causing infections to go unnoticed.
Here, we performed a systematic study to investigate the serological prevalence of SARS-CoV-2 in wild feline species in India. The three most common wild feline species of India – Asiatic lions, Bengal tigers, and leopards – were studied. As per the most recent census, the total population of Asiatic lions, Bengal tigers, and leopards in the country is 674, 2,967, and 12,852, respectively.
Sampling was conducted between March 2020 and October 2021. Samples were collected from wildlife sanctuaries, zoos, and national parks covering eight states of the country and submitted to the Center for Wildlife Conservation Management and Disease Surveillance, ICAR-Indian Veterinary Research Institute, Izatnagar UP India. Serum samples (n = 320) were collected and submitted in duplicate. These samples included 126 from leopards, 96 from Asiatic lions, and 98 from Bengal tigers. In addition, serum samples from leopards (n = 30) that were collected before the COVID-19 pandemic were used as negative controls. Serum samples collected from domestic cats (n = 21) with confirmed feline infectious peritonitis infection were also included in the panel to exclude the possibility of cross-neutralization. Similarly, 40 samples from members of the family Cervidae (spotted deer [Axis axis] and swamp deer [Rucervus duvaucelii]) and 24 samples from Indian elephants (Elephas indicus maximus) that were collected during the pandemic were also included in this study.
All of the samples were processed in the biosafety level 3 facility of the ICAR-Indian Veterinary Research Institute, following institute biosafety guidelines, and they were handled by trained personnel only.
A wild-type virus isolate of SARS-CoV-2 (SARS-CoV-2/human/IND/CAD1339/2020) (GenBank accession number MZ203529) was propagated in Vero cells. Clarified cell culture supernatant was titrated to determine the 50% tissue culture infective dose (TCID50).
A microneutralization assay was performed as described previously [14, 15]. Briefly, serum samples were heat-inactivated at 56°C for 30 minutes, followed by serial twofold dilution (starting from 1:16) in Dulbecco’s modified Eagle medium containing 2% fetal bovine serum. Single-use vials of the virus were thawed, and the dilutions were then incubated with an equal volume of 100 TCID50 SARS-CoV-2 for 1 h at 37°C. One hundred µL of this virus-antibody mixture was then transferred in triplicate to 96-well cell culture plates containing 2 × 104 Vero cells per well. The plates were then incubated at 37°C with 5% CO2 for 72 hours. The following controls were also included in the test: (1) back-titration (virus + cells), (2) uninoculated cells, and (3) negative control (samples collected before the pandemic). The plates were observed daily for 3 days for the appearance of a cytopathic effect (CPE) (Supplementary Fig. S1), and the endpoint titer was calculated using the Reed and Muench method [16]. The highest serum dilution that could protect > 90% of the cells from CPE was considered the neutralizing titer. The titer was expressed as the log of the reciprocal of the dilution in the range of < 1.5, 1.8, 2.1, and > 2.4.
All of the samples that gave a positive result in the microneutralization test were tested further using a plaque-reduction neutralization test (PRNT) as described previously [17]. The assay was performed in duplicate using 24-well tissue culture plates. A serial dilution of each serum was incubated with 30–40 plaque-forming units of the virus for 1 h at 37°C. The virus-serum mixtures were then added to a pre-formed Vero cell monolayer (maintained in DMEM with 2% FBS) and incubated for 1 h at 37°C in a 5% CO2 incubator. The cell monolayer was then overlaid with 1% agar in cell culture medium and incubated for 72 hours, after which the plates were fixed and stained as described previously [17]. Antibody titers were defined as the highest serum dilution that resulted in ≥ 90% (PRNT90) reduction in the number of virus plaques.
In total, 48 out of 320 serum samples (15% with CI 11.09–18.91% at 95% accuracy level) showed the presence of SARS-CoV-2 neutralizing antibodies with titers ranging from 2.1 to > 2.4 (Supplementary Table S1). Out of these 48 samples, 24 were from lions, 14 samples were from tigers, and 10 were from leopards (Table 1). Two lions from Etawah Lion Safari, Uttar Pradesh, India, that were confirmed to be positive for SARS-CoV-2 by RT-PCR on 30 April 2021 [10] showed decreasing titers of neutralizing antibodies in consecutive months (> 2.4 in May 2021, 2.1 in June 2021, and 1.8 in July 2021). Similarly, another three samples from lions showed titres of > 2.4 in July 2021 and 2.1 in August 2021. All 48 seropositive samples showed plaque reduction by a factor of > 90% when using 1:128-diluted serum in the PRNT assay, confirming the specificity of the antibodies against SARS-CoV-2.
Table 1.
Number of serum samples collected each month from wild feline species from March 2020 to October 2021 and the number positive in the microneutralization assay. The seropositivity was rate was the highest in Asiatic lions.
| Species | Leopard | Lion | Tiger | Total samples tested | Total positive | |||
|---|---|---|---|---|---|---|---|---|
| Month | Samples tested | Positive | Samples tested | Positive | Samples tested | Positive | ||
| Mar-20 | 4 | 0 | 3 | 0 | 4 | 0 | 11 | 0 |
| Apr-20 | 3 | 0 | 4 | 0 | 3 | 0 | 10 | 0 |
| May-20 | 4 | 0 | 5 | 0 | 4 | 0 | 13 | 0 |
| Jun-20 | 4 | 0 | 3 | 0 | 4 | 0 | 11 | 0 |
| Jul-20 | 5 | 0 | 0 | 0 | 0 | 0 | 5 | 0 |
| Aug-20 | 6 | 0 | 6 | 0 | 5 | 0 | 17 | 0 |
| Sep-20 | 7 | 0 | 4 | 0 | 6 | 0 | 17 | 0 |
| Oct-20 | 7 | 2 | 4 | 2 | 6 | 0 | 21 | 4 |
| Nov-20 | 8 | 0 | 4 | 2 | 5 | 0 | 19 | 2 |
| Dec-20 | 6 | 0 | 5 | 0 | 3 | 0 | 14 | 0 |
| Jan-21 | 6 | 0 | 6 | 0 | 4 | 0 | 16 | 0 |
| Feb-21 | 4 | 0 | 6 | 0 | 6 | 0 | 16 | 0 |
| Mar-21 | 9 | 2 | 4 | 4 | 6 | 2 | 25 | 8 |
| Apr-21 | 9 | 1 | 4 | 3 | 6 | 0 | 23 | 4 |
| May-21 | 9 | 0 | 7 | 2 | 5 | 0 | 23 | 2 |
| Jun-21 | 8 | 2 | 6 | 2 | 8 | 1 | 26 | 5 |
| Jul-21 | 8 | 2 | 6 | 4 | 6 | 4 | 26 | 10 |
| Aug-21 | 9 | 1 | 8 | 5 | 7 | 7 | 30 | 13 |
| Sep-21 | 4 | 0 | 6 | 0 | 4 | 0 | 14 | 0 |
| Oct-21 | 6 | 0 | 5 | 0 | 6 | 0 | 17 | 0 |
| Total | 126 | 10 | 96 | 24 | 98 | 14 | 320 | 48 |
Serum samples collected from domestic cats that were confirmed to be infected with feline coronavirus did not show the presence of neutralizing antibodies against SARS-CoV-2, indicating that there was no cross-neutralization reaction with SARS-CoV-2. All of the samples collected from leopards before the pandemic tested negative for neutralizing antibodies. Similarly, samples collected from spotted deer, swamp deer, and Indian elephants during the pandemic were also negative.
Pet cats have been shown to be more susceptible to natural SARS-CoV-2 infection than other pet animals, but they exhibit milder clinical signs [18]. Malayan tigers and African lions that tested positive for SARS-CoV-2 in the Bronx Zoo, New York City, USA, showed respiratory signs including dry cough and inappetence [19]. An experimental infection study showed that cats are highly susceptible to this virus and have a long period of viral shedding, which increases the likelihood of direct-contact transmission of infection to other cats [13]. The same study also showed that cats are usually asymptomatic during the infection. These reports indicate a high probability of asymptomatic infections of feline species, which were not reported at that time. Seroprevalence studies have the advantage of detecting infections even when the virus is no longer present.
Interactions between humans and wildlife are especially frequent in India. Although the number of COVID-19 cases reported in wild animals was very low, the high seropositivity observed in wild felines (15%) indicates that spillover infections from humans to wildlife have gone undetected.
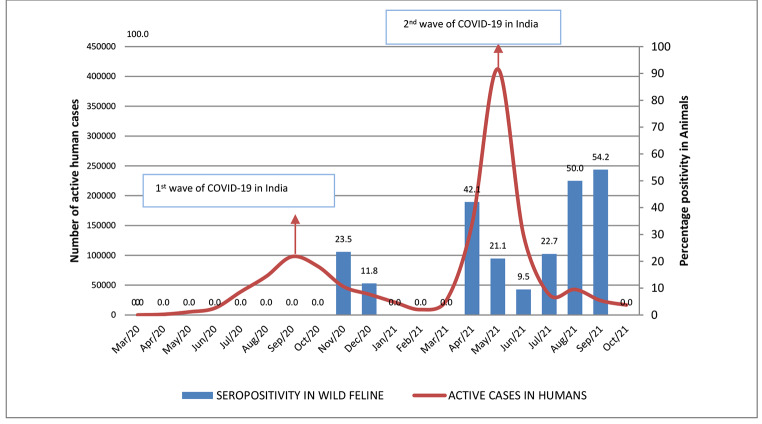
A high degree of association was observed between the temporal distribution of SARS-CoV-2 infections in humans in India and that of seropositivity in wild felines (Fig. 1). After the first wave of COVID-19, which peaked in the months of Aug-Sep 2020, two peaks of seropositivity in animals were observed in the months of Oct-Nov-2020 and March-April 2021. A very large second wave in humans was observed during the months of May and June 2021, followed by a seropositivity peak in animals in the months of July and August 2021 (Fig. 1). Retrospectively, the first case of infection in our study dates back to October 2020, after the number of COVID 19 cases in humans peaked during the first wave in September 2020 (Fig. 1).
Fig. 1.
Temporal distribution of active human cases of COVID19 in comparison to the estimated seropositivity in wild felines
This retrospective study provides new information about the seropositivity of wild felines against SARS-CoV-2. Whether all of the positive animals acquired the infection from contact with infected humans cannot be determined. Collection of samples from free-ranging wild felines is difficult, and samples were therefore collected from captive animals in zoos, national parks, and wild safari parks. The probability of transmission of virus through contact with infected human handlers is very high in captive animals, and transmission from human was confirmed in the Bronx Zoo, New York City, USA [19]. A second route of infection is through transmission from animal to animal. Although animals are kept in isolation in captivity, especially leopards and tigers, which are solitary in nature, in contrast to lions, which are known to stay in groups, they still have a certain degree of freedom of movement, which may result in animal-to-animal transmission. Although domestic cats have been shown to transmit the virus via droplets to other cats [13, 20], it is not known whether the same applies to wild feline species. Visitors might have played a role in the spillover incidents, but during the period of sample collection, most of the facilities were closed to visitors due to the nationwide lockdown.
In one study, a seropositivity rate of 14.7% (15/102) was estimated in domestic cats in Wuhan province of China during the active phase of the outbreak [21]. Similarly, a study in France showed that about 23% of domestic cats had antibodies against SARS-CoV-2, suggesting a high frequency of human-to-cat transmission [22]. In another study in Istanbul, Turkey, a seropositivity rate of 21.9% (34/155) was seen in the domestic cat population [23]. However, in a large-scale serological survey conducted in Germany, only 0.69% (6/920) of cats showed seropositivity. However, during the time period in which the samples were collected, the incidence of COVID-19 cases in Germany was also rather low (0.85%) [24].
Although the very few samples from deer species (n = 40) that were tested in this study were collected from only one location at only one time point, it is nevertheless interesting that no seropositivity was observed despite the fact that SARS-CoV-2 has been detected in free-ranging white-tailed deer (Odocoileus virginianus) in the USA [25] and a high seropositivity rate of 40% was reported [26]. Antibodies against SARS-CoV-2 persist for only a very short time [27, 28], and therefore, the time point of collection of samples is very important. In this study, neutralizing antibody titers were used to estimate seroprevalence, but, as mentioned above, the rate of decay of neutralizing antibodies is higher than that for total antibody titers against the S or N proteins. Hence, the positivity rate in this study might have been higher if a different assay had been used. In addition, other species might have also been positive for SARS-CoV-2 if total antibody titers had been measured.
So far, there have been no reports of animal-to-human transmission, but there is always this risk, as animals exhibit prolonged oral and nasal shedding of the virus [29]. In the future, if another such virus emerges, some preventive measures should be followed, such a suitable distance between infected humans and the animals, in order to protect these valuable animals. In conclusion, our study provides evidence of SARS-CoV-2 infection in wild feline species in India. Further research is required to understand the dynamics of transmission of the virus between humans and big cats.
Electronic Supplementary Material
Below is the link to the electronic supplementary material
Acknowledgments
The authors are thankful to all of the officials of the various Forest Divisions for providing the samples used in this study.
Author Contribution
RB, SKN, HRJ, SM, SN, RM: performed virus isolation, microneutralization, and plaque assay. KM, KS, SKB, VC, AP: collected the samples and prepared the figures. GS, KPS, GKS: designed the study, analysed the data, and wrote the manuscript.
Funding
Funds for this study were provided by the ICAR - National Agricultural Science Fund (NASF) and Department of Science and Technology-SERB.
Data Availability
The primary data will be provided upon request.
Declarations
Conflict of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu P, Jiang JZ, Wan XF, Hua Y, Li L, Zhou J, Wang X, Hou F, Chen J, Zou J, Chen J. Are pangolins the intermediate host of the 2019 novel coronavirus (SARS-CoV-2)? PLoS Pathog. 2020;16(5):e1008421. doi: 10.1371/journal.ppat.1008421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam SD, Bordin N, Waman VP, Scholes HM, Ashford P, Sen N, van Dorp L, Rauer C, Dawson NL, Pang CS, Abbasian M (2020) SARS-CoV-2 spike protein predicted to form stable complexes with host receptor protein orthologues from mammals, but not fish, birds or reptiles [DOI] [PMC free article] [PubMed]
- 4.Rendon-Marin S, Martinez-Gutierrez M, Whittaker GR, Jaimes JA, Ruiz-Saenz J. SARS CoV-2 Spike Protein in silico Interaction With ACE2 Receptors From Wild and Domestic Species. Front Genet. 2021;12:27. doi: 10.3389/fgene.2021.571707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garigliany M, Van Laere AS, Clercx C, Giet D, Escriou N, Huon C, van der Werf S, Eloit M, Desmecht D. SARS-CoV-2 Natural Transmission from Human to Cat, Belgium, March 2020. Emerg Infect Dis. 2020;26(12):3069–3071. doi: 10.3201/eid2612.202223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sailleau C, Dumarest M, Vanhomwegen J, Delaplace M, Caro V, Kwasiborski A, Le Poder S. First detection and genome sequencing of SARS-CoV‐2 in an infected cat in France. Transbound Emerg Dis. 2020;67(6):2324–2328. doi: 10.1111/tbed.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newman A, Smith D, Ghai RR, Wallace RM, Torchetti MK, Loiacono C, Behravesh CB. First reported cases of SARS-CoV-2 infection in companion animals—New York, March–April 2020. Morb Mortal Wkly Rep. 2020;69(23):710. doi: 10.15585/mmwr.mm6923e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAloose D, Laverack M, Wang L, Killian ML, Caserta LC, Yuan F, Diel DG. From people to Panthera: Natural SARS-CoV-2 infection in tigers and lions at the Bronx Zoo. MBio. 2020;11(5):e02220–e02220. doi: 10.1128/mBio.02220-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartlett SL, Diel DG, Wang L, Zec S, Laverack M, Martins M, Calle PP. SARS-CoV-2 infection and longitudinal fecal screening in Malayan tigers (Panthera tigrisjacksoni), Amur tigers (Panthera tigrisaltaica), and African lions (Panthera leokrugeri) at the Bronx Zoo, New York, USA. J Zoo Wildl Med. 2021;51(4):733–744. doi: 10.1638/2020-0171. [DOI] [PubMed] [Google Scholar]
- 10.Karikalan M, Chander V, Mahajan S, Deol P, Agrawal RK, Nandi S, Sharma GK (2021) Natural infection of Delta mutant of SARS-CoV‐2 in Asiatic lions of India. Transboundary and emerging diseases [DOI] [PMC free article] [PubMed]
- 11.Mishra A, Kumar N, Bhatia S, Aasdev A, Kanniappan S, Sekhar AT, Singh VP. Sars-cov-2 delta variant among asiatic lions, india. Emerg Infect Dis. 2021;27(10):2723. doi: 10.3201/eid2710.211500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahajan S, Karikalan M, Chander V, Pawde AM, Saikumar G, Semmaran M, Lakshmi PS, Sharma M, Nandi S, Singh KP, Gupta VK, Singh RK, Sharma GK. Detection of SARS-CoV-2 in a free ranging leopard (Panthera pardus fusca) in India. Eur J Wildl Res. 2022;68(5):59. doi: 10.1007/s10344-022-01608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosco-Lauth AM, Hartwig AE, Porter SM, Gordy PW, Nehring M, Byas AD, Bowen RA (2020) Experimental infection of domestic dogs and cats with SARS-CoV-2: Pathogenesis, transmission, and response to reexposure in cats. Proceedings of the National Academy of Sciences, 117(42), 26382–26388 [DOI] [PMC free article] [PubMed]
- 14.Cerutti H, Bandini T, Castria M, Cartocci A, Ricci V, Tornesi S, Brogi A. A quantitative assay for detection of SARS-CoV-2 neutralizing antibodies. J Clin Virol. 2022;147:105064. doi: 10.1016/j.jcv.2021.105064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolscheid-Pommerich R, Bartok E, Renn M, Kümmerer BM, Schulte B, Schmithausen RM, Hartmann G. Correlation between a quantitative anti‐SARS‐CoV‐2 IgG ELISA and neutralization activity. J Med Virol. 2022;94(1):388–392. doi: 10.1002/jmv.27287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed LJ, Muench H (May 1938) A simple method of estimating fifty per cent endpoints. Am J Epidemiol 27(3):493–497. 10.1093/oxfordjournals.aje.a118408
- 17.Lau EH, Tsang OT, Hui DS, Kwan MY, Chan WH, Chiu SS, Peiris M. Neutralizing antibody titres in SARS-CoV-2 infections. Nat Commun. 2021;12(1):1–7. doi: 10.1038/s41467-020-20247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdel-Moneim AS, Abdelwhab EM. Evidence for SARS-CoV-2 infection of animal hosts. Pathogens. 2020;9(7):529. doi: 10.3390/pathogens9070529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.APHIS. USDA Statement on the Confirmation of COVID-19 in a Tiger in New York. Available online: https://www.aphis.usda.gov/aphis/newsroom/news/sa_by_date/sa-2020/NY-zoo-covid-19
- 20.Mallapaty S (2020) Coronavirus can infect cats–dogs, not so much. Nature. [DOI] [PubMed]
- 21.Zhang Q, Zhang H, Gao J, Huang K, Yang Y, Hui X, Jin M. A serological survey of SARS-CoV-2 in cat in Wuhan. Emerg microbes infections. 2020;9(1):2013–2019. doi: 10.1080/22221751.2020.1817796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fritz M, Rosolen B, Krafft E, Becquart P, Elguero E, Vratskikh O, Leroy EM. High prevalence of SARS-CoV-2 antibodies in pets from COVID-19 + households. One Health. 2020;11:100192. doi: 10.1016/j.onehlt.2020.100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yilmaz A, Kayar A, Turan N, Iskefli O, Bayrakal A, Roman-Sosa G, Or E, Tali HE, Kocazeybek B, Karaali R, Bold D (2021) Presence of antibodies to SARS-CoV-2 in domestic cats in Istanbul, Turkey, before and after COVID-19 pandemic. Frontiers in veterinary science, 1123 [DOI] [PMC free article] [PubMed]
- 24.Michelitsch A, Hoffmann D, Wernike K, Beer M. Occurrence of antibodies against SARS-CoV-2 in the domestic cat population of Germany. Vaccines. 2020;8(4):772. doi: 10.3390/vaccines8040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hale VL, Dennis PM, McBride DS, Nolting JM, Madden C, Huey D, Bowman AS. SARS-CoV-2 infection in free-ranging white-tailed deer. Nature. 2022;602(7897):481–486. doi: 10.1038/s41586-021-04353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandler JC, Bevins SN, Ellis JW, Linder TJ, Tell RM, Jenkins-Moore M, Shriner SA (2021) SARS-CoV-2 exposure in wild white-tailed deer (Odocoileus virginianus). Proceedings of the National Academy of Sciences, 118(47) [DOI] [PMC free article] [PubMed]
- 27.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, Elliott J, Hofmann C, Hausner MA, Yang OO. Rapid decay of anti–SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. 2020;383(11):1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seow J, Graham C, Merrick B, Acors S, Pickering S, Steel KJ, Doores KJ. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5(12):1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernández-Bellon H, Rodon J, Fernández-Bastit L, Almagro V, Padilla-Solé P, Lorca-Oró C, Valle R, Roca N, Grazioli S, Trogu T, Bensaid A, Carrillo J, Izquierdo-Useros N, Blanco J, Parera M, Noguera-Julián M, Clotet B, Moreno A, Segalés J, Vergara-Alert J (2021) Monitoring Natural SARS-CoV-2 Infection in Lions (Panthera leo) at the Barcelona Zoo: Viral Dynamics and Host Responses. Viruses. 25;13(9):1683. 10.3390/v13091683 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The primary data will be provided upon request.